Abstract
Antimicrobial resistance has become one of the most important public health problems of our century. In addition to the spread of resistance, biofilm production also makes the treatment of infections increasingly difficult. Therefore, this study, it was aimed to investigate the effect of the predator bacterium Bdellovibrio bacteriovorus HD100 on various clinical pathogens and their biofilms. A large panel of Gram-positive and negative clinical isolates were included in the study. The double-layer agar method was used to optimize the cultivation of predatory bacteria. The effectiveness of Bdellovibrio bacteriovorus HD 100 on planktonic cells and biofilms, was determined by co-culture and crystal violet staining methods, respectively. The antibiofilm activity was also visualized via scanning electron microscopy. The predator bacteria was found effective against most of the Gram-negative isolates. But it was determined that the lowest activity among these isolates was shown to Pseudomonas aeruginosa and Acinetobacter baumannii. Although it is known that B. bacteriovorus does not predate on Gram-positive isolates, interestingly, Staphylococci species included in this study were found to be inhibited in co-culture studies. As determined in co-culture and biofilm studies, B. bacteriovorus can be used to control both bacterial growth and biofilms in most Gram-negative species. Interestingly, our data also suggest that predatory bacteria may also be effective against Gram-positive bacterial biofilms in addition to Staphylococcus aureus. Although the evaluation of different species of isolates in this study demonstrates the potential of predatory bacteria, the host specificity and the relation of prey and predator need to be demonstrated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-023-01071-y.
Keywords: Antimicrobial resistance, Bdellovibrio bacteriovorus, Biofilm, Predation
Introduction
Antibiotics, a great breakthrough in our age, have saved the lives of millions [1]. Plenty of molecules isolated from microorganisms or chemically synthesized began to be used over 80 years [2]. However, these important molecules are not as effective as before. This crisis, called antimicrobial resistance (AMR), has caused longer hospitalization of the infected individuals, economic losses, and above all, an increase in mortality and morbidity [3, 4]. Therefore, it has become very important to be able to understand and prevent the reasons causing the resistance.
The biofilm, microbial communities embedded in extracellular polymeric substances, is one of the main resistance mechanisms [5]. In this way, a barrier is created to protect the bacteria from all external influences including antibiotics and disinfectants. It also gives cells the ability to attach to surfaces and colonize medical equipment such as implants and catheters, to cause hospital infections [6]. For the reasons mentioned above, biofilm removal or prevention has gained importance as a strategy for infectious diseases [7].
The predatory bacteria Bdellovibrio bacteriovorus, which is a member of the Delta-proteobacteria class, could be an intriguing alternative [8]. These small Gram-negative bacteria can feed on plant, animal, and human pathogens [9]. The biphasic life cycle of B. bacteriovorus consists of the attack and the growth phases. The predator attaches to its prey through its flagella, then enters the host and initiates the synthesis of the necessary macromolecules [10]. Matured B. bacteriovorus cells, break down the host cell utilizing lytic enzymes eventually, allowing it to be released and seek new prey [11].
Here we evaluated the effect of B. bacteriovorus HD 100, a predatory bacterium as an alternative to antibiotics, on a large panel of clinically resistant Gram-negative and Gram-positive isolates.
Materials and Methods
Selection of Bacterial Isolates
To investigate the effectiveness of B. bacteriovorus, it was planned to use resistant and biofilm-producing clinical isolates. For this purpose, isolates from Ege University Faculty of Medicine, Department of Medical Microbiology, Bacteriology Laboratory were pre-examined for antibiotic susceptibilities and biofilm-forming capacity. The identification and antibiograms of the isolates were determined by using MALDI-TOF MS (Biomerieux, France) and VITEK 2 (Biomerieux, France) automated systems respectively. The susceptibility results were interpreted in accordance with EUCAST criteria. The biofilm-forming capacities of isolates were determined by the crystal violet method as described by Stepanović et al. [12]. Enterococcus faecalis ATCC 29212 was used as the positive control strain. Following the biofilm experiments, resistant and biofilm-producing isolates were selected for the further stages of the study. The bacteria were stored at − 80 °C in a Brain–Heart Infusion broth (BHI) (Merck) with the addition of 5% glycerine until they were studied. The experiments were done in triplicate for each isolate.
Double Layer Agar Method
Due to the life cycle of the predatory bacterium, it is necessary to use the double-layer agar method for its cultivation to use in experiments. As it was determined that different media and solutions were used in the literature for this method, firstly it was aimed to optimize the method within the scope of this study [13–15]. For this, Nutrient Agar (NA), Diluted Nutrient Agar (DNA), Enriched Nutrient Agar (ENA) (with the addition of 5% casamino acids to the DNA), 1/10 NA, 1/10 ENA media were used. Each medium was prepared as a double layer both in the presence and absence of ions using HEPES buffer (25 mM, pH: 7.2). Prey isolates were added only to the upper layer. Bdellovibrio bacteriovorus suspension were prepared using the same HEPES solution at 0.5 McFarland turbidity and different volumes of the inoculum were spread on the surface of the agar with a “drigalski spatula” and incubated at 30℃ for 48 h. HEPES solution without the addition of MgCl2 and CaCl2 were also included). The medium and HEPES solution duo, in which plaque formation was detected in the shortest time, was applied for the B. bacteriovorus production in all steps.
Effect of Predator Bacteria on Planktonic Cells
The experiments were designed as described by Garcia-Armesto et al. [16]. The number of colonies in the plates of Tryptone Soy Agar (TSA) (Merck) was calculated as CFU/mL after the incubation period [17]. The experiments were performed in triplicate for each strain.
Biofilm Removal Assay
The study was performed with minor modifications to the crystal violet method applied by Stepanović et al. [12]. Following the biofilm production of isolates, microplates were washed with PBS. Then 200 μL of B. bacteriovorus HD100 was inoculated into the wells and incubated at 30℃ for another 48 h. Biofilm fixation, drying, staining, and imaging steps were done as mentioned above. Pseudomonas aeruginosa ATCC 15442 for Gram-negative strains and Enterococcus faecalis ATCC 29212 for Gram-positive strains were used as positive controls. TSB with 0.25% glucose was used as the negative control. Experiments were done in triplicate.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was used to visualize both the attack of the predatory and the biofilm experiments. The experiment was carried out using several different studies and modifications on representative isolates of Proteus mirabilis, Listeria monocytogenes, Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis [16, 18–20]. In short, biofilms of the above-mentioned bacteria were first formed. Afterwards, predator bacteria were added and incubated again. At the end of the period, the coverslips were washed, dehydrated and dried, respectively. Each coverslip was covered with gold and visualized in SEM (Thermo Scientific Apreo S).
Results
Selection of Bacterial Isolates
Utilizing biofilm and susceptibility data, Enterococcus faecalis, Enterococcus faecium (VRE), Listeria monocytogenes, Staphylococcus hominis, Staphylococcus haemolyticus, Staphylococcus epidermidis, and MRSA as Gram-positive clinical isolates, Escherichia coli, Pseudomonas aeruginosa, Enterobacter hormaechei, Serratia marcescens, Enterobacter aerogenes, Morganella morganii, Klebsiella pneumonia, Proteus mirabilis, Shigella sonnei, Proteus rettgeri, Salmonella typhimurium, Stenotrophomonas maltophilia and Acinetobacter baumannii as Gram-negative clinical isolates, were selected to evaluate the predation characteristics. ATCC strains were also included (Escherichia coli ATCC 25922, Salmonella enterica ATCC 04059, Pseudomonas aeruginosa ATCC 15442, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 02021).
Double-Layer Agar Method
Different media (including dilute versions) were used to control the predation property of B. bacteriovorus HD100 before co-culture and biofilm studies. When 40 µL and 50 µL of predator (at the 0.5 McFarland turbidity) were inoculated onto DNA medium, the fastest predation was determined in terms of time. There was no significant difference between the two volumes. It was also determined that growth was detected in the presence of HEPES, which was used without adding ions. As the addition of ions did not make a difference in terms of time DNA medium and ion-free HEPES solution duo was used in all B. bacteriovorus production stages.
Effect of Predator Bacteria on Planktonic Cells
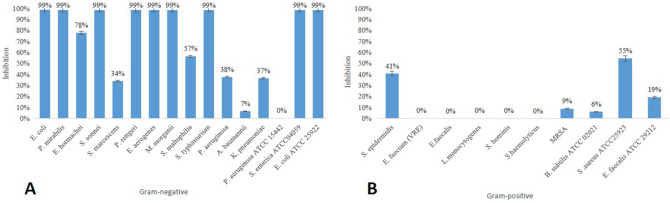
As a result of the experiments, it was found that the inhibitions vary depending on the type of bacteria. Although it is highly effective on Gram-negative isolates, it has not been determined to be effective on Pseudomonas aeruginosa ATCC 15442 (Fig. 1a). In Gram-positive isolates, inhibition of MRSA and Staphylococcus epidermidis isolates has also been identified (Fig. 1b).
Fig. 1.
Decrease in planktonic cells after predation. After the B. bacteriovorus is incubated with clinical isolate, the reduction of the bacterium compared to the control group is considered to be predation. The figure shows the inhibition rate of Gram negative pathogens and Gram positive pathogens as CFU
Biofilm Removal Assay
A reduction in biofilms of all Gram-positive clinical isolates after treatment with predator bacteria has been determined. Among the Gram-negative isolates, the highest removal was found on Morganella morganii, Stenotrophomonas maltophilia, Acinetobacter baumannii, and Escherichia coli. The effect on the isolates is shown in Table 1.
Table 1.
Change in biofilm after B. bacteriovorus predation
| Gram-negative | Gram-positive | ||
|---|---|---|---|
| Prey | Biofilm removal | Prey | Biofilm removal |
| E. coli | (+) | E. faecium (VRE) | (+) |
| E. coli ATCC 25922 | (+) | E. faecalis | (+) |
| E. hormaechei | (+) | E. faecalis ATCC 29212 | (+) |
| S. marcescens | (−) | L. monocytogenes | (+) |
| E. aerogenes | (+) | S. hominis | (+) |
| M. morganii | (+) | S. haemolyticus | (+) |
| K. pneumoniae | (−) | S. epidermidis | (+) |
| P. mirabilis | (+) | MRSA | (+) |
| S. sonnei | (−) | S. aureus ATCC 25923 | (+) |
| P. rettgeri | (+) | B. subtilis ATCC 02021 | (−) |
| S. typhimurium | (−) | ||
| S. enterica ATCC 04059 | (−) | ||
| S. maltophilia | (+) | ||
| P. aeruginosa | (−) | ||
| P. aeruginosa ATCC 15442 | (+) | ||
(+): Predation by B. bacteriovorus, (−): No predation
SEM Experiment
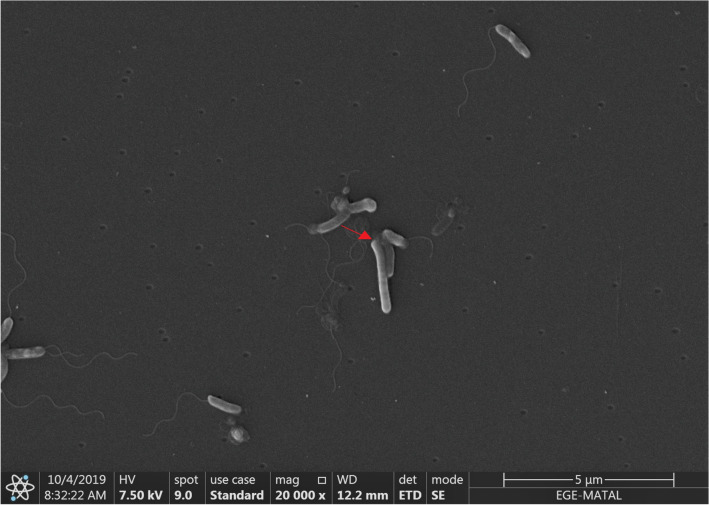
The image of the predatory bacterium was obtained in Scanning electron microscopy. Also, in Fig. 2, Bdellovibrio bacteriovorus, which is smaller and flagellated, appears to attach on to its host (Escherichia coli), which is much longer than itself.
Fig. 2.
Shows the interactions of Bdellovibrio bacteriovorus (flagellated and smaller bacillus) on the prey cell (E. coli). The arrow indicates the attachment site to the prey
Confirmation of the biofilm removal capacity of the predatory bacterium was determined by visualizing SEM. Bdellovibrio bacteriovorus inhibits the Proteus mirabilis and Listeria monocytogenes biofilms. SEM images of Proteus mirabilis and Listeria monocytogenes grown on sixteen-millimeter diameter round coverslips are shown in Fig. 3.
Fig. 3.
SEM images of antibiofilm activity. Bdellovibrio bacteriovorus reduce the number of surface-adhered P. mirabilis and L. monocytogenes biofilm cells. Shown are the SEM images of P. mirabilis (A–B) and L. monocytogenes (C–D) grown on sixteen-millimeter diameter round coverslips. Images A and C indicates the control which are not exposed to predatory bacteria, B and D shows the predatory exposed cells after 48 h of incubation
Discussion
Antimicrobial resistance is one of the most important problems of modern medicine. In addition to the deaths caused each year by this crisis, the decrease in the rate of discovery of new antibiotics will exacerbate the problem. Ever since its discovery in the sixties, the B. bacteriovorus and its ability to reduce bacterial populations appear to be an intriguing alternative [21]. First, by using the double-layer agar method, the most suitable medium, ion conditions and time for predation were optimized within the study.
Several supplemented media were used to reveal the best conditions; ion-free DNA media inoculated with B. bacteriovorus were found to be the most suitable, allowing the most rapidly inhibited bacterial reproduction. Although there are studies in the literature showing the contrary, it has also been found that the addition of ions does not result in a significant increase in predation [22–24]. Experiments were conducted based on the determined medium and ion contents in the continuation of the study.
Bdellovibrio bacteriovorus is known to be an obligate predator of Gram-negative bacteria. However, it is found that studies are usually conducted with a limited number of species; ESKAPE pathogens in particular. To the best of our knowledge, this study was the first to show the predation activity on Providencia rettgeri, Enterobacter aerogenes isolates. In addition to these, 98–99% decrease in the population of other Gram-negative clinical isolates such as Escherichia coli, Proteus mirabilis, Shigella sonnei, Salmonella typhimurium, and Morganella morganii were also determined. Although this is generally consistent with the literature, the most interesting observation emerging from our data was the Acinetobacter baumannii is slightly affected by predation [17]. Furthermore, no predation was found for P. aeruginosa ATCC 15442. Since different studies of these pathogens have reached contradictory conclusions [25, 26], it is difficult to speculate about the reasons for this host specificity; however, it is clearly not correlated with the resistance pattern of the isolate or the genus of the pathogen.
Taking into account the co-culture results of Gram-positives, it was unexpected to determine that B. bacteriovorus HD100 had a low lethal effect on Staphylococcus epidermidis and Staphylococcus aureus (MRSA). In a few studies, it has been proved that B. bacteriovorus may benefits from Staphylococcus aureus, in particularly nutrient deficient conditions [27, 28], yet, it has been determined for the first time that it can show efficacy on Staphylococcus epidermidis probably by the same mechanism.
As biofilm is related persistent infections, the interest to control this matrix has increased, it was reported that B. bacteriovorus caused a reduction in biofilm mass in some of the Gram-positive isolates such as Staphylococcus aureus [29]. However, it was discovered for the first time in this study that it could show a similar effect presumably via enzymatic activity of serine proteases, in other Gram-positive pathogens such as Listeria monocytogenes, and Enterococcus species. When the situation in gram negatives is examined, unexpectedly found to be ineffective for some of the isolates such as; Shigella sonnei, Salmonella typhimurium, Klebsiella pneumoniae, Pseudomonas aeruginosa and Salmonella enterica ATCC. The reason for this selectivity is unknown, although cases of the ineffectiveness of the predator bacteria against some Gram-negative biofilms have been also reported before [25]. However, there is a possibility that this situation may be related to the architecture of the biofilm structures of prey bacteria, as has been reported in Vibrio cholera [30].
This study has some limitations. Although the inhibitory effect of B. bacterivorus on various pathogens has been identified, the main mechanisms that cause host specificity have not yet been explained. Furthermore, it was not investigated whether resistance to predation had been observed. There is still much to be clarified; it must be fully understood the mechanisms involved in predatory behaviour and the influence of environmental conditions such as temperatures and pH, and the impact on the host's immune system in case of in vivo applications. Despite these important points, predatory bacteria can contribute to resolving the antibiotic crisis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Part of this study was presented “at the 30th European Congress on Clinical Microbiology and Infectious Diseases (ECCMID)” with the number Abstract 4598.
Funding
The Ege University Scientific Research Projects (BAP) Commission with Grant No: TYL-2019-20489 financially supported this study.
Declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Barriere SL. Clinical, economic and societal impact of antibiotic resistance. Expert Opin Pharmacother. 2015;16:151–153. doi: 10.1517/14656566.2015.983077. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36:1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- 4.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;10:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- 6.Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharani S, Kim DH, Shanks RMQ, Doi Y, Kadouri DE. Susceptibility of colistin-resistant pathogens to predatory bacteria. Res Microbiol. 2018;169:52–55. doi: 10.1016/j.resmic.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shatzkes K, Tang C, Singleton E, Shukla S, Zuena M, Gupta S, Dharani S, Rinaggio J, Connell ND, Kadouri DE. Effect of predatory bacteria on the gut bacterial microbiota in rats. Sci Rep. 2007;7:43483. doi: 10.1038/srep43483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan MC, Gillette RK, Maglasang MA, Corn EA, Tai AK, Lazinski DW, Shanks RMQ, Kadouri DE, Camilli A. High-throughput analysis of gene function in the bacterial predator Bdellovibrio bacteriovorus. MBio. 2019;10:e01040. doi: 10.1128/mBio.01040-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negus D, Moore C, Baker M, Raghunathan D, Tyson J, Sockett RE. Predator versus pathogen: how does predatory Bdellovibrio bacteriovorus interface with the challenges of killing Gram-negative pathogens in a host setting? Annu Rev Microbiol. 2017;8:441–457. doi: 10.1146/annurev-micro-090816-093618. [DOI] [PubMed] [Google Scholar]
- 11.Williams HN, Chen H. Environmental regulation of the distribution and ecology of Bdellovibrio and like organisms. Front Microbiol. 2020;11:545070. doi: 10.3389/fmicb.2020.545070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 13.Iebba V, Totino V, Santangelo F, Gagliardi A, Ciotoli L, Virga A, Ambrosi C, Pompili M, De Biase RV, Selan L, Artini M, Pantanella F, Mura F, Passariello C, Nicoletti M, Nencioni L, Trancassini M, Quattrucci S, Schippa S. Bdellovibrio bacteriovorus directly attacks Pseudomonas aeruginosa and Staphylococcus aureus Cystic fibrosis isolates. Front Microbiol. 2014;5:280. doi: 10.3389/fmicb.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnappa AK, Dwidar M, Mitchell RJ. Application of bacterial predation to mitigate recombinant bacterial populations and their DNA. Soil Biol Biochem. 2013;57:427–435. doi: 10.1016/j.soilbio.2012.09.010. [DOI] [Google Scholar]
- 15.Seidler RJ, Starr MP. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969;100:769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Armesto MR, Prieto M, García-López ML, Otero A, Moreno B. Modern microbiological methods for foods: colony count and direct count methods. A review. Microbiologia. 1993;9:1–13. [PubMed] [Google Scholar]
- 17.Sun Y, Ye J, Hou Y, Chen H, Cao J, Zhou T. Predation efficacy of Bdellovibrio bacteriovorus on multidrug-resistant clinical pathogens and their corresponding biofilms. Jpn Infect Dis. 2017;70:485–489. doi: 10.7883/yoken.JJID.2016.405. [DOI] [PubMed] [Google Scholar]
- 18.Hou W, Sun X, Wang Z, Zhang Y. Biofilm-forming capacity of Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa from ocular infections. Investig Ophthalmol Vis Sci. 2012;53:5624–5631. doi: 10.1167/iovs.11-9114. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Bumunang EW, Stanford K, Bie X, Niu YD, Mcallister TA. Biofilm formation by Shiga toxin-Producing Escherichia coli on stainless steel coupons as affected by temperature and incubation time. Microorganisms. 2019;7:95. doi: 10.3390/microorganisms7040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okajima Y, Kobayakawa S, Tsuji A, Tochikubo T. Biofilm formation by Staphylococcus epidermidis on intraocular lens material. Invest Ophthalmol Vis Sci. 2006;47:2971–2975. doi: 10.1167/iovs.05-1172. [DOI] [PubMed] [Google Scholar]
- 21.MacGowan A, Macnaughton E. Antibiotic resistance. Medicine. 2017;45:622–628. doi: 10.1016/j.mpmed.2017.07.006. [DOI] [Google Scholar]
- 22.Kadouri D, O'Toole GA. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol. 2005;71:4044–4051. doi: 10.1128/AEM.71.7.4044-4051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina AA, Kadouri DE. Biofilm formation of Bdellovibrio bacteriovorus host-independent derivatives. Res Microbiol. 2009;160:224–231. doi: 10.1016/j.resmic.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Van Essche M, Quirynen M, Sliepen I, Van Eldere J, Teughels W. Bdellovibrio bacteriovorus attacks Aggregatibacter actinomycetemcomitans. J Dent Res. 2009;88:182–186. doi: 10.1177/0022034508329693. [DOI] [PubMed] [Google Scholar]
- 25.Dashiff A, Junka RA, Libera M, Kadouri DE. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol. 2011;110:431–444. doi: 10.1111/j.1365-2672.2010.04900.x. [DOI] [PubMed] [Google Scholar]
- 26.Bonfiglio G, Neroni B, Radocchia G, Marazzato M, Pantanella F, Schippa S. Insight into the possible use of the predator Bdellovibrio bacteriovorus as a probiotic. Nutrients. 2020;12:2252. doi: 10.3390/nu12082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantanella F, Iebba V, Mura F, Dini L, Totino V, Neroni B, Bonfiglio G, Maria T, Passariello C, Schippa S. Behaviour of Bdellovibrio bacteriovorus in the presence of Gram-positive Staphylococcus aureus. New Microbiol. 2018;41:145–152. [PubMed] [Google Scholar]
- 28.Im H, Dwidar M, Mitchell RJ. Bdellovibrio bacteriovorus HD100, a predator of Gram-negative bacteria, benefits energetically from Staphylococcus aureus biofilms without predation. ISME J. 2018;12:2090–2095. doi: 10.1038/s41396-018-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnappa A, Dwidar M, Seo JK, Hur JH, Mitchell RJ. Bdellovibrio bacteriovorus inhibits Staphylococcus aureus biofilm formation and invasion into human epithelial cells. Sci Rep. 2014;4:3811. doi: 10.1038/srep03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wucher BR, Elsayed M, Adelman JS, Kadouri DE, Nadell CD. Bacterial predation transforms the landscape and community assembly of biofilms. Curr Biol. 2021;31:2643–2651. doi: 10.1016/j.cub.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.