Abstract
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are the latest addition to guideline-directed medical therapy in heart failure (HF) with reduced ejection fraction with recent trials suggesting a significant reduction in adverse cardiovascular outcomes in patients with HF with mildly reduced and preserved ejection fraction. SGLT-2 inhibitors have evolved as metabolic drugs due to their multi-system effects and are indicated for the management of HF across the ejection fraction spectrum, type 2 diabetes, and chronic kidney disease. There is ongoing research to explore the mechanistic effects of SGLT-2 inhibitors in HF and to evaluate their use in worsening HF and after myocardial infarction. This review focuses on the evidence for SGLT-2 inhibitors from type 2 diabetes cardiovascular outcome and primary HF trials and discusses ongoing research related to their use in cardiovascular disease.
Keywords: Sodium-glucose transporter 2 inhibitors, Heart failure, Systolic, Diastolic
INTRODUCTION
Traditionally known as anti-hyperglycemic drugs, sodium-glucose co-transporter 2 (SGLT-2) inhibitors have established themselves as a pivotal therapy for heart failure (HF) across the complete spectrum of left ventricular ejection fraction (LVEF). The therapeutic effect in HF of SGLT-2 inhibitors was initially signaled in the first cardiovascular outcome trial for empagliflozin in the management of type 2 diabetes mellitus (T2DM) in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients Removing Excess Glucose (EMPA-REG OUTCOME) trial1) in 2013 that revealed a significant reduction in the secondary endpoint of HF hospitalizations in the empagliflozin group compared to placebo. Further cardiovascular outcome trials further consolidated this evidence of a reduction in HF hospitalizations and cardiovascular death indicating a possible direct drug effect of SGLT-2 inhibitors in HF independent of its anti-hyperglycemic properties. This prompted several landmark clinical trials in HF irrespective of diabetes status, which affirmed the efficacy of SGLT-2 inhibitors for HF. In this review, we discuss the scope and the current evidence of SGLT-2 inhibitors in HF and the ongoing trials and prospects of this promising therapy in HF.
SGLT-2 INHIBITORS AS GLUCOSE-LOWERING AGENTS
SGLT-2 receptors are exclusively present on renal proximal convoluted tubules and are responsible for the reabsorption of almost all of the body’s glucose and the majority of sodium filtered in the glomerulus. The inhibition of this receptor caused a diuretic effect causing the excretion of both sodium and glucose, presenting a non-insulin-dependent way of reducing serum glucose levels, without increasing the risk of hypoglycemia. The first oral SGLT-2 inhibitor was found to reduce hyperglycemia in rats, indicating a potential role in the management of T2DM.2) Randomized clinical trials proved the efficacy of SGLT-2 inhibitors in causing a modest reduction in hemoglobin A1c of 0.5% to 1.1% and since the past 2 decades, empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin have been approved for use in T2DM. Similar to GLP1 agonists and DPP4 inhibitors, SGLT-2 inhibitors were required to undergo outcomes trials to ensure that the drugs did not increase the risk of cardiovascular disease, as mandated by the US Food and Drug Administration in 2008, after rosiglitazone, a promising thiazolidinedione was found to increase the risk of cardiovascular events.3)
CARDIOVASCULAR OUTCOME TRIALS IN TYPE 2 DIABETES
The EMPA-REG OUTCOME1) evaluated cardiovascular outcomes with empagliflozin in 7,020 patients with established cardiovascular disease with a median follow-up of 3.1 years. Empagliflozin was found to have a significant 14% (12.1% vs. 10.5%; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.74–0.99) reduction in the primary composite endpoint of major adverse cardiovascular events (cardiovascular mortality, nonfatal stroke, nonfatal myocardial infarction; MACE-3) compared to placebo, primarily attributed to a 38% significant reduction in cardiovascular death. All-cause mortality was also significantly reduced by 32% with empagliflozin (5.7% vs. 8.3%; HR, 0.68; 95% CI, 0.57–0.82]). In the context of HF, empagliflozin was found to significantly reduce HF hospitalizations by 35% (2.7% vs. 4.3%; HR, 0.65; 95% CI, 0.50–0.85). This finding was unexpected and required validation from other parallel studies. The Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes (CANVAS)4) and the Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes (DECLARE TIMI)5) trials reported similar findings with canagliflozin and dapagliflozin, respectively. The Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy (CREDENCE)6) trial primarily evaluated kidney-related outcomes with canagliflozin and found a significant 31% reduction in the secondary composite outcome of cardiovascular death and HF hospitalizations with canagliflozin compared to placebo as a secondary outcome. The Cardiovascular Outcomes With Ertugliflozin in Type 2 Diabetes (VERTIS) trial7) evaluated cardiovascular outcomes with ertugliflozin in a similar cohort of patients; the study did not find ertugliflozin to be superior to placebo in reducing the MACE-3 endpoint; however, a significant reduction in HF hospitalizations was reported in the ertugliflozin arm. A pooled meta-analysis of these trials8) revealed a significant reduction in MACE-3 events, all-cause mortality, cardiovascular deaths, and HF hospitalizations. The consistency of these findings, especially the reduction in HF hospitalizations indicated that SGLT-2 inhibitors may have an independent role in the management of HF.
HF WITH A REDUCED EJECTION FRACTION (HFrEF)
The findings of reduction in cardiovascular death and HF hospitalizations in cardiovascular outcome trials in patients with T2DM prompted further investigation of the drug’s potential role in the management of HF. The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial9) was the first study that evaluated the efficacy of an SGLT-2 inhibitor in patients with HFrEF. The trial enrolled 4,744 patients with stable, chronic HF with LVEF <40% that were followed over a period of 18 months. Dapagliflozin was found to significantly reduce the primary composite outcome of cardiovascular death, HF hospitalizations, and urgent HF visits compared to placebo (16.3% vs. 21.2%; HR, 0.74; 95% CI, 0.65–0.85). Moreover, all components of the primary composite outcome were individually found to be significantly reduced with dapagliflozin therapy, and the treatment effect was persistent independent of baseline diabetes status.10)
This landmark study was followed by the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trial11) that evaluated the efficacy of empagliflozin in a similar cohort, albeit with a lower mean LVEF of up to 27% vs. up to 31% in DAPA-HF. The trial enrolled 3,730 patients with a median follow-up of 16 months. Empagliflozin significantly reduced the composite outcome of cardiovascular death and HF hospitalizations compared to placebo with a relative risk reduction of 21% (19.4% vs. 24.7%; HR, 0.75; 95% CI, 0.65–0.86). The results were driven by a significant 31% relative risk reduction in HF hospitalizations (HR, 0.69; 95% CI, 0.59–0.81). A further pooled analysis from both these trials showed a significant reduction in all-cause mortality with a relative risk reduction of 13% (HR, 0.87; 95% CI, 0.77–0.98).12) Moreover, a significant reduction in the composite endpoint of cardiovascular death and HF hospitalizations was observed in the treatment group (HR, 0.74; 95% CI, 0.68–0.82) with a 14% reduction in cardiovascular death (HR, 0.86; 95% CI, 0.76–0.98) (Table 1).
Table 1. Summary of trials that evaluated the use of sodium-glucose co-transporter 2 inhibitors in heart failure.
| Study | Primary endpoint | Study drug | Number | Inclusion criteria | Median follow-up (months) | Outcomes | |
|---|---|---|---|---|---|---|---|
| HF with reduced ejection fraction | |||||||
| DAPA-HF | Primary composite outcome of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or death from cardiovascular causes | Dapagliflozin | 4,744 | Adults (≥18 years of age), LVEF of 40% or less, and NYHA class II, III, or IV symptoms | 18 | Reduction in the primary composite outcome in the dapagliflozin group (HR, 0.74; 95% CI, 0.65–0.85) | |
| Reduction in total HF hospitalizations in the dapagliflozin group (HR, 0.70; 95% CI, 0.59–0.83) | |||||||
| Reduction in death from cardiovascular causes in the dapagliflozin group (HR, 0.82; 95% CI, 0.69–0.98) | |||||||
| EMPEROR-Reduced | Primary composite outcome of death from cardiovascular causes or hospitalization for HF (first or recurrent) | Empagliflozin | 3,730 | Adults (≥18 years of age), LVEF of 40% or less, and NYHA class II, III, or IV symptoms | 16 | Reduction in the primary composite outcome in the empagliflozin group (HR, 0.75; 95% CI, 0.65–0.86) | |
| Reduction in the first hospitalization for HF in the empagliflozin group (HR, 0.69; 95% CI, 0.59–0.81) | |||||||
| No difference in death from cardiovascular causes (HR, ;95% CI, 0.75–1.12) | |||||||
| HF with preserved ejection fraction | |||||||
| EMPEROR-Preserved | Primary composite outcome of death from cardiovascular causes or hospitalization for HF (first or recurrent) | Empagliflozin | 5,988 | Adults (≥18 years of age), LVEF of more than 40%, and NYHA class II, III, or IV symptoms | 26 | Reduction in the primary composite outcome in the empagliflozin group (HR, 0.79; 95% CI, 0.69–0.90) | |
| Reduction in total HF hospitalizations in the empagliflozin group and (HR, 0.71; 95% CI, 0.60–0.83) | |||||||
| No difference in death from cardiovascular causes (HR, 0.91; 95% CI, 0.76–1.09) | |||||||
| DELIVER | Primary composite outcome of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or death from cardiovascular causes | Dapagliflozin | 10,584 | Adults (≥18 years of age), LVEF of more than 40%, and NYHA class II, III, or IV symptoms, including patients with improved LVEF | 28 | Reduction in the primary composite outcome in the dapagliflozin group (HR, 0.82; 95% CI, 0.73–0.92) | |
| Reduction in worsening HF events in the dapagliflozin group (HR, 0.79; 95% CI, 0.69–0.91) | |||||||
| No difference in death from cardiovascular causes (HR, 0.88; 95% CI, 0.74–1.05) | |||||||
| WHF | |||||||
| SOLOIST-WHF | Total number of deaths from cardiovascular causes and hospitalizations and urgent visits for HF (first and subsequent) | Sotagliflozin | 1,222 | Adults 18 to 85 years of age with type 2 diabetes hospitalized for worsening HF and received treatment with intravenous diuretic therapy | 9 | Reduction in the primary end-point events in the sotagliflozin group (HR, 0.67; 95% CI, 0.52–0.85) | |
| EMPULSE | Hierarchical composite outcome of time to all-cause death, the number of HF exacerbations, time to first HF exacerbation, and a 5 point or greater difference in change from baseline in KCCQ-TSS after 90 days of treatment | Empagliflozin | 530 | Adults (≥18 years of age who were hospitalized with worsening HF with characteristics signs and symptoms of volume overload | 3 | Empagliflozin was superior in 53.9% of paired comparisons and placebo was superior in 39.7%, whereas 6.4% of comparisons were tied (win ratio 1.36; 95% CI, 1.09–1.68) | |
DAPA-HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR-Reduced = Empagliflozin Outcome Trial in Patients With Chronic Heart Failure and a Reduced Ejection Fraction; EMPEROR-Preserved = Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; DELIVER = Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure; SOLOIST-WHF = Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure; EMPULSE = The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute Heart Failure: A Multinational Randomized Trial; HF = heart failure; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire – Total Summary Score; NYHA = New York Heart Association, LVEF = left ventricular ejection fraction; HR = hazard ratio; CI = confidence interval; WHF = worsening heart failure.
HF WITH A PRESERVED EJECTION FRACTION (HFpEF)
HFpEF is a more complex phenotype of HF than HFrEF, which is characterized by diastolic dysfunction commonly seen in the geriatric population with concomitant cardio-renal-metabolic co-morbidities, constitutes nearly half of the total HF burden, and unlike HFrEF has limited treatment options. The Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial13) was the first trial to indicate that SGLT-2 inhibitors may have a benefit in reducing cardiovascular events in patients with HFpEF and T2DM. The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved) trial14) was the first study that exclusively evaluated the efficacy of SGLT-2 inhibitors (empagliflozin) in patients with HF with mildly reduced (HFmrEF) and HFpEF irrespective of patient’s diabetes status. The trial enrolled a total of 5,988 patients with LVEF >40% with New York Heart Association (NYHA) II and NYHA III symptoms. The results were similar to those reported in EMPEROR-Reduced; empagliflozin reduced the primary composite outcome of cardiovascular death and HF hospitalizations by 19% (13.8% vs. 17.1%; HR, 0.79; 95% CI, 0.69–0.90), driven by a significant 27% risk reduction in HF hospitalizations. The reduction in the primary endpoint was observed as early as 18 days after drug initiation.15) Of note, attenuation of treatment effect was observed at LVEF >60% (HR, 0.87; 95% CI, 0.69–1.10) in the trial indicating that the drug possibly is more efficacious at the lower end of the normal LVEF spectrum in HFpEF.16) The reduction in the primary endpoint was consistent regardless of baseline diabetes status.17)
The Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial18) evaluated the efficacy of dapagliflozin in patients with HFmrEF and HFpEF. The trial was similar in design to EMPEROR-Preserved except that the inclusion criteria were broadened to include patients with improved LVEF (patients that had a reduced LVEF but had improved to LVEF >40% with medical therapy). The primary composite endpoint comprised of unplanned HF hospitalization, urgent visits for HF, and cardiovascular death. A total of 6,263 patients were enrolled with a median duration of follow-up of 2.3 years. Dapagliflozin was found to significantly reduced the primary composite endpoint by 18% compared to placebo (16.4% vs. 19.5%; HR, 0.82; 95% CI, 0.73–0.92), driven largely by a significant 21% reduction in HF hospitalizations and urgent visits for HF (11.8% vs. 14.5%; HR, 0.79; 95% CI, 0.69–0.91) (Table 1). The reduction in the primary endpoint was consistent across patients with LVEF ≥60% (HR, 0.78; 95% CI, 0.62–0.98), in contrast to what was observed in the EMPEROR-Preserved trial.
NON-SELECTIVE SGLT INHIBITION
Sotagliflozin is a non-selective SGLT-1 and SGLT-2 inhibitor. The SGLT-1 receptor is primarily located in the intestines and is responsible for mucosal sodium and glucose absorption from intestinal contents. Sotagliflozin was evaluated for early initiation following an HF hospitalization in patients with T2DM in the SOLOIST-WHF trial. This trial differed from other HF cardiovascular outcome trials in several ways; it only enrolled patients with T2DM and those with a recent worsening HF (WHF) event. Patients were randomized to treatment and placebo close to discharge from the hospital (up to 49% before and 51% within 2 days of discharge). The trial enrolled 1,222 patients that were followed for a median of 9 months before the trial ended early due to loss of funding from the sponsor. There was a 33% reduction in the primary endpoint of cardiovascular death, HF hospitalizations, and urgent HF visits compared to placebo (51.0% vs. 76.3%; HR, 0.67; 95% CI, 0.52–0.85), primarily driven by a significant reduction in WHF events (HR, 0.64; 95% CI, 0.48–0.83). This effect was observed across the complete spectrum of LVEF with statistical significance in the primary endpoint observed in both LVEF ≤50% and LVEF >50% on subgroup analysis.
The Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial19) evaluated cardiovascular outcomes with sotagliflozin in 10,584 patients at increased risk of cardiovascular events over a median follow-up of up to 14 months. The trial was initially planned for a co-primary endpoint of MACE-3 events and time to first cardiovascular death and HF hospitalization. However, the primary endpoint was later changed to a composite endpoint of total cardiovascular deaths, HF hospitalizations, and urgent HF visits due to low recruitment because of the coronavirus disease 2019 (COVID-19) pandemic. The drug was found to significantly reduce the risk of occurrence of the primary endpoint compared to placebo (5.6 events per 100 person-years [PY] vs. 7.5 events per 100 PY; HR, 0.74; 95% CI, 0.63–0.88), driven by a significant reduction in HF hospitalizations and urgent HF visits (HR, 0.67; 95% CI, 0.55–0.82). Although there was no significant difference in the overall number of adverse events between sotagliflozin and placebo (23.4% to 25.2%), patients who received sotagliflozin had a higher rate of diarrhea, diabetic ketoacidosis, genital mycotic infections, and volume depletion.
Sotagliflozin is not yet approved by the FDA for use in HF and the trials experienced serious issues due to the COVID-19 pandemic resulting in trials ending early with lower-than-anticipated numbers and modified primary endpoints. The trials did show a signal for efficacy of sotagliflozin, and it may have a future role in HF management.
WHF
Findings from major trials in chronic stable HF prompted interest in the efficacy of empagliflozin in acute decompensated HF. The SOLOIST-WHF trial13) evaluated the effect of early initiation (before or shortly after discharge) of sotagliflozin following an episode of decompensated HF on cardiovascular outcomes in patients with T2DM and has been described earlier. The The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute HF: A Multinational Randomized Trial (EMPULSE) trial20) evaluated the effect of empagliflozin initiation following an episode of decompensated HF (randomization at 24 hours of admission and empagliflozin given a median of 3 days after admission) across the LVEF spectrum irrespective of the presence of T2DM. The study of 530 participants reported a significant clinical benefit, defined as a hierarchical composite of death from any cause, the number of HF events and time to first HF event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score, of in-hospital initiation of empagliflozin after 90 days of therapy (win ratio: 1.35; 95% CI, 1.09–1.68]) (Table 1). The Efficacy and Safety of Dapagliflozin in Acute Heart Failure (DICTATE-AHF) trial21) is another ongoing trial that is evaluating the impact of in-hospital initiation of dapagliflozin in patients hospitalized for acute decompensated HF.
SAFETY EVENTS
Several adverse events have been reported with varying prevalence across large SGLT-2 inhibitor trials. There were initial concerns about the increased risk of hypoglycemia and urinary tract infections, but a significant increase in these 2 adverse events has not been reported across several trials.22,23) SGLT-2 do increase the risk of genital mycotic infections, but the absolute risk is low and considering the benefit with these drugs and the easily treatable nature of genital mycotic infections, the recommendations are to continue treatment, unless these infections are recurrent.1,11) Fournier’s gangrene, defined as necrotizing fasciitis of perineal soft issues, has also been reported as a rare adverse effect24) and has been identified as a safety warning.25) Moreover, a recent meta-analysis revealed a greater than 2-fold increased risk of diabetic ketoacidosis (DKA) with SGLT-2 inhibitors and that has been attributed to its effect on suppression of insulin secretion and increased ketogenesis.26) However, the baseline risk of DKA was low across all trials (0.2 per 1,000 patient years in the combined placebo group) and simple measures to stop the SGLT-2 inhibitor use in patients on insulin or secretagogues if their food intake or medications are altered (e.g., surgery or diarrheal illness) can further mitigate this risk. SGLT-2 inhibitors can lead to volume depletion due to its natriuretic and diuretic effect, and gastrointestinal effects in case of non-selective SGLT inhibitors,19) and may require dose adjustment of other diuretic medications.
SGLT-2 INHIBITORS AS METABOLIC DRUGS AND POSSIBLE MECHANISMS OF ACTION
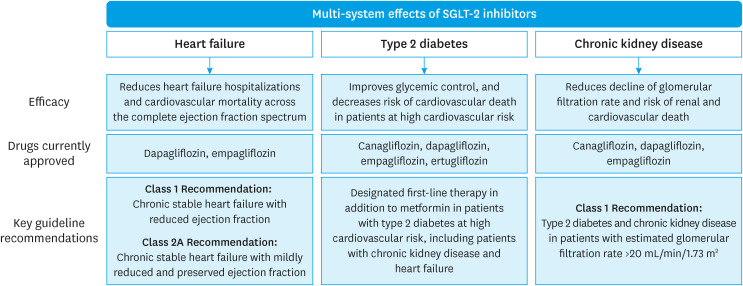
Apart from their role in HF management, SGLT-2 inhibitors have emerged as novel therapies for the management of patients with chronic kidney disease. The efficacy was initially observed in the CREDENCE trial which primarily evaluated the reno-protective effect of SGLT-2 inhibitors where canagliflozin was found to significantly reduce major cardiovascular and renal events in patients with T2DM. Since then, these findings have been further validated by Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD)27) and The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY)28) trials, demonstrating similar benefit irrespective of T2DM. Given its wide-ranging effects on multiple organ systems including the renal, cardiac, and endocrine systems, SGLT-2 are the pioneer metabolic drugs (Figure 1). SGLT-2 inhibition leads to only a modest reduction in hemoglobin A1c when used for the management of T2DM; however, it still leads to a significant reduction in cardiovascular outcomes in these patients which indicates that adverse cardiovascular events in T2DM may not be solely mediated by dysglycemia. Moreover, SGLT-2 inhibitors are the only drug effective across the entire LVEF spectrum of HF, through postulated mechanisms that are not limited to the diuretic or natriuretic effects of the drug.
Figure 1. The spectrum of clinical indications of SGLT-2 inhibitors.
SGLT-2 = sodium-glucose co-transporter 2.
There is ongoing research on the totality of metabolic targets of SGLT-2 inhibition.29) It was initially postulated that SGLT-2 inhibitors increase fasting serum ketone levels which act as an additional substrate for myocytes but was not supported by experimental data.30,31) Another hypothesis currently under investigation is the inhibition of the sodium-hydrogen exchanger-1, a potent receptor in the renal proximal tubule and the myocyte membrane.32) It is involved in the pathophysiology of both diabetes and HF where it sequesters toxic levels of calcium inside cells leading to myocardial dysfunction and is also involved in the pathophysiology of glomerular hypertension. The inhibition of sympathetic nerve trafficking to the kidneys and reduction in glomerular hypertension, which plays a role in the pathophysiology of HF and chronic kidney disease, has also been postulated for the drug’s multi-system effects.33) Stimulation of erythropoiesis as an indirect effect of SGLT-2 inhibition has been identified as a potential beneficial mechanism. This is thought to be caused by renal medullary hypoxia secondary to enhanced active reabsorption of sodium in the distal convoluted tubule leading to production of hypoxia inducible factors which in turn cause release of erythropoietin. The resultant effect is an increase in red cell mass, marked by an elevation in hematocrit, which improves oxygen delivery to the myocardium and reduces left ventricular mass.34,35) There has also been a recent interest in the evaluation of circulating proteomics with SGLT-2 inhibition which may provide further evidence for the biomolecular targets of these drugs.36) There is a possible role of SGLT-2 inhibitors in enhancing autophagic flux, a process that cells utilize to destroy impaired cells or organelles (e.g., mitochondria), nutrition deprivation signaling and attenuating reactive oxygen species causing a pronounced anti-oxidant effect.37,38) The research must remain ongoing as physician practice is driven by the understanding of the drug's mechanism of action, a better understanding of which would allow for SGLT-2 inhibitors to transition from primarily glucose-lowering medications to cardiovascular disease-modifying drugs for HF.
CURRENT PRACTICE GUIDELINES FOR HF
The American Diabetes Association (ADA) recommends the addition of SGLT-2 inhibitors in the management of T2DM with HF or patients at high risk of HF.39) The most recent European Society of Cardiology (ESC) 202140) and the American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) 202241) HF guidelines recommend the use of SGLT-2 inhibitors (currently only dapagliflozin and empagliflozin) in the management of chronic, stable HFrEF (class 1 recommendation for a reduction in cardiovascular death and HF hospitalizations) irrespective of baseline diabetes status. The AHA/ACC/HFSA HF guidelines 2022 provide a class 2A recommendation for HFmrEF and HFpEF based on findings of the EMPEROR-Preserved trial, which may be upgraded in future guidelines following the publication of the DELIVER trial. The ESC 2021 guidelines were published before the publication of the EMPEROR-Preserved and DELIVER trials and hence does not address use of SGLT-2 inhibitors in HFmrEF and HFpEF. The drug is prescribed as part of a single dose daily regimen (10 mg for both dapagliflozin and empagliflozin) with no need for dose titration and no routine laboratory monitoring required. Experts advocate for the initiation of SGLT-2 inhibitors in those hospitalized for HF as part of an aggressive approach of rapid, simultaneous optimization of medical therapies,42,43) given that the drug takes less than a month to incur clinical benefit, and has a signal of benefit in patients with recent WHF.
FUTURE DIRECTIONS
The scope of SGLT-2 inhibitor use in cardiovascular disease is currently being further explored beyond its efficacy in HF. Experimental studies have revealed a possible cardioprotective effect of SGLT-2 inhibitors after myocardial infarction. The Empagliflozin in Patients With Acute Myocardial Infarction (EMMY) trial34) reported a significant reduction in serum NT-proBNP levels over 26 weeks with empagliflozin versus placebo in patients that underwent randomization 72 hours after percutaneous coronary intervention. There are ongoing trials, Empagliflozin on Hospitalization for Heart Failure and Mortality in Patients With Acute Myocardial Infarction (EMPACT-MI)44) and Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack (DAPA-MI),45) that is evaluating the effect of SGLT-2 inhibitors on cardiovascular outcomes in patients after myocardial infarction.
GLP-1 agonist is another glucose-lowering drug class that has been effective in decreasing the risk of cardiovascular events in high-risk patients with T2DM. The drug class does not have a similar effect on HF as SGLT-2 inhibitors, but these medications cause significant weight loss and have reno-protective effects. The ADA recommends the use of GLP-1R agonists in patients with T2DM at high risk for cardiovascular events, an indication similar to that for SGLT-2 inhibitors. There is current interest in the use of simultaneous GLP-1R agonist and SGLT-2 inhibitor therapy and if dual antagonism of multiple pathological metabolic pathways would result in a synergistic effect and confer greater benefits. Both these classes of medications do not increase the risk of hypoglycemia, and 2 recent trials, Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With T2DM Inadequately Controlled With Metformin Monotherapy (DURATION-8)46) and Dulaglutide as Add-on Therapy to SGLT2 Inhibitors in Patients With Inadequately Controlled Type 2 Diabetes (AWARD-10),47) were found to be effective in achieving glycemic control with no significant increase in adverse events when co-administered.
CONCLUSIONS
SGLT-2 inhibitors are the most recent addition to drug therapies that reduce morbidity and mortality in HF and are effective across the spectrum of LVEF. The mechanisms underlying these benefits are not well-established, with current ongoing research suggesting a vast number of metabolic and biomolecular targets that may play a role in incurring a cardio-renal-metabolic effects in HF. The efficacy of these drugs extends across T2DM and chronic kidney disease, and it is imperative to ensure appropriate implementation of SGLT-2 inhibitor therapy use across these populations to improve event-free survival and reduce associated healthcare costs.
Footnotes
Funding: Dr. Anker reported grants from Abbott Vascular and Vifor International and personal fees from Abbott Vascular, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bioventrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Edwards, Impulse Dynamics, Janssen, Novartis, Occlutech, Respicardia, Servier, Thermo Fisher Scientific, Vectorious, Vifor, and V-Wave outside the submitted work. Dr Butler reported personal fees from Abbott, Adrenomed, American Regent, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Cardior, CVRx, Eli Lilly and Company, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Medtronic, Merck, Novartis, Novo Nordisk, Occlutech, Pfizer, Relypsa, Roche, Sanofi, Sequana Medical, and Vifor for consulting outside the submitted work.
Conflict of Interest: The authors have no financial conflicts of interest.
- Data curation: Talha KM, Anker SD.
- Formal analysis: Talha KM.
- Writing - original draft: Talha KM, Anker SD, Butler J.
- Writing - review & editing: Butler J, Anker SD.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Yasuda K, Okamoto Y, et al. T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats. Metabolism. 2000;49:990–995. doi: 10.1053/meta.2000.7729. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 8.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 11.Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 14.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Siddiqi TJ, Filippatos G, et al. Early benefit with empagliflozin in heart failure with preserved ejection fraction: insights from the EMPEROR-Preserved trial. Eur J Heart Fail. 2022;24:245–248. doi: 10.1002/ejhf.2420. [DOI] [PubMed] [Google Scholar]
- 16.Butler J, Packer M, Filippatos G, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippatos G, Butler J, Farmakis D, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation. 2022;146:676–686. doi: 10.1161/CIRCULATIONAHA.122.059785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon SD, McMurray JJ, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 20.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox ZL, Collins SP, Aaron M, et al. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE-AHF trial. Am Heart J. 2021;232:116–124. doi: 10.1016/j.ahj.2020.10.071. [DOI] [PubMed] [Google Scholar]
- 22.Mascolo A, Di Napoli R, Balzano N, et al. Safety profile of sodium glucose co-transporter 2 (SGLT2) inhibitors: a brief summary. Front Cardiovasc Med. 2022;9:1010693. doi: 10.3389/fcvm.2022.1010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 24.Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med. 2019;170:764–769. doi: 10.7326/M19-0085. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration. FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes [Internet] Silver Spring: US Food and Drug Administration; 2018. [cited 2023 March 10]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes. [Google Scholar]
- 26.Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 28.The EMPA-KIDNEY Collaborative Group. Herrington WG, Staplin N, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146:1383–1405. doi: 10.1161/CIRCULATIONAHA.122.061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Xie M, Li Q, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128:232–245. doi: 10.1161/CIRCRESAHA.120.317933. [DOI] [PubMed] [Google Scholar]
- 31.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 32.Katsurada K, Nandi SS, Sharma NM, Patel KP. Enhanced expression and function of renal SGLT2 (sodium-glucose cotransporter 2) in heart failure: role of renal nerves. Circ Heart Fail. 2021;14:e008365. doi: 10.1161/CIRCHEARTFAILURE.121.008365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hare GM, Zhang Y, Chin K, et al. Impact of sodium glucose linked cotransporter-2 inhibition on renal microvascular oxygen tension in a rodent model of diabetes mellitus. Physiol Rep. 2021;9:e14890. doi: 10.14814/phy2.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazer CD, Hare GM, Connelly PW, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141:704–707. doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 36.Zannad F, Ferreira JP, Butler J, et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur Heart J. 2022;43:4991–5002. doi: 10.1093/eurheartj/ehac495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/reperfusion in nondiabetic rats. Oxid Med Cell Longev. 2022;2022:1197061. doi: 10.1155/2022/1197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C, Wang W, Zhong J, et al. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol. 2018;152:45–59. doi: 10.1016/j.bcp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2020 . Diabetes Care. 2020;43:S98–110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 40.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 41.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 42.Packer M, McMurray JJ. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23:882–894. doi: 10.1002/ejhf.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure-optimizing therapy with the need for speed. JAMA Cardiol. 2021;6:743–744. doi: 10.1001/jamacardio.2021.0496. [DOI] [PubMed] [Google Scholar]
- 44.Harrington J, Udell JA, Jones WS, et al. Empagliflozin in patients post myocardial infarction rationale and design of the EMPACT-MI trial. Am Heart J. 2022;253:86–98. doi: 10.1016/j.ahj.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 45.AstraZeneca. Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack. Bethesda: U.S. National Library of Medicine; 2023. [Google Scholar]
- 46.Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 47.Ludvik B, Frías JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]