Abstract
AIM
To explore the long-term efficacy, safety, and optical mechanism of orthokeratology with increased compression factor in adolescent myopia control.
METHODS
A prospective, double-masked, and randomized clinical trial was performed from May 2016 to June 2020. Subjects aged between 8 and 16y, with myopia (-5.00 to -1.00 D), low astigmatism (≥-1.50 D) and anisometropia (≤1.00 D), were stratified into low (-2.75 to -1.00 D) and moderate (-5.00 to -3.00 D) myopia groups. Then they were randomly assigned to wear either increased compression factor (ICF; 1.75 D) orthokeratology or conventional compression factor (CCF; 0.75 D) orthokeratology. The data were recorded including axial length (AL), spherical equivalent (SE), best corrected visual acuity (BCVA), near visual acuity (NVA), corneal staining (using Efron grading scales), corneal hysteresis (CH), corneal resistance factor (CRF), higher-order aberrations (HOAs, expressed as root mean square, RMSh), and subfoveal choroidal thickness (SFChT) in the 2-year follow-up period. Pearson's correlation coefficient was conducted to analyze the association between the changes in AL and RMSh, SFChT.
RESULTS
At the 2-year visit, there were no statistical differences in all the parameters between the ICF group and the CCF group in low myopia subjects (P>0.05). For the moderate myopia subjects, the ICF group had shorter AL elongation (0.23±0.08 vs 0.30±0.11 mm, P=0.015), higher RMSh (1.94±0.50 vs 1.65±0.51 µm, P=0.041), and higher SFChT (279.04±35.72 vs 254.08±29.60 µm, P=0.008) than those in CCF group. The change in AL was negatively correlated with RMSh (r=-0.687, P<0.001) and SFChT (r=-0.464, P=0.013).
CONCLUSION
ICF orthokeratology can control the progression of moderate myopia more effectively, which might be related to greater RMSh and SFChT.
Keywords: orthokeratology, compression factor, myopia, axial length, higher-order aberrations, choroidal thickness
INTRODUCTION
Orthokeratology (ortho-k) is a popular and non-surgical method that can suppress the progression of myopia effectively in adolescents[1]–[2]. Our previous study confirmed that wearing ortho-k for one year slowed down the axial elongation by 48.28%, compared with spectacles[3]. However, the overnight ortho-k effect is temporary, and daytime refractive regression after lens removal has been reported frequently, especially in patients with moderate to high myopia. Refractive regression can be compensated by the compression factor (The extra power added onto the basic refractive error, also known as the Jessen factor)[4].
Currently, many ortho-k lens manufacturers utilize conventional compression factor (CCF; 0.75 D). But there was still 0.25 D under-correction 4h after lens removal, and 0.50-0.75 D under-correction 8-10h after lens removal[5]–[6]. Therefore, the conventional compression factor may not achieve the expected correction target. Chan et al[7] analyzed 123 retrospective subjects and recommended that an extra 1.00 D should be added to maintain satisfactory unaided vision. Several short-term (4-7wk) studies[8]–[10] have shown that the increased compression factor (ICF; 1.75 D) did not affect lens fitting, or ocular health, but did correct refractive error more rapidly. However, the lack of long-term and comprehensive studies made the conclusion and mechanism of myopia control equivocal.
Short-term changes in higher-order aberrations (HOAs) and choroidal thickness are expected to be the efficacy predictors of ortho-k. Sun et al[11] revealed that greater HOAs may relate to effective myopia control. Chen et al[12] tried to alter the HOAs profile by modifying the lens design, but to date, no research has examined the effect of HOAs on the changes in axial length (AL) systematically. Changes in choroidal thickness after wearing ortho-k remain controversial[13]. However, some of these studies did not consider the diurnal variation, age, and spherical equivalent (SE), which could affect choroidal thickness[14].
Hence, this 2-year prospective, double-masked, and randomized study assessed the efficacy by AL, SE, best corrected visual acuity (BCVA), and assessed the safety by near visual acuity (NVA), corneal staining, corneal hysteresis (CH), corneal resistance factor (CRF), and explored the correlation of changes in AL with HOAs and subfoveal choroidal thickness (SFChT).
SUBJECTS AND METHODS
Ethical Approval
This study was in accordance with the ethical standards formulated in the Helsinki Declaration and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Chengdu Medical College (2020CYFYHEC-BA-30). All subjects and their parents had been told the complications and disadvantages of wearing ICF and CCF ortho-k in detail. Also, they were informed of the trial protocol, objectives, and procedures, then signed a written informed consent before commencement.
Subjects
This randomized, double-blinded clinical trial enrolled 153 children who visited the First Affiliated Hospital of Chengdu Medical College for refraction correction from May 2016 to June 2020. The participants should conform to the criteria in Table 1.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
| 8 to 16 years old (inclusive) | An experience of myopia control |
| Myopia: -5.00 to -1.00 D | Ocular or systemic disease |
| Anisometropia: ≤1.00 D | Contraindications for ortho-k lens |
| Astigmatism: ≥-1.50 D | Poor compliance |
| BCVA and NVA (logMAR) equal to or better than 0.00 | |
BCVA: Best corrected visual acuity; NVA: Near visual acuity.
Allocations and Treatments
The subjects were divided into 2 groups according to the severity of myopia: low myopia group (-2.75 to -1.0 D) and moderate myopia group (-5.00 to -3.00 D). Then they were randomly (using a Microsoft Excel random number, and the numbers were sealed in opaque envelopes) divided into the experimental group and the control group. Patients and the examiner were both blinded to group assignments. We prepared a disclosure envelope for each randomization number in case emergency unblinding becomes necessary, such as serious adverse events (e.g., infiltrates, pannus, microcysts, microbial keratitis). The experimental group wore ICF ortho-k, and the control group wore CCF ortho-k. All subjects were fitted with the Mouldway ortho-k lenses (Autek China Inc., Sino-US Joint Venture) composed of Boston XO. The initial lens parameters including compression factor were inputted into the computer software provided by the manufacturer. All Subjects should perform an ideal lens fitting and they were instructed to wear ortho-k lenses every night for at least 8h.
Measurements
All subjects should attend baseline and subsequent visits: 1d, 1wk, 1mo, 3mo, 6mo, 1y, 1.5y, and 2y after commencing lens wear. The aftercare visits were conducted from 8:00 to 10:00 a.m. and within 2h after lens removal. At the 1-year and 2-year visits, the subjects ceased lens wear for 3wk. Cycloplegic SE examination (using Tropiamide phenylephrine) was performed after the cessation period. Unscheduled consultations were provided if necessary to ensure the safety of lens wear.
AL was measured by IOLMaster 500 (Carl Zeiss, Germany). Five mean values were recorded if each difference <0.02 mm and SNR>2.0. SE was measured by open-field WAM-5500 (Grand Seiko, Japan). The first three values with a difference ≤0.25 D were recorded. ETDRS charts 2000 (Precision Vision, USA) was used to measure low-contrast (10%) best corrected visual acuity (LC BCVA) and high-contrast (>90%) best corrected visual acuity (HC BCVA) successively. NVA was measured by Jaeger chart. Corneal staining was graded by the Efron grading system. CH and CRF were measured by Ocular Response Analyzer (ORA, Reichert Corporation, USA). Three records with each score ≥5.0 were obtained. Ocular HOAs were measured by iTrace aberration analyzer (Tracey, USA) through the natural pupil in a dark room. The pupil diameter for analysis was 6 mm. The total HOAs were calculated by Zernike polynomial as the root mean square (RMSh). SFChT was determined by Spectralis OCT (Heidelberg Engineering, Germany) with the linear scan pattern (average of the vertical and horizontal line) through the enhanced depth imaging (EDI) mode. Automatic registration aided to capture repeat scans, and follow-up mode Spectralis helped to ensure the same measured location at each visit.
Sample Size
According to the randomized tests for two means (PASS11.0) and based on the previous research[15], it was estimated to induce at least a 0.17 mm difference in AL between the two groups over 2y, with a standard deviation (SD) of 0.21 mm. A sample size of 20 subjects in each group was required to achieve a power (1-β) of 80% and a significance level (α) of 0.05. Assuming 30% dropouts, a minimum total sample size of 116 subjects (low myopia 29:29, moderate myopia 29:29) was allocated.
Statistical Analysis
IBM SPSS Statistics 22.0 was applied for data analysis, and the data from right eyes were analyzed. Chi-squared test (or continuity-corrected Chi-square test, as appropriate) was used to compare the incidence of corneal staining and subjective discomforts between the two groups. The normality of the data was evaluated by Shapiro-Wilk tests. In the case of a normal distribution or approximate normal distribution, data were presented as mean±SD. The variance homogeneity was assessed by Levene's test. Then independent sample t-test was performed to assess the significant differences between the two groups. Pearson's correlation coefficient was conducted to calculate the correlation between the ocular biometrics (RMSh, SFChT) and AL change. A P value <0.05 was considered statistically significant.
RESULTS
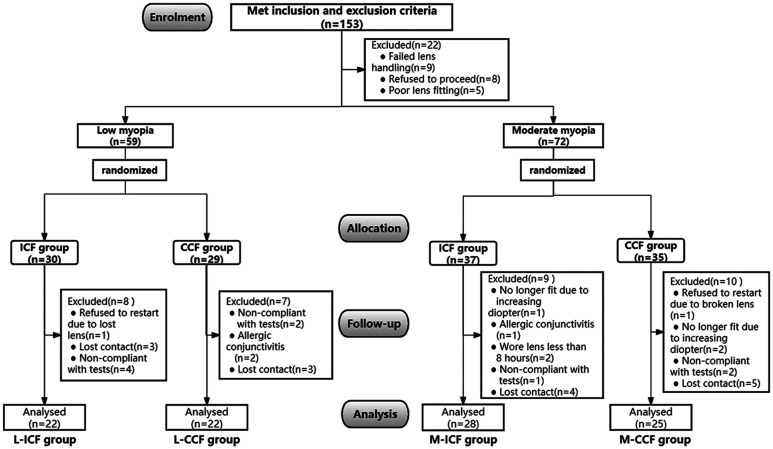
A total of 153 subjects were eligible to enroll in this research, of whom 131 subjects underwent fitting with the ortho-k lenses and proceeded to baseline data collection. A total of 59 low myopia subjects were randomly assigned to either ICF (L-ICF group, n=30) or CCF (L-CCF group, n=29) group, and 72 moderate myopia subjects were randomly assigned to either ICF (M-ICF group, n=37) or CCF (M-CCF group, n=35) group. Finally, a total of 97 (22 subjects in L-ICF group, 22 subjects in L-CCF group, 28 subjects in M-ICF group, and 25 subjects in M-CCF group) completed the 2-year follow-up (Figure 1).
Figure 1. Study flowchart.
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k.
Table 2 demonstrates the baseline biometric parameters of the analyzed subjects who completed the 2-year study. There were no statistical differences in baseline parameters between the ICF group and CCF group (all P>0.05). No serious adverse event was observed in the study period.
Table 2. Baseline data.
| Parameters | L-ICF group (n=22) | L-CCF group (n=22) | t | P | M-ICF group (n=28) | M-CCF group (n=25) | t | P |
| Age (y) | 11.32±1.78 | 10.50±1.65 | 1.578 | 0.122 | 12.04±2.36 | 12.52±2.16 | -0.775 | 0.442 |
| BCVA (logMAR) | -0.05±0.04 | -0.04±0.03 | -0.654 | 0.517 | -0.05±0.05 | -0.05±0.04 | -0.412 | 0.682 |
| SE (D) | -2.08±0.53 | -1.89±0.51 | -1.147 | 0.258 | -4.15±0.74 | -4.33±0.70 | 0.900 | 0.372 |
| AL (mm) | 23.97±0.63 | 23.95±0.61 | 0.126 | 0.901 | 24.80±0.82 | 25.02±0.96 | -0.869 | 0.389 |
| RMSh (µm) | 0.66±0.31 | 0.57±0.42 | 0.801 | 0.427 | 0.75±0.31 | 0.82±0.37 | -0.768 | 0.446 |
| SFChT (µm) | 263.05±23.59 | 259.00±16.15 | 0.664 | 0.511 | 229.07±27.54 | 224.16±25.59 | 0.670 | 0.506 |
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; BCVA: Best corrected visual acuity; SE: Spherical equivalent; AL: Axial length; RMSh: Higher-order aberrations expressed as root mean square; SFChT: Subfoveal choroidal thickness.
mean±SD
Changes in Axial Length
The AL elongation in the M-ICF group was statistically slower than that in the M-CCF group. Over 2y, the AL elongation was 0.23±0.08 mm in the M-ICF group and 0.30±0.11 mm in the M-CCF group (P=0.015). The ICF group was 23.33% more effective in slowing moderate myopia AL elongation than the CCF group. No significant differences between the L-ICF group and the L-CCF group were observed in terms of AL elongation (Table 3).
Table 3. Changes in axial length in different groups.
| Follow-ups | L-ICF group (n=22) | L-CCF group (n=22) | t | P | M-ICF group (n=28) | M-CCF group (n=25) | t | P |
| 6mo | 0.10±0.05 | 0.09±0.04 | 0.183 | 0.855 | 0.05±0.03 | 0.08±0.03 | -2.218 | 0.031 |
| 1y | 0.17±0.06 | 0.18±0.06 | -0.126 | 0.900 | 0.12±0.05 | 0.17±0.05 | -3.253 | 0.002 |
| 1.5y | 0.24±0.08 | 0.27±0.10 | -0.916 | 0.365 | 0.20±0.08 | 0.25±0.08 | -2.364 | 0.022 |
| 2y | 0.28±0.10 | 0.31±0.10 | -0.815 | 0.420 | 0.23±0.08 | 0.30±0.11 | -2.524 | 0.015 |
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k.
mean±SD, mm
Changes in Spherical Equivalent
The increases of SE (measured after lens cessation for 3wk) in the M-ICF group were lower than that in the M-CCF group (1-year cessation for 3wk: P=0.008, 2-year cessation for 3wk: P=0.023). However, there were no significant differences observed between the L-ICF group and the L-CCF group at all the yearly washout visits (Table 4).
Table 4. Changes in spherical equivalent after lens cessation for 3wk in different groups.
| Follow-ups | L-ICF group (n=22) | L-CCF group (n=22) | t | P | M-ICF group (n=28) | M-CCF group (n=25) | t | P |
| 1y | -0.36±0.28 | -0.43±0.33 | 0.744 | 0.461 | -0.22±0.20 | -0.42±0.29 | 2.794 | 0.008 |
| 2y | -0.55±0.36 | -0.63±0.45 | 0.662 | 0.512 | -0.35±0.28 | -0.57±0.40 | 2.355 | 0.023 |
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k.
mean±SD, D
Changes in Root Mean Square
Figure 2 represents changes and differences in RMSh values between the ICF group and CCF group during the study course. At the 3-month visit, RMSh differed significantly between the M-ICF group (1.96±0.49 µm) and the M-CCF group (1.61±0.46 µm, P=0.011). After 3mo of lens wear, M-ICF showed a significantly greater increase in RMSh (all P<0.05). No significant differences between the L-ICF group and the L-CCF group were observed in RMSh values.
Figure 2. the changes and differences in RMSh.
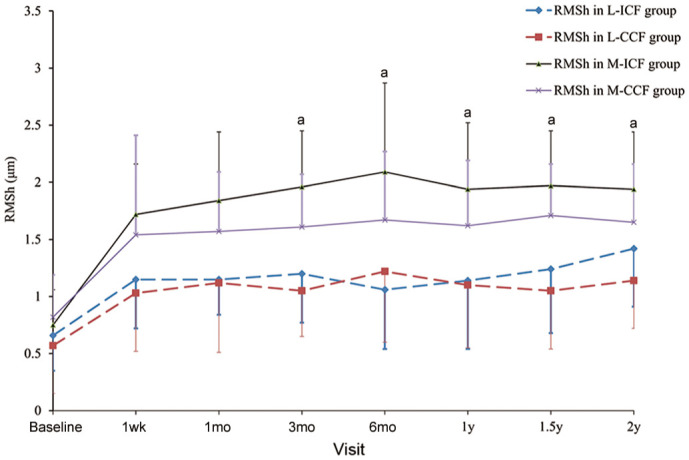
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; RMSh: Higher-order aberrations expressed as root mean square. aP<0.05, ICF group vs CCF group by independent sample t-test. Error bars represent standard deviation.
Changes in Subfoveal Choroidal Thickness
Changes and differences in SFChT for CCF and ICF groups are shown in Figure 3. SFChT in the M-ICF group was significantly thicker than that in the M-CCF group from the 6-month visit onward (P<0.05), except for the follow-up at 1.5y (P=0.086). However, there were no significant differences observed in terms of SFChT between the L-ICF group and the L-CCF group at all visits.
Figure 3. The changes and differences in SFChT.
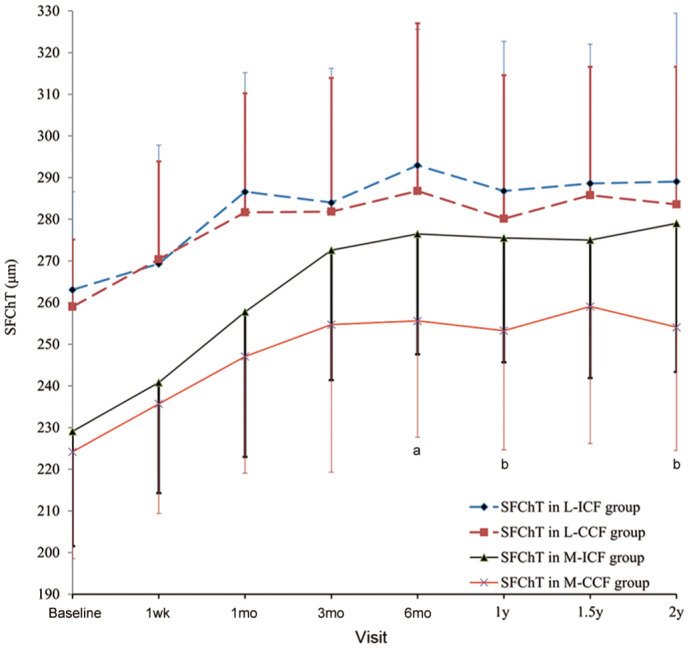
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k. SFChT: Subfoveal choroidal thickness. aP<0.05, bP<0.01, ICF group vs CCF group by independent sample t-test. Error bars represent standard deviation.
Correlation Analysis for Root Mean Square and Subfoveal Choroidal Thickness
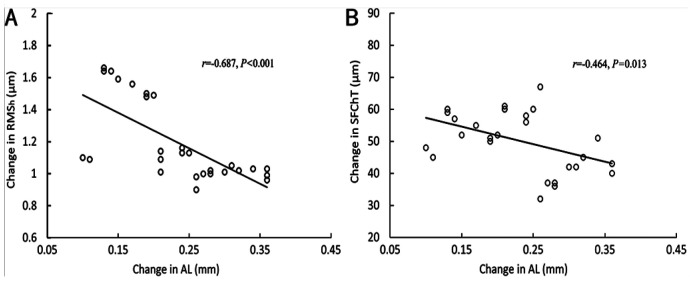
Since AL elongation differed statistically only between the M-ICF group and M-CCF group, and was shorter in the M-ICF group, correlation analysis was performed for RMSh and SFChT in the M-ICF group. Examined data from the M-ICF group revealed that change in AL over 2y had a negative association with the change in RMSh (r=-0.687, P<0.001; Figure 4A) and SFChT (r=-0.464, P=0.013; Figure 4B).
Figure 4. Scatterplots exhibit the correlation between the change in RMSh (A), SFChT (B) and the change in AL in the M-ICF group over 2y.
Regression lines indicate the coefficients of the Pearson correlation in the M-ICF group. AL: Axial length; RMSh: Higher-order aberrations expressed as root mean square; SFChT: Subfoveal choroidal thickness.
Comparisons of Other Measurements
There were no statistical differences between the ICF group and the CCF group for each parameter over 2y (all P>0.05; Table 5). No G3, G4 corneal staining (Efron's Grading Scale), poor near visual acuity, or poor dark adaptation was observed at any visit in all subjects.
Table 5. Comparisons of BCVA, NVA, CH, CRF, corneal staining, and subjective discomforts over 2y.
| Parameters | L-ICF group (n=22) | L-CCF group (n=22) | t/χ2 | P | M-ICF group (n=28) | M-CCF group (n=25) | t/χ2 | P |
| HC BCVA (logMAR) | -0.05±0.05 | -0.04±0.06 | -0.028 | 0.978 | -0.06±0.05 | -0.05±0.05 | -0.800 | 0.427 |
| LC BCVA (logMAR) | 0.24±0.13 | 0.20±0.14 | 1.124 | 0.268 | 0.23±0.19 | 0.31±0.19 | -1.729 | 0.088 |
| NVA (logMAR) | 0.03±0.02 | 0.03±0.03 | -0.437 | 0.664 | 0.06±0.03 | 0.04±0.05 | 1.602 | 0.118 |
| CH (mm Hg) | 11.15±1.72 | 10.86±1.80 | 0.544 | 0.590 | 10.94±1.69 | 10.91±1.71 | 0.048 | 0.962 |
| CRF (mm Hg) | 11.04±2.04 | 11.22±1.79 | -0.319 | 0.751 | 10.82±1.68 | 10.79±1.69 | 0.056 | 0.956 |
| Corneal staining (G1, %) | 18.18 | 13.64 | 0.000 | >0.999 | 21.43 | 20.00 | 0.016 | 0.898 |
| Corneal staining (G2, %) | 9.09 | 18.18 | 0.193 | 0.660 | 14.29 | 16.00 | 0.000 | >0.999 |
| Glare, ghosting (%) | 27.27 | 18.18 | 0.518 | 0.472 | 17.86 | 12.00 | 0.044 | 0.833 |
| Visual fluctuation (%) | 4.55 | 13.64 | 0.275 | 0.600 | 3.57 | 8.00 | 0.010 | 0.919 |
L-ICF: Low myopia subjects fitted with increased compression factor (1.75 D) ortho-k; L-CCF: Low myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; M-ICF: Moderate myopia subjects fitted with increased compression factor (1.75 D) ortho-k; M-CCF: Moderate myopia subjects fitted with conventional compression factor (0.75 D) ortho-k; LC BCVA: Low-contrast best corrected visual acuity; HC BCVA: High-contrast best corrected visual acuity; NVA: Near visual acuity; CH: Corneal hysteresis; CRF: Corneal resistance factor.
DISCUSSION
As the most important parameter for ortho-k lens design, the back optic zone radius (BOZR) was determined based on the Jessen formula. That formula is BOZR=Kf-T-CF, while Kf represents the flat keratometry, T represents the target myopic reduction and CF represents the compression factor. Lee et al[16] inferred that ICF ortho-k might steepen the mid-peripheral cornea and induce more peripheral myopic defocus to control myopia progression.
To the best of our knowledge, this is the first randomized trial to explore the efficacy and the optical mechanism of ICF ortho-k for myopic children stratified by myopia degrees over 2y. Table 6 illustrates the comparisons of current studies about ICF ortho-k.
Table 6. Comparisons of previous studies and our study.
| Study | Age range (y) | Sample size | Follow-up time | Myopia range (D) | Initial SE (D) | Change in AL (mm) | ||
| ICF | CCF | ICF | CCF | |||||
| Lau[8]–[9] | 7.8-11 | 28 | 4-7wk | -4.0 to -0.5 | -2.30±1.03 | -2.27±0.99 | -0.011 | 0.002 |
| Wan[10] | 6-10 | 25 | 4wk | -4.0 to -0.5 | -2.08±0.97 | -2.11±0.97 | - | |
| He[17] | 18-38 | 44 | 12mo | -5.0 to -0.75 | -3.46±0.90 | -3.84±0.77 | - | |
| Ren[18] | 20-36 | 50 | 1mo | -5.0 to -0.75 | -3.58±0.90 | -3.88±0.75 | - | |
| Wan[19] | 6-11 | 69 | 4wk | -4.0 to -0.5 | -2.12±0.86 | -2.17±0.81 | -0.031 | 0.003 |
| Present | 8-16 | 97 | 2y | -5.0 to -1.0 | -2.08±0.53 (L); -4.15±0.74 (M) | -1.89±0.51 (L); -4.33±0.70 (M) | 0.28 (L); 0.23 (M) | 0.31 (L); 0.30 (M) |
ICF: Increased compression factor (1.75 D) ortho-k; CCF: Conventional compression factor (0.75 D) ortho-k; SE: Spherical equivalent; L: Low myopia subjects; M: Moderate myopia.
Our results demonstrate that ICF ortho-k can correct low-moderate myopia in adolescents safely, which are basically consistent with conclusions drawn by He et al[17] and Ren et al[18].
Our most intriguing finding is that ICF ortho-k can prevent myopia progression more effectively in moderate myopia adolescents, as assessed by 2 primary endpoints (change in AL and SE). In the moderate myopia group, the ICF group was 23.33% more effective in slowing axial elongation than CCF group after 2-year therapy. The increase of SE in the M-ICF group manifested 0.22 D less than in the M-ICF group after 2-year therapy. Wan et al[19] reported that the axial elongation in ICF group (-0.031±0.06 mm) was significantly slower than that in CCF group (0.003±0.038 mm) after 4-week therapy, which was in agreement with our study. Nevertheless, Lau et al[8] concluded that there was no statistical difference in AL and SE between ICF group and CCF group. That might be caused by the different follow-up times, sample sizes, and baseline SE. While ICF ortho-k was invalid for more myopic control in low myopia adolescents. This was in accordance with the study proved by Lau et al[9]. However, we have to acknowledge that it was not so appropriate to compare these studies with ours simply because of differences in follow-up times, sample sizes, and demographic characteristics.
Our findings revealed that two factors might be responsible for slower axial growth in M-ICF group: greater higher-order aberrations (HOAs) or thicker choroidal thickness. On the one hand, this long-term study confirmed that ICF ortho-k led to a significant increase in HOAs and RMSh metrics, particularly after 3months. Recent studies investigated that children with higher levels of HOAs in the root-mean-square (RMS) values exhibited slower axial growth[20]–[22]. As the corneal shape is modified during ortho-k treatment, ocular aberrations, primarily HOAs, significantly increase[23]–[25]. The increase in HOAs may usually be asymmetric. This can provide directional cues to the retina to respond to different visual stimuli rapidly and accurately. This compensatory optimization of image quality can suppress axial elongation by improving accommodative facility[26]–[27]. In this study, HOAs increased significantly, demonstrating theoretically that the visual quality in moderate myopic children worsened after ICF ortho-k treatment. However, we found no difference in LC BCVA or subjective discomforts between the two groups. Therefore, we speculate that children with ICF ortho-k may experience a higher visual compensation process or a blur adaptation induced by increased HOAs and visual fluctuation[28]–[29]. Yet, we need to weigh the pros and cons of visual quality and myopia control.
On the other hand, this research also investigated that there was a negative correlation between the change in SFChT and AL at 2y in the M-ICF group. Therefore, it showed that choroidal thickening had a certain protective effect (r=-0.464) on axial growth. This was consistent with the results drawn by Lau et al[8] (r=-0.86), and Jin et al[30] (r=-0.4). However, Ding et al[31] held a different view that the change in SFChT was irrelevant to the change in SE. The cause might be different analysis indicators (SE vs AL), and different measurement times (10:00-15:00 vs 8:00-10:00 a.m.). Nevertheless, the mechanism remains unclear. We speculate that improvement in optical performance and steady-state structure may lead to an increase in choroidal blood circulation and thickness[32]. Choroidal thickening could change the optical imaging flat of the retina[33], or generate certain chemical substances (NO, etc.) that induce a signal of scleral remodeling to slowed axial elongation[34]. In recent years, EDI-SD OCT has been widely used in clinical practice to observe the choroidal tomographic structure and measure the thickness of the choroid due to its non-invasive, intuitive, and repeatable advantages[35]. However, the change of choroidal thickness after wearing ortho-k was slight (usually measured in tens of µm)[36]. We utilized automatic registration and follow-up mode Spectralis to guarantee the precision and identical location at each visit.
Two main limitations should be noticed. First, the sample size of this study was still insufficient to group by age or lens design. Second, we did not control for confounding factors such as genetic and environmental factors.
In conclusion, ortho-k with increased compression factor (an extra 1 D) demonstrated safe and effective myopia correction in adolescents during the observation period, and it could control moderate myopia progression more effectively, which might be related to increasing RMSh and SFChT. This study may provide a novel and effective ortho-k lens design for patients with moderate myopia.
Acknowledgments
Foundation: Supported by Education Department Foundation of Sichuan Province (No.15ZA0262).
Conflicts of Interest: Tang WT, None; Zhang L, None; Zhang HD, None; Li SB, None; Liang H, None.
REFERENCES
- 1.Sankaridurg P. Contact lenses to slow progression of myopia. Clin Exp Optom. 2017;100(5):432–437. doi: 10.1111/cxo.12584. [DOI] [PubMed] [Google Scholar]
- 2.Cho P, Cheung SW. Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO-SEE studies. Invest Ophthalmol Vis Sci. 2017;58(3):1411–1416. doi: 10.1167/iovs.16-20594. [DOI] [PubMed] [Google Scholar]
- 3.Tang WT, Li JQ, Zhou LS, Yu Q. Effect of orthokeratology on relative peripheral refraction in adolescent myopia. Guoji Yanke Zazhi (Int Eye Sci) 2021;21(4):734–737. [Google Scholar]
- 4.Maldonado-Codina C, Efron S, Morgan P, Hough T, Efron N. Empirical versus trial set fitting systems for accelerated orthokeratology. Eye Contact Lens. 2005;31(4):137–147. doi: 10.1097/01.icl.0000146170.27288.a3. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KL, Carney LG, Mountford JA, Collins MJ, Cluff S, Collins PK. Visual performance after overnight orthokeratology. Cont Lens Anterior Eye. 2007;30(1):29–36. doi: 10.1016/j.clae.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Sorbara L, Fonn D, Simpson T, Lu F, Kort R. Reduction of myopia from corneal refractive therapy. Optom Vis Sci. 2005;82(6):512–518. doi: 10.1097/01.opx.0000166772.68413.0e. [DOI] [PubMed] [Google Scholar]
- 7.Chan B, Cho P, Mountford J. The validity of the Jessen formula in overnight orthokeratology: a retrospective study. Ophthalmic Physiol Opt. 2008;28(3):265–268. doi: 10.1111/j.1475-1313.2008.00545.x. [DOI] [PubMed] [Google Scholar]
- 8.Lau JK, Wan K, Cheung SW, Vincent SJ, Cho P. Weekly changes in axial length and choroidal thickness in children during and following orthokeratology treatment with different compression factors. Transl Vis Sci Technol. 2019;8(4):9. doi: 10.1167/tvst.8.4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau JK, Vincent SJ, Cheung SW, Cho P. The influence of orthokeratology compression factor on ocular higher-order aberrations. Clin Exp Optom. 2020;103(1):123–128. doi: 10.1111/cxo.12933. [DOI] [PubMed] [Google Scholar]
- 10.Wan K, Lau JK, Cheung SW, Cho P. Refractive and corneal responses of young myopic children to short-term orthokeratology treatment with different compression factors. Cont Lens Anterior Eye. 2020;43(1):65–72. doi: 10.1016/j.clae.2019.10.134. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Wang L, Gao J, Yang M, Zhao Q. Influence of overnight orthokeratology on corneal surface shape and optical quality. J Ophthalmol. 2017;2017:3279821. doi: 10.1155/2017/3279821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Li M, Yuan Y, Me R, Yu Y, Shi G, Ke B. Interaction between corneal and internal ocular aberrations induced by orthokeratology and its influential factors. Biomed Res Int. 2017;2017:3703854. doi: 10.1155/2017/3703854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asmussen A, Smith BS, Møller F, Jakobsen TM. Repeatability and inter-observer variation of choroidal thickness measurements using swept-source optical coherence tomography in myopic Danish children aged 6-14 years. Acta Ophthalmol. 2022;100(1):74–81. doi: 10.1111/aos.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sander BP, Collins MJ, Read SA. The interaction between homatropine and optical blur on choroidal thickness. Ophthalmic Physiol Opt. 2018;38(3):257–265. doi: 10.1111/opo.12450. [DOI] [PubMed] [Google Scholar]
- 15.Vincent SJ, Cho P, Chan KY, Fadel D, Ghorbani-Mojarrad N, González-Méijome JM, Johnson L, Kang P, Michaud L, Simard P, Jones L. CLEAR - orthokeratology. Cont Lens Anterior Eye. 2021;44(2):240–269. doi: 10.1016/j.clae.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Lim DH, Chung TY, Hyun J, Han JS. Association of axial length growth and topographic change in orthokeratology. Eye Contact Lens. 2018;44(5):292–298. doi: 10.1097/ICL.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 17.He YH, Liu LQ, Vincent SJ. Compression factor and visual performance in adults treated with orthokeratology. Eye Contact Lens. 2021;47(7):413–419. doi: 10.1097/ICL.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 18.Ren Q, Yang B, Liu LQ, Cho P. Orthokeratology in adults and factors affecting success: study design and preliminary results. Cont Lens Anterior Eye. 2020;43(6):595–601. doi: 10.1016/j.clae.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Wan K, Lau JK, Cheung SW, Cho P. Orthokeratology with increased compression factor (OKIC): study design and preliminary results. BMJ Open Ophthalmol. 2020;5(1):e000345. doi: 10.1136/bmjophth-2019-000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraoka T, Kotsuka J, Kakita T, Okamoto F, Oshika T. Relationship between higher-order wavefront aberrations and natural progression of myopia in schoolchildren. Sci Rep. 2017;7(1):7876. doi: 10.1038/s41598-017-08177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau JK, Vincent SJ, Collins MJ, Cheung SW, Cho P. Ocular higher-order aberrations and axial eye growth in young Hong Kong children. Sci Rep. 2018;8(1):6726. doi: 10.1038/s41598-018-24906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes RP, Vincent SJ, Read SA, Collins MJ. Higher order aberrations, refractive error development and myopia control: a review. Clin Exp Optom. 2020;103(1):68–85. doi: 10.1111/cxo.12960. [DOI] [PubMed] [Google Scholar]
- 23.Vincent SJ, Tan Q, Ng ALK, Cheng GPM, Woo VCP, Cho P. Higher order aberrations and axial elongation in combined 0.01% atropine with orthokeratology for myopia control. Ophthalmic Physiol Opt. 2020;40(6):728–737. doi: 10.1111/opo.12730. [DOI] [PubMed] [Google Scholar]
- 24.Ding CL, Chen YY, Li X, Huang Y, Chen H, Bao J. The associations of accommodation and aberrations in myopia control with orthokeratology. Ophthalmic Physiol Opt. 2022;42(2):327–334. doi: 10.1111/opo.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau JK, Vincent SJ, Cheung SW, Cho P. Higher-order aberrations and axial elongation in myopic children treated with orthokeratology. Invest Ophthalmol Vis Sci. 2020;61(2):22. doi: 10.1167/iovs.61.2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122(1):93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Cheng X, Xu J, Chehab K, Exford J, Brennan N. Soft contact lenses with positive spherical aberration for myopia control. Optom Vis Sci. 2016;93(4):353–366. doi: 10.1097/OPX.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 28.Guo HC, Jin WQ, Pan AP, Wang QM, Qu J, Yu AY. Changes and diurnal variation of visual quality after orthokeratology in myopic children. J Ophthalmol. 2018;2018:3174826. doi: 10.1155/2018/3174826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu JJ, Tao CW, Mao XJ, Lu X, Bao J, Drobe B, Chen H. Blur detection sensitivity increases in children using orthokeratology. Front Neurosci. 2021;15:630844. doi: 10.3389/fnins.2021.630844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin WQ, Huang SH, Jiang J, Mao XJ, Shen MX, Lian Y. Short term effect of choroid thickness in the horizontal meridian detected by spectral domain optical coherence tomography in myopic children after orthokeratology. Int J Ophthalmol. 2018;11(6):991–996. doi: 10.18240/ijo.2018.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding X, Lu Y, Zhao F, Wang H, Xu J. Changes in macular choroidal thickness before and after orthokeratology in adolescents with low and moderate myopia. Journal of China Medical University. 2019;48(9):822–827. [Google Scholar]
- 32.Lee JH, Hong IH, Lee TY, Han JR, Jeon GS. Choroidal thickness changes after orthokeratology lens wearing in young adults with myopia. Ophthalmic Res. 2021;64(1):121–127. doi: 10.1159/000510715. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Zhang G, Zhou X, Xu R, Wang S, Guan Z, Lu J, Srinivasalu N, Shen M, Jin Z, Qu J, Zhou X. Changes in choroidal thickness and choroidal blood perfusion in Guinea pig myopia. Invest Ophthalmol Vis Sci. 2019;60(8):3074–3083. doi: 10.1167/iovs.18-26397. [DOI] [PubMed] [Google Scholar]
- 34.Hao Q, Zhao Q. Changes in subfoveal choroidal thickness in myopic children with 0.01% atropine, orthokeratology, or their combination. Int Ophthalmol. 2021;41(9):2963–2971. doi: 10.1007/s10792-021-01855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau JK, Cheung SW, Collins MJ, Cho P. Repeatability of choroidal thickness measurements with Spectralis OCT images. BMJ Open Ophthalmol. 2019;4(1):e000237. doi: 10.1136/bmjophth-2018-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Cui D, Hu Y, Ao S, Zeng J, Yang X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017;40(6):417–423. doi: 10.1016/j.clae.2017.09.010. [DOI] [PubMed] [Google Scholar]