Abstract
The Korean Society of Heart Failure (KSHF) guidelines aim to provide physicians with evidence-based recommendations for the management of patients with heart failure (HF). After the first introduction of the KSHF guidelines in 2016, newer therapies for HF with reduced ejection fraction, HF with mildly reduced ejection fraction, and HF with preserved ejection fraction have since emerged. The current version has been updated based on international guidelines and research data on Korean patients with HF. Herein, we present Part II of these guidelines, which comprises treatment strategies to improve the outcomes of patients with HF.
Keywords: Heart failure, Guideline, Treatment, Pharmacotherapy
INTRODUCTION
Globally, heart failure (HF) is a major public health issue that involves high medical costs. In Korea, the prevalence of patients with HF is 2.25%, and given the increasing older adult population, the burden of HF is expected to rise. Since the introduction of the Korean guidelines for the diagnosis and management of chronic HF in March 2016,1) newer strategies have emerged to improve outcomes of patients with HF. Angiotensin receptor-neprilysin inhibitors (ARNI) is more beneficial for patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with mildly reduced ejection fraction (HFmrEF). Sodium-glucose co-transporter 2 (SGLT2) inhibitors were effective in improving the prognosis of patients with HF, irrespective of the left ventricular (LV) ejection fraction (EF). Furthermore, tafamidis has demonstrated clinical benefit in patients with cardiac transthyretin amyloidosis (ATTR). This article aims to provide the most up-to-date evidence to improve outcomes in patients with HF and assist shared decision making in clinical practice. The current guidelines have been established based on previous HF research in the Korean population and international guidelines with a focus on providing the best possible care for patients with HF.2,3,4,5)
PHARMACOTHERAPY
HFrEF
Treatment algorithm of HFrEF
1. In patients with HFrEF, ARNI or angiotensin-converting enzyme inhibitors (ACEI) (or angiotensin receptor blockers [ARBs], in case of intolerance), beta-blockers, mineralocorticoid receptor antagonists (MRA; aldosterone antagonists), and SGLT2 inhibitors are the standard of care for reducing cardiovascular mortality and HF hospitalization. (Class I, LOE A)
2. Even if HF symptoms improve after the guideline directed medical therapy (GDMT) and LVEF improves to >40%, maintaining the GDMT is recommended. (Class I, LOE B)
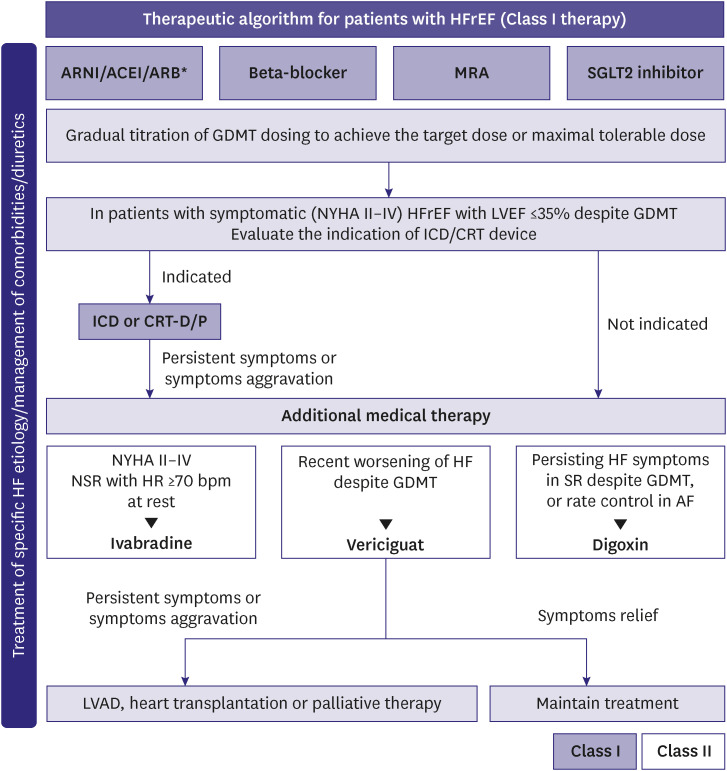
The key treatment goals for patients with HFrEF are as follows: 1) reduced mortality rate; 2) reduced readmissions due to worsening HF; and 3) improved functional clinical status and quality of life.4,5,6,7) The typical treatment strategies to achieve these goals are illustrated in Figure 1.
Figure 1. Therapeutic algorithm for HFrEF.
HFrEF = heart failure with reduced ejection fraction; ARNI = angiotensin receptor-neprilysin inhibitors; ACEI = angiotensin converting enzyme inhibitors; ARB = angiotensin receptor blocker; MRA = mineralocorticoid receptor antagonists; SGLT2 = sodium-glucose co-transporter 2; GDMT = guideline directed medical therapy; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; ICD = implantable cardioverter-defibrillator; CRT-D/P = cardiac resynchronization therapy-defibrillator/pacemaker; NSR = normal sinus rhythm; HR = heart rate; HF = heart failure; SR = sinus rhythm; AF = atrial fibrillation; LVAD = left ventricular assist device.
*If patients with chronic HFrEF are intolerant to ACEI because of cough or angioedema and when the use of ARNI is not feasible, the use of ARB is recommended to reduce morbidity and mortality.
Patients with symptoms and signs of HF and LVEF ≤40% may be classified as HF with recovered EF in the following scenarios: if the EF is >40% on post-treatment follow-up; if the EF is improved by >10% compared with the previous examination; or if the EF is improved to ≥50%. However, the term “recovery” may be inappropriate because an improvement in LVEF does not necessarily mean complete recovery of the dysfunction, and ascertaining if the patient has completely recovered from HF is often difficult.4,5,6,7) In a clinical study, comprising a small group of patients with improved EF after HF treatment, the LV function, HF symptoms, or HF worsened in 45% of patients who were randomly assigned to discontinue HF medications, within 6 months after discontinuations.8) Thus, even if the EF is improved post-treatment, HFrEF may be considered as an “improved” condition, rather than classifying it as an independent disease group, and continuation of the standard treatment is recommended.
(1) Renin-angiotensin system inhibitor
1. In patients with HFrEF, ARNI is recommended as the standard of care to reduce cardiovascular mortality and HF hospitalization. If ARNI is intolerable or unavailable, the use of ACEI is recommended. (Class I, LOE A)
2. If both ARNI and ACEI are intolerable or unusable, ARBs are recommended as alternatives. (Class I, LOE A)
3. Even If the patient is stable with ACEI or ARBs, the replacement with ARNIs is recommended to further reduce the risk of HF-related cardiovascular mortality and hospitalization. (Class I, LOE B)
4. If acutely exacerbated hospitalized HFrEF patients recovered to be hemodynamically stable, treatment with ARNI, instead of ACEI or ARBs, is reasonable. (Class IIa, LOE B)
In patients with HFrEF, ACEIs improve symptoms, reduce mortality, and readmissions. These effects have been found to be consistent regardless of previous or current symptoms of HF, severity of symptoms, and irrespective of coronary artery disease.4,5,6,7)
ARB can theoretically overcome some limitations of ACEI. ACEIs do not completely block angiotensin II formation due to presence of non-ACE pathway which continuously product low level of angiotensin II. ACEIs inhibit the breakdown of bradykinin and increase circulating bradykinin levels which is implicated in pathogenesis of cough and angioedema. According to the Korean Acute Heart failure Registry (KorAHF), there was no difference in all-cause mortality between HFrEF patients on ARBs and ACEIs during 27 months of follow up (29.1% vs. 28.9%), while ARBs significantly reduced all-cause mortality when compared to those without renin-angiotensin blockers (adjusted hazard ratio, 0.71; p<0.001). ARBs were more tolerable than ACEIs within one year follow up as discontinuation rates were lower in ARB group compared to ACEI group (20.8% vs. 33.6%, p<0.001).9)
Previously, if symptoms persisted despite standard treatment (including ACEI), ARNI was recommended as an alternative; however, recent studies have consistently confirmed the reduction in mortality and readmission rates; therefore, ARNI is currently recommended as a first-line treatment over ACEI (Class I).10,11,12,13,14)
Based on the results of recent studies, ARNI may be used as a first-line treatment in patients hospitalized for acute HF exacerbations, including newly-diagnosed HF, or in patients who have never used ACEIs or ARBs.15,16) Contraindications or precautions of ARNI are similar to that when using ACEIs or ARBs. In particular, if the patient is already using ACEIs, a washout period of 36 hours is required before switching to ARNI, to avoid the risk of angioedema.
(2) Beta-blockers
1. Beta-blockers are recommended for administration in patients with stable HFrEF to improve symptoms and reduce mortality and HF hospitalization. (Class I, LOE A)
2. Beta-blockers proven to reduce mortality in randomized clinical trial include bisoprolol, carvedilol, and metoprolol sustained-release tablets. (Class I, LOE A)
3. In patients aged ≥70 years, the use of nebivolol can be beneficial. (Class IIa, LOE B)
Beta-blockers reduce mortality and HF hospitalization in patients with HFrEF.17,18,19,20,21) In a domestically conducted registry, beta-blockers improved the prognosis in patients with HF,22) and reduced the risk of death, particularly older patients with HFrEF.23) In another nationwide prospective study, beta-blockers combined with renin-angiotensin-aldosterone antagonists reduced overall mortality at discharge in patients with HFmrEF (EF 40–49%).24) A higher adherence to beta-blockers indicates a better prognosis.25)
High-dose ACEIs or ARBs are not necessarily required when beta-blockers are being used, and even if low-dose ACEIs are used, prompt addition of beta-blockers is recommended.26) According to data from the KorAHF, even in patients hospitalized with decompensated HF requiring vasopressors, the use of beta-blockers after recovery and before discharge improves the prognosis.27) Beta-blockers prescribed before discharge reduced mortalities by 24% after one year, if the heart rate (HR) was ≥70 beats/min at discharge; however, beta-blockers were ineffective if the HR was <70 beats/min.28) In a study based on a HR of 60 beats/min at discharge, a pre-discharge HR ≥60 beats/min was effective in reducing overall mortality; however, a HR <60 beats/min was ineffective.29) Comparative studies between beta-blockers are rarely reported; however, in a comparative study of carvedilol and bisoprolol in patients with acute HFrEF, there was no difference in the mortality rates between the two drugs.30)
(3) MRA
1. The use of MRA (aldosterone antagonists) is recommended to reduce HF hospitalization and mortality in patients with HFrEF. (Class I, LOE A)
Aldosterone antagonists or MRA reduce mortality and readmission and improve HF symptoms in patients with HFrEF.31,32,33) Spironolactone is initiated at a dose of 12.5–25 mg/day and eplerenone at 25 mg/day; both drugs can be increased to 50 mg/day. Since hyperkalemia may occur as a side effect of aldosterone antagonists, a blood test should be performed before initiating the medication to check for abnormalities in kidney function and electrolyte balance, and if the estimated glomerular filtration rate (eGFR) is <30 mL/min/1.73m2 or serum potassium concentration is >5.0 mEq/L, subsequent drug administration should be cautious.
(4) SGLT2 inhibitors
1. In patients with HFrEF with or without diabetes, administration of SGLT2 inhibitors (empagliflozin or dapagliflozin) is recommended to reduce HF hospitalization or cardiovascular mortality. (Class I, LOE A)
SGLT2 inhibitors were developed as antidiabetic drugs. However, in randomized clinical trials, irrespective of diabetes, SGLT2 inhibitors have been demonstrated to reduce HF hospitalization34,35) and improve the quality of life36,37) in patients with HFrEF. Before initiating SGLT2 inhibitors, kidney function should be evaluated at an early stage and regularly monitored. The eGFR slightly decreases during treatment initiation; however, this is reversible and discontinuing the drug is not recommended. Moreover, SGLT2 inhibitors have been confirmed to have a protective effect on kidney function. Caution is advised when using SGLT2 inhibitors because SGLT2 inhibitors increase risk of urogenital infection, and may contribute to volume depletion. Although rare, hypoglycemia and ketoacidosis may occur in patients with diabetes.4,5)
(5) Diuretics
In patients with HF with fluid retention, diuretics is recommended to maintain adequate fluid volume, regardless of LV systolic function. (Class I, LOE B)
Assessment of volume status and maintenance of proper fluid balance are essential components in the treatment of patients with HF, regardless of LV systolic function. Initial treatment involves the use of diuretics, such as loop diuretics, in addition to water and salt intake restrictions.4,5) Prolonged use of excessive diuretics may cause a state of low cardiac output (CO) due to reduced body fluid, hypotension, or deterioration of kidney function; therefore, adequate care is needed. In case when high doses of oral diuretics do not improve pulmonary congestion or swelling, limiting salt intake is necessary. Furthermore, it is necessary to ensure that the patient is not receiving non-steroidal anti-inflammatory drugs or corticosteroids. In patients with severe edema, furosemide may have insufficient intestinal absorption; therefore, short-term intravenous administration or replacement with torsemide may be considered. If resistance to loop diuretics is exhibited, short-term combination therapy of thiazide diuretics may be considered to inhibit salt reabsorption in the distal tubules.36)
(6) Ivabradine
1. In symptomatic HFrEF (LVEF ≤35%) patients who are in sinus rhythm (SR) and a resting HR ≥70 beats/min, ivabradine can be useful to reduce the risk of HF hospitalization and cardiovascular mortality; if HF symptoms persist despite the use of beta-blockers, ACEI (or ARNI) and MRA. (Class IIa, LOE B)
2. In symptomatic HFrEF (LVEF ≤35%) patients who are in SR and a resting HR ≥70 beats/min, ivabradine can be useful to reduce the risk of hospitalization and cardiovascular mortality, if beta-blockers cannot be used. (Class IIa, LOE C)
HR is an important prognostic factor in patients with HFrEF. Ivabradine lowers the HR by inhibiting the If channel of the sinoatrial node. Ivabradine specifically reduces HR without affecting myocardial contractility or other cardiac ionic current in patients with HFrEF. In symptomatic HFrEF (LVEF ≤35%) patients despite GDMT including beta blockers at maximally tolerated dose and who are in SR and a HR of ≥70 beats/min, ivabradine significantly reduced cardiovascular mortality and HF hospitalization.37,38)
(7) Vericiguat
1. Vericiguat may be used to reduce cardiovascular mortality or HF hospitalization in selected high-risk patients with HF (LVEF <45%) and recent worsening of HF already on GDMT. (Class IIa, LOE B)
Vericiguat is a soluble guanylate cyclase receptor stimulator that enhances the cyclic guanosine monophosphate pathway and restores nitric oxide sensitivity. According to the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) study, vericiguat significantly reduced cardiovascular death or hospitalization in high risk pateints with HF (LVEF <45%) with recent worsening HF (35.5% vs. 38.5%; hazard ratio, 0.90; 95% confidence interval, 0.82–0.98; p=0.02).39) The absolute risk reduction by vericiguat was 4.2% per year. Vericiguat was approved by the United States Food and Drug Administration (FDA) in January 2021, the European Union European Commission in July 2021, and Korean Ministry of Food and Drug Safety in November 2021. Vericiguat may be considered in selected high-risk patients with HFrEF and recent worsening of HF already on GDMT, to reduce HF hospitalization and cardiovascular death.
(8) Digoxin
1. In patients with HFrEF with atrial fibrillation (AF), if the use of beta-blockers does not provide good HR control, or if beta-blockers are contraindicated, digoxin can be beneficial. (Class IIa, LOE B)
2. In patients with symptomatic HFrEF despite GDMT, digoxin may be used to reduce HF hospitalization. (Class IIb, LOE B)
Digoxin inhibits the sodium/potassium (Na/K) ATPase pump of sarcoplasmic reticulum and increases intracellular calcium concentration even as intracellular sodium concentration decreases. In addition, it sensitizes the Na/K ATPase in afferent vagal nerves to enhance parasympathetic activity and reduce sympathetic activity by reducing plasma norepinephrine.40) The Digitalis Investigation Group (DIG) trial reported that digoxin provided no overall mortality benefit and only a modest reduction in hospitalizations among patients with HFrEF. The post hoc analysis of DIG trial demonstrated higher serum digoxin levels were associated with increased mortality.41) Digoxin is conventionally initiated at a low dose and subsequently, continued at a maintenance dose of 0.125 or 0.25 mg/day. Attention should be paid to the occurrence of side effects due to toxicity. Typical symptoms of digoxin toxicity include digestive (loss of appetite, nausea, and vomiting) and nervous (visual and cognitive impairments, and confusion) system symptoms; fatal arrhythmias, especially in older adults (aged >70 years) and patients with renal failure, low body weight, or electrolyte abnormalities. Side effects occur when digoxin is co-administered with drugs that can affect digoxin metabolism (macrolide antibiotics, itraconazole, cyclosporin, amiodarone, quinidine, etc.).42,43,44)
(9) Tolvaptan
1. The use of vasopressin V2-receptor antagonists (tolvaptan) may be considered in patients with HF in a state of volume overload with hyponatremia refractory to other treatments. (Class IIb, LOE B)
Hyponatremia causes cognitive impairment, which can easily cause falls, and in severe cases (Na <125 mEg/L), can alter consciousness.45) In hyponatremia accompanied by volume overload, vasopressin V2-receptor antagonists have been reported to significantly improve cognitive function associated with hyponatremia.45,46,47,48) In hyponatremia, ensuring the absence of other causes, such as syndrome of inappropriate antidiuretic hormone secretion, hypothyroidism, or hypoaldosteronism, is crucial; if not, fluid intake can be limited (800–1,000 mL/day) or drugs that inhibit angiotensin II can be used. Vasopressin V2-receptor antagonists may increase serum sodium levels in hyponatremia with volume overload46,47,48); however, they have not improved survival in patients with HF.47,48)
HFmrEF and heart failure with preserved ejection fraction (HFpEF)
1. Screening and treatment for comorbidities (cardiovascular diseases such as hypertension and AF; non-cardiovascular diseases such as diabetes and renal failure) are needed. (Class I, LOE C)
2. Diuretics are necessary, if symptoms of congestion are present. (Class I, LOE C)
3. SGLT2 inhibitors (empagliflozin or dapagliflozin) are recommended for patients with HF with or without diabetes to reduce hospitalization or cardiovascular mortality. (Class I, LOE B)
4. ARNI can be beneficial to reduce hospitalization or cardiovascular mortality due to HF. (Class IIa, LOE B)
5. MRA can be useful to reduce the risk of HF hospitalization. (Class IIa, LOE C)
6. ARBs or ACEIs may be considered to reduce hospitalization or cardiovascular mortality due to HF. (Class IIb, LOE C)
7. Beta-blockers may be considered to reduce cardiovascular mortality. (Class IIb, LOE C)
Until recently, no prospective randomized clinical trials have been conducted in patients with HFmrEF, although some evidence can be gathered from sub-analyses of studies in patients with HFpEF. The survival rate of patients with HFpEF is marginally higher than that of patients with HFrEF, although it is low; furthermore, hospitalization and disease burden due to worsening HF are known to be similar.49,50) Various drugs and device treatments have been developed for HFrEF to gradually improve survival; however, no treatment has clearly demonstrated improvement in survival rates for HFpEF. Patients with HFpEF are mainly older women with concomitant cardiovascular (hypertension, AF, and ischemic heart disease) and non-cardiovascular (diabetes and renal failure) diseases.51,52,53) The clinical phenotypes of HFpEF are diverse due to varied etiologies. In particular, lung disease, anemia, and obesity may exhibit similar symptoms; therefore, first, each causative disease entity has to be diagnosed and treated individually.51,52,53) Until recently, treatment recommendations to improve the course of HFpEF were insufficient, and conventional treatments were aimed at alleviating symptoms. Diuretics should be appropriately used; loop diuretics are initially recommended for congestive symptoms,54,55) thiazide diuretics may be useful in case of concomitant hypertension.56) In patients with obesity, weight loss and exercise therapy can help alleviate symptoms and improve athletic performance.57,58)
A prespecified meta-analysis including EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved)59) and Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER)60) showed that SGLT2 inhibitors reduced the risk of cardiovascular death and hospitalizations for HF irrespective of EF and the clinical benefit extended to HF patients with LVEF≥60%.61,62,63) DELIVER trial demonstrated clinical benefit in broad spectrum of HF patients, including HF with improved EF and regardless of recent HF hospitalization.60) The SGLT2 inhibitors, empagliflozin or dapagliflozin, are recommended for reducing cardiovascular mortality and HF hospitalization in patients with HFpEF and HFmrEF, regardless of diabetes.58,59,60,61,62)
The FDA recently approved ARNI and MRA for patients with HF and EF below normal, which include both HFmrEF and HFpEF. Although primary outcomes were not met in Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial, from the exploratory analysis, there was a significant benefit for the ARNI for HF hospitalizations in patients with LVEF below the median (45–57%) compared to valsartan.63,64,65,66) Regarding MRA, the subgroup analysis of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) study suggested benefit for hospitalization for HF in symptomatic HF patients with LVEF <55%.67,68,69,70) Post hoc analyses of TOPCAT study suggest a possibility of benefit in appropriately selected patients with symptomatic HFpEF (LVEF ≥45%, elevated B-type natriuretic peptide [BNP] level or HF admission within 1 year, eGFR >30 mL/min/1.73 m2, creatinine <2.5 mg/dL, and potassium <5.0 mEq/L).69,70) The KorAHF studies have reported that the use of ARBs, ACEIs, and beta-blockers reduced in-hospital mortality, post-discharge mortality in patients with HFpEF71); therefore, the use of these agents may be considered to improve prognosis.
Cardiac implantable electronic device
(1) Implantable cardioverter-defibrillator (ICD)
1. In patients who recovered from hemodynamically unstable ventricular arrhythmias, in the absence of reversible causes or unless if the ventricular arrhythmia occurred within 48 h after myocardial infarction, and survival is expected for >1 year, an ICD is recommended to reduce the risk of sudden death and all-cause mortality. (Class I, LOE A)
2. In patients with symptomatic HF (New York Heart Association [NYHA] II–III) of ischemic origin, if LVEF is ≤35% despite ≥3 months of GDMT, and survival is expected for >1 year, an ICD is recommended to reduce sudden death and all-cause mortality. (Class I, LOE A)
3. In patients with symptomatic HF (NYHA II–III) of non-ischemic origin, if LVEF ≤35% despite ≥3 months of GDMT and survival is expected for >1 year, an ICD is reasonable to reduce the risk of sudden death and all-cause mortality. (Class IIa, LOE A)
4. Experienced cardiologists should reassess the patient before generator replacement, because the patient’s needs and clinical status may have changed. (Class IIa, LOE B)
5. An ICD insertion is not recommended within 40 days of myocardial infarction, since it does not improve clinical outcome. (Class III, LOE A)
6. In patients with NYHA class IV symptoms who do not respond to medical therapy, an ICD is not recommended, unless they are candidates for cardiac resynchronization therapy (CRT), ventricular assist devices or transplantation. (Class III, LOE C)
Patients with HF experience more sudden cardiac deaths than the general population; this is the leading cause of death in HF patients with NYHA class II and III.72) An ICD prevents sudden death and reduces the risk of mortality in such patients.72) ICDs reduce the risk of sudden cardiac death and all-cause mortality in patients who experienced sustained symptomatic ventricular arrhythmias. Therefore, an ICD is recommended for secondary prevention in patients with HF in absence of reversible cause or unless the ventricular arrhythmia occurred within 48 hours of myocardial infarction and survival is expected for >1 year. An ICD for primary prevention is recommended in symptomatic patients (NYHA II-III) with HFrEF (EF ≤35%) despite ≥3 months of GMDT. ICDs are not recommended in severe symptomatic patients (NYHA IV) who are refractory to medical treatment, unless they are candidates for mechanical circulatory support, CRT or heart transplant.
(2) CRT
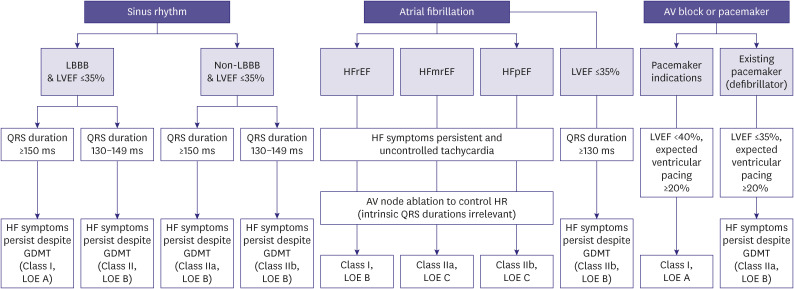
CRT uses pacemaker leads to induce coordinated contraction of the left and right ventricles (ventricular resynchronization) simultaneously, thereby improving the quality of life, reducing HF hospitalization and mortality, and inhibiting the process of LV remodeling.73,74,75)In symptomatic patients (NYHA III–IV) with chronic HF who did not respond to appropriate medical treatment, CRT was confirmed to be a crucial non-pharmacological treatment, which improved HF symptoms and quality of life; in cases HfrEF with electrical dyssynchrony, CRT significantly reduced HF hospitalization and mortality.73,74,75) The algorithm for the indications for CRT, which reflects the results of recent trials, is presented in Figure 2.
Figure 2. Indications for cardiac resynchronization therapy.
SR = sinus rhythm; AF = atrial fibrillation; AV = atrioventricular; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; HF = heart failure; GDMT = guideline directed medical therapy; HFrEF = heart failure with reduced ejection fraction; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HR = heart rate.
Treatment for specific cardiomyopathies
(1) Cardiac amyloidosis
1. Tafamidis is recommended to reduce symptoms, cardiovascular-related hospitalizations and mortality in NYHA I-II patients with wild-type or hereditary (genetic mutation) ATTR-CM. (Class I, LOE B)
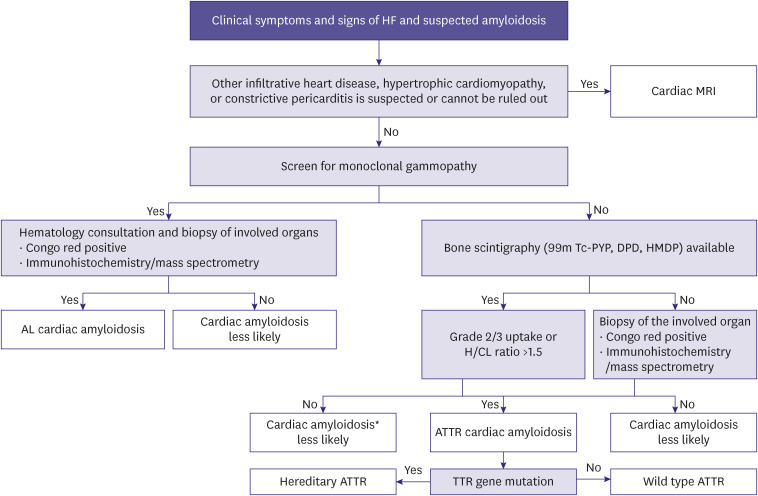
Amyloidosis is a disorder where misfolded proteins accumulate and cause organ dysfunction. It has an age-standardized incidence rate of 0.5 persons per 100,000 persons. Although it is a relatively rare disease, cardiac amyloidosis is under-recognized cause of HF. The diagnosis is often difficult and delayed.76,77,78,79,80) The typical amyloid proteins that cause cardiac amyloidosis include light chain immunoglobulin amyloidosis (AL) and transthyretin (TTR). The clinical symptoms and signs of cardiac amyloidosis are listed in Table 1. The algorithm for diagnosing cardiac amyloidosis is presented in Figure 3. In AL cardiac amyloidosis, chemotherapy or autologous stem cell transplantation is the main treatment. For TTR cardiac amyloidosis (ATTR-CM), TTR stabilization and reduction of TTR production is the basis of treatment. Tafamidis reduced all-cause mortality and cardiovascular hospitalization in hereditary and wild-type TTR cardiac amyloidosis, in patients with NYHA I or II.81,82)
Table 1. Clinical symptoms and signs of cardiac amyloidosis.
| Signs and symptoms | ||
|---|---|---|
| Cardiac | ||
| Clinical symptoms | Heart failure, intolerance to beta blockers or ACE inhibitors, hypotension or normotensive if previously hypertensive | |
| ECG | Pseudo-infarct pattern, low QRS voltage to degree of LV thickness, AV conduction disease | |
| Echocardiography | Myocardial walls-granular sparkling, increased thickness of RV wall, increased valve thickness, pericardial effusion, decreased longitudinal strain, and apical sparing pattern | |
| CMR | Subendocardial /transmural LGE, increase in native T1 value and ECV in extracellular volume | |
| Blood test | Disproportionately elevated NT-proBNP, sustainably elevated troponin | |
| Extracardiac | ||
| Peripheral neuropathy | ||
| Autonomic neuropathy | ||
| AL | Proteinuria, renal failure, bruises/periorbital purpura, macroglossia, MGUS | |
| ATTR | Lumbar spinal stenosis, family history of ATTR, vitreous deposit, biceps tendon rupture, bilateral carpal tunnel syndrome | |
ACE = angiotensin converting enzyme; ECG = electrocardiogram; LV = left ventricle; AV = atrioventricular; RV = right ventricle; CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement; ECV = extracelluar volume; NT-proBNP = N-terminal pro b type natriuretic peptide; AL = light-chain amyloidosis; MGUS = monoclonal gammopathy of undetermined significance; ATTR = transthyretin amyloidosis.
Figure 3. Diagnostic algorithm for cardiac amyloidosis.
HF = heart failure; CMR = cardiovascular magnetic resonance; Tc-PYP = technetium pyrophosphate; Tc-DPD = technetium 3,3-diphospho-1,2-propanodicarboxylic acid; Tc-HMDP = technetium-hydroxymethylene diphosphonate; H/CL = heart-to-contralateral lung; AL = amyloid light chain; ATTR= transthyretin amyloidosis; TTR = transthyretin.
*Consider cardiac biopsy if clinical suspicion is high.
(2) Myocarditis
1. In cases of acute severe HF of unknown cause that rapidly progress despite treatment, endocardial biopsy is recommended to diagnose myocarditis. (Class I, LOE B)
2. If giant cell or eosinophilic myocarditis are suspected, endocardial biopsy can be useful for diagnostic and prognostic evaluation. (Class Iia, LOE C)
3. Cardiac magnetic resonance (CMR) imaging can be beneficial in patients with suspected myocarditis. (Class Iia, LOE C)
4. In patients with myocarditis, immunosuppressive treatment may not improve survival. (Class III, LOE B)
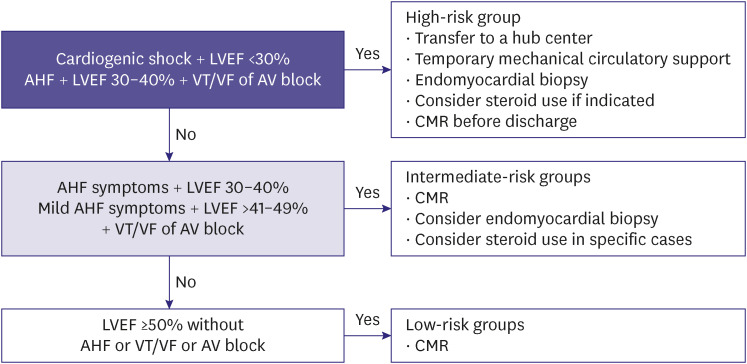
The reported cases of HF due to myocarditis vary depending on age and region. The incidence ranges from 0.5–4%.83,84,85) The etiology of acute myocarditis is varied and includes viral diseases, toxins or drugs, or systemic autoimmune diseases. Acute myocarditis typically presents with nonspecific symptoms including chest pain, dyspnea, palpitations, and fainting; in severe cases, cardiogenic shock may occur. Since myocarditis has various clinical manifestations depending on the degree and etiology, diagnosis and treatment are performed according to hemodynamic status and risk (Figure 4); furthermore, myocardial biopsy can be helpful in differential diagnosis and risk stratification.83,84,85,86,87,88)Approximately 40–60% of patients with myocarditis fully recover after the acute phase; however, approximately 20% of patients develop HF and subsequently, dilated cardiomyopathy within a few years.89)Therefore, HF treatment is recommended for at least 6 months after heart function is recovered (LVEF >50%) and the arrhythmia disappears; additionally, electrocardiogram (ECG) annual follow-up with echocardiograms are recommended for 4 years.86,87,88,89,90)
Figure 4. Risk-based approach for acute myocarditis.
LVEF = left ventricular ejection fraction; AHF = acute heart failure; VT = ventricular tachycardia; VF = ventricular fibrillation; AV = atrioventricular; CMR = cardiovascular magnetic resonance.
(3) Right HF
1. Coronary revascularization should be performed in patients with acute ST-segment elevation myocardial infarction with right ventricular myocardial infarction. (Class I, LOE A)
2. The use of vasodilators in patients with group 1 pulmonary arterial hypertension is recommended to improve survival. (Class I, LOE A)
3. During mitral valve surgery, severe tricuspid valve regurgitation should be concomitantly corrected. (Class I, LOE C)
4. In patients with right HF with congestive symptoms, diuretics are recommended. (Class I, LOE C)
5. For right HF with unclear diagnosis, hemodynamic evaluation via right cardiac catheterization is recommended. (Class I, LOE C)
6. In patients with arrhythmia-induced right ventricular cardiomyopathy, a defibrillator is recommended if there is a high probability of sudden cardiac death. (Class I, LOE C)
7. For families of patients with arrhythmia-induced right ventricular cardiomyopathy, clinical screening and genetic testing are recommended. (Class I, LOE C)
8. In patients with HF with hypotension and decreased peripheral perfusion, vasopressors and/or cardiac agents may be used. (Class Iib, LOE C)
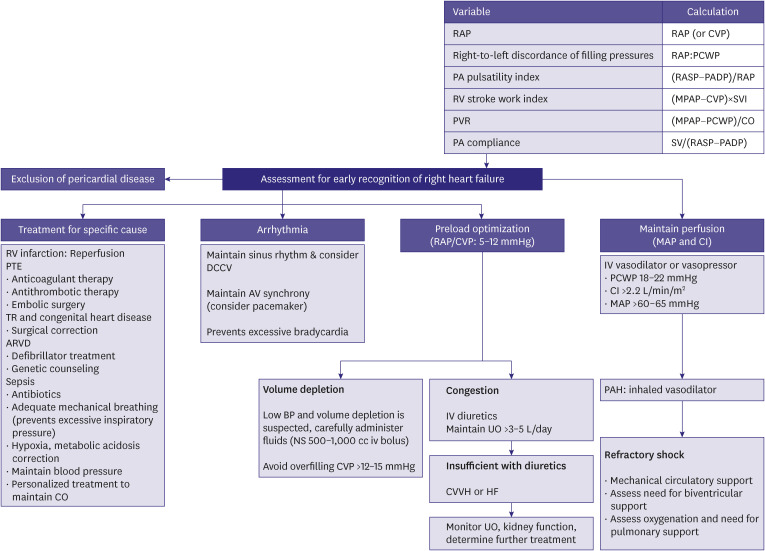
Right HF is associated with increased pressure in the right ventricle and atrium. Right HF can cause problems with LV filling, ultimately reducing systemic CO.91,92) Mechanisms and etiology of right HF are varied, relieving venous congestion is the treatment priority. Diuretics are often the first line of therapy for venous congestion. Inotropes and vasopressors are indicated for low CO and hemodynamic instability. Inotropes reducing that cardiac filling pressures are preferred (e.g., levosimendan, milrinone). Since these inotropic agents may aggravate arterial hypotension, they may be combined with norepinephrine, if needed (Figure 5).
Figure 5. Assessment and treatment of right HF.
HF = heart failure; RAP = right atrial pressure; CVP = central venous pressure; PCWP = pulmonary capillary wedge pressure; PA = pulmonary artery; PASP = pulmonary artery systolic pressure; PADP = pulmonary artery diastolic pressure; RV = right ventricle; MPAP = mean pulmonary artery pressure; SVI = stroke volume index; PVR = pulmonary vascular resistance; CO = cardiac output; SV = stroke volume; PTE = pulmonary thromboembolism; TR = tricuspid regurgitation; ARVD = arrhythmogenic right ventricular dysplasia; DCCV = direct current cardioversion; AV = atrioventricular; BP = blood pressure; IV = intravenous; UO = urine output; CVVH = continuous veno-venous hemofiltration; MAP = mean arterial pressure; CI = cardiac index; PAH = pulmonary arterial hypertension.
Multidisciplinary care
(1) Improving the quality of non-drug treatment and medical care - a multidisciplinary approach
1. A multidisciplinary approach is recommended to reduce HF hospitalization or mortality. (Class I, LOE A)
2. Patient self-management is recommended to reduce HF hospitalization or mortality. (Class I, LOE A)
3. Home- or office-based HF management programs is recommended to reduce HF hospitalization or mortality. (Class I, LOE A)
A multidisciplinary HF treatment approach should be patient-centered, based on sufficient discussion and communication, and adaptable to local- and national-level social, cultural, and economic conditions. Several clinical studies have demonstrated that compared with the standard HF treatment, a multidisciplinary approach reduces HF hospitalization and mortality and improves the quality of life.93,94,95,96,97,98,99)
(2) Cardiac rehabilitation
1. Exercise therapy is recommended to improve exercise performance and the quality of life and reduce HF hospitalization in all patients with HF. (Class I, LOE A)
2. In patients with severe disease, frailty, or with multiple comorbidities, supervised exercise-based cardiac rehabilitation programs can be beneficial. (Class IIa, LOE C)
3. Measures to increase participation in cardiac rehabilitation programs can be beneficial. (Class IIa, LOE B)
4. Home-based cardiac rehabilitation, telehealth, and mobile health intervention may be considered to increase long-term participation in cardiac rehabilitation programs. (Class IIb, LOE B)
The goals of cardiac rehabilitation in patients with HF are to improve the quality of life by improving cardiorespiratory endurance, and to reduce readmissions and mortalities due to worsening HF.100,101) The contents of cardiac rehabilitation programs includes multidisciplinary access through the followings: 1) patient evaluation; 2) diet; 3) weight management; 4) blood pressure management; 5) blood lipid management; 6) diabetic disease management; 7) smoking cessation; 8) psychosocial management; 9) physical activity counseling; and 10) all sections in cardiac rehabilitation exercise therapy should be included. Inpatient-cardiac rehabilitation programs can be initiated after stabilization of patients’ symptoms, cardiac enzymes, N-terminal pro B-type natriuretic peptide (NT-proBNP; or brain natriuretic peptide) levels, and ECG findings for >48 hours. Outpatient-cardiac rehabilitation is recommended within the first 1 week post-discharge, and approximately 4 weeks after thoracotomy.100,101) If the patient is unable to participate in hospital-based cardiac rehabilitation program, a tele-cardiac rehabilitation program using home-based cardiac rehabilitation, monitoring devices, and information and communication technology may be considered.100,101,102,103) Supervised rehabilitation should be considered in patients with ICD, CRT, or LV assist device, or those who underwent high-risk open-heart surgery or heart transplant, or those with cancer or frailty.104,105,106,107)
(3) Performance measures or clinical quality indicators for quality improvement in patients with HF
1. In patients with HF, assessment of treatment outcomes and clinical quality indicator can be beneficial to improve the quality of HF treatment and patient prognosis. (Class IIa, LOE B)
In the United States, standardized performance indicators were applied to improve readmission and mortality rates, and society-led performance indicators and checklists for HF programs revealed improvement in patient prognosis by applying quality improvement programs for each institution.108) Furthermore, the HF practice guidelines published by the European Society of Cardiology emphasize the effective applications of the guidelines and performance evaluation of quality management of HF.4) Although standardized quality management indicators have not yet been developed in Korea, verifying their usefulness while developing and applying performance indicators to Korean HF patients are essential.109) To successfully improve the quality of HF treatment and patient prognosis through Korean clinical quality indicators in the future, institutional efforts are needed to integrate health care system and medical information among HF institutions.
CONCLUSION
In this part of the guideline, we have discussed pharmacotherapy, cardiac implantable electronic devices, treatment for specific cardiomyopathies, and multidisciplinary care to improve the prognosis and provide the best care for patients with HF. We have evaluated and summarized up-to-date evidences for novel drugs including ARNI, SGLT2 inhibitors, and tafamidis. These guidelines will facilitate treatment decision making for patients with HF. Furthermore, we recognize the importance of multidisciplinary care and the necessity for assessing and reporting the quality of HF care for the best possible patient outcomes.
ACKNOWLEDGMENTS
This article has been published jointly, with consent, in both Korean Circulation Journal and International Journal of Heart Failure.
Also, the final version of this guideline was endorsed by Korean Society of Cardiology, Korean Society of Lipid and Atherosclerosis, Korean Association of Clinical Cardiology, Korean Society of Hypertension, Korean Society of Heart Failure, Korean Society of Echocardiography, Korean Society of Interventional Cardiology, Korean Heart Rhythm Society, and Korean Society of CardioMetabolic Syndrome.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Youn JC, Kim D, Cho HJ.
- Visualization: Youn JC, Kim D, Cho HJ.
- Writing - original draft: Youn JC, Kim D, Cho HJ.
- Writing - review & editing: Youn JC, Kim D, Cho JY, Cho DH, Park SM, Jung MH, Hyun J, Cho HJ, Park SM, Choi JO, Chung WJ, Yoo BS, Kang SM.
References
- 1.Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. doi: 10.4070/kcj.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Kim MS, Kim EJ, et al. KSHF Guidelines for the management of acute heart failure: Part I. Definition, epidemiology and diagnosis of acute heart failure. Korean Circ J. 2019;49:1–21. doi: 10.4070/kcj.2018.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Kim MS, Yoo BS, et al. KSHF Guidelines for the management of acute heart failure: Part II. Treatment of acute heart failure. Korean Circ J. 2019;49:22–45. doi: 10.4070/kcj.2018.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim ES, Youn JC, Baek SH. Update on the pharmacotherapy of heart failure with reduced ejection fraction. Cardiovasc Prev Pharmacother. 2020;2:113–133. [Google Scholar]
- 7.Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019;34:11–43. doi: 10.3904/kjim.2018.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2019;393:61–73. doi: 10.1016/S0140-6736(18)32484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KH, Lee GY, Choi JO, et al. Effects of angiotensin receptor blocker at discharge in patients with heart failure with reduced ejection fraction: Korean Acute Heart Failure (KorAHF) registry. Int J Cardiol. 2018;257:168–176. doi: 10.1016/j.ijcard.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 11.Senni M, McMurray JJ, Wachter R, et al. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail. 2018;20:491–500. doi: 10.1002/ejhf.1054. [DOI] [PubMed] [Google Scholar]
- 12.Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–1095. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khariton Y, Fonarow GC, Arnold SV, et al. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:933–941. doi: 10.1016/j.jchf.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myhre PL, Vaduganathan M, Claggett B, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73:1264–1272. doi: 10.1016/j.jacc.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 16.DeVore AD, Braunwald E, Morrow DA, et al. Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open-label extension of the PIONEER-HF trial. JAMA Cardiol. 2020;5:202–207. doi: 10.1001/jamacardio.2019.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 18.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 19.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 20.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF) JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 21.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 22.Lim NK, Lee SE, Lee HY, et al. Risk prediction for 30-day heart failure-specific readmission or death after discharge: data from the Korean Acute Heart Failure (KorAHF) registry. J Cardiol. 2019;73:108–113. doi: 10.1016/j.jjcc.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Seo WW, Park JJ, Park HA, et al. Guideline-directed medical therapy in elderly patients with heart failure with reduced ejection fraction: a cohort study. BMJ Open. 2020;10:e030514. doi: 10.1136/bmjopen-2019-030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KH, Choi JO, Jeon ES, et al. Guideline-directed medical therapy for patients with heart failure with midrange ejection fraction: a patient-pooled analysis from the Kor HF and Kor AHF registries. J Am Heart Assoc. 2018;7:e009806. doi: 10.1161/JAHA.118.009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn MS, Yoo BS, Yoon J, et al. Guideline-directed therapy at discharge in patients with heart failure and atrial fibrillation. Heart. 2020;106:292–298. doi: 10.1136/heartjnl-2019-315240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KA, Kim ES, Youn JC, et al. A dose-response relationship of renin-angiotensin system blockers and beta-blockers in patients with acute heart failure syndrome: a nationwide prospective cohort study. Eur Heart J Cardiovasc Pharmacother. 2022;8:587–599. doi: 10.1093/ehjcvp/pvac002. [DOI] [PubMed] [Google Scholar]
- 27.Cho MS, Kim MS, Lee SE, et al. Outcomes after predischarge initiation of β-blocker in patients hospitalized for severe decompensated heart failure requiring inotropic therapy. Can J Cardiol. 2018;34:1145–1152. doi: 10.1016/j.cjca.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Park JJ, Park HA, Cho HJ, et al. β-Blockers and 1-year postdischarge mortality for heart failure and reduced ejection fraction and slow discharge heart rate. J Am Heart Assoc. 2019;8:e011121. doi: 10.1161/JAHA.118.011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HJ, Jo SH, Lee MH, Seo WW, Choi JO, Ryu KH. The effect of beta-blockers in acute heart failure according to heart rate. Korean J Intern Med. 2021;36:898–905. doi: 10.3904/kjim.2020.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KH, Lee GY, Choi JO, et al. The mortality benefit of carvedilol versus bisoprolol in patients with heart failure with reduced ejection fraction. Korean J Intern Med. 2019;34:1030–1039. doi: 10.3904/kjim.2018.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 32.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 33.Na SJ, Youn JC, Lee HS, et al. The prescription characteristics, efficacy and safety of spironolactone in real-world patients with acute heart failure syndrome: a prospective nationwide cohort study. Front Cardiovasc Med. 2022;9:791446. doi: 10.3389/fcvm.2022.791446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 35.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 36.Ellison DH. The physiologic basis of diuretic synergism: its role in treating diuretic resistance. Ann Intern Med. 1991;114:886–894. doi: 10.7326/0003-4819-114-10-886. [DOI] [PubMed] [Google Scholar]
- 37.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 38.Swedberg K, Komajda M, Böhm M, et al. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 40.Ziff OJ, Kotecha D. Digoxin: the good and the bad. Trends Cardiovasc Med. 2016;26:585–595. doi: 10.1016/j.tcm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenblick M, Abraham AS, Meshulam Z, Eylath U. Correlation between manifestations of digoxin toxicity and serum digoxin, calcium, potassium, and magnesium concentrations and arterial pH. Br Med J (Clin Res Ed) 1983;286:1089–1091. doi: 10.1136/bmj.286.6371.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bavendiek U, Aguirre Davila L, Koch A, Bauersachs J. Assumption versus evidence: the case of digoxin in atrial fibrillation and heart failure. Eur Heart J. 2017;38:2095–2099. doi: 10.1093/eurheartj/ehw577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingelfinger JA, Goldman P. The serum digitalis concentration--does it diagnose digitalis toxicity? N Engl J Med. 1976;294:867–870. doi: 10.1056/NEJM197604152941603. [DOI] [PubMed] [Google Scholar]
- 45.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71.e1–71.e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Ghali JK, Koren MJ, Taylor JR, et al. Efficacy and safety of oral conivaptan: a V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab. 2006;91:2145–2152. doi: 10.1210/jc.2005-2287. [DOI] [PubMed] [Google Scholar]
- 47.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 48.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 49.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 50.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 51.Youn JC, Ahn Y, Jung HO. Pathophysiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17:327–335. doi: 10.1016/j.hfc.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Shim CY. Heart failure with preserved ejection fraction: the major unmet need in cardiology. Korean Circ J. 2020;50:1051–1061. doi: 10.4070/kcj.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 54.Faris R, Flather M, Purcell H, Henein M, Poole-Wilson P, Coats A. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol. 2002;82:149–158. doi: 10.1016/s0167-5273(01)00600-3. [DOI] [PubMed] [Google Scholar]
- 55.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 56.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 57.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butler J, Packer M, Filippatos G, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jhund PS, Kondo T, Butt JH, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon SD, McMurray JJ, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 62.Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. doi: 10.1016/S0140-6736(22)01429-5. [DOI] [PubMed] [Google Scholar]
- 63.Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 64.Solomon SD, Vaduganathan M, L Claggett B, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–361. doi: 10.1161/CIRCULATIONAHA.119.044586. [DOI] [PubMed] [Google Scholar]
- 65.Vaduganathan M, Claggett BL, Desai AS, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. 2020;75:245–254. doi: 10.1016/j.jacc.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connor CM. Meet me in the middle: lessons from the cardiorenal advisory committee for sacubitril/valsartan in HFpEF. JACC Heart Fail. 2021;9:161–163. doi: 10.1016/j.jchf.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 68.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 69.de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT - new insights into regional variation. N Engl J Med. 2017;376:1690–1692. doi: 10.1056/NEJMc1612601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho JH, Choe WS, Cho HJ, et al. Comparison of characteristics and 3-year outcomes in patients with acute heart failure with preserved, mid-range, and reduced ejection fraction. Circ J. 2019;83:347–356. doi: 10.1253/circj.CJ-18-0543. [DOI] [PubMed] [Google Scholar]
- 72.Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 73.O’Brien T, Park MS, Youn JC, Chung ES. The past, present and future of cardiac resynchronization therapy. Korean Circ J. 2019;49:384–399. doi: 10.4070/kcj.2019.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SH, Park SJ, Kim JS, Shin DH, Cho DK, On YK. Mid-term outcomes in patients implanted with cardiac resynchronization therapy. J Korean Med Sci. 2014;29:1651–1657. doi: 10.3346/jkms.2014.29.12.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uhm JS, Oh J, Cho IJ, et al. Left ventricular end-systolic volume can predict 1-year hierarchical clinical composite end point in patients with cardiac resynchronization therapy. Yonsei Med J. 2019;60:48–55. doi: 10.3349/ymj.2019.60.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang SY, Kim D, Choi JO, Jeon ES. Incidence, cause of death, and survival of amyloidosis in Korea: a retrospective population-based study. Int J Heart Fail. 2021;3:172–178. doi: 10.36628/ijhf.2021.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021;42:1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the american heart association. Circulation. 2020;142:e7–22. doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 79.Kim D, Choi JO, Kim K, Kim SJ, Jeon ES. Untangling amyloidosis: recent advances in cardiac amyloidosis. Int J Heart Fail. 2020;2:231–239. doi: 10.36628/ijhf.2020.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung MH, Chang S, Han EJ, Youn JC. Multimodal imaging and biomarkers in cardiac amyloidosis. Diagnostics (Basel) 2022;12:627. doi: 10.3390/diagnostics12030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 82.Damy T, Garcia-Pavia P, Hanna M, et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021;23:277–285. doi: 10.1002/ejhf.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 84.Cooper LT, Jr, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014;9:121–129. doi: 10.1016/j.gheart.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Heymans S, Eriksson U, Lehtonen J, Cooper LT., Jr The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 86.Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youn JC, Shim HS, Lee JS, et al. Detailed pathologic evaluation on endomyocardial biopsy provides long-term prognostic information in patients with acute myocarditis. Cardiovasc Pathol. 2014;23:139–144. doi: 10.1016/j.carpath.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 89.D’Ambrosio A, Patti G, Manzoli A, et al. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart. 2001;85:499–504. doi: 10.1136/heart.85.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tschöpe C, Cooper LT, Torre-Amione G, Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res. 2019;124:1568–1583. doi: 10.1161/CIRCRESAHA.118.313578. [DOI] [PubMed] [Google Scholar]
- 91.Harjola VP, Mebazaa A, Čelutkienė J, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18:226–241. doi: 10.1002/ejhf.478. [DOI] [PubMed] [Google Scholar]
- 92.Arrigo M, Huber LC, Winnik S, et al. Right ventricular failure: pathophysiology, diagnosis and treatment. Card Fail Rev. 2019;5:140–146. doi: 10.15420/cfr.2019.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wever-Pinzon O, Drakos SG, Fang JC. Team-based care for advanced heart failure. Heart Fail Clin. 2015;11:467–477. doi: 10.1016/j.hfc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 95.Rich MW, Gray DB, Beckham V, Wittenberg C, Luther P. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am J Med. 1996;101:270–276. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 96.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 97.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 98.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 99.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 100.Kim C. Overview of cardiac rehabilitation and current situations in Korea. Ann Cardiopulm Rehabil. 2021;1:6–16. [Google Scholar]
- 101.Kim WS. Exercise-based cardiac rehabilitation in heart failure. Ann Cardiopulm Rehabil. 2021;1:57–65. [Google Scholar]
- 102.Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol. 2015;22:35–74. doi: 10.1177/2047487313501093. [DOI] [PubMed] [Google Scholar]
- 103.Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 104.Pedretti RF, Iliou MC, Israel CW, et al. Comprehensive multicomponent cardiac rehabilitation in cardiac implantable electronic devices recipients: a consensus document from the European Association of Preventive Cardiology (EAPC; Secondary prevention and rehabilitation section) and European Heart Rhythm Association (EHRA) Europace. 2021;23:1336–1337o. doi: 10.1093/europace/euaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail. 2014;20:548–554. doi: 10.1016/j.cardfail.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmidt T, Bjarnason-Wehrens B, Predel HG, Reiss N. Exercise after heart transplantation: typical alterations, diagnostics and interventions. Int J Sports Med. 2021;42:103–111. doi: 10.1055/a-1194-4995. [DOI] [PubMed] [Google Scholar]
- 107.Wilhelm M, Abreu A, Adami PE, et al. EAPC core curriculum for preventive cardiology. Eur J Prev Cardiol. 2022;29:251–274. doi: 10.1093/eurjpc/zwab017. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki S, Yoshihisa A, Sato Y, et al. Clinical significance of get with the guidelines-heart failure risk score in patients with chronic heart failure after hospitalization. J Am Heart Assoc. 2018;7:e008316. doi: 10.1161/JAHA.117.008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim IC, Youn JC, Jang SY, et al. Physician adherence and patient-reported outcomes in heart failure with reduced ejection fraction in the era of angiotensin receptor-neprilysin inhibitor therapy. Sci Rep. 2022;12:7730. doi: 10.1038/s41598-022-11740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]