Abstract
In this study, infection-derived immunity in the rabbit model of Lyme disease was compared to immunity following immunization with purified outer membrane vesicles (OMV) isolated from Borrelia burgdorferi and recombinant outer surface protein A (OspA). Immunization of rabbits with OMV isolated from virulent strain B31 and its avirulent derivative B313 (lacking OspA and DbpA) conferred highly significant protection against intradermal injection with 6 × 104 in vitro-cultivated virulent B. burgdorferi. This is the first demonstration of protective immunogenicity induced by OMV. While immunization with OspA and avirulent B31 OMV provided far less protection against this challenge, rabbits with infection-derived immunity were completely protected. Protection against host-adapted B. burgdorferi was assessed by implantation of skin biopsies taken from rabbit erythema migrans (a uniquely rich source of B. burgdorferi in vertebrate tissue) containing up to 108 spirochetes. While all of the OMV- and OspA-immunized rabbits were fully susceptible to skin and disseminated infection, rabbits with infection-derived immunity were completely protected. Analysis of the antibody responses to outer membrane proteins, including DbpA, OspA, and OspC, suggests that the remarkable protection exhibited by the infection-immune rabbits is due to antibodies directed at antigens unique to or markedly up-regulated in host-adapted B. burgdorferi.
An understanding of the early steps in the pathogenesis of Borrelia burgdorferi infection has shown that the current OspA-based Lyme disease vaccine (49, 53) works by a novel mechanism. OspA is abundant in B. burgdorferi residing in the gut of unfed ticks or cultivated in vitro (20, 47). After a blood meal the spirochetes multiply, migrate to the salivary glands, and no longer express OspA (20, 47) prior to transmission. A blood meal bringing OspA antibodies to the tick gut inhibits B. burgdorferi multiplication and migration to the salivary glands (20, 27). The OspA vaccine is therefore based on a molecule that is not expressed by B. burgdorferi during vertebrate infection (18).
It is likely that the changes B. burgdorferi undergoes prior to transmission from the tick and during mammalian infection are of importance to pathogenesis and protective immunity. Barthold et al. have used the term “host-adapted” to refer to B. burgdorferi obtained from infected mouse tissue (7). There is biological evidence that these host-adapted borreliae (HAB) differ from in vitro-cultivated borreliae (IVCB) in relevant ways. Mice actively or passively immunized with OspA are protected against needle challenge with IVCB but are fully susceptible to disseminated infection upon challenge with HAB from ear implants of infected donor mice (7). This finding has highlighted the significance of the lack of OspA expression during mouse infection. Moreover, the differences between IVCB and HAB extend beyond OspA. Mice passively immunized with serum from chronically infected mice are protected against needle challenge with IVCB but are fully susceptible to infection with HAB (19). This finding is consistent with the hypothesis that immunogens that stimulate protection against IVCB are distinct from those of HAB.
There have only been two reports of protection against infection with HAB. Cassatt and colleagues reported that mice immunized with DbpA, a surface lipoprotein adhesin which mediates binding to the collagen-associated protein decorin (33, 35) and is up-regulated during infection (13), showed partial protection against infection with a challenge of six to eight spirochetes recovered from infected mouse plasma (13). Barthold found that antibiotic treatment of chronically infected mice resulted in partial protection against infection with HAB of the homologous strain; less or no protection was evident with heterologous strain challenge (4). It is therefore clear that a critical test of new Lyme disease vaccine candidates is whether protection against HAB is conferred.
We have previously reported that intradermal infection of the rabbit with B. burgdorferi uniformly results in the development of erythema migrans (EM) (28, 29) and, in the first week of infection, dissemination to spleen, liver, lymph nodes, central nervous system, and joints. Within 3 months, B. burgdorferi infection is fully cleared, and in contrast to the mouse model (6), complete infection-derived immunity results (28) without the need for antibacterial treatment. The quantitative nature of the protection is impressive in that rabbits with infection-derived immunity are fully protected against intradermal needle injection using 4 × 107 IVCB (28). By comparison, rabbits immunized with OspA and challenged with several orders of magnitude less IVCB showed only partial protection (29). In addition, serum from rabbits with complete immunity (immune rabbit serum [IRS]) confers passive protection against challenge with large numbers (6 × 106 organisms) of IVCB (C. Chong and J. N. Miller, unpublished data).
In this study, we show that rabbits with infection-derived immunity are also completely protected against challenge with up to 108 HAB, administered through implantation of infected rabbit skin. The rabbit model therefore provides a unique opportunity to study the nature of the potent complete protection against HAB infection. Proteins associated with HAB theoretically consist of those that are uniquely expressed during mammalian infection, those that are present in IVCB but up-regulated during infection, and those that are present in HAB and IVCB in relatively similar amounts. In this regard, we have described the protein constituents of outer membrane vesicles (OMV) isolated from virulent IVCB strain B31 and avirulent B31. Certain OMV proteins of IVCB are apparently expressed by HAB, as evidenced by binding antibodies found in the serum of infection-immune rabbits (52). We have also tested whether OMV can confer protection against IVCB and HAB challenge and compared it to infection-derived immunity.
MATERIALS AND METHODS
Bacterial strains.
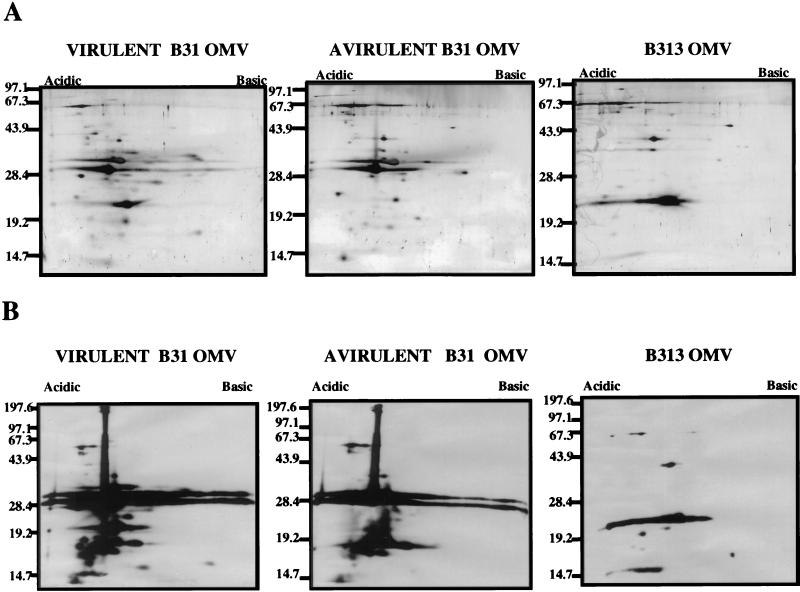
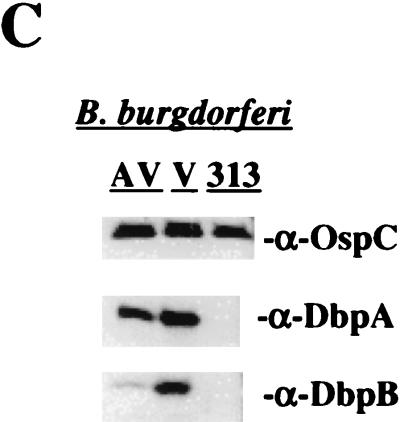
Virulent and avirulent (ATCC 35210) B. burgdorferi sensu stricto strain B31 was grown at 34°C in BSK II medium supplemented with 6% heat-inactivated normal rabbit serum (NRS) as previously described (28). Low-passage (≤4) virulent B31 used in these experiments was demonstrated to be infective and cause EM in rabbits (data not shown). B. burgdorferi strain B313, kindly provided by Alan Barbour (University of California, Irvine), is a mutant strain of B31 reported to lack multiple plasmids including the 54-kb plasmid and does not express OspA, OspB, and OspD (46, 66). In addition, Sadziene et al. have reported that B313 does not express OspC (46). However, in our laboratory, B313 was found to express OspC (Fig. 3C).
FIG. 3.
Specific antibody responses to OMV proteins. (A) Gold stain of the total protein profiles of 109 organism equivalents of virulent B31, avirulent B31, and B313 OMV separated by 2-D NEPHGE. (B) Western immunoblot analysis of similar OMV separated by 2-D NEPHGE probed with corresponding prechallenge serum. OspA (31 kDa) and OspB (32 kDa) are absent in strain B313. Markers to the left are in kilodaltons; acidic and basic ends of gels are indicated at the top. (C) Western immunoblot using anti-OspC, anti-DbpA, and anti-DbpB sera against 1.5 × 107 avirulent B31 (AV), virulent B31 (V), and B313 (313) strains of B. burgdorferi.
Isolation of B. burgdorferi OMV.
The OMV of avirulent and virulent B. burgdorferi strains B31 and B313 were isolated as previously described (52). Briefly, 1011 B. burgdorferi cells were vortexed in 90 ml of 25 mM citrate buffer for 3 h at room temperature in order to release OMV. OMV and protoplasmic cylinders were pelleted at 20,000 × g for 30 min, and the pelleted material was separated on a 25 to 56% discontinuous sucrose gradient. The OMV band was recovered, washed in phosphate-buffered saline (PBS), pH 7.4, and repurified on an additional discontinuous sucrose gradient.
Immunization of rabbits with rOspA and OMV.
Each of 10 New Zealand White rabbits was immunized intradermally and intramuscularly with a total of 35 μg of recombinant OspA (rOspA) from strain ZS7 (which differs from strain B31 by two amino acids) adsorbed onto aluminum hydroxide (alum) (kindly provided by Yves Lobet, SmithKline Beecham Biologicals, Rixensart, Belgium) as previously described (28). Rabbits were boosted twice at 2-week intervals and challenged 2 weeks after the final boost. Immunizations with B. burgdorferi OMV from avirulent B31, virulent B31, and B313 strains were administered into the popliteal lymph node of the rabbit, which we have found to elicit a strong immune response using nanogram amounts of immunogen in the absence of adjuvants. The use of adjuvants was avoided to safeguard against possible alteration of native OMV protein conformation and potential loss of conformation-dependent epitopes (9). For popliteal node injections, rabbits were anesthetized with 45 μg of ketamine and 8.8 μg of xylazine per kg of body weight. Using aseptic technique, a popliteal lymph node was located under the skin and injected with 60 μl containing OMV isolated from 109 spirochetes. Rabbits were boosted three times at 2-week intervals with 60 μl of OMV from 2 × 109 organism equivalents and challenged 1 week after the final boost. In the case of all immunizations with OspA and OMV, sera were obtained prior to immunization and immediately prior to challenge.
Infection-immune rabbits.
New Zealand White rabbits were inoculated intradermally at six sites with 107 virulent B. burgdorferi B31 as previously described (28, 29). At 8 days postinoculation, each of the rabbits developed typical EM lesions which, when biopsied and cultured in BSK II, were positive for B. burgdorferi. Cultures of skin biopsies from 2-month-postinfection rabbits were shown to be negative, and the rabbits were challenged 3 months later (28, 29) as described below.
Challenge of rabbits with B. burgdorferi.
Rabbits were challenged either by intradermal needle inoculation with IVCB or with HAB in the form of a skin biopsy taken from a rabbit EM lesion. IVCB strain B31 (passage 1) used for challenge was centrifuged at 6,800 × g for 15 min, washed with an equal volume of PBS (pH 7.4), and resuspended in 50% PBS-heat inactivated NRS. For challenge by needle inoculation (IVCB), rabbits were inoculated intradermally at six sites with 104 virulent B. burgdorferi B31 organisms per site so that each rabbit received a total of 6 × 104 IVCB. For challenge by skin implantation with HAB, six normal donor rabbits were inoculated with 107 virulent B. burgdorferi at six sites. At the time of EM development, 5-mm punches were taken at the site of the EM lesion and either cut into five 1-mm pieces for implant challenge, cultured in BSK II, or quick-frozen in dry ice-ethanol and stored at −70°C for quantitative PCR (QPCR) and reverse transcription-PCR (RT-PCR). Each rabbit to be challenged was anesthetized with ketamine and xylazine as described above, and six small horizontal subcutaneous incisions were made in the back. Five 1-mm pieces of EM skin biopsies containing 1.4 × 106 to 2.3 × 107 HAB (see below) were inserted into each incision so that each rabbit receiving implants at six sites was challenged with a total of 8.1 × 106 to 1.38 × 108 HAB. The area of the incision was then monitored daily for EM development. The rabbits were bled, and punch biopsies were taken near the site of challenge at 8 and 21 days following challenge and cultured in BSK II containing rifampin and phosphomycin as previously described (28). Rabbits were sacrificed 3 weeks following challenge, and the skin, right and left popliteal lymph nodes, right and left stifle joint tissue, spleen, and spinal cord were cultured in BSK II with antibiotics as previously described (28). All cultures were examined once a week for a total of 5 weeks for growth of B. burgdorferi.
RT-PCR analysis of OspA in HAB and IVCB.
To determine if ospA gene transcription was down-regulated in vivo, RT-PCR analysis was performed on 8-day EM skin biopsies. Total RNA was extracted directly from skin biopsies with a power tissue homogenizer (IKA Works, Inc., Wilmington, N.C.) in the presence of 1.2 ml of TRIzol reagent (GIBCO BRL, Gaithersburg, Md.). Total RNA was purified according to the manufacturer's protocol and resuspended in 20 μl of diethyl pyrocarbonate-treated water. Total RNA was also extracted from 104 in vitro-cultivated low-passage virulent strain B31, using 1.2 ml of TRIzol reagent, and the RNA pellets were resuspended in 12 μl of diethyl pyrocarbonate-treated water. As a negative control, total RNA was extracted from normal skin biopsies. Prior to performance of RT-PCR, contaminating genomic DNA was removed from RNA samples by digestion with 10 U of DNase I/2 μg of RNA. Approximately 2 μg of total RNA was reverse transcribed with random hexamers by using the Superscript preamplification system for first-strand cDNA synthesis as instructed by the manufacturer (GIBCO BRL). In these studies, flagellin subunit B (flaB) was used as a control for B. burgdorferi, and rabbit actin was used as a control for rabbit tissue extraction. One-tenth of the first-strand reaction was amplified by PCR using the following gene-specific primer pairs: OspA forward (5′-GTTAGCAGCCTTGACGAGAA-3′) and reverse (5′-CTGCTGACCCCTCTAATTTG-3′) (706-bp expected product); flaB forward (5′-CTGGCAAGATTAATGCTCAA-3′) and reverse (5′-CAGGAGAATTAACTCCACCT-3′) (567-bp expected product); and rabbit actin forward (5′-CTGAAGAACATCCAACCCTG-3′) and reverse (5′-CTGAGAGCACATTGTTAGCA-3′) (609-bp expected product). Ten percent of the amplified product was analyzed on a 1% agarose gel.
Quantitative competitive PCR.
To determine the number of copies of B. burgdorferi present in the EM skin biopsy used for challenge, QPCR was performed. Total DNA (target DNA) was extracted from each 5-mm EM skin biopsy (∼60 mg) or from 108 in vitro-cultivated virulent strain B31, using an Easy-DNA kit (Invitrogen, Carlsbad, Calif.). The DNA was precipitated, the pellet was resuspended in 98 μl of 10 mM Tris (pH 8.0)–1 mM EDTA, RNase was added to a final concentration of 40 μg/ml, and the mixture was incubated for 30 min at 37°C. Quantitation of B. burgdorferi DNA levels in a 60-mg skin biopsy was achieved by competitive PCR using DNA gyrase subunit B (gyrB) as the target DNA and a nonhomologous internal standard (PCR MIMIC) as the competitor (48). The MIMIC fragment has the same primer template sequence as the target DNA (gyrB) but contains a completely different intervening sequence (φX174 phage DNA). Two composite MIMIC primers designated 1F composite (5′ ATGAATTATGTTGCTAGTAACATTGAAGGTACGTTGCAGGCTGGCACT-3′) and 840R composite (5′ AACATGAGTTCCCCCTTCTCTTGTCAAGCATTGGGGATTGAGAAAGAG-3′) were generated, with the first 24 bases of each primer to gyrB and the last 24 bases to φX174. PCR amplification of the MIMIC fragment was performed with AmpliTaq Gold (Perkin-Elmer, Foster City, Calif.) in a standard reaction mixture with the following temperature cycling parameters: 95°C for 10 min, (1 cycle only), 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s (35 cycles), and a final extension at 72°C for 10 min. The resulting MIMIC fragment (970 bp) was cloned using a TOPO TA cloning kit (Invitrogen), and the MIMIC plasmid DNA was isolated with Qiaprep spin (Qiagen Inc.). To determine the amount (attograms) of double-stranded DNA present in one copy of MIMIC DNA, the following equation was used:
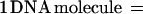 |
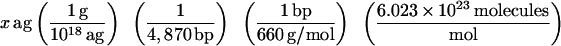 |
Therefore, there are 5.3 ag of DNA in one PCR MIMIC molecule. Tenfold serial dilution stock solutions of MIMIC were prepared ranging from 100 copies of MIMIC (5.3 ag of DNA) to 107 copies of MIMIC (53 pg of DNA).
For all subsequent QPCRs, 1 μl of 1:5 dilution of target DNA extracted from the skin biopsies was used. An initial competitive PCR titration curve was performed by coamplification of 1 μl of target DNA along with 1 μl of 10-fold serial dilutions of the PCR MIMIC (ranging from 100 to 107 copies) in a 51-μl reaction. PCR amplifications were carried out as described above, using the primers gyrB 1F (5′ ATGAATTATGTTGCTAGTAACATT-3′) and gyrB 840R (5′-AACATGAGTTCCCCCTTCTCTTGT-3′). Ten percent of the amplified products were visualized on a 0.9% agarose gel. The approximate equivalency point between the gyrB (840 bp) and MIMIC (970 bp) PCR products was determined, and a more accurate and quantitative PCR was performed using 1 μl of twofold serial dilutions (1:2 to 1:64) of the equivalency point dilution of the MIMIC along with 1 μl of target DNA. The PCR products were separated on a 0.9% agarose gel, and the resulting picture was analyzed with Scan Analysis software (BioSoft, Ferguson, Mo.). The resulting optical densities of the MIMIC and target DNA were plotted against femtograms of DNA, using Microsoft Excel. The point of equivalency was determined as the point where the MIMIC and target lines crossed. To quantitate the total number of DNA copies in the sample, the amount of DNA measured from the graph was used in the above equation, and this number was multiplied by a dilution factor of 500. Normal tissue DNA was run as a control for carryover. QPCR was also performed on 108 IVCB according to the manufacturer's protocol.
SDS-PAGE and Western blot analysis.
Two-dimensional (2-D) gel electrophoresis of OMV proteins was performed as follows. OMV samples isolated from 109 or 5 × 109 virulent and avirulent B31 and B313 organisms were solubilized in 50 μl of isoelectric focusing sample buffer, separated in the first dimension by nonequilibrium pH gel electrophoresis (NEPHGE) (42), then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in the second dimension (37), and transferred to a polyvinylidene difluoride membrane (58) as previously described (52). Blots were either stained with colloidal gold (AuroDye Forte; Amersham Corp., Arlington Heights, Ill.) or subjected to Western blot analysis as previously described (52). All rabbit sera were diluted 1:1,000, mouse anti-OspC sera were diluted 1:1,000 (antibody and rOspC was kindly provided by Steven Callister, Gundersen Lutheran Medical Center, La Crosse, Wis.) (45), anti-DbpA was diluted 1:3,000, and anti-DbpB was diluted 1:2,000 (both lipidated recombinant His6 fusion proteins and antibodies were kindly provided by Magnus Hook, Texas A&M University, Houston) (32).
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was performed as previously described (29) to determine anti-OspA, anti-OspC, and anti-DbpA titers of basal, prechallenge, and postchallenge sera against lipidated rOspA, recombinant pinpoint fusion-OspC, and lipidated recombinant His6-DbpA (100 ng of antigen/well), respectively.
Borreliacidal assay.
Borreliacidal assays were performed in duplicates in a final volume of 100 μl in 96-well microtiter plates (22). Sera were heat inactivated at 56°C for 30 min. Test sera were serially diluted 1:2 to 1:640 in NRS to a final volume of 10 μl. Each reaction suspension contained 25 μl of guinea pig complement (Sigma Chemical Co., St. Louis, Mo.), 20 μl of virulent low-passage B31 or B313 (5 × 107/ml) in 50% BSK II-PBS, and 45 μl of PBS, which resulted in a final concentration of 106 B. burgdorferi, 10% test serum or NRS, 25% guinea pig serum, 55% PBS, and 10% BSK II. Because B313 has been observed to aggregate in culture and since infection-immune rabbit serum required a longer period of time to kill strain B31, the reaction mixtures were incubated at 34°C for 6 h for assays using B. burgdorferi B313 and 20 h for assays using virulent B. burgdorferi B31. After incubation, approximately 2 μl of material was transferred aseptically to 175 μl of BSK II with a 96-prong clone maker, grown at 34°C for 2 weeks, and examined by dark-field microscopy for the presence of viable organisms. Each assay was also tested for residual complement activity by the addition of 50 μl of hemolysin-sensitized sheep red blood cells, followed by incubation at 37°C for 30 min.
Statistical analysis.
Mean log ELISA titers and symptom scores were compared by the t test. Statistical significance was determined by P < 0.05. Confidence intervals were determined for ELISA and borreliacidal titers.
RESULTS
OspA is down-regulated during rabbit infection.
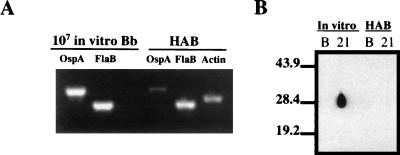
Montgomery et al. have demonstrated by RT-PCR analysis that ospA gene transcription is down-regulated in the infected mouse (40). Barthold et al. have further shown that the infected mouse ear is a source of HAB which does not elicit an antibody response to OspA (7). We used RT-PCR to determine if ospA gene transcription is also down-regulated in the skin of infected rabbits. In these studies, flaB was used as a control to represent constitutive transcription of a B. burgdorferi gene, and a rabbit actin gene was used as an internal control for extracted tissue. As shown in Fig. 1A, the levels of flaB transcription detected in HAB and 107 IVCB were similar. In contrast, the same samples showed that while ospA transcription from IVCB was readily detectable, it was almost undetectable in HAB. These results were also consistent with Western blot studies using sera from two rabbits infected with either ∼108 IVCB or ∼108 HAB (Fig. 1B). Western blots of rOspA probed with sera taken 21 days postinfection demonstrated a major antibody response to OspA in the rabbit infected with IVCB but almost undetectable OspA antibody in the rabbit infected with HAB (Fig. 1B). The lack of OspA immune stimulation in HAB-infected animals is again consistent with OspA down-regulation during the first 7 days of rabbit infection.
FIG. 1.
Down-regulation of OspA in HAB. (A) RT-PCR of ospA and flaB from 107 IVCB (Bb) versus HAB. Rabbit actin was used as a control. (B) Western immunoblot of rOspA using basal sera (lane B) or serum 21 days postinfection (lane 21) from rabbits infected either by needle inoculation of ∼108 IVCB (In vitro) or by implantation of ∼108 HAB from EM lesion skin biopsies. Markers to the left are in kilodaltons.
Quantitation of HAB in infected rabbit skin.
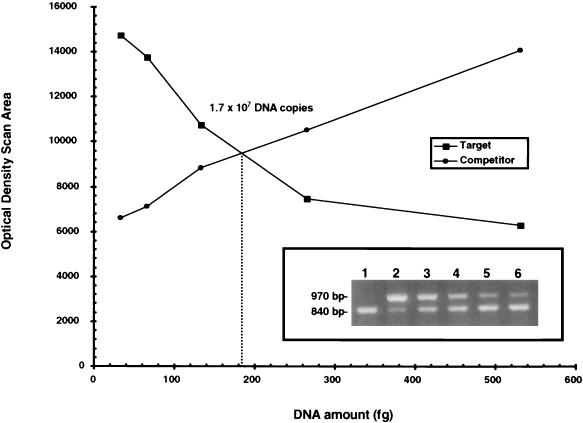
To determine the number of organisms used for implant challenge, we used QPCR to measure the number of copies of gyrB present in the donor EM biopsy. In other studies, we determined that the number of organisms in the infected rabbit skin plateaued at 7 to 14 days after infection (C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett, unpublished data). Therefore, in this study, skin punches were obtained 8 days after infection for QPCR analysis. It was determined from the initial PCR with 10-fold serial dilutions of MIMIC (107 to 100) that molar equivalence of target DNA and MIMIC occurred with 105 copies of MIMIC (data not shown). Further amplification of the target DNA with twofold serial dilutions (1:2 to 1:16) of the 105 MIMIC stock showed that as the amount of MIMIC (970 bp) decreased, a point of equivalence at which there were approximately equal amounts of target DNA (840 bp) and MIMIC DNA was reached (Fig. 2 inset, lane 4; 1:4 dilution). The resulting titration curves of MIMIC and sample DNA are shown in Fig. 2. The amount of B. burgdorferi DNA measured in the target DNA at equivalency (point of crossover) was determined to be approximately 185 fg. Therefore, there were 1.70 × 107 copies of gyrB in a 5-mm punch biopsy. We calculated that IVCB contained approximately 17 times more copies of gyrB than enumerated by dark-field microscopy and spectrophotometric analysis (data not shown), similar to previous observations that Borrelia hermsii isolated from mice contains 13 to 18 copies of the chromosome (36). However, the number of copies of the chromosome in HAB is not known. QPCR was performed on a total of seven EM skin biopsies used as the source of implant challenge; based on a potential 1 to 17 copies of gyrB per organism, there was a range of 1.4 × 106 to 2.3 × 107 HAB per skin biopsy. Therefore, in these studies where each rabbit was challenged with six skin implants, each rabbit received a total of 8.1 × 106 to 1.4 × 108 HAB.
FIG. 2.
Representative QPCR of the number of B. burgdorferi present in skin implant challenge. The inset shows results of QPCR of twofold serial dilutions of the 105 MIMIC stock. Lane 1, target sample without MIMIC; lane 2, undiluted 105 MIMIC stock; lane 3, 1:2 dilution of 105 MIMIC; lane 4, 1:4 dilution; lane 5, 1:8 dilution; lane 6, 1:16 dilution. The graph shows optical density scan areas of the MIMIC (competitor) and target DNA. The point of crossover is 185 fg of DNA, which equates to ∼1.70 × 107 organisms, assuming one copy of the chromosome per organism.
Challenge with IVCB.
The results of challenge of naive, infection-immune, and OMV- and OspA-immunized rabbits with intradermal injection of 6 × 104 IVCB are presented in Table 1. Each of the naive control rabbits developed EM, was skin biopsy positive at 8 days and 3 weeks after infection, and had positive visceral cultures at 3 weeks. In contrast, the infection-immune rabbits were completely protected against IVCB infection as previously reported (28, 29).
TABLE 1.
EM and culture results of challenge with IVCB and HAB
| Immunization | Challengea | EM lesionsb (no. positive/ no. tested) | No. of rabbits with positive cultures/ no. in group
|
Symptom scored | ||
|---|---|---|---|---|---|---|
| Skin
|
Viscerac 3 wk | |||||
| 8 day | 3 wk | |||||
| rOspA | IVCB | 5/5 | 1/5 | 4/5 | 2/5 | 12/20 |
| Avirulent B31 OMV | IVCB | 1/5 | 3/5 | 2/5 | 1/5 | 7/20 |
| Virulent B31 OMV | IVCB | 0/5 | 1/5 | 0/5 | 0/5 | 1/20 |
| B313 OMV | IVCB | 0/5 | 2/5 | 0/5 | 0/5 | 2/20 |
| Infectione | IVCB | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| Naive | IVCB | 5/5 | 5/5 | 5/5 | 5/5 | 20/20 |
| rOspA | HAB | 5/5 | 5/5 | 5/5 | 5/5 | 20/20 |
| Avirulent B31 OMV | HAB | 3/5 | 5/5 | 5/5 | 5/5 | 18/20 |
| Virulent B31 OMV | HAB | 5/5 | 5/5 | 5/5 | 5/5 | 20/20 |
| B313 OMV | HAB | 5/5 | 5/5 | 5/5 | 5/5 | 20/20 |
| Infectione | HAB | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| Naive | HAB | 5/5 | 5/5 | 5/5 | 5/5 | 20/20 |
Rabbits were challenged by intradermal inoculation with 6 × 104 IVCB or by implantation of EM skin biopsies containing approximately 8.1 × 106 to 1.4 × 108 HAB.
EM lesions appeared 6 to 13 days postchallenge; rabbits were scored positive if any of the six sites developed EM lesions.
Viscera were scored positive if any of the joints, popliteal lymph nodes, spinal cord, or spleen tissues were culture positive.
Total number of symptoms of EM, 8-day skin, and 3-week skin and visceral cultures/total possible score.
Infection-derived immunity.
Each of the rabbits immunized with OspA developed EM after challenge with IVCB. By 3 weeks postchallenge, four of the five rabbits had positive skin cultures, and two of five had disseminated infection; these findings were statistically insignificant compared to those for the naive rabbits (P = 0.34). We also considered the outcomes of intradermal challenge in individual rabbits as a four-point symptom score based on whether EM developed, whether the skin biopsies were positive at day 8 and at 3 weeks, and whether visceral infection was present at 3 weeks. Viewed in this manner, disease manifestations were milder in the rOspA-immunized rabbits than in the naive controls (P = 0.001). These results are in accord with our previous findings that OspA immunization confers only modest protection against IVCB in the rabbit model (29).
Immunization with OMV derived from avirulent B. burgdorferi strain B31 also conferred modest protection against IVCB. This group exhibited less EM (one of five rabbits) than the OspA-immunized and naive control groups. In addition, only two of five rabbits had skin infection, and one of five rabbits had disseminated infection 3 weeks postchallenge. However, the symptom score was not significantly different from that of the OspA-immunized group (P = 0.12).
By comparison, rabbits immunized with OMV from virulent strain B31 exhibited highly significant protection against IVCB, as EM did not develop and only one of the five rabbits had a positive culture at 8 days but no skin or disseminated infection at 3 weeks postchallenge. These findings indicate that although in the one animal skin infection was established 8 days postchallenge, there was an acceleration in the clearance of organisms which ultimately resulted in the resolution of infection. The symptom score of this group was not significantly different from that of the infection-immune group (P = 0.33).
Rabbits immunized with OMV from the avirulent B31 derivative B313 (46), which lacks DbpA, OspA, OspB, and OspD, also exhibited highly significant protection. While none of these rabbits developed EM, two of five had culture-positive skin biopsies at 8 days postchallenge, which was statistically significant from the result for the naive animals (P = 0.04). However, by 3 weeks postinfection, none of the rabbits had skin or visceral infection. The symptom score of the B313 OMV-immunized group also was not significantly different from that of the infection-immune group (P = 0.15).
Challenge with HAB.
Table 1 details the outcomes of HAB implant challenge of naive, infection-immune, and OMV- and OspA-immunized rabbits. Naive rabbits were fully susceptible to development of EM lesions and had positive skin cultures at 8 days after challenge. At the time of sacrifice, 3 weeks after challenge, each of the naive rabbits had positive skin biopsy cultures and culture-positive viscerally disseminated infection. The groups of rabbits immunized with OspA and with each of the three OMV preparations were also fully susceptible to HAB implant challenge. In contrast, the three rabbits with infection-derived immunity exhibited complete protection; none developed EM, and B. burgdorferi was not cultured from skin or viscera. This finding has been further confirmed in a recent study where five out of five B31 strain infection-immune rabbits were completely immune to HAB challenge (Shang et al., unpublished data).
Immunogenicity of individual OMV proteins.
To assess the specific antibody response against the OMV proteins used for immunization, 2-D NEPHGE immunoblots were prepared with OMV from 109 virulent B31, avirulent B31, and B313 organisms and then probed with corresponding prechallenge antiserum. The protein constituents of the OMV preparations are shown in Fig. 3A. We have previously described the OMV proteins uniquely found in virulent strain B31 OMV but absent in avirulent B31 OMV (52). The compositional analysis presented here includes all OMV proteins, whereas previously, only hydrophobic Triton X-114-phase protein extracts were reported (52). It is apparent from Fig. 3A and B that B313 OMV lack numerous proteins found in virulent B31 and in the avirulent B31 strain from which B313 was derived, including OspA and OspB (46). In addition, Fig. 3C confirms that B313 does not express DbpA, consistent with the lack of linear plasmid 54, which encodes DbpA (46). In contrast to the original description of B313 (46), this strain now expresses OspC (Fig. 3C). Western immunoblotting with the appropriate prechallenge sera demonstrated that immunizations with the three OMV preparations resulted in the generation of antibodies to most of their constituent proteins (Fig. 3B).
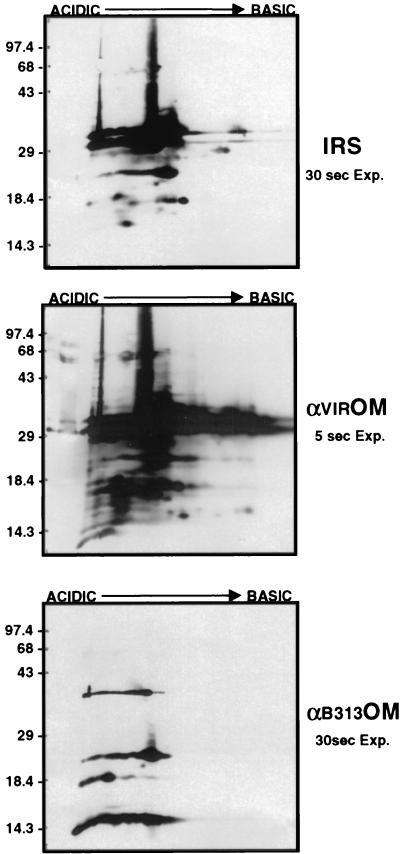
Comparison of OMV antigens recognized by IRS and prechallenge OMV antisera.
Identical immunoblots of 2-D gels containing OMV derived from 5 × 109 virulent B31 were probed with IRS, antivirulent B31 OMV, and anti-B313 OMV prechallenge sera in order to determine if there were qualitative or quantitative differences in the antigens detected that distinguished infection-derived immunity from the less protective responses elicited by immunization with B313 OMV and virulent OMV (Fig. 4). Comparison of the probed virulent OMV blots revealed that immunization with the virulent B31 OMV elicited a strong antibody response to each of the virulent B31 OMV proteins. The amount of antibody binding, as judged by the intensity of the anti-virulent OMV blot (5-s exposure), was quantitatively and qualitatively greater than that of IRS at the same dilution (30-s exposure). In contrast, immunization with B313 OMV elicited antibodies to only a small subset of virulent OMV proteins, including OspC (24 kDa). Interestingly, the protection afforded against IVCB by B313 OMV immunization was comparable to that of B31 virulent OMV immunization (Table 1). Although immunization with virulent B31 OMV resulted in higher-titered antibodies to more virulent B31 OMV proteins than found in IRS, only infection-immune rabbits were completely protected against infection with HAB, supporting the hypothesis that antigens other than those present in virulent B31 OMV were responsible for the far greater degree of protective immunity observed.
FIG. 4.
Comparison of OMV antibodies and IRS to virulent OMV proteins by Western immunoblot analysis of 2-D NEPHGE of 5 × 109 virulent B31 OMV probed with IRS, anti-virulent OMV prechallenge serum (αVIROM), or anti-B313 OMV prechallenge serum (αB313OM). IRS and αB313OM immunoblots were exposed to film for 30 s, while the αVIROM immunoblot was exposed for only 5 s. Markers to the left are in kilodaltons; acidic and basic ends of gels are indicated at the top.
Borreliacidal activity of prechallenge sera.
To determine if a correlation could be established between serum borreliacidal titers and degree of immunity, basal and prechallenge sera from each rabbit were tested for borreliacidal activity against both the IVCB virulent B31 and B313 strains. The geometric means and upper and lower confidence intervals of the borreliacidal titers of five rabbits in each group are presented in Table 2. Although the endpoint titers were not reached in some animals, the prechallenge sera from OspA, avirulent and virulent B31 OMV-immunized, and infection-immune rabbits all had relatively high killing titers (≥1:388 to 1:617) against IVCB strain B31 compared to basal sera titers (1:10). Against strain B313, anti-OspA sera showed no significant borreliacidal activity (≤1:10), consistent with the lack of OspA expression in this strain. Avirulent OMV antisera exhibited relatively low titers (≥1:40) against B313, while virulent OMV antisera and IRS showed higher titers (≥1:58 and ≥1:144, respectively). Although B313 OMV immunization generated antibodies with significant in vitro killing titers against B313 (≥1:273), the sera did not kill B31 in vitro (≤1:10). These results are similar to previous growth inhibition studies using antisera against whole cell lysates of B31 and B313 (46). The results of these borreliacidal assays indicate that high in vitro killing titers do not correlate with protective immunity against B. burgdorferi infection.
TABLE 2.
Borreliacidal titers of prechallenge sera
| Immunization | Geometric mean reciprocal borreliacidal titera (confidence interval)
|
|||
|---|---|---|---|---|
| Virulent B31
|
B313
|
|||
| Basal | Prechallenge | Basal | Prechallenge | |
| rOspA | ≤10 (≤10–10) | ≥538 (≥437–662) | ≤10 (≤10–10) | ≤10 (≤10–10) |
| Avirulent B31 OMV | ≤10 (≤10–10) | ≥617 (≥575–663) | ≤10 (≤10–10) | ≥40 (≥20–80) |
| Virulent B31 OMV | ≤10 (≤10–10) | ≥483 (≥353–661) | ≤10 (≤10–10) | ≥58 (≥23–145) |
| B313 OMV | ≤10 (≤10–10) | ≤10 (≤10–10) | ≤10 (≤10–10) | ≥273 (≥148–504) |
| Infectionb | ≤10 (≤10–10) | ≥388 (≥279–538) | ≤10 (≤10–10) | ≥144 (≥67–310) |
Determined on in vitro-cultivated virulent B31 or strain B313. Outgrowth of Borrelia was determined after 2 weeks.
Infection-derived immunity.
Relationship of OspA, OspC, and DbpA antibody titers to the outcomes of challenge.
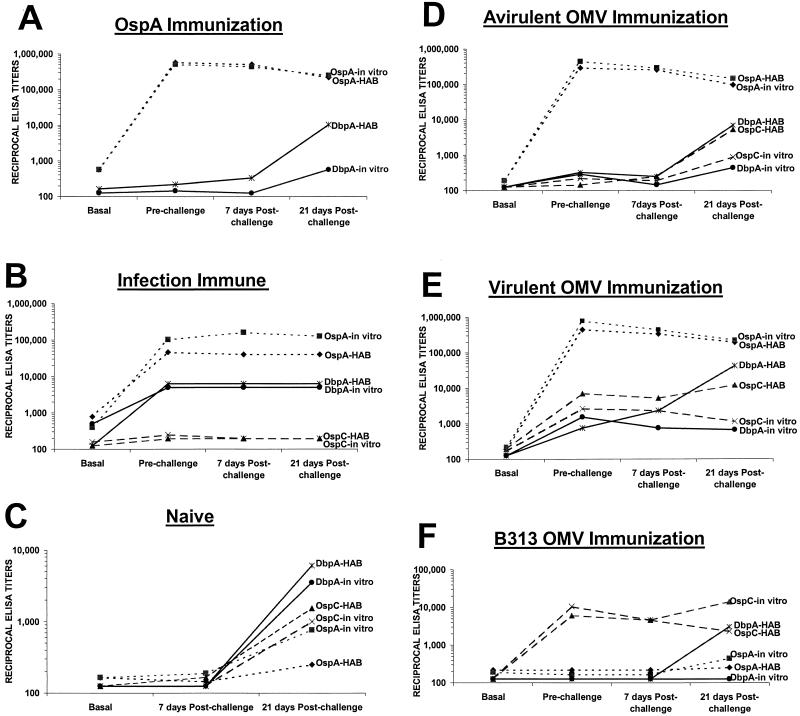
OspC (30, 31, 39, 44), DbpA (13, 35), and OspA (15, 24, 25, 50, 56) have been reported to be protective immunogens. To address their contribution to the protection conferred by immunization with OMV and by infection-derived immunity, the titers of OspA, OspC, and DbpA antibodies were determined by ELISA for each rabbit and compared to the level of protection elicited in each immunization group. Figure 5A, D, and E shows that there were high OspA titers in the average prechallenge sera of rabbits immunized with OspA (1:588,134), avirulent B31 OMV (1:294,067), and virulent B31 OMV (1:445,722), yet none of these rabbits were protected against implant challenge using HAB (Table 1). In each of these groups, OspA titers declined over the 3-week period irrespective of whether infection resulted (Fig. 5). The completely susceptible naive control group developed only a very weak response to OspA (≤1:504) 3 weeks postchallenge (Fig. 5C), providing further evidence that OspA is down-regulated during early infection. The average prechallenge titers of antibody to OspA were also relatively low in the completely protected infection-immune rabbits (1:73,873) (Fig. 5B) compared to those found in rabbits immunized with OspA or OspA containing OMV (1:610,885). Although the B313 OMV-immunized animals lacked OspA antibodies (Fig. 3A), those challenged with cultivated organisms were protected against disseminated infection. Taken together, these findings strongly suggest that although OspA can confer partial protection against needle challenge using IVCB, as previously demonstrated in the rabbit (29), OspA antibodies are not the basis of the superior protection observed in both infection-immune rabbits and in rabbits immunized with virulent B31 and B313 OMV.
FIG. 5.
Reciprocal ELISA titers of rabbits that were immunized with OspA (A), infection immune (B), naive (C), immunized with avirulent OMV (D), immunized with virulent OMV (E), and immunized with B313 OMV and challenged with either IVCB (in vitro) or HAB.
Rabbits with no protection against HAB challenge (groups immunized with OspA, avirulent and virulent B31 OMV, and B313 OMV, and naive rabbits) had an increase in OspC titers in the 21 days postchallenge period (Fig. 5C and D to F), consistent with expression of OspC during multiplication of the spirochetes. Rabbits with significant partial protection against IVCB challenge, the groups immunized with virulent B31 OMV and B313 OMV, had OspC titers which declined during the 3-week postchallenge period, suggesting limited multiplication of spirochetes. Infection-immune rabbits with complete protection against HAB challenge had no increase or decrease in OspC titers (Fig. 5B), consistent with absence of spirochetal multiplication. It is noteworthy that these infection-immune rabbits had low prechallenge (≤1:224) and 3-week postchallenge (≤1:198) OspC titers indistinguishable from the titers found in basal sera (≤1:141). Further, rabbits with the highest prechallenge OspC titers, those immunized with virulent B31 (≤1:6,964) and B313 OMV (1:6,063), were not protected against HAB challenge. These findings suggest that OspC does not play a major role in protection against HAB. Nonetheless, these rabbits did have significant partial protection against IVCB challenge, and a contribution of OspC antibodies to the protection observed cannot be ruled out at this time.
Rabbits with no protection against HAB challenge (groups immunized with OspA, avirulent and virulent B31 OMV, and B313 OMV, and naive rabbits) showed a marked increase in DbpA titers in the 21-day postchallenge period (Fig. 5A, C, and D to F). Contrasted with the serologic findings with OspA described above, which clearly relate to down-regulation of OspA synthesis in the rabbit, these findings with DbpA appear consistent with its up-regulation during rabbit infection, as is the case for infection of mice (13). Rabbits with significant partial protection against IVCB challenge included the group immunized with virulent B31 OMV. In this case, DbpA titers fell during the 3-week postchallenge period, again suggesting limited multiplication of the spirochete or possibly limited DbpA synthesis. Findings with the groups of rabbits immunized with B313 OMV are of particular interest. These rabbits had no protection against the HAB challenge, and DbpA titers rose comparably (1:3,031) (Fig. 5F) to the titers in the naive rabbits postchallenge (1:6,063). However, in the B313 group which showed significant protection against IVCB, there was no increase from basal DbpA antibody titers (≤1:125); this observation is consistent with very limited if any multiplication of the spirochete. Clearly, the significant protection conferred by B313 OMV against IVCB challenge was not due to DbpA antibodies, since B313 lacks DbpA. While infection-immune rabbits had the highest average prechallenge titers to DbpA (1:5,695), it should be noted that the groups of rabbits immunized with virulent B31 OMV had DbpA mean titers which were severalfold lower (≤1:1,137) but whose upper confidence intervals (1:2,337 to 1:3,073) almost approximated the lower confidence interval of the infection-immune groups (3,175 to 4,000). However, the biological differences between the infection-immune group and the virulent B31 OMV-immunized groups were profound, with infection-immune rabbits being completely resistant to infection with 107 to 108 HAB, while virulent B31 OMV rabbits were completely susceptible.
DISCUSSION
There has been growing recognition that there are critical differences between IVCB and mammalian HAB that are relevant to understanding pathogenesis and immunity in Lyme disease. By using mouse ear tissue as a source of HAB, Barthold and colleagues showed that OspA vaccination prevented infection with IVCB but not HAB (7). These pioneering experiments established the need to determine whether candidate vaccinogens conferred protection against HAB. Subsequent work by Barthold et al. indicated that the differences between IVCB and HAB extended beyond the down-regulation of OspA. Mice passively immunized with serum from chronically infected mice (which lack OspA antibodies) were protected against IVCB but were fully susceptible to infection with HAB. Further, immunity against HAB appears to be strain specific (4). The molecular basis for this distinction between immunity against IVCB and HAB is unknown.
Considerable effort has therefore been directed toward the identification of B. burgdorferi proteins which are up-regulated during mammalian infection, a variety of which have been reported (1, 2, 13, 14, 17, 26, 47, 54, 55, 59, 63–65). Cassatt and coworkers recently used spirochetes recovered from infected mouse plasma to demonstrate that DbpA is on the surface of HAB, which to date is the only protein defined in this manner (13). Infection of the mouse (8), like human infection (21), is characterized by the presence of HAB in numbers insufficient for isolation of B. burgdorferi from tissue for biochemical analysis. When viewed in this context, the finding presented in this report, that remarkably large numbers of HAB are present in biopsies of rabbit EM, could have significant implications for future identification and analysis of HAB molecules.
As we have previously described, the appearance of EM is a consistent feature of intradermal injection of IVCB in the rabbit (28). In this study, EM also resulted after intradermal implantation of HAB-containing rabbit skin. Assuming that there is one copy of gyrB per organism, our studies have shown that there are approximately 2.3 × 107 organisms per 5-mm punch biopsy used for implantation. Although we determined that there are approximately 17 copies of gyrB present in IVCB, without the precise enumeration of HAB in rabbit tissue, the exact number of copies of the chromosome in HAB cannot be determined. However, based on a potential 1 to 17 copies of gyrB per IVCB, there could be between 1.4 × 106 to 2.3 × 107 HAB/5-mm punch biopsy. Therefore, in these studies where rabbits were challenged with six skin implants, the minimum HAB challenge dose would be 8.1 × 106 to 1.38 × 108 organisms. The large numbers of HAB found in EM (approximately 108 organisms/cm2) have been highly reproducible. We routinely produced EM at six to eight sites on the back by injection of 103 to 106 B. burgdorferi at each site (28, 29). A large portion of the back becomes erythematous, and over 1010 HAB are present in the skin of the rabbit back at the time that EM appears (Champion et al., unpublished). In addition, abundant spirochetes have been readily demonstrable in sections of EM by immunostaining (Chong and Miller, unpublished). These numbers of B. burgdorferi in the rabbit model far surpass those found in ticks (5 × 104) (12) and mice. Barthold and coworkers reported that 2 to 8 weeks following injection with 104 IVCB into the skin, 1.5-mm punch biopsies of the mouse ear typically contained less than 640 host-adapted spirochetes, which is approximately 2 logs less than the number injected (7). The greatest numbers of B. burgdorferi previously reported have related to foci of infection in the ear (7), the ankle joint (4.3 × 105) (62), and the heart (2.2 × 106) (62). C3H/HeN mice treated with interleukin-12 monoclonal antibodies had over 105 spirochetes detected per ear by semiquantitative PCR at 2 weeks after infection, and about 105 spirochetes detected per ear in untreated mice (3); at 60 days after infection, numbers of spirochetes were approximately 1 order of magnitude less. Ma et al. (38) and Yang et al. (62), using semiquantitative PCR, reported that the average number of spirochetes in the ankle joints of C3H/HeJ mice 4 weeks postinfection ranged from 4.3 × 105 (62) to 6.4 × 105 (38). In contrast, injection of rabbits with 107 IVCB resulted in 5-mm skin biopsies containing 106 to 107 HAB, which is comparable in numbers to what was injected. Thus, the rabbit represents the only animal to date where very large numbers of HAB occur and therefore is a rich source for these organisms. It is conceivable that the large numbers of organisms in rabbit skin may be related to EM production, which is a consistent feature of the rabbit model. Whether large numbers of organisms similarly occur in human EM has not been determined but certainly can be addressed by the methods described in this study.
The numbers of HAB found in rabbit skin are of interest in relation to the basic features of the rabbit model of Lyme disease. Infection of the mouse is chronic (6) and is cleared only by antimicrobial treatment (41). As considered above, the numbers of HAB are relatively small, and HAB are concentrated in the ear, ankle joint, and heart (8, 62). Antiserum from chronically infected mice does not confer protection against HAB (4). Chronically infected mice treated with ceftriaxone were partially protected against HAB ear implant challenge with spirochetes of the homologous, but not heterologous, strains (4). In contrast, the skin of rabbits is highly permissive for multiplication of HAB, and within a few months of infection, rabbits have cleared the infection and exhibit complete protection against reinfection with 8 × 107 IVCB of the same strain (28), the highest numbers tested. The data presented in this study represent the first report of complete protection against HAB implant challenge. While infection-immune rabbits are completely protected against infection with a challenge of 8.1 × 106 to 1.4 × 108 HAB, DbpA-immunized mice were only partially protected against a challenge of six to eight HAB derived from plasma of spirochetemic mice (13). Relative to infection in mice, the rabbit is remarkably permissive for HAB multiplication yet able to clear the infection, and it becomes remarkably resistant to reinfection. Although OspA is down-regulated in both rabbit and mouse tissues, which designates the organisms as HAB, it remains to be determined whether the protein composition of HAB found in rabbit skin differs from that found in the infected mouse ear.
Infection-derived immunity in the rabbit model is based on humoral antibody (Chong and Miller, unpublished), as is passive protection against IVCB in the mouse (5). While the protection induced by immunization with virulent strain B31 OMV and B313 OMV in this study was significant, and will be considered below, the protection provided by infection-derived immunity was quantitatively far superior. Rabbits with infection-derived immunity were fully protected against challenge with at least 8.1 × 106 to 1.4 × 108 HAB; rabbits immunized with virulent strain B31 and B313 OMV had highly significant but still incomplete protection against 6 × 104 IVCB. To address the basis of the markedly different degrees of protection observed in these immune/immunized groups, we assessed their antibody levels to OMV proteins by immunoblotting and to OspA, OspC, and DbpA by ELISA.
Both the rabbits with infection-derived immunity and the rabbits immunized with virulent B31 OMV had antibodies to each of the OMV proteins found in B31 OMV. However, it is strikingly evident in the immunoblots shown in Fig. 4 that the titers of antibodies to individual OMV proteins were far lower in the case of the infection-immune rabbits. It is possible that key protective epitopes were denatured during the course of SDS-PAGE and immunoblot preparation. It is also possible that the composition of HAB OMV differs markedly from that of virulent B31 cultivated in vitro. Based on the assumption that humoral antibody is responsible for protection, the borreliacidal assay findings support the latter possibility. When virulent strain B31 was used as the killing target, high borreliacidal titers were achieved even in the case of immunization with OMV derived from avirulent strain B31. Nonetheless, very little protection was conferred by immunization with avirulent B31 OMV. The simplest explanation for these discordant findings is that the use of IVCB as a killing target is not reflective of the surface antigenic structure of HAB. The evidence presented in this study showed that OspA is down-regulated during rabbit infection, and the presence of OspA on IVCB used in the borreliacidal assays almost certainly was a factor in the generation of high borreliacidal titers by virulent and avirulent B31 OMV. The findings in this study corroborate our previous report that while high antibody titers follow immunization with OspA in the rabbit, the protection conferred against IVCB is modest (29). This study also confirmed that, as in the mouse, OspA antibodies confer no protection against infection with HAB.
While the concept that antigens unique to HAB are optimal targets of protective antibody is appealing and is supported by both Barthold and colleagues' studies (4, 7) and the findings of this report, the molecules currently known to be up-regulated or uniquely expressed during mouse infection have not shown great potential as protective immunogens. Among the best studied of such molecules is OspC, which has limited surface exposure (16) and is at least partially subsurface (10). The temperature of the blood meal induces up-regulation of OspC synthesis in the tick (47, 54). OspC is known to be highly variable among strains of B. burgdorferi (43, 57, 60, 61). While some studies showed some degree of protection against certain strains (30, 31, 39, 44), it has been shown for strains N40 and 297 that even homologous protection is not conferred (10). However, Gilmore and Mbow have recently demonstrated that OspC from strain B31 has heat-sensitive conformational protective epitopes (31). Our findings in this study corroborate those obtained for mice. OspC ELISA titers in naive rabbits rise significantly in the 3 weeks after infection, in contrast to OspA titers, which fall during the 3 weeks following infection. However, OspC titers in the infection-immune rabbits were indistinguishable from the basal titers obtained prior to the initial infection. Further, while prechallenge OspC titers were highest in rabbits immunized with B313 and with virulent B31 OMV, these groups exhibited no protection against HAB. It therefore appears that OspC antibodies are not a significant factor in protection against HAB. Nonetheless, a role of OspC in the protection conferred by B313 OMV against IVCB challenge cannot be excluded by our studies.
The temperature of a blood meal also enhances expression of the surface lipoprotein, DbpA (13), an adhesin which mediates binding to the collagen-associated protein, decorin (33, 35). Elegant proof has recently been provided that B. burgdorferi organisms isolated from the blood of spirochetemic mice have DbpA, but not OspA, on their surface (13). Partial protection against infection with HAB has been reported by Cassatt et al. (13) using plasma from spirochetemic mice as the source of HAB. Because of the low numbers of HAB in plasma, mice immunized with DbpA were challenged with six to eight spirochetes. Six of 20 DbpA-immunized mice became infected with this minimal challenge; this study also noted that the DbpA-immunized mice were susceptible to a challenge with 104 IVCB, in contrast to previous findings (23, 34, 35). In the present study, the rise in DbpA titers evident 3 weeks after infection of naive rabbits was indicative of its expression during infection. The rabbits with infection-derived immunity had the highest prechallenge DbpA titers. Rabbits immunized with virulent B31 OMV had DbpA titers approximately four times lower than those of the infection-immune rabbits, and there was almost an overlap between the upper confidence level of the former and the lower confidence level of the latter. The challenge results seem discordant with the vast disparity in degree of protection between the infection-immune and virulent strain OMV-immunized groups. It should also be noted that OMV from strain B313, which lack DbpA, conferred significant partial protection against IVCB which was indistinguishable from that conferred by virulent strain B31 OMV. Taken together, our findings imply that DbpA is not a major protective immunogen in the rabbit model, but such a conclusion could only be rigorously drawn by immunization with DbpA and challenge with HAB and IVCB.
Protection against B. burgdorferi infection by immunization with OMV has not been previously reported. We have shown that four of five virulent B31 OMV-immunized and three of five B313 OMV-immunized rabbits were completely protected against IVCB challenge. In one of five virulent B31 OMV-immunized and two of five B313 OMV-immunized rabbits, the skin infection observed at 8 days postchallenge was rapidly cleared, and at 3 weeks postchallenge, no skin or disseminated infection was observed. The finding that B313 OMV conferred significant partial protection against challenge with 6 × 104 IVCB was unexpected. For reasons considered above, we cannot exclude the possibility that OspC antibodies were a primary basis of this protection. However, the fact that OspC antibodies were clearly not a factor in protection against HAB infection does not support this conclusion. Because B313 lacks OspA and DbpA, there is a strong possibility that the protection against IVCB is conferred by a previously unrecognized protective immunogen. The protein composition of B313 OMV is a small subset of that of virulent B31 and avirulent B31 OMV, as indicated in Fig. 3A. We are currently investigating the molecular basis of the protection conferred by B313. In this regard, it should be noted that B313 OMV antisera did not exhibit bactericidal activity against OspA-expressing B31 strain organisms. It has been reported that the presence of OspA prevents binding of antibodies directed against p66 (11), a porin also known as Oms66 (51). We have recently found that antibodies against native Oms66 have potent bactericidal activity against B313 but have no killing activity against B31 (22).
The protection conferred by virulent B31 OMV was indistinguishable from that conferred by B313 OMV. OspA immunization resulted in OspA titers that did not differ from those achieved by immunization with virulent B31 OMV, yet the OspA-immunized group had far less protection. Whether the similar protection observed in the virulent B31 OMV- and B313-immunized groups has the same molecular basis remains to be determined. In this regard, immunization with B31 OMV did generate borreliacidal antibodies against B313. It is certainly possible that IVCB have several previously undescribed protective immunogens, and the amount and relative surface accessibility of these proteins may vary considerably among virulent B31 and its avirulent derivatives. There is currently no evidence to link protein constituents of IVCB to the immunogens expressed by B. burgdorferi in vivo that induce protection against HAB infection, but the possibility that minor constituents of the outer membrane of IVCB become markedly up-regulated during infection should be considered, as it could also explain our observations regarding protective immunity.
The remarkably high numbers of HAB found in rabbit EM present an opportunity to identify the B. burgdorferi surface molecules up-regulated during rabbit infection and ultimately to determine their relationship to the basis of infection-derived immunity. Such studies are in progress in our laboratory.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) grant AI-37312 to J. N. Miller and NIH grant AI-29733 to M. A. Lovett.
We thank Maurice M. Exner and James Cherry for helpful suggestions.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 3.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold S W. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco D R, Champion C I, Lewinski M A, Shang E S, Simkins S G, Miller J N, Lovett M A. Immunization with Treponema pallidum outer membrane vesicles induces high-titer complement-dependent treponemicidal activity and aggregation of T. pallidum rare outer membrane proteins (TROMPs) J Immunol. 1999;163:2741–2746. [PubMed] [Google Scholar]
- 10.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunikis J, Barbour A G. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkot T R, Piesman J, Wirtz R A. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick life cycle, doubling times, and loss while feeding. J Infect Dis. 1994;170:883–889. doi: 10.1093/infdis/170.4.883. [DOI] [PubMed] [Google Scholar]
- 13.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y F, Appel M J, Jacobson R H, Shin S J, Harpending P, Straubinger R, Patrican L A, Mohammed H, Summers B A. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect Immun. 1995;63:3543–3549. doi: 10.1128/iai.63.9.3543-3549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Barthold S W, Giles S S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Silva A M, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Silva A M, Fikrig E, Hodzic E, Kantor F S, Telford III S R, Barthold S W. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 20.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duray P H. Clinical pathologic correlations of Lyme disease. Rev Infect Dis. 1989;11(Suppl. 6):S1487–S1493. doi: 10.1093/clinids/11.supplement_6.s1487. [DOI] [PubMed] [Google Scholar]
- 22.Exner M M, Wu X-Y, Blanco D R, Miller J N, Lovett M A. Protection elicited by native outer membrane protein Oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bactericidal epitopes. Infect Immun. 2000;68:2647–2654. doi: 10.1128/iai.68.5.2647-2654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992;60:773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 26.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 27.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley D M, Gayek R J, Skare J T, Wagar E A, Champion C I, Blanco D R, Lovett M A, Miller J N. Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J Clin Investig. 1995;96:965–975. doi: 10.1172/JCI118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley D M, Wang Y P, Wu X Y, Blanco D R, Lovett M A, Miller J N. Acquired resistance to Borrelia burgdorferi infection in the rabbit. Comparison between outer surface protein A vaccine- and infection-derived immunity. J Clin Investig. 1997;99:2030–2035. doi: 10.1172/JCI119371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilmore R D, Mbow M L. Conformational nature of the Borrelia burgdorferi B31 outer surface protein C protective epitope. Infect Immun. 1999;67:5463–5469. doi: 10.1128/iai.67.10.5463-5469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo B P, Brown E L, Dorward D W, Rosenberg L C, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 33.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitten T, Barbour A G. The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics. 1992;132:311–324. doi: 10.1093/genetics/132.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mbow M L, Gilmore R D, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery R R, Malawista S E, Feen K J, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody K D, Adams R L, Barthold S W. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob Agents Chemother. 1994;38:1567–1572. doi: 10.1128/aac.38.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Farrell P Z, Goodman H M, O'Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 43.Probert W S, Crawford M, Cadiz R B, LeFebvre R B. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 44.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouselle J C, Callister S M, Schell R F, Lovrich S D, Jobe D A, Marks J A, Wieneke C A. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis. 1998;178:733–741. doi: 10.1086/515382. [DOI] [PubMed] [Google Scholar]
- 46.Sadziene A, Thomas D D, Barbour A G. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun. 1995;63:1573–1580. doi: 10.1128/iai.63.4.1573-1580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebert P D, Larrick J W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. BioTechniques. 1993;14:244–249. [PubMed] [Google Scholar]
- 49.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant outer-surface protein A Lyme disease vaccine study consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 50.Simon M M, Schaible U E, Kramer M D, Eckerskorn C, Museteanu C, Müller-Hermelink H K, Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991;164:123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- 51.Skare J T, Mirzabekov T A, Shang E S, Blanco D R, Erdjument-Bromage H, Bunikis J, Bergström S, Tempst P, Kagan B L, Miller J N, Lovett M A. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skare J T, Shang E S, Foley D M, Blanco D R, Champion C I, Mirzabekov T, Sokolov Y, Kagan B L, Miller J N, Lovett M A. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Investig. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Telford S R, III, Kantor F S, Lobet Y, Barthold S W, Spielman A, Flavell R A, Fikrig E. Efficacy of human Lyme disease vaccine formulations in a mouse model. J Infect Dis. 1995;171:1368–1370. doi: 10.1093/infdis/171.5.1368. [DOI] [PubMed] [Google Scholar]
- 57.Theisen M, Frederiksen B, Lebech A M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rössler D, Soutschek E, Johnson R C. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J R, Norris S J. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J R, Norris S J. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zückert W R, Meyer J, Barbour A G. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect Immun. 1999;67:3257–3266. doi: 10.1128/iai.67.7.3257-3266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]