Graphical abstract
Keywords: Fermented vegetables, Atherosclerosis, Molecular docking, LC-MS/MS, Network pharmacology
Highlights
-
•
Atherosclerosis is the etiology of coronary heart disease, the first rank cause of mortality worldwide in metabolic disease group.
-
•
Hyperlipidemia (High Total Cholesterol and Low-Density Lipoprotein concentration), inflammation, Endothelial Dysfunction, dysregulation of lipid transport and storage trigger the atherosclerosis plaque development.
-
•
The natural product based on vegetables and herbs fermentation is a promising candidate to be develop as a dietary supplement to prevent the disease.
-
•
Combination of metabolite screening of the extract, in silico, and in vivo study build a comprehensive mechanism in drug discovery research.
Abstract
Although positive association between fermented vegetables intake with the risk of coronary heart disease (CHD) has increased attention nowadays, the metabolite profiling and the mechanism of action are still elusive. This study designed to investigate the secondary metabolites, hypolipidemic, and anti-atherogenic effect of mixed vegetable fermentation extract (MVFE). The metabolite screening of the MVFE was assessed using the Liquid Chromatography Tandem Mass Spectrophotometer (LC-MS/MS) method. The result of LC-MS/MS was used as ligands to inhibit the binding of oxidized LDL (oxLDL) and Cluster Differentiation 36 (CD36), Scavenger Receptor A1 (SRA1), Lectin-type oxidized LDL receptor 1 (LOX1). This work was performed with molecular docking using Discovery Studio 2021, PyRx 0.9, and Autodock Vina 4.2 followed by analyzing Network Pharmacology, Protein Protein Interaction (PPI) using Cytoscape 3.9.1 and String 2.0.0. Finally, the clinical effect of MVFE was evaluated using in vivo study. Twenty rabbits were assigned to normal, negative control, and MVFE group that were fed with standard diet, high fat diet (HFD), HFD supplemented with MVFE 100, 200 mg/kg BW, respectively. The serum level of Total Cholesterol (TC) and Low-Density Lipoprotein (LDL-c) were detected at the end of week 4.
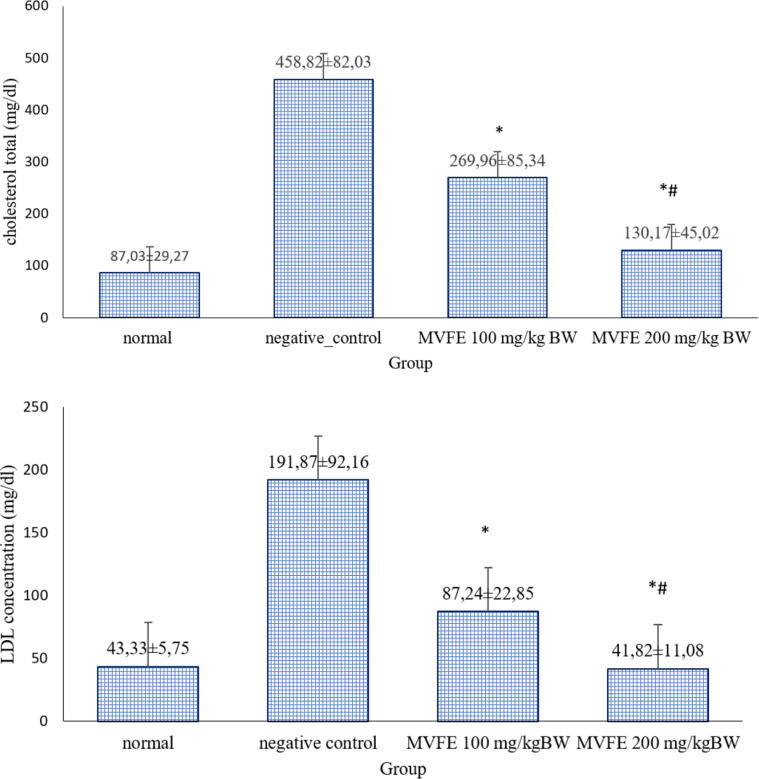
The LC-MS/MS analysis identified 17 compounds categorized as peptides, fatty acids, polysaccharides, nucleoside, flavonoids, flavanols, and phenolic compounds. Based on the docking study, more negative binding affinity was observed in the interaction between metabolites with the scavenger receptors (SR) than simvastatin. The number of nodes and edges based on Network Pharmacology analysis were 268 and 482, respectively. The PPI network showed that MVFE metabolites exerts its athero-protective effect by modulating various cellular processes including inflammation, improvement of endothelial function, and modulation of lipid metabolism. Blood TC and LDL-c concentrations in the negative control (458.82 ± 82.03; 191.87 ± 92.16 mg/dL) were higher significantly compared to the normal group (87.03 ± 29.27; 43.33 ± 5.75 mg/dL). The MVFE administration decreased the TC (100, 200 mg/kg BW MVFE: 269.96 ± 85.34; 130.17 ± 45.02 mg/dL) and LDL-c level (100, 200 mg/kg BW MVFE = 87.24 ± 22.85; 41.82 ± 11.08 mg/dL) dose-dependently (p < 0,001).
The secondary metabolites derived from fermented mixed vegetables extract might be developed as a potential strategy to prevent CHD by targeting the multiple pathways in atherosclerosis.
1. Introduction
Hyperlipidemia and atherosclerosis are the main processes associated with the risk factor of coronary heart disease (CHD) (Miller, 2009, Stein et al., 2019). Lipid abnormalities defines as alterations in lipid profiles, including high levels of low-density lipoprotein cholesterol (LDL-c), elevated triglycerides, total cholesterol (TC), and low levels of high-density lipoprotein cholesterol (HDL-c)(Stein, Ferrari and Scolari, 2019). Atherosclerosis is a multifactorial complex disease characterized by the accumulation of lipid, fibrous elements, and calcification in the prone arteries. Several arteries, such as coronary arteries, are more susceptible to develop atherosclerotic plaques (Michael A. and Guillermo, 2016). Cascade of atheroma plaque formation is initiated with foam cell formation, fatty streak, fibrous plaque formation, and advanced plaque formation(Thompson et al., 2013).
Statin therapy is the primary target of lipid-lowering therapy and could suppress the formation of atherosclerosis plaque(Lippi and Plebani, 2017, Li et al., 2019). However, statin therapy might be insufficient due to the increasing incidence of cardiovascular disease and the presence of comorbid. Moreover, few reports observed several side effects in the long-term use of statin such as rhabdomyolysis, hepatotoxicity, and cognitive impairment(Newman et al., 2019). During the last decade, the use of natural products showed a multi-target and synergistic capacity and hence safe in long-term use. These products provide beneficial effects as functional food to prevent the atherosclerosis formation(Shen, 2015, Waltenberger et al., 2016). Sambal lalapan is an Indonesian traditional side dish from mixed vegetables, herb, and spices (Surya and Tedjakusuma, 2022). A report from Ninfali et al. (2005) showed that addition of lemon balm and marjoram as herbs to salad vegetables at a concentration of 1·5% w/w increased the antioxidant capacity by 150% and 200%(Ninfali et al., 2005). In addition, the combined action of individual phytochemicals could display a higher biological effect as compared to the sum of the biological effects acquired by the individual phytochemicals in single vegetable (van Breda and de Kok, 2018). Moreover, the combination of vegetables, herbs, spices in several studies could increase the vegetable intakes in children and adults(Meengs et al., 2012, Poelman et al., 2019, van Stokkom et al., 2019).
Despite the cardioprotective effect of single ingredients in sambal lalapan, there are lack of studies explore the advantage of the mixed vegetables consumption. The main processing of Indonesia sambal with deep frying and eating raw vegetables as a preferential method could lower the nutritional value and exert potential bacterial contamination (Callejón et al., 2015). Spontaneous fermentation is an approach to enhance the benefit of plant-based food product. A range of bioactive compounds can potentially be produced during fermentation processes (Mathur, Beresford and Cotter, 2020). Furthermore, natural fermentation could also prevent the pathological bacterial growth. Therefore, the fermentation process of mixed vegetables, herbs, spices from sambal lalapan is an innovative approach to be studied. However, the mechanistic details of the mixed vegetable fermentation leading to CHD prevention are still unclear. The excess of LDL-c in circulation easily translocate to subendothelial space. Reactive oxygen species (ROS) in this location lead to modification of LDL-c with the prominent form oxidized LDL (oxLDL). The expression of vascular cell adhesion molecule 1 (VCAM1) and selectin as the sign of endothelial activation provokes the entrance of monocyte and leucocyte to sub endothelial space in tunica intima and trigger inflammation (Davignon and Ganz, 2004, Michael and Guillermo, 2016). The Cluster Differentiation 36 (CD36), Scavenger Receptor Type A1 (SRA1), lectin-type oxidized LDL receptor 1 (LOX1) are predominant SR for oxLDL uptake which the expressions are stimulated by inflammation and lead to lipid accumulation (van Eck et al., 2000, Kzhyshkowska et al., 2012, Park, 2014). The dysregulation of lipid management leads to foam cell and followed by fatty streak establishment as the early lesion of atherosclerosis. Therefore, the recent study investigated the metabolite profiling and also potential mechanism of mixed vegetable fermentation extract (MVFE) to modulate atherosclerosis by using in silico and in vivo study.
2. Material and methods
2.1. Collection and fermentation of the plants
The spices consist of onion (Allium cepa), garlic (Allium sativum), and chili (Capsicum frutescent). The only herb ingredient used was basil leaves (Occimun sanctum). The mixed vegetables were composed of white cabbage (Brassica oleraceae var. capitata f. alba), cucumber (Cucumis sativus), and tomato (Solanum lycopersicum). All the ingredients were collected from Malang traditional market and determined at Materia Medica Batu Indonesia. The composition of all the ingredients was adjusted with Indonesian sambal lalapan formula as follow: (1) 5 g onion; (2) 5 g garlic; (3) 10 g chili; (4) 50 g tomatoes; (5) 100 g white cabbage; (6) 95 g cucumber(Ji et al., 2007, Lee et al., 2018, He et al., 2021, Major et al., 2022).
Fresh cabbage was cleaned, dried, weighed, shredded, and mixed with 3% salt overnight at 4 °C. Fresh cucumber was peeled, cut into small pieces and weighed. Selection of basil leaves, followed by cleaning in fresh water, drying, weighted, and cutting into smaller sizes. The onion, garlic, chili and tomato were sorted, washed with fresh water, dried, and blended using mortar to create sambal sauce. Thus, the sugar, salt, and vegetables were mixed and added to the sambal sauce. The time for natural fermentation was 7 and 14 days. The fermentation jar was kept in 20 °C (Zabat et al., 2018).
2.2. Preparation of mixed vegetable fermentation extraction
The mixture of vegetables, herbs, and spices fermentation product were kept at −80 °C. The frozen samples were then dried using freeze-dryer (CHRIST Alpha 1–4 LSC). A pressure of 0.94 bar and a temperature of −5°C were applied for 48 hr (Fabricio et al., 2022). The end product was mashed to become powder using mortar. The fermentation powder was soaked in ethanol 70% 1:20 and extracted using ultra-assisted extraction (UAE) for 30 min at 50 °C (Nascimento et al., 2021). After extraction, the plant’s extracts were processed in a rotary evaporator at 50 °C. Subsequently, the extract was dried in a hot oven at 50 °C until the mass was stable.
2.3. LC-MS/MS analysis of MVFE
The chemical compounds of ethanol extract from mixed vegetable fermentation were evaluated using Ultra Performance Liquid Chromatography (UPLC)-QToF-Mass Spectrophotometry (MS) employed UPLC-MS systems with QToF as the analyser and positive ESI as the ionization source.
Before run, the extract (10 mg) was dissolved in 10 ml volumetric flask with absolute methanol. After that, 5 µl volumes were injected into the Acquity C18 column 1,8 µm; 2,1 × 150 mm. The gradient system of mobile phase consist of a mixture between (A) Water (HPLC grade)/formic acid (Merck, Darmstadt, Germany) 99,9/0,1 [v/v]; (B) Acetonitrile (Merck, Darmstadt, Germany)/formic acid 99,9/0,1 [v/v] (Mutiah et al., 2019). The source temperature was set at 100 °C and the desolvation temperature was 350 °C.
The area of each peak shown in the chromatogram was presented in percentage. Parameters for analysis set using positive ion mode with spectra acquired over a mass range from m/z 120 to 1000. Software Masslynx version 4.1 was used to process the chromatogram (Waters, Massachusetts, USA). The component identification was based on the ratio of measured m/z in Masslynx 4.1 tools, mz Cloud (https://beta.mzcloud.org/), and PubChem database (https://pubchem.ncbi.nlm.nih.gov/). A compound's accuracy for confirmation was determined based on MS/MS fragment matching and inaccuracy of<5 ppm(Mutiah et al., 2019).
2.4. Molecular docking
Active compound of the plant extract in several publication showed atherosclerosis inhibition by targeting inflammation, oxidative stress, and lipid metabolism process. Thus, the potency of identified compound in the fermentation extract to inhibit cardiovascular disease were analyzed using in silico and in vivo study. Molecular docking was performed to find phytochemicals' binding affinities and target protein’s active site. Several identified flavonoids which were confirmed as predominant compound in each ingredient, fatty acid, peptide, EPS were chosen as ligands. The important SR that responsible for atherosclerosis formation were selected as the receptors (Ferreira et al., 2015).
2.4.1. Ligand extraction
The LC-MS/MS tentatively identified compounds used as ligands for the molecular docking process. The 3-dimensional structure of each ligand was extracted from Pubchem https://pubchem.ncbi.nlm.nih.gov/https://pubchem.ncbi.nlm.nih.gov/. The selected ligands name and identifier were depicted as follows: Retusin (5352005), Ayanin (5280682), Apigenin (5280443), Kaempferide (5281666), 15(16) EpODE (16061062), Gamma-Glutamyl-S-Allylcysteine (11346811), Capsaicin (1548943), Linoleic Acid (5280934), Oleamide (5283387). Simvastatin was used as ligand control (54454). Two-dimensional structures of the ligands were converted to pdbqt format. The energy of the ligands was minimized using PyRx 0.9 software.
2.4.2. Receptor preparation
The the X-ray structure of the receptors CD36 (5LGD), SRA1 (7DPX), and LOX1 (1YPQ) were retrieved from https://www.rcsb.org/. The water molecules, the native ligands, and the heteroatoms molecule were removed using Biovia Discovery Studio 2021 Eberhardt et al., 2021, Trott and Olson, 2010 software. The protein's energy minimization was performed before docking using PyRx 0.9 software. Autodock Vina 4.2 tools program was used to convert protein and ligand files into pdbqt formats (Arockianathan, 2019).
2.4.3. Molecular docking and visualization
Prior to docking, the energy of the ligand molecules and the receptor were minimized. The molecular docking was performed using Autodock Vina 4.2 PyRx 0.9 tools. The site of oxLDL binding to CD36 has been identified and recently mapped to amino acids 157–171 (ILE157, LEU158, ASN159, SER160, LEU161, ILE162, ASN163, LYS164, SER165, LYS166, SER167, SER168, MET169, PHE170, GLN171) in chain A, with critical lysines at positions 164 and 166. Optimal docking box size for CD36 included the Center (X: −35,8472 Y: −35,036, Z: 49,1650) and Dimensions (X:24,0709; Y: 25.2059; Z: 22,6213). Active site of oxLDL to LOX1 lied on chain B: ILE149, TRP150, HIS151, ALA194, TYR197, SER198. The Center (X: 9,6431 Y: 6,4644, Z:23,0338) and Dimensions (X:25,000; Y: 20, 9629; Z: 17,3418) were docking box site that has been optimized for LOX. Active site of oxLDL to SRA1 was ASP29, ASP30, GLU96. SRA1 docking box size was composed of Center (X: 23.5570; Y: 42.2771; Z: 24.9941) and Dimension (X:10.3367; Y:7.9900; Z:9.6838) Amstrong (Ohki et al., 2005, Park et al., 2005, Cheng et al., 2021).
2.5. Network pharmacology
The prediction of hypolipidemic effect and anti-atherosclerosis activity of phytochemical identified from LC-MS/MS was conducted using several steps. First, the bioactive compound’s structure was searched for their gene targets in Pubchem software. On the other hand, the data of genes associated with diseases related to hyperlipidemia and atherosclerosis (dyslipidemia, endothelial dysfunction, coronary arteriosclerosis) were identified from disgenet (https://www.disgenet.org/home/). Thus, the interconnection of the compounds, genes and diseases was visualized and analyzed using Cytoscape 3.9.1 software(Su et al., 2015). Furthermore, the similar gene targets from phytochemical and disease were used for protein–protein interaction network (PPI) using String 2.0.0 application. An interaction score > 0.4 were applied to construct the PPI networks Moreover, The Gene Ontology (GO) Biological Process and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway from enrichment analysis with the threshold of adjusted p-value < 0.05 was extracted to see the predicted signaling transduction and proteins involved(Szklarczyk et al., 2015).
2.6. Animal experiment design
This study has been approved by the local ethics committee from Brawijaya University No: 104-KEP-UB-2022. Five-month-old male New Zealand White (NZW) rabbits weighing 2.2–2.5 kg were purchased from Brawijaya Lab-Animal, Indonesia. The rabbits were kept in 50x70x70 cm3 separate cages at 20 °C with a 12-hour cycle of darkness and light. After 2 weeks of acclimatization, the rabbits were randomly assigned to one of four groups (n = 5): normal, negative control, and MVFE group. The fermented vegetables used was 14th day fermentation due to the pH obtained (3.678 ± 0.06127). It was similar to other reports demonstrated that vegetables like cucumber and white cabbage reached a stable fermentation level with an average pH value of 3.47; 3.51 after 14-;12-days fermentation (Drašković Berger et al., 2020, Kiczorowski et al., 2022).
The standard diet (16.5% protein, 3.5% fat, 15.2% fibre, 7.2% ash, 52% starch, phosphorus 657 mg/kg BW, calcium 787 mg/kg BW) was supplemented to the rabbits in the normal group. The rabbits administered with the standard diet and High Fat Diet (HFD) consist of 1% cholesterol (from cow brain) served as the negative control(Rachmawati & Muhammad, 2021). The HFD supplemented with MVFE 100 mg/kg BW and 200 mg/kg BW were given to the treatment group. The fermented powder simplicial was produced with hot oven dried not freeze-dried method due to more massive, and lower cost for production. Sadiq et al. (2021) reported higher levels of amino acids, monosaccharides, and enzymatic activity in heat dried compared to freeze-dried food. In addition, other publication demonstrated that there was no significant difference of 2,2-diphenyl-1-picrylhydrazyl (DPPH) result between hot oven and freeze-dried method(Elshaafi, Musa and Abdullah Sani, 2020). Hence, the fermented food was dried in oven at 50 °C for 5 days and followed by the extraction(Sadiq et al., 2021).
Every day, fresh food was provided, and food from the day before were weighed. The rabbits had free access to diet and water. The changes of body weight were recorded weekly. All treatments were given daily and ended after 4th week (Lee et al., 2013).
2.7. Total Cholesterol (TC) and LDL-c assay
After an overnight fast at the end of 4th week treatment, blood was drawn from the central ear artery. The serum was collected and frozen at −80 °C before examination(Lozano et al., 2019). The TC level was measured using a Cholesterol assay kit from e-lab science cat. number E-BC-K109-M. Briefly, 10 µl serum was added to the tube. The tube was incubated for 10 min at 37 °C after reagent 1 (Good’s Buffer, Phenol, 4-AminoAmylPyridine, Cholesterol esterase, Cholesterol oxidase, Peroxidase) was added. Subsequently, the Optical Density (OD) value at 550 nm was recorded. The LDL-c level was measured using LDL cholesterol colorimetric assay kit Cat. Number E-BC-K205. 2,5 µl serum was added into the microplate, followed by 180 µl reagent 1(surfactant, Peroxidase, Cholesterol esterase, Cholesterol oxidase, Peroxidase, 4-AminoAmylPyridine) and incubation at 37 °C for 5 min. The OD value was measured at 546 nm. Subsequently, 60 µl of reagent 2 (Phenol, Peroxidase, surfactant, Cholesterol esterase, Cholesterol oxidase, 4-Amino Amyl Pyridine) was added to the mixture, and the absorbance was measured immediately against the prepared blank at 546 nm. The TC and LDL-c content (mg/dL) was calculated using the obtained OD according to manufacture instruction.
2.8. Statistical analysis
The in vivo data were presented as mean and standard deviation. The data analysis was performed using the one-way ANOVA followed by post hoc analysis. SPSS 26.0 was used to perform statistical analysis. p-values < 0.05 was considered statistically significant.
3. Results
3.1. Metabolite profiling of MVFE
Since, the concept of fermented vegetables with herbs and spices is relatively new, so we prefer to select the untargeted approach for hypothesis generation, followed by targeted profiling for more confident quantitation of relevant metabolites in future study(T’Kindt and van Bocxlaer, 2010). Fig. 1 illustrated the total ion chromatogram of LC-MS/MS. The MassLynk v 4.1 was used to process the chromatogram.
Fig. 1.
Chromatogram of MVFE based on LC-MS/MS analysis. (A) Chromatogram from MVFE after 7th day of fermentation; (B) Chromatogram from MVFE after 14th day of fermentation.
Several ingredients were determined in fermentation day 7 and 14. Fermentation process produced several ingredients as follows: Exopolysaccharides (EPS) and antioxidant peptides. Besides that, fermentation not only changes the structure, but also the activity of phytoconstituent of the plants, such as phenolics plant, phenolic acid, flavonoids, flavanols, and tannin. Additionally, small molecules like fatty acid, nucleoside could be identified in fermentation process due to microorganism activity. Interestingly, the result showed there were peptide (1); EPS(1); fatty acids(10); nucleoside (1); flavonoid, flavanols, or phenols (5) found in fermentation product, respectively with a total of 17 compounds. Table 1 identified several suggested metabolites from MVFE based on LC-MS/MS result.
Table 1.
Retention time, mass, molecular formula, and phytoconstituent based on LC-MS/MS results.
| Retention time | Area (%) | Graphical mass | m/z | Molecular formula | Name of compound | Classification of compound (Chem/MesH tree) | Fragmentation ion | Reference (Pubchem ID, Legacy ID) |
|---|---|---|---|---|---|---|---|---|
| Fermentation day 7 | ||||||||
| 1.303 | 1,358,353 (12.25) | 266.1251 | 266.1249 |
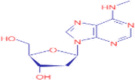 C11H15N5O3 |
N6-Me-dA | organic heteromonocyclic compound/ oxolanes/tetrahydrofuranol/ monohydroxytetrahydrofuran/ 2′-deoxyribonucleoside/purine 2′-deoxyribonucleoside |
73.0283 150.0774 |
CID 168,948 |
| 1.830 | 591,444 (5.33) | 294.1560 | 294.1557 |
 C13H21N5O4 |
GVH | – | 58.4046 121.8472 277.1292 |
– |
| 2.688 | 271,207 (2.45) | 328.1403 | 328.1391 |
 C15H18O7 |
Picrotin | organic hydroxy compound/ alcohol/ tertiary alcohol/ |
59.0496 163.0754 265.1071 |
CID 442,291 |
| 3.433 | 42,703 (0.39) | 454.1551 | 454.15 |
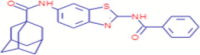 C25H25N3O2S |
NVP-231 | Organic Chemicals/ Hydrocarbons/ Hydrocarbons, Cyclic/enzyme |
– | CID 4,096,211 |
| 4.094 | 175,285 (1.58) | 457.1824 | 457.1836 |
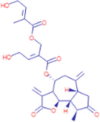 C25H30O9 |
(2E)-3-{[(4R,6aR,9S,9aR,9bR)-9-methyl-3,6-dimethylidene-2,8-dioxo-dodecahydroazuleno[4,5-b]furan-4-yl]oxy}-2-(2-hydroxyethylidene)-3-oxopropyl (2E)-4-hydroxy-2-methylbut-2-enoate | – | 81.0333 195.0644 359.1473 |
– |
| 4.445 | 79,771 (0.72) | 231.1128 | 231.1139 |
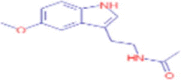 C13H16N2O2 |
Melatonin | organic heterocyclic compound/ heteroarene/ polycyclic heteroarene/ benzopyrrole/indoles/tryptamines |
– | CID 896 |
| 5.148 | 2691 (0.02) | 355.1824 | 355.1816 |
 C18H24F2N2O3 |
1,4-Anhydro-5-(benzoylamino)-2,5-dideoxy-2-[(4,4-difluorocyclohexyl)amino]-D-arabinitol | enzyme | 105.0332 178.1032 355.1757 |
CID 75,536,617 |
| 5.479 | 27,816 (0.25) | 130.0662 | 130.0651 |
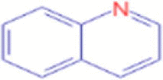 C9H7N |
Quinolline | Heterocyclic Compounds, Fused-Ring/ Heterocyclic Compounds, 2-Ring |
77.0386 103.0542 |
CID 7047 |
| 5.977 | 63,627 (0.57) | 239.1287 | 2239.1286 |
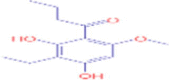 C13H18O4 |
1-(3-ethyl-2,4-dihydroxy-6-methoxyphenyl)butan-1-one | Natural products/medicine | 89.0597 179.0699 |
– |
| 6.287 | 13,589 (0.12) | 268.2029 | 268.1985 |
 C9H25N5O4 |
N-aminooxy-1-[2-[aminooxy(oxido)azaniumyl]butan-2-ylamino]-2-methylbutan-2-amine oxide | – | – | CID 164,163,701 |
| 6.659 | 1819 (0.02) | 425.1069 | 425.1868 |
 C14H26N8O6 |
NRN | – | 52.5778 173.4484 293.1326 |
– |
| 7.208 | 86.824 (0.78) | 256.0977 | 256.0965 |
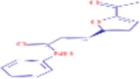 C15H13NO3 |
N1-phenyl-3-(5-acetyl-2-furyl)acrylamide | – | 94.0652 163.0388 181.0494 |
– |
| 7.630 | 19.215 (0.17) | 248.1659 | 248.1647 |
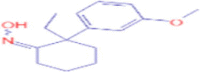 C15H21N02 |
-ethyl-2-(3-methoxyphenyl)cyclohexanone oxime | – | 81.0700 140.1072 202.1229 |
Legacy ID 4312 |
| 8.024 | 51.531 (0.46) | 301.0702 | 301.0700 |
 C16H1206 |
NP-001823 | Natural Products/Medicine | 52.1800 173.4381 269.0439 |
Legacy ID 6524 |
| 8.312 | 170.815 (1.54) | 351.2159 | 351.2153 |
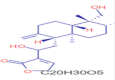 C20H30O5 |
3-{2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-decahydronaphthalen-1-yl]-1-hydroxyethyl}-2,5-dihydrofuran-2-one | Natural Products/Medicine, Endogenous Metabollite | 127.0387 207.1736 |
CID 6610 |
| 8.789 | 130.301(1.18) | 348.2747 | 348.2738 |
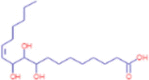 C18H34O5 |
(12Z)-9,10,11-trihydroxyoctadec-12-enoic acid | Natural Products/Medicine | 131.0855 173.1170 213.1482 313.2368 |
Legacy ID 2278 |
| 9.317 | 111,434 (1.00) | 345.0971 | 345.0955 |
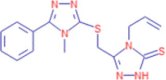 C15H16N6S2 |
4-allyl-5-{[(4-methyl-5-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione | – | 74.0055 154.0436 304.0563 |
Legacy ID 7469 |
| 9.605 | 118,210 (1.07) | 309.2066 | 309.2066 |
 C16H30O4 C16H30O4
|
NP-001596 | Natural products/medicine, Endogenous Metabollite | 58.3784 121.1011 193.1220 255.1744 273.1848 291.1954 |
Legacy ID 5629 |
| 9.894 | 986,602 (8.90) | 345.1010 | 345.1016 |
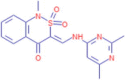 C16H16N4O3S |
3-{[(2,6-Dimethylpyrimidin-4-yl)amino]methylidene}-1-methyl-1,2,3,4-tetrahydro-2λ6,1-benzothiazine-2,2,4-trione | – | 124.0869 212.0490 |
CID 2,819,329 |
| 10.878 | 1580430(14.25) | 306.2081 | 306.2064 |
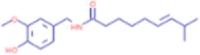 C18H27NO3 |
Capsaicin | Lipid/fatty acid derivative/ fatty amide/capsaicinoid |
137.0597 206.1903 |
CID 1,548,943 |
| 11.518 | 969.653 (8.74) | 308.2230 | 308.2220 |
 C18H29NO3 |
Dihydrocapsaicin | Lipid/fatty acid derivative/ fatty amide/capsaicinoid |
81.0705 137.0597 184.1696 |
CID 107,982 |
| 12.158 | 234,153 (2.11) | 295.2273 | 295.2278 |
 C18H34O4 |
NP-008993 | Natural Products/Medicine | 111.0811 157.0867 187.0974 277.2172 |
CID 15,159 |
| 12.769 | 220,845 (1.99) | 279.2314 | 279.2319 |
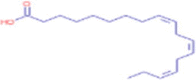 C18H30O2 |
α Linoleic acid | Lipid/ Dietary Lipid/ Dietary Fatty Acid/ Long Chain Fatty Acid Octadecatrienoic Acid |
67.0541 123.1170 261.2213 |
CID 5,280,934 |
| 13.338 | 270,131 (2.44) | 277.2197 | 277.2162 |
 C18H28O2 |
9,12-Octadecadiynoic Acid | 259.2 135.1 149.1 |
CID 1931 | |
| 13.535 | 34,957 (0.32) | 279.1603 | 279.1585 |
 C16H24O5 |
NP-022469 | Natural Products/Medicine | 107.0852 261.1483 |
Legacy ID 6483 |
| 13.979 | 61,258 (0.55) | 536.3679 | 535.7 |
 C24H49N5O8 |
2-[bis[2-[ethyl-[1-[2-(2-hydroxyethoxy)ethylamino]-1-oxopropan-2-yl]amino]ethyl]amino]acetic acid | – | – | CID 57,241,288 |
| 14.815 | 65.084 (0.59) | 524.3665 | 524.3671 |
 C28H51N3O7 |
13-(dodecan-2-yl)-6-(1-hydroxyethyl)-3-(hydroxymethyl)-12-methyl-9-(propan-2-yl)-1-oxa-4,7,10-triazacyclotridecane-2,5,8,11-tetrone | Natural Products/Medicine, Endogenous Metabollite | 173.4359 354.2989 |
Legacy ID 1413 |
| 15.448 | 353,097 (3.18) | 282.2817 | 281.5 |
 C18H35NO |
Oleamide | Lipid/fatty acid derivative fatty amide/primary fatty amide |
263.3 235 |
CID 5,283,387 |
| 15.673 | 928,303 (8.37) | 270.2805 | 270.2797 |
 C17H35NO |
N-(13-Methyltetradecyl)acetamide | Lipid /Fatty Acids/Fatty Acids, volatile /Acetates/ Acetamides |
– | CID 47,346 |
| 15.912 | 268,027 (2,42) | 792.5626 | 792.1 |
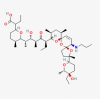 C45H77NO10 |
2-[(5S)-6-[(2S,3S,4S,6R)-6-[(3S,5S,7R,9S,10S,12R,15R)-3-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-3,10,12-trimethyl-15-(propylamino)-4,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl]-3-hydroxy-4-methyl-5-oxooctan-2-yl]-5-methyloxan-2-yl]butanoic acid | CID 130,402,727 | ||
| 16.305 | 640.179 (5.77) | 284.2959 | 284.2946 |
 C18H37NO |
Stearamide | Lipid/fatty acid derivative/ fatty amide/primary fatty amide/ octadecanamide |
72.0442 | CID 31,292 |
| 16.967 | 466.107 (4.20) | 796.5918 | 796.1 |
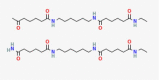 C41H77N7O8 |
N'-[6-[[6-(ethylamino)-6-oxohexanoyl]amino]hexyl]hexanediamide;N-ethyl-N'-[6-(6-oxoheptanoylamino)hexyl]hexanediamide | – | – | CID 161,770,242 |
| 18.063 | 462,622 (4.17) | 758.5691 | 758.5643 |
 C39H75N5O9 |
(2R)-2-[2-[(2S,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxypropyl-[(2S)-2-amino-3-methylbutanoyl]amino]-N'-octadecylpentanediamide | – | – | CID 22,819,456 |
| Fermentation day 14 | ||||||||
| 1.303 | 891,698 (8.58) |
175.1201 | 175.1190 |
 C6H14N4O2 |
L-(+)-Arginine | organic molecular entity/organic amino compound/amino acid/alpha-amino acid L-alpha-amino acid |
70.0652 116.0706 158.0924 |
CID 6322 |
| 1.851 | 394,954 (3.80) | 294.1560 | 294.1557 |
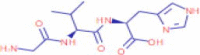 C13H21N5O4 |
GVH | – | 259.1174 121.8472 58.4046 |
– |
| 2.688 | 198,152 (1.91) | 328.1400 | 328.1391 |
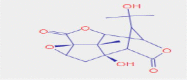 C15H18O7 |
Picrotin | Endogenous Metabolites | 265.1071 163.0754 59.0496 |
CID 442,291 |
| 3.412 | 40,646 (0.39) | 291.1011 | 291.1015 |
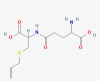 C11H18N2O5S |
N-Gamma-Glutamyl-S-(1-Propenyl)Cysteine | organic amino compound peptide oligopeptide dipeptide |
291.10275 145.0354 162.05995 164.05928 84.0461 |
CID 11,193,907 |
| 4.094 | 181,530 (1.75) | 295.1299 | 295.1281 |
 C14H18N2O5 |
6,7-bis(2-methoxyethoxy)quinazolin-4(3H)-one | – | 59.0493 92.4821 179.0448 237.0865 251.1021 |
Legacy ID 4692 |
| 4.424 | 63,488 (0.61) | 231.1143 | 231.1128 |
 C13H14N2O2 |
2,6-Dimethyl-5-(4-methylphenoxy)pyrimidin-4-ol | – | 79.0543 111.0553 132.0808 141.0659 203.1181 |
CID 2,745,089 |
| 4.881 | 39,812 (0.38) | 227.1272 | 227.1263 |
 C12H20O5 |
4-Oxododecanedioic Acid | Natural products/Medicine Endogenous metabolite |
55.0176 111.0434 191.1054 209.1158 |
CID 1865 |
| 5.169 | 51,303 (0.49) | 167.0724 | 167.0708 |
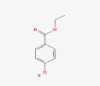 C9H10O3 |
Ethylparaben | organic hydroxy compound phenols 4-hydroxybenzoate ester paraben |
165.0553 137.0244 136.0157 166.0578 138.0275 |
CID 8434 |
| 5.521 | 59,431 (0.57) | 163.0421 | 163.0395 |
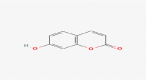 C9H6O3 |
Umbelliferone | organic heteropolycyclic compound benzopyran/1-benzopyran chromenes/chromenone coumarins/hydroxycoumarin |
163.0392 107.0493 119.0493 164.0425 135.0444 |
CID 5,281,426 |
| 5.935 | 37,159 (0.36) | 239.1265 | 239.1265 |
 C13H18O4 |
-(3-ethyl-2,4-dihydroxy-6-methoxyphenyl)butan-1-one | Natural products, endogenous metabolite | 57.4644 89.0597 151.0751 179.0699 221.1167 |
Legacy ID 2966 |
| 6.659 | 3937 (0.04) | 207.0669 | 207.0652 |
 C11H10O4 |
Sinapinic Acid | Endogenous metabolite | 73.0647 147.0441 175.0390 |
CID 637,775 |
| 7.279 | 52,650 (0.51) | 309.0871 | 309.07 |
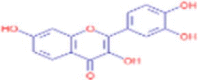 C15H10O6 |
Fisetin | Heterocyclic Compounds, 1-Ring Pyrans/Benzopyrans/Chromones Flavonoids/Flavonols |
111.13 137.00 173.00 221.15 291.05 |
CID 5,281,614 |
| 7.764 | 83,865 (0.81) | 271.0603 | 271.0601 |
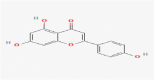 C15H10O5 |
Apigenin | eterocyclic Compounds/Heterocyclic Compounds, 1-Ring/Pyrans Benzopyrans/Chromones Flavonoids/Flavones |
117.0336 118.0351 182.04207 66.00433 89.03706 |
CID 5,280,443 |
| 8.024 | 53,853 (0.52) | 301.0714 | 301.0714 |
 C16H12O6 |
Isokaempferide | Heterocyclic Compounds/Heterocyclic Compounds, 1-Ring/Pyrans/Benzopyrans/Chromones Flavonoids |
91.0542 121.0284 165.0181 258.0523 286.0472 |
CID 5,280,862 |
| 8.375 | 88,053 (0.85) | 346.2590 | 246.2590 |
 C18H32O5 |
(11E,15Z)-9,10,13-trihydroxyoctadeca-11,15-dienoic acid | Natural products/endogenous metabollite | 109.1009 155.1061 173.1166 275.1995 311.2206 |
Legacy ID 1416 |
| 8.768 | 10,038 (0.10) | 348.2750 | 348.2739 |
 C18H34O5 |
(12Z)-9,10,11-trihydroxyctadec-12-enoic-acid | Natural products | 173.1171 213.1483 295.2265 331.2474 |
Legacy ID 2278 |
| 9.015 | 24,979 (0.24) | 226.0871 | 226.0874 |
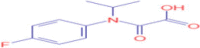 C11H12FNO3 |
Flufenacet OXA | organic acid/carboxylic acid/monocarboxylic acid | 110.0401 138.0350 180.0819 |
CID 16,212,222 |
| 9.346 | 128,084 (1.23) | 345.0970 | 345,0974 |
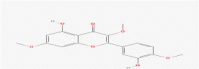 C18H16O7 |
Ayanin | Benzopurans/Chromones/Flavonoid | 340 345 350 |
CID 5,280,682 |
| 9.894 | 1,020,289 (9.82) | 345.0987 | 345.0991 |
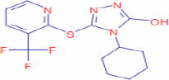 C14H15F3N4OS |
4-Cyclohexyl-5-{[3-(trifluoromethyl)-2-pyridyl}-4H-1,2,4-triazol-3-ol | – | 263.0209 243.0148 83.0855 |
CID 2,744,619 |
| 10.329 | 33,794 (0.33) | 359.1135 | 359.1125 |
 C19H18O7 |
Retusin | Heterocyclic compounds, Fused – Ring Hetercoyclic Compounds, 2-Ring/Benzopyrans/Chromones/Flavonoid |
344.1 345 359.1 |
CID 5,352,005 |
| 10.857 | 1,972,062 (18.98) | 306.2075 | 306.2064 |
 C18H27NO3 |
Capsaicin | Endogenous metabolite | 306.2064 206.1903 137.0597 |
CID 1,548,943 |
| 11.518 | 1,634,610 (15.73) | 137.0637 | 138.0662 |
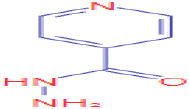 C6H7N3O |
Isoniazid | Therapeutics/Prescription Drugs | 138.0662 121.0396 79.0417 |
CID 3767 |
| 12.200 | 322,507 (3.10) | 318.3013 | 318.3003 |
 C18H39NO3 |
2-Amino-1,3,4-octadecanetriol | Endogenous Metabolites | 318.3003 282.2791 247.2422 |
CID 122,121 |
| 13.008 | 22,296 (0.21) | 295.2275 | 295.2269 |
 C18H30O3 |
9-Oxo-ODE | Endogenous Metabolites | 295.2264 277.2159 171.1015 |
CID 9,839,084 |
| 13.338 | 333,051 (3.20) | 317.2110 | 317.2101 |
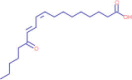 C18H30O3 |
13-OxoODE | Endogenous Metabolites | 317.2105 299.2001 179.1792 67.0535 |
CID 6,446,027 |
| 13.916 | 237,487 (2.29) | 604,3870 | 604,3876 |
 C36H45N9 |
2,4,6-Tris(4-benzylpiperazin-1-yl)-1,3,5-triazine | – | – | CID 3,393,793 |
| 14.288 | 154,813 (1.49) | 324.3857 | 324.289 |
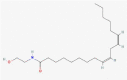 C20H37NO2 |
Linoleoyl ethanolamide | Fatty Acids Fatty Adids, unsaturated Fatty acids, essenstial Linoleic Acids |
324.2889 325.2924 306.2794 307.3640 263.2363 |
CID 5,283,446 |
| 14.569 | 9930 (0.10) | 758,5707 | 784.4609 |
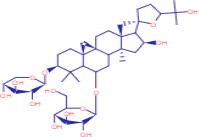 C41H68O14 |
Endogenpus Metabolites | 784.34 742.28 503.27 283.20 |
CID 122,690 | |
| 14.836 | 119,858 (1.15) | 268,2637 | 267.4 |
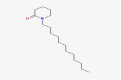 C17H33NO |
1-Dodecylpiperidin-2-one | – | – | CID 179,867 |
| 15.448 | 436,534 (4.20) | 282,2769 | 282.2791 |
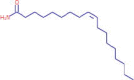 C18H35NO |
Oleamide | Endogenous Metabolites | 256.2635 135.1168 |
CID 5,283,387 |
| 15.673 | 1,385,218 (13.33) | 270.2815 | 270,2797 |
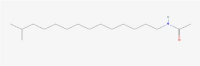 C17H35NO |
N-(13-Methyltetradecyl)acetamide | Fatty Acids, volatile Acetates Acetamides |
– | CID 47,346 |
| 16.263 | 158,962 (1.53) | 284,2979 | 284.294 |
 C18H37NO |
Octadecanamide | Carboxamide Monocarboxylic acid amide Fatty amide |
284.2943 285.2974 266.2828 84.0793 |
CID 31,292 |
| 16.812 | 24,561 (0.24) | 294.2184 | 294.1852 |
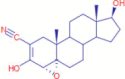 C20H27NO3 |
Trilostane | Therapeutics | 330.2064 312.1958 347.2324 352.1883 |
CID 656,583 |
| 17.402 | 8299 (0.08) | 798,6068 | 798,6036 |
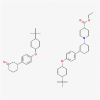 C52H79NO5 |
3-[4-(4-Tert-butylcyclohexyl)oxyphenyl]cyclohexan-1-one;ethyl 1-[3-[4-(4-tert-butylcyclohexyl)oxyphenyl]cyclohexyl]piperidine-4-carboxylate | – | – | CID 160,722,715 |
| 18.310 | 113,757 (1.09) | 798,6068 | 798,6755 |
 C38H75N7O8 |
N-[3-[2-[2-[3-(tert-butylamino)propoxy]ethoxy]ethoxy]propyl]-5-[(8S,9S)-3-[2-[2-[tert-butyl(methyl)amino]ethoxy]ethyl]-8,9-dimethoxy-5,7,8,9-tetrahydro-4H-triazolo[4,5-d]azocin-6-yl]-5-oxopentanamide;methane | – | – | CID 158,763,817 |
3.2. Potency of MVFE compounds as competitive inhibitor for oxLDL interaction with CD36, SRA1, and LOX1 using molecular docking
CD36, SRA1, LOX1 are important SR responsible for continuous uptake of oxLDL, thus contribute to the dysregulation of lipid metabolism inside the artery and atherosclerosis plaque initiation. Therefore, these SR were selected to be potential target for molecular docking analysis. Table 2 summarize the affinity binding between CD36, SRA1, LOX1 with active ingredients of MVFE from LC-MS/MS results.
Table 2.
Affinity binding score of docked secondary metabolites from MVFE with CD36, LOX1, and SRA-1.
| The receptor | Secondary metabolites (Pubchem ID) | Binding Afinity (kcal/mol) | Hydrogen Bond | Hydrophobic Bond Interaction |
|---|---|---|---|---|
| CD36 | Simvastatin (54454) | −5.3 | SER A:168, ASN A:139, PHE A:170, SER A:167 | PRO A:185 |
| Retusin (5352005) | −6.4 | GLN A:74, SER A:167, VAL A:172, ASP A:184 | GLN A;171, PRO A:185, PHE A:170, PRO A:73, MET A:77 | |
| Ayanin (5280682) | −6.2 | GLN A:74, SER A:167, VAL A:172, SER A:168, ASP A:184 | GLN A:171, PRO A:185 | |
| Apigenin (5280443) | −6.1 | SER A:167, VAL A:172 | ||
| Kaempferide (5281666) | −5.9 | SER A:167, ASP A:184, VAL A:172 | PRO A:185 | |
| 15(16)EpODE (16061062) | −5.0 | ASN A:139, SER A:167, ASP A:184 | PRO A:73. VAL A:172 | |
| Gamma-Glutamyl-S-Allylcysteine (11346811) | −4.9 | PHE A:170, SER A:167, ASN A:139, SER A:168 | ||
| Capsaicin (1548943) | −4.9 | THR B:663, PRO A:191, LEU A:161, LYS A:164, | ||
| Linoleic Acid (5280934) | −4.7 | LYS A:164, LEU A:189, LEU A:161, PRO A:191, LEU B:669 | ||
| Oleamide (5283387) | −3.2 | GLN A:171 | PRO A:73, VAL A:172, ARG A:173 | |
| LOX1 | Simvastatin (54454) | −7,2 | PHE A:158 | LEU A:157 |
| Apigenin (5280443) | −8,4 | TYR A:197, SER A:160 | ILE B:149, PHE A:158, ALA B:194, LEU A:157 | |
| Kaempferide (5281666) | −7,6 | ASP A:147, SER A:160 | ILE B:149, PHE A:158, ILE A:149, TYR A:197, ALA B:194, LEU A:157 | |
| Ayanin (5280682) | −7,4 | PHE B:190 | LEU A:157, PHE A:158, ALA B:194, ILE B:149, LEU A:175, ILE A:149, SER A:160 | |
| Retusin (5352005) | −7,4 | SER A:160 | LEU A:157, PHE A:158, LEU A:175, ALA B:194, ILE A:149, ILE B:149 | |
| Capsaicin (1548943) | −7,3 | PHE A:158, ASP A:147 | ALA A:194, PHE A:190, ILE A:149, ILE B:149, PHE B:158, TYR A:197, ALA B:194 | |
| 15(16)EpODE (16061062) | −6,2 | PHE A:158, ASP A:147, SER A:160 | PHE A:158, PHE B:190, ALA B:194, ILE B:149, LEU A:175 | |
| Linoleic Acid (5280934) | −6,2 | SER A:160, LEU A:157 | PHE A:158, ILE B:149, LEU A:157, LEU A:175, ALA B:194, PHE B:190 | |
| Gamma-Glutamyl-S-Allylcysteine (11346811) | −5,9 | ASP A:147, ALA B:194, TYR B:197 | TRP A:148, LEU A:157 | |
| Oleamide (5283387) | −5,8 | ASP A:147, TYR A:156, PHE A:158 | PHE A:158, TRP A:148, LEU A:157, PHE B:190, ALA B:194, TYR B:197 | |
| SRA1 | Simvastatin (54454) | +35 | ASP A:29, GLU A:96, LYS A:54, ASP:30 | |
| Retusin (5352005) | −6,4 | ASP A:29, GLU A:96, ASP A:30, LYS A:54 | ||
| Ayanin (5280682) | −6,2 | ASP A:29, TRP A:32 | GLU A:96, ASP A:29, LYS A:54, ASP A:30 | |
| Apigenin (5280443) | −6,1 | ASP A:29. GLU A:96, LYS A:54, ASP A:30 | ||
| Kaempferide (5281666) | −5,9 | TRP A:32. ASP A:29 | GLU A:96, ALA A:55, LYS A:54, ASP A:30 | |
| Linoleic Acid (5280934) | −4,7 | GLU A:96 | LYS A:54, ALA A:55 | |
| Oleamide (5283387) | −4,2 | LYS A:54, ALA A:55 | ||
| Gamma-Glutamyl-S-Allylcysteine (11346811) | −2.8 | ASP A:30, SER A:95, GLU A:96 | LYS A:53, ALA A:55 | |
| Capsaicin (1548943) | −1.8 | ASP A:30, GLU A:96 | ASP A:29, LYS A:54 | |
| 15(16)EpODE (16061062) | −0.5 | ALA A:91, ASP A:30, CYS A:92 | ALA A:55, LYS A:54 |
Retusin, ayanin, apigenin, kaempferide showed higher binding affinities with CD36, SRA1, LOX1 compared to simvastatin, respectively. Interestingly, although EPS and capsaicin have lower binding affinity, they interact with CD36 through lysin residu, which was an essential amino acid to bind with oxLDL. The visualization of the binding completed with the amino acid residues and also the type of the bond was depicted below (Fig. 2).
Fig. 2.
Docking visualization of mixed vegetables fermentation extract compound with CD36, SRA1, and LOX1. The left panel of each picture represented the interaction between protein and ligand. The protein was shown in a ribbon diagram while the ligand was shown in stick representation. The 2D visualization of interaction in right panel showed different color: bright green for conventional hydrogen bond, soft green for van der walls bond. Magenta, pink, purple reflected the hydrophobic bond.
3.3. Network pharmacology, PPI network, and enrichment analysis
The top six ranks of flavonoids, flavanols, and phenol derivatives from molecular docking were used in this step. A network pharmacology visualizes and analyzes possible interactions between active ingredients from mixed vegetable with dyslipidemia, endothelial dysfunction and coronary arteriosclerosis (Fig. 3). The number of nodes, number of edges, average number of neighbors, network diameter, network radius, characteristic path length, clustering coefficient, network density, network heterogeneity, network centralization was 268, 482, 2644, 5, 3, 2088, 0, 0,014, 4689, 0,948, respectively.
Fig. 3.
Network Pharmacology and PPI network of secondary metabolites from MVFE and atherosclerosis-based disease. (A) Yellow box; blue triangle; pink arrow; blue box; orange box indicated botanical plants; active ingredients; list of gene targets of active ingredients which were similar with gene targets of diseases; list of gene targets of active ingredients but not the targets of diseases; the pathological process which was associated with coronary heart disease, respectively. (B1): blue; yellow circle; the mixed color in the middle represented the total gene targets of active ingredients from MVFE LC-MS/MS analysis; gene targets of pathological processes associated with CHD; the number of similar target genes between both circles, respectively. (B2) Multicolor section in the outer of each circle reflected biological process from PPI network analysis. The pink box represented the cluster of protein which contribute in the same signaling pathway related to hyperlipidemia and atherosclerosis.
The network showed that active ingredients from previous analysis were closely related to various target genes in dyslipidemia (GO:0033993, GO:0071396, GO:0019216, GO:0010888, GO:0010883, GO:0032368, GO:0006869), endothelial dysfunction (GO:1903037, GO:0030155, GO:0022407, GO:0007159, GO:0045321, GO:0002685, GO:0002694, GO:0002688, GO:0030595, GO:0050901, hsa04670), inflammation (GO:0006954, hsa04064, hsa04620, hsa04668), and atherosclerosis (hsa03320, hsa02010). The number of similar targets between active ingredients and diseases was 117 genes and hence used for further PPI network analysis. The significant enriched signaling pathways (FDR < 0.001) were shown in Table 3.
Table 3.
Biological process and pathways from PPI network.
| ID | Number of Genes involved | Category | Description | FDR |
|---|---|---|---|---|
| DOID:1936 | 6 | DISEASES | Atherosclerosis | 6.56E-7 |
| hsa05418 | 25 | KEGG Pathways | Fluid shear stress and atherosclerosis | 6.42E-27 |
| GO:0033993 | 48 | GO Biological Process | Response to lipid | 7.53E-31 |
| GO:0071396 | 37 | GO Biological Process | Cellular response to lipid | 3.45E-26 |
| GO:0019216 | 24 | GO Biological Process | Regulation of lipid metabolic process | 1.06E-14 |
| GO:0010888 | 6 | GO Biological Process | Negative regulation of lipid storage | 2.38E-7 |
| GO:0010883 | 7 | GO Biological Process | Regulation of lipid storage | 9.38E-7 |
| GO:0032368 | 9 | GO Biological Process | Regulation of lipid transport | 1.19E-6 |
| GO:0006869 | 12 | GO Biological Process | Lipid transport | 5.38E-6 |
| GO:0006954 | 29 | GO Biological Process | Inflammatory response | 7.15E-18 |
| hsa04064 | 16 | KEGG Pathways | NF-kappa B signaling pathway | 1.33E-16 |
| hsa04620 | 16 | KEGG Pathways | TLR signaling pathway | 1.33E-16 |
| hsa04668 | 22 | KEGG Pathways | TNF signaling pathway | 3.45E-24 |
| GO:1903037 | 20 | GO Biological Process | Regulation of leukocyte cell–cell adhesion | 4.87E-13 |
| GO:0030155 | 27 | GO Biological Process | Regulation of cell adhesion | 1.07E-12 |
| GO:0022407 | 21 | GO Biological Process | Regulation of cell–cell adhesion | 8.65E-12 |
| GO:0007159 | 8 | GO Biological Process | Leukocyte cell–cell adhesion | 1.02E-7 |
| GO:0045321 | 29 | GO Biological Process | Leukocyte activation | 1.05E-11 |
| GO:0002685 | 15 | GO Biological Process | Regulation of leukocyte migration | 1.88E-10 |
| GO:0002694 | 20 | GO Biological Process | Regulation of leukocyte activation | 2.65E-9 |
| GO:0002688 | 10 | GO Biological Process | Regulation of leukocyte chemotaxis | 1.73E-7 |
| GO:0030595 | 8 | GO Biological Process | Leukocyte chemotaxis | 4.59E-5 |
| GO:0050901 | 4 | GO Biological Process | Leukocyte tethering or rolling | 1.3E-4 |
| hsa04670 | 6 | KEGG Pathways | Leukocyte trans-endothelial migration | 1.6E-4 |
| hsa03320 | 3 | KEGG Pathways | PPAR signaling pathway | 0.0221 |
| hsa02010 | 3 | KEGG Pathways | ABC transporters | 0.0061 |
FDR: false discovery rate; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Gene and Genome; NFκB: Nuclear Factor Kappa B; TLR: Toll like receptor; TNF: Tumor Necrosis Factor; PPAR: Peroxisome Proliferator Activated Receptor.
3.4. The effect of fermentation extract on TC and LDL-c level in Rabbit exposed to HFD
Atherosclerosis begins with the alteration of lipid metabolism which is determined by the increasing of TC and LDL-c content in the circulation. To confirm the previous bioinformatic results, this recent study investigated the effect of MVFE on the improvement of lipid metabolism by observing the TC and LDL-c concentration in the circulation. Fig. 4 demonstrated the TC and LDL-c level after administered with MVFE 100 mg and 200 mg/kg BW.
Fig. 4.
The effect of MVFE on TC and LDL-c level in Rabbits supplemented with HFD. Data were expressed as mean ± SD, (n = 5). Values with different superscripts are significantly different p < 0,05 (* different to negative control and # different to MVFE 100 mg/kg BW). A significant difference in TC and LDL-c content were observed in animal group treated with MVFE compared to negative control group.
The data showed normal distribution and homogenous. Based on Anova one way test, there was significant differences among the groups. The TC and LDL concentration were higher in negative control group compared to normal group. Additionally, there was a decrease of TC and LDL content obtained in animal group treated with MVFE, dose dependently.
4. Discussion
Recently, a dietary supplement derived from natural products has become an important approach to inhibit the establishment and progression of cardiovascular disease (McChesney et al., 2007, Alissa and Ferns, 2012, Shen, 2015, Waltenberger et al., 2016, Asgary et al., 2018, Trejo-Moreno et al., 2018). The cardioprotective effect (antioxidant, anti-inflammatory, lipid metabolism regulator) of onion, garlic, cucumber, white cabbage, chili, tomato, and basil leave have been studied for a long time(Rivlin et al., 2006, Yang, 2018, Rachmawati et al., 2019, Yamani et al., 2021). Interestingly, many countries including Indonesia have traditional cuisine composed of mixed vegetable, herbs, and spices called sambal lalapan. Furthermore, a study by Ninfali and colleagues showed that the supplementation of aromatic herbs into vegetable salads increased the phenolic and ORAC values of the whole salad. Thus, based on potency to exhibit additive or even synergistic effect, the efforts to mix vegetables with herbs and spices based on Indonesian sambal lalapan formula which consists of cucumber, white cabbage, tomato, basil leaves, onion, garlic, and chili in a specific proportion should be conducted(Surya and Tedjakusuma, 2022).
Although sambal lalapan as a main traditional Indonesian dish is the best example of a mixed vegetable-plant based food, the frying method in making the sambal sauce and the preferable method of eating fresh lalapan vegetables could induce several negative effects (Surya and Tedjakusuma, 2022). Frying processing could lower the nutritional value of the active compound in vegetables. A study by Koh and Surh in 2015 showed that the levels of conjugated trienes and malondialdehyde increased with the frying frequency of the vegetables in school meals (Koh and Surh, 2015). The INTERHEART study where the data was collected from 52 countries observed a positive association between fried food intake and acute myocardial infarction. The choice of frying oil, the temperature, the frequency of oil affects the adverse effect. At high temperatures (150–200 °C), highly unsaturated fatty acid has a short frying life due to their susceptibility to oxidation. In addition, high frying temperature produces Saturated Fatty Acid (SFA) and high trans-fatty acids (TFA) up to 20% (Gadiraju et al., 2015). On the other hand, the outbreaks of diarrhea, the oxidation, the low bioavailability were obtained after consuming raw vegetables (Callejón et al., 2015).
Therefore, spontaneous fermentation stands out as a useful technology to produce novel nutritional supplement agents. Fermentation is based on the action of different enzymes as well as microorganisms, which facilitates the extraction of polyphenols and other compounds(Hur et al., 2014). In this present study, we used LC-MS/MS to characterize the compound in this mixture. This approach is crucial for identifying low-molecular substances including fatty acids, sterols, nucleosides, because of its sensitivity, specificity, and selectivity. Several important flavonoids were detected including apigenin, kaempferide, ayanin, fisetin, and retusin. Besides that, several fatty acids like oleamide, α-linoleic acid, capsaicin, dihydrocapsaicin, stearamide; peptides such as cysteine and arginine; polysaccharide EPS were identified in the mixture. In addition, enzyme like NVP-231 and 1,4-Anhydro-5-(benzoylamino)-2,5-dideoxy-2-[(4,4-difluorocyclohexyl)amino]-D-arabinitol were also characterized. All of the recognized compounds were consistent with result of other studies quantifying the phytochemical from fermented vegetables. Although we did not quantify each compound's concentration, previous studies' data support our result. The concentration of apigenin-7-O-glucoside and free apigenin was noticeably higher in fermented (0.42 mg/ml, 0.25 mg/ ml) in comparison with native chamomile extract(Vukmirović et al., 2016). The lactic acid bacteria in natural fermentation mediate the conversion of flavonoid glycosides into flavonols, quercetin, and kaempferol which are commonly found in Brassica vegetables and onions (Allium cepa)(Gorinstein et al., 2009). A study by Vollmer and colleagues showed that in vitro fermentation using fecal sample lowered the concentration of kaempferol from 232 ± 16 µM (o hour) to 170 ± 8.32 µM (24 h), but increased the kaempferol metabolite content such as 3-(4-hydroxyphenyl) propionic acid (4-HPPA)(Vollmer et al., 2018). Exopolysaccharide and oleamide are synthesized exclusively by LAB during fermentation and cannot be found in fresh vegetable(Gu et al., 2020, Sørensen et al., 2022).
Many reports mentioned the cardioprotective effect of fermentation extract were caused by the action of flavonoids, peptides, fatty acid, and polysaccharide. Inhibition of cardiovascular disease targeted 2 important processes, including improving lipid profile and inhibiting atherosclerosis plaque formation. The improvement of lipid profile by extract administration were investigated using in vivo study. Dyslipidemia determines by TC > 5.17 mmol/l or TG > 1.7 mmol/l or HDL-c < 1.03 mmol/l in men, and HDL-c < 1.29 mmol/l in women or LDL-c > 4.1 mmol/l(Alshehri and Alshehri, 2010). Our findings showed that the extract of mixed vegetable fermentation could lower the TC and LDL-c level dose dependently in Rabbit fed with HFD.
Moreover, the effect of the selected compounds on atherosclerosis establishment was analyzed using in silico study. The SR contributes to atherosclerosis are CD36, SRA1, and LOX1(Kzhyshkowska, Neyen and Gordon, 2012). Macrophage CD36 have a major role in foam cell atherosclerotic arterial lesion formation through its interaction with oxLDL, which triggers signaling cascades for inflammatory responses (Park, 2014). Another receptor, SRA1, which abundantly found in macrophage-tunica intima also mediates the uptake of modified lipoproteins, including oxidized and acetylated LDL (OxLDL and AcLDL). In contrast to the LDL receptor, both CD36 and SRA1 are not downregulated by intracellular cholesteryl ester accumulation and might therefore accelerate the atherosclerosis progression(van Eck et al., 2000, Cheng et al., 2021). Although LOX1 has not contribute prominently, but many reports demonstrated that this SR has similar function like CD36 and SRA1, and three of them aggravate the inflammatory process inside the artery(de Villiers et al., 1994, Kzhyshkowska et al., 2012). Interestingly, our result showed that the interaction of these receptors with active ingredient of fermentation extract had more negative binding energy compared to statin based on molecular docking result. The negative number of the binding energy indicates that the ligand was bound spontaneously without requiring any energy, whereas a positive value indicates that the binding required energy and could only occur under certain conditions. Therefore, we suggest that the active constituent from vegetable and herbs fermentation could inhibit the interaction of oxLDL and the SR.
Network pharmacology and PPI network complemented the docking result by understanding the predicted pathways of the compound in atherosclerosis inhibition(Ferreira et al., 2015, Yuan et al., 2017, Banerjee et al., 2019). The data from recent study showed that several target genes and protein was interact with the botanical metabolite and also disease associated with atherosclerosis through modulation of lipid metabolism (GO:0033993, GO:0071396, GO:0019216, GO:0010888, GO:0010883, GO:0032368, GO:0006869), endothelial dysfunction (GO:1903037, GO:0030155, GO:0022407, GO:0007159, GO:0045321, GO:0002685, GO:0002694, GO:0002688, GO:0030595, GO:0050901, hsa04670), inflammation (GO:0006954, hsa04064, hsa04620, hsa04668), and foam cell formation (hsa03320, hsa02010).
Taken together, the metabolomic profiling, in silico and in vivo study gave extensive evidence supporting the potency of fermentation extract to be expanded as a product candidate in cardiovascular disease prevention strategy. However, this work have several limitations. We did not quantify the suggested important compounds in fermentation product. We also did not assess the antioxidant capacity of the fermentation extract. We only performed the bioinformatic study, but did not measure the direct effect of MVFE on CD36, SRA1, LOX1 expression or other molecules contribute to atherosclerosis signaling pathway. Therefore, further studies are required to more specifically address these possibilities.
5. Conclusion
In the recent study, effects of MVFE on initial process of atherosclerosis were investigated. These results suggested that the fermentation extract could be potential candidate as the prevention of CHD through an improvement of lipid profile, endothelial dysfunction, and inhibition of atherosclerosis plaque formation.
CRediT authorship contribution statement
Ermin Rachmawati: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data curation, Visualization, Writing – original draft. Suharti Suharti: Methodology, Software, Validation, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition. Djanggan Sargowo: Conceptualization, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition. Larasati Sekar Kinasih: Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Yudi Her Octaviano: Validation, Formal analysis, Investigation, Writing – review & editing, Supervision, Project administration, Funding acquisition. Roihatul Mutiah: Investigation, Resources, Data curation. Mahrus Ismail: Resources, Data curation. Ahmad Munjin Nasih: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by research grants from Riset Kolaborasi Indonesia 2022, Grant Number 17.5.38/UN32.20.1/LT/2022.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alissa, E.M. and Ferns, G.A. (2012) ‘Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases’, Journal of Nutrition and Metabolism, 2012. Available at: https://doi.org/10.1155/2012/569486 [DOI] [PMC free article] [PubMed]
- Alshehri, A.M. and Alshehri, A. (2010) ‘Metabolic syndrome and cardiovascular risk’, Journal of Family and Community Medicine, 17. Available at: https://doi.org/10.4103/1319-1683.71987 [DOI] [PMC free article] [PubMed]
- Arockianathan, P.M. (2019) ‘Proximate composition, phytochemicals, minerals and antioxidant activities of Vigna mungo L. seed coat’, Bioinformation, 15(8), pp. 579–585. Available at: https://doi.org/10.6026/97320630015579. [DOI] [PMC free article] [PubMed]
- Asgary, S., Rastqar, A. and Keshvari, M. (2018) ‘Functional Food and Cardiovascular Disease Prevention and Treatment: A Review’, https://doi.org/10.1080/07315724.2017.1410867, 37(5), pp. 429–455. Available at: https://doi.org/10.1080/07315724.2017.1410867. [DOI] [PubMed]
- Banerjee, S. et al. (2019) ‘LC–MS/MS analysis and network pharmacology of Trigonella foenum-graecum – A plant from Ayurveda against hyperlipidemia and hyperglycemia with combination synergy’, Phytomedicine, 60(March), p. 152944. Available at: https://doi.org/10.1016/j.phymed.2019.152944. [DOI] [PubMed]
- Callejón, R.M. et al. (2015) ‘Reported foodborne outbreaks due to fresh produce in the united states and European Union: Trends and causes’, Foodborne Pathogens and Disease, 12(1), pp. 32–38. Available at: https://doi.org/10.1089/fpd.2014.1821. [DOI] [PubMed]
- Cheng, C. et al. (2021) ‘Recognition of lipoproteins by scavenger receptor class A members’, Journal of Biological Chemistry, 297(2), p. 100948. Available at: https://doi.org/10.1016/j.jbc.2021.100948. [DOI] [PMC free article] [PubMed]
- Davignon, J. and Ganz, P. (2004) ‘Role of endothelial dysfunction in atherosclerosis’, Circulation, 109(23 SUPPL.). Available at: https://doi.org/10.1161/01.cir.0000131515.03336.f8. [DOI] [PubMed]
- de Villiers W.J.S., et al. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J. Exp. Med. 1994;180(2):705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drašković Berger, M. et al. (2020) ‘Cabbage (Brassica oleracea L. var. capitata) fermentation: Variation of bioactive compounds, sum of ranking differences and cluster analysis’, Lwt, 133(March). Available at: https://doi.org/10.1016/j.lwt.2020.110083.
- Eberhardt J., Santos-Martins D., Tillack A.F. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. Journal of Chemical Information and Modeling. 2021 doi: 10.1021/acs.jcim.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaafi I.M., Musa K.H., Abdullah Sani N. Effect of oven and freeze drying on antioxidant activity, total phenolic and total flavonoid contents of fig (Ficus carica L.) leaves. Food Res. 2020;4(6):2114–2121. doi: 10.26656/fr.2017.4(6).072. [DOI] [Google Scholar]
- Fabricio, M.F. et al. (2022) ‘Effect of freeze-dried kombucha culture on microbial composition and assessment of metabolic dynamics during fermentation’, Food Microbiology, 101(May 2021). Available at: https://doi.org/10.1016/j.fm.2021.103889 [DOI] [PubMed]
- Ferreira L.G., et al. Molecular docking and structure-based drug design strategies. Molecules. 2015 doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadiraju, T. v et al. (2015) ‘Fried Food Consumption and Cardiovascular Health : A Review of Current Evidence’, (September), pp. 8424–8430. Available at: https://doi.org/10.3390/nu7105404. [DOI] [PMC free article] [PubMed]
- Gorinstein, S. et al. (2009) ‘A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables’, European Food Research and Technology, 228(6), pp. 903–911. Available at: https://doi.org/10.1007/s00217-008-1003-y.
- Gu, M., Silva, C.R. and Garcia, C. (2020) ‘Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits’.
- He, J. et al. (2021) ‘Fermentation characteristics and bacterial dynamics during Chinese sauerkraut fermentation by Lactobacillus curvatus LC-20 under varied salt concentrations reveal its potential in low-salt suan cai production’, Journal of Bioscience and Bioengineering, 132(1), pp. 33–40. Available at: https://doi.org/10.1016/j.jbiosc.2021.03.009. [DOI] [PubMed]
- Hur, S.J. et al. (2014) ‘Effect of fermentation on the antioxidant activity in plant-based foods’, Food Chemistry, 160, pp. 346–356. Available at: https://doi.org/10.1016/j.foodchem.2014.03.112. [DOI] [PubMed]
- Ji, F. di et al. (2007) ‘Note. Microbial changes during the salting process of traditional pickled Chinese cabbage’, Food Science and Technology International, 13(1), pp. 11–16. Available at: https://doi.org/10.1177/1082013207075952.
- Kiczorowski, P. et al. (2022) ‘Effect of fermentation of chosen vegetables on the nutrient , mineral , and biocomponent profile in human and animal nutrition’, Scientific Reports, pp. 1–13. Available at: https://doi.org/10.1038/s41598-022-17782-z. [DOI] [PMC free article] [PubMed]
- Koh, E. and Surh, J. (2015) ‘Food types and frying frequency affect the lipid oxidation of deep frying oil for the preparation of school meals in Korea’, Food Chemistry, 174, pp. 467–472. Available at: https://doi.org/10.1016/j.foodchem.2014.11.087. [DOI] [PubMed]
- Kzhyshkowska, J., Neyen, C. and Gordon, S. (2012) ‘Role of macrophage scavenger receptors in atherosclerosis’, Immunobiology, 217(5), pp. 492–502. Available at: https://doi.org/10.1016/j.imbio.2012.02.015. [DOI] [PubMed]
- Lee, L.S. et al. (2013) ‘Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits’, Molecules, 18(10), pp. 12548–12560. Available at: https://doi.org/10.3390/molecules181012548. [DOI] [PMC free article] [PubMed]
- Lee, K.W. et al. (2018) ‘Effects of different types of salts on the growth of lactic acid bacteria and yeasts during kimchi fermentation’, Food Science and Biotechnology, 27(2), pp. 489–498. Available at: https://doi.org/10.1007/s10068-017-0251-7. [DOI] [PMC free article] [PubMed]
- Li, M. et al. (2019) ‘Statins for the Primary Prevention of Coronary Heart Disease’, BioMed Research International, 2019. Available at: https://doi.org/10.1155/2019/4870350 [DOI] [PMC free article] [PubMed]
- Lippi, G. and Plebani, M. (2017) ‘Statins for Primary Prevention of Cardiovascular Disease’, Trends in Pharmacological Sciences, 38(2), pp. 111–112. Available at: https://doi.org/10.1016/j.tips.2016.11.011. [DOI] [PubMed]
- Lozano, W.M. et al. (2019) ‘Diet-Induced Rabbit Models for the Study of Metabolic Syndrome’, Animals, 9, p. 463. Available at: https://doi.org/10.3390/ani9070463. [DOI] [PMC free article] [PubMed]
- Major, N. et al. (2022) ‘Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions’, Foods, 11(9). Available at: https://doi.org/10.3390/foods11091218 [DOI] [PMC free article] [PubMed]
- Mathur, H., Beresford, T.P. and Cotter, P.D. (2020) ‘Health benefits of lactic acid bacteria (Lab) fermentates’, Nutrients, 12(6), pp. 1–16. Available at: https://doi.org/10.3390/nu12061679. [DOI] [PMC free article] [PubMed]
- McChesney J.D., Venkataraman S.K., Henri J.T. Plant natural products: Back to the future or into extinction? Phytochemistry. 2007;68(14):2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Meengs J.S., Roe L.S., Rolls B.J. ‘Vegetable variety: an effective strategy to increase vegetable intake in adults’. J. Acad. Nutr. Diet. 2012;112(8):1211–1215. doi: 10.1016/j.jand.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A., G.J. and Guillermo, G.-C. (2016) ‘Endothelial cell dysfunction and the pathobiology of atherosclerosis.’, Circulation Research, 176(1), pp. 139–148. Available at: https://doi.org/10.1161/CIRCRESAHA.115.306301.Endothelial. [DOI] [PMC free article] [PubMed]
- Miller M. Dyslipidemia and cardiovascular risk: The importance of early prevention. QJM. 2009;102(9):657–667. doi: 10.1093/qjmed/hcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutiah, R. et al. (2019) ‘Metabolite Fingerprinting Eleutherine palmifolia (L .) Merr . Using UPLC-QTOF-MS / MS’, 24(December), pp. 139–159. Available at: https://doi.org/10.22146/mot.44883.
- Nascimento L.B.D.S., et al. Comparison between fermentation and ultrasound-assisted extraction: which is the most efficient method to obtain antioxidant polyphenols from sambucus nigra and punicagranatum fruits? Horticulturae. 2021;7(10):1–12. doi: 10.3390/horticulturae7100386. [DOI] [Google Scholar]
- Newman, C.B. et al. (2019) Statin Safety and Associated Adverse Events A Scientific Statement from the American Heart Association, Arteriosclerosis, Thrombosis, and Vascular Biology. Available at: https://doi.org/10.1161/ATV.0000000000000073 [DOI] [PubMed]
- Ninfali P., et al. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005;93(2):257–266. doi: 10.1079/bjn20041327. [DOI] [PubMed] [Google Scholar]
- Ohki I., et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure. 2005;13(6):905–917. doi: 10.1016/j.str.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Park Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014;46(6):e99–e107. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Adsit F.G., Boyington J.C. The 1.4 Å crystal structure of the human oxidized low density lipoprotein receptor lox-1. J. Biol. Chem. 2005;280(14):13593–13599. doi: 10.1074/jbc.M500768200. [DOI] [PubMed] [Google Scholar]
- Poelman A.A.M., et al. Multiple vs single target vegetable exposure to increase young children’s vegetable intake. J. Nutr. Educ. Behav. 2019;51(8):985–992. doi: 10.1016/j.jneb.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Rachmawati, E. and Muhammad, R.F. (2021) ‘The ethanolic extract of holy basil leaves (Ocimum sanctum L.) attenuates atherosclerosis in high fat diet fed rabbit’, AIP Conference Proceedings, 2353(May). Available at: https://doi.org/10.1063/5.0052561.
- Rachmawati N.A., Wasita B., Kartikasari L.R. Basil leaves (Ocimum sanctum linn.) extract decreases total cholesterol levels in hypercholesterolemia sprague dawley rats model. IOP Conf. Ser.: Mater. Sci. Eng. 2019;546(6):6–12. doi: 10.1088/1757-899X/546/6/062020. [DOI] [Google Scholar]
- Rivlin R.S., Budoff M., Amagase H. Significance of garlic and its constituents in cancer and cardiovascular disease. J. Nutr. 2006;136(3):716–725. doi: 10.1093/jn/136.3.v. [DOI] [Google Scholar]
- Sadiq, N. bin et al. (2021) ‘Postharvest drying techniques regulate secondary metabolites and anti-neuroinflammatory activities of Ganoderma lucidum’, Molecules, 26(15). Available at: https://doi.org/10.3390/molecules26154484. [DOI] [PMC free article] [PubMed]
- Shen B. A new golden age of natural products drug discovery. Cell. 2015;163(6):1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen, H.M. et al. (2022) ‘Exopolysaccharides of Lactic Acid Bacteria : Production , Purification and Health Benefits towards Functional Food’. [DOI] [PMC free article] [PubMed]
- Stein, R., Ferrari, F. and Scolari, F. (2019) ‘Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights’, Current Cardiology Reports, 21(8). Available at: https://doi.org/10.1007/s11886-019-1161-5 [DOI] [PubMed]
- Su G., et al. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinformatics. 2015;23(1):1–7. doi: 10.1002/0471250953.bi0813s47.BIOLOGICAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya, R. and Tedjakusuma, F. (2022) ‘Diversity of sambals , traditional Indonesian chili pastes’, Journal of Ethnic Foods [Preprint]. Available at: https://doi.org/10.1186/s42779-022-00142-7
- Szklarczyk D., et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(D1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T’Kindt, R. and van Bocxlaer, J. (2010) ‘LC-MS based metabolomics’, Handbook on Mass Spectrometry: Instrumentation, Data and Analysis, and Applications, 8(2), pp. 39–73. Available at: https://doi.org/10.1007/978-1-4419-9863-7_1155
- Thompson R.C., et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013;381(9873):1211–1222. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- Trejo-Moreno, C. et al. (2018) ‘Cucumis sativus Aqueous Fraction Inhibits Angiotensin II-Induced Inflammation and Oxidative Stress In Vitro’, Nutrients 2018, Vol. 10, Page 276, 10(3), p. 276. Available at: https://doi.org/10.3390/NU10030276. [DOI] [PMC free article] [PubMed]
- van Breda, S.G.J. and de Kok, T.M.C.M. (2018) ‘Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases’, Molecular Nutrition and Food Research, 62(1), pp. 1–12. Available at: https://doi.org/10.1002/mnfr.201700597. [DOI] [PubMed]
- van Eck, M. et al. (2000) ‘Effect of human scavenger receptor class A overexpression in bone marrow-derived cells on cholesterol levels and atherosclerosis in apoE-deficient mice’, Arteriosclerosis, Thrombosis, and Vascular Biology, 20(12), pp. 2600–2606. Available at: https://doi.org/10.1161/01.ATV.20.12.2600. [DOI] [PubMed]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. Journal of Computational Chemistry. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stokkom V.L., et al. Combinations of vegetables can be more accepted than individual vegetables. Food Qual. Prefer. 2019;72(October 2018):147–158. doi: 10.1016/j.foodqual.2018.10.009. [DOI] [Google Scholar]
- Vollmer M., et al. Mutual interaction of phenolic compounds and microbiota: metabolism of complex Phenolic Apigenin-C- and Kaempferol-O-derivatives by human Fecal samples. J. Agric. Food Chem. 2018;66(2):485–497. doi: 10.1021/acs.jafc.7b04842. [DOI] [PubMed] [Google Scholar]
- Vukmirović S., et al. Fermentation potentiates antimotility properties of chamomile ligulate flower extracts. Indian J. Pharm. Sci. 2016;78(5):691–694. doi: 10.4172/pharmaceutical-sciences.1000170. [DOI] [Google Scholar]
- Waltenberger, B. et al. (2016) ‘Natural Products to Counteract the Epidemic of Cardiovascular and Metabolic Disorders’, Molecules 2016, Vol. 21, Page 807, 21(6), p. 807. Available at: https://doi.org/10.3390/MOLECULES21060807. [DOI] [PMC free article] [PubMed]
- Yamani N., et al. Meta-analysis evaluating the impact of chili-pepper intake on all-cause and cardiovascular mortality: a systematic review. Ann. Med. Surg. 2021;70(September) doi: 10.1016/j.amsu.2021.102774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D.K. (2018) ‘Cabbage (Brassica oleracea var. capitata) Protects against H2O2-Induced Oxidative Stress by Preventing Mitochondrial Dysfunction in H9c2 Cardiomyoblasts’, Evidence-based Complementary and Alternative Medicine, 2018. Available at: https://doi.org/10.1155/2018/2179021. [DOI] [PMC free article] [PubMed]
- Yuan, H. et al. (2017) ‘How Can Synergism of Traditional Medicines Benefit from Network Pharmacology?’, Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry, 22(7). Available at: https://doi.org/10.3390/MOLECULES22071135 [DOI] [PMC free article] [PubMed]
- Zabat, M.A. et al. (2018) ‘Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community’, Foods, 7(5). Available at: https://doi.org/10.3390/foods7050077 [DOI] [PMC free article] [PubMed]