Abstract
Lymphatic filariasis is a major tropical disease caused by the mosquito-borne nematodes Brugia and Wuchereria. About 120 million people are infected and at risk of lymphatic pathology such as acute lymphangitis and elephantiasis. Vaccines against filariasis must generate immunity to the infective mosquito-derived third-stage larva (L3) without accentuating immunopathogenic responses to lymphatic-dwelling adult parasites. We have identified two highly expressed genes, designated abundant larval transcript-1 and -2 (alt-1 and alt-2), from each of which mRNAs account for >1% of L3 cDNAs. ALT-1 and ALT-2 share 79% amino acid identity across 125 residues, including a putative signal sequence and a prominent acidic tract. Expression of alt-1 and alt-2 is initiated midway through development in the mosquito, peaking in the infective larva and declining sharply following entry into the host. Humans exposed to Brugia malayi show a high frequency of immunoglobulin G1 (IgG1) and IgG3 antibodies to ALT-1 and -2, distinguishing them from adult-stage antigens, which are targeted by the IgG4 isotype. Immunization of susceptible rodents (jirds) with ALT-1 elicited a 76% reduction in parasite survival, the highest reported for a single antigen from any filarial parasite. ALT-1 and the closely related ALT-2 are therefore strong candidates for a future vaccine against human filariasis.
Filarial nematodes are helminth parasites which are responsible for lymphatic filariasis, a tropical disease afflicting some 119 million people (26, 28, 34). The parasites have a complex life cycle in which mosquito-borne infective third-stage larvae (L3) invade the human body, mature to adult worms, and produce large numbers of newborn larvae (microfilariae) which must transit the mosquito vector in order to develop to L3 (16). Overt disease has a major immunopathologic component, and a prominent risk of vaccination with filarial antigens is exacerbation of pathology (22, 27, 32). The target of immunopathological reactions, however, is thought to be the long-lived adult worm and not the infective larva (23, 29).
To date, strategies to identify vaccine antigens in filariasis have relied on serum antibodies to define antigens, whether by comparing apparently uninfected subjects with infected patients (11) or by using sera from animals vaccinated with radiation-attenuated parasites (19, 20). Among the antigens so discovered have been several with high levels of similarity to host antigens (such as muscle proteins), raising an additional specter of autoimmune induction by vaccination. No recombinant filarial antigen yet tested induces significant degrees of immunity to challenge infection (21, 30), indicating that an alternative criterion needs to be adopted.
We describe here a molecular biological approach, the analysis of mRNAs which are highly and selectively expressed by the mosquito-derived larva at the time that it is competent to infect the mammalian host. We sought to identify new antigens which are restricted to this stage and absent from the mature forms thought to evoke immunopathology. We also wished to discover parasite-specific genes which carry minimal risk of cross-reaction with host constituents. By using a PCR approach with the conserved nematode 5′-spliced leader and oligo(dT) (12), we have previously reported the full-length cDNA sequences of two highly expressed genes, designated abundant larval transcript-1 and -2 (13). ALT-1 and ALT-2 represent closely related proteins (79% identity) and are homologous to an abundant immunogen from larvae of the dog heartworm Dirofilaria immitis (Di-20/22L) (10) and to proteins from the additional filarial parasites Onchocerca volvulus (Ov-ALT-1) (15) and Acanthocheilonema viteae (31). Most recently, the SLAP (secreted larval acidic protein) produced by O. volvulus larvae (2, 3) has also been shown to be a member of the ALT family (Y. Wu and A. E. Bianco, personal communication).
Products associated with parasite invasion, which are tightly regulated and parasite specific, are likely to be essential to the success of parasitism (2). We describe here two related genes, expressed strongly at the larval stage, which are prime candidates for a new vaccine against filarial infection. The alt genes represent attractive vaccine antigens for three reasons: (i) they are larva specific in immunological terms; (ii) they are highly expressed, offering an abundant target; and (iii) they have no known homolog in the mammalian host.
MATERIALS AND METHODS
Parasites and infections of mosquitoes and jirds.
Brugia malayi parasites were obtained from TRS Laboratories and maintained by feeding Aedes aegypti mosquitoes with microfilariae in blood. Mosquitoes were maintained for up to 12 days and crushed to recover infective larvae by baermannization (13). Jirds were infected with 300 infective larvae intraperitoneally, and peritoneal adult worms and microfilariae were recovered 3 or more months later (1).
Genomic cloning.
B. malayi genomic DNA was prepared as described previously (24) and used as the template for PCR with alt-1- and alt-2-specific primers as follows: alt-1 forward (nucleotides [nt] 101 to 127 of cDNA), GAT GAC GAA TTC GAC GAC GAA TCC TCA; alt-1 reverse (nt 433 to 407 of cDNA), TTG TTT TGC TTG CTT TGT AAG CAT TTA; alt-2 forward (nt 102 to 128 of cDNA), GAC GAA GAG TTC GAT GAC TCC GCA GCC; and alt-2 reverse (nt 443 to 417 of cDNA), GTA GTA TCA AAG ACT GAT TCA TTC CTA.
RT-PCR.
For reverse transcription (RT)-PCR, first-strand cDNA was produced from total RNA using GeneAmp RT-PCR kits (Applied Biosystems, Cheshire, U.K.) as previously described (13). PCR used the alt-1- and alt-2-specific primers on first-strand cDNA for 35 cycles of 1 min at 99°C, 1 min at 55°C, and 1.5 min at 72°C.
Expression, antibody production, and Western blotting.
The predicted mature ALT-1 protein was expressed in the pET29 T vector (Novagen) as a fusion protein with histidine and 15-amino acid (15-aa) S tag peptides. The PCR-amplified alt-1 insert was directly cloned into the T overhang of the plasmid. Expression was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 3 h. Bacteria were pelleted and sonicated, and the supernatant was taken for metal-chelating affinity chromatography on His-Bind resin (Novagen). BALB/c and CBA/Ca mice were immunized with 20 μg of recombinant ALT-1 (rALT-1) in complete Freund's adjuvant (CFA), boosted 1 month later, and bled 7 days subsequently. Western blotting was performed with 6 μg of phosphate-buffered saline-soluble protein extract from L3, mixed-stage adults, and microfilariae.
ELISA.
Forty human sera from Sulawesi, Indonesia, were selected for testing. Recombinant Bm-ALT-1 was coated at 1 μg/ml, a concentration determined to be optimal in a pilot experiment. Isotype-specific monoclonal antibodies were used as described previously (18). Recombinant Bm-33, an aspartyl protease inhibitor (7), was expressed as a fusion protein with maltose-binding protein (MBP) in the pMAL expression vector. rBm-33 and bacterially expressed MBP were each used to coat enzyme-linked immunosorbent assay (ELISA) plates at 1 μg/ml, and anti-MBP responses were subtracted from those measured for Bm-33/MBP fusion proteins.
Immunization.
Male jirds (Meriones unguiculatus) were immunized with 75 μg of rALT-1 in CFA subcutaneously or with CFA alone (six jirds per group). At weeks 32 and 33, boosts were given of 25 μg of rALT-1 in incomplete Freund's adjuvant (IFA) or IFA alone, and at week 45 a final boost of 7.5 μg in IFA was given. Two weeks later, all jirds were challenged with 300 larvae of B. malayi introduced intraperitoneally. After 4 weeks, jirds were euthanized, and parasites were recovered from the peritoneal cavity and testes. Parasite recoveries and counting were performed without knowledge of the experimental status of each animal.
Nucleotide sequence accession numbers.
The genomic nucleotide sequences for alt-1, alt-2a (1,075-bp band), and alt-2b (1,212-bp band) have been deposited in the GenBank database under accession numbers AF183572, AF183573, and AF183574, respectively. The cDNA sequence for alt-2 has been deposited in the GenBank database under accession number U84723.
RESULTS AND DISCUSSION
Abundance of alt mRNA.
The alt-1 gene was originally identified as a prominent trans-spliced mRNA from L3 larvae of B. malayi (13), and alt-2 was identified as a closely related expressed sequence tag (EST) from the same stage. Since then, the Filarial Genome Project has deposited over 18,000 ESTs from all stages of this parasite (33). Analysis of this database reveals that alt-1 is represented by 33 of 2,378 ESTs (1.39%) from the L3, and alt-2 is represented by 67 of 2,378 (2.82%). The alt-2 and alt-1 transcripts are, respectively, first and third in abundance among all L3-expressed cDNAs in the EST dataset (J. E. Allen, J. Daub, D. Guilliano, A. McDonnell, M. Lizotte-Waniewski, D. Taylor, and M. Blaxter, submitted for publication). Remarkably, neither cDNA can be found among the 14,000-plus sequences derived from other points in the life cycle, implying that alt-1 and alt-2 are expressed at less than 0.01% of mRNAs in non-L3 stages.
alt-like gene in Caenorhabditis elegans.
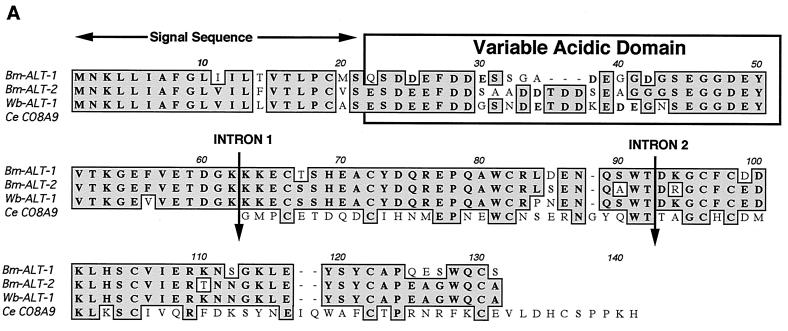
alt-1 and alt-2 cDNA sequences encode novel proteins each with signal sequences and N-terminal acidic tracts, which share 79% amino acid identity (13), and similar proteins have been described in other filarial species (10, 15, 31). Alignment of these shows that among the filarial parasites the acidic tract is highly variable, but the remainder of the protein is conserved (Fig. 1A). Among the predicted protein sequences from C. elegans deposited in WormPEP, only distant similarities could be found with low significance (P ≥ 0.18), including two genes designated as phospholipase A2. However, when the complete nucleotide sequence of C. elegans was searched, a more significant similarity (P < 10−6) was found on cosmid C08A9. This cosmid contains a short tract corresponding to the second and third exons of Bm-alt-1/2 and encodes a predicted peptide sequence with 32% amino acid identity with Bm-ALT-1 (Fig. 1A). Moreover, C08A9 shows exact alignment of all cysteine residues with Bm-ALT-1/2 and includes introns in identical positions to those determined for Bm-alt-1/2. Curiously, no upstream reading frame encoding an acidic domain could be identified in 3 kb 3′ of the homologous sequence.
FIG. 1.
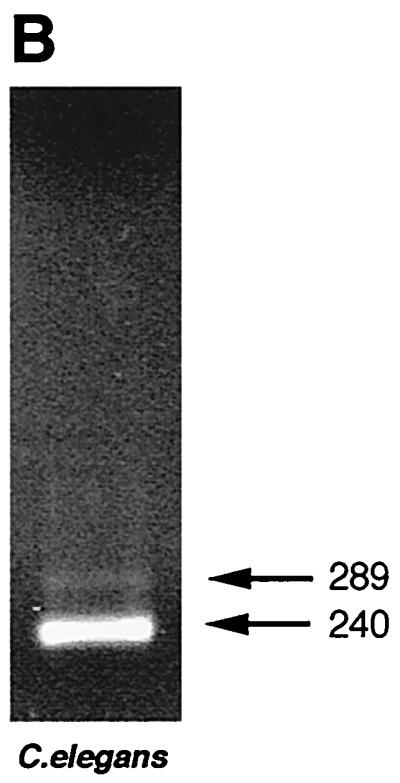
(A) Sequences of B. malayi ALT-1 (U57547) and ALT-2 (U84723) compared to W. bancrofti ALT-1 (AF084553; R. Sabarinathan and P. Kalikraj, Anna University, Madras, India, unpublished) and C. elegans cosmid CO8A9. Alignments with D. immitis and O. volvulus have been published previously (13, 15). Note that the intron position in W. bancrofti has not been determined. (B) Evidence for expression of the C. elegans alt homologue, a 240-bp PCR product representing cDNA from mixed-stage C. elegans. Note the 289-bp band representing genomic DNA.
Although not previously recognized as an open reading frame (and hence not included in WormPEP), we established that the C. elegans gene designated Ce-alt-1 is expressed in the free-living organism: amplification of mixed-stage cDNA with Ce-alt-1-specific primers gave a band with a size fully consistent with correct splicing, as well as a larger band corresponding to the genomic copy (Fig. 1B). Sequencing of the cDNA band confirmed the splicing of the predicted intron (intron 2). This may provide a route for future work aimed at discerning the biological function of the ALT proteins.
Variant intronic sequences for alt-1 and alt-2.
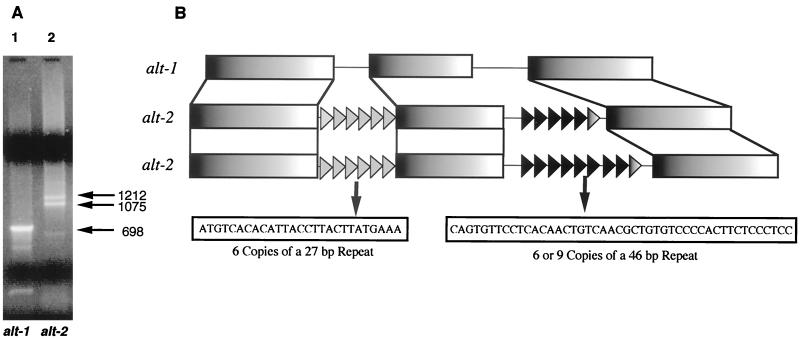
We amplified genomic PCR products for each gene to characterize gene structure, compare intronic structure, and design suitable primers for RT-PCR. Both genes contained two introns, which show no similarity between the two genes, with intron-exon boundaries conforming closely to the consensus described for Brugia genes (36). However, two products were observed from genomic PCR for alt-2 (Fig. 2A), and this was found to be due to a dimorphism in the second intron of alt-2 (Fig. 2B). Both alt-2 introns are unusual in consisting largely of 27- or 46-nt repeat units (Fig. 2B). The dichotomy in alt-2 is due to the presence of either six or nine copies of the 46-bp repeat (Fig. 2B), and individual parasites possess either or both isoforms, indicative of a Mendelian polymorphism.
FIG. 2.
(A) PCR of B. malayi genomic DNA amplified with (track 1) alt-1-specific primers and (track 2) alt-2-specific primers. Sizes are shown in base pairs. (B) Schematic of gene structure of alt-1 and two alt-2 variants, and the repeat motifs found in the alt-2 introns.
Stage-specific gene expression.
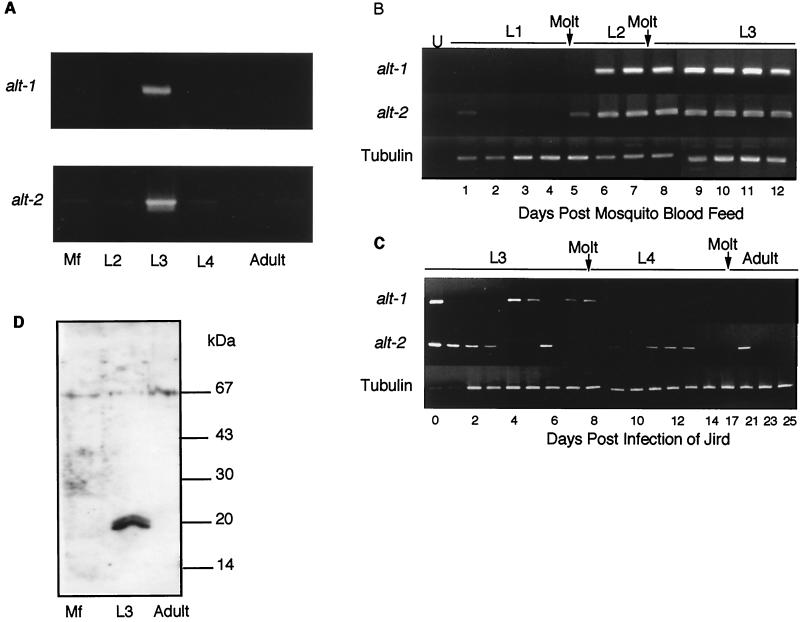
Expression of alt-1 and alt-2 at different points of the filarial life cycle was assessed by RT-PCR. Primers designed to span the introns defined above were used with the stage-specific cDNA libraries prepared by the Filarial Genome Project. From these, alt-1 expression was shown to be strictly L3 specific, and alt-2 largely so, although trace levels of amplification were evident in other stages (Fig. 3A). To provide greater detail, freshly prepared first-strand cDNA was taken at daily intervals during development of parasites from the microfilarial stage to the infective larva in A. aegypti vector mosquitoes (Fig. 3B). This showed that both alt-1 and -2 are switched on between 5 and 6 days following uptake into the mosquito vector and remain expressed for the duration of tenure in the insect. Similarly, parasites were recovered following infection of the rodent host M. unguiculatus (the jird or Mongolian gerbil). Here, alt-1 expression terminated abruptly on transfer into the jird; although brief periods of transcription were detected between days 4 and 8, no subsequent expression could be detected (Fig. 3C). alt-2 transcription was less rigorously controlled, with expression continuing for 3 days postinfection and recurring at intervals over the following 3 weeks.
FIG. 3.
Expression around the life cycle. (A) Library PCR using alt-1- and alt-2-specific primers on B. malayi cDNA libraries from microfilariae (MF), L2, L3, and adult male and female parasites. (B) RT-PCR of first-strand cDNA prepared from B. malayi parasites recovered from mosquitoes on successive days following feeding of microfilariae to A. aegypti. U, uninfected mosquitoes. (C) RT-PCR of first-strand cDNA prepared from B. malayi parasites recovered on successive days following infection of jirds with L3. Day 0, mosquito-derived L3. (D) Western blot with anti-ALT-1 and protein extracts from B. malayi L3, adult worms, and microfilariae, No reactivity was seen with normal mouse serum.
Antibodies to recombinant ALT-1 protein reacted specifically with a doublet of 20 kDa in soluble extract of L3 on Western blots (Fig. 3D), but no reactivity was detectable towards extracts of microfilariae and adult stages. Thus, at the protein level, ALT-1 and -2 are effectively L3 specific. This concords with the larva-specific expression of the related Di20/22L proteins in D. immitis (9, 10) and of the secreted larval acidic protein of O. volvulus (3), both of which are released from larvae once they are cultured under mammalian conditions. Although ALT proteins have not been identified on the surface of B. malayi larvae, immunoelectron microscopy has revealed intense staining with anti-Bm-ALT-1 antibody in the larval glandular esophagus, implying that this product may also be stockpiled ready for release within the mammalian host (A. E. Bianco, G. Egerton, W. F. Gregory, R. M. Maizels, and Y. Wu, unpublished observations).
Human recognition.
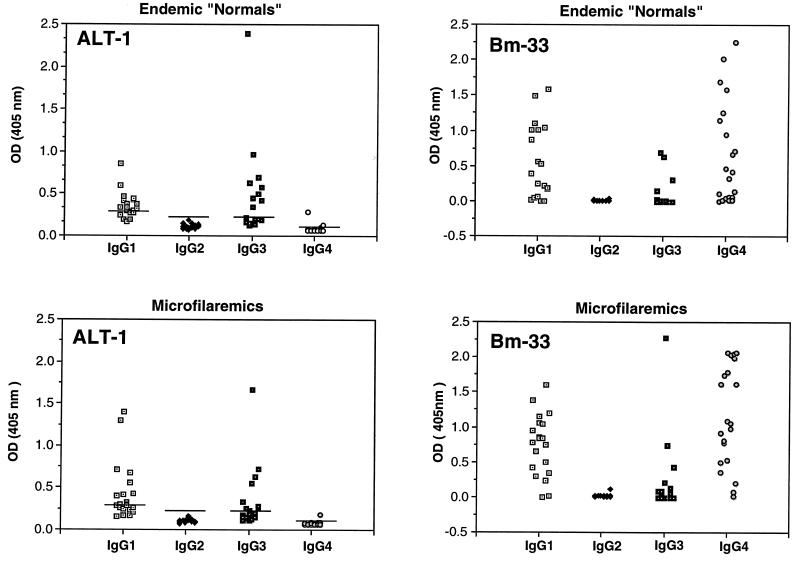
The prominence of the alt transcripts suggests that exposed humans may be serologically reactive to the ALT proteins. We tested sera from 40 patients resident in a B. malayi-endemic area of Indonesia, drawn equally from the two categories of amicrofilaremic and microfilaremic. The former group will contain both parasite-free individuals and subjects with subpatent infections; the latter group all have detectable blood-borne microfilariae. In patients with filariasis, it is well established that the overwhelming proportion of antibodies to crude adult and microfilaria-stage antigens are of the IgG4 isotype (14, 18, 25).
Antibodies to rALT-1 protein were found in members of both groups (Fig. 4). Interestingly, the antibody isotypes are predominantly IgG1 and IgG3, and no IgG4 is observed. Recombinant proteins associated with the adult stage of B. malayi, such as Bm33 (7), are generally recognized by IgG4 isotype antibodies (Fig. 4). There is also a discordance with observations on the O. volvulus protein Ov-ALT-2 (86% identical to but eight amino acids smaller than Ov-ALT-1). This is constitutively expressed (15), and human onchocerciasis patients have high levels (95%) of seropositivity, including IgG4. Thus, in O. volvulus, ALT may not represent a larva-specific antigen.
FIG. 4.
ELISA using 40 human B. malayi filariasis sera against ALT-1 and Bm-33 proteins. Upper panels show sera from amicrofilaremic normal subjects where filariasis is endemic; lower panels show sera from patients with circulating microfilariae. Horizontal bars in ALT-1 panels represent the mean value + 3 standard deviations of nonendemic control human serum reactions. Values for Bm-33 represent the net optical density (OD) following subtraction of readings for each serum against its fusion protein partner, MBP.
Protective immunization.
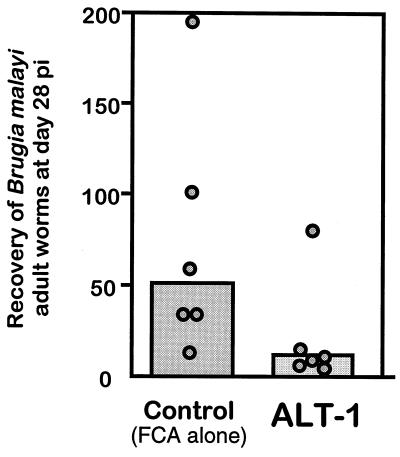
B. malayi infects only certain host species, among them the jird, which has been used as a model for human filariasis (1). We immunized jirds with four doses of ALT-1 and challenged them with 300 live L3. Four weeks later, 76% fewer live parasites were recovered from the immunized group versus the adjuvant-only controls (Fig. 5). This difference was significant (P < 0.05) by Whitney-Mann nonparametric statistics. The data reported here indicate greater than 70% protection in jirds against a challenge infection; this is substantially better than any previous filarial recombinant antigen reported and is in the range achieved by vaccination with radiation-attenuated larvae, 44 to 91% (35). Of the previously tested antigens, paramyosin has yielded disappointing results (20, 21), while heat shock protein 70, myosin, and α1-type IV collagen have recently been shown not to stimulate protective immunity (30). Thus, ALT-1 and ALT-2 offer the best vaccine candidates yet found for filariasis.
FIG. 5.
Immunization of jirds (M. unguiculatus) with ALT-1. Groups of six jirds were immunized with either ALT-1 in CFA or CFA alone, boosted with ALT-1 in IFA or IFA alone, and challenged with 300 B. malayi L3. After 28 days, the number of parasites recovered were counted. Data from individual animals are plotted, with geometric means shown as bars. The two groups are significantly different by the Mann-Whitney nonparametric test (P < 0.05).
Conclusion.
Vaccination against helminth parasite organisms has proved problematic, both in identifying likely vaccine antigens from the wide repertoire of antigens expressed and with respect to the immunopathological responses to many of these antigens (22). We report here a new approach, selecting highly expressed, stage-specific products such as the ALT proteins, which we show are not present in the mature adult stage. This, coupled with the fact that ALT proteins are parasite-specific products unrelated to any host constituent, renders less likely any adverse consequences of immunization. There is evidence from two other filarial species to associate ALT recognition with immunity (8–10, 15), but the data given here provide the first demonstration of protective immunity in a susceptible host. The high level of sequence similarity between ALT sequences from B. malayi and W. bancrofti suggests that there will be immunological cross-protection between these two species, one of which (W. bancrofti) is responsible for >90% of human infections but does not infect laboratory animals.
The human antibody profile supports, in the context of natural exposure, the concept that ALT-1 is an L3-specific antigen, as we have previously shown that responses to L3 overall are less dominated by IgG4 (17). It is notable that both microfilaria-negative and -positive groups contain individuals seropositive for ALT-1 antibodies. This finding is in keeping with the notion of age-acquired concomitant immunity, in which exposed individuals gain protection against new infection while being unable to eradicate the resident parasites (4–6). Thus, both normal subjects from endemic areas and microfilaria carriers may express protective antibodies, effectively negating the search for protective antigens by comparing antibodies from “immune” and “susceptible” individuals; such a comparison would have excluded ALT-1 from consideration.
The conservation of alt genes in all the filarial nematodes so far studied and the very weak similarity to a single C. elegans locus imply that ALT products are critical in a filaria-specific role. Moreover, this role evidently requires a remarkably high degree of expression at the point of initial entry by parasites into the mammalian host. If this role is essential to parasite survival, neutralization by the immune response may be sufficient to ensure protection. Elucidation of the function of the ALT proteins and the nature of the response induced by vaccination with these antigens should greatly enhance our understanding of the immunology of filarial infection and may lead to successful strategies for the control of filarial disease.
ACKNOWLEDGMENTS
We thank the Wellcome Trust, the Medical Research Council, and the European Commission for support under the INCO-DC programme.
We also thank Mark Blaxter, David Guiliano, Xing-xing Zang, and Maria Yazdanbakhsh for discussion and advice and Janice Murray for assistance with the filarial life cycle. Bm33 cDNA was kindly supplied by S. Dissanayake.
REFERENCES
- 1.Ash L R, Riley J M. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J Parasitol. 1970;56:969–973. [PubMed] [Google Scholar]
- 2.Bianco A E, Robertson B D, Kuo Y-M, Townson S, Ham P. Developmentally regulated expression and secretion of a polymorphic antigen by Onchocerca infective-stage larvae. Mol Biochem Parasitol. 1990;39:203–212. doi: 10.1016/0166-6851(90)90059-u. [DOI] [PubMed] [Google Scholar]
- 3.Bianco A E, Wu Y, Jenkins R E. Onchocerca spp: a “family” of secreted acidic proteins expressed by infective larvae in blackflies. Exp Parasitol. 1995;81:344–354. doi: 10.1006/expr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 4.Day K P. The endemic normal in filariasis: a static concept. Parasitol Today. 1991;7:341–343. doi: 10.1016/0169-4758(91)90215-a. [DOI] [PubMed] [Google Scholar]
- 5.Day K P, Gregory W F, Maizels R M. Age-specific acquisition of immunity to infective larvae in a Bancroftian filariasis endemic area of Papua New Guinea. Parasite Immunol. 1991;13:277–290. doi: 10.1111/j.1365-3024.1991.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 6.Day K P, Grenfell B, Spark R, Kazura J W, Alpers M P. Age specific patterns of change in the dynamics of Wuchereria bancrofti infection in Papua New Guinea. Am J Trop Med Hyg. 1991;44:518–527. doi: 10.4269/ajtmh.1991.44.518. [DOI] [PubMed] [Google Scholar]
- 7.Dissanayake S, Xu M, Nkenfou C, Piessens W F. Molecular cloning and serological characterization of a Brugia malayi pepsin inhibitor homolog. Mol Biochem Parasitol. 1993;62:143–146. doi: 10.1016/0166-6851(93)90191-y. [DOI] [PubMed] [Google Scholar]
- 8.Frank G R, Grieve R B. Metabolic labeling of Dirofilaria immitis third- and fourth-stage larvae and their excretory-secretory products. J Parasitol. 1991;77:950–956. [PubMed] [Google Scholar]
- 9.Frank G R, Grieve R B. Purification and characterization of three larval excretory-secretory proteins of Dirofilaria immitis. Mol Biochem Parasitol. 1995;75:221–229. doi: 10.1016/0166-6851(95)02533-2. [DOI] [PubMed] [Google Scholar]
- 10.Frank G R, Tripp C A, Grieve R B. Molecular cloning of a developmentally regulated protein isolated from excretory-secretory products of larval Dirofilaria immitis. Mol Biochem Parasitol. 1995;75:231–240. doi: 10.1016/0166-6851(95)02534-0. [DOI] [PubMed] [Google Scholar]
- 11.Freedman D O, Nutman T B, Ottesen E A. Protective immunity in Bancroftian filariasis: selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J Clin Invest. 1989;83:14–22. doi: 10.1172/JCI113850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gems D H, Ferguson C J, Robertson B D, Page A P, Blaxter M L, Maizels R M. An abundant, trans-spliced mRNA from Toxocara canis infective larvae encodes a 26 kDa protein with homology to phosphatidylethanolamine binding proteins. J Biol Chem. 1995;270:18517–18522. doi: 10.1074/jbc.270.31.18517. [DOI] [PubMed] [Google Scholar]
- 13.Gregory W F, Blaxter M L, Maizels R M. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Hussain R, Grögl M, Ottesen E A. IgG antibody subclasses in human filariasis: differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J Immunol. 1987;139:2794–2798. [PubMed] [Google Scholar]
- 15.Joseph G T, Huima T, Lustigman S. Characterization of an Onchocerca volvulus L3-specific larval antigen, Ov-ALT-1. Mol Biochem Parasitol. 1998;96:177–183. doi: 10.1016/s0166-6851(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 16.Kazura J W, Nutman T B, Greene B M. Filariasis: immunology and molecular biology of parasitic infections. 3rd ed. Boston, Mass: Blackwell Scientific Publications; 1993. pp. 473–495. [Google Scholar]
- 17.Kurniawan A, Sartono E, Partono F, Yazdanbakhsh M, Maizels R M. Antibody responses to filarial infective larvae are not dominated by the IgG4 isotype. Parasite Immunol. 1998;20:9–17. doi: 10.1046/j.1365-3024.1998.t01-1-00118.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurniawan A, Yazdanbakhsh M, van Ree R, Aalberse R, Selkirk M E, Partono F, Maizels R M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–3950. [PubMed] [Google Scholar]
- 19.Li B-W, Chandrashekar R, Alvarez R M, Liftis F, Weil G J. Identification of paramyosin as a potential protective antigen against Brugia malayi infection in jirds. Mol Biochem Parasitol. 1991;49:315–324. doi: 10.1016/0166-6851(91)90075-h. [DOI] [PubMed] [Google Scholar]
- 20.Li B-W, Chandrashekar R, Weil G J. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J Immunol. 1993;150:1881–1885. [PubMed] [Google Scholar]
- 21.Li B-W, Zhang S, Curtis K C, Weil G J. Immune responses to Brugia malayi paramyosin in rodents after DNA vaccination. Vaccine. 1999;18:76–81. doi: 10.1016/s0264-410x(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 22.Maizels R M, Holland M, Falcone F H, Zang X X, Yazdanbakhsh M. Vaccination against helminth parasites: the ultimate challenge for immunologists? Immunol Rev. 1999;171:125–148. doi: 10.1111/j.1600-065x.1999.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 23.Maizels R M, Lawrence R A. Immunological tolerance: the key feature in human filariasis? Parasitol Today. 1991;7:271–276. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 24.Maizels R M, Robertson B D, Blaxter M L, Selkirk M E. Parasite antigens, parasite genes: a laboratory manual for molecular parasitology. New York, N.Y: Cambridge University Press; 1991. [Google Scholar]
- 25.Maizels R M, Sartono E, Kurniawan A, Selkirk M E, Partono F, Yazdanbakhsh M. T cell activation and the balance of antibody isotypes in human filariasis. Parasitol Today. 1995;11:50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 26.Michael E, Bundy D A P, Grenfell B T. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 27.Nutman T B. Protective immunity in lymphatic filariasis. Exp Parasitol. 1989;68:248–252. doi: 10.1016/0014-4894(89)90106-9. [DOI] [PubMed] [Google Scholar]
- 28.Nutman T B, editor. Lymphatic filariasis. London, U.K: Imperial College Press; 1999. [Google Scholar]
- 29.Ottesen E A. Infection and disease in lymphatic filariasis: an immunological perspective. Parasitology. 1992;104:S71–S79. doi: 10.1017/s0031182000075259. [DOI] [PubMed] [Google Scholar]
- 30.Peralta M E, Schmitz K A, Rajan T V. Failure of highly immunogenic filarial proteins to provide host-protective immunity. Exp Parasitol. 1999;91:334–340. doi: 10.1006/expr.1998.4382. [DOI] [PubMed] [Google Scholar]
- 31.Pogonka T, Oberlander U, Marti T, Lucius R. Acanthocheilonema viteae: characterization of a molt-associated excretory/secretory 18-kDa protein. Exp Parasitol. 1999;93:73–81. doi: 10.1006/expr.1999.4445. [DOI] [PubMed] [Google Scholar]
- 32.Selkirk M E, Maizels R M, Yazdanbakhsh M. Immunity and the prospects for vaccination against filariasis. Immunobiology. 1992;184:263–281. doi: 10.1016/S0171-2985(11)80479-1. [DOI] [PubMed] [Google Scholar]
- 33.The Filarial Genome Project. Deep within the filarial genome: an update on progress in the Filarial Genome Project. Parasitol Today. 1999;15:219–224. doi: 10.1016/s0169-4758(99)01454-4. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Lymphatic filariasis: the disease and its control. Fifth Report of the WHO Expert Committee on Filariasis. Geneva, Switzerland: World Health Organization; 1992. [PubMed] [Google Scholar]
- 35.Yates J A, Higashi G I. Brugia malayi: vaccination of jirds with 60cobalt-attenuated infective stage larvae protects against homologous challenge. Am J Trop Med Hyg. 1985;34:1132–1137. doi: 10.4269/ajtmh.1985.34.1132. [DOI] [PubMed] [Google Scholar]
- 36.Zang X X, Yazdanbakhsh M, Kiang H, Kanost M R, Maizels R M. A novel serpin expressed by the blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood. 1999;94:1418–1428. [PubMed] [Google Scholar]