Abstract
Congenital malformations of the urogenital system with fully developed duplications, such as urinary bladder, are sporadic. They are often present in the setting of endogenous molecular disbalance, such as steroid metabolism disturbances. Other rare manifestations of hormonal disbalance present as intersex conditions in which the individual has karyotype-specific internal genital organs with opposite-sex signs of the external genitalia, known as ambiguous genitalia. Congenital variations and malformations are often fully recognized and understood during radiological exams. Herein we present a unique case of a 2-month-old baby with female chromosomal sex and ambiguous genitalia together with the manifestation of several anatomical malformations: urinary bladder duplication in the coronal plane, pancake kidney with supernumerary renal arteries, 2 ureters and neural tube defect.
Despite their low incidence rate, knowledge of such malformations is paramount for correct diagnosis and treatment in such cases.
Keywords: Developmental sex disorder, Ambiguous genitalia, Double urinary bladder, Pancake kidney
Introduction
The disorder of sexual development presented in this article consists of having female chromosomal sex (45, XX) with ambiguous genitalia. The classical example where the presence of this condition is found is congenital adrenal hyperplasia (CAH), which comprises of several autosomal recessive disorders affecting the synthesis of steroids in different pathways. In the typical case a 21-hydroxylase enzyme deficiency leads to disruption in cortisol and aldosterone synthesis with compensatory hypersynthesis of precursors and steroids (as testosterone) not metabolized by the abovementioned enzyme [1]. Noncongenital reasons are also known to play a role in this condition, such as excess in exogenous androgens exposure–maternal adrenal hyperplasia, maternal ovarian tumor or even placental aromatase P450 deficiency [2]. The old term female pseudohermaphroditism is associated with various inherited malformations, including urogenital and musculoskeletal deformities [3,4]. Some of the rarest urogenital malformations include coronal plane duplication of the urinary bladder and cross-fused pancake kidney.
Case presentation
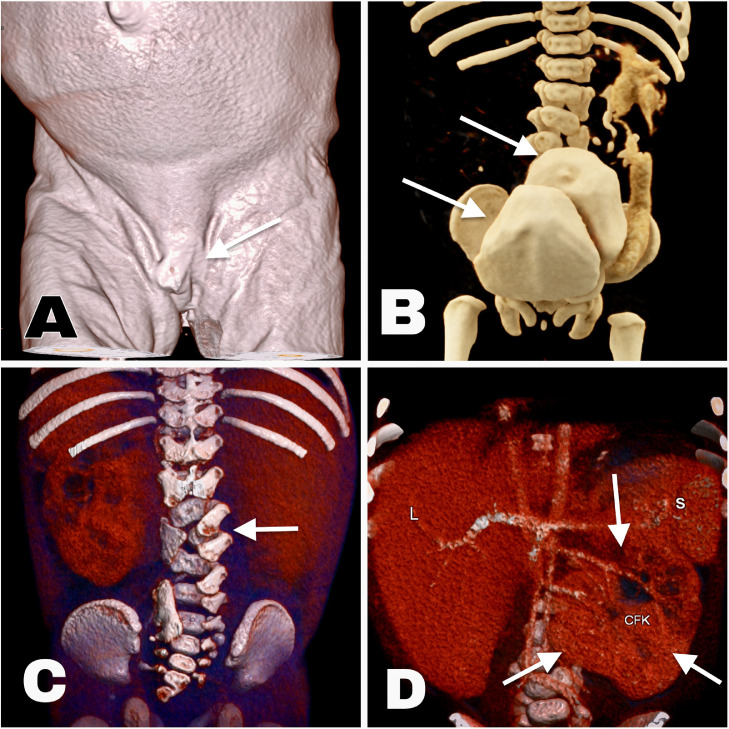
We present a case of sexual development disorder (Fig. 1A) in combination with semihydronephrotic cross-fused cake kidney (Fig. 1D) with supernumerary renal arteries (Fig. 2E), totally duplicated urinary bladder in the coronal plane (Fig. 1B), spina bifida occulta with a supernumerary lumbar wedge-shaped vertebra (Fig. 1C). The patient–a 2-month-old female baby, had already had several foetal ultrasonography exams and since 27 g.w. was known to have what was termed at that time as а megacyst–abdominal cystic finding behind the urinary bladder, right renal agenesia and left renal hydronephrosis.
Fig. 1.
(A) External genitalia with male features in a female patient. (B) Cinematic CT volume rendered image. Arrows show 2 different contrast enhanced urinary bladders one behind the other as they are duplicated in the coronal plane. (C) Volume rendered image, posterior-anterior orientation. Arrow showing wedge shaped supernumerary vertebra between L3 and L4. (D) Thick-sliced volume rendered CT image. Annotations: L – liver; S – spleen; CFK – cross-fused kidney.
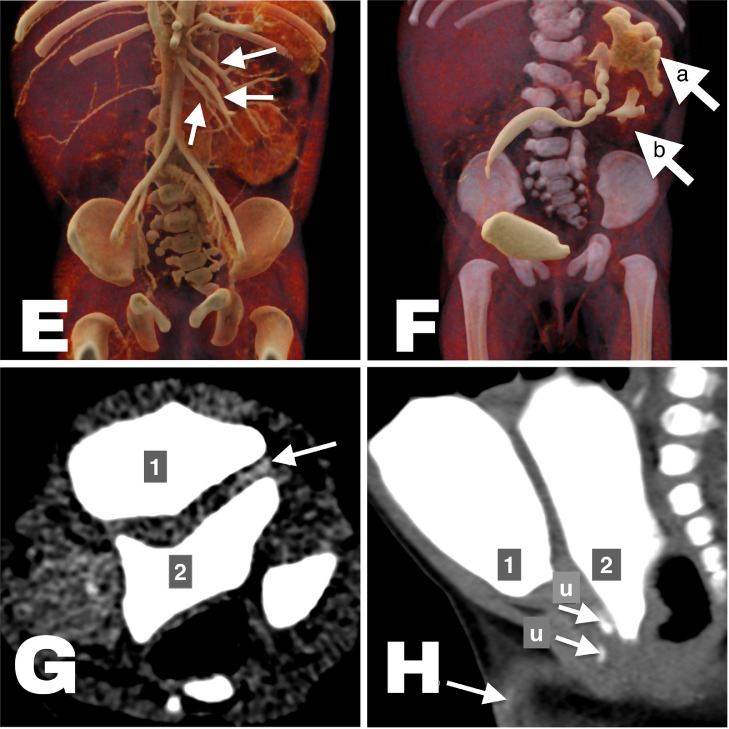
Fig. 2.
(E) Cinematic volume rendered CT image, arterial phase. Each of the 3 arrows is pointing at a renal artery. (F) Cinematic volume rendered CT image, urographic phase. Arrow “a” is pointing at a hydronephrotic kidney with its hydroureter. Arrow “b” is pointing at the collector system of a nonhydronephrotic kidney. (G). Axial CT image 4 hours after contrast injection. Urinary bladders are numbered. Arrow is pointing at the ureter draining into the posterior bladder. (H). Sagittal CT image 4 hours after contrast injection. Urinary bladders are numbered. Arrows are pointing at urethral routes (“u”).
The physical examination revealed facial dysmorphism, Mongolian spots and ambiguous external genitalia comprising of micropenis without orifice, urethra located “above” the anus (as written in the medical documentation) with the latter located atypically close to the hypoplastic scrotum. During the hospital stay, a urethral fistula was suspected due to the rectal externalization of urine.
Clinical laboratory tests showed a typical constellation of inflammation as the primary reason for hospitalization was cough and dyspnea due to respiratory bacterial infection. Later urinary infection was also proven.
The diagnostic method of choice was multidetector computed tomography using an exceptionally dedicated pediatric protocol according to ALARA (as low as reasonably achievable) principle. Intravenous contrast media was administered according to the patient's mass weight of 1.5 mg/kg as a fistula was observed. The observation of a secondary urinary bladder with slower filling imposed a CT–urography scan 4 hours after the intravenous administration of contrast media, where the diagnosis of 2 separate urinary bladders in the coronal plane together with the rest anomalies was made.
A cross-fused kidney on one side of the body was found with partial hydronephrosis, 2 ureters, supernumerary renal arteries, and lumbosacral spina bifida occulta. At the time of CT urography, only the ventral urinary bladder was opacified, where one of the ureters was draining (Fig. 2F). The ventral bladder drained through the patient's urethra (Fig. 2H). Hydronephrotic kidney with the belonging hydroureter was proven with an additional CT urographic scan after 4 hours to drain into the posterior cystic finding), now addressed as a posterior urinary bladder (Fig. 2G).
Further referral of the baby to the endocrinology department revealed disturbed endogenous cortisone synthesis, and optimal levels were maintained with external admission of hydrocortisone.
As the sexual development disorder needed to be specified, even when supported by the physical examination, imaging procedures, laboratory tests and treatment with hydrocortisone, the patient was referred for complete genetic testing and surgical correction. The authors regret to say that more than a year later, genetic testing revealing the exact mutation is still not finalized due to parents inability to cooperate.
Discussion
Disorders of sexual development are found mostly in sporadic genetic disorder caused by 21-α hydroxylase deficiency for congenital adrenal hyperplasia with a prevalence rate of 1 per 14,500 [3]. The most common reasons are genetic mutations of the genes encoding aromatize subunits, thus leading to aromatase deficiency, which further lead to adrenal insufficiency and subsequent virilization [3]. A statistically based connection between urogenital and gastrointestinal malformations is found in cases of sexual development disorders [4,5].
The complexity of embryological development of the urogenital system is a source of a great number of interesting medical cases when a disorder is present, but still, some abnormal congenital conditions are less common than others.
Generally, fusion anomalies of the kidneys are divided into 2 groups: horseshoe kidney (the inferior poles of the 2 kidneys are fused) and crossed-fused ectopia. A lump or cake kidney is a case of crossed-fused ectopia where both kidneys have a complete fusion to form a single homogenous mass [6]. According to the calculations of Miclaus et al. [7], the incidence rate of the lump kidney is between 1:65 000 and 1:375 000 people, as it is 2.5 times more common in males (male: female ratio 2.5:1).
Another sporadic developmental disorder is complete duplication of the urinary bladder, with only 50 cases described in the literature, as far as we know. There are 2 types of bladder duplication–the sagittal plane, the more common one, and the coronal plane, which is far less common [8]. In order to conclude the existence of a second urinary bladder, we first must differentiate it from a cloaca (a shared space where the urinary, genital and gastrointestinal tracts open into). The incidence rate of such malformation is approximately 1 in 50 000 births [9]. However, in order to diagnose such a malformation, several diagnostic criteria must be met–from the physical examination, only one opening must be present, instead of 3 (urethra, vagina, anus); from the radiological examination, ovarian tubes, ureters and rectum must be seen to open into a common space (the cloaca) [10]. In our case, however, there is a present urethral opening, vagina and anus. Furthermore, from the imaging tests, ureters were seen to drain into both urinary bladders without any other structures opening into the posterior bladder. Another malformation is to be noted here, as there are 2 urethras, 1 exiting from each bladder. The urethra from the anterior bladder course opens externally, whereas the urethra from the posterior bladder courses posteriorly and inferiorly, opening into the distal part of the anal canal. Complete urethral duplication is rare, especially in females [11,12]. One such case of female pseudohermaphroditism with urethral duplication was reported by D'Cunha et al. [13] However, in their case, one urethra was opening normally, and the second one was draining into the vagina. In addition, their case did not include duplication of the urinary bladder.
Moreover, neural tube development defects can also be associated with sexual development disorders [4]. The term spina bifida refers to a group of defects in the closure of the neurological tube during embryological development, causing agenesis of the vertebral arcs. There are 2 types of this disorder: spina bifida aperta–visible herniation of the meninges (meningocele) or the meanings and the spinal cord (meningomyelocele); and spina bifida occulta–the defect is being covered by skin [14].
Disturbances during embryological development result in different renal malformations. Presumably, lump kidneys are formed because of the compression of the nephrogenic blastomeres by the umbilical arteries during the cranial migration of the ureteral buds, which most likely leads to the adhesion of the buds, hence leading to their fusion–resulting in a lump kidney [15]. Furthermore, disturbances in the branching pattern of the ureteric bud are the reason for the presence of double ureters. Multiple renal arteries are a rare abnormality with an incidence rate of 1%-2% for triple renal arteries and less than 1% for quadruple renal arteries [16].
Moreover, according to Abrahamson et al., there are 2 types of complete duplication of the urinary bladder: sagittal division (the 2 urinary bladders are divided by a peritoneal fold in the sagittal plane) and frontal division (a fibromuscular septum separates the 2 urinary bladders). The frontal division is the rarer one; to our knowledge, there are only 7 other reported cases. Nevertheless, neither has described a case of sexual development disorder combined with a complete duplication of the urinary bladder and lump kidney.
The urinary bladder starts its development with the division of the cloaca between the fourth to the seventh week of gestation [17]. The cloaca is divided into 2 parts: posterior–the future rectum and anterior–the urogenital sinus [18]. With time the upper, more significant portion of the urogenital sinus will give rise to the urinary bladder [19]. Our review of the literature showed that there are many theories about the embryological defects resulting in the complete duplication of the urinary bladder, even though most of them aim to explain the development of the complete sagittal septum but fail to explain the complete frontal septum. Abrahamson et al. suggested that the frontal septum might result from an aberrant urorectal septum persistence of the fused portion of the urogenital sinus and mesonephric ducts. It remains yet to be proven which is the actual cause for the complete duplication of the urinary bladder in the frontal plane.
In conclusion sexual development disorders need a thorough investigation concerning genetic factors and endocrine background. Differentiating between different sexual development conditions can be challenging, primarily if a full genetic record cannot be obtained due to several objective and subjective factors. This case shows how imaging modalities can help getting closer to the specific condition, enabling the possibility of considering every present variation and anomaly. Diagnostic imaging procedures are of immense help also when planning surgical correction or treatment, depending on the severity of the findings. Detailed knowledge of such rare malformations is essential for future procedures and therapies aiming to optimize patients's quality of life.
This article aims to present a rare combination of radiological findings which can be of help for other authors when they come across a rarity of such magnitude.
Patient consent
I hereby give my proof that the patient's parents (as the patient is 2-month-old at the time the diagnosis was made) gave their written consent to use all images and medical data of their child medical case with which to participate in a scientific paper.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Keely E, Malcolm J. Congenital adrenal hyperplasia in pregnancy: approach depends on who is the 'patient'. Obstet Med. 2012;5(4):154–160. doi: 10.1258/om.2012.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horii M, Boyd TK, Quade BJ, Crum CP, Parast MM. In: Diagnostic gynecologic and obstetric pathology. 3rd ed. Crum CP, Nucci MR, Howitt BE, Granter SR, Parast MM, Boyd TK, editors. Elsevier; Philadelphia, PA: 2018. Chapter 1 - female genital tract development and disorders of childhood; pp. 1–21. [Google Scholar]
- 3.Bulotta A, Varetti C, Ferrara F, Giannotti G, Maggio G., Garzi A, et al. Female pseudohermaphroditism. J Siena Acad Sci. 2012;1:82. doi: 10.4081/jsas.2009.82. [DOI] [Google Scholar]
- 4.Lubinsky MS. Female pseudohermaphroditism and associated anomalies. Am J Med Genet. 1980;6(2):123–136. doi: 10.1002/ajmg.1320060206. [DOI] [PubMed] [Google Scholar]
- 5.Chadha R, Kothari SK, Tanwar US, Gupta S. Female pseudohermaphroditism associated with cloacal anomalies: faulty differentiation in the caudal developmental field. J Pediatr Surg. 2001;36(7):E9. doi: 10.1053/jpsu.2001.24772. [DOI] [PubMed] [Google Scholar]
- 6.McDonald JH, McClellan DS. Crossed renal ectopia. Am J Surg. 1957;93(6):995–999. doi: 10.1016/0002-9610(57)90680-3. [DOI] [PubMed] [Google Scholar]
- 7.Miclaus GD, Pupca G, Gabriel A, Matusz P, Loukas M. Right lump kidney with varied vasculature and urinary system revealed by multidetector computed tomographic (MDCT) angiography. Surg Radiol Anat. 2015;37(7):859–865. doi: 10.1007/s00276-014-1390-7. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamson J. Double bladder and related anomalies: clinical and embryological aspects and a case report. Br J Urol. 1961;33:195–214. doi: 10.1111/j.1464-410x.1961.tb11606.x. [DOI] [PubMed] [Google Scholar]
- 9.Hendren W.H. Cloaca, the most severe degree of imperforate anus: experience with 195 cases. Ann Surg. 1998;228(3):331–346. doi: 10.1097/00000658-199809000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warne SA, Hiorns MP, Curry J, Mushtaq I. Understanding cloacal anomalies. Arch Dis Childh. 2011;96(11):1072–1076. doi: 10.1136/adc.2009.175034. [DOI] [PubMed] [Google Scholar]
- 11.Prasad N, Vivekanandhan KG, Ilangovan G, Prabakaran S. Duplication of the urethra. Pediatr Surg Int. 1999;15:419–421. doi: 10.1007/s003830050620. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RA, Winkle DC, Borzi PA. Urethral duplication: cases of ventral and dorsal complete duplication and review of the literature. J Pediatr Urol. 2010;6:188–191. doi: 10.1016/j.jpurol.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.D'Cunha AR, Kurian JJ, Jacob TJ. Idiopathic female pseudohermaphroditism with urethral duplication and female hypospadias. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-214172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataramana NK. Spinal dysraphism. J Pediatr Neurosci. 2011;6(Suppl. 1):31–40. doi: 10.4103/1817-1745.85707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tubbs RS, Shoja MM, Loukas M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation. John Wiley & Sons; Hoboken, New Jersey: 2016. p. 1321. [Google Scholar]
- 16.Pollack HM, McClennan BL, Dyer R, Kenney PJ. 2nd ed. Vol. 3. The Saunders imprint of Elsevier; Philadelphia: 2000. (Clinical urography). Vol. 2478. [Google Scholar]
- 17.Tubbs, R. S., Shoja, M. M., & Loukas, M. (Eds.). (2016).
- 18.Sadler T.W., Langman J. 12th ed. Vol. 232. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2012. p. 296. (Langman's medical embryology). 233240. [Google Scholar]
- 19.Eubanks JD, Cheruvu VK. Prevalence of sacral spina bifida occulta and its relationship to age, sex, race, and the sacral table angle: an anatomic, osteologic study of three thousand one hundred specimens. Spine (Phila Pa 1976). 2009;34(15):1539–1543. doi: 10.1097/BRS.0b013e3181a98560. [DOI] [PubMed] [Google Scholar]