Abstract
To describe the prevalence of long COVID in children infected for the first time (n = 332) or reinfected (n = 243) with Omicron compared with test-negative children (n = 311). Overall, 12%-16% of those infected with Omicron met the research definition of long COVID at 3 and 6 months after infection, with no evidence of difference between cases of first positive and reinfected (Pχ2 = 0.17).
Keywords: post COVID-19 condition, omicron, long COVID, young people, prospective
The emergence of the Omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in November 2021 was a major change in the pandemic.1 The variant spread rapidly across the globe, with cases during December 2021 and March 2022 exceeding all previously reported cases.2 Although Omicron caused less severe acute illness in vaccinated populations compared to previous variants, including in children and young people, its longer-term impact remains relatively unknown.3 A large case-control study in adults using the National Institute for Health and Care Excellence’s long COVID criterion (ie, having symptoms for ≥4 weeks after the start of acute COVID-19) found lower odds of long COVID with Omicron than the Delta variant in the region of 0.24-0.50 depending on age (older adults had greater odds of long COVID) and time since vaccination, corresponding to 4.5% of adults having long COVID after Omicron infection compared to 10.8% after Delta.2 , 4 When considering symptoms 12-16 weeks after infection, between 4% and 5% of triple-vaccinated adults self-reported long COVID after infection with either the Omicron or Delta variants.5
There is little comparable research regarding the impact of the Omicron variant on long COVID in children and young people The original Children and young people with Long COVID (CLoCk) study, the largest national, matched longitudinal study of long COVID in children and young people, recruited participants aged 11-17 years, who had polymerase chain reaction (PCR) tests between September 2020 and March 2021 in England. These nonhospitalized children and young people self-report on post-COVID-19 health, and PCR-confirmed SARS-CoV-2-positive children and young people are compared with SARS-CoV-2 PCR-negative children and young people. Findings from CLoCk and other studies on earlier variants indicate that, although most children and young people recover well, a minority continue to have impairing symptoms 3 months after infection.6 , 7 However, the original CLoCk study was limited by retrospective recall of symptoms at testing and did not include children and young people infected with the Omicron variant.
Given that more than 90% of children and young people have now been exposed to SARs-CoV-2, with large numbers of primary infections and reinfections with Omicron, it is critical to understand the long-term impact of infection: even if a low proportion of children and young people develop long COVID, the scale of Omicron infections and reinfections indicates there could be an unprecedented impact and demand for services.8 Therefore, our primary objective was to describe the impact of Omicron infection on long COVID in children and young people. Specifically, we aimed to (1) determine the proportion of children and young people meeting the research definition of long COVID at 3 and 6 months across 3 infection status groups: always tested negative (test negative), first SARS-CoV-2 infection during the period when Omicron was dominant (first positive), and previous confirmed SARS-CoV-2 infection with reinfection when Omicron was dominant (reinfected); (2) compare symptom profiles across the 3 groups; and (3) examine differences in long COVID prevalence by age.
Methods
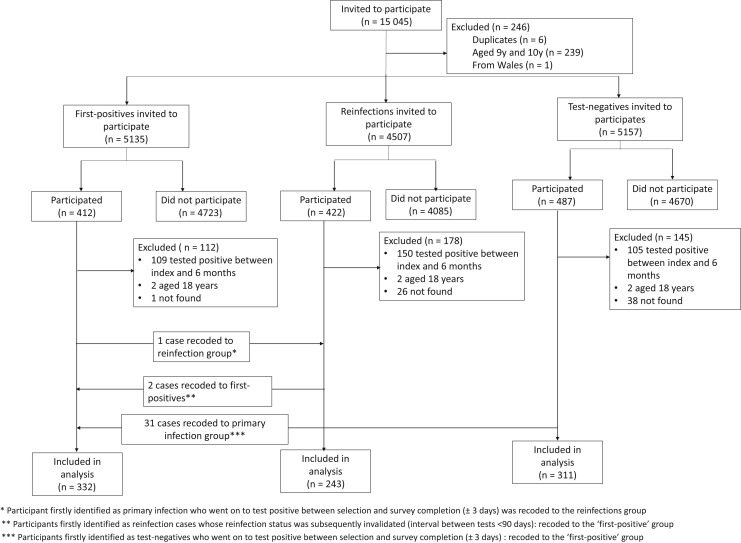
The CLoCk study methodology has been described elsewhere.9 For this sub-study, 15 045 CYP who had a PCR test in January 2022 were invited by mail to participate. The first positives were matched at study invitation to the test negatives by age (at last birthday), sex, and geographical area (based on lower super output area) using the national SARS-CoV-2 testing database held at the UK Health Security Agency; all reinfected children and young people were invited (Figure 1 ). Consenting children and young people filled in an online questionnaire at 0, 3, and 6 months after testing. The questionnaires included demographics, elements of the International Severe Acute Respiratory and Emerging Infection Consortium questionnaire, 28 symptoms (eg, shortness of breath, tiredness, brain fog), as well as validated scales including the EQ-5D-Y (which measures health-related quality of life).10 The Delphi research definition of long COVID in children and young people was operationalized as experiencing 1 or more symptoms and problems with mobility, self-care, doing usual activities, or having pain or discomfort or feeling very worried or sad, based on the EQ-5D-Y scale, at the time of questionnaire completion 3 and 6 months after testing.11 Children and young meeting this operationalized research definition were classified as having long COVID.
Figure 1.
Participant flow diagram.
We generated stacked bar charts showing the distribution of (i) individual symptoms at 0, 3, and 6 months (for 26 symptoms; runny nose and sneezing were added in to the 3- and 6-month questionnaires) and (ii) long COVID at 3 and 6 months by the 3 infection status groups, indicating when the symptom/long COVID was first reported. Using χ2 tests, we assessed whether long COVID varied by age-group (11-14 vs 15-17, based on education key stage groups in England), for each infection status group.
Results
There were 332 first-positive, 243 reinfected, and 311 test-negative children and young people who filled in the online questionnaires at 0, 3, and 6 months after testing and were included in the analytic sample, representing 5.9% of those invited (886/15 089). The analytical sample consisted of more females and was older and more affluent than the target sample (Table ). There were also some regional disparities; for example, compared with the target, the analytic sample overrepresented South-west England and under-represented North-west England. By 6 months, 3.8% (34/886) of the included children and young people reported seeking help from general practitioners (family physicians) and 6 children and young people (2 first positive, 3 reinfected, 1 test negative) had stayed overnight in hospital because of COVID-19-related problems (either acute or more long term). The proportion of children and young meeting the long COVID research definition at both 3 months and 6 months was 12.1% (first positives), 16.1% (reinfected), and 4.8% (always tested negative). The Delphi research definition of long COVID in children and young people requires a positive laboratory confirmation of SARS-CoV-2 infection.11 We excluded the need for a positive PCR test for the ‘always tested negative’ group to determine how many met this long COVID definition.11 Although the prevalence of long COVID at both 3 months and 6 months differed by infection status (P χ2 < .001), there was no evidence of difference between first positives and reinfected (P χ2 = .17). Thirteen percent of the reinfected group (ie, those who tested positive previously) met the long COVID definition at baseline (32/243); of these, 41% (13/32) had long COVID at both 3 and 6 months.
Table.
Demographics of target population and participants included in the analytical sample
| Characteristics | First positive SARS-CoV-2 test |
Reinfections SARS-CoV-2 test |
Always negative SARS-CoV-2 test |
|||
|---|---|---|---|---|---|---|
| Target |
Study |
Target |
Study |
Target |
Study |
|
| Population |
Participants |
Population |
Participants |
Population |
Participants |
|
| (n = 5135) | (n = 332) | (n = 4507) | (n = 243) | (n = 5157) | (n = 311) | |
| Percent of target population | 6.47 | 5.39 | 6.03 | |||
| Sex | ||||||
| Female | 2569 (50.03) | 193 (58.13) | 2217 (49.18) | 145 (59.67) | 2560 (49.64) | 177 (56.91) |
| Male | 2566 (49.97) | 139 (41.87) | 2280 (50.60) | 98 (40.33) | 2566 (49.76) | 134 (43.09) |
| Not known | 0 (0.00) | 10 (0.22) | 31 (0.60) | |||
| Age (years) | ||||||
| 11-14 | 2608 (50.79) | 143 (43.07) | 3745 (83.10) | 179 (73.66) | 2666 (51.70) | 152 (48.87) |
| 15-17 | 2527 (49.21) | 189 (56.93) | 762 (16.90) | 64 (26.34) | 2491 (48.30) | 159 (51.13) |
| Ethnicity | Not recorded | Not recorded | Not recorded | |||
| White | 271 (81.63) | 204 (83.95) | 259 (83.28) | |||
| Asian, Asian British | 29 (8.73) | 19 (7.82) | 31 (9.97) | |||
| Mixed | 14 (4.22) | 11 (4.53) | 8 (2.57) | |||
| Black, African, Caribbean | 11 (3.31) | 6 (2.47) | 9 (2.89) | |||
| Other | 3 (0.90) | 2 (0.82) | 3 (0.96) | |||
| Prefer not to say | 4 (1.20) | 1 (0.41) | 1 (0.32) | |||
| Region | ||||||
| East Midlands | 570 (11.10) | 36 (10.84) | 411 (9.12) | 22 (9.05) | 577 (11.19) | 33 (10.61) |
| East of England | 570 (11.10) | 34 (10.24) | 636 (14.11) | 32 (13.17) | 574 (11.13) | 36 (11.58) |
| London | 570 (11.10) | 35 (10.54) | 361 (8.01) | 14 (5.76) | 568 (11.01) | 32 (10.29) |
| North East England | 570 (11.10) | 32 (9.64) | 296 (6.57) | 20 (8.23) | 570 (11.05) | 30 (9.65) |
| North West England | 570 (11.10) | 24 (7.23) | 499 (11.07) | 25 (10.29) | 571 (11.07) | 30 (9.65) |
| South East England | 570 (11.10) | 42 (12.65) | 889 (19.72) | 49 (20.16) | 574 (11.13) | 41 (13.18) |
| South West England | 570 (11.10) | 48 (14.46) | 350 (7.76) | 27 (11.11) | 573 (11.11) | 40 (12.86) |
| West Midlands | 570 (11.10) | 38 (11.45) | 619 (13.75) | 28 (11.52) | 568 (11.01) | 41 (13.18) |
| Yorkshire and the Humber | 570 (11.10) | 43 (12.95) | 445 (9.87) | 26 (10.70) | 571 (11.07) | 28 (9.00) |
| Not Known | 5 (0.10) | 1 (0.02) | 11 (0.21) | |||
| IMD quintile | ||||||
| 1 (most deprived) | 1200 (23.37) | 54 (16.27) | 1053 (23.38) | 31 (12.76) | 1079 (20.92) | 42 (13.50) |
| 2 | 964 (18.77) | 51 (15.36) | 800 (17.75) | 38 (15.64) | 926 (17.96) | 40 (12.86) |
| 3 | 928 (18.07) | 56 (16.87) | 832 (18.46) | 42 (17.28) | 963 (18.67) | 54 (17.36) |
| 4 | 988 (19.24) | 79 (23.80) | 800 (17.75) | 62 (25.51) | 1002 (19.43) | 82 (26.37) |
| 5 (least deprived) | 1055 (20.55) | 92 (27.71) | 1022 (22.67) | 70 (28.81) | 1187 (23.02) | 93 (29.90) |
IMD, Index of Multiple Deprivation, calculated from the children and young people's small local area level based geographic hierarchy (lower super output area) at the time of the questionnaire and used as a proxy for socioeconomic status. We report IMD quintiles from most (quintile 1) to least (quintile 5) deprived.
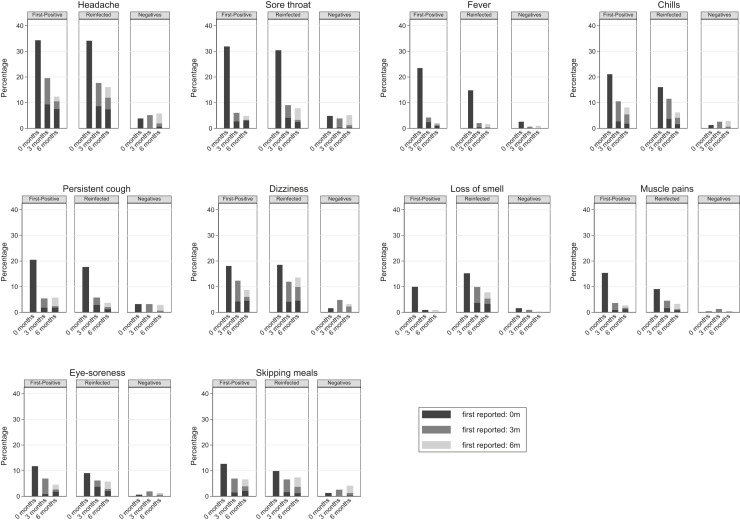
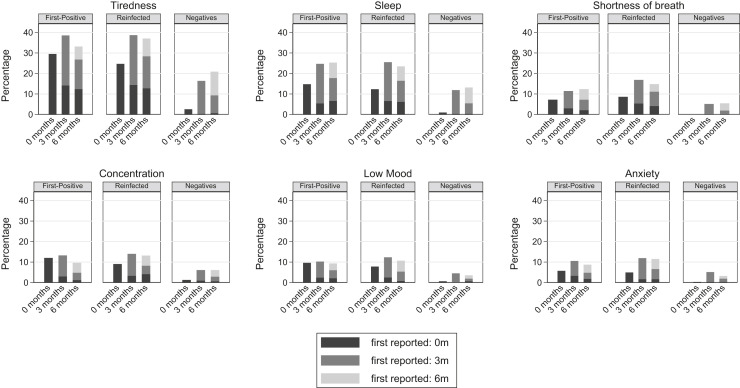
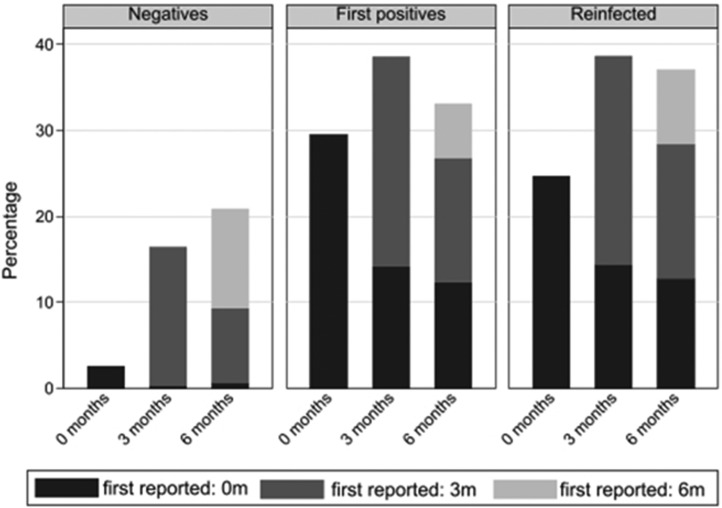
Of the 26 symptoms reported at 0, 3, and 6 months, tiredness was consistently the most commonly reported symptom across the 3 infection status groups (Figure 2, Figure 3, Figure 4 ): for example, at 6 months the overall prevalence of tiredness was 33.1% (first positives), 37.0% (reinfected), and 20.9% (test negatives). Examining within-individual variation in symptoms, it was not the case that the specific symptom was consistently reported by the same children and young at 0, 3, and 6 months. Instead, the prevalence of specific symptoms being reported by the same children and young declined from time of testing to 3 months and then declined further or remained stable at 6 months; simultaneously, those same symptoms were reported for the first time by a new cohort of children and young at 3 months and again another new cohort of children and young people first reported the symptom at 6 months (see Figure 5 for an illustration with respect to tiredness). Therefore, across the groups, although the within-individual prevalence of most reported symptoms decreased over time, the overall prevalence of tiredness, poor sleep, shortness of breath, difficulty concentrating, low mood, and anxiety increased over time with higher prevalences among first positives and reinfected than test negatives (Figure 3). Long COVID at 6 months was numerically more common in those aged 15-17 years compared with their younger counterparts (first positives, 24.3% vs 19.6%; reinfected, 28.1% vs 22.9%; test negatives, 15.1% vs 10.5%), although differences were not statistically significant (P χ2 > .22 for all infection status groups).
Figure 2.
Symptoms where the overall prevalence declined from baseline to 6 months post-test in first positives/reinfected groups.
Figure 3.
Symptoms where overall prevalence increased or generally stayed high (>10%) over time, in first positives/reinfected groups.
Figure 4.
Symptoms with very low overall prevalence (≤10%) at all time points.
Figure 5.
Prevalence of tiredness at time of testing and at 3 and 6 months after testing by infection status.
Discussion
To our knowledge, this is to investigate long COVID in children and young people first infected or reinfected with SARS-CoV-2 during the period when Omicron was dominant, with follow-up over 6 months and to examine this according to age; 12.1% of children and young people infected for the first time, 16.1% of those reinfected, and 4.8% who always tested negative (by PCR or self-report) met the research definition of long COVID at both 3 and 6 months. It may be the case that the research definition of long COVID is too broad, given that the criterion involves having 1 or more impairing symptom, because less than 4% of children and young sought help from general practitioners, which may be an important behavioral indicator of impairment. Given the scale of infection with Omicron, it is clear that a substantial number of children and young people continue to report experiencing impairing symptoms after infection, albeit few were hospitalized or sought treatment.
We found that, although all groups reported symptoms, the prevalence was always higher in the first positives and reinfected compared with test negatives. There were 2 notable observations. First, symptom profiles were similar to the profiles reported in the original and larger CLoCk study for children and young people infected with earlier variants.6 Second, for symptoms such as tiredness, poor sleep, and shortness of breath, although within-individual specific symptoms decreased over time, new cohorts experienced these symptoms at 3 and 6 months. Understanding the reason for the emergence of new symptoms months after initial infection is essential. As with previous studies, older children and young people were numerically more likely to experience long COVID than younger children and young people (although not statistically significant here).6 Much of the early work in children and young people infected with Omicron focused on very young children; here, we included those aged 11 years and older.12 Future work would benefit from a lifespan approach including younger children and young people. It should also be considered that successive infection episodes may not be independent of one another in terms of long COVID risk and we might expect some degree of intra-person correlation to exist.
Strengths and limitations of CLoCk study methodology have been described in detail elsewhere.6 Two limitations of the current report are noteworthy. First, the response rate was 5.9% and it is possible that nonresponse may introduce bias. Moreover, although there were systematic differences between the analytic and target population, the target population (by design, using UK Health Security Agency PCR testing data) might not accurately reflect the general population of children and young people in England or more broadly. Second, we initially set out to determine whether long COVID varied by vaccination status; however, because of data limitations we were unable to appropriately account for vaccine dosage or timing. The latter factor, in particular, may play an important role.2 Hence, further studies are required to accurately disentangle links between infection and vaccination status and subsequent long COVID. A strength of this study is that symptoms at testing were reported almost immediately and hence recall bias was minimal. Importantly, in this brief report we describe the symptom profiles for 3 infection status groups and do not speculate as to the underlying cause.
Our predominant finding is that children and young people with long COVID after likely infection with Omicron (both first infection and reinfection) have a similar profile to children and young people with long COVID after infection with other variants and that substantial numbers of children and young people are likely to be impacted.
Declaration of Competing Interest
This work is independent research jointly funded by the National Institute for Health and Care Research (NIHR) and UK Research and Innovation (UKRI) (Children & young people with Long COVID (CLoCk) study, Reference COV-LT-0022). All research at Great Ormond Street Hospital Charity NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. S.M.P.P. is supported by a UK Medical Research Council Career Development Award (ref: MR/P020372/1). M.G.S. is supported by The Pandemic Institute, Liverpool and the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at the University of Liverpool with UK Health Security Agency. The views expressed in this publication are those of the author(s) and not necessarily those of NIHR, The Department of Health and Social Care or UKRI.
T.S. is Chair of the Health Research Authority and therefore recused himself from the Research Ethics Application.
Acknowledgments
We thank Michael Lattimore (UK Health Security Agency) for the original suggestion of conducting this study.
Contributor Information
CLoCk Consortium:
Marta Buszewicz, Trudie Chalder, Esther Crawley, Bianca De Stavola, Tamsin Ford, Shruti Garg, Anthony Harnden, Dougal Hargreaves, Michael Levin, Vanessa Poustie, Calum Semple, Kishan Sharma, Olivia Swann, and Elizabeth Whittaker
Appendix
CLoCK Consortium Members
Marta Buszewicz, University College London, m.buszewicz@ucl.ac.uk.
Trudie Chalder, Kings College London, trudie.chalder@kcl.ac.uk.
Esther Crawley, University of Bristol, Esther.Crawley@bristol.ac.uk.
Bianca De Stavola, University College London, b.destavola@ucl.ac.uk.
Tamsin Ford, University of Cambridge, tjf52@medschl.cam.ac.uk.
Shruti Garg, University of Manchester, Shruti.Garg@mft.nhs.uk.
Anthony Harnden, Oxford University, anthony.harnden@phc.ox.ac.uk.
Dougal Hargreaves, Imperial College London, d.hargreaves@imperial.ac.uk.
Michael Levin, Imperial College London, m.levin@imperial.ac.uk.
Vanessa Poustie, University of Liverpool, v.poustie@liverpool.ac.uk.
Calum Semple, University of Liverpool, castle@liverpool.ac.uk.
Kishan Sharma, Manchester University NHS Foundation Trust (sadly deceased)
Olivia Swann, Edinburgh University, Olivia.Swann@ed.ac.uk.
Elizabeth Whittaker, Imperial College London, e.whittaker@imperial.ac.uk.
Supplementary Data
References
- 1.World Health Organization Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern n.d. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 2.Antonelli M., Pujol J.C., Spector T.D., Ourselin S., Steves C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399:2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt A.A., Dargham S.R., Chemaitelly H., Al Khal A., Tang P., Hasan M.R., et al. Severity of illness in persons infected with the SARS-CoV-2 delta variant vs Beta variant in Qatar. JAMA Intern Med. 2022;182:197–205. doi: 10.1001/JAMAINTERNMED.2021.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE n.d. https://www.nice.org.uk/guidance/NG188 [PubMed]
- 5.Self-reported long COVID after infection with the Omicron variant in the UK - Office for National Statistics n.d. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidafterinfectionwiththeomicronvariant/18july2022
- 6.Stephenson T., Pinto Pereira S.M., Shafran R., de Stavola B.L., Rojas N., McOwat K., et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Heal. 2022;6:230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behnood S.A., Shafran R., Bennett S.D., Zhang A.X.D., O’Mahoney L.L., Stephenson T.J., et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84:158–170. doi: 10.1016/J.JINF.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Office for National Statistics Coronavirus (COVID-19) latest insights - Office for National Statistics n.d. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/antibodies
- 9.Stephenson T., Shafran R., De Stavola B., Rojas N., Aiano F., Amin-Chowdhury Z., et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study) BMJ Open. 2021;11:e052838. doi: 10.1136/BMJOPEN-2021-052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wille N., Badia X., Bonsel G., Burström K., Cavrini G., Devlin N., et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res. 2010;19:875–886. doi: 10.1007/S11136-010-9648-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson T., Allin B., Nugawela M.D., Rojas N., Dalrymple E., Pinto Pereira S., et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107:674–680. doi: 10.1136/ARCHDISCHILD-2021-323624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark M., Walker B., Bennett E., Herrick A., Kenny S., Gent N. Clinical Characteristics of SARS-CoV-2 omicron infection in children under one Year. SSRN Electron J. 2022 doi: 10.2139/SSRN.4013461. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.