Abstract
In this study we established human vaginal epithelial cells (hVECs) in culture and evaluated their interaction with Trichomonas vaginalis parasites to complement previous studies using other cell types. Primary cultures of hVECs were established. Contaminating fibroblasts were separated from epithelial cells by differential trypsinization. Specific antibody staining revealed that over 92% of cells in hVEC monolayers were epithelial cells. T. vaginalis adhered to hVECs and produced severe cytotoxic effects resulting in obliteration of the monolayer within 24 h. Adherence and cytotoxicity were not observed when T. vaginalis was exposed to human vaginal fibroblasts or bovine vaginal epithelial cells. Likewise, the bovine parasite Tritrichomonas foetus had no cytotoxic effects on hVECs. We concluded that the interaction between T. vaginalis and hVECs is both cell specific (limited to epithelial cells and not vaginal fibroblasts) and species specific (limited to human vaginal cells and not bovine cells). Pretreatment of T. vaginalis with metronidazole or periodate abolished the adhesion of parasites to cell monolayers and the cytotoxic effect, suggesting involvement of carbohydrate-containing molecules in these processes. Different clinical isolates of T. vaginalis caused damage to cultured cells at different rates. Parasites separated from the vaginal cell monolayer by a permeable membrane did not produce a cytopathic effect, suggesting contact-dependent cytotoxicity.
Trichomonas vaginalis, a protozoan parasite, is the causative agent of trichomoniasis, the most common nonviral sexually transmitted disease (STD) in humans. The parasite has a worldwide distribution. An estimated 5 million to 10 million Americans and more than 170 million people worldwide are infected annually (16). In underdeveloped countries, the rates of trichomoniasis may vary between 17 and 47% (6, 37). In men, the infection is usually asymptomatic, although it may cause irritating urethritis or prostatitis. In women, the disease is associated with a wide spectrum of clinical signs ranging from a relatively asymptomatic state to severe vaginitis with a foul-smelling vaginal discharge (29).
Trichomoniasis, in addition to being a cause of serious discomfort to women, also has been associated with adverse pregnancy outcome, manifested by preterm rupture of membranes, preterm delivery, low-birth-weight infants (10, 27), infertility (20), cervical cancer (21, 24), and increase in the transmission of human immunodeficiency virus (12, 26). Newer information indicates that trichomoniasis should be taken more seriously, not only because of its prevalence but also because of its potential effect on the health of women and children.
The cellular mechanisms of pathogenesis of T. vaginalis are not well defined. Several advances have been made in understanding the interaction between T. vaginalis and host cells and in dissecting the steps in the invasion process (see review by Petrin et al. [29]). T. vaginalis adherence to host cells and damage by a contact-dependent mechanism has been reported (3, 4, 14, 25, 29). These studies, however, did not employ natural human target cells; instead, they utilized cell lines such as HeLa and HEp-2 epithelial cells, Madine-Darby canine kidney (MDCK) epithelial cells, and Chinese hamster ovary (CHO) cells. Both human and bovine trichomonads bind to these cells, and these systems have yielded valuable information. Their principal weakness, however, is lack of specificity. Alderete et al. (1) made an attempt to purify human vaginal epithelial cells (hVECs) from human vaginal swabs and studied the interaction between parasites and host cells. Recently, Fiori et al. (15, 16) reported the contact-dependent and contact-independent disruption of human erythrocytes by T. vaginalis. The critical step in establishing human trichomoniasis is interaction of T. vaginalis with human vaginal epithelial cells (hVECs). A thorough understanding of mechanisms of infection requires study of this process under defined conditions. This report describes the in vitro culture of hVECs and the study of the pathogenic effects exerted by T. vaginalis on these cells. (Preliminary studies on the cytotoxic effects of T. vaginalis on hVECs have been presented [35]).
MATERIALS AND METHODS
Culture of hVECs.
Vaginal tissue samples were obtained from patients undergoing benign gynecological surgery with informed consent. Subjects had had a normal Pap smear within a year of the procedure and had no evidence of any vaginal infection. The tissue was obtained from redundant vaginal mucosa excised to correct anterior or posterior vaginal wall prolapse. Immediately after surgery, tissue samples were placed in sterile Dulbecco's modified Eagle essential medium supplemented with penicillin and streptomycin and then transported on ice to the laboratory. Superficial vaginal tissue was carefully dissected into blocks approximately 0.5 mm in each dimension. Several such blocks were placed in a tissue culture flask and allowed to adhere for about 30 min before being covered with Williams complete medium (33, 34) supplemented with fetal bovine serum (10%), insulin, transferrin, selenium, epidermal growth factor, and antibiotic-antimycotic mixture. Flasks were incubated at 37°C in an atmosphere of 5% CO2 in humidified air. Cells (epithelial cells and fibroblasts) usually grew from the explants within 1 to 2 weeks. The two cell types typically exhibited different morphological characteristics, with the fibroblasts being spindle-shaped and the epithelial cells being more full-bodied. Once cells were approaching confluence (2 to 3 weeks), contaminating fibroblasts were removed by differential trypsinization.
The cultured cells were washed with calcium- and magnesium-free buffer and then exposed to 0.05% trypsin and 0.53 mM EDTA in calcium- and magnesium-free buffer. The cells were kept under microscopic observation while the fibroblasts rounded up and became detached. (The epithelial cells were insensitive to this concentration and duration of exposure to trypsin.) The flasks were then tapped to loosen the detached fibroblasts, which were removed by aspiration and discarded or cultured separately. The trypsin was inactivated by addition of serum-containing medium. This procedure was repeated if necessary to obtain a morphologically uniform cell population. The purity of cell preparations was determined by growing an aliquot of cells on glass slides. These cells were fixed in 95% cold ethanol (5°C) for 10 min and stained with a monoclonal antibody against cytokeratin (AE1/AE3; Boehringer Mannheim), diluted 1:100, and counterstained with AEC aminoethylcarbazole chromogen, which produced a red end product. The Histostain-SP staining kit used in this experiment was obtained from Zymed Laboratories. The presence of squamous epithelium in hVEC culture was confirmed by immunostaining with antibody C23, against human small proline-rich protein 1 (sPRP1) (5, 22, 36). The antibody was generated by Reen Wu (University of California, Davis) and was kindly obtained through S. P. Reddy (Johns Hopkins School of Public Health, Baltimore, Md.). Fibroblasts were identified by staining an aliquot of cells with a monoclonal antibody against vimentin (obtained from Dako), diluted 1:40, and treated with the same counterstain. Nonimmune mouse ascites fluid was used (at 1:40 and 1:100 dilutions) as a negative control.
Once the purity of cells had been established, the epithelial cells were subcultured in 24-well plates for experimentation. It took approximately 7 to 10 days for hVECs to become confluent (Fig. 1). The medium was changed twice a week. Epithelial cells isolated in this way were amenable to freezing and thawing via standard protocols. For adhesion studies, the confluent hVECs were equilibrated in incubation medium containing two parts of Williams complete medium (pH 7.2) and one part of Diamond's medium (W/D 2:1) for 15 min at 37°C (5% CO2) prior to the addition of parasites. This medium mixture was chosen because it supported both host cells and parasites in coincubation experiments in terms of minimizing pH changes and maintaining parasite motility.
FIG. 1.
Confluent culture of hVECs. (Phase-contrast photography, bar = 100 μm.)
Trichomonads.
T. vaginalis (BC strain) isolates were obtained recently at our clinical pathology laboratory as vaginal samples from a woman affected by trichomoniasis. Following axenization, parasites were cultured in Diamond's TYM (9) with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc.) at 37°C in screw-capped 50- or 100-ml serum bottles. Cultures were passaged every 24 h. This strain was very sensitive to metronidazole and was immobilized by 250 μg of the drug per ml. The metronidozole-resistant T. vaginalis strain CDC-85 (ATCC 50143) was also used in some experiments. A metronidazole concentration of 1,000 μg/ml was required to immobilize this strain. Besides these strains, seven other isolates were obtained recently from infected women. These isolates were from patients who ranged from asymptomatic to symptomatic. One had severe vaginitis. These clinical isolates were first subcultured in InPouch TV media (Biomed Diagnostic, Fullerton, Calif.) for 48 h and then transferred to our standard Diamond's medium supplemented with penicillin and streptomycin until cultures became axenic. After 3 to 4 days, clinical isolates were grown in Diamond's medium alone. Bacterial contamination was monitored by standard microbiological techniques. There was no evidence of contamination in the cultures used. A related bovine-pathogenic trichomonad, Tritrichomonas foetus (strain KV1), was also grown in Diamond's medium. The initial pHs were 6.2 for T. vaginalis and 7.2 for T. foetus, and the inoculum was 106 ml−1. Parasites were counted at 24 h (Coulter Counter), harvested in late log phase (24 h) by centrifugation (4,000 × g), and washed twice with cold phosphate-buffered saline (PBS; pH 7.2). The parasites were suspended in W/D 2:1.
Chemical treatment of T. vaginalis.
In some cases, PBS-washed parasites were treated with metronidazole (250 μg/ml for 5 min or, in the case of metronidazole-resistant strains, 1,000 μg/ml for 5 min) or periodate (5 and 10 mM in 50 mM sodium acetate buffer [pH 4.5] for 5 or 10 min) at room temperature. Toxic effects of drug on clinical isolates were initially evaluated by using variable concentrations (2 μg/ml to 10 mg/ml) and times (5 min to 24 h) under aerobic and anaerobic conditions as reported earlier (28). The specific time point and concentration were chosen to fit the experimental protocols used for this study. Under these conditions, parasites became immobile (>96%) but were not lysed and retained their cellular integrity, as visualized by phase-contrast microscopy. Their metabolic activity was measured using the CellTiter AQueous assay (see below). Chemically treated parasites were washed twice with PBS and once with incubation medium (W/D 2:1) before being suspended in incubation medium (W/D 2:1) and added to wells containing hVECs.
Microscopy.
Initial experiments relied on microscopic observation of the interaction between hVECs and parasites. The nature and extent of cell damage were assessed using an inverted phase-contrast microscope. For experiment 1, hVECs and human vaginal fibroblasts (hVFs) were cultured separately to confluence in 24-well culture plates. T. vaginalis parasites (approximately 4 × 106/well) were added to the confluent hVEC and hVF monolayers. At the same time, hVEC monolayers in a separate 24-well plate were incubated with the related bovine pathogen T. foetus at the same concentration. There were 12 replicates for this experiment, which was repeated twice. Data were recorded from 1 to 48 h.
In experiment 2, hVEC monolayers were incubated separately with T. vaginalis (4 × 106/well) or T. vaginalis parasites treated with metronidazole or periodate. In one set of experiments, wells were incubated for 30 min, washed three times with PBS, and then examined to determine parasite adhesion. The wells were reexamined after culture for 24 h to examine the effects of parasites on hVECs. The condition of cells throughout the incubation period was monitored by Nikon phase-contrast microscopy. There were 12 replicates for each experiment. In another set of experiments, cells were not washed at 30 min after addition of parasites but were cultured for 24 h before washing off nonadherent parasites, at which stage we assessed viability and integrity of hVECs.
In experiment 3, parasites were physically separated from the monolayers by placing them in a chamber with a permeable membrane (Transwell-COL collagen-coated membrane; 0.4-μm pore size) to test the hypothesis that the cytopathic effect of T. vaginalis on hVECs is contact dependent. Cell monolayer integrity was compared with monolayers without parasites and with parasites in direct contact with the cells. Approximately 2 × 106 T. vaginalis parasites/well were used in this experiment. An inverted phase-contrast microscope was used to evaluate contact-dependent cytotoxicity. There were four replicates for each experiment, which was repeated twice. Data were recorded from 3 to 48 h.
Cytotoxicity of hVECs mediated by T. vaginalis.
In addition to microscopic observation, we used three different quantitative assay methods to assess cytotoxicity of the parasites. One was the spectrophotometric cell enumeration by crystal violet uptake. Another was to assay the release of radioactivity from [3H]thymidine-labeled host cells, as reported earlier for T. vaginalis (3, 4). The third was use of a CellTiter 96 AQueous nonradioactive cell proliferation assay kit (Promega Corp., Madison, Wis.) as instructed by the manufacturer (Promega Technical Bulletin 169).
Spectrophotometric (crystal violet) assays.
For each experimental condition, hVECs in 24-well plates were equilibrated in W/D 2:1 medium for 15 min at 37°C (under 5% CO2) before the addition of parasites. Approximately 8 × 105 parasites were added to monolayers (5 × 105 cells) and incubated for 2 to 24 h. For control experiments, parasites were not added to the hVECs in the wells. At the end of the incubation periods, the cells were gently washed twice with warm PBS, and the remaining cells were fixed with 2% formaldehyde in PBS for 10 min. The wells were washed with PBS and stained with 0.13% crystal violet solubilized in ethanol-formaldehyde (2:1) as reported previously (4). The stained product was subsequently washed twice with distilled water and air dried. The stained cells were finally solubilized in 1% sodium dodecyl sulfate in 50% ethanol, and the intensity of staining was read at a wavelength of 570 nm. Each experiment was performed in quadruplicate, and the means of the data are presented. All measurements of experimental (E) samples were indexed to those of control (C) samples (E/C). Cytotoxity was defined as 1 − E/C.
Using this and the methods described below, we measured the cytotoxity of a related trichomonad T. foetus (8 × 105 parasites/5 × 105 hVECs/well) and T. vaginalis parasites (8 × 105) which were treated with metronidazole (250 μg/ml) or periodate (10 mM) before being added to the hVECs. We also compared the cytotoxicities of several different strains of T. vaginalis on identical cell cultures at 8 and 24 h. The clinical presentation of patients (from whom the new strains are acquired) with trichomoniasis ranged from asymptomatic carriers to symptomatic with greenish vaginal discharge to a severe case of vaginitis.
The CellTiter 96 AQueous assay.
Parasites (3 × 106) were added to confluent hVEC monolayers in 24-well plates and incubated for 4 and 22 h as described earlier. For control experiments, parasites were not added to the wells. At the end of incubation periods, the cells were gently washed four times with PBS. After washing, cells in wells were incubated with 0.4 ml of Williams medium and 80 μl of CellTiter 96 AQueous assay reagents (MTS and phenazine methosulfate solution) for 1 h at 37°C in a humidified 5% CO2 atmosphere. After 1 h, the absorbance was recorded at 490 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader. In some cases, wells were not washed with PBS, and the assay reagents were added directly to the wells (in order to measure released or detached products) followed by incubation for 1 h. In another control experiment, hVECs were subjected to three cycles of freezing and thawing to ensure the death of hVECs, and the viability of cells was measured quantitatively by this method. Data were expressed as mean absorbance values (optical density) derived from quadruplicate samples in three separate experiments. Cytotoxicity in this assay was calculated as 1 − E/C, where E/C is the ratio of absorbance of the formazan reading at 490 nm for experimental (E) versus control (C) samples.
Release of [3H] by host cells.
It has been reported that target cells labeled with radioactive DNA precursors released labeled DNA in the presence of pathogenic organisms, indicating that the microbe had damaged the membrane of host cells (4, 32). We used [3H]thymidine to label hVEC monolayers in order to assess the damage to hVECs caused by T. vaginalis. Confluent monolayers were labeled with [3H]thymidine (8 μCi/well; specific activity, 40 to 60 Ci/mmol; ICN) overnight. After gentle removal of media, wells were washed twice with warm W/D 2:1 medium prior to the addition of different numbers of parasites for the desired length of time. No parasites were added to control wells. After the experimental period, the incubation medium was collected and the release of 3H was determined by liquid scintillation counting. Each experiment was performed in quadruplicate; data means are presented.
Results are reported as means plus or minus standard errors of the mean. Differences between groups (chemical treatment of parasites, parasite concentration) in cytotoxicity were explored by one-way analysis of variance. Two-way analysis of variance was used to examine the effect of time and strain for parasites isolated from patients with symptomatic or asymptomatic trichomoniasis. Student-Neuman-Keuls post hoc test was used to illuminate differences between specific groups. Calculations were performed using commercially available statistical software (SigmaStat; Jandel Scientific, San Rafael, Calif.).
RESULTS
Specificity of T. vaginalis adherence to hVECs.
Specific staining revealed that over 92% of cells in hVEC monolayers were epithelial cells. In addition, the anti-sPRP1 antibody, a marker for small proline-rich sPRPs proteins expressed in squamous cells, reacted with hVECs, indicating that the cells in culture were squamous epithelial cells (5, 22, 36). This system was then used to evaluate the nature and specificity of parasite adherence to host target cells. T. vaginalis adhered to hVECs in much greater numbers than did T. foetus, indicating a species-specific host-parasite interaction. Incubation of T. foetus with hVECs for up to 48 h showed no clear adherence of parasites to cells; the parasites remained alive, and the monolayers were intact and viable. In contrast, incubation of T. vaginalis with hVEC resulted in adherence of parasites to the monolayer and disruption of the monolayer within 24 to 30 h, by which time no live parasites remained. Prolonged incubation of trays after the death of parasites was followed by reattachment and continued growth of only very few epithelial cells, indicating cell death or severe damage as opposed to simple detachment. T. vaginalis failed to adhere to hVFs (up to 48 h) and caused no conspicuous damage to hVF monolayers. We have also shown that T. vaginalis parasites failed to adhere to or disrupt bovine vaginal epithelial cells (bVECs); only T. foetus adhered to and disrupted BVEC monolayers (34). These results imply specific host-cell and host-parasite interactions. When T. vaginalis parasites were separated from direct contact with hVECs by means of a permeable collagen membrane (Transwell-COL), no damage to the monolayer was observed over a 48-h period. The parasites remained vigorously motile over this period.
To examine whether parasite surface glycoconjugates and metabolism of T. vaginalis were important for adherence of parasites to hVEC monolayers, the parasites were treated with metronidazole or periodate before exposure to hVECs. Viability of chemically treated parasites was examined by the CellTiter AQueous assay. Results showed that parasites retained >65% of their metabolic activity after metronidazole (1 mg/ml) treatment. Treatment of T. vaginalis with 10 mM periodate for 10 min resulted in <10% viability, while milder conditions (10 mM, 5 min; 5 mM, 5 or 10 min) resulted in viability of 50 to 60%. All of these treatments disabled the parasites' ability to destroy hVECs in 24 h. Under the same condition, untreated parasites extensively damaged hVECs. In one set of experiments, chemically treated and nontreated parasites were allowed to adhere for 30 min, washed to remove nonadherent parasites, and examined under phase-contrast microscopy. Chemical treatment with metronidazole or periodate dramatically reduced the number of adherent parasites. After 24 h, untreated T. vaginalis had completely destroyed the hVEC monolayer. In contrast, the monolayer was not damaged by metronidazole- or periodate-treated parasites. Although some metronidazole-treated parasites adhered to hVECs (<10%), they did not destroy host cells. This suggests that adherence of T. vaginalis is necessary but not sufficient to cause damage to VECs. Metronidazole does not seem to disturb the parasite membrane under the conditions used, suggesting that metabolic integrity of the parasite is important to produce a cytopathic effect on host cells. The periodate-treated parasites produced no damage to hVECs, and monolayers were intact and viable. The effect of periodate suggests that parasite surface glycoconjugates are involved in the mechanism of parasitism of host cells, during adhesion and possibly at other steps. This is based on the fact that periodate cleaves (oxidative cleavage) two or more OH or =O groups on adjacent carbon atoms. These structures are predominantly present in glycoconjugates. Trichomonads have been shown to contain a major cell surface glyconjugate, lipophosphoglycan, which is involved in adhesion of parasites to host cells (34; B. N. Singh, R. O. Gilbert, G. Hayes, and J. J. Lucas, unpublished data).
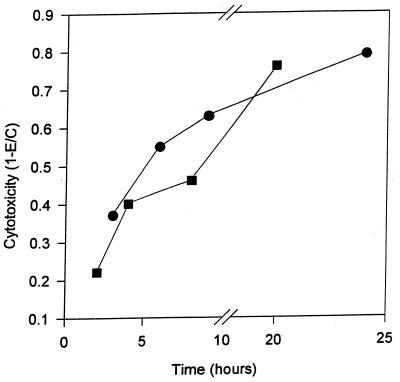
Having demonstrated severe disruption of hVEC monolayers by T. vaginalis, we sought to define this effect quantitatively. Initial studies used colorimetric enumeration of surviving epithelial cells. Figure 2 shows the kinetics of damage to hVECs caused by T. vaginalis. Cytotoxic effects are observed as early as 2 h after parasite exposure to host cells, and greater than 80% disruption occurs by 24 h. Complete destruction of monolayers occurred around 30 h as observed by microscopy. We also examined cytotoxicity using both the shorter incubation time and higher parasite densities in order to obviate significant multiplication of parasites during the experiments (2 to 24 h). Cytotoxicity increased as a function of time (P < 0.001). As shown in Fig. 3, increasing the parasite-to-host cell ratios (2:1, 4:1, and 10:1) increased the cytotoxic effects measured at 8 h (P < 0.001). This result implies that cytopathic effect is a function of T. vaginalis density.
FIG. 2.
Time course of cytotoxicity of hVEC monolayers by T. vaginalis (■, 2 to 20 h; ●, 3 to 24 h). Cytotoxicity was determined by crystal violet assay as described in the text. Each well contained 8 × 105 parasites and 5 × 105 hVECs.
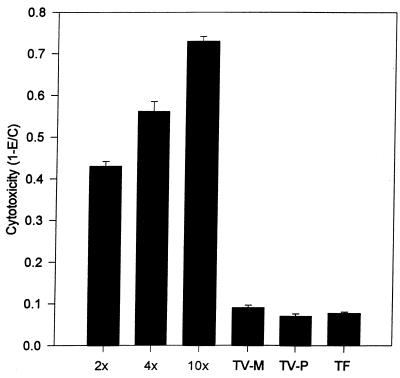
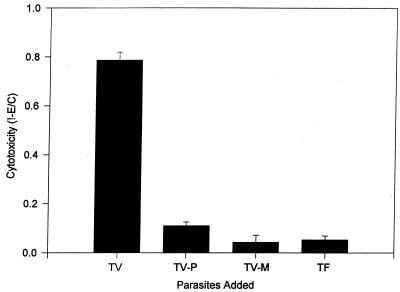
FIG. 3.
Comparison of cytotoxicity to hVEC monolayers in the presence of increasing ratios of T. vaginalis to host cells, T. vaginalis treated with periodate (TV-P) and metronidazole (TV-M), and the related bovine trichomonad T. foetus (TF). The ratios of parasites versus hVECs were 2:1 for TV-P, TV-M, and TF. The incubation time was 8 h.
The crystal violet assay was also used to study the effects of periodate and metronidazole treatment of T. vaginalis on cytotoxicity (Fig. 3). Parasites treated in these ways showed no cytotoxicity over the course of 6 to 24 h in a different set of experiments. However, microscopic examination showed that some metronidazole-treated parasites adhere to host cells, although no damage to these cells was observed. Periodate-treated T. vaginalis showed minimal adherence to host cells microscopically, suggesting involvement of carbohydrate-containing molecules in the adhesion process. In an interesting control experiment, incubation of the pathogenic bovine parasite T. foetus with hVECs showed no cytotoxic effects, indicating species-specific host-parasite interactions (Fig. 3). The chemically treated T. vaginalis parasites and the T. foetus caused essentially no measurable cytotoxicity (0.4% ± 0.8%), significantly different from the 51.2% ± 3.6% effected by untreated T. vaginalis (P < 0.001).
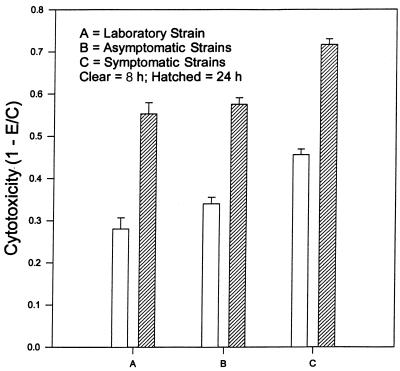
To evaluate whether the different clinical isolates obtained from asymptomatic to severe vaginitis patients produce different levels of cytotoxicity to hVECs, we used crystal violet to determine the cytopathic effects quantitatively at 8 and 24 h. The data are summarized in Fig. 4. Each experiment was performed in triplicate and repeated three times. The TV-UR1 isolate, obtained from a patient with severe vaginitis, and TV-UH2, TV-UH3, and TV-UH5, from symptomatic patients, were grouped together. They caused significantly more cell damage at both 3 and 24 h than isolates from asymptomatic patients (TV-UR3, TV-UR5, and TV-UH7). Strain TV30001 (obtained from M. Müller, Rockefeller University), which had been in culture for more than 8 months, produced less damage to VECs than the fresh isolates, a difference that was statistically significant only in comparison to isolates from symptomatic patients. The laboratory strain, asymptomatic group, and symptomatic group produced cytotoxicities of 28.0% ± 2.7%, 34.0% ± 1.5%, and 45.6% ± 1.3%, respectively, at 8 h. After 24 h, cytotoxicities were 55.3% ± 2.7%, 57.6% ± 1.5%, and 71.7% ± 1.3%, respectively. The effects of both time and group were significant (P < 0.001). The interaction term (time × group) was not significant (P = 0.58). Some of these isolates were also subjected to periodate treatment in order to evaluate their cytotoxic effect on hVECs. As expected, periodate-treated parasites produced no measurable cytotoxicity (P < 0.001).
FIG. 4.
Comparison of different levels of cytotoxicity to hVEC monolayers by various strains of T. vaginalis isolates at 8 and 24 h. Strain TV3001 had been in culture for more than 8 months, and the remainder of the isolates were obtained recently from a sexually transmitted disease clinic. UR1 was from a patient with severe vaginitis and is grouped with UH2, UH3, and UH5 from symptomatic patients (Symptomatic); UR3, UR5, and UH7 were from asymptomatic patients and are grouped together. Approximately 5 × 105 parasites were added to each well containing hVECs. Cytotoxicity was determined by crystal violet assay method as described in the text.
We also studied the release of 3H from [3H]thymidine-labeled hVECs incubated with T. vaginalis to demonstrate the kinetics of cell disruption caused by the parasite (data not shown). T. vaginalis in contact with radiolabeled hVECs produced appreciable levels of 3H release (1.14, 1.34, 3.06, and 9.1 times control level at 3, 6, 9, and 24 h, respectively) over a 24-h period. In control experiments, radiolabeled hVEC monolayers in the absence of T. vaginalis showed no release of radioactive material. An increased ratio of parasites to hVEC (2:1, 6:1, and 20:1) showed greater release of 3H (3.08, 6.55, and 7.52 times control levels, respectively; P < 0.001), consistent with our previous observations described above.
In addition to the above two assays, we used the Promega CellTiter AQueous assay to measure cytotoxicity and viability of hVECs in presence and absence of T. vaginalis parasites. This quantitative colorimetric method has been used to determine cytotoxicity, proliferation, or activation. The dehydrogenase enzymes present in metabolically active cells convert tetrazolium compound into soluble formazan. The results of this experiment are shown in Fig. 5. The coincubation of T. vaginalis with hVECs for 22 h resulted in damage to host cells of greater than 96%. The absorbance reading at 490 nm (0.287) was very close to the reading obtained from a freeze-thaw control, indicating complete death of host cells. Since washing away of detached live cells would lead to similar results, the assay was also performed in the original incubation media without washing the monolayers. Periodate- or metronidazole-treated parasites showed no cytotoxicity to hVECs. Time and concentration related data on cytotoxicity kinetics were also observed using increasing parasite/hVEC ratios (data not shown). The contact dependence of the damage as described earlier was also confirmed by this assay method. These results are consistent with our other results derived from crystal violet and 3H release assays.
FIG. 5.
Comparison of cytotoxicity of T. vaginalis (TV), T. vaginalis treated with periodate (TV-P) or metronidazole (TV-M), and the related bovine parasite T. foetus (TF) to hVECs. Cytotoxicity was determined by the Promega CellTiter AQueous system. Parasites were exposed to hVECs for 22 h. Cytotoxicity was determined as described in the text. Absorbance was recorded at 490 nm using an ELISA plate reader.
DISCUSSION
The establishment of in vitro culture of hVECs allowed us to study the specificity of host-parasite interactions with T. vaginalis as well as to quantitate the cytotoxicity to host cells by the parasites in greater detail than heretofore possible. Rasmussen et al. (31) were able to culture hVECs in vitro to study the cytotoxicity of T. vaginalis by microscopic examinations. However, these investigators did not address the purity and specificity of hVECs or the quantification of host cell damage by the parasite. The fact that the hVECs react with anti-sPRP1 antibody further suggest that the hVECs in culture are squamous epithelial cells. The sPRPs are expressed in squamous tissues (skin, trachea, vagina, esophagus, etc.) and this antibody has been used as a marker for squamous cells (5, 22, 36). This is the first report of its kind where relatively pure hVECs have been subcultured for experimental purposes. This allows the study of host-parasite interactions to take place in a convenient, easily manipulated system.
Incubation of live T. vaginalis with hVEC monolayers produced disruption of host cells within 2 h and resulted in total loss of cell viability after extended exposure to parasites. This suggests that the cytotoxicity of T. vaginalis to hVECs is a slow process requiring several hours of contact with the host target cells. Our data also point to the fact that the cytopathogenic effect is a function of parasite density. In the absence of direct contact, there is no damage to the host cell monolayers. The CellTiter AQueous assay provided a quantitative and repeatable method to measure cell survival and cell death. The dead cells are unable to form formazan, which is accomplished by dehydrogenase enzymes present in metabolically active cells. Exposure of hVECs to T. vaginalis for more than 22 h abolished detectable production of formazan, implying complete metabolic death of the epithelial cells. The hVECs not exposed to T. vaginalis or separated by Costar membrane from parasites showed abundant formation of formazan, indicating viability of these cells.
Our results indicate that all T. vaginalis isolates, whether from asymptomatic patients or from patients with vaginitis, were capable of destroying hVECs. The levels of cytotoxicity produced by different clinical isolates may be related to different levels of cytotoxic product(s) released by the organism in presence of host target cells. One of the isolates, TV30001, which had been in culture for several months, was less cytotoxic to hVECs than the fresh isolates. It is not surprising that the parasitic organisms maintained in culture for a long time lost their potential to infect host cells. It is interesting that T. vaginalis adhered to and destroyed the hVECs but T. foetus did not, indicating a species-specific host parasite relationship. Similarly, T. vaginalis parasites recognized and damaged hVECs but not hVFs or bVECs (34). The metronidazole-treated T. vaginalis showed some adherence to host cells but produced no damage to hVECs, as demonstrated by microscopy and two colorimetric assays. These results suggest that metabolic integrity of T. vaginalis is essential for the attachment of parasites to host cells and the induction of a cytopathic effect. A similar type of finding was reported earlier by Alderete and Garza (3) on the effect of metronidazole-treated T. vaginalis on HeLa cells. The absence of adhesion or cytotoxic effect of parasites treated with periodate is noteworthy and suggests that the adhesion is modulated by parasite surface glycoconjugate-like components. This is in contrast to earlier observations reported by Alderete and Garza (3), who indicated that the treatment of T. vaginalis with periodate had no effect on host cell parasitism. The fact that periodate treatment did not abolish metabolic activity of parasites, while at the same time completely eradicating any measurable cytopathic effect, supports a role for surface glycoconjugates in mediating pathogenesis (probably via a role in parasite adhesion to the host cell). We have shown both in hVECs as well as in HeLa cells (data not provided) that the periodate treatment abolishes the binding of T. vaginalis to host cells. A similar finding was also observed with binding of T. foetus to bVECs (34). In fact, our recent observations of T. foetus (34) as well as T. vaginalis (unpublished) suggest the involvement of a major cell surface glycoconjugate, lipophosphoglycan, in the adhesion of trichomonads to host target cells.
Several cell lines such as HeLa and MDCK have also been used for cell-trichomonad interaction studies. Those cell lines are parasitized by both T. vaginalis and T. foetus (3, 14). Using our hVEC culture system, we have clearly demonstrated host-parasite specificity. Filho-Silva and deSouza (14) suggested that trichomonads exert their pathogenic effects on epithelial MDCK cells in culture either by direct contact or by the release of certain components. It is possible that certain proteases and glycosidases found in trichomonad extracts play a role in modulating the interactions of trichomonads with epithelial cells. Thus, it has been reported that the addition of protease inhibitors to the incubation medium decreased epithelial cell disruption by T. foetus (7, 14).
Several investigators have proposed that some types of soluble cytotoxin may play a role in the pathogenic effect on host cells (2, 13, 29). Garber et al. (18) have reported the presence of a cell-free product of T. vaginalis, cell-detaching factor, involved in the cytopathic effects in cell cultures of McCoy, HEp-2, human foreskin fibroblasts, and CHO monolayers. Garber and Bowie (17) later suggested that very low pH associated with metabolically active T. vaginalis may be an important factor in the contact-dependent killing of mammalian cells. Pindak et al. (30) suggested that the acidic metabolites produced by T. vaginalis during coincubation of parasite and host cells lead to death of cultured cells. The observations that T. vaginalis parasites did not damage hVF monolayers, that the related pathogen T. foetus produced no cytopathic effects on hVECs, and that T. vaginalis was not cytotoxic to bVECs indicate that there must be some other mechanisms or factors involved in the cytopathic effects of T. vaginalis on hVECs. A number of microorganisms have been reported to produce extracellular components that are cytotoxic (9, 23, 32, 38). Our results agree with other investigators that cell destruction by T. vaginalis parasites is very likely a contact-dependent mechanism. It is not known whether T. vaginalis parasites produce cytotoxic material upon contact with host cells.
The establishment of relatively pure hVECs in vitro as reported here provides a model system for studying the pathogenicity of T. vaginalis in detail. Furthermore, the establishment of hVECs has important implications in studying other disease processes in women. The knowledge of T. vaginalis-host cell interactions will also provide insight into the mechanisms of host cytopathogenicity and the pathobiochemistry of trichomoniasis.
ACKNOWLEDGMENTS
We thank S. A. Gilroy and M. Urban for providing clinical parasite isolates, G. Hayes for useful advice and preparation of graphic illustrations, and J. J. Lucas for useful advice and discussions.
This work was supported in part by SUNY Health Science Center Intramural Research Grant and Women's Health Fund, Health Science Center Foundation (to B.N.S.), and by a grant from the Cooperative State Research, Education, and Extension Service, USDA Department of Agriculture's Section 1433 Animal Health and Disease Program (to R.O.G.).
REFERENCES
- 1.Alderete J F, Demes P, Gombosova A, Valent M, Fabusova M, Janoska A, Stefanovic J, Arroyo R. Specific parasitism of purified vaginal epithelial cells by Trichomonas vaginalis. Infect Immun. 1988;56:2558–2562. doi: 10.1128/iai.56.10.2558-2562.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete J F, Garza G E. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol Biochem Parasitol. 1984;13:147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- 3.Alderete J F, Garza G E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete J F, Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984;60:99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An G, Tesfaigzi J, Carlson D M, Wu R. Expression of a squamous cell marker, the spr1 gene, is posttranscriptionally down regulated by retinol in airway epithelium. J Cell Physiol. 1993;157:562–568. doi: 10.1002/jcp.1041570316. [DOI] [PubMed] [Google Scholar]
- 6.Borchardt K A, Hernandez V, Miller S, Loaiciga K, Cruz L, Naranjo S. A clinical evaluation of trichomoniasis in San Jose, Costa Rico using the InPouch TV test. Genitourin Med. 1992;68:328–330. doi: 10.1136/sti.68.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess D E, Knoblock T, Daugherty T, Robertson N P. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun. 1990;58:3627–3632. doi: 10.1128/iai.58.11.3627-3632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Increasing incidence of low birthweight—United States, 1981–1991. Morb Mortal Wkly Rep. 1994;43:335–339. [PubMed] [Google Scholar]
- 9.Clinkenbeard K D, Mosier D A, Confer W W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella hemolytica leukotoxin. Infect Immun. 1989;57:420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coteh M F, Pastorek J G, Nugent R P, Hiller S L, Gibbs R S, Martin D H, Eschenbach D A, Edelman R, Carey J C, Regan J A, Krohn M A, Klebenoff M A, Rao A V, Rhoads G G. Trichomonas vaginalis associated with low birth weights and preterm delivery. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Diamond L. Techniques of axenic cultivation of Entamoeba histolytica Schaudubb 1903 and E. histolytica-like amoebae. J Parasitol. 1968;54:1047–1056. [PubMed] [Google Scholar]
- 12.Draper D, Donohoe W, Mortimeer L, Heine R P. Cystein proteiases of Trichomonas vaginalis degrade secretary leucocyte protease inhibitor. J Infect Dis. 1998;178:815–819. doi: 10.1086/515366. [DOI] [PubMed] [Google Scholar]
- 13.Farris V K, Honigberg B M. Behaviour and pathogenicity of Trichomonas vaginalis Donne' in chick liver cells. J Parasitol. 1970;56:849–882. [PubMed] [Google Scholar]
- 14.Filho-Silva C S, deSouza W. The interaction of Trichomonas vaginalis and Tritrichomonas foetus with epithelial cells in vitro. Cell Struct Funct. 1988;13:301–310. doi: 10.1247/csf.13.301. [DOI] [PubMed] [Google Scholar]
- 15.Fiori P L, Rappelli P, Addis M F, Sechi A, Cappuccinelli P. Trichomonas vaginalis haemolysis: pH regulates a contact-dependent mechanism based on pore-forming proteins. Microb Pathog. 1996;20:109–118. doi: 10.1006/mpat.1996.0010. [DOI] [PubMed] [Google Scholar]
- 16.Fiori P L, Rappelli P, Addis M F, Mannu F, Cappuccinelli P. Contact-dependent disruption of host cell membrane skeleton induced by Trichomonas vaginalis. Infect Immun. 1997;65:5142–5148. doi: 10.1128/iai.65.12.5142-5148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber G E, Bowie W R. The effect of Trichomonas vaginalis and the role of pH on cell culture monolayer viability. Clin Investig Med. 1990;13:71–76. [PubMed] [Google Scholar]
- 18.Garber G E, Lemchunk-Favel L T, Bowie W R. Isolation of a cell-detaching factor of Trichomonas vaginalis. J Clin Microbiol. 1989;27:1548–1553. doi: 10.1128/jcm.27.7.1548-1553.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerbase A C, Rowley J T, Heymann D H L, Barkley S F B, Pist P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74:512–515. [PubMed] [Google Scholar]
- 20.Goldstein F, Goldman M B, Cramer D W. Relation of tubal infertility to a history of sexually transmitted diseases. Am J Epidemiol. 1993;137:577–584. doi: 10.1093/oxfordjournals.aje.a116711. [DOI] [PubMed] [Google Scholar]
- 21.Gram I, Macaluso M, Churchill J, Stalsberg H. Trichomonas vaginalis (TV) and human papilloma virus (HPV) infection and the incidence of cervical intraepithelial neoplasia (CIN) grade III. Cancer Causes Control. 1992;3:231–236. doi: 10.1007/BF00124256. [DOI] [PubMed] [Google Scholar]
- 22.Hu R, Hu R, Deng J, Lau D. A small proline-rich protein, spr1: specific marker for squamous lung carcinoma. Lung Cancer. 1998;20:25–30. doi: 10.1016/s0169-5002(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 23.Keen M G, Hoffman P S. Characterization of a Legionella pneumophila extracellular protease exhibiting hemolytic and cytotoxic activities. Infect Immun. 1989;57:732–738. doi: 10.1128/iai.57.3.732-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharsany A B, Hoosen A A, Moodley J, Bagaratee J, Gouws E. The association between sexually transmitted pathogens and cervical intra-epithelial neoplasia in a developing community. Genitourin Med. 1993;69:357–360. doi: 10.1136/sti.69.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger J N, Ravdin J I, Rein M F. Contact dependent cytopathogenic mechanism of Trichomonas vaginalis. Infect Immun. 1985;50:778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets E, Batter V, Alary M, Heyward W L, Ryder R W, Piot P. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Minkoff H, Grunebaum A N, Schwartz R H, Feldman J, Cummings M C, Clark W L, Pringle G, McCormack W M. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150:965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 28.Nix D E, Tyrrel R, Müller M. Pharmacodynamics of metronidazole determined by time-kill assay for Trichomonas vaginalis. Antimicrob Agents Chemother. 1995;39:1848–1852. doi: 10.1128/aac.39.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pindak F F, Mora de Pindak M, Gardner J. Contact independent cytotoxicity of Trichomonas vaginalis. Genitourin Med. 1993;59:35–40. doi: 10.1136/sti.69.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen S E, Nielsen M H, Lind I, Rhoades J M. Morphological studies of the cytotoxicity of Trichomonas vaginalis to normal vaginal epithelial cells in vitro. Genitourin Med. 1986;62:240–246. doi: 10.1136/sti.62.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravdin J E, Croft B Y, Guerrant R L. Cytopathic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152:377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiser R F. The effect of selected surfactants on baboon vaginal epithelial organ and tissue culture in vitro. Ph.D. thesis. Ithaca, N.Y: Cornell University; 1993. [Google Scholar]
- 34.Singh B N, Lucas J J, Beach D L, Shin S T, Gilbert R O. Adhesion of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect Immun. 1999;67:3847–3854. doi: 10.1128/iai.67.8.3847-3854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh B N, Elia G, Gilbert R O. Cytotoxic effects of Trichomonas vaginalis on human vaginal epithelial cells. Abstr J Soc Gynecol Investig. 1999;6(Suppl.):447. [Google Scholar]
- 36.Tesfaigzi J, An G, Wu R, Carlson D M. Two nuclear proteins in tracheal epithelial cells are recognized by antibodies specific to squamous differentiation marker, spr1. J Cell Physiol. 1995;164:571–578. doi: 10.1002/jcp.1041640315. [DOI] [PubMed] [Google Scholar]
- 37.Wawer M J, McNairn D, Wabwine-Mangen F, Paxton L, Gray R H, Kiwanuk N. Self-administered vaginal swabs for population-based assessment of Trichomonas vaginalis prevalence. Lancet. 1995;345:131. doi: 10.1016/s0140-6736(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 38.Young J D E, Cohn Z A. Molecular mechanisms of cytotoxicity modified by Entamoeba histolytica: characterizations of a pore-forming protein (PFP) J Cell Biochem. 1985;29:299–308. doi: 10.1002/jcb.240290404. [DOI] [PubMed] [Google Scholar]