Abstract
Background:
Since the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTPs) was introduced in 2016, most retrospective studies have included cases diagnosed as encapsulated follicular variant of papillary thyroid carcinoma. We investigate a cohort diagnosed with NIFTP at resection.
Methods:
Retrospective institutional cohort of NIFTP from 2016 to 2022, including clinical, cytological, and molecular data for 319 cases (6.6% of thyroid surgeries, 183 cases as NIFTP-only).
Results:
The patient cohort had unifocal or multifocal thyroid nodules. Female:male ratio was 2.7:1, mean age was 52 years and median NIFTP size was 2.1 cm. NIFTP was associated with multiple nodules in 23% patients (n = 73) and 12% of NIFTP were multifocal (n = 39). Fine needle aspiration (FNA) of NIFTP (n = 255) were designated as nondiagnostic = 5%, benign = 13%, atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) = 49%, follicular neoplasm/suspicious for follicular neoplasm (FN/SFN) = 17%, suspicious for malignancy = 12%, or malignant = 4%. Molecular alterations were identified in 93% (n = 114), RAS or RAS-like. Thyroid Imaging Reporting and Data System (TI-RADS) score 4 was recorded in 50% of NIFTP, followed by scores 3 and 5 (26% and 20%, respectively). We also investigated the factors associated with extent of surgery. In our NIFTP-only group (n = 183), 66% were identified after hemithyroidectomy (HT) and 34% after total thyroidectomy (TT). On univariate analysis, TT patients demonstrated higher Bethesda category by FNA, more often had aberrant preoperative thyroid function, and/or underwent an FNA of additional nodule(s). With multivariable regression, Bethesda V NIFTP, in the presence of other nodules being evaluated by FNA and aberrant preoperative thyroid function, independently predicts TT. Bethesda II NIFTP correlated significantly with HT. Fifty-two patients (28%) with NIFTP-only had at least one postoperative surveillance ultrasound. In the NIFTP-only cohort, no HT patients had completion thyroidectomy or received postoperative radioactive iodine. No recurrence or metastases were recorded with median follow-up of 35 months (6–76 months; n = 120).
Conclusions:
Given this large cohort of NIFTP, including a large subset of isolated NIFTP-only, some with >6 years of follow-up and no tumor recurrences, consensus practical guidelines are needed for adequate postoperative management. Given the American Thyroid Association (ATA) provides guidelines for management of low-risk malignancies, guidance regarding that for borderline/biologically uncertain tumors, including NIFTP, is a reasonable next step.
Keywords: NIFTP, FNA, molecular, management, surveillance
Introduction
In 2016, a subset of tumors previously classified as encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) was designated as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTPs). This revised nomenclature from a carcinoma to low-risk neoplasm followed convincing data using strict, but evolving diagnostic criteria and >10 years of clinical follow-up in the initial retrospective cohort.1 Despite some subjectivity regarding a diagnostic threshold of features to qualify as NIFTP, published data have confirmed limited biological potential when strict criteria are met. Given the novel NIFTP designation and concern for long-term biological potential, its validity continues to be closely scrutinized.2 To date, most published studies have retrospectively assessed EFVPTC as a surrogate for NIFTP, despite more stringent required inclusion criteria for its diagnosis.3
In assessing the notion that NIFTP have exceptionally low long-term biological potential, most published studies, except for two recent studies,4,5 have included cases diagnosed as EFVPTC from before 2016, despite discordant diagnostic criteria,6–12 and those patients were treated according to their diagnosis. Prospectively developing care plans for patients initially diagnosed with NIFTP, after 2016, requires an approach more in line with the revised conservative treatment recommendations from the American Thyroid Association (ATA) for well-differentiated thyroid cancers promoting de-escalation, including active surveillance.13
Two recent studies evaluated NIFTP cases since 2016 and exclusively diagnosed as NIFTP.4,5 Each offers unique insight into the evolution of NIFTP, although one study included cases classified as NIFTP with BRAF p.V600E4 and the other included cases with concomitant lesions.5 A pure NIFTP cohort, with attempted elimination of confounding neoplasia, is desired to assess isolated tumor behavior over time. These cases may be compared in parallel with those having concomitant low-risk and higher-risk thyroid lesions to avoid cohort bias. Stratifying NIFTP-associated carcinomas into low-risk and higher-risk biological scenarios may help clinicians to better counsel patients for optimal therapeutics and surveillance protocol development. In a large institutional cohort, we studied the clinical, pathological, and molecular characteristics of 319 patients diagnosed with NIFTP and focus on a subgroup of 183 NIFTP with no associated carcinoma.
Materials and Methods
Study population
The study was approved by the Mass General Brigham Institutional Review Board (2011P000013 to P.M.S.). The Massachusetts General Hospital Laboratory Information Systems database (CoPath Plus; Sunquest, Tucson, AZ) was searched for all patients with of the term NIFTP from 2016 until mid-2022.
A total of 319 patients (6.6%) from a total of 4803 thyroid surgeries qualified for inclusion in our study (Supplementary Fig. S1). The total NIFTP cohort was subdivided into three groups: group 1, NIFTP-only (n = 183; pTXpN0a/X); group 2, one concomitant papillary thyroid microcarcinoma (PTMC; n = 53; classic or follicular variant [≤1.0 cm] and no metastatic disease at presentation; pT1a pN0a/X); and group 3, higher-risk (n = 83; two or more PTMC, any papillary thyroid carcinoma [PTC] >1.0 cm, any follicular or medullary thyroid carcinoma, or any carcinoma with lymph node metastases at presentation). Pathologists were blinded to patient outcomes.
Pathological parameters
Tumor size, number of NIFTP, tumor sampling, monoclonal BRAF p.V600E-specific antibody (BRAFVE; Cat. No. 29002s; Cell Signaling; assess for BRAF p.V600E) and HBME1 (Cat. No. 760-4445; Ventana RTU) immunostains (as previously described),14 associated carcinomas, number of associated carcinomas (single or multiple), size of largest associated carcinoma, lymph node status, extrathyroidal extension (including microscopic), lymphatic invasion, angioinvasion, surgical margins, and staging were all obtained from pathology records.
Clinical parameters
Clinical data were retrieved from electronic medical records (Epic Systems, Verona, WI). The following variables were collected for all patient groups: age, sex, tumor location, and fine needle aspiration (FNA) results, including Bethesda System for Reporting Thyroid Cytopathology.15 Molecular testing results for Afirma Genomic Sequencing Classifier (GSC) and Xpression Atlas (XA),16 Targeted next-generation sequencing panel (ThyroSeq),17 and microRNA (miRNA) gene expression and somatic gene alterations (ThyraMIR)18 were recorded. Radioactive iodine treatment and recurrence were noted.
For Group 1 (NIFTP-only), additional variables include Thyroid Imaging Reporting and Data System (TI-RADS) score, compressive symptoms, thyroid function (thyrotropin [TSH] levels; use of medications such as methimazole, propylthiouracil, or thyroxine), patients and providers preferences for treatment; and extent of operation performed (hemithyroidectomy [HT] or total thyroidectomy <TT>). HT includes lobectomy with or without isthmusectomy. The extent of surgery (HT vs. TT) was based on the patient–provider conversation and guided by the current ATA guidelines and recommendations for well-differentiated thyroid cancers.19 Number of postoperative ultrasounds (USs) were also collected.
Statistical analysis
For the NIFTP-only group, univariate analyses comparing features from patients undergoing HT versus TT were performed with proportion tests for categorical variables and Wilcoxon rank sum tests for continuous nonparametric variables. Univariate logistic regressions were performed to identify associations between the index procedure and patient and thyroid characteristics. Covariates meeting nominal significance and those of clinical interest were tested in the multivariable logistic regression model, with the final variables selected using backward stepwise elimination. p-Values <0.05 were considered statistically significant. Statistical analysis was performed using STATA version 15.1 (StataCorp, LLC, College Station, TX). In addition, we used Fisher's exact test for values <5 to compare the proportion of people who underwent TT in the non-NIFTP Bethesda II group versus the proportion who underwent TT in the non-NIFTP III–VI Bethesda group.
Results
The clinicopathological and molecular characteristics of all NIFTP groups are demonstrated in Table 1. In our full cohort of 319 patients, 23% presented with multiple nodules (n = 73) and 12% of NIFTP were multifocal (n = 39). FNA was performed on 80% of NIFTP specimens and was either nondiagnostic or benign in 18% of cases (n = 46; 12 were nondiagnostic and 34 were benign). Around half of NIFTP were Bethesda category III (atypia of undetermined significance/follicular lesion of undetermined significance; AUS/FLUS; n = 125).
Table 1.
Clinicopathological Characteristics of the Entire Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features Cohort (319 Patients)
| Clinicopathological characteristics | |
|---|---|
| Variables | Total NIFTP (n = 319) n/N (%) |
| Age, mean | |
| Range (years) | 52 (19–87) |
| Sex | |
| Female | 233/319 (73) |
| Male | 86/319 (27) |
| Size of NIFTP, median | |
| Range (cm) | 2.1 (0.2–7.5) |
| ≤1.0 | 52/319 (16) |
| 1.1–2.0 | 105/319 (33) |
| 2.1–4.0 | 119/319 (37) |
| >4.0 | 43/319 (14) |
| Number of NIFTP | |
| 1 | 280/319 (88) |
| 2 | 32/319 (10) |
| 3 or more | 7/319 (2) |
| NIFTP FNA | |
| Yes | 256/319 (80) |
| No | 63/319 (20) |
| Bethesda system category of NIFTP | |
| Nondiagnostic | 12/256 (5) |
| Benign | 34/256 (13) |
| AUS/FLUS | 125/256 (49) |
| FN/SFN | 43/256 (17) |
| Suspicious for malignancy | 32/256 (12) |
| Malignant | 10/256 (4) |
| Molecular alterations in NIFTP | |
| Yes | 106/114 (93) |
| No | 8/114 (7) |
| Gene identified | |
| Yes | |
| Gene | |
| NRAS | 32/60 (53) |
| KRAS | 10/60 (17) |
| THADA fusion | 8/60 (13) |
| HRAS | 7/60 (12) |
| BRAF p.K601E | 2/60 (3) |
| RNF125/RNF138 | 1/60 (2) |
| Unspecified by assay | |
| Afirma suspicious | 44/106 (42) |
| BRAFVE IHC on NIFTP | |
| Positive | — |
| Negative | 193/195 (99) |
| Indeterminatea | 2/195 (1) |
| HBME1 IHC on NIFTP | |
| Positive | 148/163 (91) |
| Negative | 15/163 (9) |
| Non-NIFTP FNA | |
| Yes | 95/319 (30) |
| Bethesda system category for non-NIFTP FNA | |
| Nondiagnostic | 3/95 (3) |
| Benign | 28/95 (29) |
| AUS/FLUS | 21/95 (22) |
| FN/SFN | 8/95 (8) |
| Suspicious for malignancy | 10/95 (11) |
| Malignant | 25/95 (26) |
| Associated follicular carcinoma | |
| Yes | 8/319 (3) |
| Associated intrathyroidal PTC | |
| Yes | 132/319 (41) |
| Associated MTC | |
| Yes | 1/319 (<1) |
Indeterminate cases had high-background stain with a blush of stain both in tumor and normal cells. No positive cases were detected.
AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; BRAFVE, monoclonal BRAF p.V600E-specific antibody; FNA, fine needle aspiration; FN/SFN, follicular neoplasm/suspicious for follicular neoplasm; IHC, immunohistochemistry; MTC, medullary thyroid carcinoma; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear feature; PTC, papillary thyroid carcinoma.
Seventeen percent of cases were designated as Bethesda IV (follicular neoplasm/suspicious for follicular neoplasm; FN/SFN; n = 43). In 16% of cases (n = 42), NIFTP were designated as either suspicious for malignancy (n = 32) or malignant (n = 10). The histological, cytological, and immunohistochemical features of NIFTP, compared with circumscribed classic PTC with predominantly follicular growth, are demonstrated in (Fig. 1). The distribution of NIFTP cytological diagnoses since 2016 is demonstrated in (Fig. 2A). Thirty percent of biopsied NIFTP had FNA of a second nodule (n = 95). Bethesda categories for the second FNA are described in Table 1.
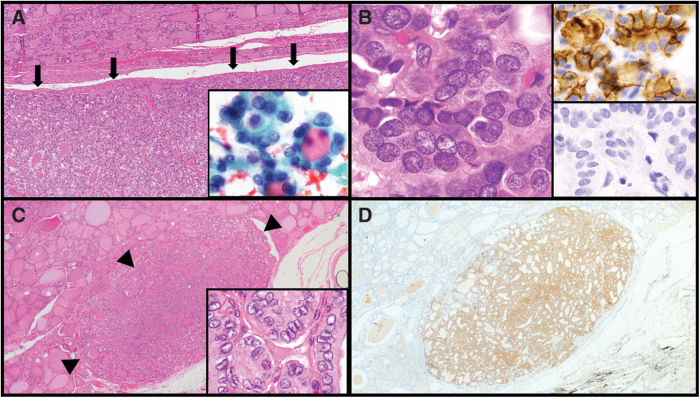
FIG. 1.
Comparison of NIFTP versus circumscribed classic PTC with predominantly follicular architecture. NIFTP is well circumscribed [200 × , H&E but unencapsulated (arrows, A), and in this example, 1.5 cm in size with NRAS p.Q61R]. Cytology of NIFTP with microfollicles and nuclear atypia (Bethesda IV; inset, 1000 × , A). Higher magnification of NIFTP (1000 × , H&E, B) highlights crowded nuclei with clearing and some grooves. Immunohistochemistry to assess for HBME1 (upper inset, 1000 × , B) showing a strong, membranous staining pattern and BRAF p.V600E (lower inset, 1000 × , B) is negative. Well-circumscribed (arrow heads) PTMC (0.4 cm) (200 × , H&E, C) shows minimal infiltration into adjacent thyroid parenchyma, has more pronounced PTC-like nuclear features (1000 × , H&E, inset, C) and is positive for BRAF p.V600E (200 × , BRAFVE immunostain, D), excluding an NIFTP diagnosis. BRAFVE, monoclonal BRAF p.V600E-specific antibody; H&E, hematoxylin and eosin; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma.
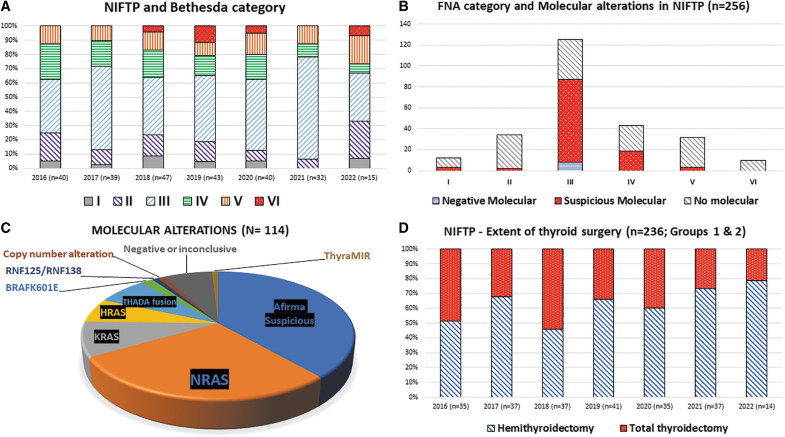
FIG. 2.
NIFTP Diagnostic and surgical parameters. Retrospective distribution of NIFTP classification using The Bethesda System for Reporting Thyroid Cytopathology, 2016-6/2022 (A, n = 256) with distribution of molecular findings for the same cases by Bethesda category (B). Distribution of molecular findings, including specific findings from ThyroSeq and categorical findings from other gene classifiers (C). Surgical choice is shown in (D).
Ninety-three percent of molecular tests were performed for Bethesda III/IV (n = 106). The proportion of NIFTP with molecular testing and associated molecular alterations are shown in Figure 2B.
The distribution of NIFTP molecular alterations is shown in Figure 2C. Most NIFTP harbor RAS mutations (82%; n = 49), with NRAS p.Q61R being most common (n = 32). One patient had two bilateral nodules (Bethesda III) with subsequent ThyroSeq testing identifying NRAS p.Q61R mutation in both nodules. The second most common alteration was THADA fusion (n = 8) with two reported partners (IGF2BP3; n = 7 and TRA2A; n = 1). In 44 cases (42%), Afirma testing was suspicious with no subsequent XA. In groups 1 and 2, there has been a trend toward more HT since NIFTP emerged in 2016 (Fig. 2D).
In the thyroid, BRAF p.V600E is exclusive to a diagnosis of carcinoma. BRAFVE immunostain (Ventana, IN) was used to rule out BRAF p.V600E-mediated PTC and was negative in 99% of NIFTP (n = 193/195). In two cases, there was high background, including on repeat stain, but no BRAFVE positive case was detected. BRAFVE did not stain any of RAS-aberrant or the BRAF p.K601E NIFTP (n = 33 and n = 2, respectively).
In group 3 NIFTP, 19 patients presented with lymph node metastases, with 6 tested metastatic lymph nodes showing positive BRAFVE immunostain. Corresponding NIFTP in three of these patients were BRAFVE negative, and the associated PTC in four patients were BRAFVE positive (Table 2). In two NIFTP cases (5.5 and 3.7 cm) resected with HT, metastatic PTC was identified in the central compartment lymph nodes (both cases BRAFVE positive nodes), but no primary PTC within the thyroid lobe was found (the entire lobe was submitted in each case).
Table 2.
Cases with Metastatic Lymph Nodes with BRAFVE Immunostain Performed on the Metastatic Lymph Nodes with Concomitant Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Feature
| Cases (positive/total LN; pN stage) and positive BRAFVE on LN | Size of largest metastatic focus, ENE | Tumor size; histology; focality | BRAFVE on PTC | FNA category of PTC | NIFTP size | FNA category of NIFTP | Molecular testing on NIFTP | BRAFVE immunostain on NIFTP |
|---|---|---|---|---|---|---|---|---|
| Case 1 (2/16; pN1a) | 0.2 cm, no ENE | 4.2 cm; PTC; classic; multifocal | Positive | VI | 0.5 | — | — | Not performed |
| Case 2 (2/7; pN1a) | 2.2 cm, no ENE | 1.9 cm; PTC; infiltrative follicular; multifocal | Positive | VI | 4.5 | III | — | Not performed |
| Case 3 (3/8; pN1b) | 3.7 cm, no ENE | 4.5 cm; PTC; tall cell; multifocal | Positive | V | 1.3 | — | — | Negative |
| Case 4 (5/15) | 3.8 cm with ENE, 0.3 cm | 1.4 cm; with diffuse sclerosing features; multifocal | Positive | VI | 1.2 | — | — | Negative |
| Case 5a (1/3) | 0.8 cm, no ENE | NA | NA | NA | 5.5 | II | — | Not performed |
| Case 6a (4/5) | 1.3 cm, with ENE, 0.1 cm | NA | NA | NA | 3.7 | III | Afirma suspicious | Negative |
Two cases presented with PTC metastatic to lymph nodes without primary thyroid PTC.
ENE, extranodal extension; LN, lymph nodes; NA, not applicable.
HBME1 (Ventana, IN) immunostain highlighted cells with nuclear membrane irregularities in both NIFTP (Fig. 1B, top inset) and PTC. HBME1 was positive in 91% of NIFTP (n = 148).
Group 1: NIFTP-only cohort
Clinicopathological and radiological characteristics
The clinicopathological, radiological, and molecular characteristics of NIFTP-only are detailed in Table 3. Female to male ratio is 2.8 to 1. The median age is 53 years. FNA was performed for most NIFTP specimens, either primarily for the NIFTP nodule and/or for a concomitant nodule (n = 163 and 10, respectively). Surgeon recommendation, patient preferences, and final treatment for NIFTP are documented from patients' medical records, and patients nearly uniformly went with surgeon recommendations for extent of surgery.
Table 3.
Clinicopathological Characteristics of 183 Patients with Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features-only (Group 1)
| Clinicopathological characteristics | |
|---|---|
| Variables | Group 1 (n = 183) NIFTP-only, n/N (%) |
| Age, median | |
| Range (years) | 53 (19–87) |
| Sex | |
| Female | 135/183 (74) |
| Male | 48/183 (26) |
| Size of NIFTP, median | |
| Range (cm) | 2.3 (0.3–7.5) |
| ≤1.0 | 21/183 (12) |
| 1.1–2.0 | 55/183 (30) |
| 2.1–4.0 | 79/183 (43) |
| >4.0 | 28/183 (15) |
| Number of NIFTP | |
| 1 | 171/183 |
| 2 | 9/183 |
| 3 or more | 3/183 |
| NIFTP FNA | |
| Yes | 163/183 (89) |
| No | 20/183 |
| Bethesda system category of NIFTP, n = 163 | |
| Nondiagnostic | 6/163 (4) |
| Benign | 20/163 (12) |
| AUS/FLUS | 83/163 (51) |
| FN/SFN | 31/163 (19) |
| Suspicious for malignancy | 18/163 (11) |
| Malignant | 5/163 (3) |
| Molecular alterations in NIFTP, n = 79 | |
| Yes | 73/79 (92) |
| No | 6/79 |
| Gene identified | |
| Yes | 46/73 |
| Gene | |
| NRAS | 24/46 (52) |
| KRAS | 7/46 (15) |
| HRAS | 7/46 (15) |
| TAHDA fusion | 8/46 (17) |
| Suspicious Afirma | 26/73 (36) |
| BRAFVE IHC; n = 108 | |
| Positive | 0/108 |
| Negative | 106/108 (98) |
| Indeterminate | 2/108 (2) |
| HBME1 IHC; n = 93 | |
| Positive | 84/93 (90) |
| Negative | 9/93 |
| TI-RADS for NIFTP; n = 148 | |
| 1 | 2/148 (1) |
| 2 | 5/148 (3) |
| 3 | 38/148 (26) |
| 4 | 73/148 (50) |
| 5 | 30/148 (20) |
| Preoperative features | |
| Compression | 30/181 (17) |
| Thyroid function; n = 181 | |
| Euthyroid | 164/181 (91) |
| Hyperthyroid | 10/181 (6) |
| Hypothyroid | 7/181 (4) |
| Thyroid medication use | |
| Methimazole or thyroxine | 9/181 (5) |
| Non-NIFTP FNA | |
| Yes | 28/183 (15) |
| Bethesda system category for non-NIFTP FNA; n = 28 | |
| Nondiagnostic | 2/28 (7) |
| Benign | 15/28 (53) |
| AUS/FLUS | 8/28 (29) |
| FN/SFN | 1/28 (4) |
| Suspicious for malignancy | 2/28 (7) |
| Malignant | 0/28 (0) |
| Procedure | |
| Lobectomy/hemithyroidectomy | 120/183 (66) |
| Total thyroidectomy | 63/183 (34) |
| Completion thyroidectomy | |
| Yes | 0/118 (0) |
| No | 118/118 |
| NA | 2/120 |
| Lymph nodes sampling | |
| Yes | 64/183 |
| Positive lymph nodes | |
| Yes | 0/64 (0) |
| No | 64/64 |
| Radioactive iodine | |
| Yes | 0/152 (0) |
| No | 152/152 |
| Unknown (follow up <4 weeks) | 31/183 |
| Recurrence | |
| Yes | 0/128 (0) |
| No | 128/152 |
| Unknown (follow up <6 months) | 54/183 |
TI-RADS, Thyroid Imaging Reporting and Data System.
Analysis of features associated with undergoing TT
Characteristics of NIFTP patients by procedure type are shown in Table 4. Patients who underwent TT more often had their NIFTP characterized as Bethesda VI by FNA (p = 0.03), had aberrant thyroid function (hyperthyroid, p = 0.02 and hyperthyroid p = 0.04), required thyroid medication (p = 0.04), or had a non-NIFTP nodule evaluated by FNA (p = 0.0015). Normal thyroid function tests correlated with HT (p = 0.005). No difference in surgical recommendation was noted based on TI-RADS score. Statistical analysis based on clinicopathological features associated with undergoing TT is presented in Table 5.
Table 4.
Clinicopathological and Radiological Characteristics for Patients with Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Feature-only (Group 1) and Correlation with Total Thyroidectomy on Univariate Analysis
| Clinicopathological and radiological characteristics | Hemithyroidectomy, N (%) or median (IQR); n = 120 | Total thyroidectomy, N (%) or median (IQR); n = 63 | p |
|---|---|---|---|
| Age (years) | 52 (39–65) | 56 (43–65) | 0.13 |
| Female | 87 (73) | 48 (76) | 0.59 |
| Bethesda system category of NIFTP | |||
| Nondiagnostic | 3 (3) | 3 (5) | 0.41 |
| Benign | 16 (13) | 4 (6) | 0.15 |
| AUS/FLUS | 59 (49) | 24 (38) | 0.15 |
| FN/SFN | 23 (19) | 8 (12) | 0.27 |
| Suspicious for malignancy | 8 (7) | 10 (16) | 0.05 |
| Malignant | 1 (1) | 4 (6) | 0.03 |
| Not performed | 10 (8) | 10 (15) | 0.11 |
| Underwent FNA of non-NIFTP nodule | 11 (9.2) | 17 (27) | 0.0015 |
| TI-RADS for NIFTP | |||
| 1 | 1 (1) | 1 (2) | 0.64 |
| 2 | 4 (3) | 1 (2) | 0.49 |
| 3 | 26 (22) | 12 (19) | 0.68 |
| 4 | 50 (42) | 23 (37) | 0.50 |
| 5 | 17 (14) | 13 (21) | 0.27 |
| TSH (mIU/mL) | 1.4 (1.0–2.3) | 1.6 (1.1–2.3) | 0.35 |
| Preoperative features | |||
| Compression | 16 (13.3) | 14 (22.2) | 0.12 |
| Euthyroid | 113 (94.2) | 51 (81.0) | 0.005 |
| Hyperthyroid | 3 (2.5) | 7 (11.1) | 0.02 |
| Hypothyroid | 2 (1.7) | 5 (7.9) | 0.04 |
| Thyroid medication use (methimazole and thyroxine) | 3 (2.5) | 6 (9.5) | 0.04 |
| Molecular testing | |||
| Suspicious | 45 (37.5) | 24 (38.1) | 0.94 |
| RAS (KRAS, NRAS, and HRAS) | 24 (20.0) | 14 (22.2) | 0.73 |
Bold: statistically significant.
IQR, interquartile range; TSH, thyrotropin.
Table 5.
Univariate and Multivariable Analysis of Factors Associated with Undergoing Total Thyroidectomy for Patients with a Single Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Feature Nodule
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Age | 1.02 | 1.00–1.04 | 0.12 | |||
| Female | 1.18 | 0.58–2.39 | 0.65 | |||
| Preoperative patient factors | ||||||
| Compression | 1.82 | 0.83–4.03 | 0.14 | 2.57 | 0.93–7.05 | 0.07 |
| Hypothyroidism or hyperthyroidisma | 5.32 | 1.78–15.9 | 0.003 | 6.46 | 1.61–26.0 | 0.009 |
| Nodule size | 0.80 | 0.64–1.01 | 0.06 | |||
| TI-RADS of NIFTP | ||||||
| 1 | Ref. | — | — | |||
| 2 | 0.48 | 0.05–4.41 | 0.52 | |||
| 3 | 0.84 | 0.40–1.92 | 0.74 | |||
| 4 | 0.82 | 0.41–1.62 | 0.38 | |||
| 5 | 1.80 | 0.79–4.12 | 0.16 | |||
| TI-RADS of non-NIFTP nodule | ||||||
| 1 | Ref. | — | — | |||
| 2 | 5.20 | 0.57–47.7 | 0.15 | |||
| 3 | 2.43 | 0.56–10.6 | 0.24 | |||
| 4 | 0.51 | 0.18–1.49 | 0.22 | |||
| 5 | 0.53 | 0.13–2.16 | 0.38 | |||
| FNA classification of dominant nodule | ||||||
| Indeterminate | Ref. | — | — | |||
| Benign | 0.48 | 0.15–1.51 | 0.21 | |||
| AUS/FLUS | 0.72 | 0.37–1.38 | 0.32 | |||
| FN/SFN | 0.67 | 0.28–1.62 | 0.38 | 0.21 | 0.05–0.83 | 0.03 |
| Suspicious for malignancy | 2.97 | 1.10–8.03 | 0.03 | 3.39 | 1.15–10.0 | 0.03 |
| Malignant | 8.90 | 0.97–81.7 | 0.05 | 9.12 | 0.88–94.3 | 0.06 |
| Underwent FNA of non-NIFTP nodule | 3.66 | 1.59–8.42 | 0.002 | 6.66 | 2.07–21.4 | 0.001 |
| Suspicious molecular testing | 0.80 | 0.43–1.51 | 0.50 | |||
Bold: statistically significant.
Patients are clinically diagnosed with hypothyroidism or hyperthyroidism with abnormal TSH levels, or either on thyroid replacement medication or thyroid suppressive therapy.
CI, confidence interval; OR, odds ratio; Ref.: Reference variable for multivariable analysis.
By multivariable analysis, preoperative aberrant thyroid function (p = 0.009, odds ratio [OR] = 6.46, confidence interval [CI] 2. 1.61–26.0), FNA Bethesda V (p = 0.03, OR = 3.39, CI 1.15–10.0) for the NIFTP nodule, and undergoing an FNA of a non-NIFTP (p = 0.001, OR = 6.66, CI 2.07–21.4) were all independently correlated with undergoing TT. HT was significantly associated with a Bethesda II NIFTP (p = 0.03, OR = 0.21, CI 0.05–0.83). Using Fisher's exact test, a non-NIFTP diagnosed as Bethesda II correlated with HT, compared with intermediate or higher Bethesda categories (III–VI), who show more correlation with TT (p = 0.006). Seventy-nine patients underwent molecular testing, 6 with benign results and 73 designated as suspicious. There was no correlation between suspicious molecular testing and recommendation for TT. Other variables such as compressive symptoms and TI-RADS score did not correlate with TT decision.
Surveillance strategies and outcomes
Postoperative surveillance US was carried out in 28% of patients in our cohort (n = 52) with a median interval of 371 days (range 210–476 days). Eighty USs were performed for the 52 patients with postsurgical imaging. Of patients with available imaging, 71% received HT and the remaining patients underwent TT (total = 52; 37 = HT and 15 = TT). Thirteen HT patients had a nodule in the other lobe (13/37; 35%). Within this HT group, no patients were recommended for completion thyroidectomy. Postoperative radioactive iodine was not administered in any patient. No recurrence or metastases were recorded with median follow-up of 35 months (6–76 months).
Group 2: NIFTP with a single additional low-risk carcinoma (PTMC)
In this group, 25% had a concomitant FNA for another nodule (n = 13). Of those, 11 nodules were resected, and 2 (Bethesda II), in the contralateral lobe, were preserved. Within the entire group (n = 53), PTMC was an incidental finding except for the two biopsied cases, one biopsied as the primary pyramidal lobe tumor (0.9 cm) with TT (0.5 cm NIFTP) and the second within the ipsilateral lobe (also 0.9 cm) but with a dominant NIFTP (3.4 cm) with HT. Lymphatic invasion was noted in 17% of PTC (n = 9). In the same group, 49% of NIFTP were identified after HT (n = 26) and 51% after TT (n = 27). Of 26 HT, none had completion thyroidectomy. Furthermore, no cases received postoperative radioactive iodine. No recurrence or metastases were recorded with median follow-up of 45 months (8–77 months; n = 35).
Group 3: NIFTP with higher-risk carcinoma
In this group, 78% (62/77) presented with multiple foci of PTC 57% with a carcinoma >1.0 cm (44/77). Eight minimally invasive follicular thyroid carcinomas (0.2–3.9 cm; including four oncocytic thyroid carcinomas) and one medullary thyroid microcarcinoma (0.4 cm) were identified. Nineteen percent of PTC (15/79) presented with lymph node metastases; of those, 20% (3/15) presented with lateral neck disease (pN1b).
In this higher-risk cohort (n = 83), 65% cases had a non-NIFTP nodule assessed by FNA (n = 54). Fifty-three percent of PTC were incidental with no prior FNA (n = 44). Lymphatic invasion was associated with 60% (49/81) of PTC. Gross extrathyroidal extension into strap muscles was seen in one patient (pT3b; 4.2 cm). Of the 23 patients with HT, six patients (five with multiple PTC and one with follicular thyroid carcinoma, 3.9 cm) underwent completion thyroidectomy. Within Group 3, follow-up was available for 67 patients. Twenty-eight percent received radioactive iodine (n = 19), and only one patient presented with local recurrence of the carcinoma after 8 months [pT3a(m) N1b]. All patients were free of disease at the time of the last follow-up and no one died of disease. The median follow-up for this cohort was 46 months (6–77 months; n = 58).
Discussion
NIFTP was first described in 20161 and recognized by the WHO in 2017.20 Although universally recognized by those involved in diagnosis and treatment of thyroid neoplasia, its novelty,1 along with modifying inclusion criteria,2,21,22 require increased data reporting. Lacking long-term outcomes data, NIFTP is described to be of low biological potential.23–25 Recently, the ATA has modified treatment recommendations regarding well-differentiated thyroid carcinomas to allow for treatment de-escalation and active surveillance for lower-risk carcinomas.13,26 As NIFTP is not classified as malignant, our data reinforce the need for new management protocols.19 We subdivided NIFTP into three groups: group 1, NIFTP-only; group 2, NIFTP with a single PTMC; and group 3, NIFTP with a more significant associated carcinoma burden.
In one study, 29% of NIFTP were incidental,27 similar to the 20% in our cohort. Pathologist interobserver variability may also be a significant factor in NIFTP incidence but was beyond the scope of our study. Carcinoma has been associated with NIFTP in 15–46% of patients,27–29 and our cohort is similar (41%). Incidental PTMC did not change the prognosis in patients with NIFTP, similar to Taneja et al. that included PTMC in their NIFTP cohort.30 We considered NIFTP with concomitant lesions separately (Groups 2 and 3).
NIFTP was Bethesda category III in 49% of cases, consistent with prior reports,31–34 and cellular morphology alone is limited in differentiating NIFTP from follicular variant papillary thyroid carcinoma (FVPTC).35 BRAF p.V600E should exclude the diagnosis of NIFTP, a finding favoring PTC.22
Molecular studies are increasingly employed for surgical planning.36 In our cohort, NRAS p.Q61R is the most common genetic variant detected, over HRAS and KRAS, consistent with prior studies.28,33,37,38 BRAF p.K601E and THADA fusion are common in follicular-patterned tumors and are considered RAS-like mutations.37,39–41 Mutations in miRNA, copy number alterations, and DNA methylation can be present in NIFTP but with unclear significance.
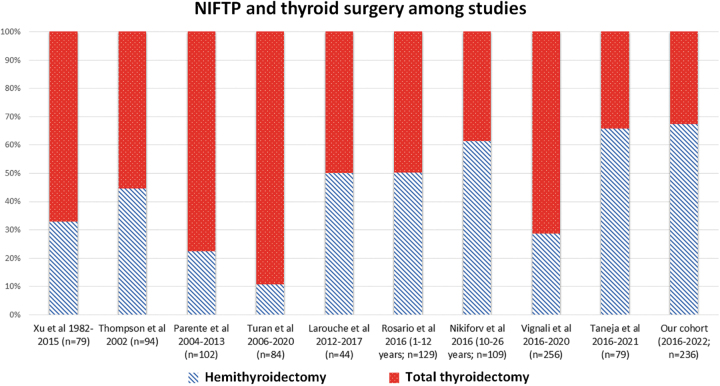
Institutional management of encapsulated nodules, including NIFTP, is geographically variable (Fig. 3), a finding that may reflect cultural approaches to nodule management. Although surgical plans are multifactorial, for isolated NIFTP, a consensus approach should be considered to optimize cost, outcomes, and quality of life.42
FIG. 3.
Distribution of surgery for NIFTP in retrospective studies.
Although HT appears sufficient treatment for NIFTP-only and NIFTP with low-risk carcinomas, several patients in our cohort underwent TT (90/236). Indication for each TT is not further commented on in this study, but unlikely considered for NIFTP in isolation, as multivariable regression shows the decision to undergo TT was independently associated with the FNA result (favoring TT in Bethesda V/VI), FNA of an additional, non-NIFTP nodule, or preoperative aberrant thyroid function.
For 68 patients (37.8%), providers document that molecular testing of the dominant nodule informed their surgical decision-making, and the majority of NIFTP were characterized as Afirma suspicious. While genetic testing is nonstandard for Bethesda V/VI lesions, indeterminate categories (Bethesda III/IV) are often sent for molecular evaluation. As genetic testing has become standard on FNA material, and as costs for testing decline, more routine testing may be considered beyond the indeterminate FNA setting and extend to lesions with suspected malignancy to facilitate longitudinal management.43
Our NIFTP-only cases (Group 1) had a median follow-up of 35 months and our NIFTP plus PTMC cases (Group 2) had a median follow-up of 46 months. No recurrences or metastases were reported for NIFTP, and partnered with earlier studies with >10 years of follow-up of noninvasive encapsulated FVPTC, our study affirms lack of NIFTP recurrence or metastasis.1,4,6–8,11,12
Postoperative follow-up for NIFTP patients has been variable. Surveillance US, 80 in total, was performed on 52 patients in group 1, those with NIFTP-only, including patients who had undergone TT HT with no contralateral nodule. The individual reasons for postoperative US in the 15 patients in Group 1 who underwent TT have not been scrutinized.
Our study has several caveats, and most glaring is the short clinical follow-up period given the typically long-term view of low-risk thyroid neoplasia. Given all cases reported in this study were diagnosed since NIFTP appeared in the literature, the maximum follow-up can be 6 years.26,44 Our cohort has no pediatric patients, and, in part, may be due to selection bias from our hospital's predominantly adult patient population, although consistent with Taneja et al.,30 where the youngest reported patient was 21 years. Other studies have found NIFTP in children and adolescents.45–48
Protocols for postoperative management of isolated NIFTP patients should be in line with those for other biologically low-risk thyroid neoplasias. Follicular adenomas and tumors categorized by the WHO as uncertain malignant potential are the most equivalent entities to NIFTP, architecturally and molecularly. With our current understanding of NIFTP, including the data presented in this study, we propose a postoperative management algorithm (Fig. 4). NIFTP alone should result in routine clinical follow-up. NIFTP plus any carcinoma should follow established management protocols for the coincidental lesion, from routine follow-up for incidental ipsilateral microcarcinomas to more lesion-specific protocols in all other cases.
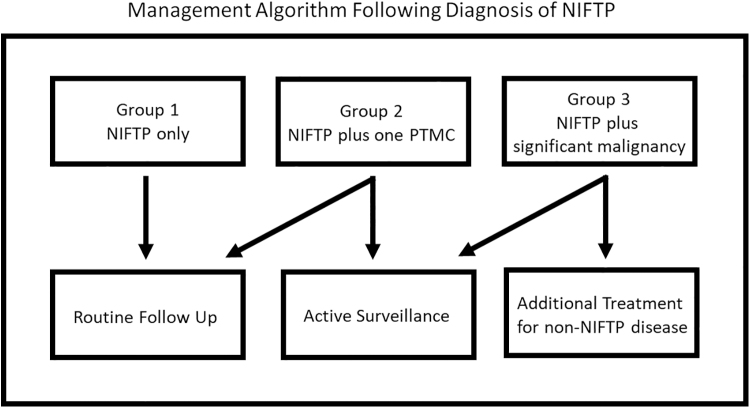
FIG. 4.
Postoperative management algorithm after NIFTP diagnosis. Tumors are divided into Groups 1–3: Group 1 being NIFTP-only, Group 2 is NIFTP plus a single PTMC, and Group 3 is NIFTP plus additional malignancies (multiple microcarcinomas, PTC >1.0 cm, non-PTCs). Group 1 patients and patients in Group 2 with incidentally detected single foci of PTMC should be followed routinely, as any patient diagnosed with a benign thyroid tumor would be followed. Patients in Group 2 with nonincidental PTMC should be followed with active surveillance. Patients in Group 3 should be followed based on their concomitant disease process, indifferent to NIFTP, some with active surveillance and some with additional treatment.
Conclusion
NIFTP, so-named due to features of circumscription (no invasion), follicular-patterned architecture and atypical nuclear features, all seem to have variant RAS or RAS-like genetics and behavior. The historical binary concept of either benign or malignant is challenged with increased frequency, and, at least for clinical management purposes, our data favor the drift of isolated NIFTP toward a benign diagnosis, knowing that any true neoplastic process is inherent with at least some biological risk. As the ATA guidelines for management of low-risk malignancies now favors a de-escalated approach, guidance regarding that for borderline/biologically uncertain tumors, including NIFTP, is a reasonable next step.
Supplementary Material
Authors' Contributions
Conceptualization, methodology, data collection, investigation, and writing—original draft preparation by B.A.A., and final draft preparation with Dr. Sadow. L.N.K., R.C., T.K., E.I.A., A.S.F., W.C.F., V.N., M.M.-L., G.W.R., R.M.G., and C.C.L. were involved with investigation and writing—reviewing and editing. Conceptualization, methodology, data collection, writing—original draft preparation and supervision, final draft preparation, and approval by P.M.S.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Support for Drs. Faquin and Sadow is provided by the National Cancer Institute of the National Institutes of Health (5P01CA240239-04).
Supplementary Material
References
- 1. Nikiforov YE, Seethala RR, Tallini G, et al. . Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2(8):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baloch ZW, Asa SL, Barletta JA, et al. . Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol 2022;33(1):27–63. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd RV, Asa SL, LiVolsi VA, et al. . The evolving diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum Pathol 2018;74:1–4. [DOI] [PubMed] [Google Scholar]
- 4. Vignali P, Proietti A, Macerola E, et al. . Clinical-pathological and molecular evaluation of 451 NIFTP patients from a single referral center. Cancers (Basel). 2022;14(2):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taneja C, Yip L, Morariu EM, et al. . Clinicopathologic characteristics and postsurgical follow-up of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the postnomenclature revision era. Thyroid 2022;32(11):1346–1352. [DOI] [PubMed] [Google Scholar]
- 6. Parente DN, Kluijfhout WP, Bongers PJ, et al. . Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: Is NIFTP truly benign? World J Surg 2018;42(2):321–326. [DOI] [PubMed] [Google Scholar]
- 7. Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol 2016;29(7):698–707. [DOI] [PubMed] [Google Scholar]
- 8. Rosario PW, Mourao GF, Nunes MB, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Endocr Relat Cancer 2016;23(12):893–897. [DOI] [PubMed] [Google Scholar]
- 9. Xu B, Tallini G, Scognamiglio T, et al. . Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2017;27(4):512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shafique K, LiVolsi VA, Montone K, et al. . Papillary thyroid microcarcinoma: Reclassification to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A retrospective clinicopathologic study. Endocr Pathol 2018;29(4):339–345. [DOI] [PubMed] [Google Scholar]
- 11. Turan G, Ozkara SK. Pathological findings of the retrospective diagnosis of NIFTP (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) in 84 cases from Turkey and systematic review. Ann Diagn Pathol 2021;53:151764. [DOI] [PubMed] [Google Scholar]
- 12. Larouche V, Pusztaszeri MP, Filimon S, et al. . Preoperative prediction of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: A Canadian single-centre experience. J Otolaryngol Head Neck Surg 2020;49(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haugen BR, Alexander EK, Bible KC, et al. . 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson DN, Sadow PM. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum Pathol 2018;82:32–38. [DOI] [PubMed] [Google Scholar]
- 15. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017;27(11):1341–1346. [DOI] [PubMed] [Google Scholar]
- 16. Hu MI, Waguespack SG, Dosiou C, et al. . Afirma Genomic Sequencing Classifier and Xpression Atlas molecular findings in consecutive Bethesda III-VI thyroid nodules. J Clin Endocrinol Metab 2021;106(8):2198–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nikiforova MN, Wald AI, Roy S, et al. . Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 2013;98(11):E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wylie D, Beaudenon-Huibregtse S, Haynes BC, et al. . Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J Pathol Clin Res 2016;2(2):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haugen BR, Sawka AM, Alexander EK, et al. . American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2017;27(4):481–483. [DOI] [PubMed] [Google Scholar]
- 20. Lloyd RV, Osamura RY, Kloppel G, et al. . WHO Classification of Tumours of Endocrine Organs. International Agency for Research on Cancer (IARC): Lyon; 2017. [Google Scholar]
- 21. Seethala RR, Baloch ZW, Barletta JA, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol 2018;31(1):39–55. [DOI] [PubMed] [Google Scholar]
- 22. Nikiforov YE, Baloch ZW, Hodak SP, et al. . Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol 2018;4(8):1125–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cipriani NA, Johnson DN, Sarne DH, et al. . The significance of RAS-like mutations and microRNA profiling in predicting malignancy in thyroid biopsy specimens. Endocr Pathol 2022;33(4):446–456. [DOI] [PubMed] [Google Scholar]
- 24. Gilani SM, Abi-Raad R, Garritano J, et al. . RAS mutation and associated risk of malignancy in the thyroid gland: An FNA study with cytology-histology correlation. Cancer Cytopathol 2022;130(4):284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong KS, Barletta JA. Challenges in encapsulated follicular-patterned tumors: How much is enough? Evaluation of nuclear atypia, architecture, and invasion. Surg Pathol Clin 2023;16(1):27–44. [DOI] [PubMed] [Google Scholar]
- 26. Alexander EK, Doherty GM, Barletta JA. Management of thyroid nodules. Lancet Diabetes Endocrinol 2022;10(7):540–548. [DOI] [PubMed] [Google Scholar]
- 27. Canini V, Leni D, Pincelli AI, et al. . Clinical-pathological issues in thyroid pathology: study on the routine application of NIFTP diagnostic criteria. Sci Rep 2019;9(1):13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seo JY, Park JH, Pyo JY, et al. . A multi-institutional study of prevalence and clinicopathologic features of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in Korea. J Pathol Transl Med 2019;53(6):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song SJ, LiVolsi VA, Montone K, et al. . Pre-operative features of non-invasive follicular thyroid neoplasms with papillary-like nuclear features: An analysis of their cytological, Gene Expression Classifier and sonographic findings. Cytopathology 2017;28(6):488–494. [DOI] [PubMed] [Google Scholar]
- 30. Taneja C, Yip L, Morariu EM, et al. . Clinicopathologic characteristics and postsurgical follow-up of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the postnomenclature revision era. Thyroid 2022;32(11):1346–1352. [DOI] [PubMed] [Google Scholar]
- 31. Lindeman BM, Nehs MA, Angell TE, et al. . Effect of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on malignancy rates in thyroid nodules: How to counsel patients on extent of surgery. Ann Surg Oncol 2019;26(1):93–97. [DOI] [PubMed] [Google Scholar]
- 32. Zhou H, Baloch ZW, Nayar R, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Implications for the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Cancer Cytopathol 2018;126(1):20–26. [DOI] [PubMed] [Google Scholar]
- 33. Paulson VA, Shivdasani P, Angell TE, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid 2017;27(4):506–511. [DOI] [PubMed] [Google Scholar]
- 34. Zhao L, Dias-Santagata D, Sadow PM, et al. . Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 2017;125(5):323–331. [DOI] [PubMed] [Google Scholar]
- 35. Hahn SY, Shin JH, Lim HK, et al. . Preoperative differentiation between noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and non-NIFTP. Clin Endocrinol (Oxf) 2017;86(3):444–450. [DOI] [PubMed] [Google Scholar]
- 36. Silaghi CA, Lozovanu V, Georgescu CE, et al. . ThyroSeq v3, Afirma GSC, and microRNA panels versus previous molecular tests in the preoperative diagnosis of indeterminate thyroid nodules: A systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:649522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chin PD, Zhu CY, Sajed DP, et al. . Correlation of ThyroSeq results with surgical histopathology in cytologically indeterminate thyroid nodules. Endocr Pathol 2020;31(4):377–384. [DOI] [PubMed] [Google Scholar]
- 38. Brandler TC, Liu CZ, Cho M, et al. . Does noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) have a unique molecular profile? Am J Clin Pathol 2018;150(5):451–460. [DOI] [PubMed] [Google Scholar]
- 39. Ravella L, Lopez J, Descotes F, et al. . Preoperative role of RAS or BRAF K601E in the guidance of surgery for indeterminate thyroid nodules. World J Surg 2020;44(7):2264–2271. [DOI] [PubMed] [Google Scholar]
- 40. Morariu EM, McCoy KL, Chiosea SI, et al. . Clinicopathologic characteristics of thyroid nodules positive for the THADA-IGF2BP3 fusion on preoperative molecular analysis. Thyroid 2021;31(8):1212–1218. [DOI] [PubMed] [Google Scholar]
- 41. Geng Y, Aguilar-Jakthong JS, Moatamed NA. Comparison of Afirma Gene Expression Classifier with Gene Sequencing Classifier in indeterminate thyroid nodules: A single-institutional experience. Cytopathology 2021;32(2):187–191. [DOI] [PubMed] [Google Scholar]
- 42. Mehta V, Naraparaju A, Liao D, et al. . What's in a name? A cost-effectiveness analysis of the noninvasive follicular thyroid neoplasm with papillary-like nuclear features' nomenclature revision. Thyroid 2022;32(4):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajab M, Payne RJ, Forest VI, et al. . Molecular testing for thyroid nodules: The experience at McGill University Teaching Hospitals in Canada. Cancers (Basel) 2022;14(17):4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159(3):676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, Correa H, Sanders M, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in children: An institutional experience and literature review. Pediatr Dev Pathol 2020;23(2):121–126. [DOI] [PubMed] [Google Scholar]
- 46. Rosario PW, Mourao GF. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in children and adolescents. Endocrine 2018;61(3):542–544. [DOI] [PubMed] [Google Scholar]
- 47. Mariani RA, Kadakia R, Arva NC. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: Should it also be reclassified in children? Pediatr Blood Cancer 2018;65(6):e26966. [DOI] [PubMed] [Google Scholar]
- 48. Rossi ED, Mehrotra S, Kilic AI, et al. . Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the pediatric age group. Cancer Cytopathol 2018;126(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.