Abstract
AIM
To examine the protection of ferulic acid (FA) against ionizing radiation (IR)-induced lens injury in rats, as well as the underlying mechanisms.
METHODS
FA (50 mg/kg) was administered to rats for 4 consecutive days before they were given 10 Gy γ-radiation, as well as for 3 consecutive days afterward. Two weeks after radiation, the eye tissues were collected. Histological alterations were evaluated by hematoxylin-eosin staining. Enzyme linked immunosorbent assay (ELISA) was utilized to assess the activities of glutathione reductase (GR) and superoxide dismutase (SOD), as well as the levels of glutathione (GSH) and malondialdehyde (MDA) in the lenses. The protein and mRNA levels of Bcl-2, caspase-3, Bax, heme oxygenase-1 (HO-1), and glutamate-cysteine ligase catalytic subunit (GCLC) were quantified using Western blot and quantitative reverse transcription polymerase chain reaction, respectively. With nuclear extracts, the nuclear factor erythroid-2 related factor (Nrf2) protein expressions in the nuclei were also measured.
RESULTS
Rats exposed to IR showed lens histological alterations which could be alleviated by FA. FA treatment reversed apoptosis-related markers in IR-induced lens, as evidenced by lower levels of Bax and caspase-3 and higher level of Bcl-2. Furthermore, IR induced oxidative damage manifested by decreased GSH level, increased MDA level, and decreased SOD and GR activities. FA boosted nuclear translocation of Nrf2 and increased the expressions of HO-1 and GCLC to inhibit oxidative stress, as evidenced by an increase in GSH, a decrease in MDA, and an increase in GR and SOD activities.
CONCLUSION
FA may work well in preventing and treating IR-induced cataract through promoting the Nrf2 signal pathway to attenuate oxidative damage and cell apoptosis.
Keywords: ferulic acid, ionizing radiation, lens, oxidative stress, Nrf2
INTRODUCTION
There have been numerous reported lens injuries from ionizing radiation (IR), particularly among atomic bomb survivors, astronauts, cyclotron workers, medical personnel, and radiotherapy patients[1]–[2]. One of the most radiosensitive tissues in the human body is the lens[3]. After IR exposure, it may suffer subtle alterations that take years to show obvious injury, that is clinically-defined cataract[4]. According to epidemiological observations, posterior subcapsular cataract has often been observed after IR exposure[3].
Cataract is the leading global cause of blindness[5]. Global data of World Health Organization (WHO) indicates that cataract accounts for 51% of global blindness. Currently, surgery is the only treatment for cataract, but it can be associated with vision-threatening complications, such as persistent inflammation, glaucoma, and endophthalmitis, especially in elderly patients and those with systematic diseases[6]–[8]. Therefore, non-surgical treatments for the prevention and management of cataract caused by IR are required.
Pathophysiology of IR-induced cataract is intricate and incompletely understood. Nevertheless, it is thought that oxidative stress and the resultant oxidative injury to the lens plays a crucial part in the beginning and progression of cataract[9]. To counteract oxidative stress, lenses contain antioxidant defense systems. However, when the amount of oxidative stress overwhelms the antioxidant defense, it will result in highly oxidized lenses and cataract[10]. One of the most crucial defenses against oxidative stress is the antioxidative system controlled by nuclear factor erythroid-2 related factor (Nrf2)[11]. In a previous study, we discovered that IR-induced cataract is associated with a dysfunctional Nrf2 antioxidative system in the lens[12]. Together, these suggest that activating the Nrf2 signal pathway might be a way to attenuate IR-induced lens injury.
Ferulic acid (FA) is a phenolic compound found in several Chinese medicines (such as Cimicifuga, Angelica sinensis, and Ligusticum chuanxiong) and edible plants (such as whole grain foods, grains, peanuts, grapefruits, oranges, and coffee) and has been found to have potent antioxidative and radioprotective effects by activating the Nrf2 signal pathway[13]–[14]. Nevertheless, the radioprotective properties of FA on lens have yet to be determined. This research aimed to examine the beneficial effect of FA on IR-induced lens injury and its underlying mechanisms, as well as to develop a novel agent to prevent or alleviate IR-induced cataract.
MATERIALS AND METHODS
Ethical Approval
All animal procedures and experimental protocols adhered to Jinling Hospital's Guidelines for Care and Use of Laboratory Animals and were approved by the hospital's Animal Ethics Committee (Nanjing, China, approval No.2020JLHGKJDWLS-109).
Animals Care and Grouping
Thirty male SD rats (weight of 200±20 g) were acquired from SiPeiFu biotech (Beijing, China, license No.SCXK 2019-0010) and randomly assigned into 3 groups: 1) control group rats received sham radiation; 2) IR group rats received 10 Gy γ-radiation and intragastric 0.9% saline solution; 3) FA+IR group rats received 10 Gy γ-radiation and intragastric FA (50 mg/kg) diluted in 0.9% saline solution. FA (Solarbio, Beijing, China) or 0.9% saline solution was given once daily for 7d: 4d prior to radiation and 3d after radiation. Two hours following the 4th administration of FA, the rats received a single dose of whole-body γ-radiation. Rats were irradiated using 60Co γ source (SQH-type, SanQiangHeLi Radiation Engineering Technology Co., Ltd., Beijing, China) at the Radiological Research Center of Nanjing University of Aeronautics and Astronautics (Nanjing, China). Rats under anesthesia were placed in well-ventilated perspex containers. They received a single dose of 10 Gy whole-body γ-radiation at a rate of 1.38 Gy/min. The γ-radiation dose was determined based on our previous study about the effect of neutron radiation on rat lens and Nrf2 signal pathway, and the biological effect of 3.6 Sv neutron radiation is equivalent to 10 Gy γ-radiation[12]. The FA dose was determined according to previous literatures[15]–[16]. On day 14 following irradiation, all the rats were sacrificed under anesthesia, and eye tissues were taken for the following investigations.
Histopathological Evaluation
The eyes were fixed, dehydrated, embedded, and then cut into sections. Following deparaffinization and rehydrated, sections were stained with hematoxylin-eosin (HE). The pathological alterations of lenses were examined using a microscope.
Biochemical Analysis
After homogenizing and centrifuging the lenses, and the supernatant was collected for further analyses. The activities of glutathione reductase (GR) and superoxide dismutase (SOD), as well as the levels of glutathione (GSH) and malondialdehyde (MDA) were evaluated with commercially available kits (Jiancheng, Nanjing, China) in accordance with the manufacturer's protocols.
Quantitative Real-time PCR Analysis
The lenses' RNA were isolated using Trizol reagent. Reverse transcription of RNA into cDNA was carried out using a 1st strand cNDA synthesis kit. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using Universal SYBR Master Mix (Vazyme Biotech, Nanjing, China) with StepOnePlus Real-Time PCR Systems. Table 1 shows the primers supplied by General Biol (Chuzhou, China). The internal parameter was GAPDH, and 2−ΔΔCT method was used to calculate the relative levels of mRNA.
Table 1. qRT-PCR primers.
| Gene | Primer sequences (5′-3′) |
| GCLC | F: ACAGCACGTTGCTCATCTCT |
| R: TGGTCAGACTCGTTGGCATC | |
| HO-1 | F: GCCTGGTTCAAGATACTACCTCT |
| R: CTGAGTGTGAGGACCCATCG | |
| Caspase-3 | F: GAGCTTGGAACGCGAAGAAA |
| R: GAGTCCATCGACTTGCTTCCA | |
| Bcl-2 | F: GGTGAACTGGGGGAGGATTG |
| R: AGAGCGATGTTGTCCACCAG | |
| Bax | F: ACGTCTGCGGGGAGTCAC |
| R: AGGCCCCTGTCTTCATGATCT |
qRT-PCR: Quantitative real-time polymerase chain reaction; HO-1: Heme oxygenase-1; GCLC: Glutamate-cysteine ligase catalytic subunit.
Western Blot Analysis
The lenses' total and nuclear protein were extracted using the whole cell lysis assay kit and nuclear protein extraction kit (KeyGen, Nanjing, China), respectively, according to the instructions provided by the manufacturer. Western blot was conducted as previously mentioned[12]. Primary antibodies (1:1000, abcam) included: Bax, caspase-3, Bcl-2, Nrf2, GCLC, and HO-1. The bands were visualized with ECL. Internal reference was GAPDH or histone H3.
Statistical Analysis
Data were shown as mean±standard deviation (SD) and analyzed using SPSS (version 17.0) with one-way analysis of variance (ANOVA) followed by least significant difference (LSD). P<0.05 was defined as statistically significant.
RESULTS
FA Improved IR-induced Histological Alterations in Rat Lens
HE staining was used to evaluate the protective impact of FA on IR-induced lens injury. Lens epithelial cells (LECs) were regularly arranged, and lens fibers were tightly packed in the control (Figure 1A, 1B). Lens in IR group revealed injuries, including irregularly arranged LECs with diversities in size and shape (Figure 1C), and vacuolization near lens' posterior pole (Figure 1D). FA improved the histological alterations of lens, however, couldn't restore them to normal (Figure 1E, 1F). These results indicated that FA attenuated IR-induced histological alterations in rat lens.
Figure 1. FA improved IR-induced morphological changes in rat lens.
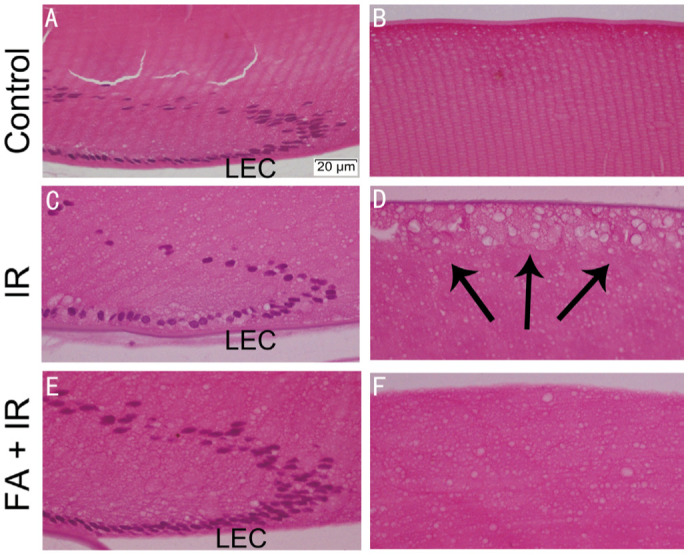
Lenses were sectioned and stained with HE (400×). Lens bow region in the control (A), IR (C), and FA+IR (E) groups. Lens posterior pole region in the control (B), IR (D), and FA+IR (F) groups. Black arrows indicate vacuolization under the posterior capsule. FA: Ferulic acid; IR: Ionizing radiation; HE: Hematoxylin and eosin; LEC: Lens epithelial cell.
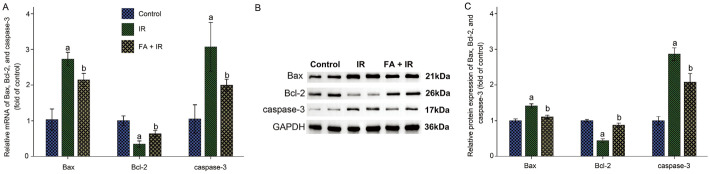
FA Regulated Expressions of Apoptosis-Related Markers in IR-induced Rat Lens
To explore the beneficial impact of FA on IR-induced cell apoptosis for rat lens, mRNA and protein expressions of apoptosis-related biomarkers (Bax, caspase-3, and Bcl-2) were measured in lens. As depicted in Figure 2, radiation induced a considerable rise in the mRNA and protein expressions of Bax and caspase-3 in rat lens, accompanied by a reduction of Bcl-2, compared to the control (P<0.05). FA administration intensely decreased mRNA and protein expressions of caspase-3 and Bax and increased Bcl-2 compared to the IR group (P<0.05). These results disclosed that FA treatment prevented IR-induced cell apoptosis in lens by reducing IR-induced apoptotic caspase-3 upregulation, preserving anti-apoptotic member Bcl-2 expression and suppressing pro-apoptotic member Bax expression.
Figure 2. FA regulated expression of apoptosis-related markers in IR-induced rat lens.
A: Bar graph of Bax, Bcl-2, and caspase-3 mRNA in each group; B: Representative bands of Bax, Bcl-2, caspase-3, and GAPDH; C: Bar graphs of Bax, Bcl-2, and caspase-3 protein expression in each group. Data were expressed as mean±SD. aP<0.05 vs control group; bP<0.05 vs IR group. FA: Ferulic acid; IR: Ionizing radiation.
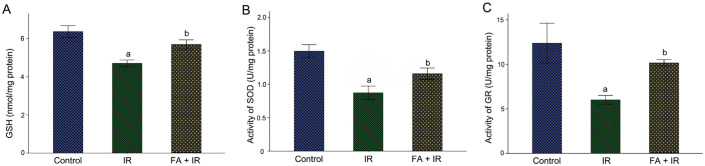
FA Ameliorated IR-induced Oxidative Stress in Rat Lens
MDA, a lipid peroxidation marker, was used to evaluate IR-induced oxidative stress in lens. As demonstrated in Figure 3, the MDA level was considerably elevated after irradiation versus the control (P<0.05); And FA treatment mitigated IR-induced MDA elevation (P<0.05). These results revealed that FA protected rat lens from oxidative damage induced by IR.
Figure 3. FA ameliorated IR-induced oxidative stress in rat lens.
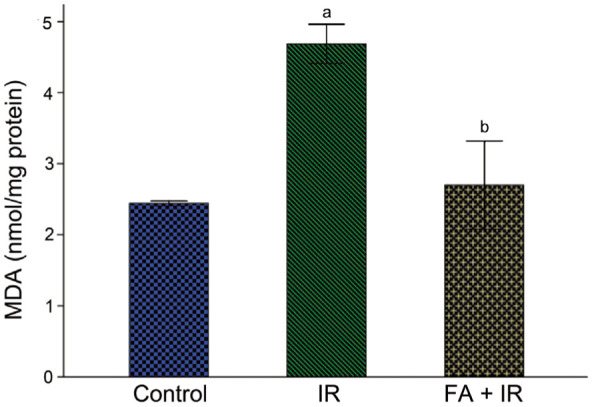
Rats exposed to radiation showed a significant increase of MDA in lens, whereas treatment with FA significantly ameliorated this alteration. Data were expressed as mean±SD. aP<0.05 vs control group; bP<0.05 vs IR group. FA: Ferulic acid; IR: Ionizing radiation; MDA: Malondialdehyde.
FA Upregulated Antioxidant Capacity of Rat Lens
We evaluated antioxidant ability in rat lens by analyzing the amount of GSH as well as activities of GR and SOD. As depicted in Figure 4, radiation significantly decreased the amount of GSH and the activities of SOD and GR in lens compared to the control (P<0.05). FA treatment enhanced the antioxidant capacity, as indicated by a higher level of GSH and upregulated activities of SOD and GR relative to the IR group (P<0.05). These results revealed that FA intervention increased lens oxidation resistance to IR.
Figure 4. FA upregulated antioxidant capacity in IR-induced rat lens.
Treatment with FA significantly ameliorated IR-induced decreased level of GSH (A), and increased activities of SOD (B) and GR (C). Data were expressed as mean±SD. aP<0.05 vs control group; bP<0.05 vs IR group. FA: Ferulic acid; IR: Ionizing radiation; GSH: Glutathione; SOD: Superoxide dismutase; GR: Glutathione reductase.
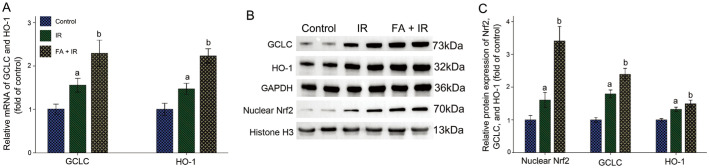
FA Activated Nrf2-mediated Antioxidative Signal Pathway in IR-induced Rat Lens
Considering the importance of Nrf2 in mitigating oxidative stress, we looked into how FA affected Nrf2 signaling in the lens of IR-induced rats. As depicted in Figure 5, FA administration promoted the translocation of Nrf2 to nuclei and upregulated mRNA and protein expressions of its downstream genes (HO-1 and GCLC) compared to the IR group (P<0.05). These results suggested that the increased antioxidant capacity in lens of FA-treated radiated rats might be related to FA-induced upregulation of Nrf2 signal pathway.
Figure 5. FA augmented Nrf2-mediated antioxidative signal pathway in IR-induced rat lens.
A: Bar graph of mRNA of HO-1 and GCLC; B: Representative bands of nuclear Nrf2, GCLC, and HO-1; C: Bar graph of protein expressions of nuclear Nrf2, GCLC, and HO-1. Data were expressed as mean±SD. aP<0.05 vs control group; bP<0.05 vs IR group. FA: Ferulic acid; IR: Ionizing radiation; HO-1: Heme oxygenase-1; GCLC: Glutamate-cysteine ligase catalytic subunit.
DISCUSSION
IR-induced cataract is a well-known complication related to radiation exposure. IR primarily affects the germinative dividing cells of LECs[17]. These affected LECs differentiate into new fiber cells which migrate to the posterior pole and cover on the old lens fiber cells[17]–[18]. Because of the avascular nature of the lens, there is no mechanism to remove these aberrant cells, and they accumulate with time to form a cataract, which is clinically manifested by posterior subcapsular opacity[3],[18]. Posterior subcapsular opacity is primarily due to the aberrations in the outermost layers of the lens[19]. Before the development of symptomatic cataract as these cells accumulate, an intermediate stage in cataract formation occurs[17]. HE staining in our study disclosed aberrant LECs and vacuolization near lens' posterior pole after γ-radiation which is consistent with these researches.
The mechanism of IR-induced cataract is complex, nevertheless, oxidative injury plays a significant role in cataract beginning and development. We propose that because of the oxidative damage induced by IR, the newly differentiated lens fiber cells from LECs may have less antioxidant capacity, and that these alterations can lead to oxidation and crystallin accumulation in the posterior areas which results in the vacuolization of the posterior subcapsular region. Therefore, activating the intracellular antioxidant defense capacity is crucial for preventing IR-induced oxidative damage to the lens. Numerous studies have shown that FA, a phenolic acid extracted from numerous edible and medicinal plants, possesses potent antioxidant and radioprotective properties due to its capacity to increase nuclear accumulation of Nrf2 and increase its downstream antioxidant genes[13]–[14]. However, there are no data regarding the impact of FA on IR-induced cataract. Thus, we assume that FA protects against IR-induced lens damage through activating the Nrf2 signaling pathway to combat oxidative stress. The findings of the current study confirm our idea. FA administration improved IR-induced histological alterations of rat lens, such as decreased vacuolization near lens' posterior pole and less irregularly arranged LECs.
All the cells in the lens are differentiated from LECs, which take an important part in sustaining the lens's transparency and the stability of the internal environment[20]. It is well-known that LECs apoptosis is associated with cataract formation[21]–[22]. In this study, radiation induced an increase of caspase-3 and Bax in the lens, along with a decrease of Bcl-2, which was also demonstrated in previous studies that radiation-induced cell apoptosis is controlled by the balance between anti-apoptotic factor Bcl-2 and pro-apoptotic factor Bax[23]. Caspase-3 participates in apoptosis as the main executor in the caspase cascade[24]. Previous study showed that FA was able to alleviate IR-induced caspase-3 expression and cell apoptosis by regulating Bax and Bcl-2 in murine duodenum[25]. Similarly, in present study, FA intervention attenuated IR-induced cellular apoptosis, manifested by decreased Bax and caspase-3 levels and increased Bcl-2 levels. These findings suggested that FA inhibited IR-induced cell apoptosis in rat lens.
LEC apoptosis and lens damage is significantly influenced by IR-induced oxidative stress[12],[26]. A normal lens has antioxidant defense systems, including antioxidant enzymes and small molecular antioxidants, to counteract oxidative stress[19]. GSH, maintaining cellular redox homeostasis, is the primary small molecular antioxidant and is synthesized and regenerated on lens cortex[19]. Upon oxidative stress, GSH is transformed into the oxidized form (GSSG) which can be reinstated through GR (an enzymatic antioxidant in lens) action[27]. SOD, another enzymatic antioxidant in lens, can transform O2- into H2O2 to scavenge free radicals and reduce oxidative stress. In our study, radiation caused a significant decrease of GSH and decreased SOD and GR activities, and an increase in MDA level in lens. This has also been demonstrated in other studies that radiation can cause oxidative stress of tissues[28]. The decline of GSH might be caused by the interaction between free radicals and GSH, and the decreased activities of SOD and GR could lead to an increase in superoxide flux in cellular compartments, which causes an increase in lipid peroxidation of membrane unsaturated fatty acids, and ultimately leads to the death of LECs and the formation of cataract[16],[18]. Furthermore, our study showed that FA administration protected lens from IR-induced downregulation of GSH and decreased activities of GR and SOD, as well as mitigated IR-induced lipid peroxidation. El-Mesallamy et al[16] and Srinivasan et al[28] also revealed the protective impact of FA on improving antioxidant state and alleviating IR-induced oxidative damage. The antioxidant effect of FA may be attributed for its ability to form a stable resonat structure of the phenoxy radical to scavenge and stop free radical chain reactions[28].
Nrf2 is a crucial transcription factor for the regulation cellular anti-oxidation-reduction and oxidative stress[11]. Normally, Nrf2 is sequestered within the cytoplasm. Once activated by signals such as IR-induced free radicals, Nrf2 translocates to nuclei and binds to antioxidant responsive element, which upregulates its downstream antioxidant genes, such as GCLC and HO-1[29]. GCLC is a catalytic subunit of glutamate-cysteine ligase (GCL) which catalyzes the initial and rate-limiting step in GSH synthesis[30]. HO-1 is an antioxidant enzyme that can breakdown heme to CO, biliverdin, and iron. Hemoglobin emits harmful free heme in response to oxidative stress, whereas HO-1 and its isoenzymes can accelerate the breakdown of free heme, exert antioxidant effects, and increase cell survival[29]. In our investigation, radiation slightly promoted the nuclear translocation of Nrf2 and increased GCLC and HO-1. This is consistent with other studies that radiation was able to activate Nrf2 signal pathway[30]. Even though the Nrf2 signal pathway was activated in the IR group, radiation still produced lens damage. This may be owing to the excessively high levels of free radicals induced by IR which overwhelmed the Nrf2 antioxidant defense protection and resulted in oxidized lens[20]. However, FA administration promoted Nrf2 nuclear translocation further, significantly upregulated HO-1 and GCLC, and attenuated histological alterations in lens. Other studies also found that FA's protection against IR-induced toxicity might contribute to the upregulation of Nrf2 signal pathway[13],[25]. These results explained the molecular mechanism of FA on protecting lens against IR-induced injury.
A limitation of this study is the short observation period due to the poor condition of rats two weeks after being exposed to radiation. Another limitation of the current study was that Nrf2-knockout rats were not employed. Further study employing Nrf2-knockout rats with lower radiation dosage and longer assessment period is necessary to validate the role of activating the Nrf2 signal pathway for FA to exert its radioprotective effects.
To summarize, we report that FA could protect rat lens against γ-radiation by inhibiting IR-induced oxidative stress and cellular apoptosis via activation of the Nrf2 signal pathway. Therefore, FA-enriched diet can be beneficial for radiation exposure related population, such as radiotherapy patients, space workers, and medical workers. However, it warrants further clinical trial.
Acknowledgments
We would like to thank Radiological Research Center of Nanjing University of Aeronautics and Astronautics for providing 60Co γ equipment.
Foundations: Supported by Medical Science Foundation of Military for Young Scholars (No.19QNP064); Natural Science Foundation of Jiangsu Province (No.BK20191233); Jiangsu Funding Program for Excellent Postdoctoral Talent (No.2022ZB702).
Conflicts of Interest: Chen Y, None; Shen J, None; Zhang X, None; Gao W, None; Cao Q, None; Yan F, None; Xue C, None.
REFERENCES
- 1.Hamada N, Azizova TV, Little MP. An update on effects of ionizing radiation exposure on the eye. Br J Radiol. 2020;93(1115):20190829. doi: 10.1259/bjr.20190829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamada N, Sato T. Cataractogenesis following high-LET radiation exposure. Mutat Res Rev Mutat Res. 2016;770(Pt B):262–291. doi: 10.1016/j.mrrev.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Ainsbury EA, Barnard S, Bright S, et al. Ionizing radiation induced cataracts: recent biological and mechanistic developments and perspectives for future research. Mutat Res Rev Mutat Res. 2016;770(Pt B):238–261. doi: 10.1016/j.mrrev.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Bahia S, Blais E, Murugkar S, Chauhan V, Kumarathasan P. Oxidative and nitrative stress-related changes in human lens epithelial cells following exposure to X-rays. Int J Radiat Biol. 2018;94(4):366–373. doi: 10.1080/09553002.2018.1439194. [DOI] [PubMed] [Google Scholar]
- 5.Hilliard A, Mendonca P, Russell TD, Soliman KFA. The protective effects of flavonoids in cataract formation through the activation of Nrf2 and the inhibition of MMP-9. Nutrients. 2020;12(12):3651. doi: 10.3390/nu12123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JV, Patel DV, Squirrell D, McGhee CN. Cystoid macular oedema following cataract surgery: a review. Clin Exp Ophthalmol. 2019;47(3):346–356. doi: 10.1111/ceo.13513. [DOI] [PubMed] [Google Scholar]
- 7.Peterson SR, Silva PA, Murtha TJ, Sun JK. Cataract surgery in patients with diabetes: management strategies. Semin Ophthalmol. 2018;33(1):75–82. doi: 10.1080/08820538.2017.1353817. [DOI] [PubMed] [Google Scholar]
- 8.Haripriya A, Baam ZR, Chang DF. Endophthalmitis prophylaxis for cataract surgery. Asia Pac J Ophthalmol (Phila) 2017;6(4):324–329. doi: 10.22608/APO.2017200. [DOI] [PubMed] [Google Scholar]
- 9.Braakhuis AJ, Donaldson CI, Lim JC, Donaldson PJ. Nutritional strategies to prevent lens cataract: current status and future strategies. Nutrients. 2019;11(5):1186. doi: 10.3390/nu11051186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T. Age-related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012;200(1):1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YQ, Feng JD, Liu JY, Zhou H, Luo HY, Xue CY, Gao WP. Effects of neutron radiation on Nrf2-regulated antioxidant defense systems in rat lens. Exp Ther Med. 2021;21(4):334. doi: 10.3892/etm.2021.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das U, Manna K, Khan A, Sinha M, Biswas S, Sengupta A, Chakraborty A, Dey S. Ferulic acid (FA) abrogates γ-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic Res. 2017;51(1):47–63. doi: 10.1080/10715762.2016.1267345. [DOI] [PubMed] [Google Scholar]
- 14.Qi MY, Wang XT, Xu HL, Yang ZL, Cheng Y, Zhou B. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats. Food Funct. 2020;11(4):3706–3718. doi: 10.1039/c9fo02398d. [DOI] [PubMed] [Google Scholar]
- 15.Das U, Biswas S, Sengupta A, Manna K, Chakraborty A, Dey S. Ferulic acid (FA) abrogates ionizing radiation-induced oxidative damage in murine spleen. Int J Radiat Biol. 2016;92(12):806–818. doi: 10.1080/09553002.2016.1230241. [DOI] [PubMed] [Google Scholar]
- 16.EL-Mesallamy HO, Gawish RA, Sallam AA M, Fahmy HA, Nada AS. Ferulic acid protects against radiation-induced testicular damage in male rats: impact on SIRT1 and PARP1. Environ Sci Pollut Res. 2018;25(7):6218–6227. doi: 10.1007/s11356-017-0873-6. [DOI] [PubMed] [Google Scholar]
- 17.Seals KF, Lee EW, Cagnon CH, Al-Hakim RA, Kee ST. Radiation-induced cataractogenesis: a critical literature review for the interventional radiologist. Cardiovasc Intervent Radiol. 2016;39(2):151–160. doi: 10.1007/s00270-015-1207-z. [DOI] [PubMed] [Google Scholar]
- 18.Elanchezhian R, Palsamy P, Madson CJ, Mulhern ML, Lynch DW, Troia AM, Usukura J, Shinohara T. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 2012;3(4):e301. doi: 10.1038/cddis.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cekić S, Zlatanović G, Cvetković T, Petrović B. Oxidative stress in cataractogenesis. Bosn J Basic Med Sci. 2010;10(3):265–269. doi: 10.17305/bjbms.2010.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JI, Kim J, Choung SY. Polyphenol-enriched fraction of Vaccinium uliginosum L. protects selenite-induced cataract formation in the lens of Sprague-Dawley rat pups. Mol Vis. 2019;25:118–128. [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Han X, Piao MJ, et al. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J Cancer Prev. 2016;21(1):41–47. doi: 10.15430/JCP.2016.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang FX, Ma J, Han F, Guo XJ, Meng L, Sun YF, Jin C, Duan HJ, Li H, Peng Y. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep. 2016;6:19396. doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu N, Liu R, He LX, Mao RX, Liu XR, Zhang T, Hao YT, Fan R, Xu MH, Li Y. Radioprotective effect of walnut oligopeptides against gamma radiation-induced splenocyte apoptosis and intestinal injury in mice. Molecules. 2019;24(8):1582. doi: 10.3390/molecules24081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Zheng. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J Inorg Biochem. 2019;193:143–151. doi: 10.1016/j.jinorgbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Das U, Sengupta A, Biswas S, Adhikary A, Dey Sharma R, Chakraborty A, Dey S. Alteration of murine duodenal morphology and redox signalling events by reactive oxygen species generated after whole body γ-irradiation and its prevention by ferulic acid. Free Radic Res. 2017;51(11-12):886–910. doi: 10.1080/10715762.2017.1388916. [DOI] [PubMed] [Google Scholar]
- 26.Chen YQ, Zhu L, Meng H, Sun XD, Xue CY. Ferulic acid protects human lens epithelial cells against ionizing radiation-induced oxidative damage by activating Nrf2/HO-1 signal pathway. Oxid Med Cell Longev. 2022;2022:6932188. doi: 10.1155/2022/6932188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai CF, Wu JY, Hsu YW. Protective effects of rosmarinic acid against selenite-induced cataract and oxidative damage in rats. Int J Med Sci. 2019;16(5):729–740. doi: 10.7150/ijms.32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology. 2006;228(2-3):249–258. doi: 10.1016/j.tox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Feng WJ, Yang XJ, Feng MP, et al. Alginate oligosaccharide prevents against D-galactose-mediated cataract in C57BL/6J mice via regulating oxidative stress and antioxidant system. Curr Eye Res. 2021;46(6):802–810. doi: 10.1080/02713683.2020.1842456. [DOI] [PubMed] [Google Scholar]
- 30.Liu KT, Singer E, Cohn W, Micewicz ED, McBride WH, Whitelegge JP, Loo JA. Time-dependent measurement of Nrf2-regulated antioxidant response to ionizing radiation toward identifying potential protein biomarkers for acute radiation injury. Proteomics Clin Appl. 2019;13(6):e1900035. doi: 10.1002/prca.201900035. [DOI] [PMC free article] [PubMed] [Google Scholar]