Abstract
There is a symbiotic relationship between gut microbiota and human beings. Imbalance of the gut microbiota will cause pathological damages to humans. Although many risk factors are associated with missed abortion (MA), the pathological mechanism of it is still unclear. Here, we analyzed gut flora of the patients with MA by S16 high-throughput sequencing. The possible pathogenic mechanisms of the MA were explored. Fecal samples from 14 healthy controls and 16 MA patients were collected to do 16S rRNA gene high-throughput sequencing analysis. The abundance of the Bacteroidetes, Proteobacteria, Actinobacteria, Escherichia, Streptococcus_ Salivarius, and Lactobacillus was significantly reduced in the MA group, while, the abundance of the Klebsiella was significantly increased in the MA patients. The Ruminococcaceae and [Eubacterium]_coprostanoligenes_group were found only in the specimens of the MA patients. The Fabrotax function prediction analysis showed that four photosynthesis function bacteria (cyanobateria, oxygenic_photoautotrophy, photoautotrophy, and phototrophy) only existed in the MA group. In the analysis of the BugBase microbiome function prediction, the Escherichia of the MA group is significantly reduced compared to that of the healthy controls in the items of that Contains_Mobile_Elements, Facultatively_Anaerobic, Forms_Biofilms, Potentially_Pathogenic.png, Gram_Nagative, and Stress_Tolerant_relabundance. These alterations may affect the stability of the host's immune, neural, metabolic and other systems by interfering with the balance of the gut microbiota or by the metabolites of those bacteria, causing the MA. This study explored the possible pathogenic factors of the gut microbiota of the MA. The results provide evidence to figure out the pathogenesis of the MA.
Keywords: Missed abortion, Gut microbiota, High-throughput sequencing, 16S rRNA
Introduction
Missed abortion (MA) is defined as a spontaneous death of the embryo or fetus [1, 2]. MA has a great impact on pregnant women and her families. It may cause anxiety, depression or stress in the relevant personnel. Many who experienced MA have a sad process [3]. About 80% of the MA occurs in the first three months of pregnancy [1, 4]. The risk of the MA within five to 20 weeks is 11% to 22%. The MA rate increases with the age of both parents [5, 6]. The MA rate was 11%, increased from 9% at the age of 22 to 84% at the age of 48 in Denmark [7]. The etiological cause of about half of the MA involves chromosomal abnormalities. Although scientists believe there are many risk factors, however, not all of them can be determined [8–10]. Therefore, it is still a challenge for scientists to clarify the pathological mechanism of the MA.
Gut flora is the bacteria living in the human digestive tract [11, 12]. The gut flora has a wide range of effects and perform many useful functions, such as fermenting unused energy substrates, training the immune system through metabolic end products (such as propionate and acetate, preventing the growth of harmful species), maintaining the intestinal epithelium, synthesizing vitamins (such as biotin and vitamin K) for the host, and producing hormones to guide the host to store fat, metabolize dietary and pharmaceutical compounds, control immune function and even influence behavior through gut-brain-axis. The relationship between gut flora and humans is not only symbiotic (harmless coexistence), but also reciprocal. Some human gut florae benefit the host by fermenting dietary fiber into short chain fatty acids (SCFAs) [13–15]. It has been proven that the imbalance of the gut flora will bring many diseases to humans. For example, ulcerative colitis [16], allergy, asthma and diabetes [14, 17], cirrhosis, nonalcoholic fatty liver [18], obesity [19], and even increase the risk of cancer [20]. Bacteria constitute the majority of the flora in the colon, accounting for 60% of the dry weight of feces [20]. It makes feces as an ideal source of gut flora for any test and experiment. Through nucleic acids extraction from fecal samples and 16S rRNA gene sequencing, the gut bacteriome information could be easily obtained. This method is also generally preferable to the invasive techniques such as biopsy.
About 15% of recurrent abortion is related to immune factors [21]. Autoimmune disorder is a risk factor for MA. Abnormal immune status will influence the developing fetus, resulting in abnormal embryos, leading to MA [22, 23]. On the other hand, the gut flora also has a profound impact on the host's immune system. Gut-brain-axis is a biochemical signal that occurs between the gastrointestinal tract and the central nervous system [24]. Gut flora can affect the host's neuroimmune system via the gut-brain-axis [24, 25]. Gut flora also plays a direct role in defending against pathogens, secreting cytokines to initiate an inflammatory response against infection by utilizing all available nutrients [26]. In this study, we analyzed gut flora of the patients with MA by S16 high-throughput sequence analysis. The possible pathogenic mechanisms of the MA were explored from the analysis of the differential gut flora between the patients with MA and healthy controls (EPs). The results provided a proof in gut microflora factors for the study of the pathogenic mechanism of MA.
Materials and Methods
Patients and Sample Collection
In this study, from June to September of 2021, 16 hospitalized early pregnant patients with the MA (average age: 29.07 ± 4.428 years old) were recruited in our hospital. 10–20 g of their fresh feces on the day of diagnosis were collected in a sterilized 50 ml covered test tube from them, kept in a − 80 °C freezer until it was submitted for gut flora detection. During the same period, the feces of 14 normal pregnant women (average age: 28.75 ± 4.235 years old) were also collected as EPs. Inclusion and diagnosis criteria of the MA were the patients whose pregnacy period were within three months and who matched the MA diagnosis of the Society of Radiologists in Ultrasound in America [27, 28]. Exclusion criteria of MA were those who had hereditary diseases, diabetes, infection, tumor, malnutrition, thyroid disease, or autoimmune diseases, who recently used antibiotics or other drugs, or who has ovarian and uterine malformations, uterine fibroids, or obesity. This study was approved by the Ethic Committee of the First Hospital of Putian (No. 2022-025). All patients and healthy controls signed the informed consents.
Gut Flora Detection
All the gut flora were detected by Biomarker (Beijing, China). The specific primer with barcode was synthesized according to the full-length primer sequence. PCR amplification was carried out using the primers with barcode. The product was purified, quantified and homogenized to form corresponding sequencing library (Single Molecule Real Time Bell), and sequenced by PacBio Sequel RS II (Pacific Biosciences, Menlo Park, CA, USA). Cutadapt method [29] was applied to recognize the primer sequences. Trimmatic means [30] was employed to filter the CodeCharge Studio (CCS) documents. USEARCH [31] and UCHIME [32] measures were used to remove chimeras. Finally high-quality sequences were obtained for subsequent analysis.
Operational Taxonomic Unit (OTU)/Amplification Sequence Variants (ASVs) Analysis
The sequences were clustered below the level 97% similarity using USEARCH to filter out the OTUs. The DADA2 [33] method in QIIME2 [34] was used to denoise the data after quality control. The reserved total sequences number was equal and above 0.005% Features (i.e. OTU) of the total number of sequences.
Diversity Analysis
Alpha diversity indexes of the gut flora of the MA and the EP groups were analyzed and evaluated by QIIME2 (https://qiime2.org/) to get indexes of Chao 1 richness estimator, ACE richness estimator, Shannon Wiener diversity index, Simpson diversity index, and PD_whole_tree, respectively. The beta diversity analysis was processed with binary Jaccard, Bray Curtis and (UN) weighted UniFrac algorithms to present the species diversity matrix. R language platform was used to prepare Principal component analysis (PCA), principal coordinate analysis (PCoA) and correlation analysis between environmental factors and sample composition (RDA/CCA).
Species Annotation and Taxonomic Analysis
The feature sequence was taxonomically annotated with Silva as the reference database, and naive Bayes classifier combined with alignment method. The species classification information corresponding to each feature and the composition of each sample colony at each level (phylum, class, order, family, genus, species) were obtained. QIIME software was applied to generate species abundance tables at different classification levels. Following that, the R language tool was used to draw the community structure map of the samples at various taxonomic levels.
Analysis of Significance of Difference Between Groups
Significant difference analysis between groups, which can be called biomarkers analysis, at genus and species levels were performed by Metastats analysis, ANOVA and Willcox rank sum test.
Correlation Analysis
Network graph correlation analysis was employed to perform Spearman rank correlation analysis. Screen data with correlation greater than 0.1 and p value less than 0.05 were used to build up a correlation network according to the abundance and change of each genus or species in each sample. The genus correlation network diagram was also drawn based on Python.
Based on the fact that the accumulation of metabolites of the microbiota may cause changes in the host microenvironment, thereby affecting the physiological and pathological activities of the host, it is necessary to study the correlation between gut flora and host metabolomic functions. On account of the analysis of network diagram, the coexistence relationship of species in environmental samples can be obtained, and the interaction and important mode information of species in the same environment can be obtained, further explaining the formation mechanism of phenotypic differences between samples.
Function Prediction Analysis
BugBase was applied to predict the biological level coverage of functional pathways within the complex microbiome and the biologically interpretable phenotypes. Kyoto Encyclopedia of Genes and Genomes (KEGG) and FAPROTAX [35] were also employed to predict metabolic pathways and ecological relevant functions.
BugBase first normalizes OTUs by predicting 16S copy numbers, and then predicts microbial phenotypes using the provided precomputed files. First, for each sample in the biological dataset, the relative abundance of traits was estimated in the whole range of coverage thresholds (0 to 1, in increments of 0.01). Then, BugBase selects the coverage threshold with the highest variance in all samples for each feature in the data. After setting the threshold, BugBase will generate the final organism level trait prediction table, which contains the predicted trait relative abundance of each sample. The predicted phenotype types included gram positive, gram negative, biofilm forming, genetic, mobile element containing, oxygen utility, and oxidative stress tolerance.
Results
OTU Analysis of the S16 Sequences from the Gut Flora of Both the MA and the EP
After 30 samples were sequenced by S16 high-throughput sequence and identified by Barcode, 389045 circular consensus sequencing (CCS) sequences were obtained. Each sample generated at least 12725 CCS sequences, with an average of 12968 CCS sequences.
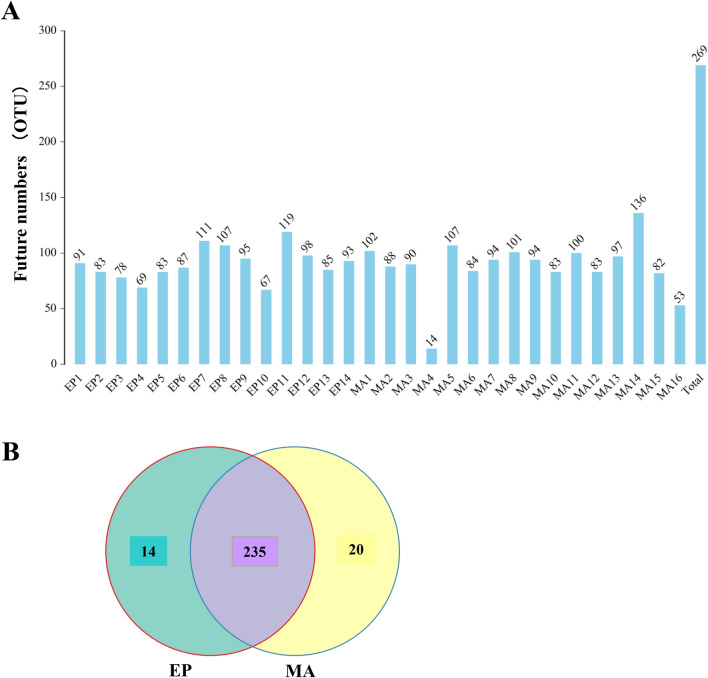
Through S16 high-throughput sequence analysis, the similarity among different sequences was calculated and compared, and the fragments with 97% similarity were clustered to form different OTU. The OTU numbers found in each MA sample and EP sample were presented in Fig. 1A, respectively. In totally 269 OTUs were found among these samples. Amongst them, 235 OTUs were shared by both the MAs and EPs. 14 OTUs were exclusively owned by healthy controls and 20 OTUs were specifically shared by MAs (Fig. 1B).
Fig. 1.
OTU analysis of the gut flora in the missed abortion (MA) samples and healthy controls (EP). A The OTU numbers in each MA and EP sample were indicated on top of the indicated bars. Total 269 OTU numbers found from these samples were showed in the right bar. B Among the 269 OTUs, 235 were shared by them (MAs and Eps). 14 were exclusively owned by Eps. 20 OTUs were specifically occupied by Mas only
Species Annotation and Taxonomic Analysis
SILVA (https://www.arb-silva.de/) (Silva.138 version) is a comprehensive database containing rRNA gene sequences of three domain microorganisms (bacteria, archaea and eukaryotes). In order to annotate the obtained OTU data, through comparing to the SILVA database, the feature taxonomic sequence of the OTUs were annotated with the naive Bayes classifier method to obtain the species classification information corresponding to each feature. Thus, the community composition of each sample at various levels (phylum, class, order, family, genus, species) was gained. Subsequently, QIIME software was employed to generate species abundance tables at different taxonomic levels. The R language tools were used to draw community structure maps of samples at various taxonomic levels.
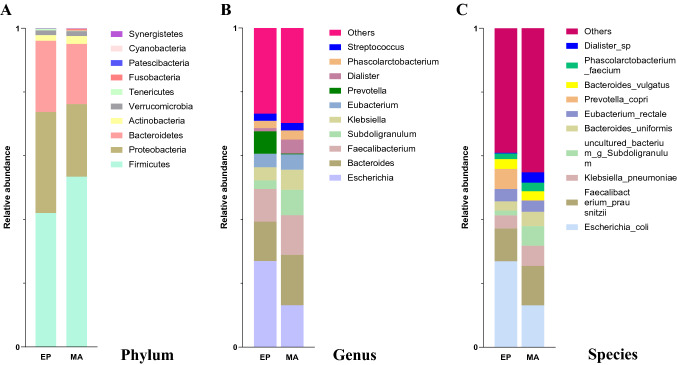
At phylum level, comparing to the EP group, the Firmicutes were comparatively enriched in the MA group, whereas the Proteobacteria and Bacteroidetes were relative enriched in the EP group (Fig. 2A). Based on the suggestion of Turnbaugh et al. [36] that the elevation of the Firmicutes/Bacteroidetes ratio is applied as a sign of pathological conditions. In our data, the ratio of the MA group was 2.83 (0.534198/0.188764) which is obviously higher than 1.883 (0.420116/0.223096) of the EP group, demonstrated that there was a pathological alteration in the MA patients.
Fig. 2.
Species annotation and taxonomic analysis at genus and specie levels. The distribution of bacterial abundance at the top ten genera (Fig. 1A) and species (Fig. 1B) were presented as indicated colors in the missed abortion (MA) group and health controls (EP)
Since the bacterial taxonomic at genus and species levels are the most abundant, in order to facilitate coherent analysis, we mainly focused on analyzing the alterations of the bacteria at the level of genus and species. In the distribution of bacterial abundance at the level of the top ten genera (Fig. 2B), compared with the EP, the relative abundance of Escherichia, Subdoligranulum, Prevotella, and Dialister in the MA group were remarkably decreased, while, the abundances of Bacteroides and Klebsiella were significantly increased in the MA group.
In the top ten distributions of bacterial abundance at specie level (Fig. 2C), compared with the EP group, the abundances of Prevotella_copri and Escherichia_coli were significantly lower, however, the abundances of Klebsiella_pneumoniae, uncultured_bacterium_g_Subdoligranulum, Bacteroides_uniformis, and Dialister_sp were obviously greater in the MA group.
Biological Diversity Analysis
Alpha-diversity indexes analysis of Shannon, Simpson, ACE, Chao1, and PD_ehole_tree did not showed any significant differences between the MA and EP groups (p > 0.05) (Table 1).
Table 1.
Alpha-index analysis between the missed abortion (MA) and health control (EP) groups (u: index; n = mean ± SE)
| Indexes | EP | MA | p value |
|---|---|---|---|
| Shannon | 3.639 ± 0.256 | 3.816 ± 0.218 | 0.6008 |
| Simpson | 0.799 ± 0.0467 | 0.8429 ± 0.0293 | 0.4203 |
| ACE | 110.6143 ± 3.6937 | 104.1973 ± 6.839 | 0.4348 |
| Chaol | 106.4329 ± 3.3567 | 103.7741 ± 7.878 | 0.7699 |
| PD whole tree | 7.9487 ± 0.2365 | 7.614 ± 0.4446 | 0.5289 |
Beta-diversity indexes analysis of Principal Component Analysis, Principal coordinates analysis, Non-MetricMulti-Dimensional Scaling, Unweighted Pair-group Method with Arithmetic Mean, combining UPGMA clustering tree with histogram, Sample thermographic analysis, and PERMANOVA/Anosim analysis did not showed any obvious difference between the groups as well (p > 0.05)(data did not show).
Analysis of Significance of Difference Between Groups
The ANOVA and Wilcox rank sum tests revealed that the abundance of the Synechococcus by ANOVA analysis at genus level and the Synechococcus_ruvescers by Wilcox rank sum test at species level are only found in the MA group. However, the abundance of the Acinetobacter by Wilcox rank sum test at genus level and the Prevotella_copri by ANOVA analysis, the Acinetobacter_lwoffii and the Synechococcus_salivarius by Wilcox rank sum test at species level were significantly lower in the MA group compared to those in the EP group (p < 0.05) (Table 2).
Table 2.
Analysis of significance of difference between groups at genus and species levels (u: relative abundance; n = mean ± SE)
| Analysis | Taxonomy | Bacteria | CTL | MA | p value |
|---|---|---|---|---|---|
| ANOVA | Genus | Synechococcus | 0 ± 0 | 0.000544 ± 0.000964 | 0.044321 |
| Wilcox rank sum | Genus | Acinetobacter | 0.001725 ± 0.004180 | 0.000272 ± 0.000282 | 0.019915 |
| ANOVA | Species | Prevotellacopri | 0.074121 ± 0.133686 | 0.001147 ± 0.003524 | 0.037164 |
| Wilcox rank sum | Species | Acinetobacterlwo ffii | 0.001525 ± 0.003893 | 0.000148 ± 0.000311 | 0.033998 |
| Wilcox rank sum | Species | Streptococcussalivarius | 0.013012 ± 0.021539 | 0.002925 ± 0.005856 | 0.019915 |
| Wilcox rank sum | Species | Synechococcusrubesce ns | 0 ± 0 | 0.000544 ± 0.000964 | 0.00882 |
The Metastats analysis at genus level indicated that the Brochothrix, the Chryseobacterium, the Dielma, and the Mitsuokella only existed in the EP group. The Ruminococcaceae_UCG_008, the Alloprevotella, the uncultured_bacterium_o_Bacteroidales, and the Anaerotignum were only found in the MA group. The abundances of the[Eubacterium] _coprostanoligenes_group, the Brevundimonas, the Prevotella, the [Eubacterium] _ruminantium_group, and the Lactobacillus were clearly lower in the MA group (p < 0.05). However, the abundances of the Gemella and the Lachnospiraceae_ND3007_group were significant higher in the MA group (p < 0.05) (Table 3).
Table 3.
Metastats analysis of abundance difference between groups at genus level (u: relative abundance; n = mean ± SE)
| Bacteria | CTL | MA | p value | q value |
|---|---|---|---|---|
| Ruminococcaceae_UCG-008 | 0 ± 0 | 0.000104 ± 0.000104 | 0.000113 | 0.012 |
| Alloprevotella | 0 ± 0 | 0.000103 ± 0.000103 | 0.00021 | 0.012 |
| Uncultured_bacterium_o_Bacteroidales | 0 ± 0 | 0.000096 ± 0.00001 | 0.000393 | 0.012 |
| Anaerotignum | 0 ± 0 | 0.00022 ± 0.00022 | 0.000999 | 0.012 |
| Brochothrix | 0.00025 ± 0.000241 | 0 ± 0 | 0.000999 | 0.012 |
| Chryseobacterium | 0.000629 ± 0.000629 | 0 ± 0 | 0.000999 | 0.012 |
| Dielma | 0.000191 ± 0.000191 | 0 ± 0 | 0.000999 | 0.012 |
| Mitsuokella | 0.00549 ± 0.000549 | 0 ± 0 | 0.000999 | 0.012 |
| Synechococcus | 0 ± 0 | 0.000544 ± 0.000241 | 0.000999 | 0.012 |
| Uncultured_bacterium_c_Bacteroidia | 0 ± 0 | 0.000194 ± 0.000194 | 0.000999 | 0.012 |
| [Eubacterium]_coprostanoligenes_group | 0.0157 ± 0.00721 | 0.00213 ± 0.000787 | 0.017 | 0.171 |
| Brevundimonas | 0.000317 ± 0.000184 | 0.000009 ± 0.000001 | 0.018 | 0.171 |
| Prevotella | 0.0823 ± 0.0381 | 0.00228 ± 0.00148 | 0.019 | 0.171 |
| Gemella | 0.000007 ± 0.000007 | 0.000169 ± 0.000104 | 0.02 | 0.171 |
| [Eubacterium]_ruminantium_group | 0.0017 ± 0.000919 | 0.000036 ± 0.000028 | 0.023 | 0.18 |
| Lactobacillus | 0.000718 ± 0.000381 | 0.000047 ± 0.000028 | 0.024 | 0.18 |
| Lachnospiraceae_ND3007_group | 0.000031 ± 0.000023 | 0.000181 ± 0.000088 | 0.046 | 0.324 |
The Metastats analysis at species level suggested that the Brochothrix_thermosphacta, the Chryseobacterium_haifense, the Dielma_fastidiosa, the Lactobacillus_iners, the Mitsuokella_sp, the Mobilibacterium_massiliense, and the Paraprevotella_xylaniphila were only observed in the EP group. The Intestinibacillus_massiliensis, the Fusobacterium_varium, the uncultured_bacterium_g_Alloprevotella, the uncultured_bacterium_o_Bacteroidales, and the Anaerotignum_lactatifermentans only existed in the MA group. The abundances of the Prevotella_copri, the uncultured_bacterium_g_[Eubacterium]_coprostanoligenes_group, the Brevundimonas_mediterranea, the uncultured_bacterium_g_[Eubacterium]_ruminantium_group, and the Acinetobacter_lwoffii were notably lower in the MA group compared to those in the EP group (p < 0.05). The abundances of the Eubacterium_sulci, the Alistipes_finegoldii, the Gemella_sanguinis, the Eubacterium_ramulus, and the uncultured_bacterium_g_Lachnospiraceae_ND3007_group were specifically higher in the MA group compared to those in the EP group (p < 0.05) (Table 4).
Table 4.
Metastats analysis of abundance difference between groups at species level (u: relative abundance; n = mean ± SE)
| Bacteria | CTL | MA | p value | q value |
|---|---|---|---|---|
| Intestinibacillus_rnassiliensis | 0 ± 0 | 0.000104 ± 0.000104 | 0.000113 | 0.0134 |
| Fus obacteriumvarium | 0 ± 0 | 0.000113 ± 0.000102 | 0.00021 | 0.0134 |
| Uncultured_bacterium_g_Alloprevotella | 0 ± 0 | 0.000103 ± 0.000103 | 0.00021 | 0.0134 |
| Uncultured_bacterium_o_Bacteroidales | 0 ± 0 | 0.000096 ± 0.000096 | 0.000393 | 0.0134 |
| Anaerotignum_lactatifermentans | 0 ± 0 | 0.00022 ± 0.00022 | 0.000999 | 0.0134 |
| Brochothrixthermosphacta | 0.00025 ± 0.000241 | 0 ± 0 | 0.000999 | 0.0134 |
| Chryseobacteriumhaifense | 0.000629 ± 0.000629 | 0 ± 0 | 0.000999 | 0.0134 |
| Dielmafastidios a | 0.000191 ± 0.000191 | 0 ± 0 | 0.000999 | 0.0134 |
| Lactobacillus_iners | 0.000372 ± 0.000372 | 0 ± 0 | 0.000999 | 0.0134 |
| Mitsuokella_sp | 0.00549 ± 0.00549 | 0 ± 0 | 0.000999 | 0.0134 |
| Mobilibacterium_mas siliens e | 0.000109 ± 0.000109 | 0 ± 0 | 0.000999 | 0.0134 |
| P araprevotellaxylaniphila | 0.00023 ± 0.00023 | 0 ± 0 | 0.000999 | 0.0134 |
| Prevotella ruminicola | 0 ± 0 | 0.000192 ± 0.000192 | 0.000999 | 0.0134 |
| Synechococcus_rubescens | 0 ± 0 | 0.000544 ± 0.00241 | 0.000999 | 0.0134 |
| Uncultured_bacterium_c_Bacteroidia | 0 ± 0 | 0.000194 ± 0.000194 | 0.000999 | 0.0134 |
| Uncultured_bacterium_g_Dialister | 0 ± 0 | 0.00124 ± 0.00124 | 0.000999 | 0.0134 |
| Eubacterium_sulci | 0.000015 ± 0.000015 | 0.000105 ± 0.000064 | 0.00976 | 0.123 |
| Alistipes_finegoldii | 0.000309 ± 0.000134 | 0.00255 ± 0.00115 | 0.016 | 0.18 |
| Prevotella_copri | 0.0741 ± 0.0357 | 0.00115 ± 0.000881 | 0.016 | 0.18 |
| Uncultured_bacterium_g_[Eubacterium]_coprostanoligenes_group | 0.0157 ± 0.00721 | 0.00213 ± 0.000787 | 0.017 | 0.182 |
| Brevundimonas_mediterranea | 0.000317 ± 0.000184 | 0.000009 ± 0.000009 | 0.018 | 0.183 |
| Gemella_sanguinis | 0.000007 ± 0.000007 | 0.000169 ± 0.000104 | 0.02 | 0.194 |
| Eubacterium_ramulus | 0.000097 ± 0.000036 | 0.00116 ± 0.000665 | 0.023 | 0.205 |
| Uncultured_bacterium_g_[Eubacterium]_ruminantium_group | 0.0017 ± | 0.000036 ± 0.000028 | 0.023 | 0.205 |
| Acinetobacter_lwoffii | 0.00153 ± | 0.000148 ± 0.000078 | 0.046 | 0.378 |
| Uncultured_bacterium_g_Lachnospiraceae_ND3007_group | 0.000031 ± 0.000024 | 0.000181 ± 0.000088 | 0.046 | 0.3789 |
Correlation Network Analysis at Genus Level
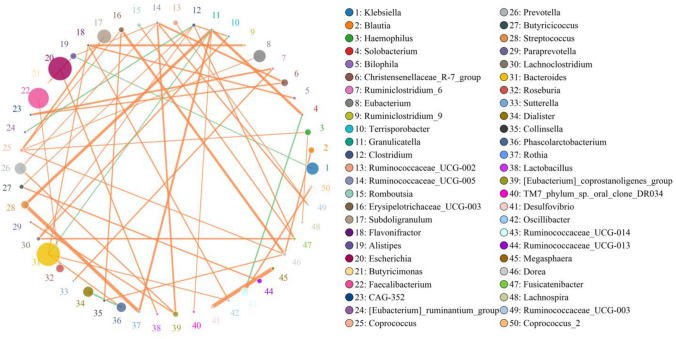
According to the abundance and change of each species in each sample, Spearman rank correlation analysis was conducted and data with correlation greater than 0.1 and p < 0.05 were screened to build a correlation network. The coexistence relationship of the species in environmental samples can be obtained. The interaction and important pattern information of the species in the same environment can be obtained to further explain the formation mechanism of phenotypic differences between samples. The highest correlation network data of the top 50 genera are summarized in Table 5(A) and (B). Most of them have positive correlation except for Klebsiella vs Alistipes, Dialister vs Phascolarctobacterium, and Clostridium vs [Eubacterium] _ruminantium_ group (Table 5).
Table 5.
The highest correlation network data of the top 50 genera (p < 0.05)
| Source | Target | Weight | Correlation |
|---|---|---|---|
| (A) | |||
| Escherichia | Lactobacillus | 0.5936 | Positive |
| Bacteroides | Phascolarctobacterium | 0.5862 | Positive |
| Bacteroides | Oscillibacter | 0.5702 | Positive |
| Bacteroides | Flavonifractor | 0.5737 | Positive |
| Faecalibacterium | Roseburia | 0.558 | Positive |
| Subdoligranulum | Coprococcus | 0.6197 | Positive |
| Eubacterium | Coprococcus | 0.5528 | Positive |
| Klebsiella | Alistipes | 0.5611 | Negative |
| Prevotella | [Eubacterium]_coprostanoligenes_group | 0.5945 | Positive |
| Prevotella | Ruminococcaceae_UCG-005 | 0.5572 | Positive |
| Dialister | Phascolarctobacterium | 0.6155 | Negative |
| Streptococcus | Rothia | 0.7849 | Positive |
| Streptococcus | Granulicatella | 0.7262 | Positive |
| Ruminococcaceae_UCG-014 | Coprococcus_2 | 0.6123 | Positive |
| Christensenellaceae_R-7_group | Ruminococcaceae_UCG-005 | 0.646 | Positive |
| Christensenellaceae_R-7_group | CAG-352 | 0.5965 | Positive |
| Haemophilus | Coprococcus | 0.5616 | Positive |
| Haemophilus | Lachnospira | 0.5527 | Positive |
| Alistipes | Ruminococcaceae_UCG-005 | 0.554 | Positive |
| Alistipes | Butyricimonas | 0.5772 | Positive |
| Blautia | Ruminococcaceae_UCG-013 | 0.5496 | Positive |
| Blautia | Romboutsia | 0.5472 | Positive |
| Blautia | Dorea | 0.5994 | Positive |
| [Eubacterium]_coprostanoligenes_group | Paraprevotella | 0.5994 | Positive |
| Ruminococcaceae_UCG-002 | Ruminococcaceae_UCG-003 | 0.5636 | Positive |
| (B) | |||
| Erysipelotrichaceae_UCG-003 | Coprococcus | 0.5615 | Positive |
| Erysipelotrichaceae_UCG-003 | Collinsella | 0.5941 | Positive |
| Ruminococcac eae_UC G- 013 | Butyricicoccus | 0.6009 | Positive |
| Butyricicoccus | Clostridium | 0.5576 | Positive |
| Coprococcus | Dorea | 0.5727 | Positive |
| Lachnoclostridium | Clostridium | 0.5581 | Positive |
| Lachnoclostridium | Fusicatenibacter | 0.6119 | Positive |
| Lachnoclostridium | Terrisporobacter | 0.6023 | Positive |
| Romboutsia | Fusicatenibacter | 0.5901 | Positive |
| Dorea | Collinsella | 0.5835 | Positive |
| Clostridium | Fusicatenibacter | 0.5875 | Positive |
| Clostridium | [Eubacterium]_ruminantium_group | 0.5864 | Negative |
| Clostridium | Terrisporobacter | 0.5963 | Positive |
| Megasphaera | Desulfo vibrio | 0.8301 | Positive |
| Megasphaera | Coprococcus_2 | 0.546 | Positive |
| Oscillibacter | Flavonifractor | 0.5888 | Positive |
| Fusicatenibacter | Flavonifractor | 0.5525 | Positive |
| Ruminococcac eae_UC G-005 | Ruminococcac eae_UC G- 003 | 0.6037 | Positive |
| Veillonella | Rothia | 0.5527 | Positive |
| Ruminiclostridium 6 | CAG-352 | 0.6372 | Positive |
| Ruminiclostridium 6 | Sutterella | 0.596 | Positive |
| Rothia | Granulicatella | 0.6639 | Positive |
| Flavonifractor | Ruminiclostridium 9 | 0.6545 | Positive |
| TM7_phylum_sp._or al_clo ne_DR034 | Granulicatella | 0.5576 | Positive |
| Granulicatella | Solobacterium | 0.708 | Positive |
The top 50 genera correlation network diagram with the highest correlation based on Python was prepared in Fig. 3.
Fig. 3.
Top 50 genera correlation network based on Python analysis. The top 50 genera correlation network diagram with the highest correlation was analyzed by python. Each genus was indicated with different colors. The circle size of the genus represents the abundance of the genus. The thickness of the lines between the genera reflects the correlation strengthen
Prediction and Analysis of Functional Genes at Genus Level
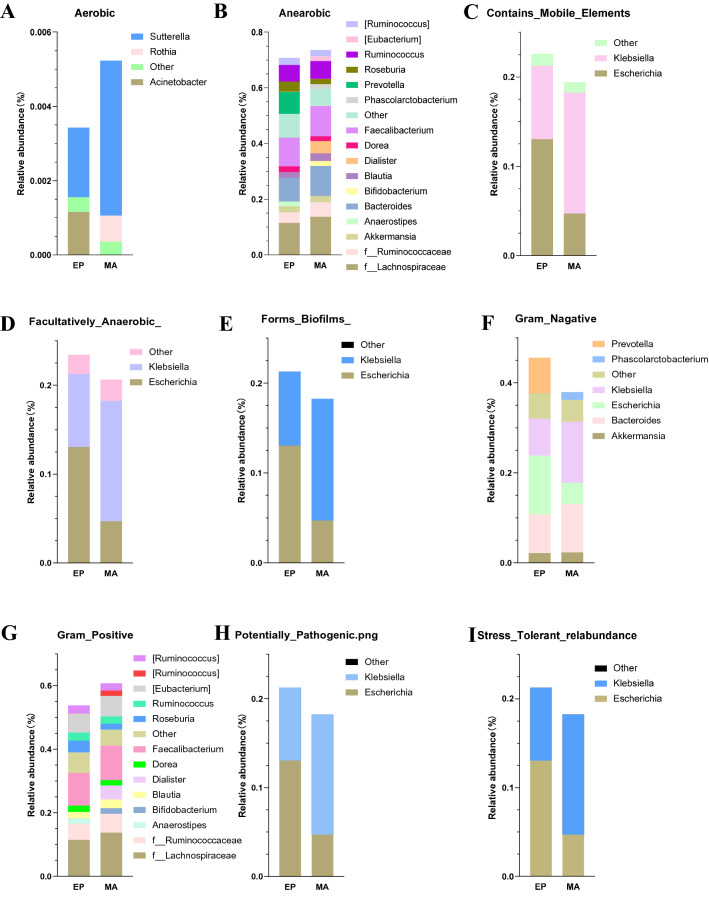
BugBase is a method to predict the biological functional pathways in complex microbiomes and the biologically interpretable phenotype. BugBase selectively carries out automatic hypothesis testing and visualization for different traits, and generates nine phenotypes (Aerobic, Anearobic, Contains_Mobile_Elements, Facultatively_Anaerobic_, Forms_Biofilms_, Gram_Nagative, Gram_Postitive, Potentially_ Pathogenic.png, and Stree_Tolerant_relabundance) to describe the relative abundance of groups with characteristic traits (Fig. 4).
Fig. 4.
BugBase microbiome functional pathway analysis at genus level. The possible functional pathways of the gut microbiota in nine phenotypes (A Aerobic, B Anearobic, C Contains_Mobile_Elements, D Facultatively_Anaerobic_, E Forms_Biofilms_, F Gram_Nagative, G Gram_Postitive, H Potentially_ Pathogenic.png, and I Stree_Tolerant_relabundance) were analyzed by BugBase in the MA and EP groups. Different colors represent the different functions. The size of the colored bar indicates the abundance of the genus
Further analysis of the KEGG metabolic pathway showed that the metabolic efficiency of the cell processes (i.e. cell mobility) in the MA group was significantly lower than that in the EP group (p < 0.05) (Table 6).
Table 6.
Significant difference in functional gene prediction between the groups by Faprotax functional prediction (mean ± SE)
| Function | EP (%) | MA (%) | p values |
|---|---|---|---|
| Cyanobacteria | 0 ± 0 | 0.014994 ± 0.026697 | 0.046020773 |
| Oxygenic_p hotoautotrop hy | 0 ± 0 | 0.014994 ± 0.026697 | 0.046020773 |
| Photoautotrophy | 0 ± 0 | 0.014994 ± 0.026697 | 0.046020773 |
| phototrophy | 0 ± 0 | 0.014994 ± 0.026697 | 0.046020773 |
Predictive analysis of functional genes with significant difference by Faprotax suggested that the cyanobacteria, the oxygenic_ photoautotrophy, the photoautrophy, and the phototrophy were only found in MA group (Table 6).
Discussion
Consisting with trillions of symbiotic microflorae, the gut microbiota provides the essential materials to maintain the host’s health [37, 38]. In our analyzed data, Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, Verrucomicrobia, Tenericutes, and Fusobacteria were the major dominant phyla, which consists of more than 90% of the total microbial population (Fig. 2A), being consistent with the findings by Jethwani and Grover [39]. Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria are the four major phyla in human adults. The Bacteroidetes and the Firmicutes possess the highest proportion, occupying ~ 48% or ~ 51%, respectively. The Proteobacteria and the Actinobacteria are of relatively lower proportions (1%) [40]. Our results revealed that the proportion of the Bacteroidetes, the Proteobacteria, and the Actinobacteria in the MA group are 19%, 22%, and 2.46%, respectively, being relatively lower than those in the EP group. However, the relative ratio of the Firmicutes (53%) of the MA group was relatively higher than that of the EP group. (Fig. 2A) The Firmicutes/Bacteroidetes ratio is suggested to be an indicator of a pathological conditions [36]. In this study, the Firmicutes/Bacteroidetes ratio was relatively high in the MA group (2.83) compared to the EP group (1.883). The tendence of these alterations are similar to Liu's findings, although the exact ratios are not the same [41]. Firmicutes, Bacteroidetes and some anaerobic gut microorganisms can metabolize indigestible carbohydrates such as hemicellulose, cellulose, pectin, resistant starch, oligosaccharides and lignin into short chain fatty acids (SCFA), such as acetic acid, propionic acid and butyric acid [42]. The interference of the SCFA biosynthesis can cause many pathological consequences to the host [43]. Thus, the higher proportion of the Firmicutes (53%) may participate in the pathogenesis of the MA. The abnormal increase of the Firmicutes has been proven to be related to diabetes and obesity [44, 45]. In fact, obesity and diabetes are both the risk factors for recurrent abortion [46]. Both the observation in this experiment and Liu's study [41] clearly showed the abnormal elevation of the Firmicutes in the MA patients. Its related pathological mechanism needs further study. On the other hand, both the EP and MA groups exhibited higher rate of the Proteobacteria (31–22%) in our observation, which might be caused by different geography and eating habits between the Spain (< ~ 1%) and Chinese [40].
In the analysis results of the top ten microbiotas at both the genus and species levels, Escherichia (or Escherichia_coli) were significantly lower in the MA group (Fig. 2B, C). Although some Escherichia_coli can cause certain diseases, most of them are harmless bacteria, which can produce vitamin K2 [47] and prevent the colonization of pathogenic bacteria in the intestine, to benefit the host and to have a reciprocal relationship [48, 49]. Vitamin K2 is an essential factor for blood coagulation [50]. Disorder of coagulation is considered as one of the causes of recurrent abortion. Therefore, the abundance reduction of the Escherichia_coli may affect the synthesis of vitamin K2 to interfere with blood coagulation function, thus inducing the MA.
Bacteroides species usually constitute the most important part of the gut microbiota of mammals. Studies have shown that long-term diet is closely related to intestinal microbiome composition. Those who eat a lot of protein and animal fat are mainly Bacteroides, while those who eat more carbohydrates predominate with Prevotella [51]. In the MA cases of this study, the abundance of the Bacteroides (Bacteroides_uniformis) is higher than that of the EP group, while the abundance of the Prevotella (Prevotella_copri) is lower than that of the control group (Fig. 2B, C). This result suggests that the population of the MA group prefers a diet of large amounts of protein and animal fat. Besten et al.have shown that the Prevotella_copri improves insulin-sensitivity [52]. A meta-analysis indicates that insulin resistance is correlated to the susceptibility to MA, it may cause the recurrent miscarriages [53]. Thus, the decline of the Prevotella (Prevotella_copri) may participate the pathogenic process of the MA.
Klebsiella usually exists in human gastrointestinal tract as normal flora. However, it is also a human pathogen. Klebsiella can cause many diseases including pneumonia, sepsis, urinary tract infection, meningitis, peritonitis, diarrhea, and soft tissue infection. Most human Klebsiella infections are caused by Klebsiella_pneumoniae [54, 55]. The distribution of the top ten species and genera showed that the abundance of the Klebsiella (genus) and Klebsiella_pneumoniae (species) are significantly greater in the MA patients (Fig. 2B, C), suggesting that the Klebsiella (Klebsiella_pneumoniae) may play a role in the pathological mechanism of the MA.
In the difference analysis between the MA and EP groups, it also showed that the abundance of the Synechococcus (genus) and Synechococcus_rubescens (species) were significantly higher in the MA patients than those in the healthy controls at the genus and species level (Table 2). Up until now, there is no report on the pathology of the Synechococcus (Synechococcus_rubescens). Whether they play a role in the pathological mechanism of the MA remains to be seen. It is interesting to note that Acinetobacter and Acinetobacter_lwoffii are the pathogen of hospital acquired pneumonia, wound infection, bacteremia and meningitis [56]. However, they are significantly reduced in the MA patients (Table 2). Streptococcus_ Salivarius was found significantly lower in the MA patients compared to that in the EP group (Table 2). It belongs to a probiotic and can produce antibacterial peptide (bacteroid inhibitor), which can inhibit the growth of Streptococcus pyogenes [57]. It is worth noting that rheumatic heart disease is an autoimmune disease caused by Streptococcus pyogenes. Autoimmune disorder is also considered as a risk factor for the MA [22]. Whether the reduction of the Streptococcus_salivarius abundance can induce an autoimmune disorder, resulting in the MA is a subject worthy of further study.
Metastats analysis showed that the Lactobacillus in the MA patients were significantly lower at genus level (Table 3) and absent at species level (Lactobacillus_iners; Table 4). The Lactobacillus has been reported to regulate L-22 to maintain the host-microorganism homeostasis on the surface of intestinal mucosa and intestinal barrier function [58], prevent intestinal inflammation [59], and elevate intestinal IgA production [60]. Inflammation is considered to be one of the causes of the MA. Therefore, the reduction or deletion of the lactobacillus may also be one of the causes of the MA.
Our data shows that there was no significant difference between MA group and EP group in either alpha- or beta-diversity analysis. The results were inconsistent with those of Liu et al. [41]. They detected the gut flora in 41 MA patients and 19 healthy controls and found that the alpha-diversity of the MA group is significantly lower than those of the control group, indicating that the abundance and evenness of the gut flora in the MA patients are low. Whether this difference is caused by different geographical regions (Shanghai vs Putian in China), or different ages (35y vs 29y) remains to be studied.
We note that the highest correlation analysis results at the genus level showed that the Ruminococcaceae_UCG-008 exhibited a significant positive correlation with the coprococcus (Table 5(A) and Fig. 3). While the Ruminococcaceae was not detectable in the gut flora of the normal controls (EP), however, it was found in the specimens of the MA patients (Table 3). Omura et al. [61] found a significant increase in the abundance of intestinal coprococcus in 35-days mice after infection with Theiler's murine encephalomyelitis virus, accompanied with increased expression of T cell receptor (TCR), IgG, IgA, various complements and major histocompatibility complex (MHC). The authors suggest that these changes are related to the damage to the nervous system caused by Theiler's murine encephalomyelitis virus infection. Therefore, the unique Ruminococcaceae of the MA is beneficial to the growth of coprococcus, which may interfere with normal fetal growth to induce MA via affecting the expression of TCR, IgG, IgA, various complements and MHC.
The highest correlation analysis results at the genus level also revealed that the [Eubacterium]_coprostanoligenes_group has significant positive correlation with the Paraprevotella (Table 5(A) and Fig. 3). Interestingly, the [Eubacterium]_coprostanoligenes_group was decreased in the specimens of the MA patients (Table 3). The decreased abundance of the [Eubacterium]_coprostanoligenes_group is not conducive to the growth of Paraprevotella, which may reduce the anti-infection ability of the Paraprevotella to pathogenic viruses. In addition, decrease of the IgA protection will also reduce the host’s immune function. All of these could be the inducing factors of the MA. Interestingly, the decrease of the Prevotella (Table 3) also accelerated the reduction of the [Eubacterium]_coprostanoligenes_group because they were positive correction (Table 5(A)), therefore, further declining the anti-infection ability in the MA patients.
It is worth noting that in the Fabrotax function prediction analysis of the difference between the two groups, only four bacteria (cyanobateria, oxygenic_photoautotrophy, photoautotrophy, and phototrophy) related to photosynthesis function existed in the MA group (Table 6). It suggests that the presence of the photosynthetic bacteria seems to be closely related to the MA. Although some cyanobaterias themselves contain substances of high biological value: such as polyunsaturated fatty acids, amino acids, proteins, pigments, antioxidants, vitamins and minerals [62]. However, some cyanobaterias can also produce cyanotoxins (including neurotoxins, cyclotoxins, endotoxins, and hepatotoxins), which are toxic to humans [63, 64]. Since the research concerning to human health and oxygenic_photoautotrophy, photoautotrophy, or phototrophy is almost inexitent, no comment can be made. However, this finding should be further studied to determine whether they play an important role in the pathological process of the MA.
In the microbial phenotype analysis of the BugBase microbiome function prediction, in terms of the Contains_Mobile_Elements, Facultatively_Anaerobic, Forms_Biofilms, Potentially_Pathogenic.png, Gram_Nagative, and Stress_Tolerant_relabundance, the Escherichia in the MA group is significantly less than that in the EP group (Fig. 4C–F, H, I). The Escherichia is considered to be related to the imbalance of female lower genital tract microbiota [65].
The limitation of this study is not able to verify the speculative pathogenic mechanism of the MA in vivo or in vitro experiments. In fact, these works need to be done in the future by multiple institutes in biochemistry, microbiology, animal model, and even clinical studies.
Conclusion
This study reveals that there are many differences in the gut microbiota in multiple aspects between the MA and healthy controls. The proportion of the Bacteroidetes, Proteobacteria, Actinobacteria, Escherichia, Streptococcus_ Salivarius, and Lactobacillus were significantly lower in the MA group. While, the abundance of the Klebsiella and Klebsiella_pneumoniae were significantly higher in the MA patients. The Ruminococcaceae and [Eubacterium]_coprostanoligenes_group were found only in the specimens of the MA patients. The Fabrotax function prediction analysis showed that four photosynthesis function bacteria (cyanobateria, oxygenic_photoautotrophy, photoautotrophy, and phototrophy) only existed in the MA group. In the analysis of the BugBase microbiome function prediction, the Escherichia of the MA group is significantly lower compared to that of the EP group in the items of the Contains_Mobile_Elements, Facultatively_Anaerobic, Forms_Biofilms, Potentially_Pathogenic.png, Gram_Nagative, and Stress_Tolerant_relabundance. These alterations may affect the stability of the host's immune, neural, metabolic and other systems by interfering with the balance of the gut microbiota or by the metabolites of those bacteria, causing the MA.
Acknowledgements
We appreciate to all doctors and nurses who were involved in the project.
Abbreviations
- ASV
Amplicon sequence variant
- CCS
Circular consensus sequencing
- EP
Healthy controls
- FAPROTAX
Functional Annotation of Prokaryotic Taxa
- HPA
Hypothalamus pituitary adrenal axis
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MA
Missed abortion
- MHC
Major histocompatibility complex
- OTU
Operational taxonomic unit
- SCFA
Short chain fatty acids
- TCR
T cell receptor
Author Contributions
Y-C, X-C, and T-Z designed the study. Y-C, P–C, X-C, L-P collected samples and clinical data of the MA patients and the healthy control. H–H prepared the samples and send them to Biomarker for gut flora detection. T-Z and Y-C wrote the manuscript.
Funding
This study is supported by Putian Science and Technology Plan Project (No. 2060499) for Y. C. and Fujian Provincial Health Research Talents Training Project (2019-ZQNB-18) for L. H., Fujian Provincial Natural Science Foundation Project (2020J01920) for L. H., and Putian City Science and Technology Program Project (2020SP004) for L. H.
Data Availability
All data could be obtained by contacting to the communication author.
Declarations
Conflict of interest
All author declares that there is no any commercial interest in this study.
Ethics Approval
This study was approved by the Ethics Committee of the First Hospital of Putian City (No. 2022-025).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Chen, Email: kch66@126.com.
Xianqian Chen, Email: cxq3000@126.com.
Tang Zhu, Email: birdbsl500@gmail.com.
References
- 1.Wilkins LW (2012) The Johns Hopkins manual of gynecology and obstetrics, 4th edn. I. 978-1451148015
- 2.Farquharson RG, et al. Updated and revised nomenclature for description of early pregnancy events. Hum Reprod. 2005;20:3008–3011. doi: 10.1093/humrep/dei167. [DOI] [PubMed] [Google Scholar]
- 3.Hunter A, Tussis L, MacBeth A. The presence of anxiety, depression and stress in women and their partners during pregnancies following perinatal loss: a meta-analysis. J Affect Disord. 2017;223:153–164. doi: 10.1016/j.jad.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 4.How many people are affected by or at risk for pregnancy loss or miscarriage? (2013)
- 5.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 6.Kleinhaus K, et al. Paternal age and spontaneous abortion. Obstet Gynecol. 2006;108:369–377. doi: 10.1097/01.AOG.0000224606.26514.3a. [DOI] [PubMed] [Google Scholar]
- 7.Nybo Andersen AM, et al. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaiman D. Genetic regulation of recurrent spontaneous abortion in humans. Biomed J. 2015;38:11–24. doi: 10.4103/2319-4170.133777. [DOI] [PubMed] [Google Scholar]
- 9.Ali O, et al. Term pegnancy on septate uterus: report of a case and review of the literature. Pan Afr Med J. 2015;22:219. doi: 10.11604/pamj.2015.22.219.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tersigni C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update. 2014;20:582–593. doi: 10.1093/humupd/dmu007. [DOI] [PubMed] [Google Scholar]
- 11.Moszak M, Szulinska M, Bogdanski P (2020) You are what you eat-the relationship between diet, microbiota, and metabolic disorders-a review. Nutrients 12:1096 [DOI] [PMC free article] [PubMed]
- 12.Engel P, Moran NA. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 13.Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y) 2013;9:560–569. [PMC free article] [PubMed] [Google Scholar]
- 14.Shen S, Wong CH. Bugging inflammation: role of the gut microbiota. Clin Transl Immunol. 2016;5:e72. doi: 10.1038/cti.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willey JM, Sherwood L, Woolverton C. Prescott's microbiology. 9. New York: McGraw Hill; 2013. [Google Scholar]
- 16.Saxena, R., Sharma VK (2016) Medical and health genomics. In: DKs. antonarakis (ed) A metagenomic insight into the human microbiome: its implications in health and disease. Elsevier Science
- 17.Gao Z, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691–1702. doi: 10.3748/wjg.v21.i6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 20.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman B (2012) Williams gynecology. New York
- 22.Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38:J266–J274. doi: 10.1016/j.jaut.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Gleicher N, Weghofer A, Barad D (2007) Female infertility due to abnormal autoimmunity: frequently overlooked and greatly underappreciated. Part II. Expert Rev Obstetr Gynecol 2(4):465–75.
- 24.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinan TG, Cryan JF. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care. 2015;18:552–558. doi: 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 26.Yoon MY, Lee K, Yoon SS. Protective role of gut commensal microbes against intestinal infections. J Microbiol. 2014;52:983–989. doi: 10.1007/s12275-014-4655-2. [DOI] [PubMed] [Google Scholar]
- 27.Doubilet PM, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443–1451. doi: 10.1056/NEJMra1302417. [DOI] [PubMed] [Google Scholar]
- 28.Xiangyin G. Expert consensus on treatment of missed abortion in early pregnancy. China Pract J Gynecol Obstetr (Chinese) 2020;1:70–73. [Google Scholar]
- 29.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J Bioinform Action 17(1)
- 30.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 32.Edgar RC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan BJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansupa C, Wahdan SFM, et al. Can we use functional annotation of prokaryotic taxa (FAPROTAX) to assign the ecological functions of soil bacteria? Appl Sci. 2021;11:688. doi: 10.3390/app11020688. [DOI] [Google Scholar]
- 36.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 37.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Jethwani P, Grover K (2019) Gut microbiota in health and diseases—a review. Int J Curr Microbiol Appl Sci (IJCMAS) 8(8):1586–1599
- 40.Villaran RF, et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, et al. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes. 2021;7:24. doi: 10.1038/s41522-021-00199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 43.Perry RJ, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley RE, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317:355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 46.Oliver A, Overton C (2014) Diagnosis and management of miscarriage. Practitioner 258: 25–8, 3 [PubMed]
- 47.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hudault S, Guignot J, Servin AL. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut. 2001;49:47–55. doi: 10.1136/gut.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid G, Howard J, Gan BS. Can bacterial interference prevent infection? Trends Microbiol. 2001;9:424–428. doi: 10.1016/S0966-842X(01)02132-1. [DOI] [PubMed] [Google Scholar]
- 50.Efsa Panel on Dietetic Products N et al (2017) Dietary reference values for vitamin K. EFSA J 15(5):e04780 [DOI] [PMC free article] [PubMed]
- 51.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Besten G, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 53.Li ZL, et al. Association between recurrent miscarriages and insulin resistance: a meta analysis. Zhonghua Fu Chan Ke Za Zhi. 2012;47:915–919. [PubMed] [Google Scholar]
- 54.Ristuccia PA, Cunha BA. Klebsiella[J] Inf Control Hospital Epidem. 1984;5(7):343–347. doi: 10.1017/s0195941700060549. [DOI] [PubMed] [Google Scholar]
- 55.Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11(4):589–603 [DOI] [PMC free article] [PubMed]
- 56.Dent LL, et al. Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis. 2010;10:196. doi: 10.1186/1471-2334-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wescombe PA, et al. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 2009;4:819–835. doi: 10.2217/fmb.09.61. [DOI] [PubMed] [Google Scholar]
- 58.Zelante T, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Lamas B, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaikiri H, et al. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int J Food Sci Nutr. 2017;68:941–951. doi: 10.1080/09637486.2017.1318116. [DOI] [PubMed] [Google Scholar]
- 61.Omura S, et al. Bioinformatics analysis of gut microbiota and CNS transcriptome in virus-induced acute myelitis and chronic inflammatory demyelination; potential association of distinct bacteria with CNS IgA upregulation. Front Immunol. 2020;11:1138. doi: 10.3389/fimmu.2020.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christaki E, Florou-Paneri P, Bonos E. Microalgae: a novel ingredient in nutrition. Int J Food Sci Nutr. 2011;62:794–799. doi: 10.3109/09637486.2011.582460. [DOI] [PubMed] [Google Scholar]
- 63.Thebault L, Lesne J, Boutin JP. Cyanobacteria, their toxins and health risks. Med Trop (Mars) 1995;55:375–380. [PubMed] [Google Scholar]
- 64.Weirich CA, Miller TR. Freshwater harmful algal blooms: toxins and children's health. Curr Probl Pediatr Adolesc Health Care. 2014;44:2–24. doi: 10.1016/j.cppeds.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Bennett J. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia: Elsevier/Saunders; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data could be obtained by contacting to the communication author.