Abstract
Objective:
This is a case report showing that transcranial photobiomodulation (tPBM) combined with traditional, speech-language therapy improved and accelerated the results from speech-language therapy, in a stroke person with aphasia (PWA).
Background:
tPBM is a safe, noninvasive technique using red and near-infrared light to improve the metabolism of cells. tPBM helps by promoting neuromodulation, while decreasing neuroinflammation and promoting vasodilation. Several studies have shown that tPBM can help individuals with stroke or traumatic brain injury achieve significant cognitive improvements.
Methods:
A 38-year-old female, who sustained an ischemic stroke on the left side of the brain, received two, 5-month series of treatments. The first series of treatments included traditional speech-language therapy, for the first 5 months poststroke. The second series of treatments included tPBM in combination with speech-language therapy, for the next 5 months. The tPBM treatments included application of red (630 and 660 nm) and near-infrared (850 nm) wavelengths of photons applied to left hemisphere scalp areas. The major cortical language areas were subjacent to the scalp placements along the line of the Sylvian fissure. At each session, first a light-emitting diode (LED) cluster head with red (630 and 660 nm) and near-infrared (850 nm) wavelengths, with an irradiance (power density) of 200 mW/cm2, a beam size of 4.9 cm2, and a fluence (energy density) of 12 J/cm2 per minute, was applied to the left side of the scalp/brain, along the Sylvian fissure for 60 sec at each at the following eight, language network target areas: frontal pole, prefrontal cortex, and inferior frontal gyrus (Broca's area); supramarginal gyrus and angular gyrus in the parietal lobe; inferior motor/sensory cortex (mouth area); and posterior superior temporal gyrus (Wernicke's area) and superior temporal sulcus in the temporal lobe, for a total of 8 min. Second, for the next 20 min (1200 sec), simultaneous with speech-language therapy, an LED PBM helmet was applied to the scalp/head. This helmet contained 256 separate LED lights, near-infrared (810 nm) wavelength, 60 mW power per LED light, total power, 15 W; energy, 72 Joules; fluence, 28.8 J/cm2; and irradiance, 24 mW/cm2.
Results and conclusions:
During the initial, 5-month treatment series with traditional speech-language therapy only, there was little to no improvement in dysarthria and expressive language. During the second, 5-month treatment series, however, with tPBM applied first, to the left hemisphere only, and second, to both hemispheres during each session plus simultaneous speech-language therapy, there was marked improvement in the dysarthria and expressive language. After the first 5-month series, this PWA had utilized a slow rate of speech with a production of ∼25 to 30 words-per-minute during conversations and spontaneous speech. Utterance length was only 4–6 words with simple, grammatical structure. After the second, 5-month series of treatment combining tPBM plus speech-language therapy, the rate of speech increased to 80+ words-per-minute and utterance length was increased to 9–10 words, with more complex grammatical structure.
Keywords: speech-language therapy, aphasia, low-level laser therapy, LLLT, photobiomodulation, PBM
Introduction
Photobiomodulation (PBM), first known as low-level laser therapy (LLLT), is a safe and noninvasive technique using red and near-infrared light to improve wound and soft tissue healing and provide pain relief.1 Additionally, PBM works directly on mitochondria, allowing oxygenation that increases neurogenesis, synaptogenesis, and growth factors, while reducing neuroexcitotoxicity and controlling inflammatory mediators. Therefore, it promotes neurorepair, neuromodulation, and neuroregeneration, and decreases neuroinflammation of cells.2
The medical applications of PBM have been extended, and are now applied in individuals with stroke, traumatic brain injury, neurodegenerative disease, and myocardial infarction with benefits.3,4 Studies have shown that PBM reduces oxidative stress and increases adenosine triphosphate (ATP), which improves metabolism of cells.1 PBM has been known for its lack of adverse side effects. Detection of some photons has been reported at a depth of 4–5 cm, from a near-infrared, 808 nm laser applied at the scalp surface in a human cadaver study.5 No negative side effect has been reported in human studies since the first clinical studies of LLLT on the body were initiated in the 1960s.6 Transcranial PBM (tPBM) applied to scalp areas located over left hemisphere language areas, plus two midline nodes of the Default Mode Network (medial prefrontal cortex and precuneus), has been associated with significant improvement in naming, in chronic, left hemisphere stroke person with aphasia (PWA).7 When the proper parameters of LLLT or a light-emitting diode (LED) therapy and appropriate treatment loci are utilized, PBM can be an excellent resource to improve brain function in individuals with stroke or brain injury.8
Transcranial Photobiomodulation and Stroke
The application of tPBM involves red and near-infrared light therapy to the scalp/brain to5 stimulate different brain areas. This technique has been used to help individuals with stroke, traumatic brain injury, Alzheimer's, Parkinson's, dementia, depression, and anxiety; several studies have reported significant cognitive improvements.9 Researchers have demonstrated improvements in executive function and verbal memory when tPBM was applied bilaterally to the frontal, parietal, and temporal lobes in individuals with traumatic brain injury.10 In addition, Naeser et al.7 reported, from magnetic resonance imaging (MRI) studies, that near-infrared photons can stimulate surface brain cortex areas of the brain when LEDs are applied on the scalp. Significant improvement in naming ability following tPBM in chronic, PWA was also reported.
For individuals who have sustained an ischemic stroke, tPBM is considered a possible therapeutic approach.4 When tPBM is applied to cells, cytochrome c oxidase (CCO; terminal enzyme of the electron transport chain), nitric oxide (NO) is released. After photons in red (600–700 nm) and near-infrared (760–940 nm) wavelengths are absorbed by CCO, the NO dissociates, and is removed from the cell, promoting increased local vasodilation. Also, more oxygen is consumed, the mitochondrial membrane potential is increased, more glucose is metabolized, and more ATP is produced by the mitochondria.11 This mechanism has been observed to promote proliferation of new neural (progenitor) cells in the peri-infarct zone in animal studies. Further, it improves the neural microenvironment by decreasing inflammatory status and promoting mitochondrial function.12
tPBM research studies with humans, who had sustained traumatic brain injury, have reported that those treated with tPBM starting at days, weeks, months, and even years after traumatic brain injury, still showed some significant improvements.9,13
Language and Speech Circuits
Language mechanisms depend on neuronal function and learning. For production of language with proper speech sounds, vocabulary, and syntactic rules, the neural 6 circuits/networks in the brain need to be activated and work together. When connections are interrupted or damaged, linguistic and nonlinguistic skills can be affected. Therefore, during rehabilitation of linguistic skills, it is important to take into account the processes for language comprehension and production, as well as the cortical/subcortical nodes of circuits/networks that interact to achieve them.14
Pulvermuller has reported that performance and perception in linguistic and nonlinguistic circuits are influenced by functional changes in superior temporal gyrus, sulcus, frontocentral motor areas, and adjacent inferior prefrontal cortex.14 Naeser et al.10 have proposed that scalp placement of transcranial LEDs over cortical nodes of specific neural networks in the brain, including the left hemisphere language network, can promote better neuromodulation (activation/deactivation) and improved behavior. In this study, neuronal circuits for articulation, phonology, and syntax suggested by Pulvermüller14 were treated with tPBM.
Methodology
A 38-year-old female who had suffered an ischemic stroke on the left side of the brain, which was suspected to be caused by her birth control pills, was treated as described in this case report. She was right handed, and completed her 1st year of college. She spoke English and Spanish and was treated in her dominant language (e.g., English, as spoken in the United States).
Per hospital reports, computed tomography (CT) scan performed at 2 days poststroke showed subacute small infarcts in the left hemisphere, including parietal lobe, temporal lobe, and the insular/subinsular region.
MRI scan performed at 2 days poststroke showed large evolving infarct in the left middle 7 cerebral artery territory, including high frontal and the majority of high parietal cortex and white matter, the entire insula, temporal operculum, focally in the posterior temporal lobe, with gyral swelling and associated sulcal effacement. At that time, this PWA was diagnosed with dysarthria and expressive aphasia. Before this study, she had received 5 months of inpatient and out-patient speech-language therapy two to three times a week by other clinicians, with limited improvements noted. After completion of that first, 5-month series of speech-language therapy alone, a combination of traditional speech-language therapy plus tPBM was provided for another 5 months with the speech-language pathologist (K.E.R., author).
The PWA was evaluated by using a Clinical Dysarthria Evaluation, parts of the Boston Diagnostic Aphasia Examination-Third Edition (BDAE),15 and the Burns Left Hemisphere Inventory of Communication and Cognition.16 All tests were administered in English. The PWA's oral-motor skills, including volitional and automatic tongue, lip, and jaw movements, were assessed. In addition, her auditory comprehension; including comprehension of words, sentences, and paragraphs, and verbal expression; including automatic speech, verbal repetition of words and sentences, and responsive and confrontational naming, were assessed. By using the Cookie Theft Picture from the BDAE, the individual's rate of speech, utterance length, voice, prosody, and intelligibility was also assessed. The individual then received 45-min sessions, 2 times a week, for a total of 30 sessions in 5 months. Treatment sessions incorporated the use of tPBM combined with traditional speech-language therapy techniques for dysarthria and aphasia.
Each tPBM treatment session was divided into two parts. Part 1: an LED cluster head with red (630 and 660 nm), and near-infrared (850 nm) wavelengths, with an irradiance (power density) of 200 mW/cm2, a beam size of 4.9 cm2, and a fluence (energy density) of 12 J/cm2 per minute (Fig. 1), was applied to the left side of the scalp/brain, primarily along the Sylvian fissure language areas, for 60 sec at each at the following eight target areas: frontal pole, prefrontal cortex, and inferior frontal gyrus (Broca's area); supramarginal gyrus and angular gyrus in the parietal lobe; inferior motor/sensory cortex (mouth area); and posterior superior temporal gyrus (Wernicke's area) and superior temporal sulcus for a total of 8 min. Part 2: for the next 20 min (1200 sec), simultaneously, during speech-language therapy, an LED tPBM helmet was applied to the scalp/head (Fig. 2).
FIG. 1.
LED Cluster Head. LED cluster head with red (630 and 660 nm) and near-infrared (850 nm) wavelengths, with an irradiance of 200 mW/cm2, a beam size of 4.9 cm2, and a fluence (energy density) of 12 J/cm2 per min, applied for 60 sec over each of the eight, left hemisphere language network target areas during the initial part of a tPBM treatment. LED, light-emitting diode; tPBM, transcranial photobiomodulation.
FIG. 2.

LED PBM Helmet. LED PBM helmet containing 256 individual LED lights delivering near-infrared (810 nm) wavelength with a power of 60 mW per LED light, for a total power of 15 W, an irradiance of 24 mW/cm2, energy of 72 Joules, and a fluence of 28.8 J/cm2 was applied to the whole brain for 20 min, while traditional speech-language therapy was provided. LED, light-emitting diode; PBM, photobiomodulation.
This helmet contained 256 separate LED lights, near-infrared (810 nm) wavelength, 60 mW power per LED light, total power, 15 W; energy, 72 Joules; fluence, 28.8 J/cm2; and irradiance, 24 mW/cm2. Traditional speech-language therapy alone was provided for the remaining 17 min of each session (Table 1).
Table 1.
Areas Treated with Transcranial Photobiomodulation Using LED Devices
| tPBM treatment protocol used during each treatment session | |
|---|---|
| Treated areas | LED device parameters |
| 1. Frontal pole 2. Prefrontal cortex 3. Left inferior frontal gyrus (Broca's area) 4. Left supramarginal gyrus 5. Left angular gyrus 6. Inferior motor/sensory cortex (mouth area) 7. Left posterior superior temporal gyrus (Wernicke's area) 8. Superior temporal sulcus |
1. An LED cluster head was applied for 60 sec at each of the treated areas for a total of 8 min. Wavelength: 2 red (630 and 660 nm) and 1 near-infrared (850 nm) lights Irradiance: 200 mW/cm2 Exposure time: 60 sec at each of the eight target areas for a total of 480 sec (8 min) |
| 9. Both hemispheres | 2. An LED PBM helmet was administered for 20 min, while speech-language therapy was provided. Wavelength: 256 individual LED lights delivering near infrared (810 nm) Power: 60 mW per LED light, for a total power of 15 W Irradiance: 24 mW/cm2 Exposure time: 1200 sec (20 min) Energy: 72 Joules Fluence: 28.8 J/cm2 0.024 J/cm2 for 1 sec = 28.8 for 1200 sec |
LED, light-emitting diode; PBM, photobiomodulation; tPBM, transcranial PBM.
Data collection consisted of treatment notes after each therapy session, and periodic assessments to evaluate and compare progress.
Results
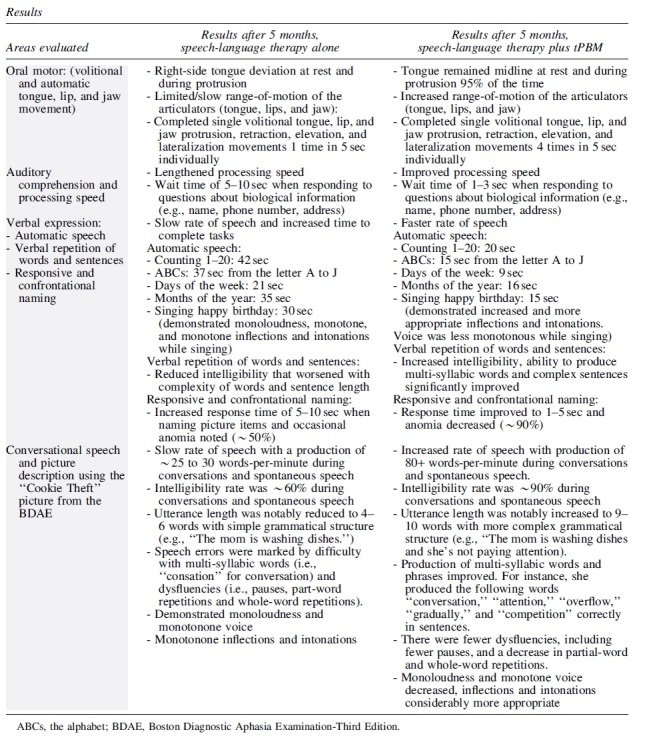
At the time of initial evaluation, the following areas were assessed: function of the oral mechanism, auditory comprehension and processing speed, and verbal expression, as well as conversational speech and picture description. At the level of oral mechanisms, she exhibited right-side tongue deviation at rest and during protrusion, with limited/slow range-of-motion of the articulators (tongue, lips, and jaw). She completed single volitional tongue, lip, and jaw protrusion, retraction, elevation, and lateralization movements, once in 5 sec individually. During auditory comprehension tasks, she demonstrated lengthened processing speed and required 5–10 sec to respond to questions about biological information (e.g., name, phone number, address). Verbal expression tasks were marked by a slow rate of speech, and she required increased time (e.g., 21–42 sec) to complete presented tasks.
She demonstrated reduced intelligibility that worsened with complexity of words and sentence length, and occasional anomia was also noted 50% of the time when naming picture items. During conversational speech and picture description using the “Cookie Theft Picture” from the BDAE, she presented with a slow rate of speech with a production of ∼25 to 30 words-per-minute during conversations and spontaneous speech. Her intelligibility rate was ∼60%. Utterance length was notably reduced to 4–6 words with simple structure (e.g., “The mom is washing dishes.”) Speech errors were marked by difficulty with multi-syllabic words (i.e., “consation” for conversation) and dysfluencies (i.e., pauses, part-word repetitions and whole-word repetitions). She also demonstrated monoloudness and monotone voice, and her inflections and intonations were monotonous (Table 2).
Table 2.
Results After 5 Months, Speech-Language Therapy Alone, and After 5 Months, Speech-Language Therapy Plus Transcranial Photobiomodulation
|
After 30 sessions of speech-language therapy plus tPBM, this PWA demonstrated significant improvement in all areas initially assessed. At the level of oral mechanisms, her tongue remained midline at rest and during protrusion, 95% of the time. She demonstrated increased range-of-motion of the articulators (tongue, lips, and jaw). She completed single volitional tongue protrusion, retraction, elevation, and lateralization movements 4 times in 5 sec. During auditory comprehension tasks, she improved her processing speed, demonstrating a wait time of only 1–3 sec to respond to questions about personal information (e.g., name, phone number, address). While completing verbal tasks, she exhibited a faster rate of speech for completion of the same initial tasks in 9–20 sec, compared to 21–42 sec, previously. She also demonstrated increased intelligibility, and her ability to produce multi-syllabic words and complex sentences also increased.
During conversational speech and picture description using the “Cookie Theft Picture” from the BDAE, she demonstrated an increased rate of speech with production of 80+ words-per-minute and her intelligibility rate was ∼90%. Utterance length was notably increased to 9–10 words with more complex grammatical structure (e.g., “The mom is washing dishes” and she's not paying attention). Production of multi-syllabic words improved. For instance, she produced the following words “conversation,” “attention,” “overflow,” “gradually,” and “competition” correctly in sentences. In addition, there were fewer dysfluencies, including fewer pauses, and a decrease in partial-word and whole-word repetitions. She also demonstrated a decrease in monoloudness and monotone voice, and her inflections and intonations were considerably more appropriate (Table 2).
Discussion
This case report differs from the tPBM methodology used with chronic, PWA (n = 6) in Naeser et al.'s 2020 study.7 In that study, there was significant improvement in naming, only when the red/near-infrared (NIR) LED cluster heads (2-inch diameter) were placed only over the left hemisphere language areas; or when the LED cluster heads were placed on those left hemisphere language areas, plus two, midline cortical nodes of the Default Mode Network—for example, the mesial prefrontal cortex (located at midline, high forehead, at junction with front hairline), and the precuneus cortex (located at junction of the sagittal suture line with the left and right lambdoid suture lines, high parietal).
When the LED cluster heads were placed all over the head, however, including both the left and right hemispheres, plus all along the sagittal suture line including the midline, vertex of the scalp/brain (left and right supplementary motor areas, 10 simultaneously), there was impaired naming or no improvement. Based on functional magnetic resonance imaging (fMRI) findings from that study,7 it was suggested that treating only the same side of the scalp/brain where the stroke had occurred was a better method for treating chronic, PWA.
tPBM has also been used with safety in acute, nonhemorrhagic stroke.17–19 That NeuroThera Effectiveness and Safety Trial applied NIR transcranial laser once, within the first 24 h poststroke to both sides of the scalp/brain. Some cases had left hemisphere lesion, and some cases had right hemisphere lesion; however, both sides of the scalp/brain were treated in all cases. Initial results showed significance for better outcome on day 90, for real versus sham, but analysis halfway into the Phase III trials did not.
Results from the Naeser et al.'s 2020 study suggested that tPBM application to both hemispheres, plus the L and R supplementary motor areas at the midline, vertex of the head simultaneously, interfered with speech and naming ability.7 In this case study, during each treatment session in the second 5-month treatment series, first, the left hemisphere language areas only were treated alone, and next, the whole head/brain was treated using a helmet simultaneous with speech-language therapy.
It is possible here that the initial tPBM treatment to only the left hemisphere language network areas somewhat “prepared” the language network to obtain more benefit from the speech-language therapy program delivered simultaneously, with the whole-head, LED helmet treatment. This is unknown. Optimal LED placement locations for tPBM treatment of acute, and/or chronic, PWA remain to be clarified. This case report shows that a combination of speech-language therapy plus tPBM within a treatment session where tPBM is first applied to the left hemisphere language areas only, and then to the whole head during simultaneous speech-language therapy was highly beneficial for this young, 38-year-old PWA, initiated at 5 months poststroke.
Limitations
It is important to note taht this article is based on a single case study and cannot be used as a representative sample. Further investigation is needed in the use of tPBM in conjunction with speech-language therapy to continue to demonstrate the benefits of tPBM as a safe modality for the neurorehabilitation of individuals with stroke.
Conclusions
This case demonstrated that traditional speech-language therapy, when combined with tPBM applied to the left hemisphere scalp areas, can be an effective technique to improve and accelerate the results of speech-language skills of individuals diagnosed with dysarthria and expressive aphasia caused by left hemisphere stroke. Previous studies have also demonstrated the positive impact of tPBM to improve speech-language skills.7,20,21 and Bacelete and Gama,21 presented the positive results of tPBM in speech-language skills from global researchers, including the United States. However, more studies focusing on the use of tPBM with speech-language therapy are needed to increase the amount of evidence-based practice.
Acknowledgments
A special acknowledgment goes to James Carroll, THOR Photomedicine, for assistance in obtaining the parameters of the two LED devices utilized in this study.
Authors' Contributions:
K.E.R.: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing-original draft, writing-reviewing and editing, visualization, and project administration. P.C.O.: conceptualization, resources, and supervision.
Author Disclosure Statement
The authors declare no conflict of interests.
Funding Information
No external funding was received for this research.
References
- 1. Huang Y-Y, Chen AC-H, Carroll JD, et al. Biphasic dose response in low level light therapy. Dose Resp 2009;7(4):358–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 2015;33(4):183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hashmi JT, Huang Y-Y, Osmani BZ, et al. Role of low-level laser therapy in neurorehabilitation. PM R 2010;2(12 Suppl. 2):S292–S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thunshelle C, Hamblin MR. Transcranial low-level laser (light) therapy for brain injury. Photomed Laser Surg 2016;34(12):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tedford CE, DeLapp S, Jacques S, et al. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue: Transcranial and intraparenchymal light penetration. Lasers Surg Med 2015;47(4):312–322. [DOI] [PubMed] [Google Scholar]
- 6. Mester E, Spiry T, Szende B. Effect of laser rays on wound healing. Bull Soc Int Chir 1973;32(2):169–173. [PubMed] [Google Scholar]
- 7. Naeser MA, Ho MD, Martin PI, et al. Increased functional connectivity within intrinsic neural networks in chronic stroke following treatment with red/near-infrared transcranial photobiomodulation: Case series with improved naming in aphasia. Photobiomodul Photomed Laser Surg 2020;38(2):115–131. [DOI] [PubMed] [Google Scholar]
- 8. Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J Neurosci Res 2018;96(4):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hennessy M, Hamblin MR. Photobiomodulation and the brain: A new paradigm. J Opt 2017;19(1):013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naeser MA, Martin PI, Ho MD, et al. Transcranial, red/near-infrared light-emitting diode therapy to improve cognition in chronic traumatic brain injury. Photomed Laser Surg 2016;34(12):610–626. [DOI] [PubMed] [Google Scholar]
- 11. de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 2016;22(3):348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arany PR. Photobiomodulation-activated latent transforming growth factor-β1: A critical clinical therapeutic pathway and an endogenous optogenetic tool for discovery. Photobiomodul Photomed Laser Surg 2022;40(2):136–147. [DOI] [PubMed] [Google Scholar]
- 13. Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J Neurotrauma 2014;31(11):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pulvermüller F. Neural reuse of action perception circuits for language, concepts and communication. Prog Neurobiol 2018;160:1–44. [DOI] [PubMed] [Google Scholar]
- 15. Goodglass H, Kaplan E.. Boston Diagnostic Aphasia Examination, 2nd ed. Lea & Febiger: Philadelphia, PA, USA; 1983. [Google Scholar]
- 16. Burns MS. Burns Brief Inventory of Communication and Cognition. Psychological Corporation, Harcourt Assessments: San Antonio, TX, USA; 1997. [Google Scholar]
- 17. Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1): Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007;38(6):1843–1849. [DOI] [PubMed] [Google Scholar]
- 18. Zivin JA, Albers GW, Bornstein N, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009;40(4):1359–1364. [DOI] [PubMed] [Google Scholar]
- 19. Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Curr Cardiol Rep 2010;12(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortiz NC. Transcraneal photobiomudulation: Application of LLLT in speech and language circuits for the rehabilitation of patients with Cranial brain trauma. Brain Stimul 2019;12(2):396. [Google Scholar]
- 21. Bacelete VSB, Gama ACC. Therapeutic effects of photobiomodulation in the speech-language-hearing clinic: An integrative literature review. Rev CEFAC 2021;23(1):e9120. [Google Scholar]



