Abstract
AIM
To analyze and calculate the relative cost of various childhood glaucoma surgical interventions per mm Hg intraocular pressure (IOP) reduction ($/mm Hg).
METHODS
Representative index studies were reviewed to quantitate the reduction of mean IOP and glaucoma medications for each surgical intervention in childhood glaucoma. A US perspective was adopted, using Medicare allowable costs to calculate cost/mm Hg IOP reduction ($/mm Hg) at 1y postoperatively.
RESULTS
At 1y postoperatively, the cost/mm Hg IOP reduction was $226/mm Hg for microcatheter-assisted circumferential trabeculotomy, $284/mm Hg for cyclophotocoagulation, $288/mm Hg for conventional ab-externo trabeculotomy, $338/mm Hg for Ahmed glaucoma valve, $350/mm Hg for Baerveldt glaucoma implant, $351/mm Hg for goniotomy, and $400/mm Hg for trabeculectomy.
CONCLUSION
Microcatheter-assisted circumferential trabeculotomy is the most cost-efficient surgical method to lower IOP in childhood glaucoma, while trabeculectomy is the least cost-efficient surgical method.
Keywords: glaucoma, cost analysis, trabeculotomy, trabeculectomy, intraocular pressure, childhood glaucoma
INTRODUCTION
Childhood glaucoma is a heterogeneous group of disorders characterized by increased intraocular pressure (IOP), which, if left untreated, leads to irreversible optic nerve head damage and vision loss[1]–[3]. It represents almost 7% of the causes of blindness in the pediatric age group worldwide[4]. And, in some countries, it is the third leading cause of blindness[4].
The Childhood Glaucoma Research Network proposed a new classification system, categorizing childhood glaucoma into seven subtypes[5]. The incidence of childhood glaucoma and glaucoma suspects has been estimated to be 2.29/100 000 in patients aged <20y, with primary congenital glaucoma (PCG) being the most common type. The incidence of PCG varies in different parts of the world, ranging from 1/10 000-20 000 newborns in the western world up to 1/8000 in the middle east[6]–[7].
The management of most types of childhood glaucoma is mainly surgical; medications are used only as adjunctive therapy before or after surgery. Angle-based surgeries (goniotomy or trabeculotomy) are the mainstay of PCG treatment. Trabeculectomy and glaucoma drainage devices (GDDs) are usually reserved for PCG patients who failed angle surgeries or secondary childhood glaucoma patients[8]–[11]. Trans-scleral cyclophotocoagulation (CPC) is usually considered in refractory childhood glaucoma cases; however, some authors used it at earlier glaucoma stages[12].
Minimally (or micro-) invasive glaucoma surgeries have been reported in managing childhood glaucoma; however, the current evidence in the literature is still limited[13]–[15].
Childhood glaucoma not only affects vision but often also affects the psychological, socioeconomic, and functional well-being of the children and their legal guardians[16]–[17]. Adult glaucoma in the US incurs an incremental economic burden of $9.2 billion per year[18]–[19]. However, limited literature is currently available on the economic burden of childhood glaucoma[20]. The current study aims to evaluate and analyze the direct costs for the most commonly used surgical intervention in childhood glaucoma using the newly introduced parameter of cost per mm Hg IOP reduction.
MATERIALS AND METHODS
Ethical Approval
This study was exempt from institutional review board approval of the University of Miami since no patient information or medical records were needed. The study conformed with the principles of the Declaration of Helsinki.
A literature search was performed in the electronic databases of PubMed, Google Scholar, Embase the Register of Controlled Trials, and Ovid Medline using the combination of the following terms: “congenital glaucoma”, “childhood glaucoma”, “pediatric glaucoma”, “tubes”, “glaucoma drainage devices”, “Ahmed glaucoma valve”, Baerveldt implant”, “trabeculectomy”, “cyclophotocoagulation”, “circumferential trabeculotomy”, “ab-externo trabeculotomy”, “microcatheter assisted trabeculotomy” “ab-interno trabeculotomy”, “gonioscopy assisted transluminal trabeculotomy (GATT)”, “goniotomy”. There were no restrictions on language or year of publication. After screening titles and abstracts, full texts were retrieved for eligible studies for review and possible inclusion in the analysis (Figure 1).
Figure 1. Flow chart of the identified, included, and excluded studies used for utilization and outcome analysis.
A systematic review of representative index prospective or retrospective studies that evaluated surgical management in childhood glaucoma patients (<18y) were included in the analysis, as indicated below. We excluded case reports, case series, and studies that did not report the age of the patients, baseline IOP or medications, or IOP and medication changes at 1y of follow-up. Studies involving combined techniques such as combined trabeculotomy-trabeculectomy and combined goniotomy and trabeculotomy were excluded. In the conventional trabeculotomy group, studies reporting outcomes of surgical techniques other than 180° degrees trabeculotomy using Harms trabeculotomy (e.g., two-site trabeculotomy technique) were excluded. In the circumferential trabeculotomy, only studies performed by microcatheter technique were included. Novel glaucoma devices and techniques such as GATT, Kahook Dual Blade (KDB, New World Medical, Rancho Cucamonga, CA), Ahmed ClearPath (New World Medical, Rancho Cucamonga, CA), Micropulse CPC, and Preserflo (Santen, Inc., Emeryville, CA, USA) have been recently tried in childhood glaucoma with promising results. However, these studies are limited by small sample sizes and lack of long-term results, so they were excluded from the current analysis.
Representative index studies were identified as bases to estimate the mean IOP and glaucoma medications reduction for each surgical treatment modality of childhood glaucoma, including goniotomy, conventional ab-externo trabeculotomy, microcatheter-assisted ab-externo circumferential trabeculotomy, trabeculectomy, CPC, and GDDs including Ahmed glaucoma valve (AGV) and Baerveldt glaucoma implant (BGI). We presumed all glaucoma surgeries had a similar postoperative drop regimen since the variation seems to be more practitioner-related than evidence-based indication-related. In this way, the comparison between modalities remains relative, based on the primary intervention, and not biased by drops that could distort costs.
The main data collected from these studies to be used in the current cost analysis included the mean reduction in the IOP and the number of glaucoma medications for each surgical modality. We limited our analysis to the first year postoperatively.
We used the Centers for Medicare and Medicaid Services to obtain US Medicare-allowable fee data for 2022 and calculate each surgical intervention's costs (in US dollars). We assumed the procedure was performed in a hospital-based setting using the operating room[21]–[22]. Medicare-allowable professional fees and anesthesia and facility fees were tabulated for each treatment regimen. The dollars per relative value unit (RVU) conversion factor was $34.61, the estimated rate for 2022. We adjusted the reimbursement for the geographic modifier for Miami, Florida (2.6% higher than the national average); however, the relative changes would not be different irrespective of the geographic modifier used.
The Current Procedural Terminology (CPT) codes used to calculate the costs of the surgeries in the current analysis were as follows: 65 820 for goniotomy, 65 850 for both conventional and microcatheter-assisted trabecuolotomy, 66 170 for trabeculectomy, 66 179 for tubes including AGV and BGI, and 66 711 for CPC. There is no consensus about the first-line medical therapy in childhood glaucoma, so we assumed the topical glaucoma medication requirement to be filled by a prostaglandin analogue at $14.8 per month as judged by a database from our hospital pharmacy[23]. Our analysis did not include the cost of clinic visits, diagnostic tests such as optical coherence tomography, and examination under anesthesia. They should be similar in all seven treatment modality groups. Postoperative drop regimen (e.g., topical steroids, antibiotics, or non-steroidal anti-inflammatory drugs) varies widely among practitioners, so for the basis of comparison we assumed that all surgeries would result in a similar regimen for each practitioner.
Data were entered and analyzed using Microsoft Excel 2020 (Microsoft Corp, Seattle, WA, USA). Quantitative data were expressed as mean ± standard deviation.
RESULTS
At one year postoperatively, the relative cost per mm Hg IOP reduction was lowest for microcatheter-assisted trabeculotomy ($226/mm Hg) followed by CPC ($284/mm Hg) and conventional 180° trabeculotomy ($288/mm Hg). The least cost-efficient surgical intervention was trabeculectomy ($400/mm Hg). Intermediate cost-efficiency modalities were for AGV ($338/mm Hg), BGI ($350/mm Hg), and goniotomy ($351/mm Hg; Figure 2). It is expected for the relative cost per mm Hg IOP reduction to be lower after the first year postoperatively as it includes both the costs of surgery and postoperative glaucoma medications; however, costs could not be accurately calculated for all comparators after the first year due to lack of long-term studies in the childhood glaucoma literature.
Figure 2. A bar graph showing the cost of different glaucoma interventions per mm Hg IOP reduction ($/mm Hg) at 1-year postoperatively.
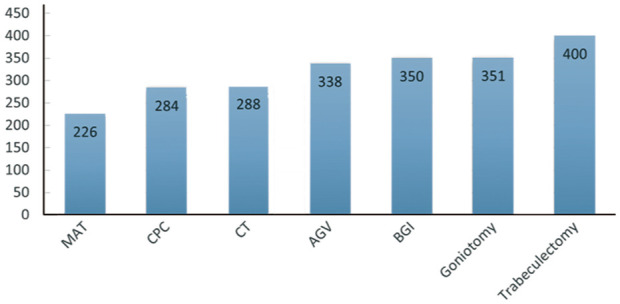
IOP: Intraocular pressure; MAT: Microcatheter-assisted trabeculotomy; CPC: Continuous-wave cyclophotocoagulation; CT: Conventional trabeculotomy using Harms trabeculotome; AGV: Ahmed glaucoma valve; BGI: Baerveldt glaucoma implant.
The effective cost of surgery is calculated by subtracting the savings as a result of glaucoma medication reduction from the total fee of the surgical procedure. The lowest cost was for CPC ($2421), followed by microcatheter catheter-assisted trabeculotomy ($3214). Tubes, whether BGI ($5181) or AGV ($5199) have the highest effective cost of surgery (Table 1).
Table 1. Summary of the Medicare allowable fees and results for surgery, including mean reduction of glaucoma medications and IOP reduction over the first year after surgery.
| Procedure | Goniotomy | Conventional trabeculotomy | Microcatheter-assisted trabeculotomy | Trabeculectomy | AGV | BGI | CPC |
| CPT code | 65820 | 65850 | 65850 | 66170 | 66179 | 66179 | 66711 |
| Total allowable fee for surgery | $3248 | $3268 | $3268 | $3530 | $5395 | $5395 | $2777 |
| Mean reduction of glaucoma medications | |||||||
| 1y | 0.1 | 0.2 | 0.3 | 1.3 | 1.1 | 1 | 2 |
| Drop savings in 1st year (reduction after surgery) | $18 | $35 | $53 | $248 | $195 | $213 | $355 |
| Imputed cost of surgery | $3230 | $3232 | $3214 | $3281 | $5199 | $5181 | $2421 |
| Mean IOP reduction (mm Hg) | |||||||
| 1y | 9.2 | 11.2 | 14.2 | 12.2 | 15.4 | 14.7 | 8.5 |
| $/mm Hg reduction | |||||||
| 1y | $351 | $288 | $226 | $400 | $338 | $350 | $284 |
CPT: Current procedural terminology; IOP: Intraocular pressure; AGV: Ahmed glaucoma valve; BGI: Barveldt glaucoma implant; CPC: Cyclophotocoagulation.
DISCUSSION
The current study is the first cost analysis study evaluating the costs of different surgical interventions in childhood glaucoma. The novel parameter ($/mm Hg IOP reduction) introduced for cost analysis in adult patients with primary open angle glaucoma was applied to childhood glaucoma cases[18]. The current study found ab-externo microcatheter-assisted trabeculotomy to be the most cost-efficient surgical intervention ($226/mm Hg), while trabeculectomy was the least cost-efficient method ($400/mm Hg). This was likely because of higher mean IOP reduction in the microcatheter-assisted trabeculotomy group. Circumferential Schlemm's canal surgery enhances the aqueous drainage outflow throughout the whole Schlemm's canal circumference resulting in better IOP reduction compared to goniotomy and conventional 180-degree trabeculotomy[8]. In contrast, for adults, selective laser trabeculoplasty was the most cost-efficient glaucoma intervention ($121/mm Hg), while iStent was the least cost-efficient surgery ($1376/mm Hg). However, the Hydrus microstent was not evaluated in that study[18].
Management of childhood glaucoma carries a lifelong economic burden considering the need for multiple surgical interventions, clinic visits, and examinations under anesthesia. Limited literature is currently available to analyze the costs and resource needs of childhood glaucoma management. A retrospective study by Liu et al[20] included 23 childhood glaucoma patients aged <21y, whether primary or secondary glaucoma, who has been followed up for at least 4y. They calculated the direct and indirect costs of childhood glaucoma care. Direct costs included the costs of the surgical interventions, anesthesia costs, examinations under anesthesia, glaucoma medications, and clinic visits. Indirect costs included estimates of caregiver lost productivity and lost wages. They estimated the mean total cost for childhood glaucoma care to be $21 441.61 per patient annually and $85 074.96 over the course of 4y. Those numbers represent 1100% of the cost for adult glaucoma per year and an 800% increase in the annual costs for average childhood patient health care[20]. The main contributing factors to the costs were the surgical interventions and examinations under anesthesia, contributing 69% and 23% to the costs over the years, respectively[20].
Creating cost-analysis models for childhood glaucoma is challenging. The main limitation is that the standard quality-adjusted life year (QALY) is mainly linked to changes in visual acuity (VA). This is not a reasonable surrogate for glaucoma, and VA is often not reported in childhood glaucoma studies but would not be expected to change with glaucoma surgeries. Some authors consider childhood glaucoma surgery as a sight-developing procedure rather than a sight-saving procedure as in the adult world, suggesting cost-utility evaluation with the assumption of visual improvement from no light perception. However, lack of information about long-term visual outcomes hinders the development of concrete assumptions for such cost-utility model. In adulthood glaucoma, some suggested converting VA to changes in the mean deviation (dB loss) on visual field (VF) testing and creating cost-analysis models based on converting VF values to QALY[24]–[25]. It is challenging to apply such a concept in childhood glaucoma because VFs are unreliable VF in children[26].
Due to such limitations, we are introducing a new parameter ($/mm Hg) for analyzing the costs of different surgical interventions instead of QALYs, which is difficult to be obtained in childhood glaucoma as has been proposed for adult glaucoma[18].
Incremental cost-effectiveness ratios (ICERs) are superior to an average cost-effectiveness ratio presented in this study because health technology assessment considers ICERs when making coverage decisions. However, applying ICERs, like cost-utility calculations, are linked to VA, as noted above, and do not capture the value of glaucoma treatments, especially in young children[27]–[28].
Given that childhood glaucoma is a slowly progressive disease with life-long care, it is important to perform cost-analysis over a long period of time. For example, a study by Sood et al[29] performed a cost-analysis model to compare the cost-effectiveness of trabecular meshwork microstents to cataract surgery using Markov model. Although trabecular meshwork microstents were not cost-effective over the first 2y compared to cataract surgery, it proved to be cost-effective after 5y. However, this may be difficult to conduct in childhood glaucoma due to lack of long-term follow-up data.
Yet another point of more importance in the childhood patient, with a much longer life expectancy than in adult studies, is the disease burden. Indirect and societal costs, such as disability-adjusted life years (DALYs) would be desirable parameters but suffer from the same restrictions of very limited outcome reporting, as noted above.
The current study has several limitations. First, we based our calculations from 51 studies with different inclusion criteria; most of them were (uncontrolled) retrospective studies due to the lack of randomized controlled trials and prospective studies in childhood glaucoma. Second, we did not include the complications in our calculation model because of a lack of sufficiently standardized data in the literature. Third, the current analysis included childhood glaucoma patients, which represents a heterogeneous group of disorders with some surgical options may work for one type better than the other. This limitation is mainly because the majority of the current literature in childhood glaucoma especially in trabeculectomy, GDD, and CPC groups evaluated the efficacy of these procedure in a heterogenous group of childhood glaucoma rather than a homogenous group of patients. Fourth, we did not include the costs of additional interventions such as needling or suture adjustment in trabeculectomy because of the lack of current literature to draw reliable assumptions.
Despite the limitations, our study could serve as a step toward reducing the economic burden of childhood glaucoma. It has been reported that vision loss is the third most common cause of DALYs impairment[30]. Given that up to 60% of childhood glaucoma patients will have a degree of visual impairment[3],[31], childhood glaucoma is expected to have a significant effect on DALYs; however, the exact estimates are difficult to determine because of the paucity of the data and relative rarity of the disease.
In conclusion, microcatheter-assisted trabeculotomy surgery was the most cost-efficient surgical modality compared to other surgical modalities, and it might represent a more appropriate primary surgical management option for childhood glaucoma patients.
Acknowledgments
Conflicts of Interest: Elhusseiny AM, None; Khodeiry MM, None; Yannuzzi NA, None; Chang TC, None; Lee RK, None; Smiddy WE, None.
REFERENCES
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.El Sayed YM, Elhusseiny AM, Gawdat GI, Esmael AF, Elhilali HM. Childhood glaucoma profile in a tertiary centre in Egypt according to the childhood glaucoma research network classification. PLoS One. 2023;18(1):e0279874. doi: 10.1371/journal.pone.0279874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam EK, Elhusseiny AM, Shah AS, Mantagos IS, VanderVeen DK. Etiology and outcomes of childhood glaucoma at a tertiary referral center. J AAPOS. 2022;26(3):117.e1–117117.e6. doi: 10.1016/j.jaapos.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Kong LK, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16(6):501–507. doi: 10.1016/j.jaapos.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin AV. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol. 2018;29(5):385–394. doi: 10.1097/ICU.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 6.Tamçelik N, Atalay E, Bolukbasi S, Çapar O, Ozkok A. Demographic features of subjects with congenital glaucoma. Indian J Ophthalmol. 2014;62(5):565–569. doi: 10.4103/0301-4738.126988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alabdulwahhab KM, Ahmad MS. Visual impairment and blindness in Saudi Arabia's school for the blind: a cross-sectional study. Clin Optom. 2020;12:169–173. doi: 10.2147/OPTO.S265293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhusseiny AM, El Sayed YM, El Sheikh RH, Gawdat GI, Elhilali HM. Circumferential schlemm's canal surgery in adult and pediatric glaucoma. Curr Eye Res. 2019;44(12):1281–1290. doi: 10.1080/02713683.2019.1659975. [DOI] [PubMed] [Google Scholar]
- 9.Elwehidy AS, Bayoumi NHL, Abd Elfattah D, Hagras SM. Surgical outcomes of visco-circumferential-suture-trabeculotomy versus rigid probe trabeculotomy in primary congenital glaucoma: a 3-year randomized controlled study. J Glaucoma. 2022;31(1):48–53. doi: 10.1097/IJG.0000000000001944. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Wang HZ, Oatts J, et al. Ab interno vs ab externo microcatheter-assisted trabeculotomy for primary congenital glaucoma with clear cornea. Clin Exp Ophthalmol. 2020;48(9):1201–1209. doi: 10.1111/ceo.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhusseiny AM, VanderVeen DK. Outcomes of glaucoma drainage devices in childhood glaucoma. Semin Ophthalmol. 2020;35(3):194–204. doi: 10.1080/08820538.2020.1781906. [DOI] [PubMed] [Google Scholar]
- 12.Sakaorat P, Mohamed-Noriega J, Sharara A, Daniel MC, Brookes J. Cyclodiode laser as the first surgical approach in childhood glaucoma under the age of 8 years. J Glaucoma. 2021;30(4):352–356. doi: 10.1097/IJG.0000000000001754. [DOI] [PubMed] [Google Scholar]
- 13.Quan AV, Chen J, Wang YE, et al. Factors associated with gonioscopy-assisted transluminal trabeculotomy (GATT) complications and failure in children. Am J Ophthalmol. 2022;241:168–178. doi: 10.1016/j.ajo.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Brandt JD. Use of a novel microshunt in refractory childhood glaucoma: initial experience in a compassionate use/early access cohort. Am J Ophthalmol. 2022;239:223–229. doi: 10.1016/j.ajo.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Elhilali HM, El Sayed YM, Elhusseiny AM, Gawdat GI. Kahook dual blade ab-interno trabeculectomy compared with conventional goniotomy in the treatment of primary congenital glaucoma: 1-year results. J Glaucoma. 2021;30(6):526–531. doi: 10.1097/IJG.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 16.Knight LSW, Ridge B, Staffieri SE, Craig JE, Prem Senthil M, Souzeau E. Quality of life in adults with childhood glaucoma: an interview study. Ophthalmol Glaucoma. 2022;5(3):325–336. doi: 10.1016/j.ogla.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Miraftabi A, Coleman AL, Nilforushan N, et al. Vision-related quality of life in patients with a history of congenital glaucoma. Eur J Ophthalmol. 2021;31(6):3074–3079. doi: 10.1177/1120672120977354. [DOI] [PubMed] [Google Scholar]
- 18.Elhusseiny AM, Yannuzzi NA, Khodeiry MM, Lee RK, Smiddy WE. Cost-analysis of surgical intraocular pressure management in glaucoma. J Glaucoma. 2021;30(11):947–951. doi: 10.1097/IJG.0000000000001938. [DOI] [PubMed] [Google Scholar]
- 19.Rasendran C, Li A, Singh RP. Incremental health care expenditures associated with glaucoma in the United States: a propensity score-matched analysis. J Glaucoma. 2022;31(1):1–7. doi: 10.1097/IJG.0000000000001957. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Huang LD, Mukkamala L, Khouri AS. The economic burden of childhood glaucoma. J Glaucoma. 2016;25(10):790–797. doi: 10.1097/IJG.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. Physician Fee Schedule. https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeesched/pfs-relativevalue-files.html. Accessed on May 30, 2022.
- 22.Centers for Medicare and Medicaid Services. Addendum B. Final OPPS Payment by HCPCS Code for CY 2019 J, 2019. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/HospitalOutpatientpps/Addendum-A-and-Addendum-B-Updates.html. Accessed on May 30, 2022.
- 23.Sacchi M, Lizzio RAU, Villani E, Monsellato G, Lucentini S, Cremonesi E, Luccarelli S, Serafino M, Nucci P. Medical management of pediatric glaucoma: lessons learned from randomized clinical trials. Graefes Arch Clin Exp Ophthalmol. 2020;258(8):1579–1586. doi: 10.1007/s00417-020-04767-9. [DOI] [PubMed] [Google Scholar]
- 24.Rein DB, Wirth KE, Johnson CA, Lee PP. Estimating quality-adjusted life year losses associated with visual field deficits using methodological approaches. Ophthalmic Epidemiol. 2007;14(4):258–264. doi: 10.1080/01658100701473267. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RI, De Moraes CG, Cioffi GA, Al-Aswad LA, Blumberg DM. Comparative cost-effectiveness of the baerveldt implant, trabeculectomy with mitomycin, and medical treatment. JAMA Ophthalmol. 2015;133(5):560–567. doi: 10.1001/jamaophthalmol.2015.44. [DOI] [PubMed] [Google Scholar]
- 26.Sinha G, Patil B, Sihota R, Gupta V, Nayak B, Sharma R, Sharma A, Gupta N. Visual field loss in primary congenital glaucoma. J AAPOS. 2015;19(2):124–129. doi: 10.1016/j.jaapos.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein EA, Khouri AS. Letter to the Editor: Cost-analysis of surgical intraocular pressure management in glaucoma. J Glaucoma. 2022;31(3):e10–e11. doi: 10.1097/IJG.0000000000001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhusseiny AM, Yannuzzi NA, Khodeiry MM, Lee RK, Smiddy WE. Cost-analysis of surgical intraocular pressure management in glaucoma. J Glaucoma. 2021;30(11):947–951. doi: 10.1097/IJG.0000000000001938. [DOI] [PubMed] [Google Scholar]
- 29.Sood S, Heilenbach N, Sanchez V, Glied S, Chen SE, Al-Aswad LA. Cost-effectiveness analysis of minimally invasive trabecular meshwork stents with phacoemulsification. Ophthalmol Glaucoma. 2022;5(3):284–296. doi: 10.1016/j.ogla.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YC, Jin GM, Fan M, Lin YF, Wen X, Li ZJ, Zeng P, Zheng DY, Lan YQ. Time trends and heterogeneity in the disease burden of glaucoma, 1990-2017: a global analysis. J Glob Health. 2019;9(2):020436. doi: 10.7189/jogh.09.020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Silva DJ, Khaw PT, Brookes JL. Long-term outcome of primary congenital glaucoma. J AAPOS. 2011;15(2):148–152. doi: 10.1016/j.jaapos.2010.11.025. [DOI] [PubMed] [Google Scholar]