Abstract
Significance:
Cardiovascular disease and drug-induced health side effects are frequently associated with—or even caused by—an imbalance between the concentrations of reactive oxygen and nitrogen species (RONS) and antioxidants, respectively, determining the metabolism of these harmful oxidants.
Recent Advances:
According to the “kindling radical” hypothesis, the initial formation of RONS may further trigger the additional activation of RONS formation under certain pathological conditions. The present review specifically focuses on a dysfunctional, uncoupled endothelial nitric oxide synthase (eNOS) caused by RONS in the setting of transportation noise exposure or chronic treatment with organic nitrates, especially nitroglycerin (GTN). We further describe the various “redox switches” that are proposed to be involved in the uncoupling process of eNOS.
Critical Issues:
In particular, the oxidative depletion of tetrahydrobiopterin and S-glutathionylation of the eNOS reductase domain are highlighted as major pathways for eNOS uncoupling upon noise exposure or GTN treatment. In addition, oxidative disruption of the eNOS dimer, inhibitory phosphorylation of eNOS at the threonine or tyrosine residues, redox-triggered accumulation of asymmetric dimethylarginine, and l-arginine deficiency are discussed as alternative mechanisms of eNOS uncoupling.
Future Directions:
The clinical consequences of eNOS dysfunction due to uncoupling on cardiovascular disease are summarized also, providing a template for future clinical studies on endothelial dysfunction caused by pharmacological or environmental risk factors.
Keywords: oxidative stress, superoxide, peroxynitrite, nitric oxide synthase uncoupling, NADPH oxidase, nitrate tolerance, transportation noise exposure
Introduction
Environmental noise is a major environmental health risk factor (Munzel et al, 2021; Munzel et al, 2018a; Webpage, 2018). A large proportion of the population is exposed to noise levels exceeding the threshold values recommended by the World Health Organization (WHO) guidelines of 55 dB(A), and there is growing evidence linking traffic noise to cardiovascular morbidity and mortality rate (Munzel et al, 2020) and also cerebral disease (Hahad et al, 2019). According to the data of the WHO environmental noise guidelines for the European region, traffic noise is associated with an increased risk of metabolic disease and with a pooled relative risk for ischemic heart disease of 1.08 (95% CI 1.01–1.15) per 10 dB(A) increase in noise exposure, starting at 53 dB(A) (Kempen et al, 2018). Railway and road noise was also associated with increased arterial stiffness, a subclinical marker of atherosclerosis and development of future cardiovascular disease (Foraster et al, 2017).
There is also epidemiological evidence that chronic exposure to road, railway, or aircraft noise is associated with elevated blood pressure, arterial hypertension (in particular nighttime noise) (Munzel et al, 2020), stroke, heart failure, arrhythmia (Munzel et al, 2018a; Munzel et al, 2014a), as well as cardiometabolic disorders such as diabetes and obesity (Munzel et al, 2021). The annual cardiovascular disease burden of noise in Europe is reflected by 1.7 million cases of hypertension, 80,000 hospital admissions, and 18,000 excess deaths (Webpage, 2014). It was estimated that reducing noise levels by 5 dB(A) could reduce hypertension by 1.4% and ischemic heart disease by 1.8%, saving 3.9 billion dollars in health costs (Swinburn et al, 2015).
Babisch et al (2001) have postulated the noise reaction model, where an “indirect pathway,” plays a crucial role in causing cardiovascular diseases (Babisch, 2003). This concept comprises the cognitive perception of noise, the subsequent cortical activation, leading to increased levels of stress hormones that become manifest in neuropsychiatric, metabolic, and cardiovascular diseases (Babisch, 2014; Munzel et al, 2021; Munzel et al, 2018a). Perturbation of the autonomic nervous system and sympathoadrenal activation (Recio et al, 2016), release of proinflammatory mediators, modified (phospho)lipids, activation of leukocytes, oxidative stress, and prothrombotic pathways, and endothelial dysfunction are crucial steps (Munzel et al, 2021; Munzel et al, 2018a; Munzel et al, 2014a).
Recent animal studies support an essential role of oxidative stress, activation and recruitment of immune cells, impairment of the circadian clock and dysregulated gene networks leading to endothelial dysfunction, and vascular/cerebral damage from aircraft noise (Kroller-Schon et al, 2018; Munzel et al, 2017a; Eckrich et al, 2021).
Organic nitrates belong to the class of nitrovasodilators and represent a mainstay in the treatment of stable and unstable angina pectoris and acute and chronic heart failure (Abrams, 1995). The phenomenon of organic nitrate tolerance is a well-recognized clinical problem (Packer, 1989; Katz, 1990; Gori and Parker, 2002). The hemodynamic and anti-ischemic effects of nitroglycerin (GTN), and also other organic nitrates such as isosorbide-5-mononitrate, are lost upon chronic administration due to the rapid development of nitrate tolerance (Munzel et al, 2013; Munzel et al, 2011). Tolerance to nitrates in response to chronic therapy was documented already in the 1970s in vivo (Packer et al, 1987; Zelis and Mason, 1975).
Later, the term cross-tolerance was introduced, after establishing that nitrate therapy also impaired endothelial function in experimental animals (Oelze et al, 2013; Munzel et al, 1995b), and also in patients with established coronary artery disease (Gori et al, 2001b; Liuni et al, 2011; Schulz et al, 2002).
We discovered that the pathophysiology leading to nitrate tolerance and cross-tolerance is mainly due to the increased formation of reactive oxygen species (ROS) and reactive nitrogen species in the mitochondria and by different additional ROS-mediated processes, including endothelial nitric oxide synthase (eNOS) uncoupling, nitration/inhibition of prostacyclin synthase, and vascular NADPH oxidase activation (Munzel et al, 2013; Munzel et al, 2011). Oxidative inhibition of the nitrate-bioactivating enzyme mitochondrial aldehyde dehydrogenase (ALDH-2) appears to play a central role for nitrate tolerance in response to GTN (Chen et al, 2002; Sydow et al, 2004).
Oxidative stress and redox signaling: implications for the cardiovascular system
Increased production of reactive oxygen and nitrogen species (RONS) leading to increased oxidative stress has been demonstrated to be a characteristic concerning many cardiovascular and cerebrovascular diseases (Sies, 2015; Sies, 1991), and also of side effects of drug therapy as exemplified by organic nitrate-induced tolerance (Munzel et al, 2014b; Munzel et al, 2005a) or environmental health risk factors such as transportation noise (Munzel et al, 2017b; Munzel et al, 2017c; Munzel et al, 2014a). Oxidative stress characterizes a state of elevated RONS formation and/or insufficient scavenging of RONS, for example, by low levels of important antioxidant proteins, with a subsequent reduction of NO bioavailability as well as depletion of low-molecular-weight antioxidants, thus causing a shift in the cellular redox balance. The most common RONS include superoxide radicals (•O2−), hydrogen peroxide, hydroxyl radicals, carbon-centered peroxides, peroxyl radicals, nitric oxide (•NO) and nitrogen dioxide radicals, peroxynitrite (ONOO−), and hypochlorite.
Some of these species act as cellular messengers by conferring redox signaling (Rhee, 1999; Sies, 2017; Stamler, 1994; Ullrich and Kissner, 2006). Nitric oxide (•NO) represents a second messenger and is probably the best-characterized radical species that plays a central role in the beneficial regulation of vascular tone and suppression of platelet activation. Xanthine oxidase (XO), NADPH oxidases, uncoupled nitric oxide synthases, and the mitochondrial respiratory chain are the most important biological sources of the superoxide anion (•O2−), an important precursor of many ROS. The detrimental biological role of •O2− was further substantiated by the discovery of superoxide dismutases (mitochondrial, Mn-SOD, and cytosolic/extracellular Cu,Zn-SOD), highly specialized enzymes with the sole biological function of detoxifying •O2− (McCord et al, 1971).
The extremely fast reaction between •NO and •O2− leads to the formation of the highly reactive peroxynitrite (ONOO−) (Beckman and Koppenol, 1996). The reaction product from •NO and •O2−, peroxynitrite, is a significantly stronger oxidant than each of the reaction partners alone and its significant contribution to cardiovascular and cerebrovascular diseases is well established (Ischiropoulos and Beckman, 2003; Turko and Murad, 2002). Since the two molecules react with each other through a diffusion-controlled reaction, in many aspects, •O2− can be regarded as a direct antagonist of •NO. In the absence of •NO, •O2− is either removed through a spontaneous disproportionation process or it is scavenged by SODs.
In general, in the presence of •NO, the disproportionation of •O2− is outcompeted by the formation of ONOO− since the reaction speed between •NO and •O2− is threefold to fivefold faster than the disproportionation process of •O2− catalyzed by SODs (Beckman and Koppenol, 1996; Kissner et al, 1997). ONOO− leads to comparable redox changes of other biomolecules such as hydrogen peroxide but is regarded ∼1000-fold more potent in causing these redox reactions.
The reactivity of the peroxynitrite anion is quite unique and specific (in the first line oxidation of thiols, thioethers, or metal-catalyzed nitration of tyrosine). Upon protonation, peroxynitrous acid (ONOOH) either isomerizes spontaneously to nitrate (the so-called inactivation route) or it undergoes homolysis to form •NO2 and •OH radicals (the so-called toxifying route) that cause one-electron oxidation reactions leading to new products via hydroxylation and nitration. The biological existence of ONOO− was demonstrated by the nitration of protein tyrosine residues in atherosclerotic lesions (Beckmann et al, 1994) and other disease conditions (Crow and Beckman, 1995; Ischiropoulos and Beckman, 2003), with prostacyclin synthase nitration and simultaneous inactivation representing a quite specific target under various pathophysiological conditions (Hink et al, 2003; Zou and Bachschmid, 1999; Zou et al, 1999).
It should, however, be kept in mind that 3-nitrotyrosine is not only an exclusive marker of peroxynitrite reactivity but can be also formed under inflammatory conditions by the reaction of myeloperoxidase (MPO) with hydrogen peroxide in the presence of nitrite leading to the formation of nitrogen dioxide radicals that can nitrate tyrosine (Brennan et al, 2002; Kettle et al, 1997; Sampson et al, 1998). The pathomechanistic features of oxidative stress and adverse redox signaling on the cardiovascular system were previously reviewed in detail (Beckman and Koppenol, 1996; Daiber and Ullrich, 2002; Radi, 2004) and have a significant impact on vascular biology, endothelial function, and importantly, cardiovascular prognosis (Fig. 1) (Daiber et al, 2017c; Heitzer et al, 2001b). This is also reflected by multiple reports on markers of oxidative stress and redox biomarkers in cardiovascular disease (Daiber et al, 2021).
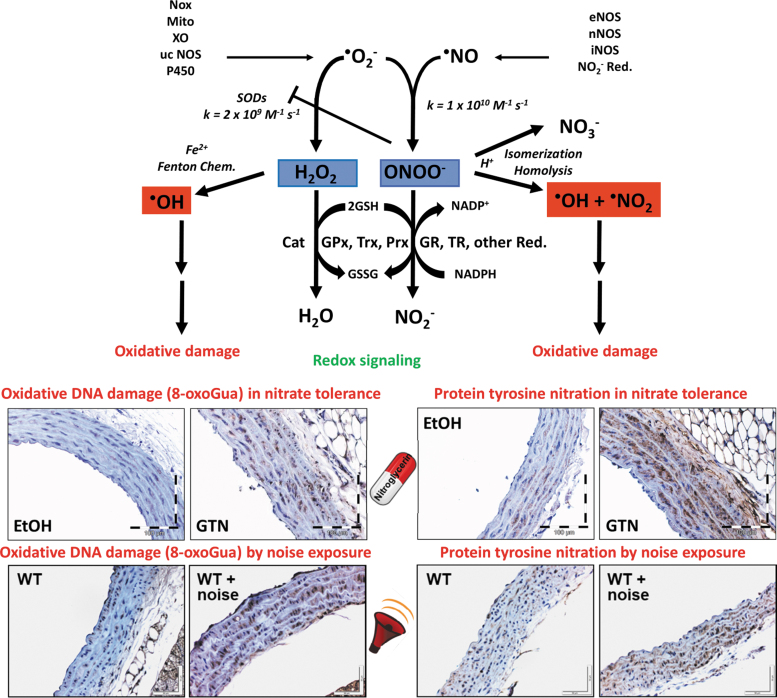
FIG. 1.
The chemical basis of vascular oxidative stress and redox signaling by superoxide and nitric oxide in animal models of nitrate tolerance and traffic noise exposure. Upper part: Superoxide (•O2−) formation from Nox, the mitochondrial respiratory chain (Mito), XO, a ucNOS, and P450 side reactions confer redox signaling mainly upon breakdown by self-dismutation or catalyzed by SODs to hydrogen peroxide. Reaction with thiol groups is a major route of detoxification for H2O2 via Prx and Trx or the selenol in GPx—these systems require energy-consuming recycling by NADPH-coupled reductases (GR, TR). Another route for H2O2 decomposition is catalyzed by catalase (Cat). When H2O2 accumulates, it may lead to the Fenton reaction (hydroxyl radical formation) upon reaction with ferrous iron (Fe2+), triggering severe oxidative damage at the protein, lipid, and DNA levels. Likewise, nitric oxide (•NO, previously termed EDRF) is formed from eNOS, nNOS, iNOS, or reduction of nitrite from nutrition conferring the diffusion-controlled reaction with superoxide to yield peroxynitrite anion (ONOO−). This rapid kinetics even outcompetes the extremely fast SOD-catalyzed breakdown of superoxide. Upon protonation, peroxynitrite undergoes either spontaneous isomerization to nitrate (representing a detoxifying route) or undergoes homolysis to form the hydroxyl radical (•OH) and the nitrogen dioxide (•NO2) radical leading to oxidative damage comparable with the Fenton reactivity of H2O2. Lower part: (Left) Hydroxyl radicals typically induce oxidative DNA damage in the form of 8-oxoGua as envisaged in GTN-treated, nitrate-tolerant rats (GTN with a dose of 50 mg/kg/day for 3.5 days) or aircraft noise-exposed mice [72 dB(A) around-the-clock for 4 days] by immunohistochemistry (brown staining). (Right) ONOOH and derived free radicals typically induce protein tyrosine nitration as envisaged in GTN-treated, nitrate-tolerant rats or noise-exposed mice by immunohistochemistry (brown staining), although MPO/H2O2/nitrite reaction will also lead to nitration. Scheme in the upper part summarized from Daiber and Ullrich (2002) and adapted from Daiber et al (2020c) and Daiber et al (2014) with permission. Images in the lower part were reproduced from Mikhed et al (2016) (for nitrate tolerance) and Kvandova et al (2020) (for noise exposure) with permission. 8-oxoGua, 8-oxoguanine; EDRF, endothelium-derived relaxing factor; eNOS, endothelial nitric oxide synthase; GPx, glutathione peroxidase; GR, glutathione reductase; GTN, nitroglycerin; iNOS, inducible nitric oxide synthase; MPO, myeloperoxidase; nNOS, neuronal nitric oxide synthase; Nox, NADPH oxidases; Prx, peroxiredoxins; SODs, superoxide dismutases; TR, thioredoxin reductase; Trx, thioredoxin; ucNOS, uncoupled nitric oxide synthase; XO, xanthine oxidase.
In the present overview, we explain the detrimental impact of oxidative stress on endothelial function in detail for the side effect of chronic treatment with organic nitrates (e.g., GTN), nitrate tolerance (Daiber and Munzel, 2015; Munzel et al, 2013; Munzel et al, 2011), and for exposure to transportation noise (Munzel et al, 2021; Munzel et al, 2018a; Munzel et al, 2018b), an environmental stressor and novel cardiovascular and cerebral health risk factor.
Oxidative stress, ROS sources, and cardiovascular disease
In the early 1990s, the Harrison's group was the first to describe in an experimental model of hypercholesterolemia that endothelial dysfunction is caused by increased superoxide production due to an activation of the XO (Harrison and Ohara, 1995; Ohara et al, 1993). The molecular proof for a decisive role of oxidative stress in cardiovascular and cerebrovascular diseases was provided by a large number of clinical studies by demonstrating that the acute administration of high dose of the antioxidant vitamin C is able to improve endothelial dysfunction (Daiber et al, 2017c; Heitzer et al, 2001b; Heitzer et al, 1996).
Also, preclinical studies using genetically engineered mice (e.g., knockout mice) elegantly demonstrated the crucial role of ROS producing or degrading enzymes with respect to the initiation and also progression of cardiovascular and cerebral diseases (Daiber and Chlopicki, 2020; Daiber et al, 2020c; Daiber et al, 2017a). For example, the deletion of the NADPH oxidase subunits p47phox and Nox-1 has been shown to have a blood pressure lowering effect and to prevent endothelial dysfunction in the model of angiotensin-II-induced hypertension in mice (Landmesser et al, 2002; Matsuno et al, 2005). In addition, GTN- or noise-induced vascular oxidative stress and endothelial dysfunction were markedly improved by genetic deletion of p47phox and/or gp91phox (Kroller-Schon et al, 2018; Wenzel et al, 2008).
This is in good agreement with multiple reports on a central role of oxidative stress in causing cardiovascular health side effects in response to chronic GTN treatment (Daiber and Munzel, 2015; Munzel et al, 2013; Munzel et al, 2011) and transportation noise exposure (Munzel et al, 2021; Munzel et al, 2018a; Munzel et al, 2018b). For both the pathophysiological conditions, oxidative DNA damage in the form of 8-oxoguanine lesions, as a footprint of in vivo hydroxyl radical formation, and protein tyrosine nitration, as a readout for peroxynitrite in vivo formation or MPO/H2O2/nitrite reactivity, were reported in preclinical studies (Fig. 1) (Mikhed et al, 2016; Kvandova et al, 2020). Likewise, these and other oxidative stress markers were also observed in human samples in response to noise exposure (Hemmingsen et al, 2015; Kroller-Schon et al, 2018) and chronic therapy with organic nitrates (Schulz et al, 2002; McGrath et al, 2002).
According to the concept of “kindling radicals” (or also “bonfire” hypothesis), the initial formation of ROS, for example, via the NADPH oxidases, will trigger further damage by causing eNOS uncoupling via quite different mechanisms (see “redox switches” below). The ROS-induced ROS production concept, originally postulated for redox activation of mitochondrial reactive oxygen species (mtROS) production by ROS from neighbored dysfunctional mitochondria (Zorov et al, 2000), can now be extended to almost any kind of source of RONS as almost all of these sources contain “redox switches.” Beyond cytoplasmic enzymes, a cross talk between mtROS formation and NADPH oxidases has been established (Daiber, 2010; Schulz et al, 2014; Kimura et al, 2005). For example, mtROS can open the mitochondrial permeability transition pore by redox regulatory mechanisms (Radi et al, 2002).
Upon release of the mtROS into the cytosol (most probably representing H2O2), these reactive species can activate the redox-sensitive zinc-finger-like complex in the protein kinase C (PKC) (Lin and Takemoto, 2005) and thereby induce the translocation of cytosolic NADPH oxidase subunits of the NOX-1 and NOX-2 isoforms, for example, p47phox or Noxa1, to the membrane thus triggering NADPH oxidase-dependent superoxide release. Prominent examples for this cross talk between mitochondrial and NADPH oxidase-derived ROS were reported for GTN-induced nitrate tolerance (Wenzel et al, 2008) and angiotensin-II-induced hypertension (Dikalova et al, 2010a; Doughan et al, 2008; Kroller-Schon et al, 2014).
Other sources of oxidative stress exhibit comparable redox switches (Daiber et al, 2017a; Schulz et al, 2014): for instance, the conversion of xanthine dehydrogenase to the oxidase form (XO) needs oxidation of critical thiol residues, and uncoupling of eNOS (and also other isoforms) can be mediated by numerous redox switches as discussed below. Of note, not only can the eNOS located in endothelial cells become dysfunctional or uncoupled, but also the eNOS in red blood cells has a significant effect on vascular tone and health (Kuhn et al, 2017; Leo et al, 2021). Of note, eNOS uncoupling and impaired endothelial function were also observed in the setting of nitrate tolerance under GTN therapy (Schulz et al, 2002; Munzel et al, 1995b) and in response to traffic noise exposure (Munzel et al, 2017a; Schmidt et al, 2013).
Of note, increased inducible nitric oxide synthase (iNOS) was reported in the brain of noise-exposed mice, which was prevented by genetic deletion of Nox-2 (Kroller-Schon et al, 2018). A role of iNOS and arginase enzymes in noise-induced hearing loss was also reported by multiple studies, but the auditory effects of noise are not in the focus of the present review. Nitrate tolerance by GTN treatment was prevented by genetic deficiency or pharmacological inhibition of arginase II (Khong et al, 2012), which may also point to a potential role for iNOS in causing this phenomenon (Wang et al, 2006).
Redox Switches in eNOS
In addition to the classical activation mechanisms of eNOS by calcium/calmodulin, caveolin, heat shock protein 90, palmitoylation and myristoylation (Forstermann and Sessa, 2012), other processes such as phosphorylation and S-glutathionylation are involved in redox-active species formation (Forstermann and Munzel, 2006). “Redox switches” of eNOS are causing alterations of enzymatic eNOS activity and may contribute to the uncoupling of eNOS (Daiber et al, 2017a; Schulz et al, 2014), as depicted in Figure 2, in response to chronic GTN treatment or exposure to transportation noise. The precise definition of eNOS uncoupling is the process by which electrons leak from the transport within the reductase domain (from NADPH over flavin mononucleotide and flavin adenine dinucleotide) and are getting transferred to molecular oxygen leading to the formation of •O2− instead of •NO.
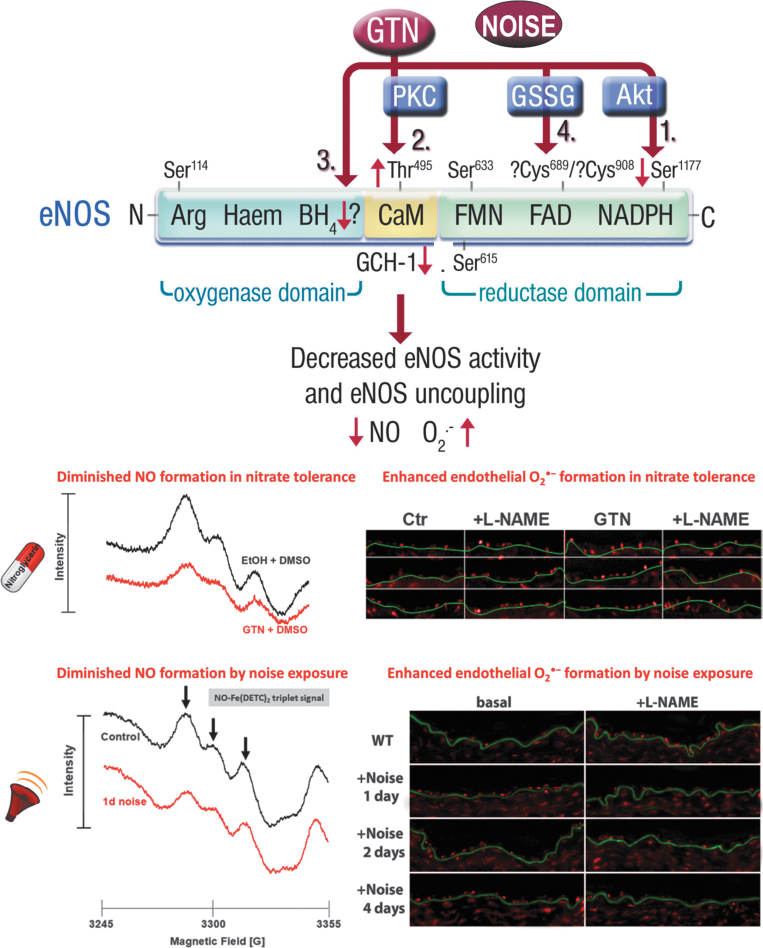
FIG. 2.
Proposed mechanisms underlying GTN-induced and traffic noise-mediated eNOS uncoupling and diminished •NO bioavailability. Upper part: GTN treatment causes a decrease in Ser1177 (1) and an increase in Thr495 (2) phosphorylation of the eNOS (Knorr et al, 2011), leading to a decreased activity and uncoupling, respectively. In addition, the key enzyme of the de novo synthetic pathway of the eNOS cofactor BH4 GCH-1 is downregulated by chronic GTN treatment (3), also leading to a dysfunctional, superoxide (•O2−)-producing nitric oxide synthase. S-Glutathionylation represents another regulatory modification of eNOS, which is increased in the setting of tolerance (Knorr et al, 2011) and upon noise exposure (Munzel et al, 2017a; Steven et al, 2020) (4). Lower part: All of these adverse regulatory pathways that are activated by GTN treatment (50 or 100 mg/kg/day for 3.5 days) (Jabs et al, 2015; Knorr et al, 2011) or aircraft noise exposure [72 dB(A) around-the-clock for 1, 2, or 4 days] (Munzel et al, 2017a), including direct scavenging of •NO by •O2−, lead to diminished •NO bioavailability and enhanced endothelial •O2− formation that was blocked by the eNOS inhibitor L-NAME, supporting eNOS uncoupling. “?” nearby Cys689/908 means that discrimination between the S-glutathionylation sites is not possible since a pan-antibody for S-glutathionylation was used. “?” nearby BH4 means that so far rather indirect evidence exists for eNOS uncoupling via BH4 depletion by GTN tolerance and noise exposure (e.g., diminished GCH-1 and improvement of FMD by vitamin C or folic acid administration). Scheme in the upper part was reproduced from Knorr et al (2011) with permission. Images in the lower part were reproduced from Jabs et al (2015) and Knorr et al (2011) (for nitrate tolerance) and Munzel et al (2017a) (for noise exposure) with permission. Akt, protein kinase B; BH4, tetrahydrobiopterin; FAD, flavin adenine dinucleotide; FMD, flow-mediated dilation; FMN, flavin mononucleotide; GCH-1, GTP-cyclohydrolase-1; GSSG, glutathione disulfide; L-NAME, NG-nitro-l-arginine methyl ester; PKC, protein kinase C.
This process is even more harmful than the consequences of inhibition of eNOS since uncoupling causes the enzyme to change from an •NO to •O2− donor, thereby changing the biological environment from a vasodilator/antiatherosclerotic and thus beneficial to a harmful/proatherosclerotic phenotype (Forstermann and Munzel, 2006; Munzel et al, 2005b). Importantly, the uncoupling process is not only restricted to eNOS but may also involve neuronal NOS (type 1) (Vasquez-Vivar et al, 1999) as well as the inducible NOS (type 2) (Xia and Zweier, 1997), also reviewed in Daiber et al (2014).
The concept of eNOS uncoupling in GTN-induced nitrate tolerance was based on the “clinical” observation that in endothelium-denuded tolerant vessels, the potency of GTN was largely improved and •O2− formation in these vessels was significantly reduced (Munzel et al, 1995b), identifying the endothelium as a powerful •O2− source. We also established that the use of eNOS inhibitors such as NG-nitro-l-arginine decreased •O2− formation in nitrate-tolerant vessels (Knorr et al, 2011; Munzel et al, 2000b). Interestingly, similar observations were made in vessels from noise-exposed mice, where endothelial •O2− formation was significantly increased and normalized by pharmacological inhibition of eNOS by NG-nitro-l-arginine methyl ester (L-NAME) (Munzel et al, 2017a; Steven et al, 2020). The eNOS-derived •O2− formation was also accompanied by diminished vascular •NO bioavailability, as depicted in Figure 2.
Oxidative depletion of tetrahydrobiopterin
Among the concepts of eNOS uncoupling, the oxidative depletion of tetrahydrobiopterin (BH4) is the most commonly accepted one. In the 1990s, many preclinical as well as clinical studies demonstrated that the presence of BH4 is essential for a normal functioning of eNOS (Bendall et al, 2014; Kinoshita et al, 1997; Tsutsui et al, 1996). For example, Milstein and Katusic (1999) showed an oxidative degradation of BH4 to dihydrobiopterin (BH2) by peroxynitrite, thereby providing a concept of how RONS (especially peroxynitrite) via functional BH4 depletion may cause oxidative uncoupling of eNOS (Milstien and Katusic, 1999).
The oxidative BH4 to BH2 depletion concept also helped to explain why vitamin C is able to improve endothelial dysfunction in human subjects with various reasons for endothelial dysfunction as measured by plethysmography or flow-mediated dilation in chronic smokers (Heitzer et al, 1996), diabetic patients (Heitzer et al, 2001a), nitrate-tolerant subjects (Gori et al, 2010), and noise-exposed individuals (Herzog et al, 2019; Schmidt et al, 2013). Possible explanations may be the direct scavenging of •O2− or recycling of oxidized BH4 intermediates by vitamin C.
Although the reaction speed between vitamin C and •O2− (1.7 × 104 M−1 s−1 for ascorbate) (Gotoh and Niki, 1992) is roughly 500,000-fold slower than the reaction speed between •NO and •O2− (6.7–10 × 109 M−1 s−1) (Kissner et al, 1997), the biological concentration of vitamin C is in the lower millimolar range in most intracellular biological fluids, the cytosol of most cell types (and at least 50–100 μM in human plasma), and accordingly may compete with •NO being present in the pico- to nanomolar range in most biological fluids (intra- and extracellular). The new concept postulates that the •BH4+ radicals (once BH2 is formed, only energy-consuming enzymatic reaction confers reduction to BH4) (d'Uscio et al, 2003; Kuzkaya et al, 2003) will react with vitamin C to form BH4, which will replenish depleted BH4 stores thereby recoupling eNOS to an •NO producing enzyme.
The essential role of BH4 for proper eNOS function was also proven by normalization of endothelial function in chronic smokers by supplementation with authentic BH4 (Heitzer et al, 2000). To exclude that solely the antioxidant properties of BH4 improved endothelial function, the smokers were also treated with tetrahydroneopterin (NH4), a structural analog of BH4 sharing the same antioxidant properties, which, however, failed to improve endothelial dysfunction in the smokers.
Likewise, supplementation with folic acid, which feeds into the de novo synthesis of BH4, improved endothelial function in nitrate-tolerant human subjects (Gori et al, 2001a). In addition, BH4 levels in vessels from tolerant rabbits were significantly decreased (Ikejima et al, 2008), whereas cotherapy of GTN-treated rats with the AT1-receptor blocker telmisartan normalized the aggravated eNOS-derived ROS formation and the diminished GTP-cyclohydrolase-1 expression (Knorr et al, 2011). The extent of the beneficial effect of vitamin C on endothelial function even turned out to be a valuable prognostic marker for cardiovascular events (predicting the risk for cardiovascular disease) (Heitzer et al, 2001b).
S-glutathionylation of the eNOS reductase domain
S-glutathionylation is a rather novel and important redox regulatory mechanism for many enzymes, and S-glutathionylation of eNOS at one or more cysteine residues of the reductase domain was able to cause uncoupling of the enzyme (Fig. 3) (Chen et al, 2010). Cysteines 689 and 908 in the reductase domain have been identified as targets for redox modifications. Subsequently, Chen et al (2011) have demonstrated superoxide-induced thiyl radical formation in eNOS and the following intracellular disulfide formation or S-glutathionylation are both leading to uncoupling of eNOS. Meanwhile, eNOS S-glutathionylation was reported for a broad spectrum of pathophysiological conditions, including hypertension, diabetes, and atherosclerosis (for review, see Daiber et al, 2019b).
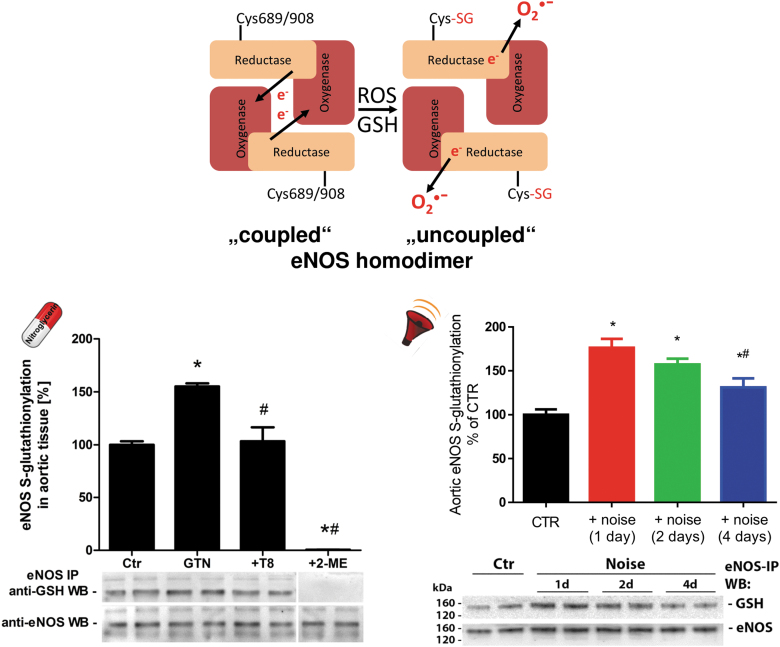
FIG. 3.
S-glutathionylation of eNOS in GTN-treated nitrate-tolerant rats and aircraft noise-exposed mice. Upper part: Oxidative stress causes S-glutathionylation of eNOS at cysteine 689 and 908, a surrogate marker for uncoupling of the protein. In the “coupled” eNOS homodimer, electrons are usually transferred from the NADPH and flavins to the heme iron. When cysteine residues 689 and/or 908 undergo S-glutathionylation, structural changes in eNOS are initiated that are followed by misdirection of the electrons to molecular oxygen with subsequent •O2− formation, termed “uncoupled” state of eNOS. Lower part: S-glutathionylation of eNOS was determined by eNOS immunoprecipitation from aortic tissue, followed by antiglutathione staining and normalization on eNOS. S-glutathionylation of eNOS was increased in nitrate-tolerant rats (GTN with a dose of 50 mg/kg/day for 3.5 days), which was normalized by telmisartan cotherapy (T8, 8 mg/kg/day for 10 days) (Knorr et al, 2011). *p < 0.05 versus Ctr, #p < 0.05 versus GTN group. S-glutathionylation of eNOS was also increased by aircraft noise exposure [72 dB(A) around-the-clock for 1, 2, or 4 days] (Munzel et al, 2017a). Disappearance of the antiglutathione staining in the presence of 2-mercaptoethanol served as a control. *p < 0.05 versus Ctr, #p < 0.05 versus noise (1 day) group. Representative blots are shown at the bottom of each densitometric quantification along with the respective loading control. Scheme in the upper part was adapted from Daiber et al (2020b) with permission. Data in the lower part were reproduced from Knorr et al (2011) (for nitrate tolerance) and Munzel et al (2017a) (for noise exposure) with permission.
As we have shown, eNOS S-glutathionylation is largely increased in GTN-treated endothelial cells and aortic tissue from GTN-infused rats, likely contributing to eNOS uncoupling and endothelial dysfunction in the setting of nitrate tolerance, which was largely prevented by AT1-receptor blocker therapy with telmisartan (Fig. 3) (Knorr et al, 2011). This mechanism of eNOS uncoupling seems to be shared by other organic nitrates since isosorbide-5-mononitrate treatment of mice leads to enhanced aortic eNOS S-glutathionylation and endothelial ROS formation as well as endothelial dysfunction (Oelze et al, 2013). Likewise, eNOS S-glutathionylation, enhanced endothelial ROS formation, and endothelial dysfunction are the result of exposure of mice to aircraft noise around-the-clock (Fig. 3) (Munzel et al, 2017a), in particular, of exposure during the sleep phase (Kroller-Schon et al, 2018).
In addition, eNOS S-glutathionylation, ROS formation, and endothelial dysfunction were also exacerbated in an additive manner by noise exposure of hypertensive mice (Steven et al, 2020) or combined exposure to noise and particulate matter (Kuntic et al, 2023). Thus, we postulate that eNOS S-glutathionylation represents a “master redox switch” determining whether the enzyme produces •NO or •O2−. This pathomechanism is also of high interest from a redox perspective since eNOS S-glutathionylation is reversed by glutaredoxin-1 (Chen et al, 2013) and is tightly connected to BH4 deficiency (Crabtree et al, 2013).
Additional mechanisms of eNOS uncoupling
Another direct redox-regulatory pathway explaining, at least in part, eNOS dysfunction due to eNOS uncoupling is the oxidative disruption of the zinc–sulfur complex (ZnCys4) in the binding region of the eNOS dimer. This will lead to a loss of eNOS dimerization, a phenomenon that has been first described by Zou et al (2002) with respect for ONOO−-mediated oxidation of eNOS. The critical role of this zinc–sulfur complex for proper eNOS dimer formation was demonstrated by a significant reduction of dimerization and thus more monomerization in a knockin mouse expressing a C101A-eNOS mutant with reduced zinc–sulfur complex building capacity (Suvorava et al, 2015). The reports on this “redox switch” reflected by a decreased eNOS dimer/monomer ratio were previously summarized and described for various disease conditions such as diabetes, hypertension, and hyperhomocysteinemia (Daiber et al, 2019b; Daiber et al, 2014; Schulz et al, 2014).
A diminished eNOS dimer/monomer ratio was also reported for GTN-treated endothelial cells, which could be prevented by pharmacological arginase-2 inhibition. In addition, nitrate tolerance was absent in aorta from animals chronically treated with GTN in the setting of genetic arginase-2 deficiency (Khong et al, 2012). However, this attractive uncoupling mechanism of eNOS was so far not detected in response to transportation noise exposure.
Another redox-sensitive regulatory pathway of eNOS activity represents the phosphorylation of the enzyme (Fig. 2). In general, there are three different important phosphorylation sites regulating the activity of eNOS. First, the activating phosphorylation at Ser1177 mediated by the protein kinase B pathway, which is calcium independent and increases the •NO producing activity of eNOS (Dimmeler et al, 1999). Second, the inactivating phosphorylation at Tyr657 mediated by the protein tyrosine kinase-2 (PYK-2), which inhibits the enzyme without evidence for uncoupling of the enzyme (Loot et al, 2009). Third, the phosphorylation at Thr495 mediated by PKC, which may contribute to uncoupling and superoxide production by eNOS (Fleming et al, 2001; Lin et al, 2003).
It should be mentioned that the phosphorylation of eNOS at Thr495 or Tyr657 is initiated by increased oxidative stress. For example, the PKC-driven phosphorylation at Thr495 is stimulated by H2O2 (Lin and Takemoto, 2005; Rathore et al, 2008). The PYK-2-triggered phosphorylation at Tyr657 is stimulated by authentic H2O2 as well as by angiotensin-II induced ROS formation in vitro and in vivo (Loot et al, 2009). Thus, both the regulatory pathways may be regarded as initiators of “redox switches” of eNOS. Interestingly, PKC, which is activated in endothelial cells in response to GTN treatment, contributes to eNOS uncoupling via phosphorylation of eNOS at Thr495, a process that has been shown for being prevented by cotherapy with the AT1-receptor blocker telmisartan (Knorr et al, 2011).
Asymmetric dimethylarginine (ADMA) is probably the most potent endogenous inhibitor of eNOS (Boger, 2003a) and it is still controversial whether ADMA can cause uncoupling of the enzyme (Sydow and Munzel, 2003). Elevated ADMA serum/plasma levels have been established to represent a reliable risk marker (and probably promoter) concerning future cardiovascular events and prognosis in patients with established cardiovascular disease (Boger, 2003b; Schnabel et al, 2005). Oxidative stress within the vasculature significantly contributes to ADMA production and/or inhibition of ADMA degradation (due to redox sensitivity of protein arginine methyltransferases and dimethylarginine dimethylaminohydrolases [DDAHs]) (Daiber et al, 2014), leading to ADMA concentrations that will significantly inhibit eNOS activity (Cooke, 2000). For example, GTN increased ADMA levels in cultured human umbilical vein endothelial cells being associated with higher ROS production and malondialdehyde levels as well as diminished cGMP formation and mitochondrial ALDH-2 activity (Zhang et al, 2008).
However, higher ADMA levels in response to chronic GTN therapy could not be confirmed in a human study (Thomas et al, 2009). Whether chronic transportation noise will increase ADMA levels remains to be established.
l-Arginine is the endogenous substrate of eNOS for the •NO synthesis. So it is not surprising that “l-arginine deficiency” has been proposed to contribute to uncoupling of eNOS (Bode-Boger et al, 2007). However, since the Km of the enzyme (the concentration of l-arginine that is necessary for half maximal saturation) is ∼2.9 μM for eNOS (Forstermann et al, 1991) and the intracellular l-arginine concentration is normally in the mM range, the concept that l-arginine depletion as a substrate is a significant regulator of eNOS activity is highly unlikely.
It remains also controversial whether l-arginine supplementation increases or decreases ROS formation from uncoupled NOS enzymes (reviewed in Daiber et al, 2014). Possible explanations for the beneficial effects of high concentrations of l-arginine may be either the direct antioxidant effects of the guanidino group in this amino acid (Lass et al, 2002) or an outcompeting of ADMA by l-arginine with respect to eNOS. The latter hypothesis becomes even more attractive if one considers the export of ADMA from endothelial cells that is driven by the cationic amino acid transporter-1 (CAT-1) in the presence of an extracellular excess of cationic amino acids such as l-arginine (Closs et al, 2012).
This concept has been described in a patient with frequent coronary artery spasms caused by a genetic defect in y+LAT expression, a major transporter for cationic amino acids, that may cause increased ADMA levels in the cytosol of endothelial cells and eNOS inhibition or uncoupling, all of which was corrected by administration of high-dose l-arginine, probably by CAT-1/l-arginine-driven export of ADMA from endothelial cells and recoupling of eNOS (Closs et al, 2012). As discussed above, the protective effects of pharmacological or genetic arginase inhibition in GTN-treated cultured cells or animals support a beneficial role of l-arginine in the setting of nitrate tolerance (Khong et al, 2012), whereas improvement of noise exposure damage by l-arginine still lacks experimental proof.
Also MPO was reported to control eNOS activity and/or nitric oxide bioavailability by direct reaction of the MPO compound 1 (highly oxidized iron intermediate during the reaction cycle of peroxidases) with nitric oxide, thereby consuming the vasodilator (Baldus et al, 2004; Eiserich et al, 2002; Rudolph et al, 2012) and thereby inducing endothelial dysfunction (Abdo et al, 2017; Stocker et al, 2004). In addition, there is a kind of pathophysiological cross talk between the eNOS inhibitor ADMA and MPO, in which ADMA activates neutrophils and the release of MPO that in turn suppresses DDAH (enzyme for breakdown of ADMA) and leads to endothelial dysfunction (von Leitner et al, 2011). Chlorinated reaction products of l-arginine and MPO were reported to inhibit eNOS activity (Zhang et al, 2001), and hypochlorous acid by vascular peroxidase 1 was also demonstrated to inhibit/uncouple eNOS enzyme (Liu et al, 2017).
Identification of Uncoupled eNOS in Vascular Cells and Tissue
Direct detection of eNOS-derived superoxide formation
According to previous reports on nitrate tolerance (Munzel et al, 2000a; Munzel et al, 2000b) and chronic transportation noise exposure (Kroller-Schon et al, 2018; Munzel et al, 2017a), •O2− formation from uncoupled eNOS is best identified by the measurement of •O2− formation in the presence and absence of NOS inhibitors. While inhibition of eNOS by eNOS inhibitors will consistently increase the •O2− signal in control tissues (excluding direct antioxidant effects of the NOS inhibitors), the use of eNOS inhibitors will decrease the •O2− signal in case eNOS is uncoupled and represents a significant •O2− source.
Methodologically, these studies used lucigenin in aortic tissue, a chemiluminescence dye that reacts with •O2− and forms a dioxetane product that decomposes to acridone and emits chemiluminescent light (Liochev and Fridovich, 1997). Lucigenin was suspected to undergo redox cycling and cause artificial •O2− production (Janiszewski et al, 2002; Tarpey et al, 1999). However, subsequent studies have shown that redox cycling of lucigenin requires artificial biological conditions (e.g., exogenous addition of NADH) and that low lucigenin concentrations (5 μM) are suitable for biological detection of •O2− (Daiber et al, 2004a). In particular, the close correlation between •O2− measurements by lucigenin and electron paramagnetic resonance (EPR) data wiped out the suspicion of artifact research by lucigenin.
Other suitable detection methods for NOS uncoupling (always in combination with a specific NOS inhibitor such as L-NAME) that can be used include the cytochrome c assay (SOD inhibitable signal) (Cai et al, 2005; Landmesser et al, 2003), high-performance liquid chromatography-based 2-hydroxyethidium quantification (the superoxide-specific oxidation product of dihydroethidium [DHE]) (Dikalova et al, 2010b; Xia et al, 2010), and DHE-dependent oxidative fluorescence microtopography in DHE-stained aortic cryosections [examples for nitrate tolerance (Jabs et al, 2015; Knorr et al, 2011) and noise exposure (Kroller-Schon et al, 2018; ; Munzel et al, 2017a; Steven et al, 2020)]. Representative staining images for the detection of eNOS uncoupling for nitrate tolerance by DHE-dependent oxidative fluorescence microtopography are shown in Figure 2, and the methodological details were explained in full depth previously (Daiber et al, 2014).
The different methods to detect vascular •O2− production have been reviewed in the past (Dikalov et al, 2007; Munzel et al, 2002) and were also discussed, highly controversial, either in favor (Daiber et al, 2017b) or in disfavor of these classical ROS detection assays (Griendling et al, 2016). As mentioned, EPR-based measurement of superoxide- or hydroxyl-adducts with different spin probes (e.g., 5,5-dimethyl-1-pyrroline N-oxide-OOH or –OH adducts) demonstrated also high specificity for •O2−, for example, in response to GTN treatment, although the sensitivity of these spin probes in vascular samples or cell culture is rather limited (Dikalov et al, 1998).
Indirect detection of eNOS uncoupling
Indirect approaches to detect eNOS uncoupling are mainly based on testing the activity of eNOS (namely, NO formation or endothelium-dependent vasodilation) in the presence of drugs that are known to ameliorate the function (coupling state) of eNOS (e.g., folic acid, sepiapterin, BH4) (Daiber et al, 2019b; Daiber et al, 2014). The substantial improvement of impaired endothelial function in chronic smokers by BH4 infusion is an important example for the recoupling of eNOS in a disease setting (Heitzer et al, 2000). In these investigations, one has to consider that BH4 is a strong antioxidant, making it difficult to differentiate whether the improvement of endothelial dysfunction is due to eNOS recoupling of just the consequence of the antioxidant properties of BH4.
Thus, as an internal control, the analog NH4 with comparable antioxidant properties such as BH4 has to be tested. For example, in the case of chronic smokers, BH4 improved endothelial dysfunction, but NH4 did not rule out unspecific antioxidant properties of the electron donor BH4.
Another example for indirect proof of an uncoupled eNOS was reported in the animal model of deoxycorticosterone acetate salt hypertension (Landmesser et al, 2003), showing the impaired •NO synthesis by a decreased EPR-NO-signal and the increase of eNOS activity in response to supplementation with BH4 or by genetic deletion of the NADPH oxidase subunit p47phox (removing the “kindling radical” for NOS uncoupling). The prevention of endothelial dysfunction in isolated vessels of diseased animals (ex vivo) by the BH4 precursor sepiapterin is another approach to prove eNOS uncoupling in vascular tissue (Laursen et al, 2001; Schuhmacher et al, 2010).
We here provide indirect proof for eNOS uncoupling in the animal models of GTN-induced nitrate tolerance (Knorr et al, 2011; Wenzel et al, 2008) and aircraft noise exposure-mediated cardiovascular damage (Eckrich et al, 2021; Kroller-Schon et al, 2018) by normalization of vascular ROS formation and improvement of endothelial dysfunction by genetic deletion of subunits of the phagocytic NADPH oxidase (NOX-2), namely p47phox or gp91phox, or pharmacological inhibition of the enzyme (Fig. 4). The phagocytic NADPH oxidase (NOX-2) represents a major trigger of oxidative eNOS uncoupling due to its abundance in inflammatory cells (Wenzel et al, 2017). Vascular ROS formation in nitrate-tolerant animals under GTN therapy could be corrected by the NADPH oxidase inhibitor apocynin (Fukatsu et al, 2007) and the PKC inhibitor chelerythrine or others (Knorr et al, 2011; Munzel et al, 1995a).
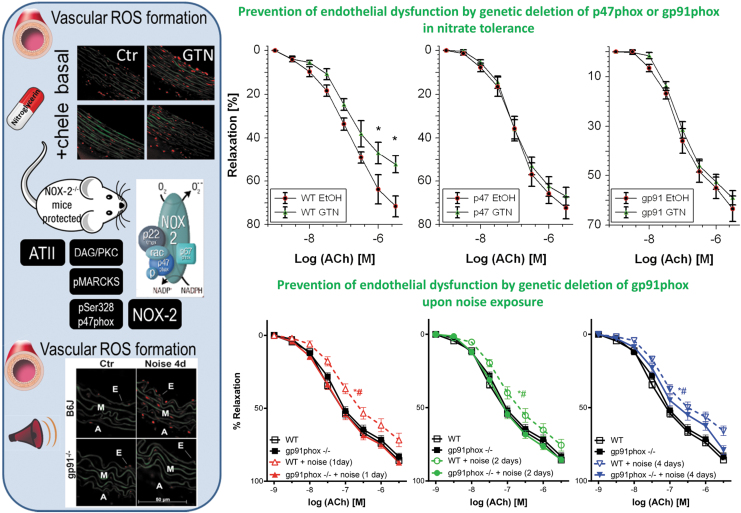
FIG. 4.
Prevention of vascular ROS formation and endothelial dysfunction by GTN or traffic noise exposure by pharmacological or genetic NADPH oxidase inhibition. Left part: Oxidative stress assessed by DHE staining (red fluorescence indicates reactive oxygen species [ROS] formation, whereas green fluorescence represents the autofluorescence of the basal lamina). E=Endothelium, M = Media, A = Adventitia. GTN (50 mg/kg/day for 3.5 days) treatment leads to aortic oxidative stress in rats, which was prevented by the PKC inhibitor chelerythrine (chele) (Knorr et al, 2011). Aircraft noise exposure [72 dB(A) around-the-clock for 1, 2, or 4 days] leads to aortic oxidative stress in rats, which was prevented by genetic deficiency of gp91phox (Kroller-Schon et al, 2018). The phagocytic NADPH oxidase (NOX-2) plays a key role in the pathomechanisms underlying nitrate tolerance and noise exposure-mediated cardiovascular damage. NOX-2 is activated by ATII receptor activation, leading to DAG formation, a strong PKC activator. PKC activation can be envisaged by phosphorylation of its target protein MARCKS. Activated PKC will cause phosphorylation of p47phox at Ser328 leading to translocation of this cytosolic regulator of NOX-2 to the multienzyme membrane complex of gp91phox with subsequent activation of NOX-2 and superoxide formation. Right part: Endothelial dysfunction (impaired ACh-dependent relaxation) by GTN treatment was prevented in mice with genetic deficiency of p47phox or gp91phox (Wenzel et al, 2008). *p < 0.05 versus WT EtOH solvent control group. Endothelial dysfunction by noise exposure was prevented in mice with genetic deficiency of gp91phox (Kroller-Schon et al, 2018). *p < 0.05 versus WT control group and #p < 0.05 versus gp91phox−/− + noise group. Scheme in the left part was adapted from Frenis et al (2021c) with permission [Copyright © 2021 the authors. Open access (CC BY)]. Data in the left part were reproduced from Knorr et al (2011) (for nitrate tolerance) and Kroller-Schon et al (2018) (for noise exposure) with permission. Data in the right part were reproduced from Wenzel et al (2008) (for nitrate tolerance) and Kroller-Schon et al (2018) (for noise exposure) with permission. ACh, acetylcholine; ATII, angiotensin-II; DAG, diacylglycerol; DHE, dihydroethidine; ROS, reactive oxygen species.
Likewise, vascular ROS formation in noise-exposed mice was completely corrected by genetic deletion of gp91phox or pharmacological NADPH oxidase inhibitors such as GSK2795039 (Kroller-Schon et al, 2018). Importantly, p47phox or gp91phox knockout mice were protected from GTN-induced cross tolerance (endothelial dysfunction), whereas the degree of nitrate tolerance (the impaired vasodilatory potency of GTN) was not improved in the knockout mice (Fig. 4) (Wenzel et al, 2008). Similar observations were made for noise exposure-induced endothelial dysfunction that was completely prevented in gp91phox knockout mice upon 1 or 2 days of noise exposure, whereas upon 4 days of noise exposure, only partial protection by gp91phox deficiency was observed, most likely because other ROS sources such as mitochondria take over after prolonged noise exposure (Fig. 4) (Kroller-Schon et al, 2018). Also noise-induced microvascular dysfunction enhanced the leukocyte–endothelium interaction, and the pro-atherothrombotic phenotype of the plasma proteome was prevented in gp91phox knockout mice (Eckrich et al, 2021).
However, as mentioned above, also other NOX isoforms may contribute to eNOS uncoupling in nitrate tolerance and transportation noise stress as detailed above for arterial hypertension in the angiotensin-II model. As for nitrate tolerance, downregulation of NOX-1 expression despite elevation of NADPH oxidase activity was reported for GTN-treated cultured smooth muscle cells (Szocs et al, 2007). In vivo GTN treatment of rats did not increase the expression of NOX-1 or NOX-4, neither at the protein nor gene level, and also, no increased ROS formation was observed by acute GTN treatment of cultured human embryonic kidney (HEK293) cells that were transfected with Nox-1, Nox-4, and the related other required subunits for full activation (Wenzel et al, 2008). In vivo treatment of mice with isosorbide-5-mononitrate increased NOX-1, NOX-2, and NOX-4 protein expression in the aorta, and NOX-4 protein expression was also increased in isosorbide-5-mononitrate-treated cultured primary human endothelial cells (Oelze et al, 2013).
As for noise-induced stress, aircraft noise exposure of mice increased aortic NOX-2 but not NOX-1 protein expression, whereas Nox-1 mRNA levels were significantly increased in mouse lung endothelial cells (Munzel et al, 2017a). NOX-1 and NOX-4 protein levels were also increased by trend in the cortex of noise-exposed mice, whereas NOX-2 was almost not changed and NOX-3 expression was even decreased (Kroller-Schon et al, 2018). Nox-1 mRNA was also upregulated in brains of mice upon sleep-phase noise exposure (Kroller-Schon et al, 2018). In addition, NOX-1 upregulation and NOX-3 downregulation were reported for the cochlea of rats exposed to loud noise in the context of hearing loss (Vlajkovic et al, 2013).
Transgenic mice overexpressing Nox-4 showed more pronounced vulnerability to noise-induced hearing loss (Morioka et al, 2018), which was in accordance with the increase in NOX-4 expression in cochlea of noise-exposed mice (Shih et al, 2021). Genetic Nox-3 deficiency protects from noise-induced sensorineural hearing loss (Rousset et al, 2022).
Clinical Perspective on the Role of Endothelial Dysfunction as a Consequence of eNOS Uncoupling in Nitrate Tolerance and Transportation Noise Exposure
Multiple mechanisms may account for endothelial dysfunction in response to chronic nitrate therapy (Munzel et al, 2013; Munzel et al, 2011) and traffic noise exposure (Munzel et al, 2021; Munzel et al, 2018a), comprising modulation of the activity and/or expression of eNOS, reduced sensitivity of vascular smooth muscle cells to •NO, or more pronounced scavenging of •NO via its reaction with •O2−. In case of inappropriately high ROS production and diminished vascular •NO bioavailability and endothelial dysfunction, vascular responses are in general improved by high doses of the antioxidant vitamin C (Duffy et al, 2001; Heitzer et al, 1996) (also reviewed in Daiber et al, 2017c; Schulz et al, 2008), which can be attributed to a direct free radical scavenging effect, and also to the recycling of oxidized BH4-radicals by vitamin C (d'Uscio et al, 2003; Kuzkaya et al, 2003).
Importantly, the degree of endothelial function amelioration by vitamin C has prognostic implications (Heitzer et al, 2001b). There are numerous reports demonstrating the prognostic importance of endothelial function, for example, for patients undergoing peripheral or coronary bypass surgery (Gokce et al, 2002), as well as for patients with essential hypertension (Perticone et al, 2001).
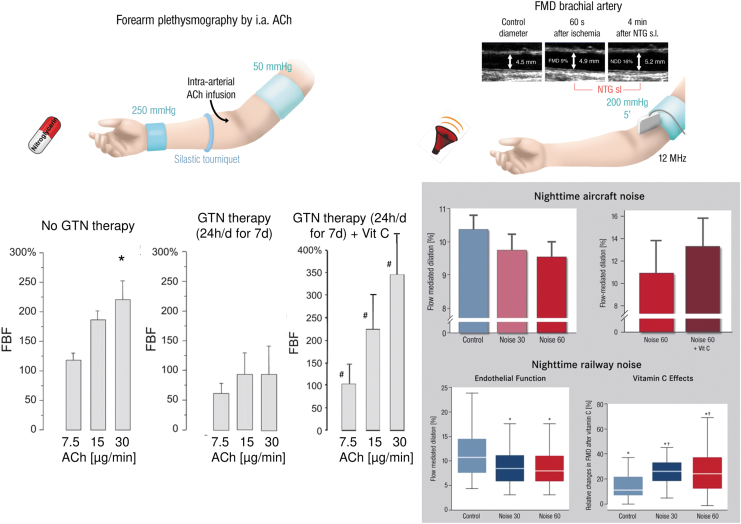
Chronic organic nitrate therapy causes endothelial dysfunction (impaired acetylcholine-dependent increase in forearm blood flow), all of which was completely prevented by coadministration of vitamin C (Fig. 5) (Gori et al, 2010). Also, several human studies documented that impaired endothelial function in response to aircraft or train noise exposure could be reversed by acute oral vitamin C administration (Herzog et al, 2019; Schmidt et al, 2013). In most instances, it is likely that an uncoupled eNOS contributes to endothelial dysfunction since the subsequent studies in animals were able to identify the uncoupling process in full detail, for example, as shown for nitrate tolerance (Knorr et al, 2011; Munzel et al, 2000a) and noise exposure (Daiber et al, 2020a; Kroller-Schon et al, 2018; Munzel et al, 2017a).
FIG. 5.
Impairment of endothelial dysfunction by GTN therapy or traffic noise exposure and improvement by vitamin C coadministration. Left part: Endothelial function was determined by forearm plethysmography, ultrasound-dependent assessment of forearm blood flow in response to increasing doses of infused ACh. FBF was expressed as the ratio of infused to noninfused arm. Each column presents the percent change in FBF from baseline in response to each infused concentration of ACh (7.5, 15, and 30 μg/min) in the untreated control group, chronic GTN treatment for 7 days, and the chronic GTN+vitamin C administration group (Gori et al, 2010). *p < 0.05 versus lowest ACh dose (7.5 μg/min), #p < 0.05 versus GTN therapy without vitamin C. Right part: Endothelial function was determined by FMD, ultrasound-dependent assessment of vasodilation (widening) of a large vessel in the arm upon reactive hyperemia after vascular occlusion for 5 min. FMD was determined in subjects without aircraft noise exposure [Leq 35.4 dB(A)] and 30 or 60 aircraft noise events for 1 night [Leq 43.1 or 46.3 dB(A)] (Schmidt et al, 2013). Likewise, FMD was determined in subjects without train noise exposure [Leq 33 dB(A)] and 30 or 60 train noise events for 1 night [Leq 52 or 54 dB(A)] (Herzog et al, 2019). The effect of vitamin C oral administration on FMD was measured in both the noise exposure studies. *p < 0.05 versus same group without vitamin C, †p < 0.05 versus unexposed control group with vitamin C. The schemes in the upper parts were reused from Daiber et al (2017c) with permission. Data in the left part were adapted from Gori et al (2010) (for nitrate tolerance) with permission. Data in the right part were adapted from Herzog et al (2019) and Schmidt et al (2013) (for noise exposure) with permission. FBF, forearm blood flow.
In summary, endothelial dysfunction, atherosclerosis, and the status of full-blown cardiovascular disease are associated with a chronic activation of the local and/or circulating renin–angiotensin–aldosterone system (RAAS) and an uncoupled eNOS. This was demonstrated in the setting of nitrate tolerance in response to chronic isosorbide-5-mononitrate therapy and GTN therapy, and also in response to transportation noise exposure, when the animals were exposed in the sleeping phase. Accordingly, in the setting of nitrate tolerance (Kurz et al, 1999) and noise stress (Daiber et al, 2019a; Munzel et al, 2021), inhibitors of the RAAS were able to improve nitrate tolerance, to reduce vascular oxidative stress, to normalize blood pressure, and to improve endothelial dysfunction most likely due to a recoupling of the eNOS enzyme.
Nitrate-tolerant animals and patients take profit from AT1-receptor blockade (Hirai et al, 2003; Knorr et al, 2011) and angiotensin-converting enzyme (ACE) inhibitor (Berkenboom et al, 1999; Heitzer et al, 1998; Watanabe et al, 1998) therapy. Similar observations were made in noise exposure animal studies, where RAAS activation was prominent as documented by elevated angiotensin-II levels (Munzel et al, 2017a), additive damage of noise in mice with arterial hypertension by angiotensin-II infusion (Steven et al, 2020), and normalization of elevated blood pressure in noise-exposed mice by the ACE inhibitor captopril (Frenis et al, 2021b). Although the molecular mechanisms of RAAS activation and increased ROS production are likely different in response to chronic nitrate therapy and noise exposure, the downstream health side effects on the cardiovascular system are likely the same (see summarizing scheme in Fig. 6).
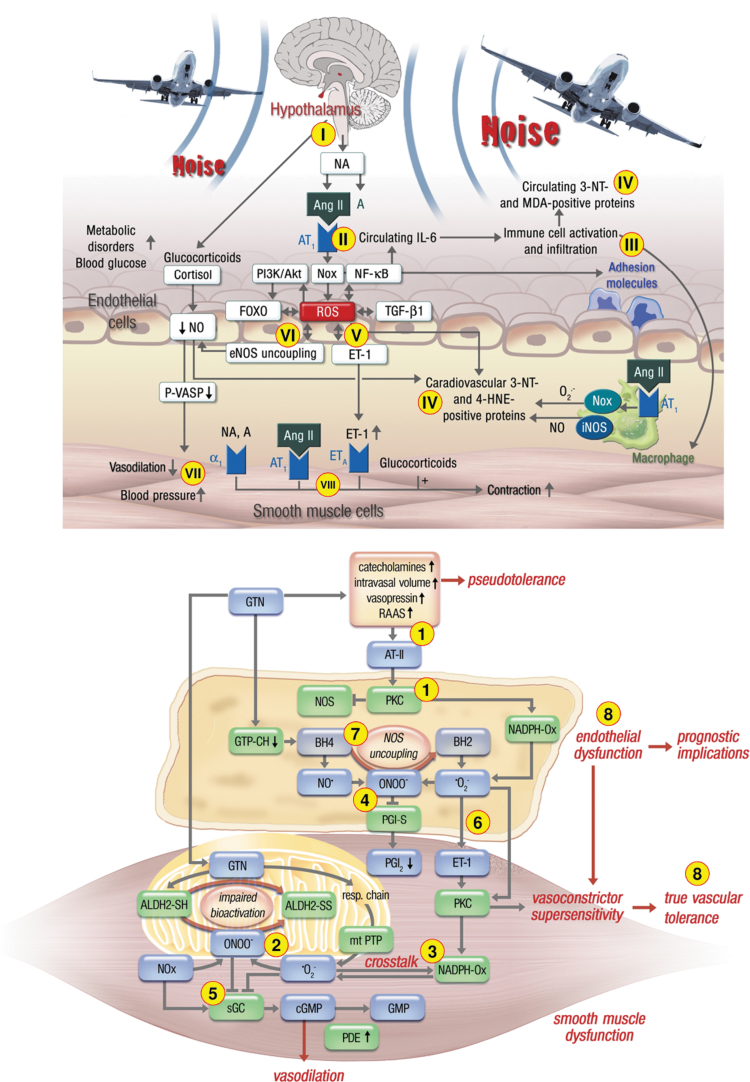
FIG. 6.
Summarizing schemes on the similarities and differences of pathomechanisms underlying nitrate tolerance (upper part) and transportation noise exposure (lower part)-mediated cardiovascular damage. (1) Chronic GTN therapy leads to activation of the RAAS via nitrovasodilator-induced hypotension with subsequent angiotensin-II release, PKC and NOX-2 activation (also called pseudotolerance). In addition, GTN therapy causes substantial mtROS formation also causing inhibition of ALDH-2, the GTN-bioactivating enzyme (2), followed by redox-dependent NOX-2 activation (3) and peroxynitrite (ONOO−) formation with subsequent dysregulation of other critical regulators of vascular tone by nitration of prostacyclin synthase (PGI-S) (4), oxidation of sGC (5), oxidative induction of ET-1 (6), and uncoupling of eNOS by BH4 depletion and S-glutathionylation (7), all of which leads to endothelial dysfunction and true vascular tolerance (8). In contrast, traffic noise exposure causes neuronal stress responses via the hypothalamic–pituitary–adrenal axis (release of cortisol/corticosterone) and the sympathetic nervous system (release of NA) (I) with subsequent RAAS and NOX-2 activation (II) along with enhanced inflammation (III). The resulting oxidative stress leads to peroxynitrite formation, protein tyrosine nitration, and lipid peroxidation (IV), as well as oxidative induction of ET-1 (V) and eNOS uncoupling (VI), all of which lead to endothelial dysfunction and hypertension (VII), in part, mediated by direct vasoconstrictor effects (VIII). The major differences of the pathomechanisms of nitrate tolerance and noise exposure are the pathways that lead to activation of the RAAS, the substantial adverse effects of GTN on mitochondrial function and mtROS formation, and the strong inflammatory component underlying noise exposure-mediated damage. The major similarities are the central role of oxidative stress in both pathomechanisms of endothelial dysfunction, with NOX-2, RAAS, and eNOS uncoupling as key players. The schemes were reused from Munzel et al (2011) (upper part) and Munzel et al (2017a) (lower part) with permission. ALDH-2, mitochondrial aldehyde dehydrogenase; ET-1, endothelin-1; mtROS, mitochondrial reactive oxygen species; NA, noradrenalin; RAAS, renin–angiotensin–aldosterone system; sGC, soluble guanylyl cyclase.
Organic nitrate therapy induces neurohormonal (counter-regulatory) activation of vasoconstrictor pathways including the RAAS as a consequence of GTN-induced hypotension, but this response may be also redox-driven as a consequence of increased endothelin-1 or angiotensin-II expression/release and signaling (Munzel et al, 2013; Munzel et al, 2011). In contrast, transportation noise induces RAAS activation via upstream induction of stress response pathways such as the hypothalamic–pituitary–adrenal axis, leading to increased cortisol release and activation of the sympathetic nervous system that are known to be tightly connected to downstream vasoconstrictor pathways based on endothelin-1 or angiotensin-II (Daiber et al, 2020a; Daiber et al, 2019a). Especially, the release of catecholamines such as noradrenaline by sympathetic activation may contribute directly to endothelial dysfunction by direct vasoconstriction via α1-receptor activation as well as downstream activation of RAAS and endothelin-1 pathway.
In the setting of nitrate tolerance, the hypotensive effects of organic nitrates and increase of intravasal volume can induce counter-regulatory effects, for example, elevated levels of noradrenaline reported for chronic GTN therapy (Parker et al, 1991) or acute nitrate infusion (Kubo et al, 1999). Likewise, chronic road noise exposure was associated with higher levels of noradrenaline in healthy women (Babisch et al, 2001), and acute exposure of healthy subjects to one night of aircraft noise caused elevated adrenaline levels (Schmidt et al, 2013), which was also supported by a chronic versus acute noise exposure study of humans (Ising and Braun, 2000) as well as animal studies (Munzel et al, 2017a; Said and El-Gohary, 2016). Catecholamines are interconnected with the RAAS and endothelin-1 pathway (reviewed in Daiber et al, 2020a; Daiber et al, 2019a; Munzel et al, 2021), with a key role for ROS derived from NADPH oxidases (Grande et al, 2011).
The similarities between the pathomechanisms underlying endothelial dysfunction in response to chronic noise exposure and GTN treatment-induced cardiovascular damage are evident (Fig. 6). They include the activation of a primary •O2− source such as the phagocytic NADPH oxidase (NOX-2) to produce the “kindling radicals” leading to uncoupling of eNOS via the above-described “redox switches” (Schulz et al, 2014). Upon RAAS activation, angiotensin-II receptor-mediated diacylglycerol formation and the subsequent PKC activation will lead to NOX-2 (phagocytic NADPH oxidase) activation. Likewise, there is an interaction between endothelin-1 and NOX-2 (Daiber et al, 2017a).
As a major difference of GTN therapy, NOX-2 activation could also be promoted by redox activation by mtROS generated by the interaction of the organic nitrate with the mitochondrial respiratory chain components (Daiber et al, 2004b; Munzel et al, 2013; Sydow et al, 2004). As a major difference of noise exposure, there is a strong inflammatory phenotype of tissues and the circulation, which was observed in noise-exposed human subjects (Herzog et al, 2019) and mice (Eckrich et al, 2021; Frenis et al, 2021a; Munzel et al, 2017a). In addition, noise exposure during the sleep phase induces sleep fragmentation and deprivation leading to dysregulation of the circadian clock (Daiber et al, 2022; Kroller-Schon et al, 2018; Munzel et al, 2020), which is per se an important cardiovascular risk factor (Lecour et al, 2022).
What mitigation strategies in the population could be applied against the cardiovascular complications by transportation noise exposure and chronic GTN and also isosorbide-5-mononitrate and dinitrate therapy-induced nitrate tolerance? First, as discussed above, RAAS inhibition (e.g., by ACE inhibitors such as captopril or AT1-receptor blockers such as telmisartan) can be used to avoid the mentioned complications such as eNOS uncoupling, and several clinical studies confirmed that inhibitors of the RAAS can indeed develop nitrate tolerance (Mehra et al, 1992). This, however, does not represent a mitigation strategy that can be applied to the general population.
Second, acute vitamin C administration improved endothelial dysfunction induced by noise or GTN therapy, but we know from large-scale clinical trials that chronic oral vitamin C administration is mostly not protective because of the rather slow reaction rate between the antioxidant and superoxide (Gori and Munzel, 2011; Schmidt et al, 2015b), except few studies where vitamin C plasma levels were well controlled (Khaw et al, 2001).
We have shown in animal models of nitrate tolerance and aircraft noise exposure, activation of the endogenous antioxidant defense system via nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase-1 activation (e.g., by hemin or dimethyl fumarate) (Bayo Jimenez et al, 2021; Schuhmacher et al, 2010; Wenzel et al, 2007), which represents a potential mitigation strategy that could be applied to large populations, for example, by dietary activators of NRF2 such as sulforaphane from broccoli and other nutraceuticals that are currently tested in clinical trials (Cuadrado et al, 2018). This approach could be promising since most environmental stressors induce damage that is responsive to NRF2 activators (Bayo Jimenez et al, 2022). Also, physical activity could be a first-line mitigation measure as cardiovascular damage by other environmental risk factors, for example, air pollution (Hahad et al, 2021; Tainio et al, 2021), is highly responsive to this nonpharmacological intervention.
Although there is evidence for improvement of noise adverse health effects by physical activity (e.g., by proximity of green space areas), large clinical trials are not available. Wearing earplugs and noise-canceling headphones (especially at night) may in theory be effective, however, large clinical studies on the protective effects of earplugs and noise-canceling headphones against (nighttime) noise exposure are currently not available. Obviously, there are still many gaps in noise research that should urgently be addressed in light of the potential additive risk of noise exposure in individuals with preexisting cardiovascular disease suggested by human (Schmidt et al, 2015a) and animal studies (Steven et al, 2020), most probably by sleep deprivation/fragmentation-mediated circadian rhythm impairment by nighttime noise (Daiber et al, 2022).
Acknowledgment
We thank Margot Neuser for graphical assistance.
Abbreviations Used
- 8-oxoGua
8-oxoguanine
- ACE
angiotensin-converting enzyme
- ACh
acetylcholine
- ADMA
asymmetric dimethylarginine
- Akt
protein kinase B
- ALDH-2
mitochondrial aldehyde dehydrogenase
- ATII
angiotensin-II
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- CAT-1
cationic amino acid transporter-1
- CI
confidence interval
- DAG
diacylglycerol
- dB(A)
decibel (A-weighted)
- DDAH
dimethylarginine dimethylaminohydrolase
- DHE
dihydroethidium
- EDRF
endothelium-derived relaxing factor
- eNOS
endothelial nitric oxide synthase (NOS-3)
- EPR
electron paramagnetic resonance
- ET-1
endothelin-1
- FBF
forearm blood flow
- FMD
flow-mediated dilation
- FMN
flavin mononucleotide
- GCH-1
GTP-cyclohydrolase-1
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSSG
glutathione disulfide
- GTN
nitroglycerin
- iNOS
inducible nitric oxide synthase (NOS-2)
- L-NAME
NG-nitro-l-arginine methyl ester
- MPO
myeloperoxidase
- mtROS
mitochondrial reactive oxygen species
- NA
noradrenalin
- NH4
tetrahydroneopterin
- nNOS
neuronal nitric oxide synthase
- NOX-1/-3/-4
NADPH oxidase isoforms 1/3/4
- NOX-2
phagocytic NADPH oxidase (gp91phox)
- NRF2
nuclear factor erythroid 2-related factor 2
- P47phox
cytosolic/regulatory subunit of NOX-2
- PKC
protein kinase C
- PYK-2
protein tyrosine kinase-2
- RAAS
renin–angiotensin–aldosterone system
- RONS
reactive oxygen and nitrogen species
- ROS
reactive oxygen species
- sGC
soluble guanylyl cyclase
- SOD
superoxide dismutase
- TR
thioredoxin reductase
- Trx
thioredoxin
- ucNOS
uncoupled nitric oxide synthase
- WHO
World Health Organization
- XO
xanthine oxidase
Authors' Contributions
T.M. performed review/editing, conceptualization and writing the original draft (lead). A.D. performed review/editing, conceptualization, and writing the original draft (lead).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Andreas Daiber and Thomas Münzel were supported by vascular biology research grants from the Boehringer Ingelheim Foundation for the collaborative research group “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutics” and through continuous research support from the Foundation Heart of Mainz and the Center for Translational Vascular Biology (CTVB). Thomas Münzel is the PI of the DZHK (German Center for Cardiovascular Research), partner site Rhein-Main, Mainz, Germany.
References
- Abdo AI, Rayner BS, van Reyk DM, et al. Low-density lipoprotein modified by myeloperoxidase oxidants induces endothelial dysfunction. Redox Biol 2017;13:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams J. The role of nitrates in coronary heart disease. Arch Intern Med 1995;155:357–364. [PubMed] [Google Scholar]
- Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health 2003;5:1–11. [PubMed] [Google Scholar]
- Babisch W. Updated exposure-response relationship between road traffic noise and coronary heart diseases: A meta-analysis. Noise Health 2014;16:1–9. [DOI] [PubMed] [Google Scholar]
- Babisch W, Fromme H, Beyer A, et al. Increased catecholamine levels in urine in subjects exposed to road traffic noise: The role of stress hormones in noise research. Environ Int 2001;26:475–481. [DOI] [PubMed] [Google Scholar]
- Baldus S, Heitzer T, Eiserich JP, et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med 2004;37:902–911. [DOI] [PubMed] [Google Scholar]
- Bayo Jimenez MT, Frenis K, Hahad O, et al. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors. Free Radic Biol Med 2022;187:72–91. [DOI] [PubMed] [Google Scholar]
- Bayo Jimenez MT, Frenis K, Kroller-Schon S, et al. Noise-induced vascular dysfunction, oxidative stress, and inflammation are improved by pharmacological modulation of the NRF2/HO-1 axis. Antioxidants (Basel) 2021;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol 1996;271:C1424–C1437. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Ye YZ, Anderson PG, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler 1994;375:81–88. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Douglas G, McNeill E, et al. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 2014;20:3040–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenboom G, Fontaine D, Unger P, et al. Absence of nitrate tolerance after long-term treatment with ramipril: An endothelium-dependent mechanism. J Cardiovasc Pharmacol 1999;34:547–553. [DOI] [PubMed] [Google Scholar]
- Bode-Boger SM, Scalera F, Ignarro LJ. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 2007;114:295–306. [DOI] [PubMed] [Google Scholar]
- Boger RH. Association of asymmetric dimethylarginine and endothelial dysfunction. Clin Chem Lab Med 2003a;41:1467–1472. [DOI] [PubMed] [Google Scholar]
- Boger RH. When the endothelium cannot say ‘NO’ anymore. ADMA, an endogenous inhibitor of NO synthase, promotes cardiovascular disease. Eur Heart J 2003b;24:1901–1902. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Wu W, Fu X, et al. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 2002;277:17415–17427. [DOI] [PubMed] [Google Scholar]
- Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res 2005;65:823–831. [DOI] [PubMed] [Google Scholar]
- Chen CA, de Pascali F, Basye A, et al. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry 2013;52:6712–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Lin CH, Druhan LJ, et al. Superoxide induces eNOS protein Thiyl radical formation: A novel mechanism regulating eNOS function and coupling. J Biol Chem 2011;286:29098–29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010;468:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A 2002;99:8306–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs EI, Ostad MA, Simon A, et al. Impairment of the extrusion transporter for asymmetric dimethyl-L-arginine: A novel mechanism underlying vasospastic angina. Biochem Biophys Res Commun 2012;423:218–223. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol 2000;20:2032–2037. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Brixey R, Batchelor H, et al. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem 2013;288:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JP, Beckman JS. Reaction between nitric oxide, superoxide, and peroxynitrite: Footprints of peroxynitrite in vivo. Adv Pharmacol 1995;35:17–43. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Manda G, Hassan A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol Rev 2018;70:348–383. [DOI] [PubMed] [Google Scholar]
- Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 2010;1797:897–906. [DOI] [PubMed] [Google Scholar]
- Daiber A, Chlopicki S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic Biol Med 2020;157:15–37. [DOI] [PubMed] [Google Scholar]
- Daiber A, di Lisa F, Oelze M, et al. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 2017a;174:1670–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Frenis K, Kuntic M, et al. Redox regulatory changes of circadian rhythm by the environmental risk factors traffic noise and air pollution. Antioxid Redox Signal 2022;37:679–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Hahad O, Andreadou I, et al. Redox-related biomarkers in human cardiovascular disease—Classical footprints and beyond. Redox Biol 2021;42:101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Kroller-Schon S, Frenis K, et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction—Signatures of the internal exposome. Biofactors 2019a;45:495–506. [DOI] [PubMed] [Google Scholar]
- Daiber A, Kroller-Schon S, Oelze M, et al. Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases. Redox Biol 2020a;34:101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Lelieveld J, Steven S, et al. Traffic-Related Environmental Risk Factors and Their Impact on Oxidative Stress and Cardiovascular Health. In: Oxidative Stress—Eustress and Distress. (Sies H. ed.) London, UK: Academic Press; 2020b:489–510. [Google Scholar]
- Daiber A, Munzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: Emphasis on redox biology and oxidative stress. Antioxid Redox Signal 2015;23:899–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Oelze M, August M, et al. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res 2004a;38:259–269. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Coldewey M, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: A comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol 2004b;66:1372–1382. [DOI] [PubMed] [Google Scholar]
- Daiber A, Oelze M, Daub S, et al. Vascular Redox Signaling, Redox Switches in Endothelial Nitric Oxide Synthase and Endothelial Dysfunction. In: Systems Biology of Free Radicals and Antioxidants. (Laher I. ed.) Berlin Heidelberg: Springer-Verlag; 2014. [Google Scholar]
- Daiber A, Oelze M, Steven S, et al. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox Biol 2017b;12:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Steven S, Vujacic-Mirski K, et al. Regulation of vascular function and inflammation via cross talk of reactive oxygen and nitrogen species from mitochondria or NADPH oxidase—Implications for diabetes progression. Int J Mol Sci 2020c;21;3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Steven S, Weber A, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 2017c;174:1591–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Ullrich V. Radikalchemie im Organismus: Stickstoffmonoxid, Superoxid und Peroxynitrit. Chemie in unserer Zeit 2002;36:366–375. [Google Scholar]
- Daiber A, Xia N, Steven S, et al. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci 2019b;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S, Fink B, Skatchkov M, et al. Formation of reactive oxygen species in various vascular cells during glyceryltrinitrate metabolism. J Cardiovasc Pharmacol Ther 1998;3:51–62. [DOI] [PubMed] [Google Scholar]
- Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007;49:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 2010a;107:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Gongora MC, Harrison DG, et al. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 2010b;299:H673–H679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008;102:488–496. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, et al. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol 2001;280:H528–H534. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Milstien S, Richardson D, et al. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 2003;92:88–95. [DOI] [PubMed] [Google Scholar]
- Eckrich J, Frenis K, Rodriguez-Blanco G, et al. Aircraft noise exposure drives the activation of white blood cells and induces microvascular dysfunction in mice. Redox Biol 2021;46:102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002;296:2391–2394. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, et al. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 2001;88:E68–E75. [DOI] [PubMed] [Google Scholar]
- Foraster M, Eze IC, Schaffner E, et al. Exposure to road, railway, and aircraft noise and arterial stiffness in the SAPALDIA study: Annual average noise levels and temporal noise characteristics. Environ Health Perspect 2017;125:097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006;113:1708–1714. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Schmidt HH, Pollock JS, et al. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol 1991;42:1849–1857. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J 2012;33:829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenis K, Helmstadter J, Ruan Y, et al. Ablation of lysozyme M-positive cells prevents aircraft noise-induced vascular damage without improving cerebral side effects. Basic Res Cardiol 2021a;116:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenis K, Kalinovic S, Ernst BP, et al. Long-term effects of aircraft noise exposure on vascular oxidative stress, endothelial function and blood pressure: No evidence for adaptation or tolerance development. Front Mol Biosci 2021b;8:814921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenis K, Kuntic M, Hahad O, et al. Redox switches in noise-induced cardiovascular and neuronal dysregulation. Front Mol Biosci 2021c;8:784910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu A, Hayashi T, Miyazaki-Akita A, et al. Possible usefulness of apocynin, an NADPH oxidase inhibitor, for nitrate tolerance: Prevention of NO donor-induced endothelial cell abnormalities. Am J Physiol Heart Circ Physiol 2007;293:H790–H797. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF Jr., Hunter LM, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- Gori T, Burstein JM, Ahmed S, et al. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: A human in vivo study. Circulation 2001a;104:1119–1123. [DOI] [PubMed] [Google Scholar]
- Gori T, Dragoni S, di Stolfo G, et al. Tolerance to nitroglycerin-induced preconditioning of the endothelium: A human in vivo study. Am J Physiol Heart Circ Physiol 2010;298:H340–H345. [DOI] [PubMed] [Google Scholar]
- Gori T, Mak SS, Kelly S, et al. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J Am Coll Cardiol 2001b;38:1096–1101. [DOI] [PubMed] [Google Scholar]
- Gori T, Munzel T. Oxidative stress and endothelial dysfunction: Therapeutic implications. Ann Med 2011;43:259–272. [DOI] [PubMed] [Google Scholar]
- Gori T, Parker JD. Nitrate tolerance: A unifying hypothesis. Circulation 2002;106:2510–2513. [DOI] [PubMed] [Google Scholar]
- Gotoh N, Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochim Biophys Acta 1992;1115:201–207. [DOI] [PubMed] [Google Scholar]
- Grande MT, Pascual G, Riolobos AS, et al. Increased oxidative stress, the renin-angiotensin system, and sympathetic overactivation induce hypertension in kidney androgen-regulated protein transgenic mice. Free Radic Biol Med 2011;51:1831–1841. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Touyz RM, Zweier JL, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the American Heart Association. Circ Res 2016;119:e39–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahad O, Kuntic M, Frenis K, et al. Physical activity in polluted air-net benefit or harm to cardiovascular health? A comprehensive review. Antioxidants (Basel) 2021;10:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahad O, Prochaska JH, Daiber A, et al. Environmental noise-induced effects on stress hormones, oxidative stress, and vascular dysfunction: Key factors in the relationship between cerebrocardiovascular and psychological disorders. Oxid Med Cell Longev 2019;2019:4623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: Implications for impaired vasomotion. Am J Cardiol 1995;75:75B–81B. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Brockhoff C, Mayer B, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ Res 2000;86:E36–E41. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Finckh B, Albers S, et al. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: Relation to parameters of oxidative stress. Free Radic Biol Med 2001a;31:53–61. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Just H, Brockhoff C, et al. Long-term nitroglycerin treatment is associated with supersensitivity to vasoconstrictors in men with stable coronary artery disease: Prevention by concomitant treatment with captopril. J Am Coll Cardiol 1998;31:83–88. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996;94:6–9. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001b;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- Hemmingsen JG, Moller P, Jantzen K, et al. Controlled exposure to diesel exhaust and traffic noise—Effects on oxidative stress and activation in mononuclear blood cells. Mutat Res 2015;775:66–71. [DOI] [PubMed] [Google Scholar]
- Herzog J, Schmidt FP, Hahad O, et al. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol 2019;114:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink U, Oelze M, Kolb P, et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol 2003;42:1826–1834. [DOI] [PubMed] [Google Scholar]
- Hirai N, Kawano H, Yasue H, et al. Attenuation of nitrate tolerance and oxidative stress by an angiotensin II receptor blocker in patients with coronary spastic angina. Circulation 2003;108:1446–1450. [DOI] [PubMed] [Google Scholar]
- Ikejima H, Imanishi T, Tsujioka H, et al. Effect of pioglitazone on nitroglycerin-induced impairment of nitric oxide bioavailability by a catheter-type nitric oxide sensor. Circ J 2008;72:998–1002. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J Clin Invest 2003;111:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising H, Braun C. Acute and chronic endocrine effects of noise: Review of the research conducted at the Institute for Water, Soil and Air Hygiene. Noise Health 2000;2:7–24. [PubMed] [Google Scholar]
- Jabs A, Oelze M, Mikhed Y, et al. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vascul Pharmacol 2015;71:181–191. [DOI] [PubMed] [Google Scholar]
- Janiszewski M, Souza HP, Liu X, et al. Overestimation of NADH-driven vascular oxidase activity due to lucigenin artifacts. Free Radic Biol Med 2002;32:446–453. [DOI] [PubMed] [Google Scholar]
- Katz RJ. Mechanisms of nitrate tolerance: A review. Cardiovasc Drugs Ther 1990;4:247–252. [DOI] [PubMed] [Google Scholar]
- Kempen EV, Casas M, Pershagen G, et al. WHO Environmental Noise Guidelines for the European Region: A systematic review on environmental noise and cardiovascular and metabolic effects: A summary. Int J Environ Res Public Health 2018;15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle AJ, van Dalen CJ, Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep 1997;3:257–258. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Bingham S, Welch A, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: A prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001;357:657–663. [DOI] [PubMed] [Google Scholar]
- Khong SM, Andrews KL, Huynh NN, et al. Arginase II inhibition prevents nitrate tolerance. Br J Pharmacol 2012;166:2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: Comparison of angiotensin II and diazoxide. Hypertension 2005;45:438–444. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Milstien S, Wambi C, et al. Inhibition of tetrahydrobiopterin biosynthesis impairs endothelium-dependent relaxations in canine basilar artery. Am J Physiol 1997;273:H718–H724. [DOI] [PubMed] [Google Scholar]
- Kissner R, Nauser T, Bugnon P, et al. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol 1997;10:1285–1292. [DOI] [PubMed] [Google Scholar]
- Knorr M, Hausding M, Kroller-Schuhmacher S, et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: Beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler Thromb Vasc Biol 2011;31:2223–2231. [DOI] [PubMed] [Google Scholar]
- Kroller-Schon S, Daiber A, Steven S, et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J 2018;39:3528–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroller-Schon S, Steven S, Kossmann S, et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 2014;20:247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Urabe Y, Suzuki S, et al. Dissociation of vascular tolerance and plasma norepinephrine adjustment during long-term nitrate therapy in human coronary arteries. Intern Med 1999;38:773–779. [DOI] [PubMed] [Google Scholar]
- Kuhn V, Diederich L, Keller TCST, et al. Red blood cell function and dysfunction: Redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal 2017;26:718–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntic M, Kuntic I, Krishnankutty R, et al. Co-exposure to urban particulate matter and aircraft noise adversely impacts the cerebro-pulmonary-cardiovascular axis in mice. Redox Biol 2023;59:102580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz S, Hink U, Nickenig G, et al. Evidence for a causal role of the renin-angiotensin system in nitrate tolerance. Circulation 1999;99:3181–3187. [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278:22546–22554. [DOI] [PubMed] [Google Scholar]
- Kvandova M, Filippou K, Steven S, et al. Environmental aircraft noise aggravates oxidative DNA damage, granulocyte oxidative burst and nitrate resistance in Ogg1(−/−) mice. Free Radic Res 2020;54:280–292. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002;40:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Suessenbacher A, Wolkart G, et al. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol 2002;61:1081–1088. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001;103:1282–1288. [DOI] [PubMed] [Google Scholar]
- Lecour S, du Pre BC, Botker HE, et al. Circadian rhythms in ischaemic heart disease: Key aspects for preclinical and translational research: Position paper of the ESC working group on cellular biology of the heart. Cardiovasc Res 2022;118:2566–2581. [DOI] [PubMed] [Google Scholar]
- Leo F, Suvorava T, Heuser SK, et al. Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. Circulation 2021;144:870–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions. J Biol Chem 2005;280:13682–13693. [DOI] [PubMed] [Google Scholar]
- Lin MI, Fulton D, Babbitt R, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem 2003;278:44719–44726. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc Natl Acad Sci U S A 1997;94:2891–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu Y, Xu Q, et al. Critical role of vascular peroxidase 1 in regulating endothelial nitric oxide synthase. Redox Biol 2017;12:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuni A, Luca MC, di Stolfo G, et al. Coadministration of atorvastatin prevents nitroglycerin-induced endothelial dysfunction and nitrate tolerance in healthy humans. J Am Coll Cardiol 2011;57:93–98. [DOI] [PubMed] [Google Scholar]
- Loot AE, Schreiber JG, Fisslthaler B, et al. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 2009;206:2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Yamada H, Iwata K, et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005;112:2677–2685. [DOI] [PubMed] [Google Scholar]
- McCord JM, Keele BB Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc Natl Acad Sci U S A 1971;68:1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LT, Dixon L, Morgan DR, et al. Production of 8-epi prostaglandin F(2alpha) in human platelets during administration of organic nitrates. J Am Coll Cardiol 2002;40:820–825. [DOI] [PubMed] [Google Scholar]
- Mehra A, Ostrzega E, Shotan A, et al. Persistent hemodynamic improvement with short-term nitrate therapy in patients with chronic congestive heart failure already treated with captopril. Am J Cardiol 1992;70:1310–1314. [DOI] [PubMed] [Google Scholar]
- Mikhed Y, Fahrer J, Oelze M, et al. Nitroglycerin induces DNA damage and vascular cell death in the setting of nitrate tolerance. Basic Res Cardiol 2016;111:52. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochem Biophys Res Commun 1999;263:681–684. [DOI] [PubMed] [Google Scholar]
- Morioka S, Sakaguchi H, Yamaguchi T, et al. Hearing vulnerability after noise exposure in a mouse model of reactive oxygen species overproduction. J Neurochem 2018;146:459–473. [DOI] [PubMed] [Google Scholar]
- Munzel T, Afanasév IB, Kleschyov AL, et al. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol 2002;22:1761–1768. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Gori T. Nitrate therapy: New aspects concerning molecular action and tolerance. Circulation 2011;123:2132–2144. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Gori T. More answers to the still unresolved question of nitrate tolerance. Eur Heart J 2013;34:2666–2673. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Mulsch A.. Explaining the phenomenon of nitrate tolerance. Circ Res 2005a;97:618–628. [DOI] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Steven S, et al. Effects of noise on vascular function, oxidative stress, and inflammation: Mechanistic insight from studies in mice. Eur Heart J 2017a;38:2838–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Daiber A, Ullrich V, et al. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 2005b;25:1551–1557. [DOI] [PubMed] [Google Scholar]
- Munzel T, Giaid A, Kurz S, et al. Evidence for a role of endothelin 1 and protein kinase C in nitroglycerin tolerance. Proc Natl Acad Sci U S A 1995a;92:5244–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Gori T, Babisch W, et al. Cardiovascular effects of environmental noise exposure. Eur Heart J 2014a;35:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Kroller-Schon S, Oelze M, et al. Adverse cardiovascular effects of traffic noise with a focus on nighttime noise and the new WHO noise guidelines. Annu Rev Public Health 2020;41:309–328. [DOI] [PubMed] [Google Scholar]
- Munzel T, Li H, Mollnau H, et al. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res 2000a;86:E7–E12. [DOI] [PubMed] [Google Scholar]
- Munzel T, Mollnau H, Hartmann M, et al. Effects of a nitrate-free interval on tolerance, vasoconstrictor sensitivity and vascular superoxide production. J Am Coll Cardiol 2000b;36:628–634. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sayegh H, Freeman BA, et al. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest 1995b;95:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Schmidt FP, Steven S, et al. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018a;71:688–697. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sorensen M, Daiber A. Transportation noise pollution and cardiovascular disease. Nat Rev Cardiol 2021;18:619–636. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sorensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: Part II-mechanistic insights. Eur Heart J 2017b;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Sorensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: Part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017c;38:550–556. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sorensen M, Schmidt F, et al. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxid Redox Signal 2018b;28:873–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Steven S, Daiber A.. Organic nitrates: Update on mechanisms underlying vasodilation, tolerance and endothelial dysfunction. Vascul Pharmacol 2014b;63:105–113. [DOI] [PubMed] [Google Scholar]
- Oelze M, Knorr M, Kroller-Schon S, et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J 2013;34:3206–3216. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993;91:2546–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M. The clinical significance of nitrate tolerance in patients with chronic heart failure. Eur Heart J 1989;10(Suppl A):20–25. [DOI] [PubMed] [Google Scholar]
- Packer M, Lee WH, Kessler PD, et al. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med 1987;317:799–804. [DOI] [PubMed] [Google Scholar]
- Parker JD, Farrell B, Fenton T, et al. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation 1991;84:2336–2345. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001;104:191–196. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 2004;101:4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, et al. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 2002;33:1451–1464. [DOI] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, et al. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio A, Linares C, Banegas JR, et al. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: An integrative model of biological mechanisms. Environ Res 2016;146:359–370. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp Mol Med 1999;31:53–59. [DOI] [PubMed] [Google Scholar]
- Rousset F, Nacher-Soler G, Kokje VBC, et al. NADPH oxidase 3 deficiency protects from noise-induced sensorineural hearing loss. Front Cell Dev Biol 2022;10:832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph TK, Wipper S, Reiter B, et al. Myeloperoxidase deficiency preserves vasomotor function in humans. Eur Heart J 2012;33:1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said MA, El-Gohary OA. Effect of noise stress on cardiovascular system in adult male albino rat: Implication of stress hormones, endothelial dysfunction and oxidative stress. Gen Physiol Biophys 2016;35:371–377. [DOI] [PubMed] [Google Scholar]
- Sampson JB, Ye Y, Rosen H, et al. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys 1998;356:207–213. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Kolle K, Kreuder K, et al. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 2015a;104:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FP, Basner M, Kroger G, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 2013;34:3508–3514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Stocker R, Vollbracht C, et al. Antioxidants in Translational Medicine. Antioxid Redox Signal 2015b;23:1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Blankenberg S, Lubos E, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: Results from the AtheroGene Study. Circ Res 2005;97:e53–e59. [DOI] [PubMed] [Google Scholar]
- Schuhmacher S, Wenzel P, Schulz E, et al. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension 2010;55:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Jansen T, Wenzel P, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 2008;10:1115–1126. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tsilimingas N, Rinze R, et al. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation 2002;105:1170–1175. [DOI] [PubMed] [Google Scholar]
- Schulz E, Wenzel P, Munzel T, et al. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 2014;20:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CP, Kuo CY, Lin YY, et al. Inhibition of cochlear HMGB1 expression attenuates oxidative stress and inflammation in an experimental murine model of noise-induced hearing loss. Cells 2021;10:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. (ed.) Oxidative Stress: Oxidants and Antioxidants. Academic Press: London, United Kingdom; 1991. [Google Scholar]
- Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol 2015;4:180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol 2017;11:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994;78:931–936. [DOI] [PubMed] [Google Scholar]
- Steven S, Frenis K, Kalinovic S, et al. Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension. Redox Biol 2020;34:101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Huang A, Jeranian E, et al. Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler Thromb Vasc Biol 2004;24:2028–2033. [DOI] [PubMed] [Google Scholar]
- Suvorava T, Nagy N, Pick S, et al. Impact of eNOS-dependent oxidative stress on endothelial function and neointima formation. Antioxid Redox Signal 2015;23:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn TK, Hammer MS, Neitzel RL. Valuing quiet: An economic assessment of U.S. environmental noise as a cardiovascular health hazard. Am J Prev Med 2015;49:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow K, Daiber A, Oelze M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest 2004;113:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl 2003;4:41–51. [DOI] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Wenzel P, et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol 2007;42:1111–1118. [DOI] [PubMed] [Google Scholar]
- Tainio M, Andersen ZJ, Nieuwenhuijsen MJ, et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ Int 2021;147:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey MM, White CR, Suarez E, et al. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res 1999;84:1203–1211. [DOI] [PubMed] [Google Scholar]
- Thomas GR, Difabio JM, Gori T, et al. Continuous therapy with transdermal nitroglycerin does not affect biomarkers of vascular inflammation and injury in healthy volunteers. Can J Physiol Pharmacol 2009;87:455–459. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Milstien S, Katusic ZS. Effect of tetrahydrobiopterin on endothelial function in canine middle cerebral arteries. Circ Res 1996;79:336–342. [DOI] [PubMed] [Google Scholar]
- Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev 2002;54:619–634. [DOI] [PubMed] [Google Scholar]
- Ullrich V, Kissner R. Redox signaling: Bioinorganic chemistry at its best. J Inorg Biochem 2006;100:2079–2086. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Martasek P, Hogg N, et al. Electron spin resonance spin-trapping detection of superoxide generated by neuronal nitric oxide synthase. Methods Enzymol 1999;301:169–177. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Lin SC, Wong AC, et al. Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear Res 2013;304:145–152. [DOI] [PubMed] [Google Scholar]
- von Leitner EC, Klinke A, Atzler D, et al. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte-derived hemoprotein myeloperoxidase. Circulation 2011;124:2735–2745. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang Y, Dwivedi S, et al. Manipulating glutathione-S-transferases may prevent the development of tolerance to nitroglycerin. Cardiovasc Toxicol 2006;6:131–144. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kakihana M, Ohtsuka S, et al. Preventive effects of angiotensin-converting enzyme inhibitors on nitrate tolerance during continuous transdermal application of nitroglycerin in patients with chronic heart failure. Jpn Circ J 1998;62:353–358. [DOI] [PubMed] [Google Scholar]
- Webpage. Health Impact Assessment for Noise in Europe ETC/ACM Technical Paper 2014/9; 2014. Available from: https://www.eionet.europa.eu/etcs/etc-atni/products/etc-atni-reports/etcacm_tp_2014_9_hia-noise_europe [Last accessed: March 21, 2022].
- Webpage. WHO report “noise and health”; 2018. Available from: https://www.who.int/europe/publications/i/item/9789289053563 [Last accessed: March 21, 2022].
- Wenzel P, Kossmann S, Munzel T, et al. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol Med 2017;109:48–60. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Mollnau H, Oelze M, et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 2008;10:1435–1447. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Oelze M, Coldewey M, et al. Heme oxygenase-1: A novel key player in the development of tolerance in response to organic nitrates. Arterioscler Thromb Vasc Biol 2007;27:1729–1735. [DOI] [PubMed] [Google Scholar]
- Xia N, Daiber A, Habermeier A, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther 2010;335:149–154. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A 1997;94:6954–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelis R, Mason DT. Isosorbide dinitrate. Effect on the vasodilator response to nitroglycerin. JAMA 1975;234:166–170. [DOI] [PubMed] [Google Scholar]
- Zhang C, Reiter C, Eiserich JP, et al. L-arginine chlorination products inhibit endothelial nitric oxide production. J Biol Chem 2001;276:27159–27165. [DOI] [PubMed] [Google Scholar]
- Zhang GG, Shi RZ, Jiang DJ, et al. Involvement of the endothelial DDAH/ADMA pathway in nitroglycerin tolerance: The role of ALDH-2. Life Sci 2008;82:699–707. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, et al. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 2000;192:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Bachschmid M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J Exp Med 1999;190:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol 1999;154:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 2002;109:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]