Abstract
Background
Epidemiological research on late effects of therapy shows the necessity to aggregate chemotherapy agents to substance classes. This requires using conversion factors by substance classes.
Aims
The aim of this study was to identify previously used conversion factors from the literature, to present a novel approach for additional factors, and to compare these approaches.
Methods and Results
A literature review was performed, which identified two main principles of deriving conversion factors: effect‐equivalence and equimolar. Thirty‐five articles presenting effect equivalence‐based factors in the widest sense were found in the literature. Ten articles presented the equimolar approach which can be applied to almost all chemotherapy substances. Based on a comprehensive list of treatment protocols used in German pediatric oncology, we derived alternative conversion factors from typical doses. We compared the conversion factors using Pearson correlation coefficients and linear regression. At least two types of conversion factor were available for each of the 49 substances included. The equivalent effect‐based and the typical dose‐based factors were highly correlated with a regression coefficient close to 1. The equimolar factors are independent.
Conclusions
For substances for which no conversion factor based on some type of effect equivalence has been published so far, a factor based on a typical doses‐approach may be used in epidemiological late effects research. Doses aggregated based on the equimolar approach may not be compatible with doses aggregated based on equivalent effects.
Keywords: chemotherapeutics, childhood cancer, conversion factors, doses, second tumor
1. INTRODUCTION
Our working group is working on a case–control study on second neoplasms after childhood cancer (second tumors after tumor therapy (STATT)) using data from the German Childhood Cancer Registry (GCCR) and German clinical therapy trials in pediatric oncology, soon to be published). For this we obtained retrospective cumulative chemotherapy dose data for the former patients. It became clear that the number of different substances is too large for joint statistical analysis and some substances are applied rarely and therefore allow no statistical analysis. Other groups working on late effects of chemotherapy had been using the solution of grouping substances by pharmacologic principles, 1 , 2 usually using a conversion factor before aggregating cumulative doses in a substance group (e.g., References 3, 4, 5, 6, 7, 8). Clinical replacement rules require conversion factors, too. 9 , 10 Given the sometimes very different dose range of substances in a substance group, aggregating them without conversion is not indicated.
However, a comprehensive list of substances used in pediatric oncology and conversion factors for them turned out not to be available in the pertinent literature. Therefore, we initiated a very broad literature search aiming to collect factors having been used before in this field of late effects research, with a special focus on childhood cancer survivors. We are presenting the results of this search here.
In addition, we developed an algorithm to fill in conversion factors for which conversion factors cannot be found in our literature search. This approach is based on typical doses determined from a comprehensive list of treatment protocols of the German Society for Pediatric Oncology and Hematology (GPOH) from the years 1970 to 2018. 11 , 12 We are presenting these factors here, too. The final question was whether it is justified using conversion factors based on different principles in the same analyses; for this we compared the factors statistically.
2. METHODS
2.1. Inclusion and grouping of substances
We included all substances with reported conversion factors in the pertinent literature and which have been used in treatment protocols for pediatric oncology in Germany since the 1970s. 11 , 12 They were included if they are considered as antineoplastic agents (Group L01) according to the Anatomical Therapeutic Chemical (ATC) code, 2 excluding immunotherapy and supportive substances. We also examined glucocorticoids which are used as antineoplastic agents in pediatric oncology although they are not listed as such according to the ATC (Group H02AB). 13 Doses were given in or converted to the unit mg/m2 (except for asparaginase (L01XX), where International Units (IU)/m2 are generally used). The substances were classified into 12 substance groups according to the ATC. 2 For each class, a reference substance was chosen. Based on the ATC, procarbazine and estramustine belong to the group ‘other antineoplastic agents’ (L01X). However, due to their mode of action, they are usually grouped with alkylating agents (L01A) in oncology literature. 3 , 8
2.2. Literature review
The literature review was performed as a scoping review according to the PRISMA‐ScR (Preferred Reporting Items for Systematic reviews and Meta‐Analyses extension for Scoping Reviews) checklist. 14 The literature search was performed in Medline via PubMed on December 13th, 2022 and in Web of Science Core Collection on November 29th, 2022.
The search strategy with criteria for inclusion and exclusion was defined a priori (see Table 1). Given that we were mainly interested in applying this to research on secondary carcinogenicity in treated children, we used the following search terms: ‘childhood second cancer AND chemotherapy AND dose’ (Search 1). In order to include articles examining glucocorticoids as well, we performed an additional specific search using ‘cortisone AND equivalence dose’. The resulting queries are provided in detail in Supplementary Table 1.
TABLE 1.
Inclusion and exclusion criteria for the literature review
| Step | Inclusion criteria | Exclusion criteria |
|---|---|---|
| First step: Title and abstract |
|
– |
|
||
|
||
| Second step: Full text |
|
|
|
|
Articles were included if they had been published since 1985 because of incomplete availability of older publications. Inclusion of adults in the respective studies was no exclusion criterion, as we were generally not interested in the respective study results, but in the method sections. The first author screened the titles and abstracts and evaluated the full texts of the remaining articles.
As we had started with generally researching the topic of carcinogenic effects of chemotherapy in children when preparing the STATT study, literature previously available to us, which was not found by the formal literature search, was added to the review.
A conversion factor is here defined as a factor the dose of a specific chemotherapeutic drug is multiplied with to obtain the equivalent dose of the reference drug in the respective substance class. We extracted or calculated these factors based on our literature search for the substances mentioned. If necessary, units were harmonized before calculating the conversion factors. Additionally, we extracted general information on the article in which the respective factor had been used (study design and study objective, study population, time period, age group and study size).
If available in the literature, we recorded the basis for the equivalence (such as equipotency, hematotoxicity or cardiotoxicity) and the evidence behind it. The term equivalence usually refers to the treatment effect or to different kinds of toxicity.
For each substance group, the reference drug was chosen based on what was usually used in the literature. If a publication used a different reference drug, we recalculated the respective conversion factors accordingly. For glucocorticoids, prednisone was used as reference drug. Hydrocortisone‐equivalents were recalculated into prednisone‐equivalents by using a conversion factor of 0.25 according to the ‘Arzneimittelkommission der Deutschen Apotheker’, 15 which means that a hydrocortisone dose of 1 mg/m2 is equivalent to a prednisone dose of 0.25 mg/m2. For anthracyclines, daunorubicin‐equivalents were considered equal to doxorubicin‐equivalents if only daunorubicin‐equivalents were available.
Wherever we found more than one conversion factor based on the same underlying principle for the same substance, we needed to select a factor for our purpose. We applied the following criteria (defined a priori) in this order: (1) most recent publication year and (2) articles which developed their own conversion factor based on their own literature review of equivalence. We present all factors found, indicating the one we selected (see section 3).
2.3. Conversion factors based on typical doses
This simple approach assumes that the ratio of typical doses of two substances in a group probably comes close to a conversion factor based on therapeutical equipotency.
For deriving the typical doses per substance we used a comprehensive list of all treatment protocols used in German pediatric oncology from the years 1970 to 2018, 11 , 12 which included 97 protocols with 678 treatment arms. Only doses given in mg/m2 were included (except for asparaginase, where only IU (International Units)/m2 were considered). The list contains cumulative doses per therapy block for each individual substance, each therapy arm and each protocol. This mainly excludes doses given in mg/kg. As a typical dose for a substance, we consider the mode, that is, the most frequently used dosage, for all doses across all therapy blocks and therapy arms of all protocols where that substance had been used. The substance Methotrexate, that is, has been used in 1515 therapy blocks over all treatment protocols and treatment arms with cumulative dosages from 12.5 to 24 000 mg/m2. Three hundred and seventy‐six out of these 1515 cumulative doses, and thus the most frequently used dosage (=mode), were 1000 mg/m2. The median dose was also 1000 mg/m2.
Dividing the typical doses of a reference substance and a substance yields the alternative conversion factor.
The literature search did not provide conversion factors for all substances needed in our project. In order to decide whether we can justify filling the gaps with the typical dose‐approach, we compared them using Pearson correlation coefficients and linear regressions on a log scale for all substances where factors from different approaches were available. We provide regression coefficients with confidence limits for the individual (CLI) values and the mean predicted values (CLM). The statistical analyses were performed with SAS 9.4 (proc corr and proc reg).
3. RESULTS
3.1. Literature review
Figure 1 gives an overview of the article selection process using the above mentioned search strategy and inclusion and exclusion criteria. In total, we identified 479 articles after removing duplicates.
FIGURE 1.
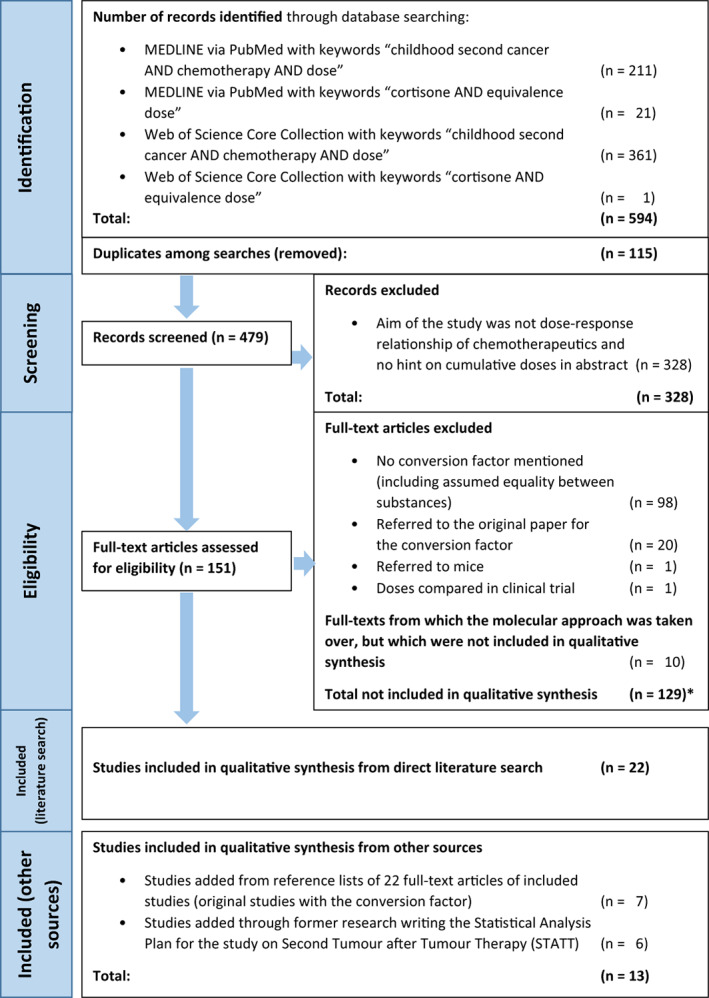
Flow chart of inclusion and exclusion of identified articles. *One study is mentioned twice because it referred to molecular weights and in a second analysis assumed equality between substances
The articles were rather diverse regarding the study designs and the study populations. Most of them included children or adolescents with cancer or childhood/adolescent cancer survivors and referred to study populations in Europe or North America. Except for three articles, 8 , 16 , 17 the information we sought was mostly part of the methods section of the respective article as the articles were not explicitly about the factors as such.
In 10 out of the 151 articles screened which met all inclusion criteria, the authors suggested converting mg/m2 of chemotherapeutics to moles/m2 to quantify the total dose of a drug in each drug class (equimolar approach). 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27
As molecular weights are easily available for almost all chemotherapeutic substances, we were able to calculate additional factors using this approach ourselves. The factors were calculated using the molecular weights, independently from any article. The higher the molecular weight, the fewer active molecules are included per weight of a substance. Under this assumption, we calculated factors derived from the molecular weights for each substance as described above for the other factors.
Twenty‐two further studies not (only) using the equimolar approach met all inclusion criteria. Additionally, we identified seven more articles of this type, which had been cited by the articles identified in the original search, 16 , 17 , 28 , 29 , 30 , 31 , 32 and added another six articles which had been known from our former general research 5 , 9 , 10 , 15 , 33 , 34 on late effects of childhood cancer. Hence, 35 articles with conversion factor suggestions other than those based on the equimolar approach were included in the literature review. Tables 2 and 3 list all 24 studies examining chemotherapeutics other than glucocorticoids (Table 2) and 11 articles examining glucocorticoids (Table 3) separately.
TABLE 2.
Overview of 24 articles included in the literature review examining chemotherapeutics other than glucocorticoids a : summary of study characteristics, study design and stated basis for equivalence
| Article | Study population | Date of recruitment | Children/Adults b | Country | Number of patients | Study design | Study objective | Stated basis for equivalence and respective reference (only references since the year 1985) | Conversion factors provided for | Remark |
|---|---|---|---|---|---|---|---|---|---|---|
| Allodji (2021) 6 | Childhood cancer survivors; Cases: secondary leukemia | 1930–2000 | Children at diagnosis | France, United Kingdom, United States, Canada, Italy, and the Netherlands | 147 cases/522 controls | Pooled data from four nested case–control studies | Risk of secondary leukemia in relation to chemotherapy | Hematological toxicity based on Green et al. 2014 8 | AA | Additional factor for dacarbazine in article |
| Blanco (2012) 33 | Childhood cancer patients treated at member organization of Children's Oncology Group (COG); Cases: with additional cardiomyopathy | 1966–2008 | Children and adolescents ≤21 years at diagnosis | Several | 170 cases/317 controls | Case–control study | Dose‐dependent risk of anthracycline‐related cardiomyopathy | Cardiotoxicity based on Lehmann et al. 2000 32 | A | Supplement of Blanco 2012 33 presents factors different from Lehmann 2000 32 |
| Casagranda (2016) 7 | Cohort of Childhood Cancer Registry Rhônes‐Alpes Region with all types of first and second primary | 1987–2004 (diagnosis of first primary) | Children | France | 64 cases/190 controls | Nested case–control‐study | Secondary neoplasia comparing chemotherapy from different groups | “hematologic toxicity or rules of substitution” based on Guérin 2003 23 and Tucker 1987 35 | AA, A, E, PD | Factors presented in article are identical to Guérin 2007, 3 not to Guérin 2003 23 |
| Children's Oncology Group (2018) 10 | Childhood Cancer Survivors | – | Children and adolescents | – | – | Guideline developed from a literature review | – | Cardiotoxicity based on literature review for this guideline | A | – |
| Creutzig (2007) 36 | Children with primary or secondary AML | 1993–2003 (treatment) | Children | Germany | 1207 | Follow‐up after clinical trial | Cardiotoxicity | Cardiotoxicity (not explicitly stated, assumed due to outcome of the study) based on 37 , 38 , 39 | A | References in article not mentioning a definitive factor 37 , 38 , 39 |
| Feig (1996) 40 | Children with acute lymphatic leukemia at first bone marrow relapse from Children's Cancer Group (CCG) | 1990–1992 | Children and adolescents <21 years at diagnosis | USA, Canada | 92 | Randomized Clinical Trial | Event‐free survival | “Isodose conversion factor” based on Berman 1991 41 and Wiernik 1992 42 | A | Conversion factors used in references of the article were slightly different from those used in article itself; references in article refer to clinical trials comparing idarubicin versus daunorubicin— > references not included additionally |
| Feijen (2015) 30 | Childhood cancer survivors | 1962–2002 | Children and adolescents <25 years at diagnosis | Several | 15 815 | Cohort studies | Hazard Ratio for cardiotoxicity (Heart failure) comparing Daunorubicin to Doxorubicin | Cardiotoxicity assumed based on references stated in introduction | A | Conversion factors concluded from calculated hazard ratio not included |
| Feijen (2019) 34 | Childhood cancer survivors | 1962–2005 | Children and adolescents <23 years at diagnosis | Several | 28 423 | Cohort studies | Hazard Ratio for cardiotoxicity (Heart failure) comparing different anthracyclines | Cardiotoxicity assumed based on references stated in introduction | A | Conversion factors concluded from calculated hazard ratio not included |
| Green (2014) 8 | Literature review | – | Not stated | Several | Literature review | Literature review | Literature review on hematological toxicity, comparison of Alkylating Agent Dose (AAD) with developed CED (cyclophosphamide equivalent dose) | Hematological toxicity based on literature review in article | AA | – |
| Guerin (2007) 3 | Childhood cancer survivors from solid tumor | 1942–1986 | Children at diagnosis | France, UK | 153 cases/442 controls | Nested case–control study in a European cohort | Risk of secondary neoplasia in relation to chemotherapy | “Hematological toxicity or substitution rules” based on Le Deley 2003 4 | AA, A, E, PD, VA | Four additional factors not mentioned in Le Deley 2003, 4 but in Guerin 2007 3 |
| Henderson (2012) 43 | Childhood cancer survivors with (cases) or without (controls) secondary sarcomas | 1970–1986 (first diagnosis), 2000 (baseline) | Children and adolescents <21 years at diagnosis | USA, Canada | 105 cases/422 controls | Nested case–control study in the Childhood Cancer Survivor Study | Secondary sarcoma comparing chemotherapy from different groups | Not stated | A, E, PD | – |
| Launchbury (1993) 17 | Literature review | – | Not stated | Several | Literature review | Literature review | Comparison of characteristics, therapeutic activity and toxicity of epirubicin and daunorubicin | Antitumour efficacy based on literature review in the article | A | – |
| Le Deley (2003) 4 | Childhood cancer survivors of a solid tumor with (cases) or without (controls) secondary leukemia | 1980 (first neoplasia), secondary neoplasia up to 1999 | Children at diagnosis | France | 61 cases/196 controls | Case–control study | Secondary leukemia comparing chemotherapy from different groups | “Hematologic toxicity or substitution rules”; reference not stated | AA, A, E, PD | – |
| Lehmann (2000) 32 | Consecutive patients with AML, ALL, CML, multiple myeloma or breast cancer and hematopoetic stem cell transplantation | 1985–1994 | Adolescents and Adults between 17 and 62 years old | Sweden | 148 | Case series | Cardiac systolic function with anthracycline dose as risk factor | “Equipotency of anthracycline doses and the cardiotoxic potential of the drugs” based on two references after the year 1985 | A | References in the article are literature reviews without a definitive factor 44 , 45 |
| Messinger (1999) 46 | Patients with acute lymphoblastic leukemia | – | Children and adolescents up to 21 years (most studies) | Several | Literature review | Literature review | Benefit of anthracyclines | Equipotency assumed due to reference to 17 | A | Reference 17 in the article contains only factor for epirubicin |
| Mouridsen (1990) 16 | Patients with breast cancer | – | Adults (not stated, but most likely because of breast cancer) | Literature review | Literature review | Review of phase II and phase III trials | Efficacy of anthracyclines (comparison of response rates) | Hematological and non‐hematological toxicity and cardiotoxicity based on review performed in the article | A | – |
| Mulrooney (2009) 28 | Survivors of childhood cancer in the Childhood Cancer Survivor Study (CCSS) | 1970–1986 (enrolment), 2000–2002 (follow‐up) | Children and adolescents <21 years at diagnosis | USA | 14 358 survivors and 3899 siblings | Retrospective cohort study | Cardiac outcomes | Basis for equivalence not stated; factors based on Pai 2000 47 | A | Reference in the article is review without explicit factors 47 |
| Mulrooney (2016) 5 | Survivors of childhood cancer in the St. Jude Lifetime Cohort Study | 2013 (follow‐up) | Children (“childhood cancer”) at diagnosis | USA | 1853 | Cross‐sectional study | Cardiotoxicity | “Isotoxic equivalents” based on Le Deley 2003 4 : “hematologic toxicity or substitution rules” | A | Idarubicin mentioned in Mulrooney 2016, 5 but not in Le Deley 2003 4 |
| Ozols (1985) 31 | Advanced ovarian cancer patients refractory to standard therapy | – | Adults (assumed) | USA (assumed) | Several clinical trials | Several clinical trials and in‐vitro studies comparing high dose cisplatin and high dose carboplatin | Clinical activity and toxicity, in vitro and clinical trial | “Clinically active” based on literature before 1985 | PD | – |
| Schramm (2019) 9 | Children with acute lymphatic leukemia | 2003–2010 (inclusion) | Children | Germany | 773 | Clinical trial | Survival (Overall Survival, Event‐free survival) | Not stated | Asp | – |
| Smibert (2004) 29 | Childhood cancer patients with anthracycline exposure more than 12 month after anthracycline treatment; controls from healthy siblings and patient peers | 1977–1995 (diagnosis) | Children at diagnosis | Australia (authors) | 110 cases/31 controls | Cross‐sectional, non‐randomized study | Cardiac outcome of different doses of anthracyclines and alkylating agents | Anthracyclines: “Myelosuppressive potency”; no reference stated | A | – |
| Sorensen (2003) 48 | Acute lymphoblastic leukemia survivors and Wilms tumor survivors | 1970–1990 (treatment of Wilms Tumor) | Children at diagnosis | UK | 101 acute lymphoblastic leukemia survivors, 83 Wilms tumor survivors and 100 controls | Prospective, longitudinal study | Cardiac toxicity of different doses of anthracyclines | Cardiac toxicity based on the assumption of equivalence of cardiac toxicity between daunorubicin and doxorubicin | A | – |
| Wang (2022) 49 | Childhood Cancer survivors included in seven cohorts | 1946–2012 (diagnosis)—2021 (follow‐up) | Children and adolescents <21 years at diagnosis | Europe, North America | 21 892 | Pooled Individual patient data cohort | Subsequent breast cancer | Hematological toxicity assumed due to reference to cyclophosphamide equivalent dose; factors based on cyclophosphamide equivalent dose | AA | – |
| Winick (1993) 50 | Patients with acute lymphoblastic leukemia treated with etoposide | 1986–1991 (diagnosis) | Children | USA | 205 | Follow‐up of consecutive patients | Event‐free survival, secondary AML, no comparison group | Not stated; factors based on literature before 1985 | E | – |
Abbreviations: A, anthracyclines; AA, alkylating agents; ALL, acute lymphatic leukemia; AML, acute myelocytic leukemia; Asp, asparaginase; CML, chronic myelocytic leukemia; E, epipodophyllotoxins; PD, platinum derivates; VA, vinca alkaloids.
Articles which (1) mentioned a conversion factor or doses which permitted the calculation of a conversion factor, (2) have been published since the year 1985 and (3) of which a full‐text in English or German was available (see Table 1).
In studies on childhood cancer survivors, the inclusion in the study might have taken place as adults. The age groups were defined as follows: children: below the age of 18; adults: above the age of 18; adolescents: age 16–25 (only mentioned if there were mixed groups of either adolescents and adults or children and adolescents).
TABLE 3.
Overview of 11 articles included in the literature review examining glucocorticoids a : summary of study characteristics, study design and stated basis for equivalence
| Article | Study population | Date of recruitment | Children/adults b | Country | Number of patients | Study design | Study objective | Stated basis for equivalence and respective reference (only references since the year 1985) | Remark |
|---|---|---|---|---|---|---|---|---|---|
| Arteaga (1999) 51 | Patients with persistent hypokalemia after successful adrenalectomy due to Cushing's syndrome due to ectopic ACTH secretion | Not stated | Adults | Chile | 1 | Case report | Hypokalaemia | Not stated | – |
| Arzneimittelkommission der Deutschen Apotheker 15 | – | – | Adults | Germany | – | Tables of equivalent doses | – | Antiinflammatory potency based on literature research of the “Arzneimittelkommission der Deutschen apotheker” | – |
| Bostrom (2003) 52 | Patients with acute lymphoblastic leukemia (ALL) in the Children's Cancer Group | 1993–1995 | Children | USA, Canada | 1060 | Randomized controlled trial (2 × 2 factorial design) comparing dexamethasone versus prednisone | Relapse and event free survival | “Dexamethasone is approximately 7‐fold more potent than prednisone”; statement based on “published equivalency tables” from in‐vitro studies | – |
| Ekstrand (2020) 53 | Patients with hypopituitarism receiving growth hormone replacement | 1990–2002 | Adults | Sweden (authors) | 229 | Prospective trial (post‐hoc analysis): 1 switch group (cortisone acetate— > hydrocortison), two control groups (cortisone acetate only and no glucocorticoid replacement) | Metabolic effects | Antiinflammatory potency based on Filipsson 2006 54 and on literature from before 1985 | – |
| Filipsson (2006) 54 | Hypopituitary patients from KIMS (Pfizer International Metabolic Database) | 2004 (inclusion) | Adults | 28 countries in database, non‐European patients excluded | 2424 | Longitudinal survey, examination at baseline and one year after growth hormone treatment | Metabolic outcome comparing three groups (hydrocortisone, cortisone acetate, and prednisolone/dexamethasone) | ”Previous antiinflammatory comparisons” based on references from before 1985 | – |
| Lovas (2006) 55 | Patients with Addison's disease and healthy controls | Not stated | Adults | Norway | 31 patients with Addison's disease and 20 healthy controls | Correlational study | Correlation of serum and saliva cortisol | “Conventional glucocorticoid replacement therapy” based on Arlt 2003 56 | Reference in article 56 is literature review without definitive factor |
| Pfeiffer (1992) 57 | Patients with avascular osteonecrosis of the femoral head after steroid therapy for cerebral trauma | 1981–1987 (accident) | Adolescents | Germany | 3 | Case series | Avascular osteonecrosis of the femoral head | “prednisone equivalent” based on textbooks 58 , 59 | – |
| Puglisi (2021) 60 | Patients with an established diagnosis of adrenal insufficiency | 1995–2018 | Adults | Italy | 203 | Case series | Influence of the etiology of adrenal insufficiency on the types of glucocorticoid used | Hydrocortisone equivalent dose (HEC); no reference stated | – |
| Sandrini (1993) 61 | Patients with salt‐losing form of congenital adrenal hyperplasia due to 21‐hydroxylase deficiency | Not stated | Children and adolescents | USA | 19 | Case series | Variation of cortisol dose with age | “Equivalent dose of oral cortisol” based on two articles before 1985 and two textbooks 62 , 63 | – |
| Swords (2003) 64 | Hypopituitary patients | Not stated | Adults | UK (authors) | 10 | Prospective, cross‐over study | Influence on growth hormone therapy comparing different glucocorticoids | Not stated | – |
| Tabone (2021) 65 | Childhood cancer survivors from acute leukemia included in follow‐programme LEA and fulfilling certain criteria | Since 1980 | Children at diagnosis, Adults at follow‐up | France | 89 | Follow‐up of a cohort | Factors influencing bone mineral density | Not stated | – |
Abbreviations: ALL, acute lymphatic leukemia; AML, acute myelocytic leukemia; CML, chronic myelocytic leukemia.
Articles which (1) mentioned a conversion factor or doses which permitted the calculation of a conversion factor, (2) have been published since the year 1985 and (3) of which a full‐text in English or German was available (see Table 1).
In studies on childhood cancer survivors, the inclusion in the study might have taken place as adults. The age groups were defined as follows: children: below the age of 18; adults: above the age of 18; adolescents: age 16–25 (only mentioned if there were mixed groups of either adolescents and adults or children and adolescents).
Two 30 , 34 out of these 35 articles set out to challenge the idea that factors based originally on hematologic toxicity can be used for studying cardiologic late effects, citing a large number of such factors previously used. We extracted these factors stated in the methods section of the articles according to our criteria in Table 1. As the same literature was cited in both articles, we only included the factors derived from the literature and used in the study in the later article. 34
3.2. Principles for effect equivalence—chemotherapeutics other than glucocorticoids
The basis of assessment of the different principles for effect equivalence other than the equimolar approach was usually not entirely clearly stated and rather diverse. For chemotherapeutics except glucocorticoids (24 articles), the conversion factors in the literature were mostly (15 out of 24) based on a principle which can be summarized by the term isotoxic. Toxicity referred to cardiotoxicity (n = 6), hematotoxicity (n = 8), or hematological toxicity, non‐hematological toxicity and cardiotoxicity (n = 1). An isotoxicity factor of, for example, four for a substance means that one unit of the substance was considered four times more toxic than one unit of the reference substance.
Three articles (3 out of 24) referred to the intended effects of the chemotherapeutics, using the terms antitumor efficacy (n = 2) or potency (n = 1), respectively. These can be summarized by the term equipotency. One additional article justified a factor with both cardiotoxicity (isotoxicity) and potency (equipotency).
Five articles out of 24 did not explicitly state a basis for their conversion factors; we conclude an underlying assumption of isotoxocity or equipotency from the context and usage of the factors in the respective studies.
For 17 substances, more than one factor was found in the literature. For all substances except Thiotepa, these factors were generally rather similar; however, the basis stated could still differ. As an example: Epirubicin was presented with a factor of 0.67 based on hematological toxicity 4 , 5 , 7 as well as on cardiotoxicity. 10 , 33 Another article mentioned similar factors for epirubicin based on hematological toxicity, cardiotoxicity or non‐hematological toxicity, respectively. 16 One article justified the factors for anthracyclines with both cardiotoxicity (isotoxicity) and potency (equipotency). 32
3.3. Principles for effect equivalence—glucocorticoids
For glucocorticoids (11 articles), all factors in the literature were based on the concept of equipotency (n = 8). In these articles, the following principles were used: potency (either general or inflammatory) (n = 4), conventional glucocorticoid replacement therapy (n = 1), hydrocortisone‐equivalent dose (n = 2) or prednisone equivalent (n = 1). In three articles, the basis of the conversion factor was not stated explicitly. We conclude an underlying assumption of equipotency from the context and usage of the factors in the respective study.
The usage of and stated bases for conversion factors in the literature seem to suggest that at least some authors assume the concepts of isotoxicity, isotoxicity for a specific outcome, and equipotency are sufficiently similar for general usage in late effects research. We concur for now and will refer to both concepts (isotoxicity and equipotency) as effect equivalence below. These factors are listed in Table 4 column 3.
TABLE 4.
List of substances used in pediatric oncology with respective conversion factors to convert the dose of a substance into the dose of the respective reference substance
| Anatomical therapeutic chemical (ATC) Code 2 | Drug | Different types of conversion factors | ||||||
|---|---|---|---|---|---|---|---|---|
| Factors based on effect equivalence from literature review | Novel approach: factors considering mode of all doses in respective German trials 11 , 12 | Factors from molecular weight of the respective substances | ||||||
| Conversion factor to reference drug | Reference | Preferred a | Typical Dose (mg/m2) | Conversion factor to reference drug | Molecular weight (g/mol) | Conversion factor to reference drug | ||
| Corticosteroids (H02AB) | ||||||||
| H02AB02 | Dexamethasone | 7.25 | 15 | Yes | 100.0 | 18.40 | 392.50 | 0.91 |
| 7.70 | 54 | – | ||||||
| 6.67 | 52, 65 | – | ||||||
| 10.00 | 51 | – | ||||||
| 7.5 | 57 | – | ||||||
| H02AB04 | Methylprednisolone | 1.25 | 15 | Yes | – | – | 374.50 | 0.96 |
| H02AB06 | Prednisolone | 1.00 | 15, 54 | Yes | 300.0 | 6.10 | 360.40 | 0.99 |
| H02AB07 | Prednisone | 1.00 | reference drug | Yes | 1837.5 | 1.00 | 358.40 | 1.00 |
| H02AB09 | Hydrocortisone | 0.25 | 15, 54, 61 | Yes | – | – | 362.50 | 0.99 |
| H02AB10 | Cortisone | 0.20 | 15, 53, 54, 55, 60, 64 | Yes | – | – | 360.50 | 0.99 |
| 0.21 | 61 | – | ||||||
| Alkylating agents (L01A) | ||||||||
| L01AA01 | Cyclophosphamide | 1.00 | reference drug | Yes | 1000.0 | 1.00 | 261.08 | 1.00 |
| L01AA02 | Chlorambucil | 14.29 | 6, 8, 49 | Yes | – | – | 304.20 | 0.85 |
| 10.00 | 3 | – | ||||||
| L01AA03 | Melphalan | 40.00 | 6, 8, 49 | Yes | 140.0 | 7.14 | 305.20 | 0.85 |
| 43.00 | 3, 4, 7 | – | ||||||
| L01AA05 | Chlormethine | 83.30 | 3, 4, 7 | – | – | – | 156.05 | 1.67 |
| 100.00 | 6, 8, 49 | Yes | ||||||
| L01AA06 | Ifosfamide | 0.24 | 6, 8, 49 | Yes | 6000.0 | 0.17 | 261.08 | 1.00 |
| 0.25 | 3, 4, 7 | – | ||||||
| L01AA07 | Trofosfamide | – | – | – | 150.0 | 6.67 | 323.58 | 0.81 |
| L01AB01 | Busulfan | 8.82 | 6, 8, 49 | Yes | 600.0 | 1.67 | 246.30 | 1.06 |
| 10.00 | 3, 4, 7 | – | ||||||
| L01AB02 | Treosulfan | – | – | – | 12000.0 | 0.08 | 278.30 | 0.93 |
| L01AC01 | Thiotepa | 50.00 | 6, 8, 49 | Yes | 30.0 | 33.30 | 189.22 | 1.37 |
| 6.67 | 3, 4, 7 | – | ||||||
| L01AD01 | Carmustine | 15.00 | 6, 8, 49 | Yes | – | – | 214.50 | 1.22 |
| 10.00 | 3, 4, 7 | – | ||||||
| L01AD02 | Lomustine | 16.00 | 6, 8, 49 | Yes | 600.0 | 1.67 | 233.69 | 1.11 |
| 10.00 | 3, 4, 7 | – | ||||||
| L01AX03 | Temozolomide | – | – | – | 3150.0 | 0.32 | 194.15 | 1.35 |
| L01AX04 | Dacarbazine | 2.00 | 3, 4, 7 | – | 750.0 | 1.33 | 182.18 | 1.43 |
| 3.77 | 6 | Yes | ||||||
| L01XB01 b | Procarbazine | 0.86 | 6, 8, 49 | Yes | 3000.0 | 0.33 | 221.30 | 1.18 |
| 1.00 | 3, 4, 7 | – | ||||||
| L01XX11 c | Estramustine | 0.15 | 3 | Yes | – | – | 440.40 | 0.59 |
| Antimetabolites (folic acid analogues) L01BA | ||||||||
| L01BA01 | Methotrexate | – | – | – | 1000.0 | 1.00 | 454.40 | 1.00 |
| Antimetabolites (purine analogues) L01BB | ||||||||
| L01BB02 | Mercaptopurine | – | – | – | 525.0 | 1.00 | 152.18 | 1.00 |
| L01BB03 | Thioguanine | – | – | – | 500.0 | 1.10 | 167.19 | 0.91 |
| L01BB04 | Cladribine | – | – | – | 12.0 | 43.80 | 285.69 | 0.53 |
| L01BB05 | Fludarabine | – | – | – | 150.0 | 3.50 | 365.21 | 0.42 |
| L01BB06 | Clofarabine | – | – | – | 200.0 | 2.63 | 303.68 | 0.50 |
| Antimetabolites (pyrimidine analogues) L01BC | ||||||||
| L01BC01 | Cytarabine | – | – | – | 600.0 | 1.00 | 243.22 | 1.00 |
| L01BC02 | Fluorouracil | – | – | – | 3600.0 | 0.17 | 130.08 | 1.89 |
| Vinca alkaloids L01CA | ||||||||
| L01CA01 | Vinblastine | 0.25 | 3 | Yes | 12.0 | 0.13 | 811.00 | 1.02 |
| L01CA02 | Vincristine | 1.00 | reference drug | Yes | 1.5 | 1.00 | 825.00 | 1.00 |
| L01CA03 | Vindesine | 0.50 | 3 | Yes | 3.0 | 0.50 | 753.90 | 1.09 |
| Epipodophyllotoxins L01CB | ||||||||
| L01CB01 | Etoposide (VP‐16) | 1.00 | reference drug | Yes | 450.0 | 1.00 | 588.60 | 1.00 |
| L01CB02 | Teniposide | 1.00 | 3, 4, 7, 43 | Yes | 165.0 | 2.73 | 656.70 | 0.89 |
| 2.00 | 50 | – | ||||||
| Topoisomerase inhibitors (other than epipodophyllotoxines) L01CE | ||||||||
| L01CE01 | Topotecan | – | reference drug | – | 7.0 | 1.00 | 457.91 | 1.00 |
| Antibiotics except anthracyclines L01D | ||||||||
| L01DA01 | Dactinomycin | – | reference drug | – | 1.5 | 1.00 | 1255.40 | 1.00 |
| L01DC01 | Bleomycin | – | – | – | 30.0 | 0.05 | 1415.60 | 0.88 |
| L01DC03 | Mitomycin | – | – | – | 8.0 | 0.19 | 334.30 | 3.70 |
| Anthracyclines L01DB | ||||||||
| L01DB01 | Doxorubicin | 1.00 | reference drug | Yes | 60.0 | 1.00 | 543.50 | 1.00 |
| L01DB02 | Daunorubicin | 1.00 | 28, 34, 36, 43, 48 | Yes | 120.0 | 0.50 | 527.50 | 1.03 |
| 0.83 | 3, 4, 5, 7, 33 | – | ||||||
| 0.75 | 32, 46 | – | ||||||
| 0.67 | 29 | – | ||||||
| 0.50 | 10 | – | ||||||
| L01DB03 | Epirubicin | 1.00 | 17, 34, 46 | Yes | 150.0 | 0.40 | 543.50 | 1.00 |
| 0.67 | 4, 5, 7, 10, 33 | – | ||||||
| 0.75 | 32 | – | ||||||
|
0.83 d 0.67 e 0.56 f |
16 | – | ||||||
| L01DB04 | Aclarubicine | – | – | – | 125.0 | 0.48 | 811.88 | 0.67 |
| L01DB05 | Zorubicine | 0.50 | 4, 7 | Yes | – | – | 645.7 | 0.84 |
| L01DB06 | Idarubicin | 5.00 | 5, 10, 33, 34, 36 | Yes | 14.0 | 4.29 | 497.50 | 1.09 |
| 3.00 | 28, 32, 43 | – | ||||||
| 2.78 | 7 | – | ||||||
| 4.50 | 40 | – | ||||||
| L01DB07 | Mitoxantrone | 4.00 | 4, 5, 7, 10, 34 | Yes | 20.0 | 3.00 | 444.50 | 1.22 |
| 3.00 | 32 | – | ||||||
| 5.00 | 36 | – | ||||||
| Platinum derivates L01XA | ||||||||
| L01XA01 | Cisplatin | 1.00 | reference drug | Yes | 100.0 | 1.00 | 300.00 | 1.00 |
| L01XA02 | Carboplatin | 0.25 | 3, 4, 7, 31, 43 | Yes | 600.0 | 0.17 | 371.25 | 0.81 |
| Asparaginase L01XX | ||||||||
| L01XX02 | Asparaginase | 1.00 | reference drug | Yes | 25000.0 g | 1.00 | – | – |
| L01XX24 | Pegylated asparaginase | 18.00 | 9 | Yes | 2500.0 g | 10.00 | – | – |
Note: Reference substances are printed in bold.
Based on two criteria (defined a priori), which were applied in the following order: (1) most recent publication year, (2) articles which developed their own conversion factor based on their own literature review.
According to the ATC index, Procarbazine is a Methylhydrazine (L01XB) and belongs to the group “other antineoplastic agents” (L01X). However, due to its mode of action, it is usually grouped with the alkylating agents (L01A) in oncology literature. 3 , 8
According to the ATC index, Estramustine belongs to the group “other antineoplastic agents” (L01X). However, due to its mode of action, it is usually grouped with the alkylating agents (L01A) in oncology literature. 3 , 8
Hematological toxicity.
Non‐hematological toxicity.
Cardiac toxicity.
IU (International Units)/m2.
3.4. Equimolar principle
The rationale behind the equimolar principle is that ‘a molecule of a given drug generally has one active site, whatever its weight. Even if a particular drug may have more than one active site per molecule, the error introduced by this hypothesis is probably lower than that introduced when summing the weights’. 27 The molecular weights (g/mol) of substances with an ATC code are readily available for all substances from the Website PubChem, 66 not only for the substances included in the papers found in the literature search. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27
This permitted directly calculating equimolar conversion factors for all substances (save one, see below), presented in column 9 in Table 4.
For asparaginase (ATC‐code L01XX02) and pegylated asparaginase (L01XX24), we could not present factors derived from molecular weights because this chemical approach is not applicable to enzymes.
3.5. Conversion factors based on typical dose
Typical doses were available for 41 (of the 49) substances, including the 11 substances for which no conversion factor based on effect equivalence had been found in the literature. The remaining eight (49 minus 41) substances have not been used in treatment protocols in German pediatric oncology since the 1970s. The resulting conversion factors are presented in Table 4, column 7.
3.6. Comparing factors based on different principles
Table 4 presents the conversion factors for each substance by substance group for the principles of assumed effect equivalence (as found in the literature), based on typical dose, 11 , 12 and based on the equimolar principle. 66
The conversion factors based on effect equivalence derived from the literature ranged from 0.15 to 100, those derived from typical doses ranged from 0.05 to 43.8. More than 80% or 90%, respectively, of these conversion factors were between 0.1 and 10. The range of the factors based on molecular weights, 0.42–3.70, was much narrower.
Comparing the factors, the correlation closest to 1 was found between the factors based on the principle of effect equivalence and the typical dose principle, r = 0.83. Figure 2 shows the corresponding scatter plot (factors on a log scale). The slope from the linear regression model was 0.74, so the factors from typical dose were on average closer to one than the ones suggested in the literature based on effect equivalence. A slope of 1 would mean factors from both principles would be fully comparable on average. Sensitivity analyses and subgroups are presented in Supplementary Figures 1 and 2. Results differed slightly when excluding glucocorticoids (Supplementary Figure 2).
FIGURE 2.
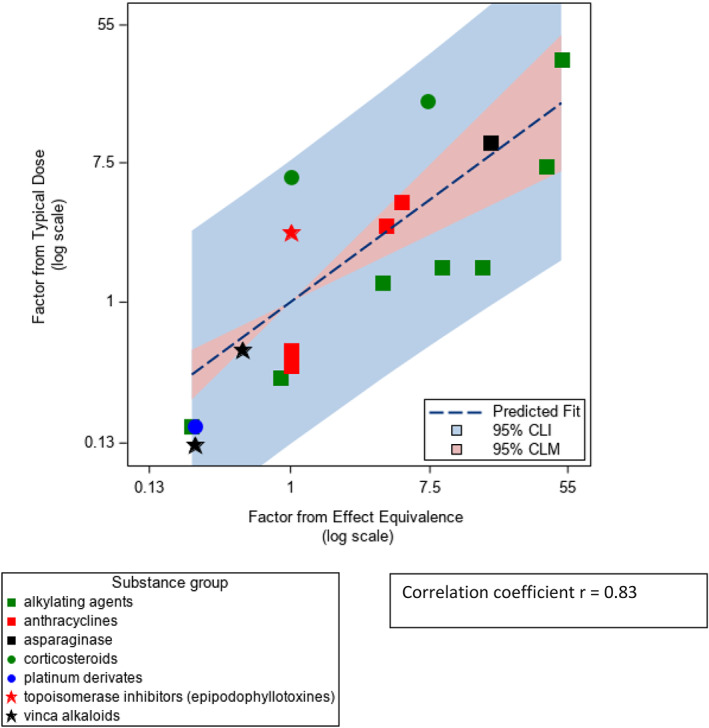
Scatter plot and regression line of the factors based on effect equivalence from literature review and the factors based on typical dose (derived from treatment protocols in pediatric oncology in Germany). CLI, confidence limits for the individual predicted values; CLM, confidence limits for the mean predicted values
The correlations of the factors based on the equimolar principle with the other factors were considerably lower (r = 0.54 equimolar versus the principle of effect equivalence, and −0.32 equimolar compared to factors based on the typical doses). Supplementary Figures 3 and 4 present the corresponding scatterplots.
4. DISCUSSION
In epidemiological research on late effects of the therapy of childhood cancer, it can be necessary to aggregate chemotherapy agents into substance classes, as in our study on second tumors after tumor therapy (STATT). We started out by considering aggregating drugs without using any conversion inappropriate because it is important to adjust for the different potencies or toxicities of the drugs, as stated in the literature, for example References 4, 8. Additionally, results obtained from aggregating doses without conversion are not transferable to other studies with another mix of drugs.
According to the criteria from Munn et al. 67 a scoping review seemed appropriate for our research aim to identify conversion factors for chemotherapeutic substance classes used in literature.
In a literature search, 35 articles were identified which used or justified conversion factors for 26 substances (excluding the 12 reference substances) based on principles which can be summarized as effect equivalence. The literature review did not yield such conversion factors for 11 relevant chemotherapeutic substances used in treatment protocols in Germany. Further 10 papers suggested the equimolar principle using molecular weights. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 For 41 substances we were able to derive a factor based on a typical dose approach based on a comprehensive list of substances used in German pediatric oncology.
Comparing these three types of conversion factors, we found the effect equivalence‐based and the typical dose‐based factors to be highly correlated (r = 0.83) and on average close to being identical. The correlation of the factors derived from molecular weights with the other factors was moderate or close to zero.
The literature search was not straightforward, as only three articles 8 , 16 , 17 were explicitly about the factors as such. All other articles mentioned the factors briefly in their respective methods sections. In a second step we searched the reference lists of the articles found by literature search in order to identify the original source for the factor mentioned in them. If the factor was the same, the original article was included instead of the article found by literature search. The search on glucocorticoids could not be restricted to second tumors after childhood cancer as we found only 22 articles with our broad search on equivalence dose for glucocorticoids.
The literature search was rather broad in scope to ensure we would not miss any relevant papers. However, the fact that we identified 13 additional papers from reference lists or through prior knowledge indicates that there were potential blind spots. These 13 articles applied substance conversion outside the topic we were primarily interested in (second tumors after childhood cancer, n = 11) or were not listed in PubMed or Web of Science as they were a guideline 10 and a table of equivalent doses 15 (n = 2).
The typical doses‐approach was feasible and had a very broad information basis, as pediatric oncology in Germany has been using nationwide, centralized treatment protocols since the 1970s, 68 and we had access to a complete overview over all these protocols until 2018. 11 , 12
We needed a criterion to select a factor when more than one was available in the literature. Using the latest information and one which stated its basis clearly seemed sensible, but they are still somewhat arbitrary and readers may make a different selection from Table 4. As factors are relatively similar, this does not influence results considerably. The only exception is Thiotepa (ATC code L01AC01), where the factors differ considerably and the one not originally selected (6.67 3 , 4 , 7 ) yielded more plausible converted doses than the one we would have selected by our criteria (50 6 , 8 , 49 ), as the larger factor created extreme outliers in the distribution of aggregated alkylating agents.
The method used by the authors of two articles 30 , 34 to derive factors from their own data was based on substance specific regression coefficients after applying a factor from the literature to then compare the effect sizes per dose. The authors suggest to use these ratios for obtaining a different conversion factor for a joint estimate; they do not apply this factor to obtain a joint dose–response estimate for their outcome, however. This is an interesting approach. One must be aware, however, that small studies and substances with small numbers of exposed patients are likely to randomly produce outlying regression coefficients, which could provide these substances randomly with an outlying weight (although the bootstrap approach chosen would render such estimates less likely). Moreover, it is questionable whether such sets of weights derived from one set of patients (or sets of patients) can be applied to another set of patients. Interestingly, the factors they derived from the data were not wildly different from the ones they cited from the literature (except for mitoxantrone). Nevertheless, we decided to err on the side of caution and to exclude these factors from our table and to focus on factors based on more general principles. Thus, we included the factors the authors of the articles 30 , 34 cited from literature. Our readers may come to a different conclusion.
The factors derived from typical doses were mainly based on the doses in single treatment blocks of a respective clinical study protocol. The cumulative doses for the whole therapy concept were not included in the compendium. 11 , 12 It does not contain any rules of replacement either. We calculated the mode of all doses listed for each substance in the compendium. We considered the mode, the dose which was used most frequently, to describe the typical dose best, independently from the number of chemotherapy blocks included in the compendium in which the respective substance was applied. We noted that the mode was almost always identical to (32 out of the 41 substances extracted from the compendium) or very close to the median.
The scientific basis for the various types of effect equivalence was often not stated (9 articles), did not yield a definitive factor (n = 4) or presented factors additional to or different from the ones cited in the original articles (n = 5). It is open for discussion to which extent conversion factors, specifically referring to cardiotoxic or hematotoxic side effects, are transferable to other endpoints in late effects research. No article referred explicitly to an equivalence of effect regarding second neoplasms, unless we equate hematoxicity with second leukemia. However, the literature search also showed that authors referring to different bases for their respective conversion factors nevertheless came to rather similar factors.
We are aware of further limitations. When applying the conversion factors in research, characteristics like dose frequency or the combination of drugs or drugs and other components of therapy, such as radiotherapy, may influence the effect equivalence. Substance combinations and dosages strongly depend on the cancer type. Conversion factors may possibly be age‐ or sex‐specific, which was however not mentioned in any of the papers found. There was no information in the available literature to calculate different conversion factors for different modes of application. For instance, for dexamethasone, the study 57 referring to intravenous application used almost the same conversion factor as the other studies generally referring to oral application. No conversion factors for intrathecal application were mentioned in the literature. Bioavailability of intrathecal application is considered the same as intravenous. 69 As to the factors from typical doses, Methotrexate was the only substance with information on intrathecal application and a dose given in mg/m2. 11 , 12 As only 22 therapy blocks were involved, neither the median nor the mode changed when including or excluding these doses. The typical dose was derived from German data only. Applying the principle to an overview of therapy protocols from another setting might yield different factors.
As we decided to use all information available to us for our own calculations, the literature review was based on a slightly different period than the typical dose‐approach (1985—November/December 2022 and 1970–2018, respectively): Besides technical reasons (incomplete availability of publications before 1985), we wanted to include the latest available literature in the literature review.
When comparing typical dose‐based factors to effect equivalence‐based factors from the literature, the slope from linear regression was 0.74; however, ideally the slope should have been even closer to 1.
It needs to be stated that all approaches described here are not meant for a clinical setting, for example, when replacing one substance with another for the treatment of an individual patient. This is also true for the typical dose approach. The protocols where the typical doses were derived from are used in clinical setting. However, the calculation of the typical doses was across all protocols and therefore all diagnoses, age groups and combination of drugs and was based on typical doses of single therapy blocks. Therefore, they need not be valid in all special clinical settings. Hence, all factors presented here are meant for and are particularly useful for population‐based epidemiological research. Practical application requires harmonizing units before applying the conversion factor. If height and weight of a patient are available, mg per kg can be converted into mg per m2.
Most of the literature cited here was about post‐hoc treatment assessment in a late effects research setting.
This study gives an overview over dose conversion factors of anticancer agents to a reference substance within their class by mode of action with an emphasis on usage in childhood cancer late effects research. We were able to present factors for 49 substances.
As a first step we present results from a literature review. The factors based on effect‐equivalence seem to be more widely used and well justified for late effects research. For substances for which no such conversion factors could be found in the literature, we proposed factors from a rather simple approach, relating typical doses. Our original question had been whether we could justify filling in these factors for the 11 substances where we could not find an effect‐equivalence factor in the literature. Based on our comparison results we consider this justified. The data base for the typical dose approach was specific for pediatric oncology in Germany; therefore, our factors may not be directly applicable to adults or in other countries.
A smaller number of articles suggested factors derived from molecular weights (equimolar). Obtaining such factors is straightforward using publicly available mole weights. These factors were basically independent from the other approaches. Results in terms of dose effects in late effects research using these factors may not be comparable to results based on data using effect equivalence‐based factors.
These conversion factors in general and their underlying principles potentially have great value for research with aggregated data, such as epidemiological late effects research.
AUTHOR CONTRIBUTIONS
Meike Ressing: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (lead); writing – review and editing (lead). Cornelia Becker: Conceptualization (equal); investigation (equal); methodology (equal); project administration (equal); writing – review and editing (supporting). Christian Müller: Investigation (equal); resources (equal); validation (equal); writing – review and editing (supporting). Seyed Hamidreza Mahmoudpour: Methodology (equal); validation (equal); writing – review and editing (supporting). Gabriele Calaminus: Investigation (equal); resources (equal); writing – review and editing (supporting). Thorsten Langer: Investigation (equal); resources (equal); writing – review and editing (supporting). Friederike Erdmann: Funding acquisition (equal); supervision (equal); writing – review and editing (supporting). Mathias Voigt: Data curation (equal); writing – review and editing (supporting). Melanie Kaiser: Data curation (equal); writing – review and editing (supporting). Peter Kaatsch: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing – review and editing (supporting). Maria Blettner: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing – review and editing (supporting). Claudia Spix: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); visualization (equal); writing – original draft (supporting); writing – review and editing (supporting).
FUNDING INFORMATION
This study was funded by Deutsche Krebshilfe, Grant Number: 70112099.
CONFLICT OF INTEREST STATEMENT
Seyed Hamidreza Mahmoudpour is currently an employee of Merck KGaA, Darmstadt, Germany. All other authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS STATEMENT
The authors declare that this work has been done in accordance to Wiley “Best Practice Guidelines on Research Integrity and Publishing Ethics” and that is has been performed in an ethical and responsible way, with no research misconduct, which includes, but is not limited to data fabrication and falsification, plagiarism, image manipulation, unethical research, biased reporting, authorship abuse, redundant or duplicate publication, and undeclared conflicts of interest
Supporting information
Data S1: Supporting Information.
ACKNOWLEDGMENTS
We thank Marleen van den Berg, Amsterdam University Medical Center, Department of Pediatrics, Division of Pediatric Oncology, for approving our usage of an extensive list of chemotherapeutics, including brand name and substance name/name of active ingredient, which was compiled for the EU‐project PanCareLIFE (funded by the European Union's Seventh Framework Programme for research, technological development and demonstration, grant agreement no 602030). We thank Dorothée Malonga Makosi, MPH, Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), University Medical Center of the Johannes Gutenberg‐University Mainz, for providing advice on data on chemotherapy. Open Access funding enabled and organized by Projekt DEAL.
Ressing M, Becker C, Müller C, et al. Equivalent doses for anticancer agents used in pediatric oncology: A literature review and evaluation of a novel approach for conversion factors. Cancer Reports. 2023;6(5):e1811. doi: 10.1002/cnr2.1811
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Niemeyer C, Eggert A. Pädiatrische Hämatologie und Onkologie. Springer; 2017:592. [Google Scholar]
- 2. WIdO . ATC‐Klassifikation für den deutschen Arzneimittelmarkt. 2021. Accessed May 14, 2021 https://www.wido.de/publikationen-produkte/arzneimittel-klassifikation/
- 3. Guerin S, Guibout C, Shamsaldin A, et al. Concomitant chemo‐radiotherapy and local dose of radiation as risk factors for second malignant neoplasms after solid cancer in childhood: a case‐control study. Int J Cancer. 2007;120(1):96‐102. [DOI] [PubMed] [Google Scholar]
- 4. Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case‐control study by the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol. 2003;21(6):1074‐1081. [DOI] [PubMed] [Google Scholar]
- 5. Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross‐sectional study. Ann Intern Med. 2016;164(2):93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allodji RS, Tucker MA, Hawkins MM, et al. Role of radiotherapy and chemotherapy in the risk of leukemia after childhood cancer: an international pooled analysis. Int J Cancer. 2021;148(9):2079‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casagranda L, Oriol M, Freycon F, et al. Second malignant neoplasm following childhood cancer: a nested case‐control study of a recent cohort (1987–2004) from the childhood cancer registry of the Rhone‐Alpes region in France. Pediatr Hematol Oncol. 2016;33(6):371‐382. [DOI] [PubMed] [Google Scholar]
- 8. Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure. A report from the childhood cancer survivor study. Pediatr Blood Cancer. 2014;61(1):53‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schramm F, Zur SU, Zimmermann M, et al. Results of CoALL 07‐03 study childhood ALL based on combined risk assessment by in vivo and in vitro pharmacosensitivity. Blood Adv. 2019;3(22):3688‐3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long‐Term Follow‐up Guidelines for Survivors of Childhood . Adolescent and Young Adult Cancers. Children's Oncology Group; 2018. [Google Scholar]
- 11. Müller C. Kompendium der Therapieoptimierungsstudien bei Kindern und Jugendlichen mit Krebserkrankungen. Band 1: Maligne hämatologische Erkrankungen. Shaker Verlag; 2016:170. [Google Scholar]
- 12. Müller C. Kompendium der Therapieoptimierungsstudien bei Kindern und Jugendlichen mit Krebserkrankungen. Band 2: Solide Tumoren. Shaker Verlag; 2017:123. [Google Scholar]
- 13. Ahmad SS, Reinius MA, Hatcher HM, Ajithkumar TV. Anticancer chemotherapy in teenagers and young adults: managing long term side effects. BMJ. 2016;354:i4567. [DOI] [PubMed] [Google Scholar]
- 14. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 15. AMK . 15.2_Glucocorticoide_Tabelle_FINAL.pdf 2021 [updated 10.02.2021]. Accessed January 7, 2022 https://www.abda.de/fileadmin/user_upload/assets/Arzneimittelkommission/Aequivalenzdosistabellen_NEU/15.2_Glucocorticoide_Tabelle_FINAL.pdf
- 16. Mouridsen HT. New cytotoxic drugs in treatment of breast cancer. Acta Oncol. 1990;29(3):343‐347. [DOI] [PubMed] [Google Scholar]
- 17. Launchbury AP, Habboubi N. Epirubicin and doxorubicin: a comparison of their characteristics, therapeutic activity and toxicity. Cancer Treat Rev. 1993;19(3):197‐228. [DOI] [PubMed] [Google Scholar]
- 18. Svahn‐Tapper G, Garwicz S, Anderson H, et al. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: a population‐based case‐control study in the five Nordic countries. Acta Oncol. 2006;45(4):438‐448. [DOI] [PubMed] [Google Scholar]
- 19. Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23(1):197‐204. [DOI] [PubMed] [Google Scholar]
- 20. Menu‐Branthomme A, Rubino C, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of soft tissue sarcoma after solid tumours during childhood. Int J Cancer. 2004;110(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 21. Little MP, de Vathaire F, Shamsaldin A, et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer. 1998;78(3):269‐275. [DOI] [PubMed] [Google Scholar]
- 22. de Vathaire F, Hawkins M, Campbell S, et al. Second malignant neoplasms after a first cancer in childhood: temporal pattern of risk according to type of treatment. Br J Cancer. 1999;79(11–12):1884‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guérin S, Dupuy A, Anderson H, et al. Radiation dose as a risk factor for malignant melanoma following childhood cancer. Eur J Cancer. 2003;39(16):2379‐2386. [DOI] [PubMed] [Google Scholar]
- 24. Kony SJ, de Vathaire F, Chompret A, et al. Radiation and genetic factors in the risk of second malignant neoplasms after a first cancer in childhood. Lancet. 1997;350(9071):91‐95. [DOI] [PubMed] [Google Scholar]
- 25. Haddy N, Le Deley MC, Samand A, et al. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer. 2006;42(16):2757‐2764. [DOI] [PubMed] [Google Scholar]
- 26. Haddy N, El‐Fayech C, Guibout C, et al. Thyroid adenomas after solid cancer in childhood. Int J Radiat Oncol Biol Phys. 2012;84(2):e209‐e215. [DOI] [PubMed] [Google Scholar]
- 27. Le Vu B, de Vathaire F, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumours during childhood. Int J Cancer. 1998;77(3):370‐377. [DOI] [PubMed] [Google Scholar]
- 28. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339(dec08 1):b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smibert E, Carlin JB, Vidmar S, Wilkinson LC, Newton M, Weintraub RG. Exercise echocardiography reflects cumulative anthracycline exposure during childhood. Pediatr Blood Cancer. 2004;42(7):556‐562. [DOI] [PubMed] [Google Scholar]
- 30. Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33(32):3774‐3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozols RF, Behrens BC, Ostchega Y, Young RC. High dose cisplatin and high dose carboplatin in refractory ovarian cancer. Cancer Treat Rev. 1985;12(Suppl A):59‐65. [DOI] [PubMed] [Google Scholar]
- 32. Lehmann S, Isberg B, Ljungman P, Paul C. Cardiac systolic function before and after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;26(2):187‐192. [DOI] [PubMed] [Google Scholar]
- 33. Blanco JG, Sun CL, Landier W, et al. Anthracycline‐related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children's Oncology Group. J Clin Oncol. 2012;30(13):1415‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late‐onset cardiotoxicity. JAMA Oncol. 2019;5(6):864‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tucker MA, Meadows AT, Boice JD, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78(3):459‐464. [PubMed] [Google Scholar]
- 36. Creutzig U, Diekamp S, Zimmermann M, Reinhardt D. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr Blood Cancer. 2007;48(7):651‐662. [DOI] [PubMed] [Google Scholar]
- 37. Buzdar AU, Marcus C, Smith TL, Blumenschein GR. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55(12):2761‐2765. [DOI] [PubMed] [Google Scholar]
- 38. Creutzig U, Körholz D, Niemeyer CM, et al. 3 × 14 mg/m2 idarubicin during induction: results of a pilot study in children with AML. Leukemia. 2000;14(2):340‐342. [DOI] [PubMed] [Google Scholar]
- 39. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. AML Collaborative Group. Br J Haematol. 1998;103(1):100‐109. [PubMed] [Google Scholar]
- 40. Feig SA, Ames MM, Sather HN, et al. Comparison of idarubicin to daunomycin in a randomized multidrug treatment of childhood acute lymphoblastic leukemia at first bone marrow relapse: a report from the Children's Cancer Group. Med Pediatr Oncol. 1996;27(6):505‐514. [DOI] [PubMed] [Google Scholar]
- 41. Berman E, Heller G, Santorsa J, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77(8):1666‐1674. [PubMed] [Google Scholar]
- 42. Wiernik PH, Banks PL, Case DC, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79(2):313‐319. [PubMed] [Google Scholar]
- 43. Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84(1):224‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hollingshead LM, Idarubicin FD. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs. 1991;42(4):690‐719. [DOI] [PubMed] [Google Scholar]
- 45. Neri B, Cini‐Neri G, Bandinelli M, Pacini P, Bartalucci S, Ciapini A. Doxorubicin and epirubicin cardiotoxicity: experimental and clinical aspects. Int J Clin Pharmacol Ther Toxicol. 1989;27(5):217‐221. [PubMed] [Google Scholar]
- 46. Messinger Y, Uckun FM. A critical risk‐benefit assessment argues against the use of anthracyclines in induction regimens for newly diagnosed childhood acute lymphoblastic leukemia. Leuk Lymphoma. 1999;34(5–6):415‐432. [DOI] [PubMed] [Google Scholar]
- 47. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263‐302. [DOI] [PubMed] [Google Scholar]
- 48. Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97(8):1991‐1998. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Kremer LCM, van Leeuwen FE, et al. Cohort profile: risk and risk factors for female breast cancer after treatment for childhood and adolescent cancer: an internationally pooled cohort. BMJ Open. 2022;12(11):e065910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winick NJ, McKenna RW, Shuster JJ, et al. Secondary acute myeloid leukemia in children with acute lymphoblastic leukemia treated with etoposide. J Clin Oncol. 1993;11(2):209‐217. [DOI] [PubMed] [Google Scholar]
- 51. Arteaga E, Fardella C, Campusano C, Cárdenas I, Martinez P. Persistent hypokalemia after successful adrenalectomy in a patient with Cushing's syndrome due to ectopic ACTH secretion: possible role of 11beta‐hydroxysteroid dehydrogenase inhibition. J Endocrinol Invest. 1999;22(11):857‐859. [DOI] [PubMed] [Google Scholar]
- 52. Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard‐risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101(10):3809‐3817. [DOI] [PubMed] [Google Scholar]
- 53. Ekstrand E, Esposito D, Ragnarsson O, Isgaard J, Johannsson G. Metabolic effects of cortisone acetate vs hydrocortisone in patients with secondary adrenal insufficiency. J Endocr Soc. 2020;4(12):bvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Filipsson H, Monson JP, Koltowska‐Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91(10):3954‐3961. [DOI] [PubMed] [Google Scholar]
- 55. Løvås K, Thorsen TE, Husebye ES. Saliva cortisol measurement: simple and reliable assessment of the glucocorticoid replacement therapy in Addison's disease. J Endocrinol Invest. 2006;29(8):727‐731. [DOI] [PubMed] [Google Scholar]
- 56. Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361(9372):1881‐1893. [DOI] [PubMed] [Google Scholar]
- 57. Pfeiffer M, Griss P. Craniocerebral trauma and aseptic osteonecrosis. Steroid‐induced sequelae after therapy of brain edema. Unfallchirurg. 1992;95(6):284‐287. [PubMed] [Google Scholar]
- 58. Forth W, Henschler D, Rummel W. Allgemeine und Spezielle Pharmakologie und Toxikologie. Wissenschaftsverlag; 1987:896. [Google Scholar]
- 59. Kaiser H. Cortisonderivate in Klinik und Praxis. Thieme; 1987:257. [Google Scholar]
- 60. Puglisi S, Rossini A, Tabaro I, et al. What factors have impact on glucocorticoid replacement in adrenal insufficiency: a real‐life study. J Endocrinol Invest. 2021;44(4):865‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sandrini R, Jospe N, Migeon CJ. Temporal and individual variations in the dose of glucocorticoid used for the treatment of salt‐losing congenital virilizing adrenal hyperplasia due to 21‐hydroxylase deficiency. Acta Paediatr Suppl. 1993;388:56‐60. discussion 61. [DOI] [PubMed] [Google Scholar]
- 62. Migeon CJ. Diagnosis and treatment of adrenogenital disorders. In: De Groot LJ, ed. Endocrinology. W.B. Saunders Company; 1989:1676‐1704. [Google Scholar]
- 63. Bondy PK. Disorders of the adrenal cortex. In: Wilson JDF, ed. Williams Textbook of Edocrinology. 7th ed. W.B. Saunders Company; 1985:816‐890. [Google Scholar]
- 64. Swords FM, Carroll PV, Kisalu J, Wood PJ, Taylor NF, Monson JP. The effects of growth hormone deficiency and replacement on glucocorticoid exposure in hypopituitary patients on cortisone acetate and hydrocortisone replacement. Clin Endocrinol (Oxf). 2003;59(5):613‐620. [DOI] [PubMed] [Google Scholar]
- 65. Tabone MD, Kolta S, Auquier P, et al. Bone mineral density evolution and its determinants in long‐term survivors of childhood acute leukemia: a Leucémies enfants adolescents study. Hema. 2021;5(2):e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Medicine National Center for Biotechnology Information . U.S. National Library of. PubChem Structure Search. Accessed December 12, 2022 https://pubchem.ncbi.nlm.nih.gov/search/search.cgi
- 67. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rossig C, Juergens H, Schrappe M, et al. Effective childhood cancer treatment: the impact of large scale clinical trials in Germany and Austria. Pediatr Blood Cancer. 2013;60(10):1574‐1581. [DOI] [PubMed] [Google Scholar]
- 69. Louis S. Goodman & Gilman's Pharmacological Basis of Therapeutics. McGraw‐Hill Education LLC; 2011:2084. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information.
Data Availability Statement
Data available on request from the authors.


