Abstract
Cervical cancer has killed millions of women over the past decade. In 2019 the World Health Organization launched the Cervical Cancer Elimination Strategy, which included ambitious targets for vaccination, screening, and treatment. The COVID-19 pandemic disrupted progress on the strategy, but lessons learned during the pandemic – especially in vaccination, self-administered testing, and coordinated mobilization on a global scale – may help with efforts to achieve its targets. However, we must also learn from the failure of the COVID-19 response to include adequate representation of global voices. Efforts to eliminate cervical cancer will only succeed if those countries most affected are involved from the very start of planning. In this article we summarize innovations and highlight missed opportunities in the COVID response, and make recommendations to leverage the COVID experience to accelerate the elimination of cervical cancer globally.
Research organism: None
Introduction
Cervical cancer is the fourth most common cancer in women (after breast, colorectal and lung cancer), and resulted in 311, 000 deaths in 2018 (Arbyn et al., 2020). The burden of cervical cancer also continues to rise – despite being a preventable disease – and this burden falls disproportionately on women in low- and middle-income countries (LMICs) (Cohen et al., 2019; Zhang et al., 2021). Many countries also lack the resources to treat cervical cancer, resulting in unnecessary death and suffering (Union for International Cancer Control, 2023). Moreover, cervical cancer is most often diagnosed in relatively young women who are often the primary wage earners in their household (Zhang et al., 2021; American Society of Clinical Oncology, 2023; Reed et al., 2000).
The goal of the Cervical Cancer Elimination Strategy, launched by the World Health Organization in 2019, is to reduce incidence of cervical cancer from about 15 per 100,000 women to less than 4 per 100,000 by the year 2030 (World Health Organization, 2020). The strategy has identified three targets to help it reach this goal: to provide HPV vaccination to 90% of girls by age 15; offer 70% women cervical screening with a high precision assay at least twice (by age 35, and again by age 45); and to treat 90% of women with pre-cancer and manage 90% of women with invasive cancer. HPV vaccination will likely have the biggest impact on the incidence of cervical cancer in the long run, with screening and treatment having bigger impacts in the short-term.
Unfortunately, progress on vaccination, screening and treatment was disrupted shortly after launch by the COVID-19 pandemic. In this article, on behalf of the Policy Committee of the International Papillomavirus Society (IPVS), we summarize the impact of the pandemic on efforts to eliminate cervical cancer and discuss how lessons learned during the pandemic can be applied to a different global public health threat – cervical cancer.
Cervical cancer prevention before the COVID-19 pandemic
Despite early demonstration of acceptability and feasibility, only 60% of WHO member states have incorporated HPV into their national vaccination schedule and only 13% of girls have completed HPV vaccination (World Health Organization, 2022a). Coverage had not reached the 90% target in most countries, attributed to various and different health systems capacity challenges (Amponsah-Dacosta et al., 2020). LMICs were also disadvantaged by limitations in the international supply chain, leaving many LMICs without adequate access to vaccine in the years leading up to the COVID-19 pandemic (Garland et al., 2020; Colzani et al., 2021).
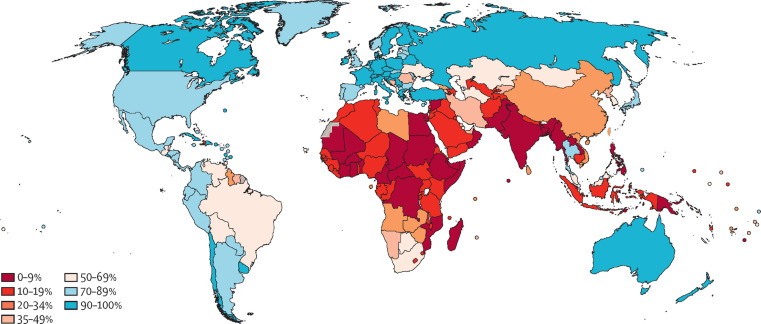
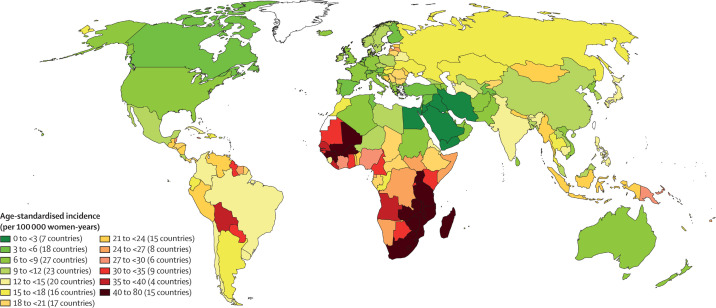
The WHO elimination strategy ambitiously aims to increase global screening coverage to 70% with two HPV-based screens by the age of 45. Prior to the start of the pandemic, only 37% of countries had achieved lifetime screening coverage of 70% or higher, and none of these were low-income countries. Lifetime screening coverage was proportional to the country income strata with lifetime screening coverage reaching 70% in 58% of high-income countries, 28% of upper-middle-income countries, 6% of lower middle-income countries and 0% of low-income countries (Figure 1; Bruni et al., 2022). Within these numbers lie wide socioeconomic disparities within countries, particularly in opportunistic rather than population-based screening programs (Phaswana-Mafuya and Peltzer, 2018; Corrêa et al., 2022). Unsurprisingly, screening coverage is inversely correlated to incidence and mortality from cervical cancer (Figure 2; Arbyn et al., 2018).
Figure 1. Map showing ever-in-lifetime cervical cancer screening coverage in women aged 30–49 years in 2019 by country.
This map demonstrates that only 75 of the 202 countries surveyed in this study had achieved screening coverage of 70% or higher. Most countries in Africa and South Asia have lifetime screening coverage less than 20%. From Bruni et al., 2022. Lancet Global Health 10:e1115–1127. (CC BY 4.0).
Figure 2. Map showing the age-standardised incidence of cervical cancer by country, estimated for 2018.
The countries with the highest incidence of cervical cancer (in parts of sub-Saharan Africa, south Asia and South America) largely correspond to the countries with low screening coverage. From Arbyn et al., 2020. Lancet Global Health 8:e191–e203. (CC BY 4.0).
The impact of the COVID-19 pandemic on efforts to prevent cervical cancer
The pandemic impacted vaccination, screening and treatment of cervical cancer globally. In places with established HPV vaccination programs, there were severe interruptions, as school-based programs were hindered by school closures, routine clinical services were suspended for variable time frames and frequencies, and funds for HPV vaccination programming were redistributed (Rao et al., 2022; Pillai, 2022; Daniels et al., 2021). Additionally, roll-out of new HPV vaccination programs was delayed (Newton, 2021).
Similarly, there were drastic reductions in cervical cancer screening and treatment services across the globe related to the COVID-19 pandemic (Mayo et al., 2021; Carcopino et al., 2022; Dennis et al., 2021; Istrate-Ofiteru et al., 2021). Health facilities themselves had to limit care to sick patients and individuals feared the risk of exposure at health facilities (Santos et al., 2021; Baaske et al., 2022; Schad et al., 2021). COVID-19 resulted in greater disruptions in cervical cancer screening programs than some of the most damaging natural disasters (Ortiz et al., 2021). While some countries only experienced temporary disruption of services, even after lifting COVID-19 related movement restrictions, screening remained lower in many places than baseline levels (Basu et al., 2021; Liu et al., 2022; Miller et al., 2020). There are little published data from LMICs, but it appears that preventive health services in LMICs took a severe hit and have been the slowest to resume baseline services (Amouzou et al., 2021; Murewanhema, 2021). Widespread burn-out has hindered efforts to return to pre-pandemic screening levels (Smith and Perkins, 2022).
We have yet to see how the disruption in health services due to the COVID-19 pandemic will impact cervical cancer burden on a global scale. Diagnoses of all cancers were reduced during the COVID-19 pandemic relative to pre-pandemic rates, resulting in a backlog of diagnoses (Eskander et al., 2022). A study in 2022 in Romania reported presentation of cervical cancer at more advanced stages during the pandemic, which accompanied significant changes in treatment courses due to interruptions in surgical and radiation services (Popescu et al., 2022). Recent modeling studies from the United Kingdom and United States estimate that the burden of cervical cancer will increase regardless of the length of time taken to catch up on missed screening, diagnosis and treatment due to COVID-19 (Castanon et al., 2021b; Burger et al., 2021). It is expected that greater disparity in these effects will be seen in settings without capacity to augment screening, diagnostic and treatment services to account for the backlog (Bonadio et al., 2021; Castanon et al., 2021a).
Why was cervical screening so vulnerable during the pandemic?
Cervical cancer screening was particularly vulnerable to losing traction during the COVID-19 pandemic because at the start of the pandemic, screening in most countries required a pelvic examination. Pelvic examination is difficult to make “COVID-friendly”. Counseling with examinations and treatment takes up to 30 minutes in a closed space. Disinfection protocols require additional time between patients. Waiting times for services are thus long, and a risk of exposure to a high volume of potential contacts is inherent to the process. Clinics were forced to compensate with reduced patient volume (Sormani et al., 2021).
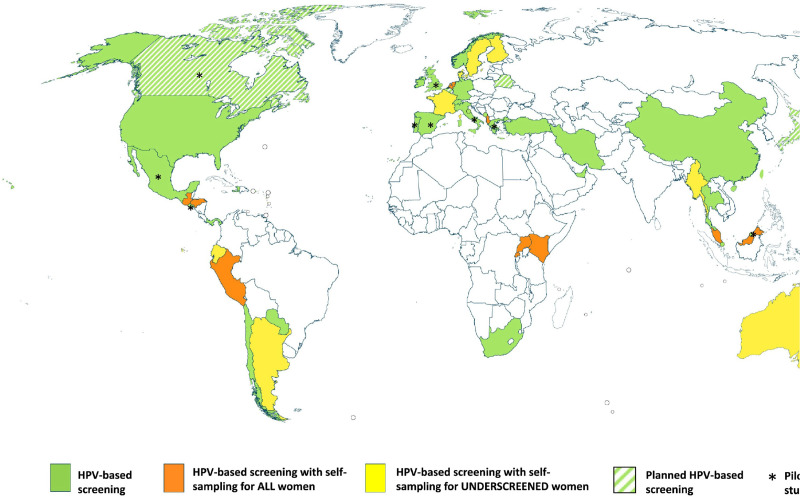
In a review of cervical cancer screening guidelines in 139 countries across income strata, cytology was the most prevalent screening modality in HICs (78%), while visual inspection with acetic acid (VIA) was the most common method in LMICs and often a component of a screen-and-treat program (Bruni et al., 2022). While 35% (48 of 139) of country strategies noted plans to include HPV testing in their recommendations, only 17 countries had introduced HPV self-sampling into their national programs or guidelines by October 2020 and of those, half reserved the use of self-sampling for under-screened populations only. Countries with HPV self-sampling were mostly concentrated in HICs (Figure 3; Serrano et al., 2022).
Figure 3. Map showing the self-sampling approach in countries that officially recommend HPV-based screening.
As of October 2020, few countries had introduced HPV primary screening, and most were concentrated in high-income countries. Only 17 countries had introduced HPV self-sampling into their national programs or guidelines and of those, half reserved the use of self-sampling for under-screened populations only. From Serrano et al., 2022. Preventive Medicine 154:106900. (CC BY 4.0).
The need for a new cervical cancer prevention paradigm
While it was clear prior to the COVID-19 pandemic that a new model for delivery of cervical cancer prevention services was needed, this need has been magnified post-pandemic by an unquantified and urgent backlog of screening and treatment services. Population-level cervical cancer screening with HPV self-testing is the most cost-effective approach in the long-run to addressing these issues, however the upfront affordability and the need for cost-effective triage strategies remain challenges (Mezei et al., 2017).
In considering cost-effectiveness, a critical consideration of outcomes is essential. Beyond the directs costs of implementation of cervical cancer programs lie the less tangible population-level economic, social and health costs of not improving screening. Programs focused on short-term measurable costs and outcomes likely miss the mark on establishing effective programming.
Perhaps the delay in focus on cervical cancer elimination due to the pandemic could be an opportunity to reframe cervical screening strategy with insight from the COVID-19 response. COVID-19 laid bare the existing inequities in global access to health resources, from vaccination to treatment (McGowan and Bambra, 2022; Pasquale et al., 2021; Stolberg, 2022; Foundation for Innovative New Diagnostics, 2023). However, the COVID-19 joint resource mobilization effort provided a model for coordinated funding and policy development for vaccination, testing and treatment that was likely the most successful approach and may provide a template for a coordinated response to cervical screening (World Health Organization, 2022b).
Many countries failed to get enough COVID-19 vaccine in a timely fashion as high-income countries had most of the early global supply of vaccine Tatar et al., 2021. However, the acute need to rapidly scale up vaccination drove increased and more efficient manufacturing. Coordination of global partners allowed identification of bottlenecks in vaccine manufacturing and led to a variety of globally coordinated interventions, from creating international trade agreements, to procuring essential equipment, to developing and scaling-up manufacturing in countries without prior capacity (World Health Organization, 2022c).
In order to expand access to diagnostics, global efforts were made to diversify the supply chain and support emergency use listing of tests to get them quickly to market. Global actors negotiated more affordable rapid diagnostics to increase global accessibility (The Global Fund, 2022). Countries rapidly implemented testing protocols, which were in many places available on demand without a health provider visit or referral. Screening outside of health facilities, often delivered in community settings and at home, and often done by individuals themselves became routine (Feletto et al., 2020). There was new engagement with the private sector for public health programming roll-out of both testing and vaccination.
There was a broad increase in individual self-efficacy in health engagement. The reporting of results directly to patients became routine. Population acceptance of swabs and self-testing was achieved through public health messaging, on-line and community-based communications (Covid-19 Information Center, 2022). There was increased utilization of telehealth, internet- and phone-based dissemination of accurate health information. Community health workers were engaged and programs were revitalized.
There was a movement to reduce the dependence of LMICs on global production by way of increasing testing capacity locally. This resulted in initiatives to start and scale up production of rapid diagnostics in Latin America, Africa and Asia, under their own branding and at more affordable prices (UNITAID, 2021). The great technical capacity of laboratories across the globe to engage in viral-based screening was front and center on the international stage. Twenty African countries were able to sequence SARS-CoV-2 by April 2020, and Botswana and South Africa were the first countries to detect the Omicron variant through genomic sequencing (Viana et al., 2022). Investment in training of both laboratory and bioinformatic personnel contributed to lasting and translatable capacity (Tegally et al., 2022).
Recommendations for future action
In the rest of this article we will consider how the lessons learnt from the global response to the COVID-19 pandemic could be applied to efforts to eliminate cervical cancer globally, and we make 14 recommendations for further action (see Box 1).
Box 1. Recommendations for accelerating the elimination of cervical cancer.
1. Leverage supply chain opportunities developed during COVID-19 to distribute vaccines and diagnostics.
2. Maximize school-based and community-based vaccination for both eligible children and those that require "catch up" vaccination.
3. Invest in laboratory infrastructure and training to support HPV-based screening.
4. Establish local vaccine and diagnostic manufacturing.
5. Facilitate rapid evaluation and approval of HPV diagnostics.
6. Invest in diagnostic research and development to develop accurate point-of-care HPV tests.
7. Negotiate prices of HPV tests currently on the market to increase accessibility.
8. Increase investment on high-performance triage of positive HPV results.
9. Leverage social media, community networks and other indigenous methods for educating women and men about vaccination, screening and follow-up.
10. Use telehealth and web-based apps to report results and engage in follow-up.
11. Implement web-based electronic surveillance systems for cervical cancer screening program monitoring.
12. Evaluate programs on process indicators with the opportunity to rapidly respond to feedback and improve care.
13. Develop meaningful long-term outcomes for cervical cancer prevention, including health, economic and social metrics.
14. Support countries to develop tailored approaches to HPV-based screening and management.
The disruption of HPV vaccination programs will require a catch-up period with expanded age cohorts in order to reach children who missed vaccination during the global shortage and COVID-19 pandemic. These programs must be designed at national levels, according to national prespecified target ages groups for HPV vaccination. For countries that did not have established national HPV vaccination programming prior to the COVID-19 pandemic, logistical capacity created by COVID-19 vaccination programming may facilitate this process through having created stable supply chains and development of community-based vaccination teams (recommendation 1). Innovation and rapid development of COVID-19 vaccines may have created momentum to bolster the supply of effective prophylactic HPV vaccines. Increased supply and logistical capacity, coupled with new data supporting single dose HPV schedules may all facilitate the implementation of school-based and community-based HPV vaccination for both eligible children and those that require "catch up" vaccination (recommendation 2) (The Lancet Oncology, 2022; Toh et al., 2021). The development of COVID-19 vaccine registries may be capitalized upon to create vaccine registration for all age groups.
Learning from COVID-19 could accelerate implementation of primary HPV screening with self-testing both in clinical and community settings (Arbyn et al., 2018). There are currently multiple HPV assays on the market that can be used for self-sampling but await clinical validation (Wentzensen et al., 2021). The increase in production of molecular tests, the boom in molecular and nucleic acid testing platforms, alongside investment in laboratory capacity and expansion of manufacturing capacity for COVID-19 could contribute to increased capacity for HPV testing in the future (recommendation 3) (Poljak et al., 2021). Innovation in the development of point-of-care molecular diagnostics platforms designed to be used in non-laboratory setting by non-laboratory technicians may also facilitate broadly accessible test-and-treat cervical screening services (Foundation for Innovative New Diagnostics, 2021). Manufacturing diagnostics and vaccines to LMICs has the potential to expand accessibility of essential commodities for cervical cancer prevention (recommendation 4).
We have seen some examples of acceleration of HPV self-sampling programming in diverse settings, largely thanks to the disruption of routine services caused by COVID-19. Innovative strategies that had been successful in demonstration projects include community-based screening and mail-in HPV self-collection to maintain accessibility of cervical cancer screening services (Woo et al., 2022; Castle et al., 2011; Ejegod et al., 2022; Winer et al., 2019; Ngu et al., 2022).
This momentum in HPV testing could move screening beyond a resourced-oriented approach and to high-performance HPV testing, or even test-and-treat, for all. As demonstrated by the COVID-19 diagnostic response, this will require a huge amount of coordinated work in research and development, product development, intellectual property management, pre-qualification processes, regional manufacturing, and in-country regulatory approval (recommendation 5) (World Health Organization, 2022c). Targeted investment in point-of-care HPV testing platforms and regulatory approval of self-sampling are needed to enable screen-and-treat programs to move to test-and-treat programs (recommendation 6) (Lim, 2021; World Health Organization, 2022d). Point-of-care HPV diagnostics would ideally replace visual inspection screening programs currently operating, and enable countries without existing cervical screening programs to launch screening services offering high-performance testing. Test-and-treat programs which offer same-day treatment of pre-cancerous lesions would be more efficient by narrowing the pool of women screened with HPV who then need pelvic examination for triage. Same-day treatment with either ablative or excisional procedures should concurrently be expanded, leveraging on-line or hybrid trainings and using of emerging technology such as artificial intelligence aided colposcopy. Coordinated innovation needs to be accompanied by price negotiations of currently available and pipeline diagnostics and treatment devices to ensure affordable testing and promote equitable distribution (recommendation 7).
Academic, program implementation and industry players need to coordinate efforts to optimize available triage strategies while also pushing the envelope in research and accessibility of novel triage strategies. Coordinated research on HPV triage with pooled genotyping, biomarkers, methylation, and artificial intelligence is necessary for effective programming (recommendation 8). Cost analysis at the country level using existing open-access tools will be necessary to ensure integration of more effective technologies (Herrick et al., 2022). Patient navigation to ensure that women who screen HPV positive return for triage and treatment is essential. Development of a risk-based approach to triage and diagnosis may further increase the efficiency and ability to implement effective screening programs (Ribeiro et al., 2021).
Cervical screening programs must capitalize on the momentum of individual engagement in attaining health services and health information that COVID-19 has created. Approaches to sustainably and actively engage women in cervical cancer prevention services and enhance their sense of autonomy in the process are essential. Information, education and communication needs to be innovative and tailored to how different groups obtain information across various settings. Information must be understandable directly by individuals without requiring complex explanations and delivered outside of the healthcare setting through community health workers, word-of-mouth, informal peer counseling and on-line platforms (Eala and Tantengco, 2022; Ciceron et al., 2022; De Bocanegra et al., 2022; Christie-de Jong et al., 2022). Working with traditional leaders and within established societal structures is critical to disseminating accurate information to communities and engendering trust in the screening process (recommendation 9).
Finally, COVID-19 forced the rapid development of web-based systems that could be adapted to engage individuals and monitor cervical screening programs (Dixon et al., 2022). These advances could create options that complement existing technological solutions that already exist. Brazil has demonstrated the success of an automated call and recall system to invite women due for screening, provide results directly and schedule follow-up appointments (Corrêa et al., 2022). Bangladesh monitored cervical screening during the pandemic through customization of the District Health Information Software (DHIS2), a platform that demonstrated its flexibility in adapting to pandemic tracking during the pandemic (Basu et al., 2021). Such technological innovation can optimize the implementation of population-level screening with HPV self-sampling and facilitate monitoring and evaluation of cervical cancer programs (recommendations 10 and 11) (Woo et al., 2021).
Reimagining cervical cancer prevention
The success of cervical cancer elimination lies beyond innovation, technology and commodities – it must be one component of strong health systems with long-term strategies for managing endemic diseases. Integration into existing health systems was an area where the global coordinated response to COVID failed – it gathered international organizations without representation of global voices and failed to include sufficient perspective on delivery within variable health systems (World Health Organization, 2022b). A robust international network can support an equitable allocation of commodities and implementation for cervical cancer elimination. However, top-down global policy that is not contextualized in individual national planning is not effective long-term and perpetuates disparities. We need to examine the model by which externally-funded programs are designed and implemented, and focus on sustained success beyond project and strategy cycles. We need to work with countries to develop meaningful process indicators of successful programming that will lead to long-term progress towards eliminating cervical cancer. Pre-specified indicators must be subject to routine and rapid adjustment based on feedback that will improve programming (recommendation 12). Furthermore, long-term outcomes assessing progress on cervical cancer prevention, must include not only health indicators, but also consider economic and social metrics (recommendation 13).
Without meaningful country involvement, and national planning rooted in dynamic health systems, cervical cancer elimination will fail. Prioritizing national autonomy in designing programming that aligns with national health strategic goals, and then leveraging global financial, technical, and operational resources to support such programming is much more likely to be successful (recommendation 14). However, this global coordination will only succeed if those countries most impacted by cervical cancer are at the table.
Conclusions
The COVID-19 pandemic demonstrated that it is possible to control an infectious disease when local, national, and global entities respond to the needs of the people they serve. The successes of the COVID-19 response have provided momentum and importantly, the failures have forced a re-evaluation of what meaningful engagement is. In this moment, we can reimagine equitable cervical cancer prevention for all and move forward on a more successful path towards elimination.
Biographies
Rebecca Luckett is at the Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, United States
Sarah Feldman is at the Brigham and Women’s Hospital, Harvard Medical School, Boston, United States
Yin Ling Woo is at the University of Malaya, Kuala Lumpur, Malaysia
Anna-Barbara Moscicki is at the University of California, Los Angeles, Los Angeles, United States
Anna R Giuliano is at the H Lee Moffitt Cancer Center and Research Institute, Tampa, United States
Silvia de Sanjosé is at the National Cancer Institute, Bethesda, United States and ISGlobal, Barcelona, Spain
Andreas M Kaufmann is at Charité - Universitätsmedizin Berlin Berlin, Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany
Shuk On Annie Leung is at McGill University Health Center, Montreal, Canada
Francisco Garcia Deputy County Administrator for Community and Health Services and Chief Medical Officer, Pima County, Tuscon, United States
Karen Chan is at the University of Hong Kong, Hong Kong, China
Neerja Bhatla is at the All India Institute of Medical Sciences, New Delhi, India
Margaret Stanley is a Reviewing Editor at eLife and is at the University of Cambridge, Cambridge, United Kingdom
Julia Brotherton is at the Australian Centre for the Prevention of Cervical Cancer Melbourne, Australia
Joel Palefsky is at the University of California, San Francisco, San Francisco, United States
Suzanne Garland is at Melbourne Medical School, Royal Women’s Hospital, Melbourne, Australia
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Rebecca Luckett, Email: rluckett@bidmc.harvard.edu.
Peter Rodgers, eLife, United Kingdom.
Eduardo L Franco, McGill University, Canada.
Funding Information
This paper was supported by the following grant:
National Cancer Institute K08CA271949 to Rebecca Luckett.
Additional information
Competing interests
received a grant from NIH NCI 1K08CA271949-01. The author has no other competing interests to declare.
received grants from NCI/NIH and the Society to Improve Diagnosis in Medicine, and has received royalties from Uptodate. They have received payment for post-graduate talks at the Indian Health Service and Harvard Medical School, and for a community talk at Team Maureen. They received support for attending meetings of the ASCCP and the American Cancer Society. The author participated on the Mitre CDC sponsored initiative to integrate cervical cancer screening results into EHR and the American Cancer Society Advisory Committee ACS Cervical Cancer Roundtable. They are a Board Member of the IPVS and co-chair for ACS. The author has no other competing interests to declare.
is a committee member for policy as part of IPVS. The author has no other competing interests to declare.
has received from consulting fees from the Merck Advisory Board. The author participated on a Data Safety Monitoring Board/Advisory Board for CVIA 087 DSMB funded by PATH, and is an International Papillomavirus Society Board member. The author has no other competing interests to declare.
has received grants and consulting fees from Merck & Co, Inc No other competing interests to declare.
is a consultant at the National Cancer Institute (NIH, United States). No other competing interests to declare.
has received grants from the EU EUROSTARS Program; has received payment for consultation from Paul-Ehrlich Gesellschaft e.V; has been issued with patent WO 2020/161285 A1 (Inventor); member of the Data Safety Monitoring Board/Advisory Board for the German Cancer Research Center (DKFZ). No other competing interests to declare.
No competing interests declared.
member of Hong Kong SAR cancer coordinating committee (advisory board ) and the HK SAR cancer expert working group; President of the Hong Kong College of Obstetricians & Gynaecologists; council member of the Asian Society of Gynaecological Oncology (ASGO); board member for the Asia-Oceania Research Organisation in Genital Infection and Neoplasia. No other competing interests to declare.
Has received consulting fees from MSD Merck UK; has participated in a Global Advisory Board for HPV vaccines for Merck. No other competing interests to declare.
has received donated HPV tests and swabs for validation and research from Cepheid, Abbott, Seegene, Roche, AusDiagnostics, BD, and Copan. No other competing interests to declare.
has received grants from Merck & Co., Roche Diagnostics, Antiva Biosciences, Vir Biotechnologies and Virion Therapeutics; has received consulting fees from Merck & Co., Roche Diagnostics, Antiva Biosciences and Vir Biotechnologies; has received payment for consultation from Gilead Pharmaceuticals, Merck & Co. and Janssen Pharmaceuticals; has received support for attending meetings from Merck & Co. and Roche Diagnostics; participates on the Data Safety Monitoring Board/Advisory Board for the IPVS; is Chair of the International HPV Awareness Day Campaign; has stock or stock options in Virion Therapeutics; has received resources/services from Atila Biosystems. No other competing interests to declare.
has received consulting fees and lecture fees from Merck; has participated in an advisory Board for Merck; is President of the International Papillomavirus Society; has received an education grant for a study of HPV in young women. No other competing interests to declare.
Author contributions
Conceptualization, Writing – original draft.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Conceptualization, Writing – review and editing.
Writing – review and editing.
Writing – review and editing.
Writing – review and editing.
Conceptualization, Writing – review and editing.
Additional files
Data availability
No data was generated.
References
- American Society of Clinical Oncology Cervical Cancer: Statistics. 2023. [April 18, 2023]. https://www.cancer.net/cancer-types/cervical-cancer/statistics
- Amouzou A, Maiga A, Faye C. Health service utilisation during the COVID-19 pandemic in sub-Saharan Africa in 2020: A Multicountry empirical assessment with a focus on maternal, newborn and child health services. BMJ Global Health. 2021;7:e008069. doi: 10.1136/bmjgh-2021-008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amponsah-Dacosta E, Kagina BM, Olivier J. Health systems constraints and facilitators of human papillomavirus immunization programmes in sub-Saharan Africa: A systematic review. Health Policy and Planning. 2020;35:701–717. doi: 10.1093/heapol/czaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on Self-Sampling and HPV Testing Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. The Lancet Global Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaske A, Brotto LA, Galea LAM, Albert AY, Smith L, Kaida A, Booth A, Gordon S, Sadarangani M, Racey CS, Gottschlich A, Ogilvie GS. Barriers to Accessing contraception and cervical and breast cancer screening during COVID-19: A prospective cohort study. Journal of Obstetrics and Gynaecology Canada. 2022;44:1076–1083. doi: 10.1016/j.jogc.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu P, Lucas E, Zhang L, Muwonge R, Murillo R, Nessa A. Leveraging vertical COVID-19 investments to improve monitoring of cancer screening programme – A case study from Bangladesh. Preventive Medicine. 2021;151:106624. doi: 10.1016/j.ypmed.2021.106624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio RC, Messias AP, Moreira OA, Leis LV, Orsi BZ, Testa L, Estevez-Diz MDP. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience. 2021;15:1299. doi: 10.3332/ecancer.2021.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni L, Serrano B, Roura E, Alemany L, Cowan M, Herrero R, Poljak M, Murillo R, Broutet N, Riley LM, de Sanjose S. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. The Lancet Global Health. 2022;10:e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger EA, Jansen EE, Killen J, Kok I de, Smith MA, Sy S, Dunnewind N, G Campos N, Haas JS, Kobrin S, Kamineni A, Canfell K, Kim JJ. Impact of COVID-19 related care disruptions on cervical cancer screening in the United States. Journal of Medical Screening. 2021;28:213–216. doi: 10.1177/09691413211001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcopino X, Cruickshank M, Leeson S, Redman C, Nieminen P. The impact of COVID-19 pandemic on screening programs for cervical cancer prevention across Europe. Journal of Lower Genital Tract Disease. 2022;26:219–222. doi: 10.1097/LGT.0000000000000677. [DOI] [PubMed] [Google Scholar]
- Castanon A, Rebolj M, Pesola F, Pearmain P, Stubbs R. COVID-19 disruption to cervical cancer screening in England. J Med Screen. 2021a;29:1–6. doi: 10.1177/09691413221090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon A, Rebolj M, Pesola F, Sasieni P. Recovery strategies following COVID-19 disruption to cervical cancer screening and their impact on excess diagnoses. British Journal of Cancer. 2021b;124:1361–1365. doi: 10.1038/s41416-021-01275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle PE, Rausa A, Walls T, Gravitt PE, Partridge EE, Olivo V, Niwa S, Morrissey KG, Tucker L, Katki H, Scarinci I. Comparative community outreach to increase cervical cancer screening in the Mississippi Delta. Preventive Medicine. 2011;52:452–455. doi: 10.1016/j.ypmed.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie-de Jong F, Kotzur M, Amiri R, Ling J, Mooney JD, Robb KA. Qualitative evaluation of a Codesigned faith-based intervention for Muslim women in Scotland to encourage uptake of breast, colorectal and cervical cancer screening. BMJ Open. 2022;12:e058739. doi: 10.1136/bmjopen-2021-058739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceron AC, Jeon MJ, Monroe AK, Clausen ME, Magnus M, Le D. HPV knowledge, screening barriers and facilitators, and sources of health information among women living with HIV: Perspectives from the DC community during the COVID-19 pandemic. BMC Women’s Health. 2022;22:110. doi: 10.1186/s12905-022-01689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Jhingran A, Oaknin A. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Euro Surveillance. 2021;26:50. doi: 10.2807/1560-7917.ES.2021.26.50.2001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa FM, Migowski A, de Almeida LM, Soares MA. Cervical cancer screening, treatment and prophylaxis in Brazil: Current and future perspectives for cervical cancer elimination. Frontiers in Medicine. 2022;9:945621. doi: 10.3389/fmed.2022.945621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 Information Center Coronavirus. 2022. [November 30, 2022]. https://www.facebook.com/coronavirus_info
- Daniels V, Saxena K, Roberts C, Kothari S, Corman S, Yao L, Niccolai L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: A model based analysis. Vaccine. 2021;39:2731–2735. doi: 10.1016/j.vaccine.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bocanegra H, Goliaei Z, Khan N, Banna S, Behnam R, Mody S. Refugee women’s receptiveness for virtual engagement on reproductive health during the COVID-19 pandemic. Int J Behavior Med. 2022;1:1–10. doi: 10.1007/s12529-022-10097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis LK, Hsu C-H, Arrington AK. Reduction in standard cancer screening in 2020 throughout the US. Cancers. 2021;13:5918. doi: 10.3390/cancers13235918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon EL, Joshi SM, Ferrell W, Volpp KG, Merchant RM, Guntuku SC. COVID-19 contact tracing app reviews reveal concerns and motivations around adoption. PLOS ONE. 2022;17:e0273222. doi: 10.1371/journal.pone.0273222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eala MAB, Tantengco OAG. Global online interest in cervical cancer care in the time of COVID-19: An infodemiology study. Gynecologic Oncology Reports. 2022;41:100998. doi: 10.1016/j.gore.2022.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejegod DM, Pedersen H, Pedersen BT, Serizawa R, Bonde J. Operational experiences from the general implementation of HPV self-sampling to Danish screening non-attenders. Preventive Medicine. 2022;160:107096. doi: 10.1016/j.ypmed.2022.107096. [DOI] [PubMed] [Google Scholar]
- Eskander A, Li Q, Yu J, Hallet J, Coburn NG, Dare A, Chan KKW, Singh S, Parmar A, Earle CC, Lapointe-Shaw L, Krzyzanowska MK, Hanna TP, Finelli A, Louie AV, Look Hong N, Irish JC, Witterick IJ, Mahar A, Noel CW, Urbach DR, McIsaac DI, Enepekides D, Sutradhar R. Incident cancer detection during the COVID-19 pandemic. Journal of the National Comprehensive Cancer Network. 2022;20:276–284. doi: 10.6004/jnccn.2021.7114. [DOI] [PubMed] [Google Scholar]
- Feletto E, Grogan P, Nickson C, Smith M, Canfell K. How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Research & Practice. 2020;30:3042026. doi: 10.17061/phrp3042026. [DOI] [PubMed] [Google Scholar]
- Foundation for Innovative New Diagnostics US$21 million investment brings molecular diagnostic testing for COVID-19 and other infectious diseases closer to patients in Low- and middle-income countries. 2021. [November 4, 2022]. https://www.finddx.org/publications-and-statements/us21-million-investment-brings-molecular-diagnostic-testing-for-covid-19-and-other-infectious-diseases-closer-to-patients-in-low-and-middle-income-countries
- Foundation for Innovative New Diagnostics SARS-Cov-2 test Tracker. 2023. [April 18, 2023]. https://www.finddx.org/covid-19/test-tracker
- Garland SM, Stanley MA, Giuliano AR, Moscicki A-B, Kaufmann A, Bhatla N, Woo YL, IPVS Policy Committee IPVS statement on temporary HPV vaccine shortage: Implications globally to achieve equity. Papillomavirus Research. 2020;9:100195. doi: 10.1016/j.pvr.2020.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick T, Thomson KA, Shin M, Gannon S, Tsu V, de Sanjosé S. Acting on the call for cervical cancer elimination: Planning tools for low- and middle-income countries to increase the coverage and effectiveness of screening and treatment. BMC Health Services Research. 2022;22:1246. doi: 10.1186/s12913-022-08423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istrate-Ofiteru AM, Berbecaru EIA, Ruican D, Nagy RD, Rămescu C, Roșu GC, Iovan L, Dîră LM, Zorilă GL, Țieranu ML, Iliescu DG. The influence of SARS-Cov-2 pandemic in the diagnosis and treatment of cervical dysplasia. Medicina. 2021;57:1101. doi: 10.3390/medicina57101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AWW. Will COVID-19 be the tipping point for primary HPV self-sampling. Cancer Epidemiology, Biomarkers & Prevention. 2021;30:245–247. doi: 10.1158/1055-9965.EPI-20-1538. [DOI] [PubMed] [Google Scholar]
- Liu H, Yao Q, Li D, Zhao Z, Li Y. Impact of COVID-19 outbreak on the gynecological outpatients HPV infection rate in Wuhan, China: A retrospective observational study. Frontiers in Medicine. 2022;9:799736. doi: 10.3389/fmed.2022.799736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M, Potugari B, Bzeih R, Scheidel C, Carrera C, Shellenberger RA. Cancer screening during the COVID-19 pandemic: A systematic review and meta-analysis. Mayo Clinic Proceedings. 2021;5:1109–1117. doi: 10.1016/j.mayocpiqo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan VJ, Bambra C. COVID-19 mortality and deprivation: Pandemic, syndemic, and endemic health inequalities. The Lancet Public Health. 2022;7:e966–e975. doi: 10.1016/S2468-2667(22)00223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezei AK, Armstrong HL, Pedersen HN, Campos NG, Mitchell SM, Sekikubo M, Byamugisha JK, Kim JJ, Bryan S, Ogilvie GS. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: A systematic review. International Journal of Cancer. 2017;141:437–446. doi: 10.1002/ijc.30695. [DOI] [PubMed] [Google Scholar]
- Miller M, Xu L, Qin J, Hahn E, Ngo-Megzer Q, Mittman B. Impact of COVID-19 on cervical cancer screening rates among women aged 21-65 years in a large integrated health care system. MMWR. 2020;1:109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murewanhema G. The COVID-19 pandemic and its implications for cervical cancer treatment and prevention in Zimbabwe: Perspectives and recommendations. Pan African Medical Journal. 2021;39:149. doi: 10.11604/pamj.2021.39.149.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton T. Adapting HPV vaccine programmes in the COVID-19 pandemic. 2021. [November 30, 2022]. https://www.uicc.org/blog/adapting-hpv-vaccine-programmes-covid-19-pandemic
- Ngu S-F, Lau LSK, Li J, Wong GCY, Cheung ANY, Ngan HYS, Chan KKL. Human papillomavirus self-sampling for primary cervical cancer screening in under-screened women in Hong Kong during the COVID-19 pandemic. International Journal of Environmental Research and Public Health. 2022;19:2610. doi: 10.3390/ijerph19052610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz AP, Gierbolini-Bermúdez A, Ramos-Cartagena JM, Colón-López V, Sonawane K, Deshmukh AA, Ortiz-Ortiz KJ. Cervical cancer screening among Medicaid patients during natural disasters and the COVID-19 pandemic in Puerto Rico, 2016-2020. JAMA Network Open. 2021;4:e2128806. doi: 10.1001/jamanetworkopen.2021.28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale S, Gregorio GL, Caterina A, Francesco C, Beatrice PM, Vincenzo P, Caterina PM. COVID-19 in Low- and middle-income countries (LMICs): A narrative review from prevention to vaccination strategy. Vaccines. 2021;9:1477. doi: 10.3390/vaccines9121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaswana-Mafuya N, Peltzer K. Breast and cervical cancer screening prevalence and associated factors among women in the South African general population. Asian Pacific Journal of Cancer Prevention. 2018;19:1465–1470. doi: 10.22034/APJCP.2018.19.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S. Pandemic disrupted HPV vaccination for over 560,000 girls in Malaysia. 2022. [November 3, 2022]. https://codeblue.galencentre.org/2022/08/30/pandemic-disrupted-hpv-vaccination-for-over-560000-girls-in-malaysia
- Poljak M, Cuschieri K, Waheed D, Baay M, Vorsters A. Impact of the COVID-19 pandemic on human papillomavirus-based testing services to support cervical cancer screening. Acta Dermatovenerologica Alpina Pannonica et Adriatica. 2021;30:21–26. doi: 10.15570/actaapa.2021.5. [DOI] [PubMed] [Google Scholar]
- Popescu A, Craina M, Pantea S, Pirvu C, Radu D, Marincu I, Bratosin F, Bogdan I, Hosin S, Citu C, Bernad E, Neamtu R, Dumitru C, Mocanu AG, Gluhovschi A. COVID-19 pandemic impact on surgical treatment methods for early-stage cervical cancer: A population-based study in Romania. Healthcare. 2022;10:639. doi: 10.3390/healthcare10040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SR, Kampan N, Chew KT, Shafiee MN. The impact of the COVID-19 pandemic on the National HPV immunization program in Malaysia. Frontiers in Public Health. 2022;10:907720. doi: 10.3389/fpubh.2022.907720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed HE, Koblinsky MA, Mosley H. The Consequences of Maternal Morbidity and Mortality: Report of a Workshop. NCBI; 2000. https://www.ncbi.nlm.nih.gov/books/NBK225434/pdf/Bookshelf_NBK225434.pdf [PubMed] [Google Scholar]
- Ribeiro A, Corrêa F, Migowski A, Leal A, Martins S, Raiol T, Marques CP, Torres KL, Novetsky AP, Marcus JZ, Wentzensen N, Schiffman M, Rodriguez AC, Gage JC. Rethinking cervical cancer screening in Brazil post COVID-19: A global opportunity to adopt higher impact strategies. Cancer Prevention Research. 2021;14:919–926. doi: 10.1158/1940-6207.CAPR-21-0110. [DOI] [PubMed] [Google Scholar]
- Santos LD, Stevanato KP, Roszkowski I, Pedroso RB, Pelloso FC, Freitas KMS, de B Carvalho M, Pelloso SM. Impact of the COVID-19 pandemic on women’s health in Brazil. Journal of Multidisciplinary Healthcare. 2021;14:3205–3211. doi: 10.2147/JMDH.S322100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad LA, Brady LA, Tumiel-Berhalter LM, Bentham A, Vitale K, Norton A, Noronha G, Swanger C, Morley CP. Impact of COVID-19 on screening rates for colorectal, breast, and cervical cancer: Practice feedback from a quality improvement project in primary care. Journal of Patient-Centered Research and Reviews. 2021;8:347–353. doi: 10.17294/2330-0698.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano B, Ibáñez R, Robles C, Peremiquel-Trillas P, de Sanjosé S, Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Preventive Medicine. 2022;154:106900. doi: 10.1016/j.ypmed.2021.106900. [DOI] [PubMed] [Google Scholar]
- Smith DL, Perkins RB. Low rates of HPV vaccination and cervical cancer screening: Challenges and opportunities in the context of the COVID-19 pandemic. Preventive Medicine. 2022;159:107070. doi: 10.1016/j.ypmed.2022.107070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani J, Datchoua AM, Petignat P, Kenfack B, Schmidt NC. Effects of the COVID-19 pandemic on an urban cervical cancer screening program in West Cameroon. International Journal of Gynecological Cancer. 2021;31:1297–1298. doi: 10.1136/ijgc-2021-002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolberg S. As poor nations seek COVID pills, officials fear repeat of AIDS crisis. 2022. [November 4, 2022]. https://www.nytimes.com/2022/05/08/us/politics/covid-pills-global-aids-hiv.html
- Tatar M, Shoorekchali JM, Faraji MR, Wilson FA. International COVID-19 vaccine inequality amid the pandemic: Perpetuating a global crisis. Journal of Global Health. 2021;11:03086. doi: 10.7189/jogh.11.03086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H, San JE, Cotten M, Moir M, Tegomoh B, Mboowa G, Martin DP, Baxter C, Lambisia AW, Diallo A, Amoako DG, Diagne MM, Sisay A, Zekri A-RN, Gueye AS, Sangare AK, Ouedraogo A-S, Sow A, Musa AO, Sesay AK, Abias AG, Elzagheid AI, Lagare A, Kemi A-S, Abar AE, Johnson AA, Fowotade A, Oluwapelumi AO, Amuri AA, Juru A, Kandeil A, Mostafa A, Rebai A, Sayed A, Kazeem A, Balde A, Christoffels A, Trotter AJ, Campbell A, Keita AK, Kone A, Bouzid A, Souissi A, Agweyu A, Naguib A, Gutierrez AV, Nkeshimana A, Page AJ, Yadouleton A, Vinze A, Happi AN, Chouikha A, Iranzadeh A, Maharaj A, Batchi-Bouyou AL, Ismail A, Sylverken AA, Goba A, Femi A, Sijuwola AE, Marycelin B, Salako BL, Oderinde BS, Bolajoko B, Diarra B, Herring BL, Tsofa B, Lekana-Douki B, Mvula B, Njanpop-Lafourcade B-M, Marondera BT, Khaireh BA, Kouriba B, Adu B, Pool B, McInnis B, Brook C, Williamson C, Nduwimana C, Anscombe C, Pratt CB, Scheepers C, Akoua-Koffi CG, Agoti CN, Mapanguy CM, Loucoubar C, Onwuamah CK, Ihekweazu C, Malaka CN, Peyrefitte C, Grace C, Omoruyi CE, Rafaï CD, Morang’a CM, Erameh C, Lule DB, Bridges DJ, Mukadi-Bamuleka D, Park D, Rasmussen DA, Baker D, Nokes DJ, Ssemwanga D, Tshiabuila D, Amuzu DSY, Goedhals D, Grant DS, Omuoyo DO, Maruapula D, Wanjohi DW, Foster-Nyarko E, Lusamaki EK, Simulundu E, Ong’era EM, Ngabana EN, Abworo EO, Otieno E, Shumba E, Barasa E, Ahmed EB, Ahmed EA, Lokilo E, Mukantwari E, Philomena E, Belarbi E, Simon-Loriere E, Anoh EA, Manuel E, Leendertz F, Taweh FM, Wasfi F, Abdelmoula F, Takawira FT, Derrar F, Ajogbasile FV, Treurnicht F, Onikepe F, Ntoumi F, Muyembe FM, Ragomzingba FEZ, Dratibi FA, Iyanu F-A, Mbunsu GK, Thilliez G, Kay GL, Akpede GO, van Zyl GU, Awandare GA, Kpeli GS, Schubert G, Maphalala GP, Ranaivoson HC, Omunakwe HE, Onywera H, Abe H, Karray H, Nansumba H, Triki H, Kadjo HAA, Elgahzaly H, Gumbo H, Mathieu H, Kavunga-Membo H, Smeti I, Olawoye IB, Adetifa IMO, Odia I, Ben Boubaker IB, Muhammad IA, Ssewanyana I, Wurie I, Konstantinus IS, Halatoko JWA, Ayei J, Sonoo J, Makangara J-CC, Tamfum J-JM, Heraud J-M, Shaffer JG, Giandhari J, Musyoki J, Nkurunziza J, Uwanibe JN, Bhiman JN, Yasuda J, Morais J, Kiconco J, Sandi JD, Huddleston J, Odoom JK, Morobe JM, Gyapong JO, Kayiwa JT, Okolie JC, Xavier JS, Gyamfi J, Wamala JF, Bonney JHK, Nyandwi J, Everatt J, Nakaseegu J, Ngoi JM, Namulondo J, Oguzie JU, Andeko JC, Lutwama JJ, Mogga JJH, O’Grady J, Siddle KJ, Victoir K, Adeyemi KT, Tumedi KA, Carvalho KS, Mohammed KS, Dellagi K, Musonda KG, Duedu KO, Fki-Berrajah L, Singh L, Kepler LM, Biscornet L, de Oliveira Martins L, Chabuka L, Olubayo L, Ojok LD, Deng LL, Ochola-Oyier LI, Tyers L, Mine M, Ramuth M, Mastouri M, ElHefnawi M, Mbanne M, Matsheka MI, Kebabonye M, Diop M, Momoh M, da L Lima Mendonça M, Venter M, Paye MF, Faye M, Nyaga MM, Mareka M, Damaris M-M, Mburu MW, Mpina MG, Owusu M, Wiley MR, Tatfeng MY, Ayekaba MO, Abouelhoda M, Beloufa MA, Seadawy MG, Khalifa MK, Matobo MM, Kane M, Salou M, Mbulawa MB, Mwenda M, Allam M, Phan MVT, Abid N, Rujeni N, Abuzaid N, Ismael N, Elguindy N, Top NM, Dia N, Mabunda N, Hsiao N-Y, Silochi NB, Francisco NM, Saasa N, Bbosa N, Murunga N, Gumede N, Wolter N, Sitharam N, Ndodo N, Ajayi NA, Tordo N, Mbhele N, Razanajatovo NH, Iguosadolo N, Mba N, Kingsley OC, Sylvanus O, Femi O, Adewumi OM, Testimony O, Ogunsanya OA, Fakayode O, Ogah OE, Oludayo O-E, Faye O, Smith-Lawrence P, Ondoa P, Combe P, Nabisubi P, Semanda P, Oluniyi PE, Arnaldo P, Quashie PK, Okokhere PO, Bejon P, Dussart P, Bester PA, Mbala PK, Kaleebu P, Abechi P, El-Shesheny R, Joseph R, Aziz RK, Essomba RG, Ayivor-Djanie R, Njouom R, Phillips RO, Gorman R, Kingsley RA, Neto Rodrigues RM, Audu RA, Carr RAA, Gargouri S, Masmoudi S, Bootsma S, Sankhe S, Mohamed SI, Femi S, Mhalla S, Hosch S, Kassim SK, Metha S, Trabelsi S, Agwa SH, Mwangi SW, Doumbia S, Makiala-Mandanda S, Aryeetey S, Ahmed SS, Ahmed SM, Elhamoumi S, Moyo S, Lutucuta S, Gaseitsiwe S, Jalloh S, Andriamandimby SF, Oguntope S, Grayo S, Lekana-Douki S, Prosolek S, Ouangraoua S, van Wyk S, Schaffner SF, Kanyerezi S, Ahuka-Mundeke S, Rudder S, Pillay S, Nabadda S, Behillil S, Budiaki SL, van der Werf S, Mashe T, Mohale T, Le-Viet T, Velavan TP, Schindler T, Maponga TG, Bedford T, Anyaneji UJ, Chinedu U, Ramphal U, George UE, Enouf V, Nene V, Gorova V, Roshdy WH, Karim WA, Ampofo WK, Preiser W, Choga WT, Ahmed YA, Ramphal Y, Bediako Y, Naidoo Y, Butera Y, de Laurent ZR, Ouma AEO, von Gottberg A, Githinji G, Moeti M, Tomori O, Sabeti PC, Sall AA, Oyola SO, Tebeje YK, Tessema SK, de Oliveira T, Happi C, Lessells R, Nkengasong J, Wilkinson E, Africa Pathogen Genomics Initiative The evolving SARS-Cov-2 epidemic in Africa: Insights from rapidly expanding genomic surveillance. Science. 2022;378:eabq5358. doi: 10.1126/science.abq5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Fund The global fund achieves significant price reductions for antigen rapid diagnostic tests (Ag RDTS) 2022. [November 4, 2022]. https://www.theglobalfund.org/en/covid-19/news/2021-12-02-the-global-fund-achieves-significant-price-reductions-for-antigen-rapid-diagnostic-tests-ag-rdts
- The Lancet Oncology HPV vaccination in South Asia: New progress, old challenges. The Lancet Oncology. 2022;23:1233. doi: 10.1016/S1470-2045(22)00567-8. [DOI] [PubMed] [Google Scholar]
- Toh ZQ, Russell FM, Garland SM, Mulholland EK, Patton G, Licciardi PV. Human papillomavirus vaccination after COVID-19. JNCI Cancer Spectrum. 2021;5:pkab011. doi: 10.1093/jncics/pkab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Union for International Cancer Control Cervical cancer elimination in Africa: Where are we now and where do we need to be? 2023. [April 18, 2023]. https://www.uicc.org/sites/main/files/atoms/files/UICC-Cervical_Cancer_in_Africa_FA_Single.pdf
- UNITAID FIND and UNITAID invest to support technology transfer and boost local production of COVID-19 rapid tests in low- and middle-income countries. 2021. [November 4, 2022]. https://unitaid.org/news-blog/find-unitaid-technology-transfer-covid-19/#en
- Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, Anyaneji UJ, Bester PA, Boni MF, Chand M, Choga WT, Colquhoun R, Davids M, Deforche K, Doolabh D, du Plessis L, Engelbrecht S, Everatt J, Giandhari J, Giovanetti M, Hardie D, Hill V, Hsiao N-Y, Iranzadeh A, Ismail A, Joseph C, Joseph R, Koopile L, Kosakovsky Pond SL, Kraemer MUG, Kuate-Lere L, Laguda-Akingba O, Lesetedi-Mafoko O, Lessells RJ, Lockman S, Lucaci AG, Maharaj A, Mahlangu B, Maponga T, Mahlakwane K, Makatini Z, Marais G, Maruapula D, Masupu K, Matshaba M, Mayaphi S, Mbhele N, Mbulawa MB, Mendes A, Mlisana K, Mnguni A, Mohale T, Moir M, Moruisi K, Mosepele M, Motsatsi G, Motswaledi MS, Mphoyakgosi T, Msomi N, Mwangi PN, Naidoo Y, Ntuli N, Nyaga M, Olubayo L, Pillay S, Radibe B, Ramphal Y, Ramphal U, San JE, Scott L, Shapiro R, Singh L, Smith-Lawrence P, Stevens W, Strydom A, Subramoney K, Tebeila N, Tshiabuila D, Tsui J, van Wyk S, Weaver S, Wibmer CK, Wilkinson E, Wolter N, Zarebski AE, Zuze B, Goedhals D, Preiser W, Treurnicht F, Venter M, Williamson C, Pybus OG, Bhiman J, Glass A, Martin DP, Rambaut A, Gaseitsiwe S, von Gottberg A, de Oliveira T. Rapid epidemic expansion of the SARS-Cov-2 Omicron variant in Southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen N, Clarke MA, Perkins RB. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improving resilience and reduce disparities. Preventive Medicine. 2021;151:106596. doi: 10.1016/j.ypmed.2021.106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer RL, Lin J, Tiro JA, Miglioretti DL, Beatty T, Gao H, Kimbel K, Thayer C, Buist DSM. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment. JAMA Network Open. 2019;2:e1914729. doi: 10.1001/jamanetworkopen.2019.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YL, Gravitt P, Khor SK, Ng CW, Saville M. Accelerating action on cervical cancer screening in lower- and middle-income countries (LMICs) post COVID-19 era. Preventive Medicine. 2021;144:106294. doi: 10.1016/j.ypmed.2020.106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YL, Khoo SP, Gravitt P, Hawkes D, Rajasuriar R, Saville M. The implementation of a primary HPV self-testing cervical screening program in Malaysia through program ROSE – lessons learnt and moving forward. Current Oncology. 2022;29:7379–7387. doi: 10.3390/curroncol29100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Global strategy to accelerate the elimination of cervical cancer as a public health problem. 2020. [November 8, 2022]. https://www.who.int/initiatives/cervical-cancer-elimination-initiative#cms
- World Health Organization Global Market Study: HPV. 2022a. [April 18, 2023]. https://cdn.who.int/media/docs/default-source/immunization/mi4a/who-hpv-vaccine-global-market-study-april-2022.pdf?sfvrsn=6acb4c98_1&download=true
- World Health Organization External evaluation of the access to COVID-19 tools accelerator (ACT-A) 2022b. [November 7, 2022]. https://www.who.int/publications/m/item/external-evaluation-of-the-access-to-covid-19-tools-accelerator-(act-a)
- World Health Organization The ACT-accelerator: Two years of impact. 2022c. [November 4, 2022]. https://www.who.int/publications/m/item/the-act-accelerator--two-years-of-impact
- World Health Organization Use of SARS-Cov-2 antigen-detection rapid diagnostic tests for COVID-19 self-testing. 2022d. [November 11, 2022]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Ag-RDTs-Self_testing-2022.1
- Zhang X, Zeng Q, Cai W, Ruan W. Trends of cervical cancer at global, regional, and national level: Data from the global burden of disease study 2019. BMC Public Health. 2021;21:6. doi: 10.1186/s12889-021-10907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]