Abstract
We have used a previously described rodent model to examine the influence of hormonal environment on susceptibility and immune responses to genital Chlamydia infection. Ovariectomized rats were administered estradiol, progesterone, or a combination of both, infected with Chlamydia trachomatis via the intrauterine route, and sacrificed 5 days later. Histopathological examination showed severe inflammation in the uteri and vaginae of progesterone-treated animals, whereas animals receiving estradiol or a combination of both hormones showed no inflammation. Large numbers of chlamydiae were found in vaginal secretions of progesterone-treated and combination-treated animals, while estradiol-treated animals had none. Tissue localization showed that numerous chlamydial inclusions were present in the uterine epithelium of the progesterone group and the cervicovaginal epithelium of the combination group. Examination of the acute immune responses of the infected animals showed that maximum activation was present in the draining lymph node cells from the progesterone-treated group, and these cells were producing large amounts of interleukin-10 and gamma interferon compared to other hormone-treated groups. In contrast, spleen cell proliferation was suppressed in progesterone-treated animals compared to other hormone-treated groups. We conclude that progesterone increases and estradiol decreases susceptibility to intrauterine chlamydial infection in this rat model. Our data demonstrate that hormone environment, at the time of infection, has a profound effect on the outcome of microbial infection in the female reproductive tract.
Mucosal surfaces represent the largest area of contact of microbial agents with the body's immune system. Among the mucosal surfaces, the reproductive tract has possibly the most specialized immune system, since it has evolved to meet the dual challenge of providing continuous protection against potential pathogens while providing a receptive environment for allogeneic sperm and embryo. These requirements are met partly by the precise and differential regulation of the immune system in the reproductive tract by the ovarian hormones estradiol and progesterone. Studies from our laboratory have shown that antigen presentation, immunoglobulin A (IgA) transport, and presence of immune cells in the uterus and vagina are under organ-specific hormonal regulation (5, 6, 9, 29–31).
Chlamydia trachomatis is an obligate intracellular, gram-negative bacteria and the cause of the world's most common sexually transmitted bacterial infection (20). Genital chlamydial infections are a major public health concern because of the severe pathologic sequelae of the infection, including pelvic inflammatory disease, scarring of the fallopian tubes, infertility, and ectopic pregnancy. Clinical symptoms range from acute asymptomatic infection to chronic conditions characterized by a severe inflammatory response (13).
In the last decade there has been an exponential growth in our understanding of immune responses to chlamydial infection. Studies in mouse models have shown that the immune response to genital chlamydial infection is very complex: it clears infection and confers short-term protection but at the same time sensitizes the host for development of immunopathological changes (13). There is strong evidence that CD4 T-cell-mediated immunity plays a critical role in clearing infection (3, 18, 21). Other studies in the mouse as well as other animal models also show that hormones play an important role in controlling immune responses to chlamydial infection and in determining the outcome of infection (17). In general, mouse models require pretreatment with progesterone prior to exposure, to enhance infections, especially with human serovars (16). Others have shown that in the absence of progesterone pretreatment, establishment of infection is dependent on stage of the estrous cycle or requires high infectious doses (4). Guinea pigs do not require progesterone and are easily infected with C. psittaci, but develop heavier infection following estradiol treatment (2, 15). In human studies, an association has been observed between onset of chlamydial infection and stage of menstrual cycle (24). Enhanced susceptibility was observed in the proliferative part of the menstrual cycle when estradiol levels were high. Oral contraceptives have also been shown to increase susceptibility to chlamydial infections and other sexually transmitted diseases (27).
Our studies in the rat model have shown that under the influence of progesterone, intrauterine exposure to C. trachomatis can induce infection in the genital tract (7). Immune responses were detected locally and systemically, and the results indicated that clearance of infection in this model involves immune cells from the lymph nodes draining the reproductive tract. In the present study, we examined the role played by the endocrine environment in determining susceptibility of the genital tract and if this in turn could have an effect on the outcome of infection. We also examined early immune responses locally and systemically to assess whether the hormonal environment influences the immune responses at the time when infection was induced.
MATERIALS AND METHODS
Animal and hormone administration.
Adult female Lewis rats (Charles River Laboratories, Kingston, N.Y.) weighing 150 to 200 g were maintained under standard temperature-controlled conditions on a 12-h light:12-h dark cycle. Ovariectomies were performed 7 to 10 days before each experiment as previously described (8). Estradiol and progesterone were purchased from Calbiochem (La Jolla, Calif.). Estradiol was initially dissolved in ethanol, evaporated to dryness, and then resuspended in 0.9% saline. Progesterone was suspended in saline by glass-glass homogenization. Control ovariectomized animals received only saline. All hormones were administered by subcutaneous injection. To correct for the alcohol present in estradiol preparation, an equivalent amount of ethanol was evaporated in flasks used to prepare progesterone and saline.
Infection of animals.
C. trachomatis (mouse pneumonitis nigg-II strain [MoPn]) was purchased from the American Type Culture Collection (Manassas, Va.). Females were administered either 2 mg of progesterone, 10 μg of estradiol, or a combination of both for 3 consecutive days. On the second day of the hormone treatment, animals were infected with MoPn (5 × 106 inclusion-forming units [IFU]/uterine horn) via the intrauterine route as described elsewhere (7).
In vitro infectivity assay to quantitate chlamydial shedding in vaginal washes.
Vaginal swabs from infected animals were collected in 0.5 ml of sucrose-phosphate-glutamate (SPG) buffer at various days postinfection. McCoy cells were grown to confluency in 24-well plates. Vaginal washing samples were diluted starting at 1:50 and added to monolayers in duplicate. A positive control consisting of a previously titrated MoPn stock, as well as mock infection as a negative control, was included on each plate. Infected monolayers were incubated for 72 h and then stained with rabbit anti-Chlamydia antibody (Biodesign Inc., Kennebunk, Maine) and an Immunopure ABC Rabbit Ig staining kit (Pierce Inc., Rockford, Ill.). Inclusions were revealed using an Immunopure Metal Enhanced DAB kit (Pierce) and counted under a light microscope. Four to eight fields of vision were counted for each sample, and IFU per milliliter was calculated.
Immunohistochemical staining.
For immunohistochemical analysis, reproductive tract tissues were excised, rinsed in cold saline (0.9%) prior to processing with acetone, methyl alcohol, and xylene, paraffin embedded, and stained as previously described (9). Briefly, 6- to 8-μm sections were cut with a microtome and placed on silane-coated slides. Sections were deparaffinized in xylene, rehydrated, and washed in 0.01 M phosphate-buffered saline–bovine serum albumin (1 mg/ml). Nonspecific staining was blocked by incubating sections with 1% rabbit serum for 20 min at room temperature. To detect chlamydial infection, sections were stained with rabbit anti-C. trachomatis (Biodesign) polyclonal antiserum (1:200) for 60 min. Antiserum from normal rabbits was substituted for primary antibody at an equivalent concentration for control staining. Avidin-biotin coupled to alkaline phosphatase (ABC Elite kit; Vector Laboratories, Burlinghame, Calif.) followed by Vector Red (alkaline phosphatase kit; Vector Laboratories) was used to reveal antigen localization. Monoclonal antibody ED1 (Serotec Laboratories, Oxford, England) was used for staining myeloid cells (macrophages and blood granulocytes) in the reproductive tract. A horse anti-mouse Ig coupled to biotin was used as a secondary antibody. Slides were counterstained with methyl green and mounted in Permount medium prior to microscopic examination.
Lymphocyte proliferation assays.
To measure lymph node and spleen cell proliferation, para-aortic lymph nodes (PALN) draining the genital tract and spleens were removed aseptically from animals. Single-cell suspensions were prepared by teasing with sterile forceps. Debris was allowed to settle for 2 min, and supernatant containing single cells was recovered and spun down at 500 × g for 10 min. Spleen cells were treated with ammonium chloride solution for 10 min to lyse the erythrocytes as previously described (7). Cells were washed three times with RPMI 1640 medium containing 10% bovine serum albumin and plated at a concentration of 105 cells/well in a 96-well plate together with either 1 μg of concanavalin A (ConA) per ml, 5 μg of phytohemagglutinin (PHA) per ml, 10 μg of lipopolysaccharide (LPS) per ml, or 1 and 5 μg of major outer membrane protein (MOMP) per ml. Proliferative responses were measured by uptake of 1 μCi of [3H]thymidine per well for the last 18 to 24 h of a 3-day culture. Results are reported as mean counts per minute ± standard error of triplicate cultures. Each experiment was repeated at least two times. Data were analyzed using Student's t test.
Cytokine analysis.
Cytokine levels were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits from Endogen Inc. (Woburn, Mass.) according to the protocol recommended in the kits. Briefly, 50- to 100-μl aliquots of supernatants were collected from spleen or lymph node cultures 48 h after the start of incubation. Supernatants were run in duplicates in ELISA assays for rat interleukin (IL-10) and rat gamma interferon (IFN-γ). Absorbency was read at 450 nm in an ELISA reader (Dynex Technologies), and data were analyzed by Dynex Revelation software. The sensitivity of both assays was <10 pg/ml with an inter- and intra-assay coefficient of variation of <10%.
RESULTS
Histopathological evaluation of rats infected with Chlamydia following hormone treatment.
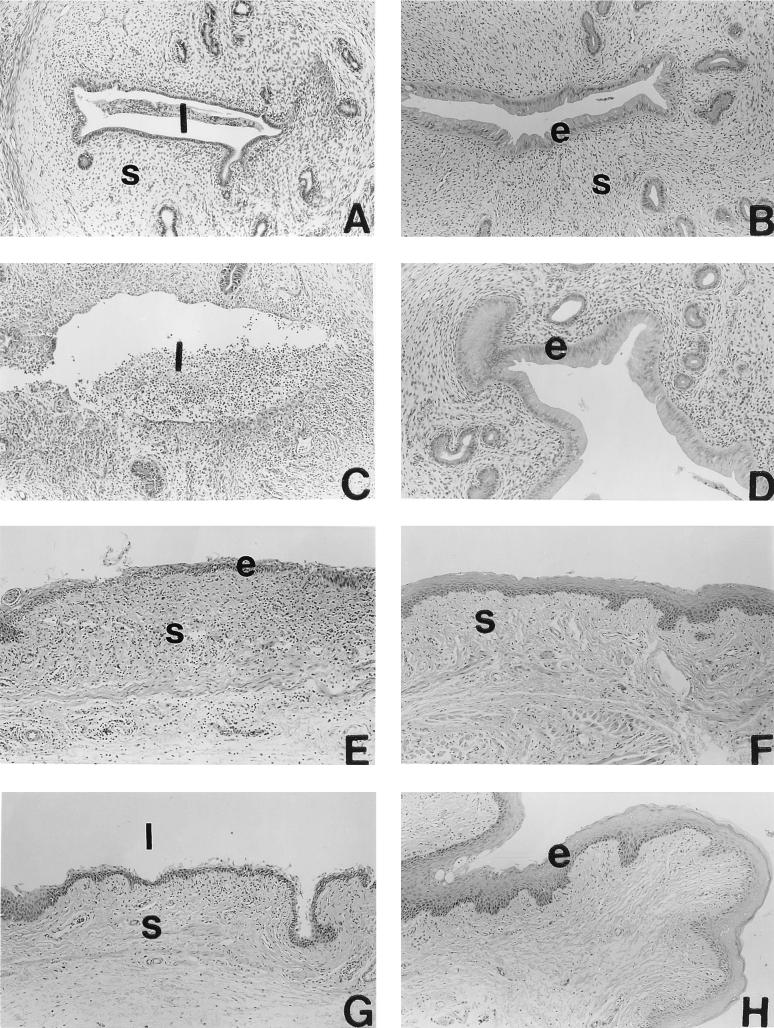
Female Lewis rats were injected with either saline, estradiol, progesterone, or estradiol plus progesterone for 3 consecutive days. On the second day of hormone treatment, rats were infected with C. trachomatis MoPn (5 × 106 IFU/uterine horn) via the intrauterine route. Rats were sacrificed 5 days later and examined for infection, tissue pathology, and immune response. Control rats (hormone treated, not infected) were also examined (results not shown). Figure 1 shows the histology of the uterus (A to D) and vagina (E to F) from the four groups of animals given hormone treatment and infected with Chlamydia. The control group (ovariectomized, saline treated, infected) (Fig. 1A and E) showed mild to moderate inflammation in the uterus and vagina characterized by an infiltration of granulocytes and a few lymphocytes into the subepithelial stroma. Rats that were estradiol treated and infected with Chlamydia had no inflammation in either uterus or vagina (Fig. 1B and F). The progesterone-treated, Chlamydia-infected group had the most severe inflammation. The uterine lumen of these rats had massive infiltrations of polymorphonuclear (PMN) cells (Fig. 1C). The glandular lumen (not shown) and the subepithelial stroma also had PMN infiltration. The vaginae of these animals had moderate inflammation with PMN infiltration (Fig. 1G). As was the case with the estradiol-treated group, no inflammation was observed in the combined hormone treatment group (Fig. 1D and H). Control rats (hormone treated, not infected) did not show any inflammatory infiltration in any of the treatment groups (data not shown). In the hormone-treated, uninfected control groups, a slight increase in number of leukocytes in the uteri of estradiol-treated rats and vaginae of progesterone-treated rats was noted. This was an expected effect of hormones, described by us previously (5), not part of an inflammatory process; we took these increases into account when grading for inflammation in infected rats. Results from two separate experiments where the uterus and vagina of each infected animal treated with hormone were histopathologically evaluated are summarized in Table 1.
FIG. 1.
Histopathology of uteri (A to D) and vaginae (E to F) of rats infected with C. trachomatis following intrauterine treatment with saline (A and E), estradiol (B and F), progesterone (C and G), or estradiol and progesterone (D and H). Animals were sacrificed 5 days postinfection; reproductive tract tissues were removed, fixed, processed for histology, and stained with hematoxylin and eosin. Representative tissue sections from each treatment groups are shown. Estrogenic effects on epithelial cells of uteri and vaginae of rats receiving estradiol can be observed (B, D, F, and H). Note the acute inflammation in the uterus of a progesterone-treated rat, characterized by large number of infiltrating leukocytes (C). s, stroma; l, lumen; e, epithelium. Original magnification, ×100.
TABLE 1.
Histopathology of hormone-treated rats, day 5 postinfection
| Groupa | Tissue | Histopathology gradeb
|
Overall assessment | |||
|---|---|---|---|---|---|---|
| Lymphocytes | Granulocytes | Tissue necrosis | Inflammation | |||
| Saline | Uterus | ++ | ++ | + | ++ | Mild/moderate inflammation |
| Vagina | ++ | +++ | + | ++ | Moderate inflammation | |
| E | Uterus | + | + | − | − | Normal |
| Vagina | + | − | − | − | Normal | |
| P | Uterus | + | ++++ | +++ | ++++ | Moderate/severe inflammation |
| Vagina | ++ | +++ | + | +++ | Moderate inflammation | |
| E+P | Uterus | +/− | + | − | − | Normal |
| Vagina | +/− | +/− | − | − | Normal | |
E, estradiol; P, progesterone.
−, no inflammatory cells/HPF (high-power field), no epithelial invasion, no tissue necrosis; −/+, <5 inflammatory cells/HPF, no epithelial invasion, no tissue necrosis; +/−, <10 inflammatory cells/HPF, minimal epithelial invasion, no tissue necrosis, focal apoptosis present; +, <20 inflammatory cells/HPF, focal epithelial invasion, no tissue necrosis, focal apoptosis present; ++, >20 inflammatory cells/HPF, focal epithelial invasion, focal tissue necrosis; +++, >20 inflammatory cells/HPF, moderate epithelial invasion, focal tissue necrosis, apparent tissue edema, purulent exudate; ++++, >20 inflammatory cells/HPF, marked epithelial invasion, marked tissue destruction, pus or abscess formation, marked tissue edema. The assessment was made from tissue samples of all animals in the same group (n = 4). The experiment was repeated twice.
Cervicovaginal shedding of C. trachomatis in hormone-treated animals.
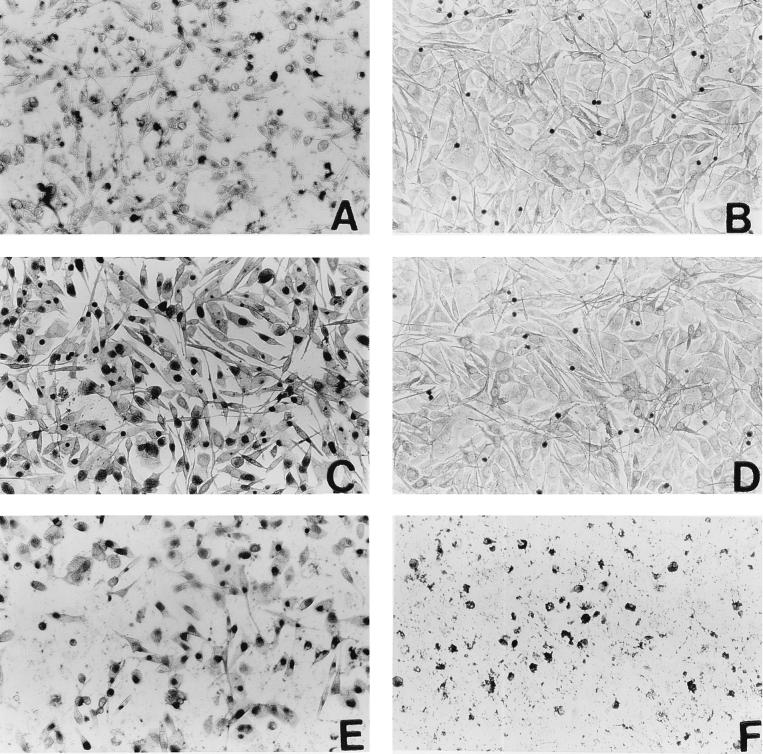
In vitro infectivity assays, described in Materials and Methods, were carried out to assess the level of infection in hormone-treated rats. The detection of inclusion bodies is illustrated in Fig. 2. As can be seen, cultures which received vaginal washes from estradiol-treated animals (Fig. 2D) were essentially similar to negative control cultures (Fig. 2B). Cultures exposed to washes from saline-treated infected animals showed inclusion bodies in large numbers (Fig. 2C). All cultures which were exposed to vaginal washes from the progesterone and combination groups, at the same dilution as for estradiol- and saline-treated groups, had either partially destroyed or completely destroyed monolayers demonstrating large numbers of shed IFU (Fig. 2E and F). These assays were repeated with further dilutions of vaginal flushes, and the quantitative results are shown in Table 2. Six of the eight saline-treated animals had titers of 109 IFU/ml or less. Titers higher than 1010 IFU/ml were observed in the cervicovaginal washings of all of eight animals from the progesterone-treated group and in seven of the eight animals in the combined hormone treatment group. We detected no organisms in the vaginal secretions from rats in the estradiol-treated group. These results are from two separate experiments, each time with four rats per group; vaginal secretions of each rat were monitored separately.
FIG. 2.
In vitro infectivity assay to assess bacterial shedding in vaginal washes of hormone-treated, infected rats. Vaginal washes from day 5 postinfection were layered on monolayers of McCoy cells and stained with an anti-Chlamydia antibody as described in Materials and Methods. Micrographs show stained monolayers from cultures layered with washings (all at same dilution) from animals infected with Chlamydia after treatment with saline (C), estradiol (D), progesterone (E), and estradiol plus progesterone (F). Positive (previously titered stock; A) and negative (mock infection; B) controls are also shown. Darkly stained inclusion bodies can be clearly distinguished in infected monolayers. Note that panels E and F are partially destroyed monolayers, indicating presence of too many IFU. These and similar samples were diluted further and retitered to obtain final titers. Results from estradiol-treated animals (D) were found to be similar to negative control results in all samples. The dark staining seen in panels B and D was due to staining of dead cells and was subtracted as background when titers were calculated.
TABLE 2.
Detection of Chlamydia in vaginal washes from hormone-treated, infected ratsa
| Group | No. of rats
|
|||
|---|---|---|---|---|
| ND | 108 IFU/ml | 109 IFU/ml | >1010 IFU/ml | |
| S | 0 | 1 | 5 | 2 |
| E | 8 | 0 | 0 | 0 |
| P | 0 | 0 | 0 | 8 |
| E + P | 0 | 0 | 1 | 7 |
Experimental groups consisted of ovariectomized female rats given saline (S), estradiol (E), progesterone (P), or a combination of both (E+P), as described in Materials and Methods. Each group had a total of eight rats in two experiments, and vaginal wash from each animal was monitored separately. ND, not detected. 1010 was the highest detection limit for this assay at a vaginal wash dilution of 1:10,000.
Tissue localization of chlamydial infection.
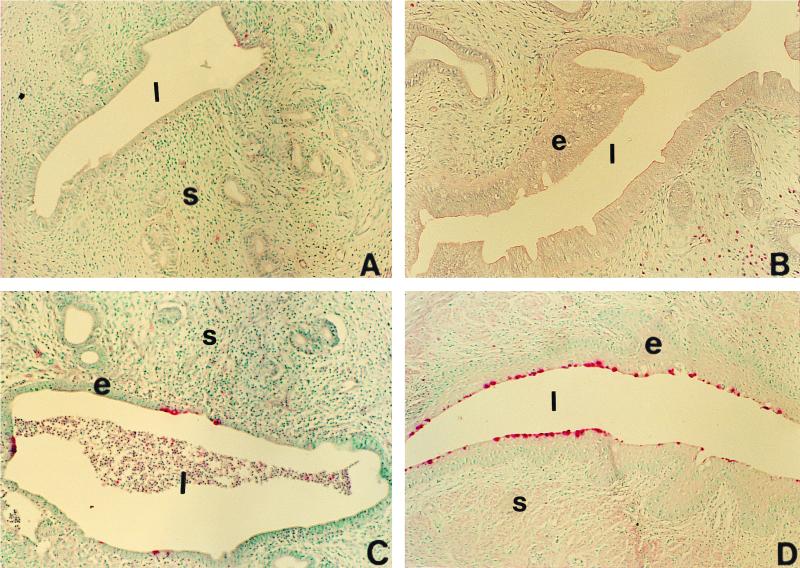
Immunohistochemical staining was done to localize chlamydial inclusion bodies in the tissue sections from hormone-treated rats, using a rabbit polyclonal antibody to Chlamydia (Fig. 3). While occasional chlamydial inclusions were detected in the uteri of saline-treated, infected rats (Fig. 3A), the uterine epithelium of progesterone-treated rats had extensive infection (Fig. 3C). Numerous inclusions were localized in the cervicovaginal epithelium of the combined hormone treatment group (Fig. 3D). There was no sign of infection in the uterine and vaginal epithelium of the estradiol-treated rats, as evidenced by lack of any detectable staining of inclusion bodies (Fig. 3B). The results from immunohistochemical assessment of all animals are summarized in Table 3. No specific staining for chlamydial antigens was observed below the epithelium in any of the groups.
FIG. 3.
Localization of infection in the reproductive tracts of hormone-treated, infected rats by immunohistochemical staining of chlamydial antigens. Polyclonal antibody was used to detect Chlamydia-specific staining as described in Materials and Methods. Representative tissue sections showing typical staining for each group are shown in the micrographs. Positive (pink) staining can be seen in the uterine epithelium of progesterone-treated animals (C) and cervicovaginal epithelium of estradiol-progesterone-treated animals (D). Very little positive staining was seen in saline-treated animals (A). Uteri and vaginae of estradiol-treated animals were negative in all sections examined. s, stroma; l, lumen; e, epithelium. Original magnification, ×100.
TABLE 3.
Immunohistochemical analysis of inclusion bodies in hormone-treated rats
| Groupa | Tissue | No. of IBb | Overall histologic assessment |
|---|---|---|---|
| S | Uterus | 3 | Mild infection with inflammation |
| Vagina | 28 | Extensive infection with inflammation | |
| E | Uterus | 0 | No infection, no inflammation |
| Vagina | 0 | No infection, no inflammation | |
| P | Uterus | 8 | Moderate infection with inflammation |
| Vagina | 30 | Extensive infection with inflammation | |
| E+P | Uterus | 7 | Faint staining, no inflammation |
| Vagina | 48 | Extensive focal infection, no inflammation |
Experimental groups consisted of ovariectomized rats treated with saline (S), estradiol (E), progesterone (P), or estradiol and progesterone (E+P).
Total number of inclusion bodies (IB) counted on each slide stained with anti-Chlamydia antibody, as described in Materials and Methods. Each slide had at least one representative tissue section from each animal (n = 4).
Characterization of tissue inflammation in hormone-treated, Chlamydia-infected rats.
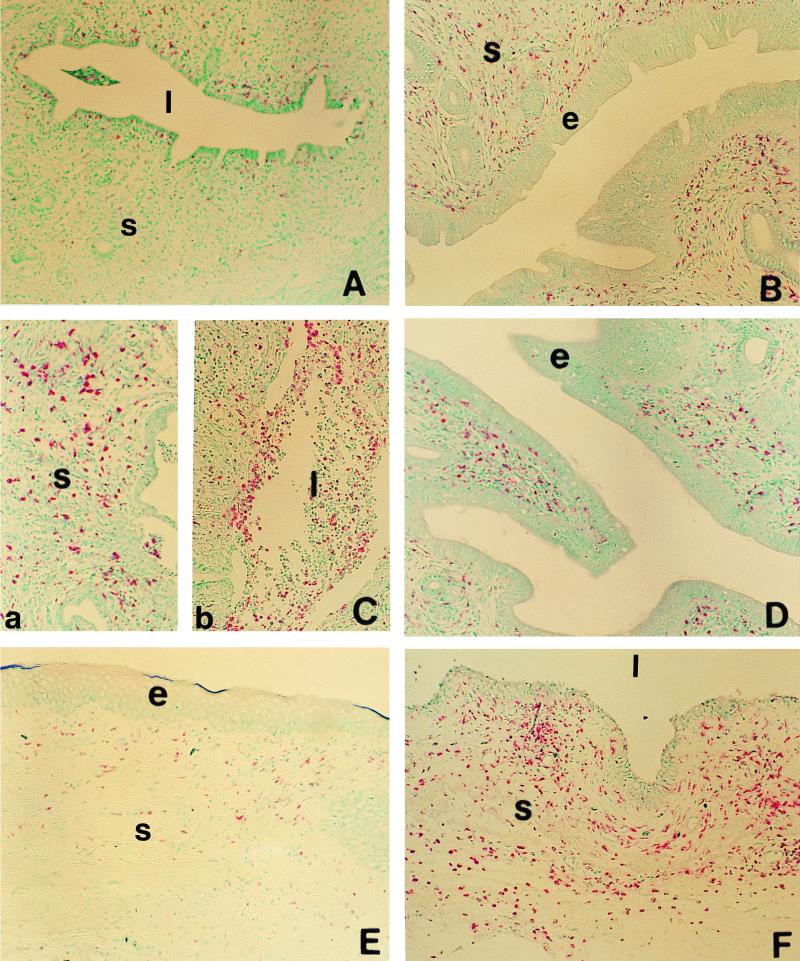
To characterize the inflammatory response seen in the hormone-treated animals infected with C. trachomatis, we examined tissue sections from the uteri and vaginae of the four experimental groups, using immunohistochemical staining for ED1, a marker for macrophages and blood granulocytes, as described in Materials and Methods (Fig. 4). Very few ED1-positive cells were found within the uterine subepithelial stroma of saline-treated, infected rats (Fig. 4A). Chlamydia-infected rats from the estradiol-treated group and combined hormone group had an increased numbers of ED1-positive cells compared to the saline group (Fig. 4B and D). These cells were uniformly scattered throughout the stroma and did not appear to be part of a localized inflammatory response. Increase in the local immune cell population under the influence of estradiol has been previously reported (5). In contrast, progesterone-treated, Chlamydia-infected animals had large numbers of ED1-positive cells in the tissue and in the uterine lumen (Fig. 4C). These cells were concentrated in the subepithelial layer (Fig. 4C, a) and in the leukocytic infiltration present in the lumen (Fig. 4C, b). A similar increase in the distribution of ED1 cells was observed in the subepithelial stroma of the vagina of saline-treated rats (not shown) and progesterone-treated infected rats (Fig. 4F), while no increase in ED1-positive cells was observed in the vaginal mucus of estradiol-treated (Fig. 4E) and combination-treated (not shown) groups. These results indicate that an acute inflammatory response characterized by the presence of granulocytes and macrophages is present in the genital tract of progesterone-treated, infected animals and to a lesser extent in saline-treated, infected animals.
FIG. 4.
Localization of ED1-positive cells (macrophages and blood granulocytes) in the uteri (A to D) and vaginae (E and F) of hormone-treated, infected rats. Positively stained cells (pink) can be seen in uteri of animals treated with saline (A), estradiol (B), progesterone (C), or estradiol and progesterone (D). Micrographs of representative tissue sections from vaginae of estradiol-treated (E) and progesterone-treated (F) animals stained for ED1-positive cells are also shown. Note the accumulation of large number of positively stained cells in the subepithelial stroma (C, a) and the leukocytic infiltration in the lumen (C, b) of uteri of progesterone-treated animals. s, stroma; l, lumen; e, epithelium. Original magnification, ×100.
Lymphocytic proliferation in hormone-treated, Chlamydia-infected rats.
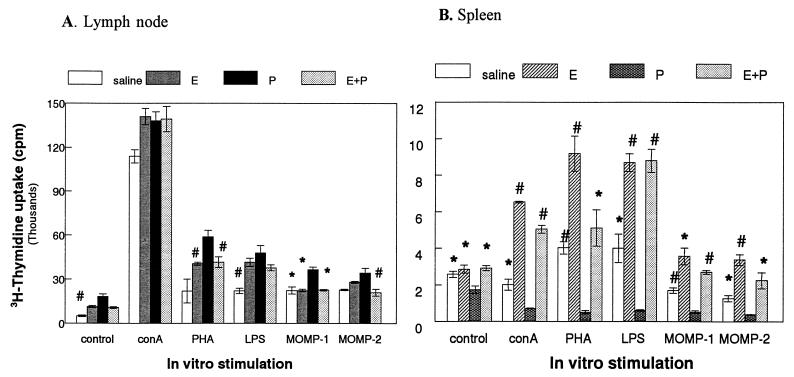
To examine the immune response in hormone-treated, Chlamydia-infected rats, PALN, which drain the genital tract, and spleen cells were isolated and stimulated by mitogens or chlamydial MOMP antigen. Figure 5A shows the lymphocyte proliferation response by the PALN cells of the different experimental groups. Maximum mitogenic as well as MOMP-specific responses were observed in PALN cells from progesterone-treated, infected rats. Enhanced mitogenic proliferation was also observed in the estradiol and combination groups compared to lymph node cells from saline-treated, infected rats that were the least activated. Spleen cells (Fig. 5B), on the other hand, gave much lower proliferation to both mitogens (ConA, LPS, and PHA) and MOMP compared to PALN cells. Proliferation was significantly suppressed in spleen cell cultures from progesterone-treated, Chlamydia-infected rats. Spleen cells from estradiol-treated and combined hormone treatment rats had the highest proliferation to mitogens as well as MOMP-specific challenge.
FIG. 5.
Lymph node (A) and spleen (B) cell proliferation assay in response to mitogens and MOMP in hormone-treated, infected animals. Four animals were used for each treatment group. Results shown are representative of two separate experiments. Spleen or lymph node cells were isolated as described in Materials and Methods and incubated in the presence of medium alone (control), ConA (1 μg/ml), PHA (5 μg/ml), LPS (10 μg/ml), MOMP-1 (1 μg/ml), and MOMP-2 (5 μg/ml). ∗, P < 0.01; #, P < 0.05 compared to progesterone-treated group. E, estradiol; P, progesterone.
Cytokine secretion by lymph node and spleen cells of hormone-treated, Chlamydia-infected rats.
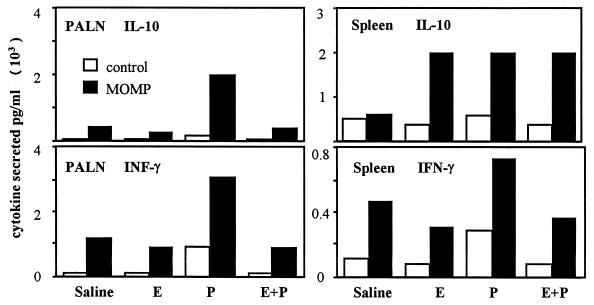
In experiments run in parallel with the proliferation assays, PALN cells and spleen cells were cultured from the four groups of infected rats, sacrificed 5 days postinfection. The cells were cultured for 48 h in the presence or absence of MOMP and the supernatants examined for IL-10 and IFN-γ. As shown in Fig. 6, large amounts of IL-10 and IFN-γ were produced by PALN cells from progesterone-treated, infected rats, following MOMP challenge relative to other hormone-treated groups. Cells from the progesterone-treated rats also had elevated IFN-γ production in the absence of MOMP compared to cells from other groups. PALN cells from estrogen and combined hormone-treated rats produced IFN-γ at levels similar to untreated controls. PALN cells produced very little IL-10 in the absence of MOMP. Supernatants from spleen cells showed elevated levels of IL-10 in all three hormone-treated group following MOMP challenge. The highest IFN-γ levels were seen with MOMP challenge of spleen cells from progesterone-treated, Chlamydia-infected rats. IFN-γ produced by spleen cells taken from the estrogen or combined hormone treatment rats was similar to that produced by spleen cells from untreated, infected rats. These results indicate that Chlamydia infection in progesterone-treated rats increased the Chlamydia-specific production of IFN-γ and IL-10 in the draining nodes and to a lesser extent IFN-γ production by spleen cells.
FIG. 6.
IFN-γ and IL-10 measurements in supernatants from PALN and spleen cells of hormone-treated, infected animals. Both IFN-γ and IL-10 were in measured in PALN and spleen cell cultures from day 5 postinfection, incubated with or without MOMP (1 μg/ml). E, estradiol; P, progesterone.
DISCUSSION
These studies provide direct evidence that the hormonal environment at the time of pathogen exposure can have a distinct effect on the outcome of a microbial infection in the genital tract. We found that in the absence of any endogenous hormones, ovariectomized rats were susceptible to genital chlamydial infection, as indicated by mild infection and low-grade inflammation in uterus and vagina. Giving estradiol to ovariectomized rats led to complete protection from chlamydial infection, and none of the animals showed any signs of inflammation in the uterus or the vagina. When progesterone alone was administered, animals became heavily infected, and infection was accompanied by an acute inflammatory response. In marked contrast, in the presence of both estradiol and progesterone, the rats became heavily infected but did not show any inflammatory response. These results suggest that estradiol and progesterone have separate and distinct effects on susceptibility to infection and inflammation. While estradiol decreases susceptibility, it also seems to have a strong anti-inflammatory response. This effect seems to be distinct from the protective effect, because inflammation was absent even in the presence of infection in the combination hormone group. Progesterone, on the other hand, seems to enhance both susceptibility and inflammatory responses. Alternatively, progesterone may enhance infection, leading to increased inflammation. With the combination of hormones used in these experiments, estradiol effect on inflammation predominates whereas progesterone affects primarily susceptibility. These findings have important implications in view of the fact that in women, chlamydial infections are often silent, and subsequent reinfections lead to inflammatory responses with pathological sequelae such as pelvic inflammatory disease, scarring of fallopian tubes, and ectopic pregnancy (12, 13). Further characterization of this model could provide a new approach to understanding asymptomatic chlamydial infections, which have been difficult to study because of the lack of an appropriate animal model.
Previous studies have shown that sex hormones influence susceptibility to microbial infections in the reproductive tract in a number of species. Mice treated with progesterone were found to have higher mortality rates when infected intravaginally with herpes simplex virus type 2 (1). In other studies, genital tract susceptibility to Neisseria gonorrhoeae was enhanced in mice at proestrus, when estrogen levels are high (10). Rank et al. have shown that guinea pigs are more susceptible to chlamydial infection following estradiol treatment, while other studies show that infection can be established in mice only after progesterone pretreatment (17, 19). Studies of humans show that women are also more susceptible to infection under estradiol influence, since more chlamydial organisms can be isolated from the proliferative part of the cycle (24). The exact mechanism by which different hormones affect susceptibility in a species-specific manner is not understood. In our studies, rats infected at estrus and diestrus stages of the cycle without prior progesterone priming did not show any signs of active infection, although enhanced local immune response was noted (7). However, following progesterone priming, rats are susceptible to genital C. trachomatis infection. Chlamydial inclusions were detected in the uteri and vaginas of infected animals, and at the same time immune responses could be detected locally and systemically (7). In other studies, we have found that rats are susceptible to genital chlamydial infection when exposed by both intrauterine and intravaginal route, following progesterone pretreatment (C. Kaushic and C. R. Wira, unpublished data). However, intravaginal exposure led to a lower infection and a faster clearance compared to intrauterine exposure.
The mechanisms by which progesterone enhances susceptibility and estradiol protects against genital tract infections in rodents are not clear. It has been suggested that under the influence of progesterone, the epithelial lining of the genital tract is thinned and stabilized in a diestrus condition, allowing infection to become established. While this is possible, there may be additional factors influencing susceptibility and type of immune response following infection. Studies from our laboratory have shown that sex hormones have profound effects, on both the inductive and the effector arm of the mucosal immune system in the reproductive tract, that may affect the outcome of infections (29). We have shown that antigen presentation in the uterus and vagina is influenced by estradiol and progesterone (30, 31). IgA and IgG levels, IgA transport, and immune cell trafficking in the genital tract are also regulated by estradiol and progesterone (5, 6, 8, 32). Moreover, this regulation is tissue specific because, while estradiol enhances and progesterone suppresses immune response in the uterus, they affect the lower reproductive tract in opposite manners. Recently similar results have been reported for the reproductive tract of women. Antigen-independent CD3+ T-lymphocyte cytolytic activity was found to be higher during the proliferative phase under the influence of estradiol and absent in the secretory phase of the menstrual cycle when progesterone levels are high (28). Thus, depending on hormonal environment at the site of exposure in the reproductive tract, the local mucosal immune system may be altered, which could lead to increased or decreased protection, with or without inflammation as seen in the present study.
One of the possible mechanisms which may account for the differences in susceptibility seen under different hormone influences may be differential expression of receptors on epithelial cells in the genital tract which mediate chlamydial entry (33). Chlamydial infection is initiated by contact, attachment and entry of infectious elementary bodies (EBs) into the host epithelial cell. The exact mechanism of attachment and entry is still not clear, although several likely possibilities have been suggested. Of these, the most promising one involves a glycosaminglycan-mediated mechanism (36). Studies show that a heparan sulfate-like glycosaminglycan on the surface of EBs binds to a heparan sulfate receptor on the epithelial cells. Other studies have provided evidence for the possibility that chlamydial MOMP is the adhesion molecule that binds to heparan sulfate proteoglycans on the epithelial surface (22). Interestingly, reproductive tract tissues have been shown to express a whole family of proteoglycans belonging to epidermal growth factor (EGF) family, including EGF, transforming growth factor β, heparin-binding EGF, and their receptors (11, 26, 34). Some of these, like heparin-binding EGF and heparin affin regulatory peptide, HARP have been demonstrated to be under estradiol and/or progesterone regulation (11, 34, 35). Examining the expression of these proteoglycans in this system under different hormone conditions and correlating with infection could provide important information on the mechanism of chlamydial susceptibility in the reproductive tract.
When early immune responses were examined in the present study, lymphocyte activation and enhanced cytokine release were observed in the draining lymph node cells of progesterone-treated animals, in response to both mitogenic and chlamydial antigen-specific stimulation. At the same time, proliferation of spleen cells was suppressed. These results show that in the absence of any other hormone effect, infection with Chlamydia, following progesterone pretreatment, leads to induction of local immune response (seen in draining lymph nodes) while at the same time there is a systemic suppression (seen in the spleen). That progesterone enhanced local immune response in the uterus and draining lymph nodes is somewhat surprising, since others have shown that it is associated with immunosuppressive effects on immune responses (for a review, see reference 14). We have shown in previous studies that progesterone treatment of ovariectomized animals leads to decreased IgA and polymeric Ig receptor mRNA and protein levels in the uterus (8). Under the influence of progesterone, antigen presentation by uterine cells is decreased; fewer immune cells are present in the rat uterus at diestrus when progesterone levels are high (5, 31). An alternate explanation to the contrast observed in local lymph node and spleen cell responses in this study may be that while progesterone has a suppressive effect on both, the suppressive effect is obvious only in the spleen; in the lymph nodes, it is masked by the local immune response to Chlamydia. The present data show that any local immunosuppression by progesterone is overridden in the presence of microbial infection in the genital tract. These results extend our previous finding that nonovariectomized, intact rats infected with chlamydia exhibited a modest activation of local immune system and suppression of the systemic immune system following progesterone treatment (7).
The present study also indicates that under the influence of estradiol, there is a greater systemic immune response to genital infection whereas progesterone leads to a specific local immune activation (7). This can be concluded from the results where enhanced mitogenic responses were found in the draining lymph node cells of the estradiol and combination hormone-treated group, but these cells did not show enhanced activation or produce significant amounts of the cytokines IFN-γ and IL-10 in response to chlamydial challenge. On the other hand, spleen cells from both groups that received estradiol showed high proliferation rates as well as enhanced IFN-γ and IL-10 secretion in response to chlamydial challenge.
Studies in murine models of MoPn infection have shown that resistance to and clearance of infection are heavily dependent on Th1-like cytokines. IL-12 is important for initial clearance of bacteria, while IFN-γ is important for long-term resolution of infection (18). Other studies have demonstrated that while cell-mediated immune responses are capable of resolving chlamydial infection, mice are more susceptible to reinfection in the absence of specific antibody (23). It is less clear if there is an association between a Th1 response and immunopathology. One study showed that in a monkey model, repeated C. trachomatis infection of fallopian tubes led to a Th1 cytokine response associated with fibrosis and scarring (25). Chlamydial LPS, endotoxin, and heat shock hsp60 and -70 have also been implicated with inflammation and pathological consequences (25). The results of the present study show that the early immune response in progesterone-treated rats infected with Chlamydia appears to be a mixed type, with both IFN-γ and IL-10 being secreted by the local lymph nodes in large amounts on day 5 postinfection. Further studies are needed to characterize the cytokine profile at a later stage in infection under different hormonal conditions to see if inflammation persists and correlates with Th1-type profile in this model.
To summarize, our results show that estradiol decreases susceptibility to genital chlamydial infection and reduces accompanying inflammation, while progesterone increases susceptibility and promotes an inflammatory response following infection. The differences seen under different hormone conditions in the infectivity, inflammation, and early immune responses in the present study make this a very useful system to identify the mechanism of susceptibility and inflammation. These findings have important implications for future vaccine strategies against genital infections. Using knowledge about the hormonal environment most likely to induce protective immune responses without the associated immunopathology may lead to more effective vaccines.
ACKNOWLEDGMENTS
We are grateful to Richard Rossoll and Dathao Ho for technical assistance; we thank Denis P. Snider for valuable suggestions and critical reading of the manuscript.
Photomicroscopy was done in the Herbert C. Englert Cell Analysis Laboratory, which is supported in part by a core grant from Norris Cotton Cancer Center (CA 23108) and by equipment grants from the Fannie E. Rippel Foundation. This work was supported by research grant AI-13541 from NIH (C.R.W.) and a research grant from the Hitchcock Foundation (C.K.).
REFERENCES
- 1.Baker D A, Plotkin S A. Enhancement of vaginal infection in mice by herpes simplex virus type II with progesterone. Proc Soc Exp Biol Med. 1978;158:131–134. doi: 10.3181/00379727-158-40156. [DOI] [PubMed] [Google Scholar]
- 2.Barron A, Pasley J N, Rank R G, White H J, Mrak R E. Chlamydial salpingitis in female guinea pigs receiving oral contraceptives. Sex Transm Dis. 1988;15:169–173. doi: 10.1097/00007435-198807000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito J J I, Harrison R, Alexander E R, Billings L J. Establishment of genital tract infection in the CF-1 mouse by intravaginal inoculation of a human oculogenital isolate of chlamydia trachomatis. J Infect Dis. 1984;150:577–582. doi: 10.1093/infdis/150.4.577. [DOI] [PubMed] [Google Scholar]
- 5.Kaushic C, Frauendorf E, Rossoll R M, Richardson J M, Wira C R. Influence of estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39:209–216. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaushic C, Frauendorf E, Wira C R. Polymeric immunoglobulin A receptor in the rodent reproductive tract: influence of estradiol in the vagina and differential expression of messenger ribonucleic acid expression in rodent uteri. Biol Reprod. 1997;57:958–966. doi: 10.1095/biolreprod57.5.958. [DOI] [PubMed] [Google Scholar]
- 7.Kaushic C, Murdin A D, Underdown B J, Wira C R. Chlamydia trachomatis infection in the female reproductive tract of rat: influence of progesterone on infectivity and immune response. Infect Immun. 1998;66:893–898. doi: 10.1128/iai.66.3.893-898.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushic C, Richardson J M, Wira C R. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology. 1995;136:2836–2844. doi: 10.1210/endo.136.7.7789308. [DOI] [PubMed] [Google Scholar]
- 9.Kaushic C, Wira C. Effect of sex hormones on immune cells and cytokines in the female reproductive tract. Clin Immunol Pathol. 1995;76:S57. [Google Scholar]
- 10.Kita E, Matsuura H, Kashiba S. A mouse model for the study of gonococcal genital infection. J Infect Dis. 1981;143:67–70. doi: 10.1093/infdis/143.1.67. [DOI] [PubMed] [Google Scholar]
- 11.Milhiet P E, Vacherot F, Caruelle J-P, Barritault D, Caruelle D. Upregulation of the angiogenic factor heparin affin regulatory peptide by progesterone in rat uterus. J Endocrinol. 1998;158:389–399. doi: 10.1677/joe.0.1580389. [DOI] [PubMed] [Google Scholar]
- 12.Morell V. Attacking the causes of “silent” infertility. Science. 1995;269:775–777. doi: 10.1126/science.7638588. [DOI] [PubMed] [Google Scholar]
- 13.Morrison R P, Manning D S, Caldwell H D. Immunology of Chlamydia trachomatis infections. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 57–84. [Google Scholar]
- 14.Olsen N J, Kovacs W J. Gonadal steroids and immunity. Endocrine Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 15.Pasley J N, Rank R G, Hough A J J, Cohen C, Barron A L. Effects of various doses of estradiol on chlamydial genital infection in ovariectomized guinea pigs. Sex Transm Dis. 1985;12:8–13. doi: 10.1097/00007435-198501000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Patton D L, Lichtenwalner A B. Animal models for the study of chlamydial research. In: Stephens R S, Byrne G I, Christiansen G, et al., editors. Chlamydial infections. Proceedings of Ninth International Symposium on Human Chlamydial Infection. Cambridge, England: Cambridge University Press; 1998. pp. 641–650. [Google Scholar]
- 17.Patton D L, Rank R G. Animal model for study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press; 1992. pp. 85–111. [Google Scholar]
- 18.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 19.Rank R G, White H J, Hough A J J, Pasley J N, Barron A L. Effect of estradiol on chlamydial genital infection of female guinea pigs. Infect Immun. 1982;38:699–705. doi: 10.1128/iai.38.2.699-705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schachter J, Grayston J T. Epidemiology of human chlamydial infections. In: Stephens R S, Byrne G I, Christiansen G, et al., editors. Chlamydia infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. Cambridge, England: Cambridge University Press; 1998. pp. 3–10. [Google Scholar]
- 21.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su H, Caldwell H D. Sulfated polysaccharides and a synthetic sulfated polymer are potent inhibitors of Chlamydia trachomatis infectivity in vitro but lack protective efficacy in an in vivo murine model of chlamydial genital tract infection. Infect Immun. 1998;66:1258–1260. doi: 10.1128/iai.66.3.1258-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweet R L, Blankfort-Doyle M, Robbie M O, Schachter J. The occurrence of chlamydial and gonococcal salpingitis during the menstrual cycle. JAMA. 1986;255:2246–2250. [PubMed] [Google Scholar]
- 25.Van Voorhis W C, Barrett L K, Cosgrove Sweeney Y T, Kuo C C, Patton D L. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X-N, Das S K, Damm D, Kagsburn M, Abraham J A, Dey S K. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- 27.Washington A E, Gove S, Schachter J, Sweet R L. Oral contraceptives, Chlamydia trachomatis infection, and pelvic inflammatory disease. A word of caution about protection. JAMA. 1985;1985:2062–2065. [PubMed] [Google Scholar]
- 28.White H D, Crassi K M, Givan A L, Stern J E, Gonzales J L, Memoli V A, Green W R, Wira C R. CD3+CD8+ CTL activity within the human female reproductive tract: influence of stage and menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 29.Wira C R, Kaushic C. Mucosal immunity in the female reproductive tract: effect of sex hormones on immune recognition and responses. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines: new trends in immunization. New York, N.Y: Academic Press; 1996. pp. 375–386. [Google Scholar]
- 30.Wira C R, Rossoll R. Antigen presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- 31.Wira C R, Rossoll R M. Antigen presenting cells in the female reproductive tract: influence of estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526–4534. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]
- 32.Wira C R, Sandoe C P. Sex steroid hormone regulation of immunoglobin G (IgG) and A (IgA) in rat uterine secretions. Nature. 1977;268:534–536. doi: 10.1038/268534a0. [DOI] [PubMed] [Google Scholar]
- 33.Wyrick P B. Cell biology of chlamydial infection: a journey in the host epithelial cell by the ultimate cellular microbiologist. In: Stephens R S, Byrne G I, Christiansen G, et al., editors. Chamydial infections. Proceedings of Ninth International Symposium on Human Chlamydial Infection. Cambridge, England: Cambridge University Press; 1998. pp. 69–78. [Google Scholar]
- 34.Zhang J P, Funk C, Glasser S, Mulholland J. Heparin-binding epidermal growth factor-like growth factor is differentially regulated by progesterone and estradiol in rat uterine epithelial and stromal cells. Endocrinology. 1994;134:1089–1094. doi: 10.1210/endo.134.3.8119147. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J P, Funk C, Glasser S, Mulholland J. Progesterone regulation of heparin-binding epidermal growth factor-like growth factor gene expression during sensitization and decidualization in the rat uterus: effects of the antiprogestin, ZK 98.299. Endocrinology. 1994;135:1256–1263. doi: 10.1210/endo.135.3.8070371. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J P, Stephens R S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]