Abstract
Streptococcal pyrogenic exotoxins (SPEs) are superantigens that have been implicated in causing streptococcal toxic shock syndrome (STSS). Most notably, SPE serotype A is made by nearly all M-protein serotype 1 and 3 streptococci, the M types most associated with the illness (these strains contain one or more other SPEs, and those proteins are likely also to contribute to disease). We have prepared double-, triple-, and hexa-amino-acid mutants of SPE A by PCR and other mutagenesis procedures. The sites chosen for mutation were solvent-exposed residues thought to be important for T-cell receptor (TCR) or major histocompatibility complex (MHC) class II interaction. These mutants were nonsuperantigenic for human peripheral blood mononuclear cells and rabbit and mouse splenocytes and were nonlethal in two rabbit models of STSS. In addition, these mutants stimulated protective antibody responses. Interestingly, mutants that altered toxin binding to MHC class II were more immunogenic than mutants altering TCR binding. Collectively, these studies indicate that multiple-site mutants of SPE A are toxoids that may have use in protecting against the toxin's effects in STSS.
The reemergence of invasive streptococcal disease, specifically streptococcal toxic shock syndrome (STSS), has been attributed to an epidemiological shift in Streptococcus pyogenes strains (8, 13). M-protein serotype 1 and 3 streptococci are the most prominently associated with this illness (3, 4, 10, 13, 24, 34, 35), and it has been suggested that the increase in severity of streptococcal illnesses (13) may be linked to the increase in isolation of these types of streptococci. Among the numerous virulence factors available to group A streptococci to cause disease are streptococcal pyrogenic exotoxins (SPEs). These toxins were formerly known as scarlet fever toxins or erythrogenic toxins, and they were thought to be the causative agents of the scarlet fever rash. Today it is well known that the SPEs have a far more complex role in the pathogenesis of TSS illnesses. SPEs are pyrogenic toxin superantigens (PTSAgs). The PTSAgs are among the most potent pyrogens known, they are superantigens, they induce the release of massive amounts of host cytokines, and they are all highly lethal (5). Moreover, the PTSAgs can amplify host susceptibility to endotoxin lethality by a factor of greater than 105. To date, nine distinct SPE types (and related molecules) have been identified; these are designated SPEs A, B, C, F, G, H, and J, streptococcal superantigen, and streptococcal mitogenic exotoxin Z (5, 28, 32).
To date several epidemiological and laboratory findings have indicated that SPE A, among the SPEs, is most significantly associated with STSS. The majority of streptococcal M1 and M3 types produce SPE A or carry the SPE A gene (speA) (8, 10, 18, 21, 24, 31, 32, 34). For example, Cleary et al. (8) have shown that invasive, but not noninvasive, M1 streptococci carry the gene speA, although this may not be the only genetic difference between the strains. Only 15% of streptococci isolated from uncomplicated infections carry speA (36). Comparison of sera from healthy donors and patients affected by streptococcal infections indicates that severe disease is associated with a lack of antibodies to SPEs, including type A (11, 21, 27). Schlievert et al. (31) showed that of isogenic streptococcal strains differing in the presence of the speA-carrying bacteriophage T12, the bacteriophage-positive strain was able to cause STSS in a rabbit model, whereas the toxin-negative strain was not. Purified SPE A by itself was shown to induce STSS in humans and in a rabbit model in which SPE A was administered in subcutaneously implanted miniosmotic pumps (18, 29). Lastly, SPE A purified from Staphylococcus aureus was used to immunize rabbits (31). These animals were significantly more resistant than controls to lethal challenge with live M1 and M3 streptococci. It is important to note that many TSS streptococci have the genes for one or more additional toxins, and these SPEs may also contribute significantly to illness.
The study by Schlievert et al. (31) showed that SPE A vaccination was effective in protecting rabbits from all symptoms of STSS, including the necrotizing fasciitis-myositis aspect of the syndrome. This suggested that although SPE A is not the only factor having a role in STSS and does not itself induce necrotizing fasciitis-myositis, antibodies to wild-type SPE A are sufficient to prevent bacterial invasion of deeper tissues by the strains used. In previous work, we generated and characterized single-site directed mutants of SPE A with reduced superantigenic activity in vitro and reduced lethal activity in vivo in rabbit models (29). In the present work, we explored the possibility of using selected multiple-site mutants of SPE A as toxoids for use as vaccines. We show that double, triple, and hexamutants of SPE A lacked significant superantigenicity and lethality, elicited production of antibodies specific for SPE A in rabbits, and could be used to immunize rabbits against lethal challenge with native SPE A.
MATERIALS AND METHODS
Mutants.
Table 1 lists the SPE A mutants analyzed in this work. The single-amino-acid mutant N20D and double mutant N20D/C98S SPE A were obtained as described previously (29). Mutant D45N was obtained by the in vitro site-directed mutagenesis system Altered Sites II (Promega, Madison, Wis.). The 1.75-kb BamHI-SalI fragment of speA, which contains the structural gene as well as upstream and downstream putative regulatory regions, was subcloned in vector pAlter provided in the mutagenesis kit (Promega). The mutagenic oligonucleotide primer was designed to be homologous to a region of DNA surrounding the D45N codon and contained the nucleotide changes necessary to generate the desired amino acid mutation. The mutagenesis reactions were performed as suggested by the manufacturer. The plasmid product of mutagenesis was purified, and speA was sequenced to confirm the presence of only the desired nucleotide mutation. Subsequently, mutant speA-containing pAlter plasmid was digested with BamHI and SalI. The DNA fragment was cloned at the corresponding sites in shuttle vector pMIN164 (23), for replication in both Escherichia coli and S. aureus. Ligated DNA was transformed in E. coli DH5α. Ampicillin-resistant clones were tested for SPE A expression in a double immunodiffusion assay using rabbit polyclonal antibodies raised against wild-type SPE A. The plasmid with mutant D45N speA (pMIN170) was then moved by protoplast transformation (7) into S. aureus RN4220. This staphylococcal host is devoid of known genes encoding enterotoxins or TSST-1. Toxin expression and secretion by erythromycin resistant colonies were detected by a double immunodiffusion assay.
TABLE 1.
SPE A mutants used in this work and plasmids carrying them
| Mutant | Amino acid change(s) | Plasmid(s) | Reference |
|---|---|---|---|
| Vector only | None | pMIN164 | 5 |
| Wild-type SPE A | None | pMIN165 | 5 |
| N20D | Asn→Asp | pMIN166 | 29 |
| D45N | Asp→Asn | pMIN170 | This work |
| C98S | Cys→Ser | pMIN173 | 29 |
| N20D/C98S | Asn→Asp, Cys→Ser | pMIN167 | 29 |
| N20D/D45N/C98S | Asn→Asp, Asp→Asn, Cys→Ser | pMIN169, pLP613 | This work |
| Q19H/N20D/L41A/L42A/D45N/C98S | Gln→His, Asn→Asp, Leu→Ala, Leu→Ala, Asp→Asn, Cys→Ser | pLP635 | This work |
The triple mutant N20D/D45N/C98S was generated by successive subcloning, by use of unique restriction sites. First, the SalI-BstEII fragment from plasmid pMIN166 (Table 1) was exchanged with the corresponding fragment of plasmid pMIN170 (Table 1), giving the double mutant N20D/D45N. Then, the BamHI-BfrI fragment of plasmid pMIN173 (Table 1) was exchanged with the corresponding fragment in the plasmid carrying the double mutant gene to generate the triple mutant gene (pMIN169). The presence of the mutations was confirmed by sequencing as described above.
The hexamutant Q19H/N20D/L41A/L42A/D45N/C98S was prepared by the Quik Change mutagenesis procedure (Stratagene, La Jolla, Calif.). The triple mutant gene (pMIN169) served as the template. The presence of the six mutations was confirmed by nucleotide sequencing after cloning into E. coli DH5α.
Both the triple and hexamutants were subsequently cloned into purified pET28a, giving rise to plasmids pLP613 and pLP635, respectively. These plasmids were transformed into E. coli BLR(DE3) or BL21(DE3) for expression. Both mutants were expressed without their signal peptides. This very significantly increased the yield of mutant proteins from approximately 1 mg/liter in S. aureus RN4220 to 10 mg/liter in E. coli.
Toxin production and purification.
Toxins were produced and purified from S. aureus RN4220 or E. coli (in the case of the triple and hexamutants) as previously described (33). Briefly, S. aureus strains carrying wild-type or mutant speA genes in pMIN164 were grown to stationary phase at 37°C with vigorous shaking for 18 h in pyrogen-free dialyzed beef heart medium containing 5 μg of erythromycin per ml. Cell culture supernatants were treated with ethanol (80% final volume) to precipitate toxins, and pellets were resuspended in small volumes of pyrogen-free water and cleared by centrifugation. Supernatants were dialyzed and subjected to successive flat-bed isoelectric focusing separations in pH gradients of 3.5 to 10 and 4 to 6. Toxin-containing fractions were extensively dialyzed in pyrogen-free water, aliquoted, and stored. Prior to performance of assays for superantigenicity, the mutant proteins were subjected to reverse-phase high-pressure liquid chromatography (22).
E. coli-derived triple and hexamutants were obtained as follows. BLR(DE3)(pLP613) and BL21(DE3)(pLP635) were grown overnight in the presence of kanamycin. The next morning each culture was diluted 1:100 into fresh medium containing kanamycin. When the absorbance of each culture at 600 nm reached 1.5, 1.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added. The cultures were grown for an additional 3 h, and cells were harvested by centrifugation at 4°C.
Triple and hexamutant SPE A proteins were purified from E. coli after cell lysis, dialysis of the soluble material against 0.1 M sodium acetate buffer (pH 4.5), removal of insoluble material by centrifugation, and cation exchange (SP-Sepharose [Pharmacia Fine Chemicals] eluted with 0 to 1 M NaCl in 0.1 M sodium acetate [pH 4.5]) and gel filtration (Superdex 75 [Pharmacia] equilibrated with 0.02 M Tris [pH 7.4]–0.15 M NaCl).
Wild-type SPE A toxin for challenge experiments was purified from S. pyogenes strain 594 (25), essentially by the protocol described above, except that toxin precipitated with ethanol was resuspended in pyrogen-free acetate saline buffer (pH 4.5) prior to isoelectric focusing.
Animals.
American Dutch belted rabbits weighing 1.5 to 2.0 kg were purchased from Birchwood Farms, Grantsburg, Wis. BALB/c mice, 4 to 6 weeks old, were purchased from Jackson Laboratories. All animals were housed in the University of Minnesota animal care facilities, and were given food and water ad libitum.
Lethality determinations.
Lethal effects of the mutant and wild-type toxins were assessed by use of miniosmotic pumps (Alzet; Alza, Palo Alto, Calif.) implanted subcutaneously in the flanks of American Dutch belted rabbits. The pumps were preloaded with 200 or 500 μg of wild-type or mutant toxin in phosphate-buffered saline (PBS) (0.005 M NaPO4, 0.15 M NaCl) at pH 7.2. These devices release a constant amount of toxin over a time period of 7 days, calculated to be equal to 20 μg/day when 200 μg/pump was used (29). Animals were monitored for symptoms of STSS, and deaths were recorded over a 15-day period. Residual toxic effects were evaluated by gross necroscopic examination of the surviving animals.
Endotoxin shock enhancement.
American Dutch belted rabbits were pretreated with sublethal doses of wild-type or mutant SPE A (5 μg/kg of body weight) administered in the marginal ear vein (33). Four hours later animals were challenged with sublethal doses of endotoxin from Salmonella enterica serovar Typhimurium (10 μg/kg of body weight, which is equal to 1/50 50% lethal dose [LD50]) via the same route. Deaths were recorded for the 48 h after the injection of endotoxin. Residual toxic effects were evaluated by gross necroscopic examination of the surviving animals.
In one experiment, three rabbits were administered 300 μg of hexamutant per kg followed at 4 h by 100 μg of endotoxin per kg. Again, deaths were recorded over a 48-h period.
Lymphocyte proliferative activity.
The ability of SPE A and mutants to stimulate proliferation of rabbit and murine splenocytes and human peripheral blood mononuclear cells (PBMCs) was assayed in a 4-day in vitro [3H]thymidine incorporation assay (2). Splenocytes from American Dutch belted rabbits or BALB/c mice were suspended in RPMI cell culture medium completed by addition of 2% fetal calf serum and 2 mM glutamine. Cells were dispensed in 96-well microtiter plates in amounts of 2 × 105 cells/well for rabbit cells and 5 × 105 cells/well for mouse cells. The cells were incubated at 37°C and 7% CO2 with serial 10-fold dilutions of wild-type or mutant SPE A for 72 h (2). Cells were then pulsed for 18 h with [3H]thymidine (Amersham Corporation, Arlington Heights, Ill.) at 1 μCi/well. Finally, cells were harvested on fiberglass filters, and counts per minute due to [3H]thymidine incorporation into DNA of proliferating cells were measured in a scintillation counter. Each sample was tested in quadruplicate. Counts from untreated cells were considered background.
Human PBMCs were obtained by separation of whole blood in Percoll gradients (Histopaque; Sigma Chemical Company, St. Louis, Mo.). Cells were suspended in complete RPMI medium at a concentration of 2 × 105 cells/well and then treated as described above for rabbit and murine splenocytes.
Immunization of animals.
American Dutch belted rabbits were used for all vaccination studies. Toxins for immunizations were produced from streptococci strain 594, S. aureus RN4220, or E. coli carrying the appropriate plasmids and purified as described above. Lyophilized toxins were dissolved immediately before use in sterile PBS at a toxin concentration of 25 μg/ml. Toxin solutions were emulsified in equal volumes of incomplete Freund's adjuvant (Gibco BRL, Gaithersburg, Md.). The emulsions were administered to rabbits in two subcutaneous injection sites on the nape of the neck. Each animal received 25 μg of toxin three times (once each on days 0, 14, and 28).
Antibody titer determination.
Titers of anti-wild-type SPE A antibodies in immunized rabbits were determined by enzyme-linked immunosorbent assay (ELISA) (12). Nunc Immunolon I 96-well microtiter plates were precoated with wild-type SPE A (1 μg/well). Bound anti-SPE A was detected by goat anti-rabbit immunoglobulin G conjugated with peroxidase (Sigma). Absorbance of the reacted substrate was read in an ELISA spectrophotometer at a wavelength of 495 nm. The reciprocal of the last dilution with an absorbance reading of greater than 0.25 was considered the titer for each sample.
In one set of experiments, antisera were collected from rabbits immunized as described above with either wild-type, triple mutant, or hexamutant SPE A. Equal volumes of sera from rabbits in each group were pooled. The pooled sera were individually treated with ammonium sulfate (33 1/3% final saturation) to precipitate immunoglobulins and were then resuspended to their original volumes in PBS and dialyzed overnight against PBS. The fractions were clarified by centrifugation (10,000 × g, 15 min) and filter sterilized (0.45-μm-pore-size filter; Acrodisc; Gelman Sciences, Ann Arbor, Mich.). The titer of each fraction against wild-type SPE A was determined by ELISA, and volumes were adjusted such that each pool had the same titer.
The ability of each pooled fraction to neutralize rabbit splenocyte mitogenicity of wild-type SPE A (1 μg/well) was assessed with use of the 4-day assay described above. Dilutions of each fraction (20 μl) were mixed with SPE A (1 μg in 20-μl volumes) in 96-well microtiter plates for 1 h at 37°C in a 7% CO2 incubator. Splenocytes were then added, and the plates were treated as described above in standard lymphocyte proliferation assays.
RESULTS
Choice of mutants for use as toxoids.
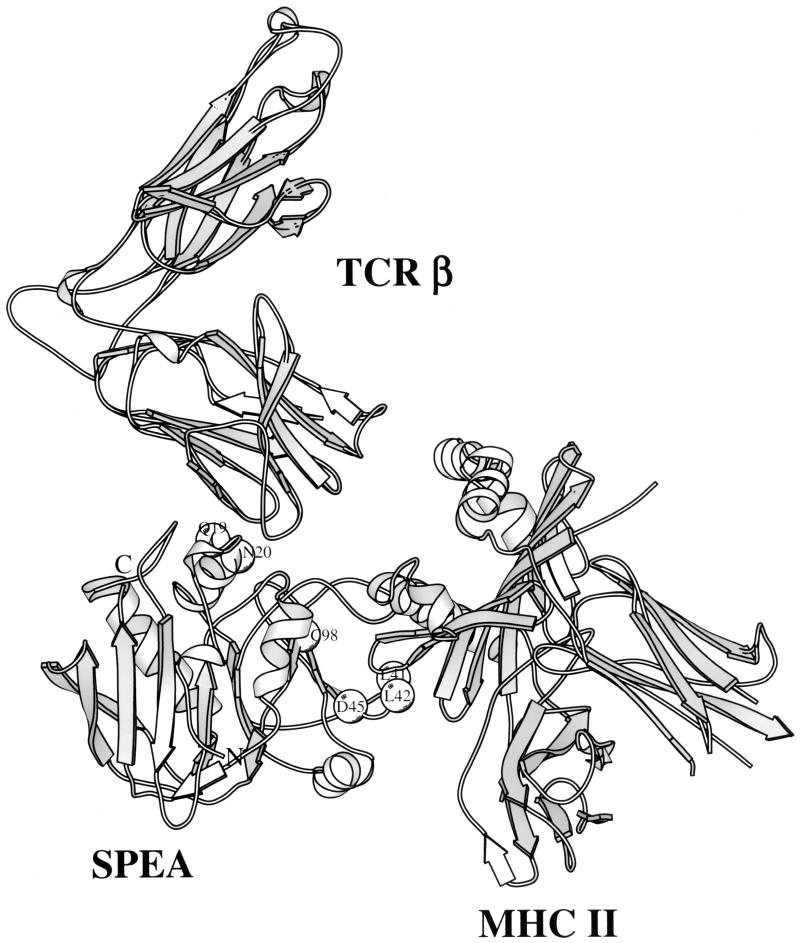
Preliminary experiments indicated that the single-amino-acid mutant of SPE A, designated N20D, was relatively nontoxic to rabbits and provoked significant antibody responses that reacted strongly with native SPE. However, we believed it necessary to further reduce both residual protein toxicity and the possibility of reversion of the mutant gene to the wild type. We decided to test multiple amino acid mutants for use as toxoids for vaccination. These included one double, one triple, and one hexamutant of SPE A. Sites on the molecule chosen for mutagenesis included residues in the putative T-cell receptor (TCR) and major histocompatibility complex (MHC) class II binding sites, as shown in the model structure in Fig. 1. The complex structure shown is based on the structures of staphylococcal enterotoxin B (SEB) complexed with murine TCR Vβ8 (19) and SEB complexed with human MHC class II HLA DR1 (14); SEB and SPE A share approximately 50% sequence identity.
FIG. 1.
Ribbon diagram of SPE A showing the locations of amino acid residues chosen for mutagenesis.
Biological properties of the mutants.
Each of the multiple-site mutants was evaluated for the ability to stimulate human PBMCs and rabbit and mouse splenocytes as superantigens in comparison to wild-type SPE A. None of the mutants caused significant stimulation of human PBMCs at any dose tested (up to 10 μg/well) (Table 2), whereas wild-type SPE A was superantigenic at doses as low as 10−4 μg/well. Similarly, each mutant was nonsuperantigenic for rabbit splenocytes (Table 3), the best model for TSS, or murine splenocytes (data not shown). In both of these model systems, wild-type SPE A was significantly stimulatory.
TABLE 2.
Superantigenicity of wild-type SPE A and mutants with use of human PBMCsa
| Protein tested | Mean cpm ± SE (103) at the following dose (μg):
|
|||||
|---|---|---|---|---|---|---|
| 10 | 1 | 0.1 | 0.01 | 0.001 | 0.0001 | |
| Wild-type SPE A | 119 ± 2.7 | 103 ± 14 | 135 ± 10 | 126 ± 13 | 142 ± 8.7 | 156 ± 3.9 |
| N20D/C98S | 16 ± 1.7 | 24 ± 3.3 | 21 ± 0.7 | 19 ± 0.7 | 21 ± 0.8 | 21 ± 0.6 |
| N20D/D45N/C98S | 25 ± 4.0 | 27 ± 1.2 | 21 ± 1.5 | 20 ± 2.4 | 22 ± 1.5 | 22 ± 2.3 |
| Q19H/N20D/L41A/L42A/D45N/C98S | 20 ± 1.7 | 23 ± 0.5 | 20 ± 3.5 | 15 ± 2.0 | 18 ± 1.3 | 20 ± 3.0 |
Human PBMCs (2 × 105/well) were incubated with proteins, in quadruplicate, for 3 days at 37°C with 7% CO2, and then 1 μCi of [3H]thymidine was added to each well. DNA was harvested after another 24 h of incubation, and counts per minute were determined by scintillation counting. The background was 16 ± 0.5 cpm for quadruplicate wells containing lymphocytes only.
TABLE 3.
Superantigenicity of wild-type SPE A and mutants with use of rabbit splenocytesa
| Protein tested | Mean cpm ± SE (103) at the following dose (μg):
|
|||||
|---|---|---|---|---|---|---|
| 10 | 1.0 | 0.1 | 0.01 | 0.001 | 0.0001 | |
| Wild-type SPE A | 211 ± 5.3 | 181 ± 8.6 | 131 ± 8.5 | 105 ± 1.6 | 79 ± 1.0 | 55 ± 4.9 |
| N20D/C98S | 13 ± 0.9 | 13 ± 0.7 | 15 ± 1.1 | 10 ± 0.8 | 14 ± 1.3 | 13 ± 1.1 |
| N20D/D45N/C98S | 23 ± 4.0 | 20 ± 2.1 | 20 ± 2.8 | 17 ± 2.1 | 18 ± 1.8 | 18 ± 2.3 |
| Q19H/N20D/L41A/L42A/D45N/C98S | 18 ± 0.8 | 19 ± 1.4 | 19 ± 2.0 | 18 ± 1.0 | 13 ± 1.4 | 17 ± 1.1 |
Rabbit splenocytes (2 × 105/well) were incubated with proteins, in quadruplicate, for 3 days at 37°C with 7% CO2, and then 1 μCi of [3H]thymidine was added to each well. DNA was harvested after another 24 h of incubation, and counts per minute were determined by scintillation counting. The background was 17 ± 0.4 cpm.
Each multiple-site mutant was also evaluated for lethality as tested in two ways (Table 4), compared to wild-type toxin. All PTSAgs share the ability to enhance greatly rabbit susceptibility to the lethal effects of endotoxin (5). None of the mutants had this property, whereas wild-type SPE A was highly lethal. None of the mutants induced demonstrable toxicity upon gross histologic examination. The hexamutant was also administered to three rabbits at 300 μg/kg, followed by endotoxin at 100 μg/kg. None of the three animals succumbed. Similarly, PTSAgs induce lethality in rabbits when administered in subcutaneously implanted miniosmotic pumps. None of the mutants induced lethality (or any sign of illness) when evaluated by this method (Table 4), in contrast to the significant toxicity seen with wild-type toxin.
TABLE 4.
Lethality of wild-type SPE A and mutants as tested by endotoxin enhancement and miniosmotic pump model
| Protein tested | No. dead/total when tested by:
|
|
|---|---|---|
| Endotoxin enhancementa | Miniosmotic pumpb | |
| Wild type SPE Ac | 11/11 | 9/11 |
| N20D/C98S | 0/3 | 0/5 |
| N20D/D45N/C98S | 0/3 | 0/5 |
| Q19H/N20D/L41A/L42A/D45N/C98S | 0/5 | 0/5 |
Animals were intravenously administered 5 μg of wild-type or mutant SPE A per kg. Four hours later, the rabbits were given 10 μg of S. enterica serovar Typhimurium endotoxin intravenously. Animals were monitored for 48 h for lethality.
Miniosmotic pumps contained 500 μg of wild-type or mutant SPE A.
Cumulated total control rabbits.
Immunogenicity of mutants.
Rabbits received three immunizations with mutants subcutaneously in adjuvant. The animals were bled prior to and 1 week after immunization and evaluated for antibody response. Significant antibody titers developed in all immunized animals, with the most immunogenic being the hexamutant (Table 5).
TABLE 5.
Immunogenicity of SPE A mutants in rabbits
| Immunizing agent | Titera
|
|
|---|---|---|
| Preimmuneb | Immunec | |
| None | 18 | 24 |
| Wild-type SPE A | 40 | 1,280 |
| N20D | 60 | 1,600 |
| D45N | 26 | 6,400 |
| N20D/C98S | 32 | 1,220 |
| N20D/D45N/C98S | 16 | 3,400 |
| Q19H/N20D/L41A/L42A/D45N/C98S | 80 | 28,800 |
Average titer of sera from five rabbits.
Rabbits were bled prior to administration of the first toxin dose.
Rabbits were bled 7 days after administration of the third immunizing dose. Each immunization consisted of 25 μg/rabbit emulsified in Freund's incomplete adjuvant administered subcutaneously on days 0, 14, and 28.
We hypothesized that the reason the hexamutant was most immunogenic was because of the presence of multiple mutations in the MHC class II binding domain. It was thus possible that wild-type SPE A binding to MHC class II as an intact superantigen slowed processing and presentation as a classic antigen. Mutations altering the ability to bind MHC class II as a superantigen may then make the toxin a typical antigen. To test this, we evaluated the single-amino-acid mutants N20D (a TCR mutant) and D45N (an MHC class II mutant) for the ability to induce antibody responses (Table 5). Consistent with our hypothesis, D45N was significantly more immunogenic than SPE A N20D, although neither mutant exhibited significant superantigenicity (data not shown).
Protective ability of immune responses to mutants.
All rabbits that were immunized with SPE A, as well as nonimmunized controls, were challenged with 500 μg of wild-type SPE A administered in subcutaneously implanted miniosmotic pumps (Table 6). Animals immunized with any of the mutants survived and showed no signs of illness (except that one animal immunized against the triple mutant showed signs of fever) and no signs of tissue damage upon gross histologic examination. In contrast, all nonimmune animals succumbed. Three animals previously immunized against the hexamutant were challenged with 200 μg of TSST-1 administered in miniosmotic pumps. All three animals succumbed in less than 8 days. This indicates that immunity to SPE A did not provide cross protection against this heterologous PTSAg.
TABLE 6.
Protective ability of SPE A mutants against challenge with wild-type SPE Aa
| Immunizing agent | No. of animals with feverb/total | No. dead/total | Pc |
|---|---|---|---|
| None | 9/10 | 10/10 | |
| N20D/C98S | 0/5 | 0/5 | 0.004 |
| N20D/D45N/C98S | 1/5 | 0/5 | 0.004 |
| Q19H/N20D/L41A/L42A/D45N/C98S | 0/5 | 0/5 | 0.004 |
Miniosmotic pumps were loaded with 500 μg of wild-type SPE A.
Rectal temperatures were taken at before miniosmotic pump implantation (baseline) and on day 2. Fevers were considered significant if temperatures were greater than 0.5°C above baseline.
By Fisher's exact test for nonimmunized compared to immunized animals.
In a final study, pooled sera from rabbits immunized against the triple or hexamutant were compared with sera from animals immunized against wild-type toxin for the ability to neutralize SPE A superantigenicity. Serum samples were first adjusted to the same titer as tested by ELISA reactivity against wild-type SPE A. Sera from rabbits immunized against either mutant or wild-type toxin were essentially equal in ability to neutralize rabbit splenocyte proliferation induced by wild-type SPE A (Fig. 2).
FIG. 2.
Ability of antibodies against either wild-type SPE A or mutants to neutralize the superantigenicity of SPE A. Dilutions of antibodies were mixed with wild-type SPE A (1 μg/well) 1 h prior to addition of rabbit splenocytes. Cultures were incubated for 3 days at 37°C in a 7% CO2 incubator, and then 1 μCi of [3H]thymidine was added per well. DNA was harvested after an additional day of incubation. Error bars indicate standard deviations.
DISCUSSION
Group A streptococci make a large number of virulence factors, both cell associated and secreted. Among the secreted factors are the SPEs (synonyms are scarlet fever toxins and erythrogenic toxins). These toxins are related to the staphylococcal PTSAgs, TSST-1, and enterotoxins, all of which have the capacity to cause TSS in a susceptible host. The toxins cause massive cytokine release through both superantigenicity and enhancement of endotoxin shock, with the result being capillary leakage and consequent hypotension and shock, among numerous other effects (5, 20, 32). Thus far, nine SPEs and related PTSAgs have been identified.
In this study we have prepared multiple-site mutants of SPE serotype A, a major toxin associated with STSS with or without necrotizing fasciitis. We prepared one double mutant, one triple mutant, and one hexamutant. The multiple-site mutants were evaluated for suitability for use as toxoid vaccines against STSS. Multiple residue changes were incorporated into the toxin gene both to prevent reversion to wild type and to reduce toxicity to negligible levels.
In selection of sites for mutagenesis, residues that were known to be important for superantigenicity, enhancement of endotoxin shock, and lethality (when administered subcutaneously to rabbits in miniosmotic pumps) were identified (16, 17, 29). For example, in this study, residue Asn 20 was changed to Asp. This residue corresponds to residue Asn 23 in both SEB and SEC and is in an absolutely conserved spatial position across all PTSAgs (19). The residue is important in TCR binding in both SEB and SEC as well as SPE A (9, 19). Similarly, SPE A residues 41, 42, and 45 are in the O/B fold region of the molecule and are important for MHC class II binding (16, 17, 29). Importantly, by selecting such key residues, we obtained three multiple-site mutants, N20D/C98S, N20D/D45N/C98S, and Q19H/N20D/L41A/L42A/D45N/C98S, that had all of the desired properties of toxoids.
The three mutants were negative for superantigenicity as tested in three systems (human, rabbit, and mouse), they were nonlethal when administered in miniosmotic pumps, and the proteins did not enhance host susceptibility to endotoxin shock. This last endotoxin enhancement assay is the most sensitive method to evaluate mutants for loss of lethal activity. There is a log-log relationship between the dose of PTSAg pretreatment and the log LD50 of endotoxin such that for each 1-log-unit increase in PTSAg there is a 1-log-unit decrease in endotoxin LD50 (30). Thus, if PTSAg pretreatment (y axis) is plotted versus endotoxin LD50 (x axis), a graph with a slope of −1 is obtained. At a dose of wild-type SPE A of 300 μg/kg, the expected LD50 of endotoxin in rabbits would be 0.0017 μg/kg. In the present study 300 μg of hexamutant per kg followed by 100 μg of endotoxin per kg resulted in no lethality. This represents at least a 2 × 105-fold reduction in hexamutant toxicity compared to wild-type SPE A (the LD50 of endotoxin alone is approximately 500 μg/kg). Finally, the three mutants were immunogenic, and the resultant antibodies that were formed equally neutralized wild-type SPE A toxicity. This last experiment was performed to compare the SPE A-neutralizing qualities of antibodies raised against the mutants. The data indicated that all three mutants elicited antibodies with comparable abilities to neutralize SPE A superantigenicity. It is not completely clear how this in vitro superantigen neutralization ability correlates with prevention of lethality in rabbits. However, only very small amounts of antibodies in vivo are likely to be required, since active immunization of rabbits every other day for five injections by injection of 3 μg of wild-type SPE A per kg provided protection from lethality in prior endotoxin enhancement experiments (15).
Because of the increasing number of SPEs and other PTSAgs identified, there have been important attempts made to cross neutralize the toxins after immunization with only one PTSAg (1, 6). SPE A, SEB, and SEC form a subgroup among the PTSAgs based upon their 50% shared primary sequence similarity and their highly related three-dimensional structures (5, 32). For example, Bohach et al. (6) showed that antibodies raised against each of these PTSAgs partially cross neutralized the activities of the other two. In a recent study (1), it was suggested that a highly conserved dodecapeptide in PTSAgs could be used to stimulate cross-protective immunity among all PTSAgs. In that study no explanation was given for how treatment of mice with such a peptide plus a PTSAg could lead to protection against highly heterologous PTSAgs, such as TSST-1 and SPE A. In the present study, it was observed that immunity against the SPE A mutants provided immunity against wild-type SPE A but not against the distantly related PTSAg TSST-1, despite the fact that the SPE A mutants contained that dodecapeptide in its native position in the molecule. Furthermore, we have previously shown that immunity against TSST-1 did not cross protect rabbits from challenge with either SEB or SPE A (data not shown). Thus, further studies, beyond the scope of the present study, are necessary to resolve the extent of cross protection that may be afforded by immunization against one or more PTSAgs. Such studies are necessary because of the increasing number of PTSAgs being identified and because of their increasing associations with serious illness. Finally, it is important to remember that the ability of streptococci to cause serious infections is the result of complex interactions of streptococci with the host, and it is likely that several toxins and other virulence factors will contribute to disease causation. Thus, it would not be expected that immunization against any one PTSAg would protect against all cases of STSS or serious streptococcal disease.
During the immunization studies with the mutants, it was noted that mutants with more residue changes in the MHC class II binding site were more immunogenic than mutants with fewer changes. This was more directly tested by selecting mutants with a single-site mutation in TCR (N20D) and MHC class II (D45N) binding. The mutant with altered MHC class II binding was more immunogenic than the mutant with altered TCR binding. These data suggest that superantigens may be intrinsically less immunogenic than other antigens, in part because of their ability to bind both externally and in intact form to MHC class II rather than being processed and then presented to T cells.
Finally, it is noteworthy that all mutants tested were similar when tested for superantigenicity in three species. It has previously been suggested that mutations in superantigens do not always affect different species in the same way (26). While this is likely to be the case for certain residue changes, our study suggests that the important regions of superantigens required for T-cell proliferation are generally conserved across species.
In sum, our studies have shown that three multiple-site mutants of the PTSAg SPE A have the desired properties of toxoids, suitable for use in vaccination against the toxin. It is hoped that such toxoids can be used in the future to reduce the risk of STSS.
ACKNOWLEDGMENTS
This work was supported by NIH grant HL36611 and by a grant from Wyeth Lederle Vaccines.
We gratefully acknowledge Melodie Bahan for help in preparation of the manuscript and Gregory Vath for preparation of Fig. 1.
REFERENCES
- 1.Arad G, Levey R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T cell activation. Nat Med. 2000;6:414–421. doi: 10.1038/74672. [DOI] [PubMed] [Google Scholar]
- 2.Barsumian E L, Schlievert P M, Watson D W. Nonspecific and specific immunologic mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun. 1978;22:681–688. doi: 10.1128/iai.22.3.681-688.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begovac J, Gmajnicki B, Schlievert P M, Johnson D R, Kaplan E L. Production of pyrogenic exotoxins in group A streptococci isolated from patients in Zagreb, Croatia. Eur J Clin Infect Dis. 1992;11:540–543. doi: 10.1007/BF01960810. [DOI] [PubMed] [Google Scholar]
- 4.Belani K, Schlievert P M, Kaplan E L, Ferrieri P. Association of exotoxin producing group A streptococci and severe disease in children. J Pediatr Infect Dis. 1991;10:351–354. doi: 10.1097/00006454-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 6.Bohach G A, Houde C J, Handley J P, Schlievert P M. Cross-neutralization of staphylococcal and streptococcal pyrogenic toxins by monoclonal and polyclonal antibodies. Infect Immun. 1988;56:400–404. doi: 10.1128/iai.56.2.400-404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Cohen S N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979;168:111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- 8.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 9.Fields B A, Malchiodi E L, Li H, Ysern X, Stauffacher C V, Schlievert P M, Karjalainan K, Mariuzza R A. Crystal structure of a T-cell receptor b chain complexed with a superantigen. Nature. 1996;384:188–192. doi: 10.1038/384188a0. [DOI] [PubMed] [Google Scholar]
- 10.Hauser A R, Stevens D L, Kaplan E L, Schlievert P M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991;29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm S E, Norrby A, Bergholm A-M, Norgren M. Aspects of pathogenesis in serious group A streptococcal infections in Sweden. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Hudson L, Hay F C. Practical immunology. 2nd ed. Oxford, England: Blackwell Scientific Publications; 1980. pp. 137–138. [Google Scholar]
- 13.Johnson D R, Stevens D L, Kaplan E L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Urban R G, Strominger J L, Wiley D C. Crystallographic structure of toxic shock syndrome toxin-1 complexed with a human class II major histocompatibility complex molecule, HLA-DR1. Science. 1994;266:1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y B, Watson D W. A purified group A streptococcal pyrogenic exotoxin. Physicochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970;131:611–628. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline J B, Collins C M. Analysis of the superantigenic activity of mutant and allelic forms of streptococcal pyrogenic exotoxin A. Infect Immun. 1996;64:861–869. doi: 10.1128/iai.64.3.861-869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kline J B, Collins C M. Analysis of the interaction between the bacterial superantigen streptococcal pyrogenic exotoxin A (Spe A) and the human T-cell receptor. Mol Microbiol. 1997;24:191–202. doi: 10.1046/j.1365-2958.1997.3381696.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee P K, Schlievert P M. Quantification and toxicity of group A streptococcal pyrogenic exotoxins in an animal model of toxic shock syndrome-like illness. J Clin Microbiol. 1989;27:1890–1892. doi: 10.1128/jcm.27.8.1890-1892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Llera A, Tsuchiya D, Leder L, Ysern X, Schlievert P M, Karjalainen K, Mariuzza R A. Three dimensional structure of the complex between a T cell receptor β chain and the superantigen staphylococcal enterotoxin B. Immunity. 1998;9:807–816. doi: 10.1016/s1074-7613(00)80646-9. [DOI] [PubMed] [Google Scholar]
- 20.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 21.Mascini E M, Jansze M, Schellekens J F P, Musser J M, Faber J A J, Verhoef-Verhage L A E, Schouls L, van Leeuwen W J, Verhoef J, van Dijk H. Invasive group A streptococcal disease in the Netherlands: evidence for a protective role of anti-exotoxin A antibodies. J Infect Dis. 2000;181:631–638. doi: 10.1086/315222. [DOI] [PubMed] [Google Scholar]
- 22.Monday S R, Vath G M, Ferens W A, Deobald C, Rago J V, Gahr P J, Monie D D, Iandolo J J, Chapes S K, Davis W C, Ohlendorf D H, Schlievert P M, Bohach G A. Unique superantigen activity of staphylococcal exfoliative toxins. J Immunol. 1999;162:4550–4559. [PubMed] [Google Scholar]
- 23.Murray D L, Prasad G S, Earhart C A, Leonard B A B, Kreiswirth N V, Novick R P, Ohlendorf D H, Schlievert P M. Immunobiological and biochemical properties of mutants of toxic shock syndrome toxin-1. J Immunol. 1994;152:87–95. [PubMed] [Google Scholar]
- 24.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauciel C, Blass J, Mangalo R, Raynaud M. Evidence for two molecular forms of streptococcal erythrogenic toxin. Conversion to a single form by 2-mercaptoethanol. Eur J Biochem. 1969;11:160–164. doi: 10.1111/j.1432-1033.1969.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 26.Neill R J, Jett M, Crane R, Wootres J, Welch C, Hoover D, Gemski P. Mitogenic activities of amino acid substitution mutants of staphylococcal enterotoxin B in human and mouse lymphocyte cultures. Infect Immun. 1996;64:3007–3015. doi: 10.1128/iai.64.8.3007-3015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norrby-Teglund A, Pauksens A K, Holm S E, Norgren M. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis. 1994;170:585–591. doi: 10.1093/infdis/170.3.585. [DOI] [PubMed] [Google Scholar]
- 28.Proft T, Moffatt S L, Berkahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–101. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roggiani M, Stoehr J A, Leonard B A B, Schlievert P M. Analysis of toxicity of streptococcal pyrogenic exotoxin A mutants. Infect Immun. 1997;65:2868–2875. doi: 10.1128/iai.65.7.2868-2875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlievert P M. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–128. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlievert P M, Assimacopoulos A P, Cleary P P. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J Lab Clin Med. 1996;127:13–22. doi: 10.1016/s0022-2143(96)90161-4. [DOI] [PubMed] [Google Scholar]
- 32.Schlievert P M, Kotb M Y, Stevens D L. Streptococcal superantigens: streptococcal toxic shock syndrome. In: Cunningham M W, Fujinami R S, editors. Effects of microbes on the immune system. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000. pp. 25–39. [Google Scholar]
- 33.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 34.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 35.Talkington D F, Schwartz B, Black C M, Todd J K, Elliot J, Breiman R F, Facklam R R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C E, Ferretti J J. Molecular epidemiologic analysis of the type A streptococcal exotoxin (erythrogenic toxin) gene (speA) in clinical Streptococcus pyogenes strains. Infect Immun. 1989;57:3715–3719. doi: 10.1128/iai.57.12.3715-3719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]