Abstract
Purpose:
Spinal pathology is very common with advancing age and can cause dysphagia; however, it is unclear how frequently these pathologies affect swallowing function. This study evaluates how cervical spinal pathology may impact swallowing function in dysphagic individuals observed during videofluoroscopic swallowing studies (VFSSs).
Method:
A retrospective case–control study was performed on 100 individuals with dysphagia as well as age-/gender-matched healthy controls (HCs) with available VFSS. Spinal anatomy of patients was classified into two predetermined categories, and a consensus decision of whether spinal pathology influenced swallowing physiology was made. Validated swallow metrics, including Modified Barium Swallow Impairment Profile (MBSImP) component scores, Penetration–Aspiration Scale (PAS) maximum scores, and 10-item Eating Assessment Tool (EAT-10) scores, were compared between the spine-associated dysphagia (SAD), non-SAD (NSAD), and HC groups using Kruskal–Wallis one-way analysis of variance.
Results:
Most patients with dysphagia had spinal pathology. Spinal pathology was judged to be the primary etiology of dysphagia in 16.9% of patients with abnormal spine pathology. Median EAT-10 scores were statistically different among the three groups, with the NSAD group scoring the highest and the HC group scoring the lowest. Similarly, median PAS scores were significantly different between dysphagic groups and HCs. Median MBSImP Oral Total scores were significantly different only between the NSAD group and HCs, whereas Pharyngeal Total score was not significantly different among the groups.
Conclusions:
Spinal pathology is commonly observed during VFSS and can contribute to dysphagia, resulting in worse swallowing-related outcomes when compared with HCs. Patients judged to have SAD tended to have better outcomes than patients with dysphagia from other etiologies, perhaps due to the progressive nature of spinal disease that allows for compensatory swallowing physiology over time.
Cervical degenerative bony spine disease is very common with advancing age, affecting approximately 60% of those over the age of 40 years (Boden et al., 1990; Fakhoury & Dowling, 2022) and 75%–90% of patients over 60 years of age (Matsumoto et al., 1998; Resnick, 1985; Seidler et al., 2009). Videofluoroscopic swallowing studies (VFSSs) are considered a gold standard method to evaluate oropharyngeal swallowing (Egerter et al., 2015; Lee et al., 2017) and have the benefit of direct observation of the cervical spine. Because advancing age is also a major risk factor for dysphagia, or difficulty swallowing, cervical degenerative vertebral spinal pathology is likely prevalent in patients undergoing VFSS; however, there are currently no studies examining the mean age of adults undergoing VFSS in an outpatient setting.
A proposed mechanism for degenerative cervical spine-associated dysphagia (SAD) is the mechanical compression on the posterior pharynx, leading to altered swallowing function and poor bolus clearance, which may cause bolus airway invasion and adverse health outcomes (Ladenheim & Marlowe, 1999; Papadopoulou et al., 2013; Shoffel-Havakuk et al., 2016). Seidler et al. found that C3–C4 osteophytes can restrict epiglottic mobility and more inferior cervical osteophytes can cause mechanical obstruction and limit the elevation and anterior movement of the larynx (Seidler et al., 2009). Furthermore, several authors have shown improvement of swallowing function after surgical resection of cervical osteophytes (Bakshi & Ramesh, 2021; Barker et al., 2021; Kolz et al., 2021; Ruetten et al., 2019; Vodicar et al., 2016). Along this spectrum of bony cervical spine disorders, there is also often improvement after surgery for diffuse idiopathic skeletal hyperostosis (DISH; Clark et al., 2003; Ohki, 2012; Oppenlander et al., 2009).
Anterior cervical spine surgery can result in postoperative dysphagia, with several studies demonstrating substantial pharyngeal swallowing impairment (Frempong-Boadu et al., 2002; Kang et al., 2016; Leonard & Belafsky, 2011; Ziegler et al., 2021) likely related to direct or retraction trauma to the vagus and/or glossopharyngeal nerves (Murry & Ricardo, 2006; Papadopoulou et al., 2013). Swallowing abnormalities, such as pharyngeal wall thickening and poor epiglottic inversion, can persist in some patients for several months after surgery (Leonard & Belafsky, 2011; Ziegler et al., 2021).
Although these conditions are relatively common, particularly in the aging population, there is a paucity of studies delineating the prevalence of cervical vertebral spinal pathology on VFSS. It is also unclear how frequently these spinal pathologies affect swallowing function. The purpose of this retrospective study was to evaluate how spinal pathology may impact swallowing function in dysphagic individuals undergoing VFSS. Based on these prevalence studies, we hypothesized that spinal disease would be present in the majority of the videofluoroscopic studies and may contribute to dysphagia through mechanical disruption. Furthermore, because C3–C4 is the spinal level at which epiglottic inversion takes place and C4–C6 is the level at which the pharyngoesophageal segment lies, we also hypothesized that osteophytes at these levels would impair the corresponding Modified Barium Swallow Impairment Profile (MBSImP) components, 10 (epiglottic inversion) and 14 (pharyngoesophageal segment opening [PESO]).
Method
For the purposes of this article, spinal disease or pathology refers to bony anatomical malformations such as osteophyte formation, spinal curvature abnormalities, degenerative disease, and/or prior cervical spine surgery. Institutional review board approval from the Medical University of South Carolina (Pro00112258 and Pro00011566 for controls) was ascertained before study procedures. Outpatients aged > 18 years who underwent VFSS for complaints of dysphagia were eligible for inclusion. Studies were performed in tertiary care nonhospital clinic settings. VFSSs that had been previously complete as part of clinic care were evaluated from November 13, 2020, to February 8, 2021, in the order they had been performed. For patients with multiple studies, only the initial VFSS was included. Patients were age matched (±3 years) and gender matched to HCs, and the spines of patients and controls were reviewed. Patients were excluded if there was no age-matched control available (n = 5) or if the swallow study could not be adequately performed due to patient immobility (n = 1).
Data from HCs were obtained from a larger normative dataset examining the effect of aging on oropharyngeal swallowing function, including the prevalence of airway invasion (Garand et al., 2019). A healthy participant was defined as someone without current or a history of dysphagia, upper aerodigestive tract surgical procedures, hiatal hernia, pulmonary disease, head and neck cancer, or neurological disease (e.g., stroke, Parkinson's disease). All HCs consumed a full oral diet without compensations or restrictions. If a dysphagic patient had several matched controls, a control was randomly assigned. If a control had been previously assigned to a patient, another control was selected if available. In some cases (n = 39), the same control had to be used for two or three patients.
Spine Characterization
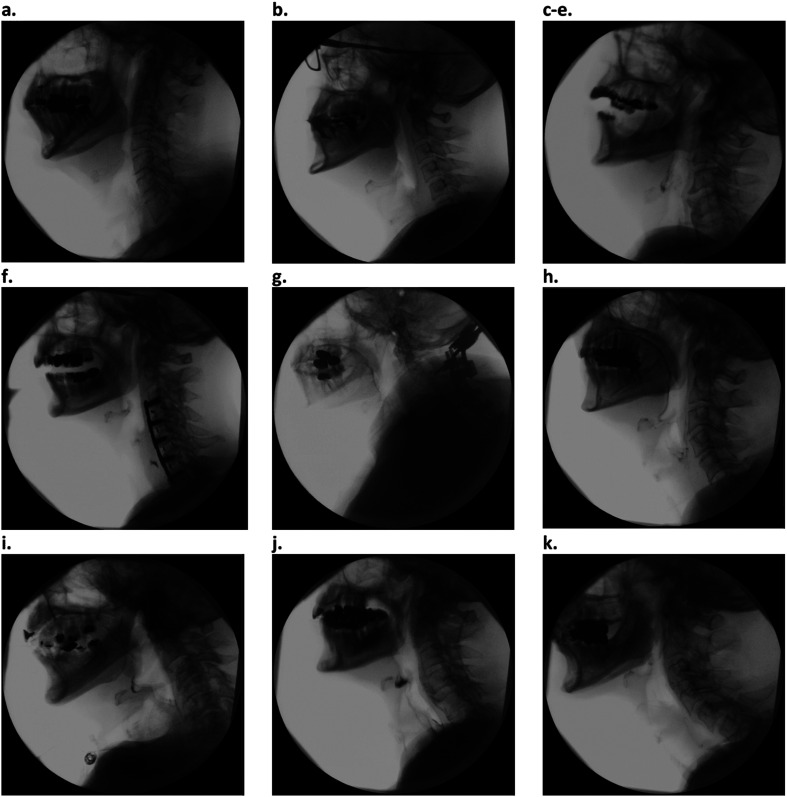
Spinal categories were predetermined and intended to be comprehensive and easily recognizable by medical professionals who many not have extensive training in spine interpretation. These categories were peer reviewed a priori by a radiologist trained in head and neck pathology (M. M.). MBSImP, Penetration–Aspiration Scale (PAS), and Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) scores were not available to the raters at the time of spinal characterization. The list of spinal categories with examples is shown in Figure 1. Spinal anatomy of dysphagic patients and controls was assessed for spinal pathology and categorized into up to two predetermined categories. Spinal categorization of each patient was performed as a group (R. G., K. D., M. C., J. B., D. S., and A. K. O.). Two categories were allowed due to the difficulty of creating discreet categories for complex diseases that can co-occur (e.g., osteophyte formation and early degenerative disc disease). The dominant two features were selected.
Figure 1.
Example images of spine disease categories. (a) Normal cervical spine. (b) Loss of normal cervical lordosis. (c) Minor/nonobstructive osteophyte. (d) Partially obstructive osteophyte. (e) Obstructive osteophyte. (f) History of anterior cervical spine surgery. (g) History of posterior cervical spine surgery. (h) Early degenerative disc disease. (i) Advanced degenerative disc disease. (j) Diffuse idiopathic skeletal hyperostosis. (k) Pronounced lordosis.
The Impact of Vertebral Spinal Pathology on Swallowing
If spinal pathology was present, the spinal level was noted, and the pathology was evaluated for its effect on swallowing function in dysphagic patients. This was determined in real time by simultaneous evaluation of, and consensus agreement between, two laryngology practitioners with extensive experience in the interpretation of videofluoroscopy (A. K. O. and D. S.) and two speech-language pathologists (K. D. and M. C.). Individuals with cervical spinal pathology without any other identifiable etiology of dysphagia upon history and clinical exam were classified as having SAD. The remaining dysphagic individuals either had normal spinal anatomy or spinal changes with another established cause of disordered swallowing on VFSS (e.g., stroke, stricture, diverticula) from the patient's medical record or were classified as non-SAD (NSAD). This classification method allowed for a stringent evaluation of the impact of spinal pathology in dysphagic patients.
Spinal Categorization Reliability
One quarter of patient spinal images, including two to three images of each spinal category, were rated independently by the laryngologist (A. K. O.) who performed the original categorization and a radiologist (M. G. M.) experienced in spine radiography. Raters were blinded to the other raters' categorization as well as the consensus categorization.
MBSImP Protocol
Participants were administered standardized consistencies of commercially prepared barium (Varibar, Bracco Diagnostics, Inc) following the MBSImP. Fluoroscopy was set to continuous, and videofluoroscopic recordings were made with a resolution of 60 fields (30 frames) per second. A medical video-recording device (TIMS DICOM System, TIMS Medical, or Kay PENTAX) was used for signal acquisition, digital storage, and retrieval of the swallowing data.
VFS Outcome Measures
Videofluoroscopic data were analyzed using the MBSImP (Martin-Harris et al., 2008), an ordinal validated rating system designed to evaluate multiple physiologic components of swallowing. Each swallow was evaluated by the consensus of two raters (K. D. and M. C. for dysphagic patients; K. D. and K. L. G. for HCs), who completed the MBSImP training (Northern Speech Services), for MBSImP, PAS, and DIGEST scoring. VFSS scoring was completed before the categorization of spinal disease; therefore, raters were blinded to final spinal disease categorization.
The presence and degree of airway invasion were measured using the maximum score of the validated 8-point ordinal PAS (Rosenbek et al., 1996). The Functional Oral Intake Scale (FOIS) was used to document the patients' oral intake level (Crary et al., 2005). The DIGEST scale was used to grade safety and efficiency of pharyngeal swallowing in dysphagic patients (Hutcheson et al., 2017). DIGEST scores were not recorded in the HC group. The 10-item Eating Assessment Tool (EAT-10) was used as a patient-reported outcome measure to capture symptoms of dysphagia (Belafsky et al., 2008).
Statistical Analysis
All statistical analyses were performed using Sigma Plot (Version 12.5) and SPSS Version 27.0.1.0 (IBM Corporation). Categorical variables (e.g., gender, race, or spinal characterizations) were summarized by frequency (n) and percentage (%). Continuous variable (age) was assessed for normality using the Shapiro–Wilk test, and ordinal variables (e.g., PAS max or MBSImP scores) were summarized by M ± SD and median and interquartile range (IQR; 25–75). Comparison of categorical variables was performed using a chi-square test or Fisher's exact test. Comparison of ordinal variables among three groups was performed with either a one-way analysis of variance or a Kruskal–Wallis test, as appropriate, with post hoc comparisons using a Dunn's test. Comparison of two groups was done using a t test or Mann–Whitney U test. Interrater reliability was determined with percentage agreement and weighted Cohen kappa (κ). The degree of interobserver agreement based on the kappa values was as follows: slight (0.01–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.81–0.99; Viera & Garrett, 2005). A p value of < .05 was considered significant for all statistical tests.
Results
A total of 100 dysphagic patients and 100 age- and gender-matched HCs who underwent VFSS were analyzed. The average age of the dysphagic patients was 67.7 years, whereas the mean age in the HCs was 66.8 years. Patients in the SAD group had an average age of 71.3 years, and those in the NSAD group had a mean age of 67.1 years. Other demographic information is presented in Table 1.
Table 1.
Patient demographics.
| Characteristics | All dysphagic patients | Spine-associated dysphagia | Non–spine-associated dysphagia | Healthy controls | p value |
|---|---|---|---|---|---|
| Total patients (N) | 100 | 15 | 85 | 100 | |
| Age (years) | .34 | ||||
| M (SD) | 67.7 (13.1) | 71.3 (7.2) | 67.1 (13.9) | 66.8 (13.0) | |
| Median (IQR) | 70.0 (63.8–76.3) | 73.0 (65.0–76.0) | 69.0 (59.0–77.0) | 70.0 (61.8–72.5) | |
| Gender | .15 | ||||
| Female | 50 | 4 | 46 | 50 | |
| Male | 50 | 11 | 39 | 50 | |
| Race | .41 | ||||
| African American | 13 | 3 | 10 | 8 | |
| Asian | 1 | 0 | 1 | 1 | |
| White | 86 | 12 | 74 | 91 | |
| Ethnicity | .92 | ||||
| Hispanic | 1 | 0 | 1 | 1 | |
| Non-Hispanic | 99 | 15 | 84 | 99 |
Note. IQR = interquartile range.
Spinal Characterization
Abnormal spinal anatomy was observed in 89 of the 100 dysphagic individuals and 84 of the 100 matched HCs. Spine disease was identified as impacting swallowing for 36 individuals. Spine disease was determined to be the sole etiology of dysphagia in 15 individuals (SAD group; see Table 2). These individuals were classified as SAD, whereas the remaining patients had one or more non–spine-related causes of dysphagia and were classified as having NSAD. None of the HCs were judged to have spinal pathology significantly impacting swallowing function, as all HCs consumed a full oral diet without compensation and denied current or past dysphagia, consistent with FOIS Level 7 (Crary et al., 2005).
Table 2.
Spine-associated and non–spine-associated etiologies for altered swallowing in dysphagic patients.
| Category | n |
|---|---|
| Spine-associated dysphagia etiologies | 15 patients |
| Osteophytes | 6 |
| Anterior cervical discectomy and fusion surgery | 6 |
| Advanced degenerative disc disease | 4 |
| Diffuse idiopathic skeletal hyperostosis | 3 |
| Kyphosis | 2 |
| Pronounced lordosis | 1 |
| Non–spine-associated dysphagia etiologies | 85 patients |
| Oropharyngeal dysphagia | 48 |
| Neurogenic (e.g., amyotrophic lateral sclerosis, Parkinson's, and stroke) | 23 |
| Structural (e.g., anterior cervical web, Killian–Jameson diverticulum, and cricopharyngeal dysfunction) | 17 |
| Myopathic (connective tissue disease) | 1 |
| Oropharyngeal dysfunction (e.g., UES restriction, delayed initiation, reduced pharyngeal stripping) | 7 |
| Esophageal dysfunction | 17 |
| Head and neck cancer and radiation changes | 8 |
| Trauma | 2 |
| Unknown (no determinant etiology identified) | 12 |
Note. UES = upper esophageal sphincter.
All spinal abnormalities were characterized into up to two categories, and the spinal characterizations for these groups as well as HCs are listed in Table 3. Osteophytes were the most common spinal pathology identified on VFSS and were present in six of 15 (40%) individuals with SAD, 38 of 85 (45%) individuals with NSAD, and 49 of 100 (49.0%) HCs. Degenerative disc disease was also common with four of 15 (27%) of the SAD group, 43 of 85 (51%) of the NSAD group, and 63 of 100 (63%) of the HC group. History of anterior cervical discectomy and fusion (ACDF) was present in six of 15 (40%) of the SAD group, five of 85 (6%) of the NSAD group, and none of the HCs as this was an exclusion criterion. The SAD cohort was significantly more likely to have a history of ACDF than the NSAD or HC cohorts (p < .001). The remaining comparisons were not statistically significant.
Table 3.
Spinal characterizations for spine-associated dysphagia (SAD), non–spine-associated dysphagia (NSAD), and healthy control (HC) groups.
| Spine characterization | SAD n (%) |
NSAD n (%) |
HC n (%) |
p value |
|---|---|---|---|---|
| 1. Normal cervical spine | 0 (0) | 11 (9.5) | 16 (11.9) | .75 a |
| 2. Loss of normal lordosis | 0 (0) | 6 (5.2) | 4 (3.0) | .52 a |
| 3. Minor/nonobstructive osteophyte | 0 (0) | 14 (12.2) | 19 (14.2) | .78 a |
| 4. Partially obstructive osteophyte | 5 (21.7) | 20 (17.4) | 28 (21.0) | .75 |
| 5. Obstructive osteophyte | 1 (4.3) | 4 (3.5) | 2 (1.5) | .52 |
| 6. History of ACDF | 6 (26.1) | 5 (4.3) | 0 (0) | .002 a |
| 7. History of PCDF | 1 (4.3) | 3 (2.6) | 2 (1.5) | .64 |
| 8. Early degenerative disc disease | 0 (0) | 22 (19.1) | 40 (29.9) | .071 a |
| 9. Late degenerative disc disease | 4 (17.4) | 21 (18.3) | 23 (17.2) | .97 |
| 10. DISH | 3 (13.0) | 0 (0) | 0 (0) | — |
| 11. Pronounced lordosis | 1 (4.3) | 6 (5.2) | 0 (0) | .99 a |
| 12. Other | 1 (4.3) | 3 (2.6) | 0 (0) | .52 a |
| Total | 23 | 115 | 134 |
Note. Dash indicates Fisher's was not able to be performed. ACDF = anterior cervical discectomy and fusion; PCDF = posterior cervical discectomy and fusion; DISH = diffuse idiopathic skeletal hyperostosis.
Columns with zero values were excluded for chi-square/Fisher's calculation.
Swallowing Outcome Measures
The complete results of swallowing outcomes are detailed in Table 4. Median (IQR) EAT-10 scores were statistically different among the three groups: SAD, 6.0 (4.0–14.5); NSAD, 13.0 (6.0–26.0); and HC, 0 (0–1.0; p < .001). Median (IQR) PAS scores were also different between both dysphagic groups and controls (SAD: 2.0 [1.0–5.0]; NSAD: 2.0 [1.0–6.0]; HC: 1.0 [1.0–2.0]; p < .001). Prestudy FOIS was as follows: SAD, 7.0 (7.0–7.0); NSAD, 7.0 (6.0–7.0); and HC, 7.0 (7.0–7.0), p < .001, with a significant difference between the NSAD group and both the SAD and HC groups. Poststudy FOIS and DIGEST total comparisons were not statistically significant between the SAD and NSAD groups. These measures were not available for HCs.
Table 4.
Swallowing outcomes for spine-associated dysphagia (SAD), non–spine-associated dysphagia (NSAD), and healthy control (HC) groups.
| Characteristics | SAD | NSAD | HCs | p value |
|---|---|---|---|---|
| Number of patients (n) | 15 | 85 | 100 | |
| Prestudy FOIS | ||||
| M (SD) | 6.7 (0.6) | 6.1 (1.6) | 7.0 (0) | < .001 |
| Median (IQR) | 7.0 (7.0–7.0) a | 7.0 (6.0–7.0) a , b | 7.0 (7.0–7.0) b | |
| Poststudy FOIS | ||||
| M (SD) | 6.5 (0.7) | 6.1 (1.4) | < .40 | |
| Median (IQR) | 7.0 (6.0–7.0) | 7.0 (6.0–7.0) | ||
| DIGEST total | ||||
| M (SD) | 1.1 (1.1) | 1.2 (1.2) | < .96 | |
| Median (IQR) | 1.0 (0–1.0) | 1.0 (0–2.0) | ||
| PAS max score | ||||
| M (SD) | 2.9 (2.1) | 3.1 (2.7) | 1.6 (1.0) | < .001 |
| Median (IQR) | 2.0 (1.0–5.0) a | 2.0 (1.0–6.0) b | 1.0 (1.0–2.0) a , b | |
| EAT-10 | ||||
| M (SD) | 9.9 (8.2) | 16.3 (11.6) | 1.0 (1.9) | < .001 |
| Median (IQR) | 6.0 (4.0–14.5) a , c | 13.0 (6.0–26.0) b , c | 0 (0–1.0) a , b | |
| MBSImP Oral Total (Components 1–6) | ||||
| M (SD) | 5.4 (3.2) | 6.7 (3.4) | 5.4 (2.1) | .034 |
| Median (IQR) | 5.0 (3.0–7.0) | 7.0 (4.0–9.0) b | 5.0 (4.0–7.0) b | |
| MBSImP Pharyngeal Total (Components 7–16) | ||||
| M (SD) | 7.9 (4.8) | 7.6 (4.9) | 6.0 (2.3) | .16 |
| Median (IQR) | 8.0 (3.0–12.0) | 6.0 (4.0–11.0) | 6.0 (5.0–8.0) | |
| MBSImP Component 1 (lip closure) | ||||
| M (SD) | 0.27 (0.59) | 0.61 (0.73) | 1.1 (0.34) | < .001 |
| Median (IQR) | 0 (0–0) a | 0 (0–1.0) b | 1.0 (1.0–1.0) a , b | |
| MBSImP Component 2 (tongue control during bolus hold) | ||||
| M (SD) | 1.0 (0.85) | 0.8 (0.90) | 0.40 (0.65) | .001 |
| Median (IQR) | 1.0 (0–2.0) a | 0.50 (0–2.0) b | 0 (0–1.0) a , b | |
| MBSImP Component 3 (bolus preparation/mastication) | ||||
| M (SD) | 0.60 (0.91) | 0.83 (1.1) | 0.20 (0.65) | < .001 |
| Median (IQR) | 0 (0–2.0) | 0 (0–2.0) b | 0 (0–0) b | |
| MBSImP Component 4 (bolus transport/lingual motion) | ||||
| M (SD) | 0.20 (0.78) | 0.88 (1.2) | 0.90 (1.2) | .069 |
| Median (IQR) | 0 (0–0) | 0 (0–2.0) | 0 (0–2.0) | |
| MBSImP Component 5 (oral residue) | ||||
| M (SD) | 1.4 (0.62) | 1.6 (0.62) | 1.8 (0.46) | .005 |
| Median (IQR) | 1.0 (1.0–2.0) a | 2.0 (1.0–2.0) b | 2.0 (2.0–2.0) a , b | |
| MBSImP Component 6 (initiation of the pharyngeal swallow) | ||||
| M (SD) | 2.7 (0.80) | 2.7 (0.65) | 2.2 (0.93) | < .001 |
| Median (IQR) | 3.0 (3.0–3.0) a | 3.0 (3.0–3.0) b | 2.0 (1.0–3.0) a , b | |
| MBSImP Component 7 (soft palate elevation) | ||||
| M (SD) | 0 (0) | 0.08 (0.32) | 0 (0) | .015 |
| Median (IQR) | 0 (0–0) | 0 (0–0) b | 0 (0–0) b | |
| MBSImP Component 8 (laryngeal elevation) | ||||
| M (SD) | 0.73 (0.59) | 0.61 (0.60) | 0.51 (0.50) | .34 |
| Median (IQR) | 1.0 (0–1.0) | 1.0 (0–1.0) | 1.0 (0–1.0) | |
| MBSImP Component 9 (anterior hyoid excursion) | ||||
| M (SD) | 0.27 (0.46) | 0.45 (0.72) | 0.85 (0.36) | < .001 |
| Median (IQR) | 0 (0–1.0) | 0 (0–1.0) | 1.0 (1.0–1.0) a , b | |
| MBSImP Component 10 (epiglottic movement) | ||||
| M (SD) | 0.73 (0.70) | 0.66 (0.80) | 0.25 (0.44) | < .001 |
| Median (IQR) | 1.0 (0–1.0) a | 0 (0–1.0) b | 0 (0–0.75) a , b | |
| MBSImP Component 11 (laryngeal vestibular closure) | ||||
| M (SD) | 0.33 (0.49) | 0.35 (0.50) | 0.22 (0.50) | .075 |
| Median (IQR) | 0 (0–1.0) | 0 (0–1.0) | 0 (0–1.0) | |
| MBSImP Component 12 (pharyngeal stripping wave) | ||||
| M (SD) | 0.73 (0.59) | 0.60 (0.64) | 0.37 (0.49) | .015 |
| Median (IQR) | 1.0 (0–1.0) a | 1.0 (0–1.0) b | 0 (0–1.0) a , b | |
| MBSImP Component 13 (pharyngeal contraction) | ||||
| M (SD) | 0.93 (0.96) | 0.80 (1.2) | 0.17 (0.51) | < .001 |
| Median (IQR) | 1.0 (0–2.0) a | 0 (0–1.0) b | 0 (0–0) a , b | |
| MBSImP Component 14 (pharyngoesophageal segment opening) | ||||
| M (SD) | 1.2 (0.56) | 1.0 (0.76) | 0.85 (0.36) | .063 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (0–1.0) | 1.0 (1.0–1.0) | |
| MBSImP Component 15 (tongue base retraction) | ||||
| M (SD) | 1.7 (0.59) | 1.8 (0.78) | 1.6 (0.50) | .24 |
| Median (IQR) | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | |
| MBSImP Component 16 (pharyngeal residue) | ||||
| M (SD) | 1.9 (0.74) | 1.9 (0.86) | 1.8 (0.50) | .73 |
| Median (IQR) | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | |
| MBSImP Component 17 (esophageal clearance) | ||||
| M (SD) | 1.9 (1.5) | 1.4 (1.1) | 1.2 (1.4) | .015 |
| Median (IQR) | 1.0 (1.0–4.0) a | 1.0 (1.0–2.0) b | 1.0 (0–2.0) a , b |
Note. FOIS = Functional Oral Intake Scale; IQR = interquartile range; DIGEST = Dynamic Imaging Grade of Swallowing Toxicity; PAS = Penetration–Aspiration Scale; EAT-10 = 10-item Eating Assessment Tool; MBSImP = Modified Barium Swallow Impairment Profile.
Statistically significant difference between medians of SAD and HC.
Statistically significant difference between medians of NSAD and HC.
Statistically significant difference between medians of SAD and NSAD.
Median (IQR) MBSImP Oral Totals of the three groups were as follows: SAD, 5.0 (3.0–7.0); NSAD, 7.0 (4.0–9.0); and HC, 5.0 (4.0–7.0), with only the NSAD group having significantly worse scores than HCs (p = .034). Median (IQR) MBSImP Pharyngeal Totals of the three groups were as follows: SAD, 8.0 (3.0–12.0); NSAD, 6.0 (4.0–11.0); and HC, 6.0 (5.0–8.0), p < .16. Individual MBSImP component scores were significantly worse in both dysphagic groups than HCs for Components 2, 6, 10, 12, 13, and 17. Components 3 and 7 had only a significant difference between the NSAD and HC groups. For Components 1, 5, and 9, HCs had significantly worse scores than dysphagic groups (see Table 4).
Those with an osteophyte at C3–C4 compared with other dysphagic patients did not have worse swallowing outcomes for epiglottic inversion (Component 10, p = .99). Similarly, those with osteophytes at C4–C6 did not have worse swallowing outcomes for PESO (Component 14) when compared with the other dysphagic patients at these levels (p = .94).
Reliability
Interrater reliability consisted of 92% agreement overall and moderate-to-strong agreement for the spinal categorizations. Categories with strong agreement included normal cervical spine (κ = 0.65), osteophytes (κ = 0.60), DISH (κ = 0.70), pronounced lordosis (κ = 0.78), and history of ACDF (κ = 1.0). Areas of moderate agreement included degenerative disc disease (κ = 0.52) and history of posterior cervical discectomy and fusion (κ = 0.52). Loss of lordosis had poor agreement (κ = 0.29), but this is mainly due to the limitations of viewing a single image for a category dependent on movement and patient positioning.
Discussion
This study systematically evaluated spinal anatomy observed on VFSS to elucidate the association between spine disease and swallowing with predetermined spinal categories. Spine disease was highly prevalent in all groups most likely due to advanced age. Spine disease is very common in older adults with a prevalence ranging from 28% to 89% (Boden et al., 1990; Matsumoto et al., 1998). Our study population had a 90% prevalence of spinal disease, slightly greater than previous established literature; however, this is likely due to the advanced age of our patients. Prior literature evaluated patients 40–60 years of age, whereas the average age of our dysphagic cohort was 67.7 years (Boden et al., 1990; Matsumoto et al., 1998). The majority of dysphagic patients (89/100, 89%) had abnormal spinal anatomy. This finding is to be expected, as both spinal disease and risk for disease and injury where dysphagia is a symptom increase with age (Fakhoury & Dowling, 2022; Resnick, 1985). Matsumoto et al. (1998) found that disc degeneration is present in 86% and 89% of men and women, respectively, above the age of 60 years when evaluating asymptomatic magnetic resonance images. Other studies have cited that approximately 75% of patients have cervical spine changes (Kumaresan et al., 2001; Resnick, 1985; Seidler et al., 2009). This age-related association of cervical pathology explains why only 16 of 100 of the age-matched HCs had a normal cervical spine. Osteophytes were very common in all groups, and these were most frequently nonobstructive (33/200, 16.5%) or partially obstructive (54/200, 26.1%). Both early (22/200, 11%) and late (25/200, 13%) degenerative disc diseases were also common. These findings are in line with the previous studies that show high rates of these pathologies in older adults (Boden et al., 1990; Matsumoto et al., 1998).
We found that the presence of abnormal spinal cervical features does not necessarily correspond with impaired swallowing. Whereas 89% (89/100) of dysphagic patients were characterized with at least one spinal pathology, only 15 of 89 patients (16.9%) were determined to have SAD. Several patients in the HC group also had spine changes, such as partially obstructive osteophytes, and two patients were noted to have obstructive osteophytes. Yet, these patients did not complain of dysphagia and had no reported diet alterations. Because spine changes are commonly seen in asymptomatic individuals, it is imperative to consider the clinical context and investigate other etiologies thoroughly before attributing dysphagia to spine disease alone.
Conversely, it is evident that sometimes isolated spine pathology can be the sole cause of dysphagia. The most common spinal pathologies affecting swallowing identified in our study were osteophytes (26/36, 72%) and degenerative disc disease (12/36, 33%). The etiologies of dysphagia in the SAD group were osteophytes (6/15, 40%), history of ACDF surgery (6/15, 40%), and degenerative disc disease (4/15, 27%). Previous studies have demonstrated that osteophytes can contribute to dysphagia and aspiration as well as increase the likelihood of foreign body impaction (Choi et al., 2019; Seidler et al., 2009; Shoffel-Havakuk et al., 2016). Choi et al. (2019) found that out of 1,866 videofluoroscopic studies of dysphagic patients, 23 patients were found to have dysphagia only attributable to anterior cervical osteophytes. These patients had pharyngeal phase dysphagia including penetration, decreased laryngeal elevation, and reduced epiglottic inversion (Choi et al., 2019). Seidler et al. (2009) found that the mechanism of dysphagia was dependent on the level of the osteophyte. We hypothesized that because C3–C4 is the spinal level at which epiglottic inversion takes place and C4–C6 is the spinal level at which the upper esophageal sphincter opens, spinal disease at these levels would impair the corresponding MBSImP components: 10 (epiglottic inversion) and 14 (PESO), respectively. However, these results were not statistically significant. MBSImP and PAS scores were similar between both dysphagic groups and, in some cases, better in the SAD group. We concluded that although spine disease can play an important role in dysphagia, it is not necessarily worse than other forms of dysphagia. These results may suggest that the progressive nature of spine disease allows for increased compensation over time, whereas the sudden onset of some neurogenic issues (e.g., stroke) does not. For example, a dysphagic patient in the NSAD group with lower cervical osteophytes incidentally utilized a self-learned Mendelsohn maneuver-like swallow (volitionally holding the hyolaryngeal complex at its maximum height during the swallow) that improved passage of the bolus through the pharyngoesophageal segment.
Symptomatic osteophytes resulting in dysphagia can be addressed with measures such as diet modification, compensatory strategies (e.g., head turn), or surgical treatment. Kolz et al. (2021) reported 19 patients who underwent anterior cervical osteophyte resection. The level of C3–C4 was most commonly addressed (69% of patients), and swallowing was reported to be improved in 95% of patients, although no validated swallow outcome measures were used to assess improvement (Kolz et al., 2021). Several other case reports and studies describe cases of moderate-to-severe dysphagia from spine-related causes that were clinically improved after spine surgery (Erdur et al., 2017; Kolz et al., 2021; Ruetten et al., 2019; Song et al., 2012; Urrutia & Bono, 2009; von der Hoeh et al., 2015). However, surgical intervention must be weighed with the risks, including those of potentially worsening dysphagia. Kolz et al. reported an overall complication rate of 42%, including diskitis and vertebral osteomyelitis, gastrostomy tube placement, laryngeal nerve injury, and aspiration pneumonia.
To obviate the risks of open surgical intervention, Jamal et al. (2015) proposed partial epiglottoplasty as a treatment of anterior cervical spine osteophytes. Although all nine patients in their study reported improvement in swallowing, concerns regarding methodology warrant careful consideration before this procedure is recommended for widespread adoption (Patel et al., 2016). Patel et al. (2016) commented that patients with previous cervical spine surgery should have been excluded from the study and not offered epiglottoplasty. It is critical that patients undergo a comprehensive multidisciplinary evaluation to confirm that spinal pathology is indeed the primary cause of dysphagia and conservative options are considered before the recommendation of surgical intervention.
Limitations
This study is limited by the imbalance in sample size across groups because only 15 individuals were determined to have solely SAD. This limited comparisons to the other dysphagic patient group and HCs, and it is possible we were unable to identify significant differences in spinal characterization frequencies or swallowing outcomes due to sample size. Future evaluation into SAD with larger sample sizes will further support or refute our findings.
Another limitation of this study is the creation of discreet categories for the classification of complex disease processes. We dichotomized degenerative disc disease into early versus late, but these changes exist along a spectrum. Some of these disease processes can also be interrelated, such as osteophytes developing in the context of DISH and degenerative changes to the spine (Kumaresan et al., 2001; Resnick, 1985). However, we created our categories based on common spinal pathology and changes that could be easily identified by the reading physician (radiologist) and recognized by speech-language pathologists and laryngologists without extensive background in radiology or spinal anatomy. These categories represent a gestalt of an individual's spinal pathology, and none of our analyses were contingent on this categorization. Most categories had moderate-to-strong interrater agreement, supporting the reliability of the classification system.
The association between the spinal pathology and dysphagia seen on VFSS for each subject was determined based on individual clinician judgment. Although consensus between two raters was required, this method does come with a level of inherent bias. Furthermore, in cases where more than one possible etiology for dysphagia was identified, it could be that multiple factors, including spinal pathology, were likely contributing to the dysphagia. Our method of classification attempted to isolate spinal causes of dysphagia by isolating patients with only spinal pathology in the SAD group. Therefore, patients who had spinal pathology impacting their dysphagia could have been missed within the NSAD group.
Conclusions
Cervical spine disease is a common finding in healthy adults and in individuals with dysphagia undergoing outpatient VFSS. Our findings suggest that cervical spinal disease can significantly impact swallowing function in a subset of patients. The gradual, progressive nature of spinal disease may allow patients to adapt to and compensate for swallowing difficulty over time. A multidisciplinary approach should be taken when drawing conclusions regarding the contribution of spinal disease to dysphagia symptoms and the resulting recommendations for intervention. Further studies evaluating spinal pathology in dysphagic patients would enable us to draw more definitive conclusions regarding the impact of SAD and guide appropriate treatment decisions.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This work was partially supported by the Veterans Affairs (RR&D 1IK1RX001628-01A to Kendrea L. Garand); American Speech-Language-Hearing Foundation (Kendrea L. Garand); National Institutes of Health (NIH/NCATS TL1R000061 to Kendrea L. Garand and NIH/NIDCD 1K24DC12801 to Bonnie Martin-Harris); Evelyn Trammell Trust (to Bonnie Martin-Harris); and Biostatistics Shared Resource, Hollings Cancer Center at the Medical University of South Carolina (P30 CA138313).
Funding Statement
This work was partially supported by the Veterans Affairs (RR&D 1IK1RX001628-01A to Kendrea L. Garand); American Speech-Language-Hearing Foundation (Kendrea L. Garand); National Institutes of Health (NIH/NCATS TL1R000061 to Kendrea L. Garand and NIH/NIDCD 1K24DC12801 to Bonnie Martin-Harris); Evelyn Trammell Trust (to Bonnie Martin-Harris); and Biostatistics Shared Resource, Hollings Cancer Center at the Medical University of South Carolina (P30 CA138313).
References
- Bakshi, S. S. , & Ramesh, S. (2021). Cervical osteophytes causing dysphagia. American Journal of the Clinical Sciences, 361(5), e43. https://doi.org/10.1016/j.amjms.2020.10.014 [DOI] [PubMed] [Google Scholar]
- Barker, T. , Gill, D. , Khatun, F. , & Lutchman, L. (2021). Anterior cervical osteophytes causing dysphagia and dyspnoea. Annals of the Royal College of Surgeons of England, 103(7), e209–e211. https://doi.org/10.1308/rcsann.2020.7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belafsky, P. C. , Mouadeb, D. A. , Rees, C. J. , Pryor, J. C. , Postma, G. N. , Allen, J. , & Leonard, R. J. (2008). Validity and reliability of the Eating Assessment Tool (EAT-10). Annals of Otology, Rhinology & Laryngology, 117(12), 919–924. https://doi.org/10.1177/000348940811701210 [DOI] [PubMed] [Google Scholar]
- Boden, S. D. , McCowin, P. R. , Davis, D. O. , Dina, T. S. , Mark, A. S. , & Wiesel, S. (1990). Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. Journal of Bone and Joint Surgery, 72(8), 1178–1184. https://doi.org/10.2106/00004623-199072080-00008 [PubMed] [Google Scholar]
- Choi, H. E. , Jo, G. Y. , Kim, W. J. , Do, H. K. , Kwon, J. K. , & Park, S. H. (2019). Characteristics and clinical course of dysphagia caused by anterior cervical osteophyte. Annals of Rehabilitation Medicine, 43(1), 27–37. https://doi.org/10.5535/arm.2019.43.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, E. , Preston, P. , Wates, A. , & Merry, P. (2003). DISHphagia—A difficult problem to swallow. Rheumatology (Oxford), 42(11), 1422–1423. https://doi.org/10.1093/rheumatology/keg353 [DOI] [PubMed] [Google Scholar]
- Crary, M. A. , Mann, G. D. C. , & Groher, M. E. (2005). Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of Physical Medicine and Rehabilitation, 86(8), 1516–1520. https://doi.org/10.1016/j.apmr.2004.11.049 [DOI] [PubMed] [Google Scholar]
- Egerter, A. C. , Kim, E. S. , Lee, D. J. , Liu, J. J. , Cadena, G. , Panchal, R. R. , & Kim, K. D. (2015). Dysphagia secondary to anterior osteophytes of the cervical spine. Global Spine Journal, 5(5), 78–83. https://doi.org/10.1055/s-0035-1546954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdur, O. , Tasli, H. , Polat, B. , Sofiyev, F. , Tosun, F. , Colpan, B. , Birkent, H. , & Ozturk, K. (2017). Surgical management of dysphagia due to anterior cervical osteophytes. Journal of Craniofacial Surgery, 28(1), e80–e84. https://doi.org/10.1097/SCS.0000000000003241 [DOI] [PubMed] [Google Scholar]
- Fakhoury, J. , & Dowling, T. J. (2022). Cervical degenerative disc disease. StatPearls. [PubMed] [Google Scholar]
- Frempong-Boadu, A. , Houten, J. K. , Osborn, B. , Opulencia, J. , Kells, L. , Guida, D. D. , & Le Roux, P. D. (2002). Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: A prospective, objective preoperative and postoperative assessment. Journal of Spinal Disorders & Techniques, 15(5), 362–368. https://doi.org/10.1097/00024720-200210000-00004 [DOI] [PubMed] [Google Scholar]
- Garand, K. L. F. , Hill, E. G. , Amella, E. , Armeson, K. , Brown, A. , & Martin-Harris, B. (2019). Bolus airway invasion observed during videofluoroscopy in healthy, non-dysphagic community-dwelling adults. Annals of Otology, Rhinology & Laryngology, 128(5), 426–432. https://doi.org/10.1177/0003489419826141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson, K. A. , Barrow, M. P. , Barringer, D. A. , Knott, J. K. , Lin, H. Y. , Weber, R. S. , Fuller, C. D. , Lai, S. Y. , Alvarez, C. P. , Raut, J. , Lazarus, C. L. , May, A. , Patterson, J. , Roe, J. W. , Starmer, H. M. , & Lewin, J. S. (2017). Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): Scale development and validation. Cancer, 123(1), 62–70. https://doi.org/10.1002/cncr.30283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal, N. , Erman, A. , & Chhetri, D. K. (2015). Partial epiglottoplasty for pharyngeal dysphagia due to cervical spine pathology. Otolaryngology—Head and Neck Surgery, 153(4), 586–592. https://doi.org/10.1177/0194599815601025 [DOI] [PubMed] [Google Scholar]
- Kang, S. H. , Kim, D. K. , Seo, K. M. , Lee, S. Y. , Park, S. W. , & Kim, Y. B. (2016). Swallowing function defined by videofluoroscopic swallowing studies after anterior cervical discectomy and fusion: A prospective study. Journal of Korean Medical Science, 31(12), 2020–2025. https://doi.org/10.3346/jkms.2016.31.12.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolz, J. M. , Alvi, M. A. , Bhatti, A. R. , Tomov, M. N. , Bydon, M. , Sebastian, A. S. , Elder, B. D. , Nassr, A. N. , Fogelson, J. L. , Currier, B. L. , & Freedman, B. A. (2021). Anterior cervical osteophyte resection for treatment of dysphagia. Global Spine Journal, 11(4), 488–499. https://doi.org/10.1177/2192568220912706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan, S. , Yoganandan, N. , Pintar, F. A. , Maiman, D. J. , & Goel, V. K. (2001). Contribution of disc degeneration to osteophyte formation in the cervical spine: A biomechanical investigation. Journal of Orthopaedic Research, 19(5), 977–984. https://doi.org/10.1016/S0736-0266(01)00010-9 [DOI] [PubMed] [Google Scholar]
- Ladenheim, S. E. , & Marlowe, F. I. (1999). Dysphagia secondary to cervical osteophytes. American Journal of Otolaryngology, 20(3), 184–189. https://doi.org/10.1016/s0196-0709(99)90070-4 [DOI] [PubMed] [Google Scholar]
- Lee, J. W. , Randall, D. R. , Evangelista, L. M. , Kuhn, M. A. , & Belafsky, P. C. (2017). Subjective assessment of videofluoroscopic swallow studies. Otolaryngology—Head and Neck Surgery, 156(5), 901–905. https://doi.org/10.1177/0194599817691276 [DOI] [PubMed] [Google Scholar]
- Leonard, R. , & Belafsky, P. (2011). Dysphagia following cervical spine surgery with anterior instrumentation. Spine (Phila Pa 1976), 36(25), 2217–2223. https://doi.org/10.1097/BRS.0b013e318205a1a7 [DOI] [PubMed] [Google Scholar]
- Martin-Harris, B. , Brodsky, M. B. , Michel, Y. , Castell, D. O. , Schleicher, M. , Sandidge, J. , Maxwell, R. , & Blair, J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. https://doi.org/10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, M. , Fujimura, Y. , Suzuki, N. , Nishi, Y. , Nakamura, M. , Yabe, Y. , & Shiga, H. (1998). MRI of cervical intervertebral discs in asymptomatic subjects. The Journal of Bone and Joint Surgery, 80(1), 19–24. https://doi.org/10.1302/0301-620x.80b1.7929 [DOI] [PubMed] [Google Scholar]
- Murry, T. C. , & Ricardo, L. (2006). Clinical management of swallowing disorders (2nd ed.). Plural. [Google Scholar]
- Ohki, M. (2012). Dysphagia due to diffuse idiopathic skeletal hyperostosis. Case Reports in Otolaryngology, 2012, 123825–123823. https://doi.org/10.1155/2012/123825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenlander, M. E. , Orringer, D. A. , La Marca, F. , McGillicuddy, J. E. , Sullivan, S. E. , Chandler, W. F. , & Park, P. (2009). Dysphagia due to anterior cervical hyperosteophytosis. Surgical Neurology, 72(3), 266–270. https://doi.org/10.1016/j.surneu.2008.08.081 [DOI] [PubMed] [Google Scholar]
- Papadopoulou, S. , Exarchakos, G. , Beris, A. , & Ploumis, A. (2013). Dysphagia associated with cervical spine and postural disorders. Dysphagia, 28(4), 469–480. https://doi.org/10.1007/s00455-013-9484-7 [DOI] [PubMed] [Google Scholar]
- Patel, A. , Tang, C. G. , & Blitzer, A. (2016). In reference to “partial epiglottoplasty for pharyngeal dysphagia due to cervical spine pathology.” Otolaryngology—Head and Neck Surgery, 154(4), 773–774. https://doi.org/10.1177/0194599816631500 [DOI] [PubMed] [Google Scholar]
- Resnick, D. (1985). Degenerative diseases of the vertebral column. Radiology, 156(1), 3–14. https://doi.org/10.1148/radiology.156.1.3923556 [DOI] [PubMed] [Google Scholar]
- Rosenbek, J. C. , Robbins, J. A. , Roecker, E. B. , Coyle, J. L. , & Wood, J. L. (1996). A penetration–aspiration scale. Dysphagia, 11(2), 93–98. https://doi.org/10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- Ruetten, S. , Baraliakos, X. , Godolias, G. , & Komp, M. (2019). Surgical treatment of anterior cervical osteophytes causing dysphagia. Journal of Orthopaedic Surgery (Hong Kong), 27(2), 230949901983742. https://doi.org/10.1177/2309499019837424 [DOI] [PubMed] [Google Scholar]
- Seidler, T. O. , Perez Alvarez, J. C. , Wonneberger, K. , & Hacki, T. (2009). Dysphagia caused by ventral osteophytes of the cervical spine: Clinical and radiographic findings. European Archives of Oto-Rhino-Laryngology, 266(2), 285–291. https://doi.org/10.1007/s00405-008-0735-4 [DOI] [PubMed] [Google Scholar]
- Shoffel-Havakuk, H. , Cahanovitc, S. , Adi, M. , Cohen, O. , Haimovich, Y. , Lahav, Y. , & Halperin, D. (2016). Cervical osteophytes increase the risk for foreign body impaction: A 171-patient case–control study. Dysphagia, 31(6), 749–756. https://doi.org/10.1007/s00455-016-9731-9 [DOI] [PubMed] [Google Scholar]
- Song, A. R. , Yang, H. S. , Byun, E. , Kim, Y. , Park, K. H. , & Kim, K. L. (2012). Surgical treatments on patients with anterior cervical hyperostosis-derived dysphagia. Annals of Rehabilitation Medicine, 36(5), 729–734. https://doi.org/10.5535/arm.2012.36.5.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia, J. , & Bono, C. M. (2009). Long-term results of surgical treatment of dysphagia secondary to cervical diffuse idiopathic skeletal hyperostosis. The Spine Journal, 9(9), e13–e17. https://doi.org/10.1016/j.spinee.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Viera, A. J. , & Garrett, J. M. (2005). Understanding interobserver agreement: The kappa statistic. Family Medicine, 37(5), 360–363. https://www.ncbi.nlm.nih.gov/pubmed/15883903 [PubMed] [Google Scholar]
- Vodicar, M. , Kosak, R. , & Vengust, R. (2016). Long-term results of surgical treatment for symptomatic anterior cervical osteophytes: A case series with review of the literature. Clinical Spine Surgery, 29(9), E482–E487. https://doi.org/10.1097/BSD.0b013e31829046af [DOI] [PubMed] [Google Scholar]
- von der Hoeh, N. H. , Voelker, A. , Jarvers, J. S. , Gulow, J. , & Heyde, C. E. (2015). Results after the surgical treatment of anterior cervical hyperostosis causing dysphagia. European Spine Journal, 24(Suppl. 4), 489–493. https://doi.org/10.1007/s00586-014-3507-4 [DOI] [PubMed] [Google Scholar]
- Ziegler, J. , Davidson, K. , Cooper, R. , Garand, K. L. , Nguyen, S. , Yuen, E. , Martin-Harris, B. , & O'Rourke, A. K. (2021). Characterization of dysphagia following anterior cervical spine surgery. Advances in Communication and Swallowing, 24(1), 55–62. https://doi.org/10.3233/ACS-210034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.