Summary
The modified 5-item frailty index (mFI-5), as a measure of frailty and biological age, has been shown to be a reliable predictor of complications and mortality in a variety of surgical specialties. However, its role in burn care remains to be fully elucidated. We, therefore, correlated frailty with in-hospital mortality and complications after burn injury. The medical charts of all burn patients admitted between 2007 and 2020 who had ≥ 10 % of their total body surface area affected were retrospectively reviewed. Data on clinical, demographic, and outcome parameters were collected and evaluated, and mFI-5 was calculated on the basis of the data obtained. Univariate and multivariate regression analyses were used to investigate the association between mFI-5 and medical complications and in-hospital mortality. A total of 617 burn patients were included in this study. Increasing mFI-5 scores were significantly associated with increased in-hospital mortality (p < 0.0001), myocardial infarction (p = 0.03), sepsis (p = 0.005), urinary tract infections (p = 0.006), and perioperative blood transfusions (p = 0.0004). They were also associated with an increase in the length of hospital stay and the number of surgical procedures, albeit without statistical significance. An mFI-5 score of ≥ 2 was a significant predictor of sepsis (odds ratio [OR] = 2.08; 95% confidence interval [CI]: 1.03 to 3.95; p = 0.04), urinary tract infection (OR = 2.82; 95% CI: 1.47 to 5.19; p = 0.002), and perioperative blood transfusions (OR = 2.61; 95% CI: 1.61 to 4.25; p = 0.0001). Multivariate logistic regression analysis revealed that an mFI-5 score of ≥ 2 was not an independent risk factor for in-hospital mortality (OR = 1.44; 95% CI: 0.61 to 3.37; p = 0.40). mFI-5 is a significant risk factor for only a few select complications in the burn population. It is not a reliable predictor of in-hospital mortality. Therefore, its utility as a risk stratification tool in the burn unit may be limited.
Keywords: Burns, Burn intensive care, Frailty, Frailty index, mFI-5
Introduction
Burn injuries are considered one of the most devastating forms of trauma and are associated with severe morbidity and mortality. Globally, 11 million burn injuries and nearly 200,000 burn-related deaths are recorded annually.1 In Europe, burns rank among the leading causes of accidental fatality.2 Accordingly, burn injuries have a substantial health economic impact. High mortality rates and frequent perioperative complications remain a major concern in severely burned patients, leading to prolonged length of hospital stay, increased healthcare costs, and the early suspension of clinical and social rehabilitation.3,4
Therefore, prediction of mortality and adverse events has become an essential component of clinical burn care, with continuous efforts being dedicated to establish objective and accurate proxies for the risk of complications after severe burn injuries. In this context, throughout the last decades, various prognostic indices and scores have been proposed, aiming to identify high-risk patients preemptively.5, 6, 7, 8 Such models commonly classify elderly patients as a particularly vulnerable subpopulation.
In recent years, the concept of frailty has additionally been implemented as a surrogate for biological age to enhance mortality prediction. Briefly, frailty describes a loss in physiological function and reserves beyond normal aging, causing a state of susceptibility and diminished resistance to systemic or external stressors, such as surgical procedures.9 Several frailty indices have been developed to facilitate the assessment of perioperative risk factors for postoperative adverse events and mortality. One of the earliest and most extensive risk assessment tools was the 70-item scale frailty index, which was based on the Canadian Study of Health and Aging (CSHA) and has shown high prognostic value in predicting adverse surgical outcomes.10 Accordingly, a similar 11-item frailty index was created on the basis of the American College of Surgeons National Surgical Quality Improvement Program database, which was further reduced to a 5-factor index (the modified 5-item frailty index [mFI-5]).11 This simplified version has been shown to yield consistent predictions of postoperative complications and mortality and has been validated in different cohorts undergoing various surgical procedures.12, 13, 14 Despite this well-documented role of mFI-5 as an accurate risk stratification tool, its applicability and utility for predicting outcomes in patients with severe burns are yet to be determined. Therefore, we aimed to assess the predictive value of mFI-5 for complications and in-hospital mortality in a single-center burn population and to evaluate its potential role in burn-related risk stratification.
Methods
Study design and data extraction
In this retrospective analysis, all data were retrieved from the records of burn patients admitted to the burn unit at Hannover Medical School from March 2007 to December 2020. All patients aged ≥ 16 years who presented with an affected total body surface area (TBSA) of ≥ 10% were included. All included patients were stratified according to mFI-5, which yielded 3 cohorts: mFI-5 = 0, mFI-5 = 1, and mFI-5 ≥ 2.
Clinical and demographic variables, including age, sex, TBSA, abbreviated burn severity index score, burn location, inhalation injury, burn severity, comorbidities, and the cause of the burn accident at the time of the burn injury, were analyzed. Similarly, outcome parameters, such as medical complications, in-hospital mortality, date and cause of death, blood transfusions, number of surgical procedures, total and intensive care unit length of stay, and mechanical ventilation, were also evaluated. To calculate mFI-5, the following comorbidities were considered: hypertension requiring medication, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, and totally or partially dependent functional health status (Table 1). Consistent with previous literature,15, 16, 17 the mFI-5 score was calculated by dividing the sum of all positive variables by the total number of input variables in the database, with a higher mFI-5 score indicating a higher degree of frailty. This research was approved by the Ethics Committee of Hannover Medical.
Table 1.
Variables included in the modified 5-item frailty index.
| Hypertension requiring medication |
| History of chronic obstructive pulmonary disease |
| History of congestive heart failure |
| Diabetes mellitus |
| Totally or partially dependent functional health status |
Statistical analysis
All data were processed and saved using Microsoft Excel (Version 16, Microsoft Corporation, Redmond, WA). All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA). Categorical variables are expressed as numbers and percentages, and their differences were measured by the χ2 or binomial test. Continuous variables are expressed as means and standard deviations, and their differences were assessed by one-way analysis of variance. All complications were evaluated by univariate logistic analysis to decipher the independent effect of an mFI-5 score of ≥ 2 on complications as outcome parameters. Multivariate logistic regression analysis was performed to evaluate potential risk factors, including mFI-5, for in-hospital mortality. These were reported as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Kaplan–Meier curves were plotted to show the survival probabilities of the patient cohorts. Statistical difference was calculated using the log-rank (Mantel–Cox) test. A p value of <0.05 was considered significant for all statistical purposes.
Results
A total of 617 patients admitted to our burn center for burn injuries during the aforementioned study period were included. Detailed descriptive statistics of the patients’ demographic and burn variables are shown in Tables 2 and 3.
Table 2.
Descriptive statistics of demographic and clinical parameters.
| Variable | mFI-5 = 0 (n = 405) | mFI-5 = 1 (n = 135) | mFI-5 ≥ 2 (n = 77) | p value |
|---|---|---|---|---|
| Age (years), mean (SD) | 43.1 (16.5) | 55.9 (18.5) | 67.7 (15.2) | <0.0001 |
| Age group (years) | ||||
| 16–24 | 63 (15.6) | 9 (6.7) | 2 (2.6) | 0.001 |
| 25–44 | 161 (39.8) | 25 (18.5) | 3 (3.9) | <0.0001 |
| 45–64 | 142 (35.1) | 60 (44.4) | 23 (29.9) | 0.17 |
| ≥ 65 | 39 (9.6) | 41 (30.4) | 49 (63.6) | <0.0001 |
| Male sex | 299 (73.8) | 95 (70.4) | 48 (62.3) | 0.54 |
| mFI-5 | ||||
| Hypertension | 0 (0.0) | 69 (51.1) | 60 (77.9) | 0.02 |
| COPD | 0 (0.0) | 5 (3.7) | 14 (18.2) | 0.001 |
| CHF | 0 (0.0) | 5 (3.7) | 12 (15.6) | 0.005 |
| Diabetes | 0 (0.0) | 8 (5.9) | 45 (58.4) | <0.0001 |
| Dependent functional status | 0 (0.0) | 48 (35.6) | 42 (54.5) | 0.05 |
| Coronary artery disease | 9 (2.2) | 9 (6.7) | 12 (15.6) | <0.0001 |
| Peripheral arterial disease | 5 (1.2) | 1 (0.7) | 3 (3.9) | 0.15 |
| Arrhythmia | 37 (9.1) | 23 (17.0) | 29 (37.7) | <0.0001 |
| Renal insufficiency | 25 (6.2) | 18 (13.3) | 16 (20.8) | 0.0002 |
Bold values indicate statistically significant differences.
Abbreviations: mFI-5, modified 5-item frailty index; COPD, chronic obstructive pulmonary disease; CHF, chronic heart failure; SD, standard deviation. Data are presented as n (%), unless otherwise stated.
Table 3.
Burn characteristics.
| Variable | mFI-5 = 0 (n = 405) | mFI-5 = 1 (n = 135) | mFI-5 ≥ 2 (n = 77) | p value |
|---|---|---|---|---|
| TBSA (%), mean (SD) | 22.6 (15.2) | 27.2 (19.6) | 19.9 (10.9) | 0.002 |
| TBSA | ||||
| 10–19.9 | 230 (56.8) | 59 (43.7) | 40 (51.9) | 0.19 |
| 20–29.9 | 78 (19.3) | 34 (25.2) | 28 (36.4) | 0.01 |
| ≥30 | 97 (24.0) | 42 (31.1) | 9 (11.7) | 0.02 |
| ABSI score, mean (SD) | 6.2 (2.1) | 7.4 (2.4) | 7.6 (1.7) | <0.0001 |
| Full-thickness burns | 157 (38.8) | 62 (45.9) | 45 (58.4) | 0.04 |
| Inhalational injury | 60 (14.8) | 19 (14.1) | 19 (24.7) | 0.12 |
| Burn etiology | ||||
| Flame/Contact | 223 (55.1) | 77 (57.0) | 41 (53.2) | 0.93 |
| Scalding | 60 (14.8) | 29 (21.5) | 26 (33.8) | 0.001 |
| Explosion/Deflagration | 104 (25.7) | 26 (19.3) | 10 (13.0) | 0.06 |
| Chemical | 9 (2.2) | 2 (1.5) | 0 (0.0) | 0.39 |
| Electricity | 9 (2.2) | 2 (1.5) | 0 (0.0) | 0.39 |
| Body area | ||||
| Face/Neck/Scalp | 247 (61.0) | 79 (58.5) | 34 (44.2) | 0.21 |
| Hands | 219 (54.1) | 68 (50.4) | 26 (33.8) | 0.071 |
| Arms | 292 (72.1) | 100 (74.1) | 44 (57.1) | 0.32 |
| Feet | 48 (11.9) | 27 (20.0) | 12 (15.6) | 0.09 |
| Legs | 218 (53.8) | 73 (54.1) | 40 (51.9) | 0.98 |
| Thorax | 189 (46.7) | 71 (52.6) | 31 (40.3) | 0.44 |
| Abdomen | 101 (24.9) | 40 (29.6) | 16 (20.8) | 0.44 |
| Back/Flanks | 112 (27.7) | 57 (42.2) | 30 (39.0) | 0.02 |
| Genital area | 28 (6.9) | 12 (8.9) | 11 (14.3) | 0.11 |
| Burn incident location | ||||
| Home | 193 (47.7) | 86 (63.7) | 62 (80.5) | 0.0006 |
| Workplace | 80 (19.8) | 13 (9.6) | 3 (3.9) | 0.0008 |
| Recreational | 111 (27.4) | 25 (18.5) | 10 (13.0) | 0.02 |
| Other | 17 (4.2) | 2 (1.5) | 1 (1.3) | 0.19 |
| Suicide attempt | 17 (4.2) | 13 (9.6) | 1 (1.3) | 0.02 |
Bold values indicate statistically significant differences.
Abbreviations: mFI-5, modified 5-item frailty index; TBSA, total body surface area; ABSI, abbreviated burn severity index; SD, standard deviation. Data are presented as n (%), unless otherwise stated.
Stratification by mFI-5 revealed that the majority of the included patients presented with an mFI-5 score of 0 (65.6 %). Of the total 617 patients, 21.9% presented with an mFI-5 score of 1 and 12.5% presented with an mFI-5 score of ≥ 2. With an increasing mFI-5 score, patients were more likely to suffer from domestic burn injuries (p < 0.001), whereas work-related burns and injuries during recreational activities were mostly observed in patients with an mFI-5 score of 0. Our results demonstrated a progressive increase in the age range and proportional incidence of most comorbidities with increasing mFI-5 score. Although the mean affected TBSA was highest in patients with an mFI-5 score of 1 (27.2), the abbreviated burn severity index score showed an increase with higher mFI-5 scores (p < 0.001). Accordingly, the number of full-thickness burns (p = 0.04) increased with higher mFI-5 scores (see Table 4).
Table 4.
Perioperative outcomes.
| Variable | mFI-5 = 0 (n = 405) | mFI-5 = 1 (n = 135) | mFI-5 ≥ 2 (n = 77) | p value |
|---|---|---|---|---|
| LOS (days), mean (SD) | 24.9 (25.0) | 26.1 (20.9) | 28.9 (17.2) | 0.37 |
| LOS in ICU (days), mean (SD) | 14.8 (20.8) | 16.6 (19.9) | 20.4 (16.9) | 0.07 |
| Surgical intervention rate, mean (SD) | 3.5 (3.9) | 3.4 (3.3) | 3.6 (2.9) | 0.93 |
| Mechanical ventilation | 131 (32.3) | 63 (46.7) | 33 (42.9) | 0.04 |
| Mechanical ventilation (hours), mean (SD) | 58.6 (221.8) | 119.8 (352.2) | 71.5 (181.4) | 0.05 |
| Complications | ||||
| Myocardial infarction | 5 (1.2) | 6 (4.4) | 4 (5.2) | 0.03 |
| Pneumonia | 44 (10.9) | 19 (14.1) | 13 (16.9) | 0.31 |
| Pulmonary embolism | 3 (0.7) | 2 (1.5) | 1 (1.3) | 0.72 |
| Thrombosis | 15 (3.7) | 3 (2.2) | 4 (5.2) | 0.53 |
| Sepsis | 28 (6.9) | 20 (14.8) | 13 (16.9) | 0.005 |
| Urinary tract infection | 33 (8.1) | 13 (9.6) | 16 (20.8) | 0.006 |
| In-hospital mortality | 36 (8.9) | 32 (23.7) | 21 (27.3) | <0.0001 |
| Perioperative blood transfusions | 110 (27.2) | 54 (40.0) | 41 (53.2) | 0.0004 |
| Red blood cells (units), mean (SD) | 3.0 (7.8) | 4.4 (8.9) | 4.2 (6.4) | 0.14 |
| Platelets (units), mean (SD) | 0.2 (1.8) | 0.2 (0.7) | 0.1 (0.4) | 0.86 |
| Fresh frozen plasma (units), mean (SD) | 1.7 (8.2) | 2.5 (7.2) | 1.5 (4.8) | 0.52 |
Bold values indicate statistically significant differences.
Abbreviations: mFI-5, modified 5-item frailty index; LOS, length of stay; ICU, intensive care unit; SD, standard deviation. Data are presented as n (%), unless otherwise stated.
Similarly, medical complications, such as myocardial infarction, pneumonia, pulmonary embolism, thrombosis, sepsis, and urinary tract infection, were observed more frequently with higher frailty scores. Significant differences in the occurrence of complications among the cohorts were noted for myocardial infarction (p = 0.03), sepsis (p = 0.005), urinary tract infection (p = 0.006), and perioperative blood transfusions (p = 0.0004).
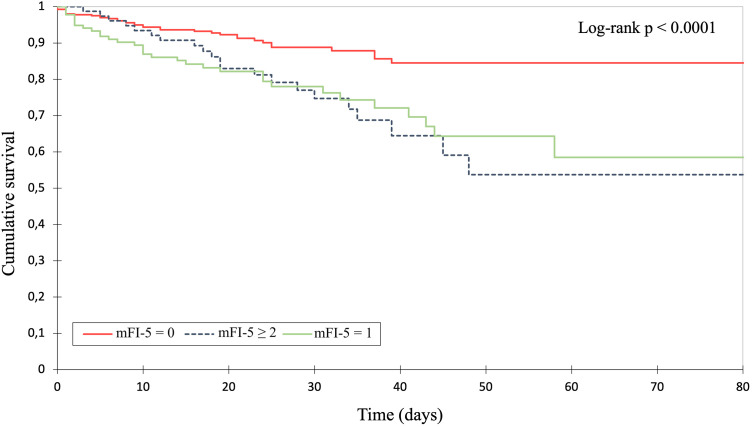
Regarding the outcome parameters, the total and intensive care unit length of stay as well as the surgical intervention rate were higher with an increasing mFI-5 score. Yet, this correlation showed no statistical significance (see Table 4). In-hospital mortality analysis revealed a significant decline in overall survival probability in patients with increasing frailty (p < 0.001). Figure 1 demonstrates the corresponding Kaplan–Meier curves of the stratified patient cohorts up to 80 days after admission (p < 0.0001, log-rank test).
Fig. 1.
Kaplan–Meier survival analysis of overall in-hospital survival after burn unit admission.
A univariate analysis was performed to assess the predictive value of an mFI-5 score of ≥ 2 for the occurrence of any complications (Table 5). Significant results were found for sepsis (OR = 2.08; 95% CI: 1.03 to 3.95; p = 0.04), urinary tract infection (OR = 2.82; 95% CI: 1.47 to 5.19; p = 0.002), and perioperative blood transfusions (OR = 2.61; 95% CI: 1.61 to 4.25; p = 0.0001).
Table 5.
Univariate regression analysis of a modified 5-item frailty index score of ≥ 2 with complications.
| Complications | OR | 95% CI | p value |
|---|---|---|---|
| In-hospital mortality | 0.92 | 0.63 to 1.33 | 0.67 |
| Myocardial infarction | 2.64 | 0.72 to 7.93 | 0.13 |
| Pneumonia | 1.83 | 0.95 to 3.34 | 0.067 |
| Pulmonary embolism | 1.41 | 0.073 to 8.88 | 0.77 |
| Thrombosis | 1.59 | 0.45 to 4.40 | 0.44 |
| Sepsis | 2.08 | 1.034 to 3.95 | 0.04 |
| Urinary tract infection | 2.82 | 1.47 to 5.19 | 0.002 |
| Perioperative blood transfusions | 2.61 | 1.61 to 4.25 | 0.0001 |
Bold values indicate statistically significant differences.
Abbreviations: OR, odds ratio; CI, confidence interval.
When adjusted for confounders, multivariate regression analysis identified age (OR = 1.05; 95% CI: 1.02 to 1.09; p = 0.005), renal insufficiency (OR = 9.39; 95% CI: 4.26 to 21.44; p < 0.0001), TBSA (OR = 1.08; 95% CI: 1.03 to 1.14; p = 0.002), and full-thickness burns (OR = 4.07; 95% CI: 1.53 to 11.54; p = 0.006) as independent risk factors with significantly higher odds of in-hospital mortality (Table 6). An mFI-5 score of ≥ 2 was not an independent predictor of in-hospital mortality (OR = 1.44; 95% CI: 0.61 to 3.37; p = 0.40).
Table 6.
Multivariate regression analysis of risk factors for in-hospital mortality.
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| mFI-5 ≥ 2 | 1.44 | 0.61 to 3.37 | 0.40 |
| Age | 1.05 | 1.02 to 1.09 | 0.005 |
| Coronary artery disease | 1.45 | 0.45 to 4.34 | 0.52 |
| Arrhythmia | 1.79 | 0.83 to 3.83 | 0.13 |
| Renal insufficiency | 9.39 | 4.26 to 21.44 | <0.0001 |
| TBSA | 1.08 | 1.03 to 1.14 | 0.0017 |
| ABSI | 1.06 | 0.66 to 1.70 | 0.82 |
| Full-thickness burns | 4.07 | 1.53 to 11.54 | 0.006 |
Bold values indicate statistically significant differences.
Abbreviations: mFI-5, modified 5-item frailty index; OR, odds ratio; CI, confidence interval; TBSA, total body surface area; ABSI, abbreviated burn severity index.
Discussion
Rising life expectancy in high-income countries is echoed by prolonged healthy lifespans.18 Yet, as life expectancy increases, so does the incidence of comorbidities and functional impairments. As such, the burden of age-related pathologies may become a growing public health concern in future years. In this context, burn units will face the challenge of admitting increasing numbers of elderly burn patients with complex comorbidity profiles. Strikingly, in the field of burn care, senescence is widely linked to poor outcomes.19, 20, 21 Multimorbidity, immobility, and polypharmacy, all common phenomena that befall elderly patients in particular, have traditionally been hypothesized to underlie this observation.
Historically, age has been considered an integral pillar of survival prediction in burn care, linking advanced age with a decline in survival probability. Along with burn injury severity, it has been included as a mono-perspective physiologic variable in the most commonly used burn scores.6, 7, 8,22
Although differences in overall health status may significantly affect the capacity to recover from a burn wound, to date, most prognostic scores do not take into account medical impairments that may interfere with the patient's physiologic reserves and their ability to adequately respond to stressors. Accordingly, profound discrepancies between a patient's chronological age and biological health status are not reflected in the aforementioned scores. Owing to this nonconsideration of the mismatch between biological and chronological age, the prognostic value of commonly applied scores may be limited. Although it may not be surprising that advanced age is associated with poor outcomes, previous reports have indicated that the burden of frailty exceeds that of chronologic age alone by more than 28-fold.23
To date, the concept of frailty remains elusive and has, therefore, been the subject of controversy in the scientific literature over the past three decades, with a fluid boundary between physiologic and pathological aging.24 Still, the concept of frailty has gained popularity in recent years and has gradually been included in the preoperative evaluation of various surgical fields as a holistic alternative to chronologic age.
In Germany, the effect of frailty on overall health, morbidity, and survival probability has been recognized by the national Study on Adult Health in Germany (DEGS1) conducted by the Robert Koch Institute on behalf of the Federal Ministry of Health.25 The report emphasizes that the assessment of frailty helps identify high-risk cohorts, thereby providing an avenue to initiate preventative measures to counteract the further loss of functional abilities. These conclusions are reiterated in studies that have found an association between frailty and perioperative complications, higher rates of mortality, dependency, and prolonged hospital stay.26, 27, 28, 29 Similarly, an emergent body of evidence has shown the association of frailty with the need for critical care and discharge complexity.30, 31, 32
A wide array of frailty assessment tools have been proposed in the past, with the FRAIL index,9 the Frailty Index by Rockwood et al.,33 and the Edmonton Frailty Scale34 being the most frequently used. In principle, frailty measurement tools should provide evidence-based predictions while being easy to use and reliable in their application.
In the present study, we employed the validated mFI-5 tool owing to its previously documented wide and broad applicability and aimed to evaluate the prognostic value of the score on perioperative outcomes in severely burned patients. We found that patients were significantly more likely to experience myocardial infarction, sepsis, urinary tract infection, and perioperative blood transfusions with increasing frailty during hospitalization. In addition, higher mFI-5 scores were associated with a significant increase in mortality rates. Interestingly, we found that our most frail cohort experienced the lowest TBSA rates in the study population. Nonetheless, the effects of the patient's comorbidity profile and burn characteristics appeared to outweigh the effect of the affected TBSA given the higher mortality rates observed in this cohort.
In our burn population, an mFI-5 score of ≥ 2 seemed to be a valid outcome predictor of sepsis, urinary tract infection, and perioperative blood transfusions, which may render it a surrogate parameter for potential clinical resource strain. In contrast to renal insufficiency, TBSA affected, or full-thickness burns, an mFI-5 score of ≥ 2 was not found to be an independent risk factor for in-hospital mortality in our cohort. Likewise, it was also not found to be a reliable predictor of myocardial infarction, pneumonia, pulmonary embolism, or thrombosis, thus limiting its utility as an overall outcome and complication predictor in the burn population. Therefore, our findings suggest that the physiologic reserve quantified by mFI-5 cannot be considered a major determinant of significant complications or in-hospital mortality in burn patients, underscoring that risk stratification in acute burn care is much more complex.
To the best of our knowledge, this is the second study on the utility of mFI-5 in a burn unit population. Previously, Sen et al.35 performed a secondary analysis of the Transfusion Requirement in Burn Care Evaluation study and similarly assessed the predictive value of mFI-5. They included a total of 347 patients with an affected TBSA of ≥ 20%. The authors reported that an mFI-5 score of ≥ 2 was not independently associated with in-hospital mortality in their analysis, which is in line with our results. A further analysis of medical complications was not performed. Interestingly, they found that an 11-item frailty index score of >1 was independently associated with in-hospital mortality.
Regarding the predictive value of complications, our observations are consistent with the findings of previous studies that have evaluated large-scale datasets to assess the predictive value of mFI-5 with regard to selected perioperative complications, particularly in the field of orthopedic surgery.36
We acknowledge that the simplified mFI-5 score may be convenient to assess various elective surgical populations given its straightforward composition and easy calculation. Accordingly, we anticipate that mFI-5 will be increasingly adopted as a risk stratification tool in elective surgical procedures. However, in the acute setting, where the necessary parameters cannot be evaluated in a timely manner owing to unavailability, inability to verify, or lack of patient responsiveness, it may serve as a suboptimal assessment tool. As such, the utility of the mFI-5 score for clinical decision making may be limited in the burn unit. This may also explain why the scoring of frailty in burn patients has not yet been implemented in the standardized outcome assessments.37,38 Further large-scale studies investigating a potential improvement in the prognostic value of established burn severity scores by integrating frailty assessments are required.
In addition to frailty, other facets may play a crucial role in risk stratification in elderly burn patients and, therefore, warrant further research. For instance, Sgonc et al.39 found that elderly patients—despite the absence of frailty—may present with severe age-related skin alterations. Such changes in the dermal (micro)structure may manifest as loss of collagen, increase in the number of inflammatory cells, and reduced microvascularization, ultimately affecting wound healing and the depth of the burn wound. Several biomarkers have been suggested as objective measures of skin aging, e.g., inflammatory or apoptosis markers, hormonal measures, and length assessment of the telomeres.40 Therefore, the consideration of biological age using quantifiable biomarkers may be of interest for future studies, possibly providing a more universal tool for risk stratification in burn care.
Limitations
Our study has several limitations. First, all data, including frailty assessment data, were analyzed retrospectively. As a result, the effect of confounding factors or bias cannot be ruled out. Second, all results are based on in-hospital data, limiting our conclusions to short-term observations. Accordingly, robust long-term conclusions cannot be drawn. In addition, the present analysis only evaluated the association between frailty and in-hospital mortality and selected complications. The effects of burn patient frailty on further outcome parameters, such as readmission rates, functional levels after discharge, and costs of health burden, may be an imperative avenue for future research.
Conclusion
mFI-5 as a frailty assessment tool seems to reliably identify burn patients at risk of sepsis, urinary tract infection, and perioperative blood transfusions. However, it was not found to be an independent risk factor for in-hospital mortality and other serious medical complications. Its utility as an overall outcome and complication predictor in the burn population is, therefore, limited. Despite its limitations in the acute setting, it may help improve the prognostic value of established burn scores, particularly for elderly and frail patients. As an acquired deficit model, which accounts for functional ability and medical comorbidities, further studies on its possible incorporation in established burn prognostication tools are warranted.
Conflicts of Interest
None.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Ethical Approval
Not required.
References
- 1.“Burns”.WHO.WHO,Mar.2018.Web.31Mar.2022. https://www.who.int/news-room/fact-sheets/detail/burns.
- 2.Eurostat: Health Statistics: Atlas on Mortality in the European Union Luxembourg 2009.
- 3.Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedler V, Künzi W, Bürgi U, Meyer VE. Care of burns victims in Europe. Burns J Int Soc Burn Inj. 1999;25(2):152–157. doi: 10.1016/s0305-4179(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri M, Fuchs PC, Lefering R, et al. The BUrn Mortality Prediction (BUMP) Score—An improved mortality prediction score based on data of the German burn registry. Burns. February 2022 doi: 10.1016/j.burns.2022.02.007. Published online. [DOI] [PubMed] [Google Scholar]
- 6.The Belgian Outcome in Burn Injury Study Group. Blot S. Development and validation of a model for prediction of mortality in patients with acute burn injury. Br J Surg. 2008;96(1):111–117. doi: 10.1002/bjs.6329. [DOI] [PubMed] [Google Scholar]
- 7.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: Extending and updating the Baux score. J Trauma Inj Infect Crit Care. 2010;68(3):690–697. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 8.Ryan CM, Schoenfeld DA, Thorpe WP, Sheridan RL, Cassem EH, Tompkins RG. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338(6):362–366. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 9.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: Report from the Canadian Study of Health and Aging. J Gerontol Ser A. 2004;59(12):1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 11.Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173–181. doi: 10.1016/j.jamcollsurg.2017.11.005. e8. [DOI] [PubMed] [Google Scholar]
- 12.AL-Khamis A, Warner C, Park J, et al. Modified frailty index predicts early outcomes after colorectal surgery: An ACS-NSQIP study. Colorectal Dis. 2019;21(10):1192–1205. doi: 10.1111/codi.14725. [DOI] [PubMed] [Google Scholar]
- 13.Dammeyer K, Alfonso AR, Diep GK, et al. Predicting postoperative complications following mastectomy in the elderly: Evidence for the 5-factor frailty index. Breast J. 2021;27(6):509–513. doi: 10.1111/tbj.14208. [DOI] [PubMed] [Google Scholar]
- 14.Yagi M, Michikawa T, Hosogane N, et al. The 5-item modified frailty index is predictive of severe adverse events in patients undergoing surgery for adult spinal deformity. Spine. 2019;44(18):E1083–E1091. doi: 10.1097/BRS.0000000000003063. [DOI] [PubMed] [Google Scholar]
- 15.Conlon M, Thommen R, Kazim SF, et al. Risk analysis index and its recalibrated version predict postoperative outcomes better than 5-factor modified frailty index in Ttraumatic spinal injury. Neurospine. 2022;19(4):1039–1048. doi: 10.14245/ns.2244326.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bludevich BM, Emmerick I, Uy K, et al. Association between the modified frailty index and outcomes following lobectomy. J Surg Res. 2022;283:559–571. doi: 10.1016/j.jss.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Cole KL, Kassicieh AJ, Rumalla K, et al. Frailty predicts worse outcomes for spine surgery patients with interhospital transfer status: Analysis of 295,875 patients from the National Surgical Quality Improvement Program (NSQIP) 2015-2019. Clin Neurol Neurosurg. 2022;224 doi: 10.1016/j.clineuro.2022.107519. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs J, Scheidt-Nave C, Gaertner B, et al. Frailty in Deutschland: Stand und perspektiven: Ergebnisse eines workshops der Deutschen Gesellschaft für Epidemiologie. Z Für Gerontol Geriatr. 2016;49(8):734–742. doi: 10.1007/s00391-015-0999-4. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren RS, Kramer CB, Rivara FP, et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rani M, Schwacha MG. Aging and the pathogenic response to burn. Aging Dis. 2012;3(2):171–180. [PMC free article] [PubMed] [Google Scholar]
- 21.Keck M, Lumenta DB, Andel H, Kamolz LP, Frey M. Burn treatment in the elderly. Burns. 2009;35(8):1071–1079. doi: 10.1016/j.burns.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med. 1982;11(5):260–262. doi: 10.1016/S0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JRF, Badhiwala JH, Moghaddamjou A, Yee A, Wilson JR, Fehlings MG. Frailty is a better predictor than age of mortality and perioperative complications after surgery for degenerative cervical myelopathy: An analysis of 41,369 patients from the NSQIP database 2010–2018. J Clin Med. 2020;9(11):3491. doi: 10.3390/jcm9113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: A review. Age Ageing. 2012;41(2):142–147. doi: 10.1093/ageing/afr182. [DOI] [PubMed] [Google Scholar]
- 25.Gößwald A, Lange M, Kamtsiuris P, Kurth BM. DEGS: Studie zur gesundheit erwachsener in Deutschland: Bundesweite quer- und längsschnittstudie im rahmen des gesundheitsmonitorings des Robert Koch-Instituts. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2012;55(6-7):775–780. doi: 10.1007/s00103-012-1498-z. [DOI] [PubMed] [Google Scholar]
- 26.Ko FC. Preoperative frailty evaluation: A promising risk-stratification tool in older adults undergoing general surgery. Clin Ther. 2019;41(3):387–399. doi: 10.1016/j.clinthera.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han B, Li Q, Chen X. Frailty and postoperative complications in older Chinese adults undergoing major thoracic and abdominal surgery. Clin Interv Aging. 2019;14:947–957. doi: 10.2147/CIA.S201062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panayi AC, Orkaby AR, Sakthivel D, et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am J Surg. 2019;218(2):393–400. doi: 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo L, Gallo M, Augustine H, et al. Assessing patient frailty in plastic surgery: A systematic review. J Plast Reconstr Aesthetic Surg JPRAS. 2022;75(2):579–585. doi: 10.1016/j.bjps.2021.09.055. [DOI] [PubMed] [Google Scholar]
- 30.Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer JP, Lederer DJ, Baldwin MR. Frailty in pulmonary and critical care medicine. Ann Am Thorac Soc. 2016;13(8):1394–1404. doi: 10.1513/AnnalsATS.201512-833FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuijt HJ, Morin ML, Allen E, Weaver MJ. Does the frailty index predict discharge disposition and length of stay at the hospital and rehabilitation facilities? Injury. 2021;52(6):1384–1389. doi: 10.1016/j.injury.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen S, Romanowski KS, Andre JA, Greenhalgh DG, Palmieri TL. Modified frailty index is an independent predictor of death in the burn population: A secondary analysis of the Transfusion Requirement in Burn Care Evaluation (TRIBE) study. J Burn Care Res. 2022:irac164. doi: 10.1093/jbcr/irac164. Published online October 31. [DOI] [PubMed] [Google Scholar]
- 36.Chotai S, Gupta R, Pennings JS, et al. Frailty and sarcopenia: Impact on outcomes following elective degenerative lumbar spine surgery. Spine. 2022;47(20):1410–1417. doi: 10.1097/BRS.0000000000004384. [DOI] [PubMed] [Google Scholar]
- 37.Madni TD, Nakonezny PA, Wolf SE, et al. The relationship between frailty and the subjective decision to conduct a goals of care discussion with burned elders. J Burn Care Res. May 2017:1. doi: 10.1097/BCR.0000000000000594. Published online. [DOI] [PubMed] [Google Scholar]
- 38.Romanowski KS, Barsun A, Pamlieri TL, Greenhalgh DG, Sen S. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res. 2015;36(1):1–6. doi: 10.1097/BCR.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 39.Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: A mini-review. Gerontology. 2013;59(2):159–164. doi: 10.1159/000342344. [DOI] [PubMed] [Google Scholar]
- 40.Kanaki T, Makrantonaki E, Zouboulis CC. Biomarkers of skin aging. Rev Endocr Metab Disord. 2016;17(3):433–442. doi: 10.1007/s11154-016-9392-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.