Abstract
AIM
To derive a Malaysia guideline and consensus as part of the Malaysia Retina Group's efforts for diagnosis, treatment, and best practices of diabetic macular edema (DME). The experts' panel suggests that the treatment algorithm to be divided into groups according to involvement the central macula. The purpose of DME therapy is to improve edema and achieve the best visual results with the least amount of treatment load.
METHODS
On two different occasions, a panel of 14 retinal specialists from Malaysia, together with an external expert, responded to a questionnaire on management of DME. A consensus was sought by voting after compiling, analyzing and discussion on first-phase replies on the round table discussion. A recommendation was deemed to have attained consensus when 12 out of the 14 panellists (85%) agreed with it.
RESULTS
The terms target response, adequate response, nonresponse, and inadequate response were developed when the DME patients' treatment responses were first characterized. The panelists reached agreement on a number of DME treatment-related issues, including the need to classify patients prior to treatment, first-line treatment options, the right time to switch between treatment modalities, and side effects associated with steroids. From this agreement, recommendations were derived and a treatment algorithm was created.
CONCLUSION
A detail and comprehensive treatment algorithm by Malaysia Retina Group for the Malaysian population provides guidance for treatment allocation of patients with DME.
Keywords: diabetic macular edema, guidelines, consensus, diabetic retinopathy, Malaysia
INTRODUCTION
Diabetic Macular Edema and Vision Loss
Diabetic macular edema (DME) is a retinal thickening involving the central fovea close to macula which is one of the most prevalent causes of visual loss worldwide[1]–[2]. It is characterized by a buildup of fluid in the central region of the retina because of fail blood-retinal barrier[1]. In the healthy people, the central retinal thickness (CRT) varies between 212±19 and 289±16 µm[3] while in DME patients, the CRT can vary from 225 to >450 µm[1]. Localized edema is caused by leakage from clusters of microaneurysms, whereas diffuse edema is caused by broad capillary leakage[1]. DME can manifest as the appearance of hard exudates which produce blurring and distortion of central vision, and can be measured by a reduction in best-corrected visual acuity (BCVA)[1]. Diabetic retinopathy (DR) is the presence of microvascular abnormalities in the fundus of diabetic patients which can be seen during clinical examination or color fundus photography. The earliest and least serious form of DR is the dot-like microaneurysms, which is discrete saccular outpouchings of the capillary wall that have sharp edges and look like a small red dot. DR is a one of the ocular diabetic complication caused by long-term diabetes. Prolong or uncontrolled diabetic can cause damage to the blood vessels in the eyes, which may lead to vision loss[4]. Globally, 34.6% of diabetic patients have DR[5], while in Malaysia, 10.4% of ageing diabetic have it, which results in blindness[6]. Active screening and early diagnosis of DR are crucial to preventing vision loss since higher prevalence of diabetes is one of the primary causes of blindness globally[7]. DR screening is one of the starting points for continuous and effective management of DR to minimized the incidence of vision loss[8]. Optical coherence tomography (OCT) imaging has provided novel diagnostic measures and clinical information that have been used to stage the illness. The ophthalmologist has several treatment options which consist of various procedures with different outcomes measure, including laser photocoagulation, anti-vascular endothelial growth factor (VEGF), steroids, and surgical therapy. These strategies have heralded the beginning of a new era in DME therapy. When laser treatment and anti-VEGF fail to give an adequate impact in DME patients, the pars plana vitrectomy operation is done[9]. Pars plana vitrectomy reduces the macula's thickness and improves visual acuity (VA) by mechanically removing vitreous fluid.
How to Detect Diabetic Macular Edema
Optical coherence tomography
OCT scan is the industry-recognized gold standard for DME diagnosis. It may be used to recognize different types of DME, detect macula traction, and locate edema to specific retina layers[10]. Diverse retinal thickening, cystoid macula edema, serous retinal detachment without posterior hyaloidal traction, and posterior hyaloidal traction with tractional retinal detachment are morphologic manifestations of DME on OCT[11]. Subretinal fluid and/or small intraretinal cystoid fluid and/or external limiting membrane and inner segment/outer segment integrity and vitreomacular adhesion are a good baseline predictor for good treatment response with high vision gains and/or good final VA which is a prognostic marker from OCT for DME[1],[12]. Contrarily, baseline abnormalities of the retinal inner layers, disruption of the inner and outer photoreceptor segments, and/or the external limiting membrane, which may result in irreversible photoreceptor destruction and loss, as well as baseline subfoveal choroid thinness, are predictors of poor visual outcomes post-treatment[1].
After dexamethasone implants in eyes with DME, biomarkers such as subretinal fluid, inner segment/outer segment continuity, the lack of hyperreflective foci, and an attached vitreoretinal interface indicated improved visual results. Anti-VEGF (ranibizumab) biomarkers such as ellipsoid zone disruption and the lack of epiretinal membrane have been linked to superior therapy outcomes[13]–[14].
A potential predictive biomarker for the visual consequences of DME is the disorganization of the retinal inner layers. Disorganization of the inner layer of retinal has been connected to both disruption of the outer retina and an increase in the severity of DR[15]. Cystoid macular edema, serous retinal detachment of subretinal fluid and retinal enlargement or thickening were all seen on OCT in individuals with DME[16].
Hyperreflective foci is another prognostic sign for DME, which forms plexiform layer's outer confluent plaques and is found within the walls of intraretinal microaneurysm[17]. The foci may be an early sign of DME barrier failure since they are believed to be extravasated proteins and/or lipoproteins. In DME patients, higher baseline hyperreflective foci levels indicate therapy response as measured by VA improvement and CRT decrease after three months[18].
Fluorescein Angiography
When OCT angiography is not available[1], fluorescein angiography plays important role in identifying treatment failure or inadequate response[19], identifying the foveal avascular zone[1], guiding supplemental laser therapy[20] and diagnosing of co-existing peripheral DR[1]. In order to evaluate the central and peripheral retina, fluorescein angiography may be employed.
SUBJECTS AND METHODS
Ethical Approval
This study used all the published data. Ethical approval is not required. This study was registered with National Medical Research Register (NMRR) with registration number NMRR ID-22-01045-RUP.
In early 2021, a team of experts from Malaysia comprising 14 ophthalmologist who were medical retina specialist with an external reviewer convened together to discuss recent research and developed a consensus guideline for the treatment of DME and how they relate to international trends and practices.
Consensus Development
The Malaysia Retina Group's efforts to establish local treatment guidelines and consensus for the management of DME and to get recommendations based on the best-updated practice resulted in the present current consensus. Prior to the conference, 11 recommended statements and one management algorithm were created using existing guideline recommendations, regional health care reimbursement policies, and treatment trends. A thorough discussion of each statement was followed by a secret vote. When ≥85% of experts voted, it was considered that consensus had been reached. Discussions were repeated, statements were changed, and voting continued until an agreement was reached.
RESULTS
Recommendation
The DME patients were characterized according to their therapy response patterns to provide the recommendations. Table 1 is a summary of the consensus guidelines for DME management.
Table 1. Consensus guidelines for managing DME.
| Consensus | Supporting data |
| Treatment goal | RISE/RIDE (2012)[21] |
| MEAD (2014)[22] | |
| RESTORE (2011)[20] | |
| VIVID/VISTA (2014)[23] | |
| DRCR.net Protocol I (2010)[24] | |
| DRCR.net Protocol T (2015) [25] | |
| DME treatment options | |
| Anti-vascular endothelial growth factor | RESTORE (2011)[20] |
| Robert & Gabriel (2016)[28] | |
| Curry et al (2020)[26] | |
| Zabrin et al (2017)[29] | |
| DRCR.net Protocol T (2015)[25] | |
| Ademan & Garg (2017)[27] | |
| Jain et al (2017)[30] | |
| Steroid implant | MAED (2014)[21] |
| Bucolo et al (2018)[31] | |
| Focal/grid laser | EURETINA (2017)[1] |
| Ademan & Garg (2017)[27] | |
| Pai et al (2010)[32] | |
| Pars plana vitrectomy + membrane peeling | Gary et al (2017)[33] |
| Special conditions | |
| Blood sugar control | Mathew et al (2015)[34] |
| Rajalakshmi et al (2016)[35] | |
| Cataract surgery | Chhablani et al (2020)[36] |
| Treatment in pregnant DME patients | Peracha et al (2015)[37] |
| Rosenthal & Johnson (2018)[38] | |
| Mathew et al (2015)[34] | |
| Yoo et al (2016)[39] | |
| Vitrectomized eyes | Chhablani et al (2020)[36] |
| Medeiros et al (2014)[40] | |
| Stable pre-existing glaucoma | Chhablani et al (2020)[36] |
| Lakhani et al (2020)[41] | |
| Endophthalmitis | Pai et al (2010)[32] |
| McCannel (2011)[42] | |
| Kiss et al (2018)[43] | |
| Goel (2017)[44] | |
| Treatment algorithm for DME | Chhablani et al (2020)[36] |
DME: Diabetic macular edema.
Treatment Goal
Although available treatments can retain and improve vision for the great majority of patients, it can be associated with significant expenses and visit burdens; hence, identifying the best treatment regimen is crucial. In significant pilot investigations of DME treatment agents, it is decided that BCVA will be the main endpoint. Various studies using ranibizumab injection in patients with clinically significant macular edema with center involvement linked to DME in RISE (registered on ClinicalTrials.gov as NCT00473330)/RIDE (NCT00473382)[21] and randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with DME [MAED (NCT00168337 and NCT00168389)] studies[22], where the proportion of patients acquiring >15 letters in BCVA from baseline was identified as the primary objective. However, DME resolution occurs when ranibizumab is used alone or in combination with laser treatment when the primary outcome was the mean change in BCVA from baseline [RESTORE (NCT01609374)][20], VIVID (NCT01363440)/VISTA (NCT01331681)[23], Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol I[24] and DRCR.net Protocol T studies[25]. From the clinical setting, improvement of VA may not be achieved, due to the Snellen is the commonest tool us for VA testing instead of Early Treatment Diabetic Retinopathy Study chart. In other cases, VA improvement is achieved after the disappearance of macular edema. Therefore, these factors led to the development of this set of guidelines, which determine the most effective treatments for the disease and inform ophthalmologists about the most recent advancements in clinical practice and the necessity of prompt suggestion to retina specialist for additional management when required.
Diabetic Macular Edema Treatment Options
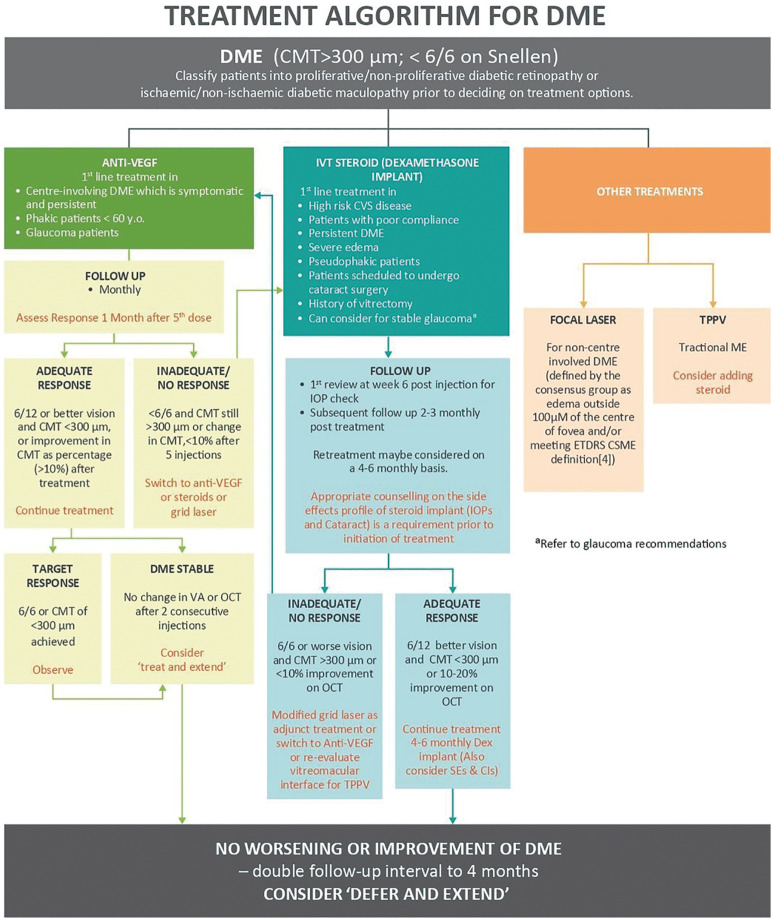
The consensus's recommendations were simplified into an algorithm (Figure 1) and overview (Table 2) to offer a straightforward treatment protocol for DME maintenance.
Figure 1. Treatment algorithm for DME adapted from Chhablani et al (2020)[36].
aFollow-up intervals can be doubled to 4mo if there is no worsening or improvement in DME after anti-VEGF or steroid treatment, and a “defer and extend” strategy may be used. DME: Diabetic macular edema.
Table 2. The overview for management of DME.
| Condition | Treatment options |
| Central involved DME with vision impairment (6/9 or worse) | In the ideal situation, authorized anti-VEGF with proven efficacy and safety is used. |
| Steroid implant. | |
| Focused/grid laser is the next step if anti-VEGF or steroids are not an option. | |
| DME with central involvement and excellent vision (VA greater than 6/9), (three alternatives currently, with community consensus on the best management) | If DME continues, observation until visual deterioration, followed by either focal/grid laser or anti-VEGF treatment. |
| Focal/grid laser up to a loss of vision, followed by anti-VEGF treatment if DME continues. | |
| Non-central involved DME | Observation until central-involved DME, then check the Focal/grid laser in selected cases where observation is judged to be inferior, such as in pregnancy or rapidly worsening cataract, or when macula edema rapidly extending towards the center of the macula |
DME: Diabetic macular edema; VA: Visual acuity; VEGF: Vascular endothelial growth factor.
The following points provide a concise description of the algorithm and overview:
Anti-vascular endothelial growth factor
The primary line of treatment for patients with symptomatic DME is anti-VEGF therapy (defined as edema within 100 µm of fovea center), phakic patients 60 years age and older and glaucoma patients.
The existing anti-VEGF recommendations prescribe a loading dose of three injections. However, in certain DME patients, loading phase is slower as VA improvement persists up to five injections[26]. Depending on financial resources, early intensive therapy will be advised. Early intensive therapy with minimum five to six first monthly dosages, with a maximum of eight to nine injection in the first year, may provide positive outcomes and enable for lessen burden of therapy in upcoming years[20],[25].
The VA can be significantly improved with monthly injection of anti-VEGF drugs with every reactivation of the disease pro pre nata protocol (PRN protocol) or treat-and-extend injectable treatment. DRCR.net Protocol I study using ranibizumab with a pro pre nata basis regimen has demonstrated a reduced number of injections at a mean of eight to nine in the first year, two to three in second year and one to two in year three while maintaining good visual outcomes[27]. VA is considered stable when there is no change after two successive injections and there is a change of under 10% in the central macular thickness on the OCT. Therefore, when DME is stable during follow up, it will be advisable to consider treating and extending the regime. However, steroid (dexamethasone) injection can be considered if there is no response after five to six injections, especially in pseudo phakic patients.
All anti-VEGF agents are equally effective and any of them can be used as a first-line treatment. However, Protocol T results indicate that aflibercept can be started in patients a worse VA (20/50 or less) who had baseline fluorescein angiography readings of 20/32 to 20/40. Treatment with anti-VEGF agents reportedly improved patients' vision by one to two lines at two years with no significant differences among agents[25].
The intravitreal use of VEGF inhibitors may increase the incidence of arterial thromboembolic events. Nonfatal myocardial infarction, nonfatal stroke, and vascular death are all considered to be arterial thromboembolic events. A Meta-analysis of anti-VEGF drugs for patients with DME who received intensive monthly anti-VEGF for two years suggests that the risk may be related to cumulative medication exposure. The analysis revealed a possible increased risk of fatalities and cerebrovascular accidents[28].
Ranibizumab with doses of 0.5 mg and 0.3 mg was found to have hazard ratios of 1.05 and 0.78 for arterial thromboembolic events, 0.84 and 0.94 for myocardial infarction 0.94 and 0.53 for stroke or transient ischemic attack, 1.63 and 0.59 for stroke (excluding transient ischemic attack), and 2.17 and 2.51 for vascular death when compared to sham[29]. Study on off label bevacizumab showed that the overall systematic side effect of intravitreal injections range from 0 to 39.3%. The majority of these occurrences are minor, unmanaged and retrospective[30].
Steroid implant
Before beginning treatment, steroid implants should be given to patients with high-risk cardiovascular disease. Potential candidates for treatment include patients with a history of vitrectomy, severe edema (greater than 500 µm), pseudophakic patients (steroid implant is contraindicated in patients with anterior chamber intraocular lens, patients who are scheduled for cataract surgery, and patients with low compliance.
After six weeks of the injection, regular intraocular pressure (IOP) should be measured in patients with no other ocular comorbidities and followed-up at months two to three after therapy. Retreatment may be recommended every four to six monthly, depending on the results. If the reaction is positive, it may be possible to consider additional evaluation and therapy at 4- to 6-montly intervals while keeping an eye on side effects and contraindications. If the reaction us insufficient or no reaction is given, switch to another anti-VEGF drug.
According to MEAD study, patients receiving 0.7 dexamethasone exhibited IOP increases of at least 10 mm Hg in 27.7% of cases and >35 mm Hg in 6.6% of cases, and with 41.5% requiring IOP-lowering medications. Only one patient required surgical intervention[22]. In the majority of clinical trials, 20% of patients/eye had IOP increase of interest. IOP-lowering agents and anti-glaucoma mediation were not necessary in the majority of IOP increase cases[31]. Retinal/vitreous/subconjunctival hemorrhage was another minor problem that occurred in 1%-2% of patients[31]. Problems relating to cataracts were observed in nearly half of phakic patients in several studies[31]. The dose of 0.7 g Ozurdex arm of the MEAD trial gave an unfavorable event relating to cataracts occur at an incidence of 67.9% mostly in phakic patients. Following cataract surgery, the patient's eyesight improved from baseline[22].
Focal/grid laser
For non-centre involving DME (defined as edema outside 100 µm radius of the foveal centre and/or meeting the Early Treatment Diabetic Retinopathy Study-Clinically Significant Macular Edema definitions[32] and rescue laser for DME involving the centre is indicated after at least six months of anti-VEGF treatment. Other indications include DME affected eyes with a CRT <300 µm[1].
Both Protocol I from the DRCR.net and Protocol T suggested postponing focal/grid laser therapy for at least 24wk. The visual outcomes of Protocol I were better to those of rapid laser with ranibizumab[27].
Ideally, focal laser should be used in conjunction with grid laser to treat diffuse macular leakage, leaky microvascular abnormalities, and non-perfusion in thicker retinas[1]. Every three to four months, the focal/grid laser should be redone. If edema continues or does not improve despite receiving anti-VEGF therapy (if available, if it is thought that using more laser could be beneficial).
There are several potential side effects of laser treatment, including the growth of laser scars (atropic creep), secondary choroidal neovascularization central scotoma, deterioration of colour vision, night vision, and contrast sensitivity, as well as subretinal fibrosis and visual-field sensitivity deterioration[1].
Pars plana victrectomy + membrane peeling
In situations with tractional macular edema, therapy is indicated with or without additional intravitreal steroid treatment[33].
Special Conditions
Blood sugar control patient, cataract surgery, pregnant DME patients, vitrectomized eyes, stable pre-existing glaucoma and endophthalmitis should all be considered while managing DME.
Blood sugar control
Poor glycemic control is linked to DME degeneration[34]. Glycemic management prevents diabetes from developing and slows down its course[35]. Education on the importance of managing diabetes is crucial for DME patients.
Cataract surgery
Prior to cataract surgery DME should preferably be treated and stabilized. Cataract surgery might provide the highest BCVA[36]. The potential development of cataracts should be discussed with patients using steroids for DME. Steroid therapy causes cataracts to worsen in those who already have them, requiring cataract surgery to attain the maximum BCVA. In DME patients who undergoing peri-operatively, anti-VEGF treatment can be given prior to cataract surgery or steroid treatment may be taken into consideration during cataract surgery[36].
Treatment in pregnant DME patients
Diabetic pregnant women are more prone to develop retinopathy and disease progression[37]. Despite substantial improvement in DME management, the recommended therapy for DME during pregnancy has remained the same throughout the years.
One of the therapies options is laser therapy. Other options include subthreshold laser and intravitreal corticosteroids. Due to concerns about fetal safety, anti-VEGF therapy should be avoided[38]. Intravitreal steroids are regarded as a pregnancy-safe treatment option for DME resistant to laser therapy[34]. Intravitreal dexamethasone implantation is effective treatment option for pregnant women with DME[39].
Vitrectomized eyes
The effectiveness of anti-VEGF in vitrectomized eyes is inconclusive[36]. There are no significant changes in BCVA and central macular thickness between vitrectomized and non-vitrectomized eyes after anti-VEGF injections in some studies, whilst others imply decreased intravitreal efficacy because of increased molecular clearance. In both vitrectomized and non-virectomized eyes, intravitreal dexamethasone implants are beneficial in treating chronic DME[40].
Stable pre-existing glaucoma
Presently there is no definition of “stable glaucoma”. However, the patient's clinician will consider glaucoma as ‘stable’ when the IOP remains below the target IOP which will be determined by the patient's clinician or when patients is on less than three medications and requiring no medication changes over a 48-month period during which no further visual field loss monitored[41]. Anti-glaucoma treatment should be continued throughout the course of steroid treatment in DME patients with stable pre-existing glaucoma. If IOP is not controlled, treatment should be stopped, and the patient should be sent to a glaucoma specialist[36].
Endophthalmitis
According to large scale meta-analyses, the incidence of endophthalmitis following intravitreal injections range from between 0.035% to 0.065%[42]. Out of 2928 injections of dexamethasone for DME, only two occurrences of acute endophthalmitis were recorded after treatment in the MEAD research[32]. Retrospective studies conducted in the United States found that the incidences of endophthalmitis following the use of aflibercept, bevacizumab, and ranibizumab were 0.100%, 0.056% and 0.046% respectively[43]. If patients did not react to intravitreal antibiotics, there are anecdotal situations where the implants were removed following vitrectomy in the lack of recommendations[44].
DISCUSSION
Appropriate treatment is required to prevent vision loss in DME. Prior to creating an individualized treatment plan for DME, it is crucial to consider risk factors such as the disease's severity, risk of cataracts, presence of exudates, history of vitrectomy, use of anti-VEGF and steroids, and patient compliance. DME can be treated with intravitreal corticosteroids, vitreoretinal surgery when required, anti-VEGF medications, and retinal laser photocoagulation. Several circulating proinflammatory cytokines such as hyperreflective retinal spots and subfoveal neuroretinal detachment have recently been explored as serum biomarkers for response in individuals with refractory DME, and their possible link with the DR and DME development[45]–[46]. DR severity is correlated to cytokine levels but not VEGF levels.
The effectiveness of anti-VEGF in vitrectomized eyes is uncertain. According to several research, that higher clearance of the molecule reduces intravitreal effectiveness[47], while in another research, patients with DME with vitrectomized and non vitrectomized eyes did not significantly vary in BCVA or central macular thickness following anti-VEGF injection[48]. Triamcinolone acetonide also shown similar outcomes[49].
In both vitrectomized and nonvitrectomized eyes, intravitreal dexamethasone implants are beneficial in treating chronic DME[40]. However, vitrectomy has no impact on the effectiveness or safety profile of dexamethasone implants for DME[50]. Spectral domain-OCT offers potential criteria for predicting dexamethasone implants response; nevertheless, more research is needed. When macular thickening cannot be detected clinically but can be measured by OCT, the disease is known as the subclinical DME. Lobo et al[51] found patients with evidence of subclinical DME have relatively small percentage to develop clinically severe DME with continuous monitoring, glycemic control, and comprehensive treatment for other risk factors such as hypertension and hyperlipidemia. The current gold standard of treatment is anti-VEGF therapy however, the use in different type of patients may result in interindividual differences[52].
The pathogenesis of DME is usually complicated by inflammation. In DME patients whose pathophysiology includes inflammation as a major factor, steroids may result in more favorable treatment results. A number of DME inflammatory pathways are also targeted by corticosteroids, particularly intravitreal dexamethasone, beside to VEGF. This involves retinal leukostasis, and synthesis of proinflammatory mediators (interleukin 6, monocyte chemoattractant protein-1), both of which are important in DME development[53].
A study conducted Sudhalkar et al[54] to determine the relationship between the position of dexamethasone intravitreal implants in the vitreous cavity and ocular hypertension, found that the treatment satisfaction of DME patients who received dexamethasone intravitreal implants had a statistically significant improvement. However, a study conducted in Sweden with anti-VEGF injections and additional; treatment such as laser and dexamethasone implants showed no change at four years when compared with baseline[55].
Proliferative DR and DME have different retinal microvascular patterns that indicate small-vessel disease. When DME is present in proliferative DR, patients on oral anti hyperglycemic medications may be at an increase chance of developing cardiovascular disease[56]. Pan-retinal photocoagulation should be used to treat naive proliferative DR. Patients with non-proliferative DR have two clinical options: the exudate production stage in DME or the proliferative changes of DR[57]. The first-line therapy is anti-VEGF injection in conjunction with pan-retinal photocoagulation in patients with severe non-proliferative DR who have progressed to the proliferative stage of DR. In the past several years, DME management has changed as a result of advancements in imaging technology and the introduction of new drugs. Therefore, in the future, in light of new research, our recommendations may need to be changed.
In conclusion, laser coagulation is the first line treatment for individuals without central macular disease. Patients with central macular involvement who have not recent experienced cardiovascular disease should be advised to start using anti-VEGF drugs. Steroids or changing to different anti-VEGF medication should be thought about in the event of non-responders. The safety measures that should be taken during steroid/intravitreal dexamethasone treatment owing to its potential side effects, including IOP spike, glaucoma, and cataract development, are well covered by the consensus recommendation.
Acknowledgments
We thank the Director General of Health, Malaysia for permission to publish this report. We thank all the stakeholders involved in this study.
Conflicts of Interest: Ngah NF, None; Muhamad NA, None; Mohamed SO, None; Abdul Aziz RA, None; Ma'amor NH, None; Ahmad Tarmidzi NA, None; Hashim H, None; Ishak H, None; Wan Muda WN, None; Muda R, None; Adnan A, None; Saleh RM, None; Wong HS, None; Mohd Jamil N, None; George TM, None; Koh A, None.
REFERENCES
- 1.Chauhan MZ, Rather PA, Samarah SM, Elhusseiny AM, Sallam AB. Current and novel therapeutic approaches for treatment of diabetic macular edema. Cells. 2022;11(12):1950. doi: 10.3390/cells11121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurreri A, Pazzaglia A. Diabetic macular edema: state of art and intraocular pharmacological approaches. Adv Exp Med Biol. 2021;1307:375–389. doi: 10.1007/5584_2020_535. [DOI] [PubMed] [Google Scholar]
- 3.Xiong K, Gong X, Li WT, Yuting L, Meng J, Wang LH, Wang W, Wenyong H. Comparison of macular thickness measurements using swept-source and spectral-domain optical coherence tomography in healthy and diabetic subjects. Curr Eye Res. 2021;46(10):1567–1573. doi: 10.1080/02713683.2021.1908566. [DOI] [PubMed] [Google Scholar]
- 4.Wykoff CC, Khurana RN, Nguyen QD, Kelly SP, Lum F, Hall R, Abbass IM, Abolian AM, Stoilov I, To TM, Garmo V. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care. 2021;44(3):748–756. doi: 10.2337/dc20-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas RL, Halim S, Gurudas S, Sivaprasad S, Owens DR. IDF Diabetes Atlas: a review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pract. 2019;157:107840. doi: 10.1016/j.diabres.2019.107840. [DOI] [PubMed] [Google Scholar]
- 6.Chew FLM, Salowi MA, Mustari Z, Husni MA, Hussein E, Adnan TH, Ngah NF, Limburg H, Goh PP. Estimates of visual impairment and its causes from the National Eye Survey in Malaysia (NESII) PLoS One. 2018;13(6):e0198799. doi: 10.1371/journal.pone.0198799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale MJ, Scruggs BA, Flaxel CJ. Diabetic eye disease: a review of screening and management recommendations. Clin Exp Ophthalmol. 2021;49(2):128–145. doi: 10.1111/ceo.13894. [DOI] [PubMed] [Google Scholar]
- 8.Murthy GVS. Situational analysis of diabetic retinopathy screening in India: how has it changed in the last three years? Indian J Ophthalmol. 2021;69(11):2944–2950. doi: 10.4103/ijo.IJO_1242_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bopp S, Kellner U. Pars plana vitrectomy. Klin Monbl Augenheilkd. 2019;236(5):705–722. doi: 10.1055/a-0849-0148. [DOI] [PubMed] [Google Scholar]
- 10.Kwan CC, Fawzi AA. Imaging and biomarkers in diabetic macular edema and diabetic retinopathy. Curr Diab Rep. 2019;19(10):95. doi: 10.1007/s11892-019-1226-2. [DOI] [PubMed] [Google Scholar]
- 11.Hagenau F, Vogt D, Ziada J, Guenther SR, Haritoglou C, Wolf A, Priglinger SG, Schumann RG. Vitrectomy for diabetic macular edema: optical coherence tomography criteria and pathology of the vitreomacular interface. Am J Ophthalmol. 2019;200:34–46. doi: 10.1016/j.ajo.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Koc F, Güven YZ, Egrilmez D, Aydın E. Optical coherence tomography biomarkers in bilateral diabetic macular edema patients with asymmetric anti-VEGF response. Semin Ophthalmol. 2021;36(5-6):444–451. doi: 10.1080/08820538.2021.1907423. [DOI] [PubMed] [Google Scholar]
- 13.Chou HD, Wu CH, Chiang WY, Chen NN, Hwang YS, Chen KJ, Lai CH, Wu PC, Chen YH, Yeung L, Shao SC, Lai CC, Wu WC. Optical coherence tomography and imaging biomarkers as outcome predictors in diabetic macular edema treated with dexamethasone implant. Sci Rep. 2022;12(1):3872. doi: 10.1038/s41598-022-07604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai CT, Hsieh YT, Lin CJ, Wang JK, Lin CY, Hsia NY, Bair H, Chen HS, Chiu CY, Weng SW. Age, initial central retinal thickness, and OCT biomarkers have an influence on the outcome of diabetic macular edema treated with ranibizumab- tri-center 12-month treat-and-extend study. Front Med (Lausanne) 2021;8:668107. doi: 10.3389/fmed.2021.668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202–208. doi: 10.1001/jamaophthalmol.2017.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YT, Chang YC, Meng PP, et al. Optical coherence tomography biomarkers in predicting treatment outcomes of diabetic macular edema after dexamethasone implants. Front Med (Lausanne) 2022;9:852022. doi: 10.3389/fmed.2022.852022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragiotta S, Abdolrahimzadeh S, Dolz-Marco R, Sakurada Y, Gal-Or O, Scuderi G. Significance of hyperreflective foci as an optical coherence tomography biomarker in retinal diseases: characterization and clinical implications. J Ophthalmol. 2021;2021:6096017. doi: 10.1155/2021/6096017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreur V, Altay L, van Asten F, et al. Hyperreflective foci on optical coherence tomography associate with treatment outcome for anti-VEGF in patients with diabetic macular edema. PLoS One. 2018;13(10):e0206482. doi: 10.1371/journal.pone.0206482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Călugăru D, Călugăru M. Detailed analysis of retinal morphology in patients with diabetic macular edema (DME) randomized to ranibizumab or triamcinolone treatment. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1035–1037. doi: 10.1007/s00417-018-3901-4. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, and RIDE Research Group RISE Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: rise and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer D, Holz FG, Heier JS, Midena E, Kaiser P, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Network DRCR, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Network DRCR, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curry BA, Sanfilippo PG, Chan S, Hewitt AW, Verma N. Clinical outcomes of a treat and extend regimen with intravitreal aflibercept injections in patients with diabetic macular edema: experience in clinical practice. Ophthalmol Ther. 2020;9(1):87–101. doi: 10.1007/s40123-019-00224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aderman CM, Garg SJ. Intravitreal anti-VEGF injection treatment algorithms for DME. Retina Today. 2017;4:53–55. [Google Scholar]
- 28.Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134(1):21–29. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 29.Zarbin MA, Dunger-Baldauf C, Haskova Z, Koovejee P, Mousseau MC, Margaron P, Snow H, Beaumont PE, Staurenghi G, Francom S. Vascular safety of ranibizumab in patients with diabetic macular edema: a pooled analysis of patient-level data from randomized clinical trials. JAMA Ophthalmol. 2017;135(5):424–431. doi: 10.1001/jamaophthalmol.2017.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain P, Sheth J, Anantharaman G, Gopalakrishnan M. Real-world evidence of safety profile of intravitreal bevacizumab (Avastin) in an Indian scenario. Indian J Ophthalmol. 2017;65(7):596–602. doi: 10.4103/ijo.IJO_992_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138(4):219–232. doi: 10.1016/j.jphs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Pai A, El Shafei MM, Mohammed OAZ, Al Hashimi M. Current concepts in intravitreal drug therapy for diabetic retinopathy. Saudi J Ophthalmol. 2010;24(4):143–149. doi: 10.1016/j.sjopt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yau GL, Silva PS, Arrigg PG, Sun JK. Postoperative complications of pars Plana vitrectomy for diabetic retinal disease. Semin Ophthalmol. 2018;33(1):126–133. doi: 10.1080/08820538.2017.1353832. [DOI] [PubMed] [Google Scholar]
- 34.Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res. 2015;2015:794036. doi: 10.1155/2015/794036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajalakshmi R, Prathiba V, Mohan V. Does tight control of systemic factors help in the management of diabetic retinopathy? Indian J Ophthalmol. 2016;64(1):62–68. doi: 10.4103/0301-4738.178146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chhablani J, Wong K, Tan GS, Sudhalkar A, Laude A, Cheung CMG, Zhao P, Uy H, Lim J, Valero S, Ngah NF, Koh A. Diabetic macular edema management in Asian population: expert panel consensus guidelines. Asia Pac J Ophthalmol (Phila) 2020;9(5):426–434. doi: 10.1097/APO.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 37.Peracha ZH, Rosenfeld PJ. Managing DME during pregnancy-Intravitreal steroids may be considered in cases refractory to blood glucose control and laser. Retina Specialist. 2015.
- 38.Rosenthal JM, Johnson MW. Management of retinal diseases in pregnant patients. J Ophthalmic Vis Res. 2018;13(1):62–65. doi: 10.4103/jovr.jovr_195_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo R, Kim HC, Chung H. Dexamethasone intravitreal implant for diabetic macular edema in a pregnant patient. Int J Ophthalmol. 2016;9(10):1524–1527. doi: 10.18240/ijo.2016.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medeiros MD, Alkabes M, Navarro R, Garcia-Arumí J, Mateo C, Corcóstegui B. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2014;30(9):709–716. doi: 10.1089/jop.2014.0010. [DOI] [PubMed] [Google Scholar]
- 41.Lakhani BK, Giannouladis K, Leighton P, King AJ. Seeking a practical definition of stable glaucoma: a Delphi consensus survey of UK glaucoma consultants. Eye (Lond) 2020;34(2):335–343. doi: 10.1038/s41433-019-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 43.Kiss S, Dugel PU, Khanani AM, Broder MS, Chang E, Sun GH, Turpcu A. Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis. Clin Ophthalmol. 2018;12:1625–1635. doi: 10.2147/OPTH.S169143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel N. Acute bacterial endophthalmitis following intravitreal dexamethasone implant: a case report and review of literature. Saudi J Ophthalmol. 2017;31(1):51–54. doi: 10.1016/j.sjopt.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med (Zagreb) 2020;30(3):030502. doi: 10.11613/BM.2020.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vujosevic S, Toma C, Villani E, Muraca A, Torti E, Florimbi G, Leporati F, Brambilla M, Nucci P, De Cilla' S. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020;57(3):287–296. doi: 10.1007/s00592-019-01424-4. [DOI] [PubMed] [Google Scholar]
- 47.García-Quintanilla L, Luaces-Rodríguez A, Gil-Martínez M, et al. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics. 2019;11(8):365. doi: 10.3390/pharmaceutics11080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edington M, Connolly J, Chong NV. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol. 2017;13(12):1217–1224. doi: 10.1080/17425255.2017.1404987. [DOI] [PubMed] [Google Scholar]
- 49.Orii Y, Gozawa M, Takamura Y, Takeuchi Y, Morioka M, Yamada Y, Matsumura T, Sugimoto M, Inatani M. Comparison of the intraocular pressure following an intravitreal triamcinolone acetonide injection for diabetic macula oedema in vitrectomised and non-vitrectomised eyes. BMJ Open Ophthalmol. 2021;6(1):e000620. doi: 10.1136/bmjophth-2020-000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishore K, Bhat PV, Venkatesh P, Canizela CC. Dexamethasone intravitreal implant for the treatment of macular edema and uveitis: a comprehensive narrative review. Clin Ophthalmol. 2022;16:1019–1045. doi: 10.2147/OPTH.S209395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lobo C, Santos T, Marques IP, Madeira MH, Santos AR, Figueira J, Cunha-Vaz J. Characterisation of progression of macular oedema in the initial stages of diabetic retinopathy: a 3-year longitudinal study. Eye (Lond) 2023;37(2):313–319. doi: 10.1038/s41433-022-01937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaytseva OV, Neroeva NV, Okhotsimskaya TD, Bobykin EV. Anti-VEGF therapy for neovascular age-related macular degeneration: causes of incomplete response. Vestn Oftalmol. 2021;137(5):152–159. doi: 10.17116/oftalma2021137051152. [DOI] [PubMed] [Google Scholar]
- 53.Minaker SA, Mason RH, Lahaie Luna G, Farahvash A, Garg A, Bhambra N, Bapat P, Muni RH. Changes in aqueous and vitreous inflammatory cytokine levels in diabetic macular oedema: a systematic review and meta-analysis. Acta Ophthalmol. 2022;100(1):e53–e70. doi: 10.1111/aos.14891. [DOI] [PubMed] [Google Scholar]
- 54.Sudhalkar A, Kodjikian L, Chhablani J, Bhojwani D, Vasavada A. Intraocular dexamethasone implant position in situ and ocular hypertension. Retina. 2018;38(12):2343–2349. doi: 10.1097/IAE.0000000000001883. [DOI] [PubMed] [Google Scholar]
- 55.Granstam E, Rosenblad A, Modher Raghib A, Granström T, Eriksson JW, Lindholm Olinder A, Leksell J. Long-term follow-up of antivascular endothelial growth factor treatment for diabetic macular oedema: a four-year real-world study. Acta Ophthalmol. 2020;98(4):360–367. doi: 10.1111/aos.14290. [DOI] [PubMed] [Google Scholar]
- 56.Obadă O, Pantalon AD, Rusu-Zota G, Hăisan A, Lupuşoru SI, Chiseliţă D. Choroidal assessment in patients with type 2 diabetes mellitus and non-proliferative diabetic retinopathy by swept-source ocular coherence tomography and image binarization. Medicina (Kaunas) 2022;58(7):918. doi: 10.3390/medicina58070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Fee JR, Juliano J, Moshfeghi AA. Factors associated with diabetic macular edema in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2022;260(7):2191–2200. doi: 10.1007/s00417-022-05595-9. [DOI] [PubMed] [Google Scholar]