Abstract
Congenital abnormalities of the iliac artery are uncommon and often discovered incidentally during the diagnosis or treatment of peripheral vascular diseases such as abdominal aortic aneurysm (AAA) and peripheral arterial diseases. The endovascular treatment of infrarenal AAA can be complicated by anatomic abnormalities in the iliac arteries, such as the absence of the common iliac artery (CIA) or overly short bilateral common iliac arteries. We present a case of a patient with a ruptured AAA and bilateral absence of the CIA, successfully treated by endovascular intervention combined with preservation of the internal iliac artery using the sandwich technique.
Keywords: Ruptured abdominal aortic aneurysm, Iliac arterial congenital anomaly, EVAR, Sandwich technique
Introduction
Endovascular aneurysm repair (EVAR) is currently considered the first-line treatment for standard abdominal aortic aneurysm (AAA) with suitable anatomy and reasonable life expectancy [1]. The procedure offers several advantages over open surgery, including reduced mortality and morbidity rates and a shorter recovery time in the first 30 days. EVAR is particularly beneficial in emergency situations where a ruptured AAA (rAAA) requires urgent intervention [2,3]. Contraindications for this approach primarily stem from anatomical abnormalities of the aorta and iliac arteries. In standard EVAR, the distal landing zone must have a minimum length of 10 mm in the common iliac arteries (CIAs) for the placement of the stent graft (SG) limbs [1]. However, anatomical anomalies, such as the bilateral absence of the CIA, can limit the length and healthy zone available for SG placement, resulting in challenges for treatment strategy and technique selection, especially in the case of a ruptured aortic aneurysm.
We present a case of a patient with an AAA and anatomic abnormalities, lacking bilateral CIAs, who underwent emergency endovascular intervention using a combination of the EVAR and sandwich technique for preservation of the right internal iliac artery (IIA). Written informed consent for the publication of this case report was obtained from the patient.
Case report
A 79-year-old male presented to the emergency department with a sudden loss of consciousness for 15 minutes, followed by persistent periumbilical abdominal pain on the day of admission. Vital signs at admission: alert, responsive, heart rate 90 beats per minute, blood pressure 100/60 mmHg, and pale pink skin. His medical history included poorly controlled grade II hypertension, stented chronic coronary artery disease, and dyslipidemia. At the emergency department, the patient received fluid resuscitation, blood pressure was stabilized at 110-120 mmHg and was urgently scheduled for a CT angiography of the aorta which showed an infrarenal, spindle-shaped AAA with a maximum diameter of 50 mm that had ruptured into the retroperitoneal space. The scan also revealed an anatomical abnormality without bilateral CIAs (Fig. 1). The patient was indicated for an emergency endovascular intervention using a combination of EVAR and the sandwich technique, which aimed to preserve the right IIA and occlude the left IIA. The patient was positioned supine and received a combination of intravenous and local anesthesia. We accessed the bilateral common femoral arteries using 7F sheaths and the left brachial artery using a 6F sheath under ultrasound guidance. Two percutaneous closure systems (ProGlide; Abbott, Chicago, IL) were used in the right femoral artery, while one was used in the left side. A 6F, 100 cm long Fortress sheath (Biotronik, Dresden, Germany) was inserted through the left brachial artery into the abdominal aorta (Fig. 2A). Subsequently, the left IIA was cannulated and embolized using a 6/8 × 7 mm Cocoon Duct Occluder vascular plug (Vascular Innovations Co. Ltd., Nonthaburi, Thailand).
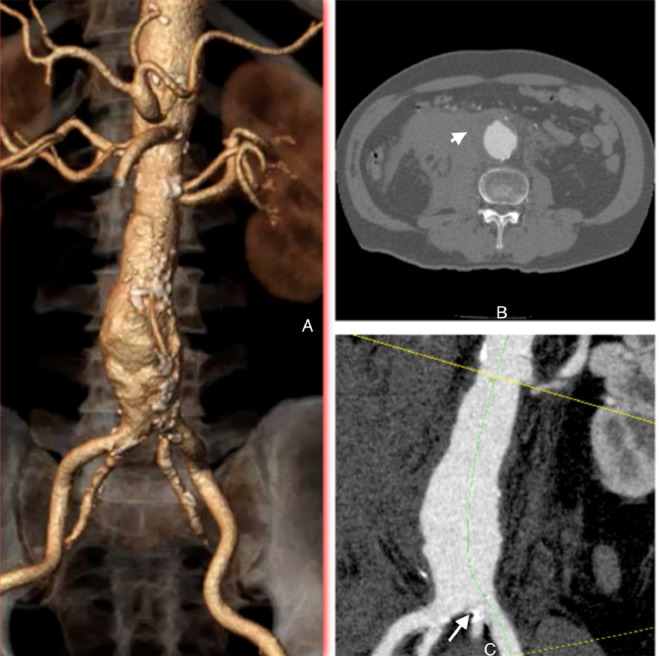
Fig. 1.
Preoperative computed tomography imaging: Axial imaging (B) of the fuso-saccular abdominal aortic aneurysm. The curved MPR image (C) highlights severe stenosis at the origin of the left internal iliac artery due to calcification. The 3-dimensional reconstruction (A) depicts the distal aorta branching into 4 arteries: 2 external iliac arteries and 2 internal iliac arteries. The AAA had the largest diameter of 50.23 mm and a cylindrical proximal neck that was nonangulated.
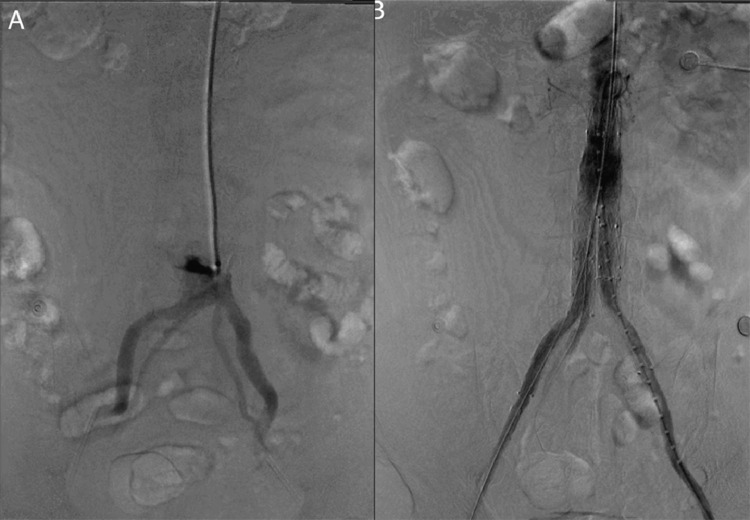
Fig. 2.
Digital subtraction angiography: the preoperative angiography (A) reveals 4 branches of the iliac arteries arising from the aorta. The completion angiogram (B) shows the successful exclusion of the abdominal aortic aneurysm with no evidence of endoleaks.
Next, an 18F hydrophilic main body sheath and a 16F hydrophilic sheath over an extra-stiff 0.035-inch wire (Lunderquist; Cook Medical, Bloomington, IN) were inserted into the right and left common femoral arteries, respectively. The 28 × 14 × 103 mm Endurant IIs stent graft (Medtronic, Minneapolis, MN) was then advanced into the aorta via the right femoral artery. The main body of the stent graft was deployed after the anatomical landmarks and position of the renal arteries were identified through angiography. The left iliac extension (Endurant IIs; Medtronic, Minneapolis, MN), measuring 16 × 10 × 93 mm, was utilized to connect the main body's short limb to the left EIA. Next, the right IIA was successfully catheterized using a hydrophilic 0.035-inch wire, and a 7-58 mm balloon-expandable covered stent (BeGraft; Bentley InnoMed, Hechingen, Germany) was placed from the left brachial artery. The iliac extension was inserted through the right femoral artery and into the long limb of the main body SG. We adjusted the position of the covered stent so that its distal landing zone was 15 mm within the right IIA and the extension was 5 mm below the proximal marker. It was necessary to ensure that the extension and the covered stent overlapped the long limb of the main body by 30 mm and that the stents were in a fixed position. The extension was deployed first, followed by the covered stent. The completion angiography showed successful exclusion of the aneurysm with no evidence of endoleaks (Fig. 2B). The patient was discharged 5 days after the intervention and had made a full recovery after 2 weeks, with no symptoms related to the occlusion of the left IIA. A CT scan indicated that the stent was functioning well with good flow into the right IIA, the aneurysm was completely isolated, and there was no evidence of endoleaks (Fig. 3).
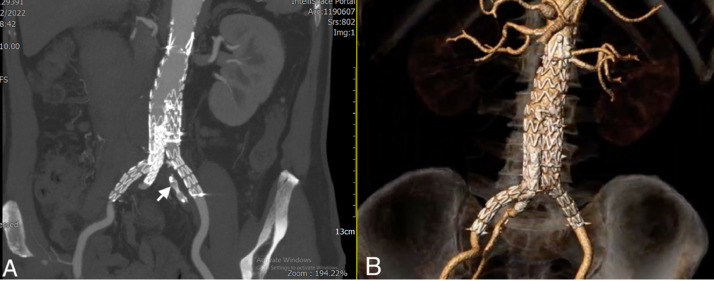
Fig. 3.
Postoperative computed tomography imaging: Coronal image (A) showing the vascular plug in the left internal iliac artery (arrowhead). Three-dimensional reconstruction (B) showing complete exclusion of the abdominal aortic aneurysm and patency of the right internal iliac artery.
Discussion
From 1964 to 2021, only 12 cases of congenital anomalies without CIA have been documented [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]–16], with one case treated for an AAA through an EVAR combined with preservation of the left IIA by E-iliac graft JOTEC branch [15]. However, there is no recorded instance of a rAAA accompanied by anatomical abnormalities in the bilateral iliac arteries requiring urgent intervention.
EVAR is considered the first-choice emergency intervention option for rAAA due to its minimally invasive nature, reduction in mortality and morbidity rates in the first 30 days, and faster recovery time [[1], [2]–3]. However, according to the standard and extended instructions for use of currently available devices (such as Medtronic Endurant IIs, Medtronic Talent, and Cook Zenit), the minimum length of the distal landing zone at the nonpathological site of the iliac artery should be at least 10 mm. If the CIA is absent or very short bilaterally, it is crucial to extend the limbs of the SG to the EIA and cover the origin of the IIA to ensure the minimum length of the distal landing zone. In general, occlusion of one IIA is well-tolerated. However, bilateral IIA occlusion has a higher incidence of causing buttock claudication and pelvic ischemia [[17], [18]–19]. The 2019 European Society for Vascular Surgery guidelines recommend preserving at least one IIA [1].
Numerous techniques have been reported to preserve the flow of the IIA, including open surgical options such as bypass surgery or repositioning the internal iliac, femoral-femoral bypass following AUI stent graft, and endovascular interventions such as stent graft with branches into the internal iliac vessels or the sandwich technique.
Compared to complete endovascular interventions, open surgery is more invasive, complex, and time-consuming. It is not suitable for emergency interventions, such as rAAA. The technique of placing an AUI stent graft combined with femoral-femoral bypass is a viable option in an emergency situation. However, in our case, there was no suitable location to deploy the CIA occlusion device due to the absence of bilateral CIAs. This was not considered an ideal option due to the high risk of a type II endoleak.
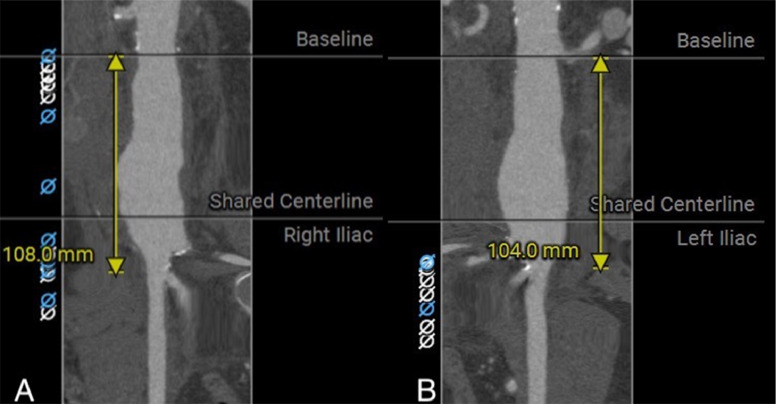
Utilizing an iliac branch device (IBD; Zenith Zebis, Cook Inc, Bloomington, IN) to preserve flow in the IIA is a useful technique, but it has drawbacks such as technical complexity and limited availability of the device. In addition, this method is inappropriate in emergency situations such as rAAA or in cases of anatomical abnormalities that increase the risk of complications, such as a straight aortic bifurcation, tortuous or calcified iliac axes, the presence of thrombus in the CIA lumen, an IIA ostial stenosis, or a calcified or aneurismal IIA [20]. In our case, the left IIA was severely calcified, resulting in a severe stenosis at its origin. The available IBD on the market by Jotec has a short body length of 53 mm, including 27 mm overlap on the limb of the SG main body, with a short limb length of the SG main body at 85 mm. To achieve successful deployment of the IIA branch of IBD, a total length ≥111 mm is required. According to the instructions for use of IBD, the minimum diameter for the internal-external iliac artery bifurcation is 18 mm. However, in our case, the total length from the left lowest renal artery to the orifice of the right IIA was 108 mm, and on the left side, it was 104 mm. The diameter of right iliac bifurcation was only 12 mm, making it completely anatomically inappropriate for the IBD (Fig. 4). Additionally, the high cost of the device is prohibitive.
Fig. 4.
Imaging study by computed tomography: the length from the lower renal artery to the orifice of the right (A) and left external iliac artery (B) measured at 108 mm and 104 mm, respectively.
The sandwich technique is an alternative method to a custom-made device, used to maintain perfusion in branch vessels originating in the region to be treated. In cases where bilateral CIA is absent, it may be possible to use the sandwich technique to simultaneously preserve both IIAs. However, in this particular case, the patient had stenosis at the origin of the left IIA and an emergency rAAA, so we decided to occlude the left IIA and then perform arterial preservation on the right side. According to a 2013 study by Lobato et al. [21], this technique has shown a very favorable technical success rate and primary patency rate of 100% and 93.8%, respectively. The sandwich technique may be preferred due to its simple procedure, which involves commonly used stents by vascular surgeons and the brachial artery approach that simplifies cannulation of the IIA. Although there were concerns about possible complications such as endoleak type III and stenosis or occlusion of iliac arteries, these fortunately did not occur on the last angiogram and 2-week postoperative CT in our case. Nevertheless, further follow-up is necessary need to evaluate long-term outcomes.
Conclusion
EVAR combined with the sandwich technique for preservation of the IIAs can be an effective treatment strategy for rAAAs with anatomical anomalies such as bilateral absence or insufficient length of the CIAs. The use of the sandwich technique can provide a suitable landing zone for the SG and prevent potential complications associated with sacrificing both IIAs. Further studies are necessary to validate the safety and effectiveness of this technique, but this case report demonstrates the potential benefit of this approach in emergency situations where alternative treatment options may be limited.
Authors’ contribution
Luong Cong Hieu and Nguyen Minh Duc contributed to write original draft. Luong Cong Hieu, Pham Minh Anh, Nguyen Thanh Hung, and Nguyen Duc Nghia contributed to undergo diagnostic procedure, collect, and interpret the imaging. Luong Cong Hieu, Pham Minh Anh, Nguyen Thanh Hung, Nguyen Duc Nghia, and Nguyen Minh Duc made substantial contributions to collect patient data and clinical data analysis. All authors have read, revised, and approved the final published version of the manuscript. All authors were responsible for submission of our study for publication.
Ethics statement
Ethical approval was not necessary for the preparation of this article.
Data availability statement
All data generated or analyzed during this study are included in this article and/or its online supplementary material files. Further enquiries can be directed to the corresponding author.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Competing Interests: The authors do not report any conflicts of interest.
References
- 1.Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor's choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Jones M, Koury H, Faris P, Moore R. Impact of an emergency endovascular aneurysm repair protocol on 30-day ruptured abdominal aortic aneurysm mortality. J Vasc Surg. 2022;76(3):663–670.e2. doi: 10.1016/j.jvs.2022.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Speicher PJ, Barbas AS, Mureebe L. Open versus endovascular repair of ruptured abdominal aortic aneurysms. Ann Vasc Surg. 2014;28(5):1249–1257. doi: 10.1016/j.avsg.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield AO, Howard JM. Absence of both common iliac arteries. A case report. Anat Rec. 1964;150(4):363–364. doi: 10.1002/ar.1091500404. [DOI] [PubMed] [Google Scholar]
- 5.Patel M, Chonat S, Olomu I, Arrington S, Kadrofske M. Absent left common and left external iliac artery presenting in a neonate. J Perinatol. 2013;33(5):407–409. doi: 10.1038/jp.2012.132. [DOI] [PubMed] [Google Scholar]
- 6.Oduro G, Cope L, Rogers I. Case report: lower limb arterial blood supply arising from the renal artery with congenital absence of the ipsilateral iliac arteries. Clin Radiol. 1992;45(3):215–217. doi: 10.1016/S0009-9260(05)80649-X. [DOI] [PubMed] [Google Scholar]
- 7.Palkhi E, Pathak S, Hostert L, Morris-Stiff G, Patel JV, Ahmad N. Complete absence of iliac arteries in the left hemipelvis in a case of deceased donor renal transplantation. Case Rep Transplant. 2015;2015:138170. doi: 10.1155/2015/138170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George JM, Ilonzo N, Choinski KN, Grossi RJ. Congenital absence of bilateral common iliac arteries. J Vasc Surg Cases Innov Tech. 2021;7(2):266. doi: 10.1016/j.jvscit.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doita T, Yamakura T, Yamasumi T, Nakamura T. Congenital absence of left common and external iliac arteries. J Vasc Surg Cases Innov Tech. 2022;8(1):16–18. doi: 10.1016/j.jvscit.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabydeen DA, Shabashov A, Shaffer K. Congenital absence of the right common iliac artery. Radiol Case Rep. 2008;3(1):47. doi: 10.2484/rcr.v3i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llauger J, Sabate J, Guardia E, Escudero J. Congenital absence of the right common iliac artery: CT and angiographic demonstration. Eur J Radiol. 1995;21(2):128–130. doi: 10.1016/0720-048X(95)00701-Q. [DOI] [PubMed] [Google Scholar]
- 12.Dumanian AV, Frahm CJ, Benchik FA, Wooden TF. Intermittent claudication secondary to congenital absence of iliac arteries. Arch Surg. 1965;91(4):604–606. doi: 10.1001/archsurg.1965.01320160058013. [DOI] [PubMed] [Google Scholar]
- 13.Tay CM, Siew EPY, Ng T-K, Vathsala A, Tiong HY. Kidney transplantation in a patient with absent right common iliac artery and congenital renal abnormalities. Int J Surg Case Rep. 2015;10:138–141. doi: 10.1016/j.ijscr.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green CS, Helmy MA. Novel, congenital iliac arterial anatomy: absent common iliac arteries and left internal iliac artery. Radiol Case Rep. 2014;9(3):978. doi: 10.2484/rcr.v9i3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham M-A, Le T-P. Preservation of internal iliac artery flow during endovascular aortic aneurysm repair in a patient with bilateral absence of common iliac artery. J Vasc Surg Cases Innov Tech. 2021;7(1):108–112. doi: 10.1016/j.jvscit.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radhakrishnan V, Kumar R, George D, Abraham GP. Absent right side iliac arterial system, an intraoperative surprise during live related recipient renal transplantation. Case Rep Transplant. 2015;2015:894786. doi: 10.1155/2015/894786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouvelos G, Katsargyris A, Antoniou G, Oikonomou K, Verhoeven E. Outcome after interruption or preservation of internal iliac artery flow during endovascular repair of abdominal aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. 2016;52(5):621–634. doi: 10.1016/j.ejvs.2016.07.081. [DOI] [PubMed] [Google Scholar]
- 18.Bosanquet D, Wilcox C, Whitehurst L, Cox A, Williams I, Twine C., et al. Systematic review and meta-analysis of the effect of internal iliac artery exclusion for patients undergoing EVAR. Eur J Vasc Endovasc Surg. 2017;53(4):534–548. doi: 10.1016/j.ejvs.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Jean-Baptiste E, Brizzi S, Bartoli MA, Sadaghianloo N, Baqué J, Magnan P-E, et al. Pelvic ischemia and quality of life scores after interventional occlusion of the hypogastric artery in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg. 2014;60(1):40-49. e1. doi: 10.1016/j.jvs.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Delay C, Deglise S, Lejay A, Georg Y, Roussin M, Schaeffer M, et al. Zenith bifurcated iliac side branch device: mid-term results and assessment of risk factors for intraoperative thrombosis. Ann Vasc Surg. 2017;41:141–150. doi: 10.1016/j.avsg.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Lobato AC, Camacho-Lobato L. The sandwich technique to treat complex aortoiliac or isolated iliac aneurysms: results of midterm follow-up. J Vasc Surg. 2013;57(2):26S–34S. doi: 10.1016/j.jvs.2012.09.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and/or its online supplementary material files. Further enquiries can be directed to the corresponding author.