Abstract
Background
Old age, obesity, and certain chronic conditions are among the risk factors for severe COVID-19. More information is needed on whether inherited metabolic disorders (IMD) confer risk of more severe COVID-19. We aimed to establish COVID-19 severity and associated risk factors in patients with IMD currently followed at a single metabolic center.
Methods
Among all IMD patients followed at a single metabolic referral center who had at least one clinic visit since 2018, those with accessible medical records were reviewed for SARS-CoV-2 tests. COVID-19 severity was classified according to the WHO recommendations, and IMD as per the international classification of IMD.
Results
Among the 1841 patients with IMD, 248 (13.5%) had tested positive for COVID-19, 223 of whom gave consent for inclusion in the study (131 children and 92 adults). Phenylalanine hydroxylase (48.4%) and biotinidase (12.1%) deficiencies were the most common diagnoses, followed by mucopolysaccharidoses (7.2%). 38.1% had comorbidities, such as neurologic disabilities (22%) or obesity (9.4%). The majority of COVID-19 episodes were asymptomatic (16.1%) or mild (77.6%), but 6 patients (2.7%) each had moderate and severe COVID-19, and two (0.9%) had critical COVID-19, both of whom died. 3 patients had an acute metabolic decompensation during the infection. Two children developed multisystem inflammatory syndrome (MIS-C). Long COVID symptoms were present in 25.2%. Presence of comorbidities was significantly associated with more severe COVID-19 in adults with IMD (p < 0.01), but not in children (p = 0.45). Compared to other categories of IMD, complex molecule degradation disorders were significantly associated with more severe COVID-19 in children (p < 0.01); such a significant IMD category distinction was not found in adults.
Discussion
This is the largest study on COVID-19 in IMD patients relying on real-word data and objective definitions, and not on merely expert opinions or physician surveys. COVID-19 severity and long COVID incidence in IMD are probably similar to the general population, and the risk of acute metabolic decompensation is not likely to be greater than that in other acute infections. Disease category (complex molecule degradation) in children, and comorbidities in adults may be associated with COVID-19 severity in IMD. Additionally, the first documented accounts of COVID-19 in 27 different IMD are recorded. The high occurrence of MIS-C may be coincidental, but warrants further study.
Keywords: Inherited metabolic diseases, Disorders of complex molecule degradation, Comorbidity, SARS-CoV-2, Coronavirus disease 2019 (COVID-19), Pneumonia
1. Introduction
It has been more than 3 years since coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, was declared a pandemic by the World Health Organization (WHO). Although preventive and therapeutic advances have curbed the devastating toll, the pandemic is still ongoing, constantly evolving with long-term effects and novel viral variants of concern, indicating that further understanding of COVID-19 will be relevant for many years to come.
Risk stratification is crucial for pandemic response, and it became clear early in the course of the pandemic that individuals with advanced age, male gender, and certain common chronic conditions including obesity, diabetes mellitus, hypertension, immune suppression and heart, lung or kidney disease have a higher risk of severe SARS-CoV-2 infection [1]. However, risk determination for rare disorders is difficult. For example, the international classification of inherited metabolic disorders (ICIMD) includes 1450 mostly rare or extremely rare disorders in 24 categories [2]. Individuals with inherited metabolic disorders (IMD) can be considered a vulnerable group because of their multisystem involvement and some may have risk of metabolic decompensation triggered by infections. Reports of COVID-19 in individuals with IMD began to emerge in June 2020 [3]. With more cases subsequently reported, the possibility of an increased risk due to the underlying metabolic defect and or to associated comorbidities arose. Additionally, as the pandemic and the knowledge around it evolved, other issues emerged, including long-term complications of COVID-19, and secondary problems related to disruption of health services, such as inability to access drugs or hospitals, which was shown to be associated with disease progression as shown by our group and others [4,5]. Availability of vaccines marked a shift in the dynamics of the pandemic and eventually paved the way for transitioning to a “new normal”. In Turkey, where the present study was performed, COVID-19 vaccines have been available for individuals over −65 years old and high-risk population, all adults (> 18 years), and children aged 12–18 years since February 2021, June 2021, and September 2021, respectively.
According to a physician survey conducted by the European Reference Network for Hereditary Metabolic Disorders (MetabERN), adult patients were reported to have a higher rate of hospitalization and a more severe course compared to general population and pediatric patients [6]. Similarly, Hasnat et al. demonstrated that children had milder clinical symptoms than adults [7]. Mütze et al. reported that hospitalization rates did not differ by diagnosis or age group, and no complications specific to IMD or related to COVID-19 have been observed [8]. More information is needed on whether inherited metabolic disorders (IMD) confer risk of more severe COVID-19. In this study, we aimed to answer this question and describe the features of COVID-19 in IMD by analyzing the COVID-19 histories and long-term complications of a large cohort of patients at a single center.
2. Material and methods
2.1. Study design and participants
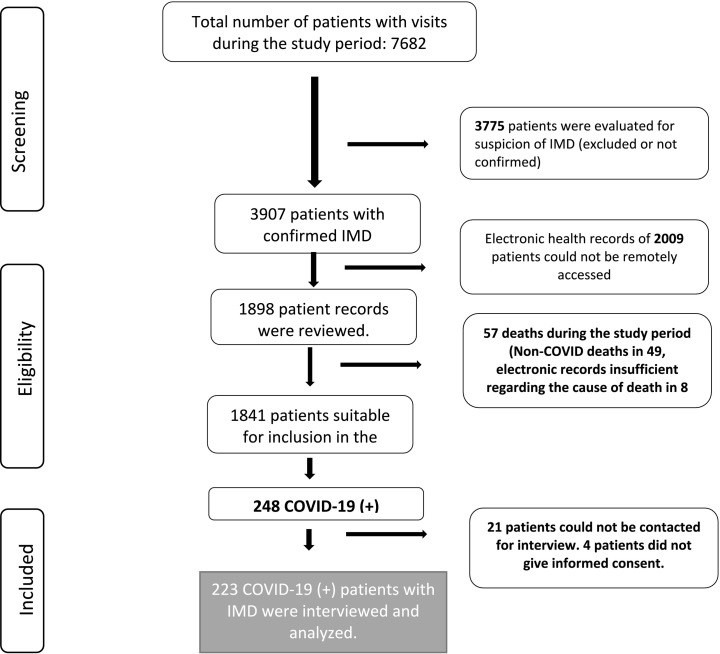
This study was approved by Hacettepe University Ethics Committee for Non-Interventional Clinical Studies (GO 22/176, 2022/08–23). Informed consent was obtained from all patients or their parents/guardians. The study population consisted of pediatric and adult patients followed at the Pediatric Metabolism Unit of Hacettepe University İhsan Dogramacı Children's Hospital with a definitive diagnosis of IMD. Among all IMD patients with at least one in-person or remote visit between 1 January 2018 and 30 June 2021, those with accessible electronic health record data were reviewed retrospectively for SARS-CoV-2 PCR or antibody tests. The flowchart of participant inclusion is shown in Fig. 1 .
Fig. 1.
Flowchart of patient selection. IMD: inherited metabolic disorders.
2.2. Data collection
The patients were contacted via phone calls and asked to participate in a survey including demographic data, medical history (comorbidities, medications etc.), and features of COVID-19 infection (timing, vaccination status, symptoms, treatment etc.). Hospital records were also reviewed retrospectively.
2.3. Definitions and classifications
Patients with records of SARS-CoV-2 antibody positivity before vaccination or PCR positivity at any time were defined to have had COVID-19. COVID-19 severity was classified according to the WHO recommendations as asymptomatic, mild, moderate, severe, or critical illness. In summary, the presence of symptoms without evidence of viral pneumonia or hypoxia defines mild COVID-19, non-severe pneumonia defines moderate COVID-19, severe pneumonia (eg. hypoxia, respiratory distress) defines severe COVID-19 and acute respiratory distress syndrome defines critical COVID-19 [9]. Determination of odds ratios for susceptibility to more severe COVID-19 was based on the development of pneumonia (asymptomatic-mild vs. moderate-severe-critical COVID-19). Death due to COVID-19 was defined based on WHO recommendations, as death resulting from a compatible illness in a patient with COVID-19 (only confirmed cases were included) without a period of complete recovery from COVID-19 between illness and death, unless there is a clear alternative cause of death unrelated to COVID-19 [10]. Long COVID was defined according to Centers for Disease Control and Prevention (CDC) recommendations as symptoms (sensory, neurologic, cardiorespiratory, and mental) that persist at least four weeks after infection. CDC recommendations were used to define multisystem inflammatory syndrome in children (MIS-C), a severe condition characterized by hyperinflammation and hypercoagulability [11]. IMD were classified according to the ICIMD [2]. If a patient experienced two or more episodes of COVID-19, it was counted as 1 patient and the characteristics of the more severe infection was recorded.
2.4. Statistical analyses
The data were analyzed by SPSS v25.0 (SPSS Inc., Chicago, USA). Normality of the variables was investigated by visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov and Shapiro-Wilk tests). Descriptive statistics were presented as mean ± standard deviation, median, range (minimum-maximum), interquartile range (IQR), and frequencies. The chi-square test or Fisher's exact test (based on whether chi-square test assumptions hold) was used to compare proportions in different groups. p < 0.05 was considered to show a statistically significant result.
3. Results
There were 1841 patients with confirmed IMD treated at our center during the study period, whose electronic health records could be accessed remotely, 248 (13.5%) of whom had tested positive for SARS-CoV-2. 223 of these patients gave consent for inclusion in the study (131 children and 92 adults) (Fig. 1).
3.1. General patient characteristics
The demographic characteristics and comorbidities of the whole study population, and also separately as children and adults are shown in Table 1 . Phenylalanine hydroxylase (PAH, n = 108, 48.4%) and biotinidase (n = 27, 12.1%) deficiencies were the most common diagnoses, followed by mucopolysaccharidoses (n = 16, 7.2%). Diagnoses and ICIMD classification of all COVID-19 positive patients are shown in Table 2 . 98 (90.7%) of PAH and 24 (88.9%) of biotinidase deficiency patients were diagnosed via newborn screening. Newborn screening is not available in Turkey for the other IMD mentioned in this study.
Table 1.
Characteristics of the patients and COVID-19 (N = 223, unless indicated otherwise).
| Patient characteristics | Child |
Adult |
All group | |
|---|---|---|---|---|
| 58.7% (n = 131) | 41.1% (n = 92) | |||
| Age (years) | Median (IQR) | 9 (5–13) | 25 (21−30) | 14 (7–23) |
| Min-max | 0.5–17 | 18–72 | 0.5–72 | |
| Sex | Male | 48.1% (n = 63) | 43.5% (n = 40) | 51.6% (n = 115) |
| Female | 51.9% (n = 68) | 56.5% (n = 52) | 48.4% (n = 108) | |
| Educational attainment of the patients | None | 40.5% (n = 53) | 18.5% (n = 17) | 31.4% (n = 70) |
| Primary school | 29% (n = 38) | 9.9% (n = 9) | 21.1% (n = 47) | |
| Secondary school | 19.1% (n = 25) | 6.5% (n = 6) | 13.9% (n = 31) | |
| High school | 11.5% (n = 15) | 44.6% (n = 41) | 25.1% (n = 56) | |
| University or higher | – | 20.7% (n = 19) | 8.5% (n = 19) | |
| Highest educational attainment of the parents (n = 217) | (n = 128) | (n = 89) | ||
| None: | 1.6% (n = 2) | 2.2% (n = 2) | 1.8% (n = 4) | |
| Primary school: | 27.3% (n = 35) | 55.1% (n = 49) | 38.7% (n = 84) | |
| Secondary school: | 12.5% (n = 16) | 11.2% (n = 10) | 12.0% (n = 26) | |
| High school: | 30.5% (n = 39) | 19.1% (n = 17) | 25.8% (n = 56) | |
| University or higher: | 28.1% (n = 36) | 12.4% (n = 11) | 21.7% (n = 47) | |
| Specific metabolic treatment | Metabolic diet only | 35.1% (n = 46) | 68.5% (n = 63) | 48.9% (n = 109) |
| Medication only | 30.5% (n = 40) | 8.7% (n = 8) | 21.5% (n = 48) | |
| Metabolic diet and medication | 12.2% (n = 16) | 14.1% (n = 13) | 13.0% (n = 29) | |
| None | 22.1% (n = 29) | 8.7% (n = 8) | 16.6% (n = 37) | |
| Comorbidities | No | 66.4% (n = 87) | 55.4% (n = 51) | 61.9% (n = 138) |
| Yes | 33.6% (n = 44) | 44.6% (n = 41) | 38.1% (n = 85) | |
| Neurologic impairment | 19.1% (n = 25) | 26.1% (n = 24) | 22.0% (n = 49) | |
| Obesity | 5.3% (n = 7) | 15.2% (n = 14) | 9.4% (n = 21) | |
| Cardiovascularδ | 7.6% (n = 10) | 3.3% (n = 3) | 5.8% (n = 13) | |
| Endocrine system | 3.1% (n = 4) | 2.2% (n = 2) | 2.7% (n = 6) | |
| Respiratory systemρ | 3.8% (n = 5) | 1.1% (n = 1) | 2.7% (n = 6) | |
| Renal and urinary systemγ | 2.3% (n = 3) | 2.2% (n = 2) | 2.2% (n = 5) | |
| Rheumatologicω | 0.8% (n = 1) | 3.3% (n = 3) | 1.8% (n = 4) | |
| Hypertension | 1.5% (n = 2) | 2.2% (n = 2) | 1.8% (n = 4) | |
| Lymphoma | 0.8% (n = 1) | – | 0.4% (n = 1) | |
| Immunosuppressive treatment (Post- HSCT) | 0.8% (n = 1) | – | 0.4% (n = 1) | |
| History related to COVID-19 | ||||
| Source of SARS-CoV-2 infection | Home | 51.9% (n = 68) | 40.2% (n = 37) | 47.1% (n = 105) |
| School/work | 12.2% (n = 16) | 5.4% (n = 5) | 9.4% (n = 21) | |
| Nosocomial | 3.1% (n = 4) | 6.5% (n = 6) | 4.5% (n = 10) | |
| Unknown | 32.8% (n = 43) | 47.8% (n = 44) | 39.0% (n = 87) | |
| Timing of SARS-CoV-2 infection | During delta variant wave | 29.8% (n = 39) | 28.3% (n = 26) | 29.1% (n = 65) |
| During omicron variant wave | 31.3% (n = 41) | 22.8% (n = 21) | 27.8% (n = 62) | |
| Other | 38.9% (n = 51) | 48.9% (n = 45) | 43.0% (n = 96) | |
| Type of SARS-CoV-2 test | PCR: | 96.2% (n = 126) | 100% (n = 92) | 97.8% (n = 218) |
| Antibody: | 3.8% (n = 5) | – | 2.2% (n = 5) | |
| Hospitalization | Yes | 13% (n = 17) | 8.7% (n = 8) | 11.2% (n = 25) |
| No | 87% (n = 114) | 91.3% (n = 84) | 88.8% (n = 198) | |
| Duration of hospitalization (days) (n = 25) | Median: 6 | Median: 6 | Median: 6 | |
| IQR: 5–7 | IQR: 1–9 | IQR: 5–8 | ||
| Min-max: 2–30 | Min-max: 1–13 | Min-max:1–30 | ||
| Oxygen requirement | Yes | 3.8% (n = 5) | 3.3% (n = 3) | 3.5% (n = 8) |
| No: | 96.2% (n = 126) | 96.7% (n = 89) | 96.5% (n = 215) | |
| Intensive care unit admission | Yes | 1.5% (n = 2) | 1.1% (n = 1) | 1.3% (n = 3) |
| No | 98.5% (n = 129) | 98.9% (n = 91) | 98.7% (n = 220) | |
| Invasive mechanical ventilation | Yes | 0.8% (n = 1) | 1.1% (n = 1) | 0.9% (n = 2) |
| No | 99.2% (n = 130) | 98.9% (n = 91) | 99.1% (n = 221) | |
| COVID-19 related death | Yes | 0.8% (n = 1) | 1.1% (n = 1) | 0.9% (n = 2) |
| No | 99.2% (n = 130) | 98.9% (n = 91) | 99.1% (n = 221) | |
| Medication during COVID-19 infection | No | 51.1% (n = 67) | 33.7% (n = 31) | 43.9% (n = 98) |
| Yes | 48.9% (n = 64) | 66.3% (n = 61) | 56.1% (n = 125) | |
| Antipyretic/analgesic | 43.5% (n = 57) | 35.9% (n = 33) | 40.5% (n = 90) | |
| Favipiravir | 4.6% (n = 6) | 52.2% (n = 48) | 24.3% (n = 54) | |
| Antibiotics | 16.0% (n = 21) | 7.6% (n = 7) | 12.6% (n = 28) | |
| Hydroxychloroquine sulfate | none | 13% (n = 12) | 5.4% (n = 12) | |
| Anticoagulant/antithrombotic | 1.5% (n = 2) | 4.3% (n = 4) | 2.7% (n = 6) | |
| Dexamethasone | 2.3% (n = 3) | 1.1% (n = 1) | 1.8% (n = 4) | |
| Intravenous immune globulin | 2.3% (n = 3) | – | 1.4% (n = 3) | |
| Oseltamivir | 1.5% (n = 2) | – | 0.9% (n = 2) | |
| Feeding difficulties | Yes | 28.2% (n = 37) | 28.3% (n = 26) | 28.3% (n = 63) |
| No | 71.8% (n = 94) | 71.7% (n = 66) | 71.7% (n = 160) | |
| Interruption of metabolic diet (n = 119) | (n = 53) | (n = 66) | ||
| Yes | 32% (n = 17) | 31.8% (n = 21) | 31.9% (n = 38) | |
| No | 68% (n = 36) | 68.2% (n = 45) | 68.1% (n = 81) | |
| Interruption of metabolic medication (n = 100) | (n = 71) | (n = 29) | ||
| Yes | 16.9% (n = 12) | 13.7% (n = 4) | 16.0% (n = 16) | |
| No | 83.1% (n = 59) | 863% (n = 25) | 84.0% (n = 84) | |
| Vaccination status before contracting COVID-19 (≥12 years, n = 138) | (n = 45) | (n = 93) | ||
| Vaccinated | 15.6% (n = 7) | 41.9% (n = 39) | 33.3% (n = 46) | |
| Unvaccinated | 84.4% (n = 38) | 58.1% (n = 54) | 67.7% (n = 92) | |
| Vaccination status, overall (≥ 12 years, n = 138) | (n = 45) | (n = 93) | ||
| Vaccinated | 35.6% (n = 16) | 77.4% (n = 72) | 63.8% (n = 88) | |
| Unvaccinated | 64.4% (n = 29) | 22.6% (n = 21) | 36.2% (n = 50) | |
COVID-19: coronavirus disease 2019, HSCT: hematopoietic stem cell transplantation, IQR: interquartile range, PCR: polymerase chain reaction, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Congestive heart failure (n = 11), coronary artery disease (n = 1), mitral valve prolapse (n = 1).
asthma (n = 1), tracheostomy (n = 2), non-invasive mechanical ventilation (n = 3).
Familial Mediterranean fever (n = 3), systemic lupus erythematosus (n = 1).
renal failure (n = 5).
Table 2.
The diagnosis and ICIMD classifications of COVID-19-positive patients.
| IMD Diagnosis | ICIMD subcategory | ICIMD category | Child n (%) | Adult n (%) | Overall n (%) |
|---|---|---|---|---|---|
| Phenylalanine hydroxylase deficiency: | Disorders of phenylalanine and tyrosine metabolism | Disorders of amino acid metabolism | 108 (48.4) | ||
| PKU on dietary treatment(n = 77) | n = 26 (50) | n = 51 (91.1) | |||
| HPA not requiring treatment (n = 21) | n = 16 (30.8) | n = 5 (8.9) | |||
| BH4-responsive PKU (n = 10) | n = 10 (19.2) | ||||
| Biotinidase deficiency | Disorders of biotin metabolism | Disorders of vitamin and cofactor metabolism | 24 (18.3) | 3 (3.3) | 27 (12.1) |
| Mucopolysaccharidosis | Disorders of glycosaminoglycan degradation | Disorders of complex molecule degradation | 10 (7.6) | 6 (6.5) | 16 (7.2) |
| Dyslipidemia | Disorders of lipoprotein metabolism | 5 (3.8) | 5 (5.4) | 10 (4.5) | |
| Maple syrup urine disease# | Branched-chain amino acids | Disorders of amino acid metabolism | 6 (4.6) | 3 (3.3) | 9 (4.0) |
| Methylmalonic acidemia# | Organic acidurias | Disorders of amino acid metabolism | 4 (3.1) | 2 (2.2) | 6 (2.7) |
| Homocystinuria⁎ | Sulfur-containing amino acids | Disorders of amino acid metabolism | 2 (1.5) | 3 (3.3) | 5 (2.2) |
| Glutaric aciduria type 1# | Organic acidurias | Disorders of amino acid metabolism | 2 (1.5) | 2 (2.2) | 4 (1.8) |
| Hereditary fructose intolerance⁎ | Disorders of galactose and fructose metabolism | Disorders of carbohydrate metabolism | 2 (1.5) | 2 (2.2) | 4 (1.8) |
| Fructose-1,6-diphosphatase deficiency⁎, # | Disorders of gluconeogenesis | Disorders of carbohydrate metabolism | 2 (1.5) | – | 2 (0.9) |
| Ornithine transcarbamylase deficiency⁎, # | Disorders of the urea cycle and hyperammonemias | Disorders of amino acid metabolism | – | 2 (2.2) |
2 (0.9) |
| Isovaleric acidemia⁎, # | Organic acidurias | Disorders of amino acid metabolism | 1 (0.8) | 1 (1.1) | 2 (0.9) |
| Lysinuric protein intolerance⁎# | Amino acid transport | Disorders of amino acid metabolism | 1 (0.8) | 1 (1.1) | 2 (0.9) |
| HMG-CoA lyase deficiency# | Disorders of ketone body metabolism | Disorders of fatty acid and ketone metabolism | 2 (1.5) | – | 2 (0.9) |
| X-linked adrenoleukodystrophy⁎ | Disorders of peroxisomal fatty acid oxidation | Disorders of lipid metabolism | 1 (0.8) | – | 1 (0.4) |
| Alkaptonuria⁎ | Disorders of phenylalanine and tyrosine metabolism | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Mitochondrial acetoacetyl-CoA thiolase deficiency⁎# | Disorders of ketone body metabolism | Disorders of fatty acid and ketone metabolism | 1 (0.8) | – | 1 (0.4) |
| Chanarin-Dorfman syndrome⁎ | Disorders of glycerolipid metabolism | Disorders of lipid metabolism | 1 (0.8) | – | 1 (0.4) |
| Dihydropteridine reductase deficiency⁎ | Disorders of tetrahydrobiopterin metabolism | Disorders of vitamin and cofactor metabolism | 1 (0.8) | – | 1 (0.4) |
| Mitochondrial and cytoplasmic glycyl-tRNA synthetase deficiency⁎ | Disorders of mitochondrial aminoacyl-tRNA synthetases | Disorders of mitochondrial gene expression | – | 1 (1.1) |
1 (0.4) |
| Hepatic glycogen synthase deficiency⁎, # | Disorders of glycogen metabolism | Disorders of carbohydrate metabolism | 1 (0.8) | – | 1 (0.4) |
| Hyperprolinemia type 2⁎ | Disorders of ornithine, proline and hydroxyproline metabolism | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| HUPRA syndrome (SARS2)⁎ | Disorders of mitochondrial aminoacyl-tRNA synthetases | Disorders of mitochondrial gene expression | 1 (0.8) | – | 1 (0.4) |
| ITPA deficiency⁎ | Disorders of purine metabolism | Disorders of nucleobase, nucleotide and nucleic acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Krabbe disease⁎ | Disorders of sphingolipid degradation | Disorders of complex molecule degradation | 1 (0.8) | – | 1 (0.4) |
| L-2-hydroxyglytaric aciduria⁎ | Disorders of mitochondrial metabolite repair | Disorders of metabolite repair/proofreading | – | 1 (1.1) |
1 (0.4) |
| LCHAD deficiency# | Disorders of mitochondrial fatty acid oxidation | Disorders of fatty acid and ketone metabolism | 1 (0.8) | – | 1 (0.4) |
| LPIN1 deficiency⁎, # | Disorders of glycerolipid metabolism | Disorders of lipid metabolism | 1 (0.8) | – | 1 (0.4) |
| MEGDEL syndrome⁎, # | Disorders of mitochondrial membrane biogenesis and remodeling | Disorders of organelle biogenesis, dynamics and interactions | 1 (0.8) | – | 1 (0.4) |
| Pompe disease | Other disorders of complex molecule degradation | Disorders of complex molecule degradation | 1 (0.8) | – | 1 (0.4) |
| Propionic acidemia# | Organic acidurias | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| PTPS deficiency⁎ | Disorders of tetrahydrobiopterin metabolism | Disorders of vitamin and cofactor metabolism | 1 (1.1) | 1 (0.4) | |
| Hypomyelinating leukodystrophy-10 (PYCR2)⁎ | Disorders of ornithine, proline and hydroxyproline metabolism | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Trimethylaminuria⁎ | Disorders of methylamine metabolism | Disorders of peptide and amine metabolism | 1 (0.8) | – | 1 (0.4) |
| Tyrosinemia type 1⁎ | Disorders of phenylalanine and tyrosine metabolism | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Tyrosinemia type 2⁎ | Disorders of phenylalanine and tyrosine metabolism | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Citrullinemia type-1⁎, # | Disorders of the urea cycle and hyperammonemias | Disorders of amino acid metabolism | 1 (0.8) | – | 1 (0.4) |
| Combined oxidative phosphorylation deficiency 20 (VARS2)⁎, # | Disorders of mitochondrial aminoacyl-tRNA synthetases | Disorders of mitochondrial gene expression | 1 (0.8) | – | 1 (0.4) |
| Total | 131 (100) | 92 (100) | 223 (100) |
BH4: Tetrahydrobiopterin, HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A, HPA: hyperphenylalaninemia, HUPRA: Hyperuricemia, pulmonary hypertension, renal failure, and alkalosis, ICIMD: International Classification of Inherited Metabolic Disorders, IMD: Inherited metabolic disorders, ITPA: Inosine triphosphatase, LCHAD: Long-chain 3-hydroxyacyl-CoA dehydrogenase, MEGDEL: 3-methylglutaconic aciduria, deafness, encephalopathy, and Leigh-like disease, PKU: phenylketonuria, PTPS: 6-pyruvoyl-tetrahydropterin synthase.
This paper is the first published record of COVID-19 in these disorders.
Disorders in which acute symptomatic metabolic decompensations are possible. Hereditary fructose intolerance, biotinidase deficiency and tyrosinemia type 1 were not included because the risk of decompensation is very low under treatment.
3.2. Features of COVID-19 in IMD
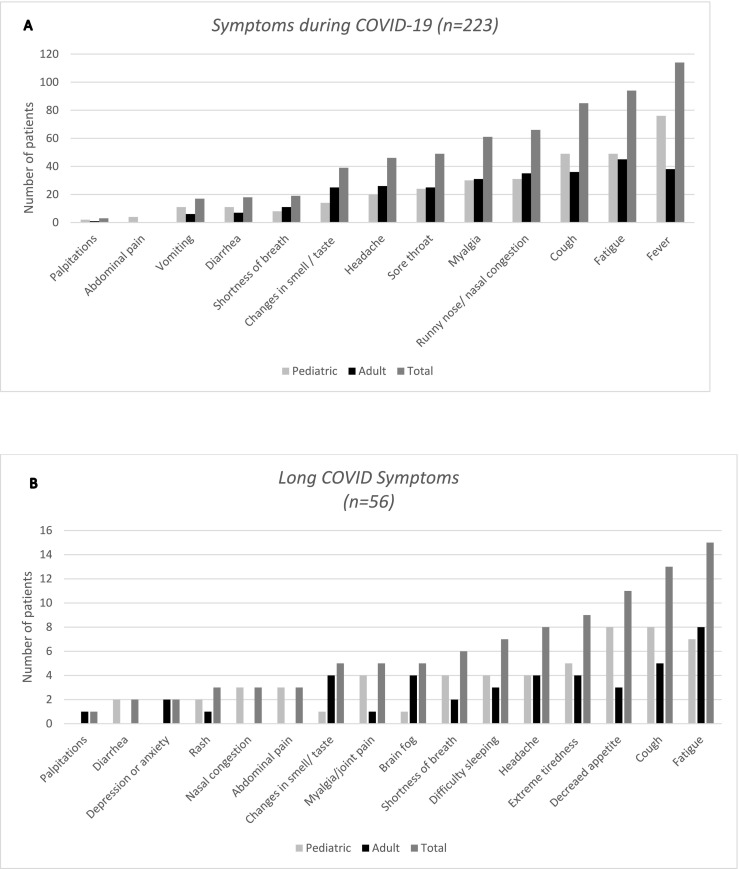
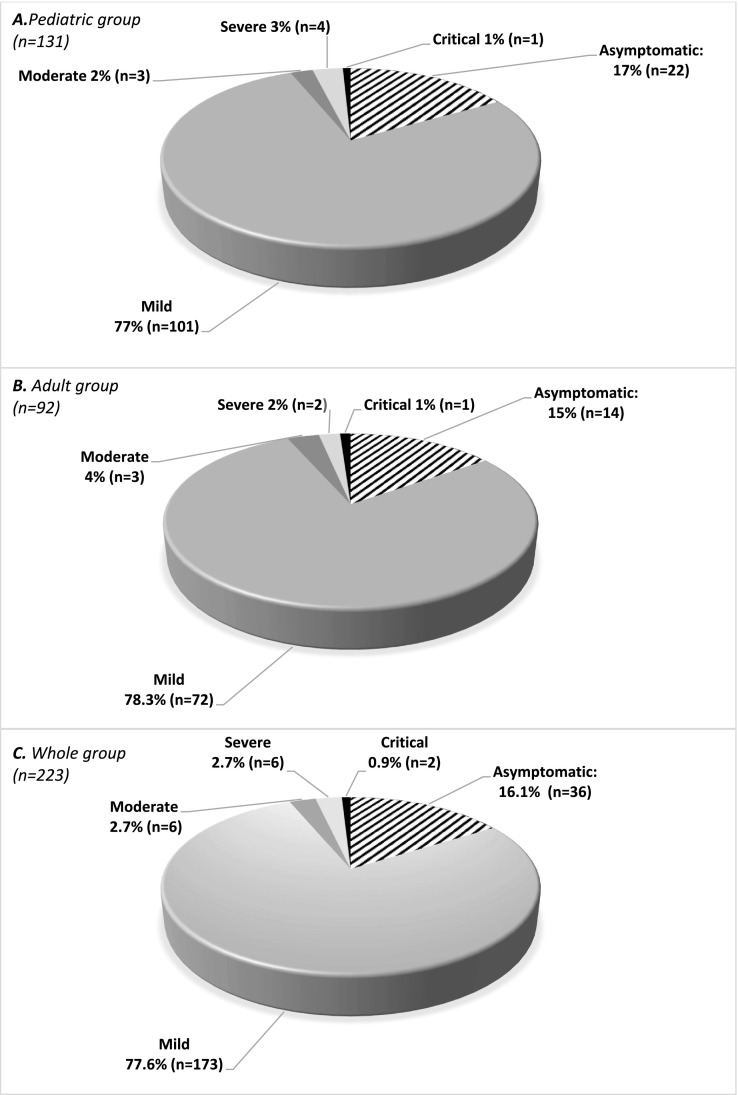
83.9% (n = 187) of patients had symptoms during COVID-19 and 25.1% (n = 56) had long COVID symptoms (Fig. 2 ). 12 patients (5%) contracted COVID-19 twice. The majority of the cases were either asymptomatic (16.1%, n = 36) or had a mild illness (77.6%, n = 173), but 6 patients (2.7%) had moderate, 6 had severe, and two (0.9%) had critical COVID-19 (Fig. 3 ), both of whom died. More detailed information regarding the 14 patients with moderate or more severe COVID-19 are presented in Table 3 . 13.0% of children and 8.7% of adults with IMD were hospitalized during COVID-19. The patients with comorbid conditions had 6.5 times higher odds of being hospitalized than those without (p < 0.001). The significantly higher risk of hospitalization in patients with comorbidities persisted when patients were analyzed separately as children and adults: The odds of hospitalization were 4.4 times higher in the pediatric (p < 0.01), and 20.5 times higher in adult (p < 0.01) patients with comorbidities. Three patients (one each of 3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] lyase deficiency, long-chain 3-hydroxyacyl-CoA dehydrogenase [LCHAD] deficiency, and methylmalonic academia [MMA]) had an acute metabolic decompensation during the infection. All of these three patients were hospitalized because of the metabolic decompensation, and were treated successfully during the course of COVID-19. Standard treatment protocols for metabolic decompensation were administered. Two children (1.5% of children) developed MIS-C and recovered with treatment (one with LCHAD and the other with biotinidase deficiency) with intravenous immunoglobulin, anakinra, favipiravir, anticoagulants anti-inflammatory agents and antibiotics.
Fig. 2.
Occurrence of symptoms during A) COVID-19 infection, and B) long COVID shown separately in the pediatric and adult groups, and in the whole patient group. A. There were a total of 223 patients (131 children and 92 adults), 187 of whom were symptomatic (108 children and 79 adults). B. 56 patients (35 children and 21 adults) experienced long COVID symptoms.
Fig. 3.
Severity distribution of COVID-19 according to the World Health Organization severity classification in A) pediatric patients, B) adult patients, and C) all the patients with inherited metabolic disorders in the study population.
Table 3.
Features of patients with moderate, severe or critical COVID-19.
| Case No | Age, Gender | Diagnosis | Comorbidities | Fully vaccinated at the time of COVID-19 | COVID-19 severity | ICU admission | Hospitalization (days) | Oxygen requirement | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 y, F | MPS-VI | Cardiac failure | No | Moderate | No | 7 | No | Alive |
| 2 | 20 y, M | Classical PKU⁎ | Obesity | No | Moderate | No | 5 | No | Alive |
| 3 | 30 y, M | Classical PKU | Neurologic impairment | No | Moderate | No | 6 | No | Alive |
| 4 | 2 y, M | HMG-CoA lyase deficiency | None | No | Moderate | No | 5 | No | Alive |
| 5 | 25 y, M | Classical PKU⁎ | Obesity | No | Moderate | No | 3 | No | Alive |
| 6 | 9 y, M | BH4-responsive PKU⁎ | None | No | Moderate | No | 0 | No | Alive |
| 7 | 2 y, M | Mild HPA⁎ | Prematurity and asthma | No | Severe | No | 7 | Yes | Alive |
| 8 | 25 y, M | HFI | FMF | Yes | Severe | No | 13 | Yes | Alive |
| 9 | 18 y, M | FH (AD) | Obesity | No | Severe | No | 10 | Yes | Alive |
| 10 | 16 y, F | MPS-III | Neurologic impairment, tracheostomy | No | Severe | No | 5 | Yes | Alive |
| 11 | 3 y, F | Krabbe disease | Neurologic impairment, tracheostomy | No | Severe | Yes | 30 | Yes | Alive |
| 12 | 6 mo, M | MPS-I (Hurler) | None | No | Severe | No | 6 | Yes | Alive |
| 13 | 12 mo, F | Tyrosinemia type I | Liver failure, rachitic pneumopathy | No | Critical | Yes | 11⁎⁎ | Yes | Exitus |
| 14 | 72 y, M | Classical PKU | Obesity, ID, HT, PD | No | Critical | Yes | 1 | Yes | Exitus |
COVID-19: coronavirus disease 2019, FH (AD): familial hypercholesterolemia (autosomal dominant), FMF: familial Mediterranean fever, HFI: hereditary fructose intolerance, HMG-CoA: 3-hydroxy-3-methylglutaryl-coenzyme A, HPA: hyperphenylalaninemia, HT: hypertension, ICU: intensive care unit, ID: intellectual disability, MPS: mucopolysaccharidosis, PD: Parkinson's disease, PKU: phenylketonuria.
Patients diagnosed via newborn screening.
She was already hospitalized at the time of contracting COVID-19. 11 days is the duration of hospitalization after the diagnosis of COVID-19.
Mean duration of interruption of school or work was 13 ± 5 days. The presence of symptoms, clinical spectrum of COVID-19, long COVID symptoms, hospitalization, and intensive care unit admission status were not significantly different between children and adults (Table 4 ). In order to determine which ICIMD categories were more likely to experience more severe COVID-19, the patients were grouped into two, according to the absence or presence of pneumonia (asymptomatic-mild vs. moderate-severe-critical COVID-19). There was a statistically significant difference between those with disorders of complex molecule degradation and the others (p = 0.018). The patients with complex molecule degradation disorders had 4.5 times higher odds of COVID-19 pneumonia compared to the other groups. When analyzed separately for age groups, the significance persisted in the pediatric group, as there was a statistically significant difference between complex molecule degradation disorders and the others (p < 0.01). The children with complex molecule disorders had 9.7 times higher odds of clinical severity. However, in adult patients (n = 92), there was no statistically significant difference between ICIMD categories in terms of COVID-19 severity (p = 1). There were no significant differences between the other categories of IMD. Within the whole group, IMD patients with comorbidities had 4.4 times higher odds to experience more severe COVID-19 (pneumonia) than those without (p = 0.011). This difference was more evident in adult IMD patients with comorbidities (odds ratio: 20.7, p < 0.01), but was not statistically significant in the pediatric age group (p = 0.45).
Table 4.
Comparison of COVID-19 features between children and adults with IMD.
| Child (n = 131) | Adult (n = 92) | p | ||
|---|---|---|---|---|
| 1 | Presence of symptoms | Yes: 82.4% (n = 108) No: 17.6% (n = 23) |
Yes: 85.9% (n = 79) No: 14.1% (n = 13) |
0.49 |
| 2 | Clinical Spectrum of SARS-CoV-2 Infection | |||
| Asymptomatic: | 16.8% (n = 22) | 15.2% (n = 14) | 0.97 | |
| Mild illness: | 77.1% (n = 101) | 78.3% (n = 72) | ||
| Moderate illness: | 2.3% (n = 3) | 3.3% (n = 3) | ||
| Severe illness: | 3.1% (n = 4) | 2.2% (n = 2) | ||
| Critical illness: | 0.8% (n = 1) | 1.1% (n = 1) | ||
| 3 | Long COVID | Yes:26.8% (n = 35) No:73.2% (n = 96) |
Yes: 22.8% (n = 21) No: 77.2% (n = 71) |
0.48 |
| 4 | Hospitalization | Yes:13.0% (n = 17) No: 87.0% (n = 114) |
Yes: 8.7% (n = 8) No: 91.3% (n = 84) |
0.32 |
| 5 | ICU admission | Yes: 1.5% (n = 2) No: 98.5% (n = 129) |
Yes: 1.1% (n = 1) No: 98.9% (n = 91) |
1 |
COVID-19: coronavirus disease 2019, ICU: intensive care unit, IMD: inherited metabolic disorder, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Status of vaccination is shown in Table 1. 46 patients (33.3% of those eligible) had been vaccinated before they contracted COVID-19. Among all the vaccinated patients (n = 88), 72.7% (n = 64) had been vaccinated with the nucleoside modified messenger RNA vaccine BNT162b2 (Comirnaty®) by BioNTech, 25.0% (n = 22) with inactivated SARS-CoV-2 vaccine CoronaVac® by Sinovac, and 2.3% (n = 2) with both. Only 1 out of 14 patients with moderate-severe-critical COVID-19 had been fully vaccinated at the time of infection (Table 3). Patient or parental education level was not significantly different between those who were and were not vaccinated (p = 0.22).
4. Discussion
In this paper, we have presented the summary of the features of COVID-19 in a large cohort of IMD patients from a single center. Our findings suggest that the risk of metabolic decompensation is not disproportionately high, and children with disorders of complex molecule degradation may be under higher risk of more severe COVID-19. To the best of our knowledge, this is the largest single-center series so far reported, and the first account of COVID-19 in 27 different IMD (Table 2), the first reports of detailed COVID-19 symptomatology, vaccination status and MIS-C in IMD. We demonstrate that the symptomatology of COVID-19 in IMD is similar to the general population [12], but the occurrence of feeding difficulties may be slightly higher in children with IMD (28.2% vs. 20%) [13].
One critical finding of our study is the demonstration of increased risk of more severe COVID-19 in children with disorders of complex molecule degradation, many of which are lysosomal storage diseases (LSD) that are often characterized by progressive multisystem involvement [2,14]. In adults with IMD, the severity of COVID-19 was associated more significantly with comorbidities rather than the category of IMD. While critical comorbidities (involvement of the lungs, heart etc.) commonly associated with LSD are known risk factors for severe COVID-19, our data on children with IMD suggest that there may be further susceptibility to SARS-CoV-2 at the cellular or molecular level in patients with inherited disorders of complex molecule degradation.
Infections are well-known triggers of acute metabolic decompensation in some metabolic disorders, especially intoxication-type and energy-deficit disorders [15]. In pre-COVID studies of patients with MMA and propionic acidemia, infections triggered metabolic decompensation in 29% and 44% of the time, respectively [[16], [17], [18]]. We had 37 COVID-19-positive patients at risk of an acute metabolic decompensation (marked with # in Table 2), but SARS-CoV-2-related decompensation occurred only in three (8.1%). Zubarioğlu et al. reported four counts of SARS-CoV-2-related decompensation in 22 IMD patients (18.1%) [19]. While the vulnerability of some IMD to infections is evident, we argue that SARS-CoV-2 itself does not pose an extra burden of an acute decompensation compared to other infectious agents circulating before the pandemic [8,20]. It should be recognized that immune-naïve patients may experience a more severe illness and therefore carry a higher risk of metabolic decompensation. It is important to underline that preventive measures such as vaccination, hygiene and social distancing should be recommended as in any other infectious disease (eg. influenza), which are a part of routine care in the IMD that carry such risks [21].
While the hospitalization and death rates of children with COVID-19 were < 2% and < 0.03% in a general population study [22], they were 13% and 0.8% in our study, respectively. In a large survey study from Brazil, nine out of 358 patients (2.5%) with IMD were hospitalized, three in the intensive care unit [23]. 12 of our patients were hospitalized not due to the severity of COVID-19, but primarily because of their IMD; two due to acute decompensation, and the 10 remaining patients due to the perception of higher risk of COVID-19 or for close observation anticipating a decompensation. In-hospital care may have contributed to the low attack rate of an acute decompensation in our study, which emphasizes the importance of continued health care for rare disease patients during the pandemic. The concern raised by MetabERN regarding higher COVID-19 risk and IMD exacerbation in patients with amino acid and organic acid-related disorders was formulated at a time when no vaccines were available for a disease we had merely begun to understand [24]. Now, with the tools and information acquired in the meantime, we propose that efforts should focus not pre-emptive intensive treatment of IMD patients contracting COVID-19, but rather on application of well-established “sick-day” regimens, and on vaccinating individuals with IMD, even young children, to prevent as many acute decompensations as possible.
There were two COVID-19-related deaths in the study population (Table 3), but it is hard to claim that the deaths are directly related to the IMD. It is known that elderly people and infants may have a critical disease course [25]. The deceased phenylketonuria patient was a 74-year-old obese man with hypertension and Parkinson's disease. The other deceased patient was an infant who already had the complications of tyrosinemia type 1 (acute liver failure, chronic liver disease and hypophosphatemic rickets) when she was diagnosed at the age of nine months. She had respiratory muscle weakness, rachitic beads, massive ascites and hepatomegaly, reducing her vital lung capacity. Both patients were diagnosed with IMD after exhibiting clinical symptoms. It is likely that newborn screening and early treatment for tyrosinemia type 1 may have prevented the patient's death due to COVID-19.
All of our eight patients with severe or critical COVID-19 (3 adults and 5 children) had risk factors (eg. old age, infancy, neurological impairment, obesity, prematurity), some of which were caused by the underlying IMD (eg. neurological impairment in MPS-III and Krabbe disease). It is well known that pediatric patients in the general population have milder COVID-19 than adults [7,26,27], attributed to multiple factors [28]. Severe or critical disease risk is reported as 3.9% in the general Turkish pediatric population with SARS-CoV-2 infection [29], and we calculated the same risk as 3.8% in our pediatric cohort. Risk of severe disease was similar to adults with IMD, and the hospitalization rate was higher in children (Table 4), although this may be partly related to physician preference, as discussed above. The MetabERN survey conducted in early 2020 when vaccines were unavailable and children were largely staying at home reported that adult patients required more hospitalization and had a more severe course compared to the general population and pediatric patients [6], but most of our cases coincide with delta and omicron waves of the pandemic, when many adults were vaccinated and children were attending school. While the inadequate numbers of severe and critical cases and the fluidity of the pandemic conditions make it difficult to draw definitive conclusions, children with IMD in general do not seem to have increased risk of severe COVID-19 than their healthy counterparts. Studies with larger numbers of IMD patients are needed for this comparison.
The long-term effects of SARS-CoV-2 infection are being increasingly recognized and emphasized [30]. Studies from different countries have reported highly variable incidences of long-COVID, ranging from 1.6% to 77% [30]. In our cohort, 25.2% of patients had long COVID, and this rate was similar between children and adults. The most common long-COVID symptom in our patients was fatigue. These findings are in line with previously published work regarding the general population [31,32]. While long-COVID is common, MIS-C is rare, with a reported incidence 1:4000 [33]. MIS-C developed in two out of 131 children included in our study. These may be the first published accounts of MIS-C in IMD. The high occurrence of MIS-C in our cohort may be coincidental, but warrants further study.
This study has several limitations, firstly related to patient interviews and the retrospective design, making it prone to recall bias. Screening protocols for asymptomatic contacts of a case changed during the course of the pandemic, possibly missing asymptomatic infections. The intended study population could not be completely enrolled because the electronic records of some patients were inaccessible, and some patients could not be contacted. Still, from a large group of patients using established, objective definitions and classifications, describing COVID-19 and long-COVID symptomatology in IMD, presenting first accounts of COVID-19 in many IMD and of MIS-C.
In conclusion, children with IMD may have a higher rate of hospitalization during COVID-19, perhaps partly because of physicians' preference of precautionary close follow-up. However, we think that the IMD patients under risk of decompensations can be assured that there is no evidence to suggest that the occurrence risk or the severity of decompensations is increased in SARS-CoV-2 infection compared to other infections, and adherence to standard emergency protocols and infection prevention measures, such as vaccination, should be recommended. Facilitated access to health services when needed remains an important challenge. Children with disorders of complex molecule degradation have a higher risk of more severe COVID-19, possibly due to multisystem involvement, but increased susceptibility to COVID-19 at the cellular or molecular should be investigated in future studies. Patients, especially adults with IMD may experience more severe COVID-19 if they have high-risk comorbidities.
Individuals with IMD and other rare diseases cannot be ignored. They should be kept in mind while formulating recommendations and making policy decisions. All patients with IMD older than 6 months should be eligible for vaccination against SARS-CoV-2 if their condition is a complex molecule degradation disorder, or is characterized by acute metabolic decompensations or chronic high-risk organ involvement. Every effort should be made to sustain acute and long-term health services for these vulnerable individuals during the pandemic.
Ethics approval and consent to participate
This study was approved by Hacettepe University Ethics Committee for Non-Interventional Clinical Studies (GO 22/176, 2022/08-23). Informed consent was obtained from all patients or their parents/guardians.
Consent for publication
All patients provided consent to publish clinical data.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors received no financial support for the research.
Authors' contributions
Conception and design: ABK, YY, SS.
Clinical evaluation and records: ABK, YY, KÇ, İE, HTA, AD, AT, SS.
Data collection: ABK, KÇ, İE, HTA.
Data analysis: ABK.
Data interpretation: ABK, YY.
Drafting the manuscript: ABK, YY.
Editing the manuscript: YY, KÇ, İE, HTA, AD, AT, SS. All authors read and approved the final manuscript.
Declaration of Competing Interest
None.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira C.R., Rahman S., Keller M., Zschocke J., Group I.A., Abdenur J., Ali H., Artuch R., Ballabio A., Barshop B. An international classification of inherited metabolic disorders (ICIMD) J. Inherit. Metab. Dis. 2021;44:164–177. doi: 10.1002/jimd.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercolini F., Donà D., Girtler Y., Mussner K.A., Biban P., Bordugo A., Molinaro G. First paediatric COVID-19 associated death in Italy. J. Paediatr. Child Health. 2021 May;57(5):736–737. doi: 10.1111/jpc.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahraman A.B., Yildiz Y., Ciki K., Akar H.T., Erdal I., Dursun A., Tokatli A., Sivri H.S. Invisible burden of COVID-19: enzyme replacement therapy disruptions. J. Pediatr. Endocrinol. Metab. 2021;34:539–545. doi: 10.1515/jpem-2021-0067. [DOI] [PubMed] [Google Scholar]
- 5.Koç Yekedüz M., Doğulu N., Sürücü Kara İ., Öncül Ü., Bakirarar B., Kullu P., Ar Y., Köse E., Eminoğlu F.T. 2022. Pros and Cons of Telemedicine for Inherited Metabolic Disorders in a Developing Country during the COVID-19 Pandemic Telemedicine and e-Health. [DOI] [PubMed] [Google Scholar]
- 6.Paneghetti L., Bellettato C.M., Sechi A., Stepien K.M., Scarpa M. One year of COVID-19: infection rates and symptoms in patients with inherited metabolic diseases followed by MetabERN. Orphan. J. Rare Dis. 2022;17:1–8. doi: 10.1186/s13023-022-02247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasnat F., Noman F., Moben A., Sarker A., Morshed J., Mutanabbi M. Difference in clinical patterns between COVID-19. Affected Childr. Adults Mymensingh Med. J. 2021;30:1093–1099. [PubMed] [Google Scholar]
- 8.Mütze U., Gleich F., Barić I., Baumgartner M., Burlina A., Chapman K.A., Chien Y.H., Cortès-Saladelafont E., De Laet C., Dobbelaere D. Impact of the SARS-CoV-2 pandemic on the health of individuals with intoxication-type metabolic diseases—data from the E-IMD consortium. J. Inherit. Metab. Dis. 2023;46:220–231. doi: 10.1002/jimd.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Living guidance for clinical management of COVID-19. 2021. [Google Scholar]
- 10.International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death. 2020. [Google Scholar]
- 11.Centers for Disease Control and Prevention . 2020. Emergency Pre- Paredness and Response: Health Alert Network. [Google Scholar]
- 12.Cui X., Zhao Z., Zhang T., Guo W., Guo W., Zheng J., Zhang J., Dong C., Na R., Zheng L., Li W., Liu Z., Ma J., Wang J., He S., Xu Y., Si P., Shen Y., Cai C. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assa A., Benninga M.A., Borrelli O., Broekaert I., de Carpi J.M., Saccomani M.D., Dolinsek J., Mas E., Miele E., Thomson M., Tzivinikos C., E. Gastrointestinal Committee of Gastrointestinal perspective of coronavirus disease 2019 in children-an updated review. J. Pediatr. Gastroenterol. Nutr. 2021;73:299–305. doi: 10.1097/MPG.0000000000003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platt F.M., d’Azzo A., Davidson B.L., Neufeld E.F., Tifft C.J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 2018;4:1–25. doi: 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A., Morris A.A. Acute presentations of inherited metabolic disorders: investigation and initial management. Paediatr. Child Health. 2019;29:99–104. [Google Scholar]
- 16.Zwickler T., Riderer A., Haege G., Hoffmann G.F., Kolker S., Burgard P. Usefulness of biochemical parameters in decision-making on the start of emergency treatment in patients with propionic acidemia. J. Inherit. Metab. Dis. 2014;37:31–37. doi: 10.1007/s10545-013-9621-3. [DOI] [PubMed] [Google Scholar]
- 17.Zwickler T., Haege G., Riderer A., Horster F., Hoffmann G.F., Burgard P., Kolker S. Metabolic decompensation in methylmalonic aciduria: which biochemical parameters are discriminative? J. Inherit. Metab. Dis. 2012;35:797–806. doi: 10.1007/s10545-011-9426-1. [DOI] [PubMed] [Google Scholar]
- 18.Forny P., Hörster F., Ballhausen D., Chakrapani A., Chapman K.A., Dionisi-Vici C., Dixon M., Grünert S.C., Grunewald S., Haliloglu G. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: first revision. J. Inherit. Metab. Dis. 2021;44:566–592. doi: 10.1002/jimd.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubarioglu T., Hopurcuoglu D., Ahmadzada S., Uzunyayla-Inci G., Cansever M.S., Kiykim E., Aktuglu-Zeybek C. Inborn errors of metabolism and COVID-19: Evaluation of the metabolic outcome, Pediatr Int. 2022 Jan;64(1):e14938. doi: 10.1111/ped.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur S., Campbell S.L., Stockton D.W. Management of COVID-19 infection in organic acidemias. Am. J. Med. Genet. A. 2021;185:1854–1857. doi: 10.1002/ajmg.a.62161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner M.R., Hörster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A., Huemer M., Hochuli M., Assoun M., Ballhausen D. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphan. J. Rare Dis. 2014;9:1–36. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann P., Pittet L.F., Curtis N. How common is long COVID in children and adolescents? Pediatr. Infect. Dis. J. 2021;40 doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz I.V.D., Randon D.N., Monsores N., Moura de Souza C.F., Horovitz D.D.G., Wilke M.V.M.B., Brunoni D. SARS-CoV-2 pandemic in the Brazilian community of rare diseases: a patient reported survey. Am. J. Med. Genet. C: Semin. Med. Genet. 2021:301–311. doi: 10.1002/ajmg.c.31883. Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampe C., Dionisi-Vici C., Bellettato C., Paneghetti L., Van Lingen C., Bond S., Brown C., Finglas A., Francisco R., Sestini S. The impact of COVID-19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphan. J. Rare Dis. 2020;15:1–14. doi: 10.1186/s13023-020-01619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi J.H., Choi S.-H., Yun K.W. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. Journal of Korean Medical Science. 2022;37 doi: 10.3346/jkms.2022.37.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen A., Huang J.-x., Liao Y., Liu Z., Chen D., Yang C., Yang R.-m., Wei X. Vol. 2. 2020. Differences in Clinical and Imaging Presentation of Pediatric Patients with COVID-19 in Comparison with Adults Radiology: Cardiothoracic Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Vol. 145. 2020. Epidemiology of COVID-19 among Children in China Pediatrics. [DOI] [PubMed] [Google Scholar]
- 28.Dhochak N., Singhal T., Kabra S., Lodha R. Pathophysiology of COVID-19: why children fare better than adults? Indian J. Pediatr. 2020;87:537–546. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yayla B.C.C., Aykac K., Ozsurekci Y., Ceyhan M. Characteristics and Management of Children with COVID-19 in a tertiary Care Hospital in Turkey. Clin. Pediatr. (Phila) 2021;60:170–177. doi: 10.1177/0009922820966306. [DOI] [PubMed] [Google Scholar]
- 30.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022;43:1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludvigsson J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110:914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne A.B., Gilani Z., Godfred-Cato S., Belay E.D., Feldstein L.R., Patel M.M., Randolph A.G., Newhams M., Thomas D., Magleby R., Hsu K., Burns M., Dufort E., Maxted A., Pietrowski M., Longenberger A., Bidol S., Henderson J., Sosa L., Edmundson A., Tobin-D'Angelo M., Edison L., Heidemann S., Singh A.R., Giuliano J.S., Jr., Kleinman L.C., Tarquinio K.M., Walsh R.F., Fitzgerald J.C., Clouser K.N., Gertz S.J., Carroll R.W., Carroll C.L., Hoots B.E., Reed C., Dahlgren F.S., Oster M.E., Pierce T.J., Curns A.T., Langley G.E., Campbell A.P., M.-C.I.A. Group, Balachandran N., Murray T.S., Burkholder C., Brancard T., Lifshitz J., Leach D., Charpie I., Tice C., Coffin S.E., Perella D., Jones K., Marohn K.L., Yager P.H., Fernandes N.D., Flori H.R., Koncicki M.L., Walker K.S., Di Pentima M.C., Li S., Horwitz S.M., Gaur S., Coffey D.C., Harwayne-Gidansky I., Hymes S.R., Thomas N.J., Ackerman K.G., Cholette J.M. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16420. e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holm M, Hartling UB, Schmidt LS, Glenthøj JP, Kruse A, Rytter MH, Lindhard MS, Lawaetz MC, Zaharov T, Petersen JJ, Andersen RM, Lemvik G, Marcinski P, Thaarup J, Jensen LH, Borch L, Nielsen AB, Vissing NH, Schmiegelow K, Nygaard U. Multisystem inflammatory syndrome in children occurred in one of four thousand children with severe acute respiratory syndrome coronavirus 2. Acta Paediatr. 2021 Sep;110(9):2581–2583. doi: 10.1111/apa.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.