Abstract
The proinflammatory cytokine tumor necrosis factor alpha (TNF-α) induces lethal hepatitis when injected into d-(+)-galactosamine-sensitized mice on the one hand or systemic inflammatory response syndrome (SIRS) in normal mice on the other hand. We studied whether serum amyloid P component (SAP), the major acute-phase protein in mice, plays a protective role in both lethal models. For this purpose, we used SAP0/0 mice generated by gene targeting. We studied the lethal response of SAP0/0 or SAP+/+ mice to both lethal triggers but found no differences in the sensitivity of both types of mice. We also investigated whether SAP is involved in establishing two types of endogenous protection: one using a single injection of interleukin-1β (IL-1β) for desensitization and clearly involving a liver protein, the other by tolerizing mice for 5 days using small doses of human TNF-α. Although after IL-1β or after tolerization the SAP levels in the serum had risen fourfold in the control mice and not in the SAP0/0 mice, the same extents of desensitization and tolerization were achieved. Finally, we observed that the induction of hemorrhagic necrosis in the skin of mice by two consecutive local injections with TNF-α was not altered in SAP0/0 mice. We conclude that the presence or absence of SAP has no influence on the sensitivity of mice to TNF-α-induced hepatitis, SIRS, and hemorrhagic necrosis or on the endogenous protective mechanisms of desensitization or tolerization.
Tumor necrosis factor alpha (TNF-α) is a pleiotropic cytokine which exhibits a pronounced antitumor activity in vitro as well as in vivo (2). However, administration of TNF-α has proven to induce a systemic inflammatory response syndrome (SIRS) (41). Administration of TNF-α in humans and in experimental animals gives rise to a shock-like response associated with hypotension and liver damage (5, 8, 19, 36). So far, the application of TNF-α in a systemic treatment remains impossible. Local treatment, however, has been achieved with considerable success using the technique of isolated limb perfusion (23, 30). Also, the use of TNF-α in isolated hepatic perfusion has recently been reported (10, 24).
In order to study TNF-α-induced hepatitis and SIRS, we used murine TNF-α (mTNF-α) in d-(+)-galactosamine (GalN)-sensitized or normal mice. GalN is a hepatotoxin which specifically inhibits transcription and translation in hepatocytes (9). In combination with TNF-α, extreme apoptosis and necrosis of hepatocytes are observed (22, 25, 42). The mice die about 6 to 8 h after the challenge. TNF-α injection into normal mice leads to lethal SIRS characterized by hypotension. In normal mice, TNF-α causes lethality between 24 and 48 h after the challenge, by a process resembling septic shock.
We are interested in identifying endogenous protective molecules and are focusing on the possible protective roles of acute-phase proteins. In this context, we already described protection conferred by α1-acid glycoprotein (25) and α1-antitrypsin (29). To induce feedback systems, we use two different methods: (i) injection of a single dose of interleukin-1β (IL-1β), which causes “desensitization,” which is most pronounced against TNF-α–GalN (28, 43), and (ii) injection of a low dose of human TNF-α (hTNF-α) twice daily for 5 days, which leads to “tolerization” against TNF-α-induced SIRS (37). The mechanism of IL-1β-induced desensitization is not fully understood but clearly involves induction of one or more factors in the liver. An involvement of the liver in establishing tolerization has not yet been demonstrated.
As IL-1β is a strong inducer of acute-phase proteins and since serum amyloid P (SAP) component is one of the major acute-phase proteins in mice, we investigated the role of SAP in both TNF-α models and in the induction of desensitization and tolerization. We also studied the effect of SAP presence or absence in the induction of hemorrhagic necrosis by TNF-α. To this end, we used recently generated SAP0/0 mice that have no circulating SAP but are fertile and develop normally (3). When such mice are treated with casein, they do not display amyloid deposition, which is a typical feature of Alzheimer's disease (3).
MATERIALS AND METHODS
Animals.
SAP0/0 mice were kindly provided by M. Botto and M. B. Pepys (Immunological Medicine Unit, Royal Postgraduate Medical School, London, United Kingdom). The SAP0/0 allele was backcrossed in a C57BL/6 background for seven generations, after which heterozygotes were intercrossed; homozygous SAP0/0 animals and homozygous wild types were identified by SAP enzyme-linked immunosorbent assay (ELISA). No SAP was detected in the serum of SAP0/0 mice by ELISA (3). ELISA has a sensitivity of approximately 10 ng/ml, whereas normal basal levels found in wild-type C57BL/6 mice are approximately 10 μg/ml. Mutant and wild-type mice were then further bred as inbred couples. Female offspring was used at the age of 8 to 12 weeks. The animals were housed in a temperature-controlled, air-conditioned room with 12-h light-dark cycles and received food and water ad libitum.
Reagents.
mTNF-α and hTNF-α were expressed in Escherichia coli, produced and purified to homogeneity in our laboratory, and had specific activities of 8.0 × 107 and 1.8 × 107 IU/mg, respectively. The endotoxin levels, assessed with a chromogenic Limulus amebocyte lysate assay (Coatest; Chromogenix, Stockholm, Sweden), were less than 10 endotoxin units (EU)/mg for both cytokine preparations. mIL-1β was expressed in E. coli, was purified at our facilities, and had a specific activity of 3.65 × 108 U/mg and an endotoxin contamination of <10 EU/mg of protein. GalN was purchased from Sigma Chemical Co. (St. Louis, Mo.).
Injections, blood collections, and SAP ELISA.
Before injection, all reagents were diluted in lipopolysaccharide-free phosphate-buffered saline (PBS). Intraperitoneal (i.p.) injections were 0.5 ml, and intravenous (i.v.) injections were 0.2 ml. Subcutaneous (s.c.) injections of TNF-α were 0.1 ml. Mice were bled at the retroorbital plexus under light ether anesthesia. Serum was kept at −20°C.
Skin necrosis was induced by two s.c. injections of TNF-α into shaved mice. To this end, mice were anesthetized using Avertine (tribromoethanol), and their backs were shaved using an electric shaver and Veet 3 days before the injections.
SAP was measured by a sandwich ELISA as previously described (39). Briefly, microtiter plates were coated overnight with a 1/1,000 dilution of a sheep anti-mouse SAP (Calbiochem-Novabiochem International, San Diego, Calif.). After washing, free places were blocked using 1% bovine serum albumin solution in PBS (1 h at 37°C). Serum and a standard (Calbiochem-Novabiochem International) were diluted 25-fold, titrated in 1/5 steps in the assay in triplicates, and incubated at 37°C for 3 h. After washing, the second antibody (a rabbit anti-mouse SAP; Calbiochem-Novabiochem International) was added in a 1/5,000 dilution; the plates were incubated 1 h at 37°C, after which an anti-rabbit antibody alkaline phosphatase-conjugated (Sigma Chemical Co.) was added and incubated for another hour at 37°C. The assay was developed using p-nitrophenylphosphate; absorption was measured at 405 nm.
Body temperatures and skin necrosis lesion size.
Rectal body temperatures were recorded with an electronic thermometer (model 2001; Comark Electronics, Littlehampton, United Kingdom). The size of the skin lesions (in square millimeters) was determined using calipers; the largest diameter and the perpendicular diameter were measured and multiplied.
Statistics.
Mean values and the standard deviations were compared using an unpaired Student's t test. Final lethality was evaluated with a χ2 test.
RESULTS
Response of SAP0/0 mice to TNF-α-induced lethality in GalN-sensitized and normal mice.
Before we studied the involvement of SAP in desensitization and tolerization, we examined the effect of SAP deficiency in both models of TNF-α-induced lethality. Therefore, mice were treated with 20 mg of GalN given i.p. in combination with different doses of mTNF-α. In C57BL/6 mice, the 100% lethal dose (LD100) is usually obtained with 0.3 to 0.5 μg of TNF-α per mouse. In Table 1, we show that both SAP+/+ and SAP0/0 mice are killed by TNF-α in combination with GalN. No sensitization or protection were observed by the deletion of SAP.
TABLE 1.
Response of SAP0/0 and SAP+/+ mice to TNF-α–GalN- or mTNF-α-induced lethality
| Challenge dose(s)a | Lethalityb (no. of animals killed/total no.)
|
|
|---|---|---|
| SAP+/+ | SAP0/0 (P) | |
| 0.01 μg of mTNF-α + 20 mg of GalN | 0/6 | 0/6 (NS) |
| 0.03 μg of mTNF-α + 20 mg of GalN | 4/6 | 5/6 (NS) |
| 0.1 μg of mTNF-α + 20 mg of GalN | 5/6 | 5/6 (NS) |
| 0.3 μg of mTNF-α + 20 mg of GalN | 6/6 | 6/6 (NS) |
| 2.5 μg of mTNF-α | 0/5 | 0/5 (NS) |
| 10 μg of mTNF-α | 8/10 | 5/6 (NS) |
| 20 μg of mTNF-α | 10/10 | 6/6 (NS) |
| 30 μg of mTNF-α | 10/10 | 6/6 (NS) |
TNF-α–GalN was given i.p., and mTNF-α was given i.v. (doses are per 20 g of body weight).
Lethality was scored for up to 48 h (no further deaths occurred). NS, not significantly different from SAP+/+ control mice.
To test the sensitivity to mTNF-α (without GalN sensitization), SAP+/+ and SAP0/0 mice were injected i.v. with 2.5, 10, 20, or 30 μg of mTNF-α (the LD100 for C57BL/6 mice is usually around 20 μg per mouse). Both SAP+/+ and SAP0/0 mice appeared to be equally sensitive to mTNF-α-induced lethal shock (Table 1). These data show that the absence of SAP does not lead to a changed response of mice to TNF-α-induced hepatitis or TNF-α-induced lethal SIRS.
Role of SAP in IL-1β-induced desensitization.
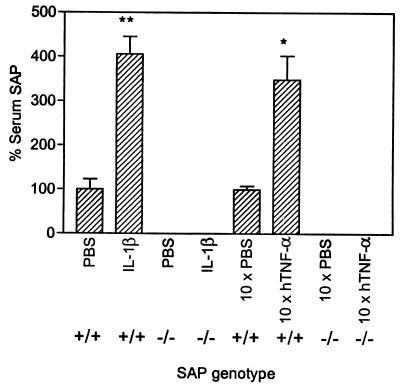
Desensitization of mice to TNF-α–GalN is obtained by pretreatment with IL-1β 12 h before the lethal challenge. As demonstrated in Fig. 1, serum levels of SAP are significantly (P < 0.0001) increased, 12 h after injection of 0.3 μg of IL-1β, in SAP+/+ mice only. In order to study the role of SAP in IL-1β-induced desensitization, SAP+/+ and SAP0/0 mice were injected i.p. with PBS or different doses of IL-1β (3 to 300 ng per mouse), followed 12 h later by an i.p. injection with 0.5 μg of mTNF-α/20 mg of GalN. For this batch of mice, this dose appeared to be slightly less than LD100. As demonstrated in Table 2, SAP0/0 mice can be desensitized just as well as SAP control mice. Also, regarding protection against a TNF-α–GalN-induced drop in body temperature, IL-1β was as active in SAP0/0 mice as in SAP+/+ mice (data not shown). These data indicate that in the absence of SAP, IL-1β is also perfectly able to desensitize to TNF-α–GalN-induced lethal hepatitis.
FIG. 1.
Induction of SAP in the serum of IL-1β-desensitized or hTNF-α-tolerized mice. SAP was determined by ELISA and is expressed as a percentage of the control (PBS-treated mice = 100%). A total of 0.3 μg of IL-1β was injected i.p., and SAP was measured 12 h later. hTNF-α was injected twice per day for 5 days, and SAP was measured 3 days later. ∗, P = 0.001; ∗∗, P < 0.001 (n = 6 in all groups).
TABLE 2.
IL-1β desensitizes SAP+/+ as well as SAP0/0 mice to TNF-α–GalN-induced lethality
| Pretreatmenta | Lethalityb (no. of animals killed/total no.)
|
|
|---|---|---|
| SAP+/+ (P) | SAP0/0 (P) | |
| PBS | 10/12 | 7/11 |
| 3 ng of IL-1β | 6/7 (NS) | 4/6 (NS) |
| 30 ng of IL-1β | 2/7** (0.0085) | 3/5 (NS) |
| 300 ng of IL-1β | 0/6** (0.0004) | 0/6* (0.0054) |
Pretreatment was given i.p. 12 h before the challenge.
Challenge was 0.5 μg of mTNF-α plus 20 mg of GalN given i.p. (doses are per 20 g of body weight). Lethality was scored for up to 48 h (no further deaths occurred). NS, not significantly different from PBS-pretreated mice. ∗ and ∗∗, P < 0.01 and P < 0.001, respectively, versus PBS-pretreated mice.
SAP is not involved in hTNF-α-induced tolerization.
Repetitive injections of small doses of TNF-α for 1 week result in tolerance to a normally lethal dose of TNF-α for a period of about 10 days (37). Tolerization was induced by hTNF-α injection (6 μg per injection) twice per day (9 a.m. and 6 p.m.) for 5 consecutive days (Monday to Friday). The lethal challenge was given on day 8 (Monday). Just before the challenge, blood was withdrawn and the serum SAP levels were measured. As demonstrated in Fig. 1, SAP levels had significantly risen (P = 0.001) in SAP+/+ mice and not in SAP0/0 mice. Furthermore, SAP+/+ mice were indeed found to resist mTNF-α-induced lethality by tolerization. In Table 3 we demonstrate that hTNF-α is also able to induce tolerance in SAP0/0 mice. These data indicate that SAP is not a necessary serum factor for the induction of tolerance to TNF-α-induced SIRS.
TABLE 3.
hTNF-α-induced tolerization to mTNF-α-induced lethal shock in SAP+/+ and SAP0/0 mice
| Pretreatmenta | Lethalityb (no. of animals killed/total no.)
|
|
|---|---|---|
| SAP+/+ (P) | SAP0/0 (P) | |
| PBS | 6/6 | 6/6 |
| 6 μg of hTNF-α | 0/6** (0.0003) | 1/6* (0.0017) |
Pretreatment was given i.p. twice a day for 5 consecutive days.
Challenge was 25 μg of mTNF-α given i.v. at day 8 (doses are per 20 g of body weight). Lethality was scored for up to 48 h (no further deaths occurred). ∗ and ∗∗, P < 0.01 and P < 0.001, respectively, versus PBS-pretreated mice.
Effect of SAP deficiency on TNF-α-induced hemorrhagic skin necrosis.
Two s.c. injections (with a 24-h interval) of 1 μg of mTNF-α into the back of mice resulted in the appearance of a local hemorrhagic necrotic spot 24 h after the second injection. This is a variation of the Shwartzman reaction as previously described (33). We treated SAP+/+ as well as SAP0/0 mice and measured the size of necrosis. We found that both types of mice equally developed the typical erythema formation 24 h after the first injection, which developed further to a necrotic spot 24 h after the second challenge. The size of the spot was slightly (but not significantly, P = 0.1774) less in SAP0/0 mice than in controls (data not shown).
DISCUSSION
Phase I and phase II clinical trials, but also experiments using laboratory animals, have revealed that the major dose-limiting toxicities of a treatment with TNF-α are hypotension, liver damage, and bowel necrosis (5, 8, 19, 36, 41). TNF-α is indeed a powerful proinflammatory cytokine, and inhibition of its inflammation-inducing properties will most likely also lead to an increased therapeutic value of TNF-α in cancer treatment. In our laboratory, we are predominantly studying two different mouse models of TNF-α-induced lethality. In the first model, recombinant mTNF-α is administered i.v. This leads to SIRS associated with hypotension, hypothermia, massive adhesion to the endothelium of neutrophils, and bowel necrosis, eventually leading to lethal shock about 24 h after the challenge. In the second model, mice are treated with a combination of TNF-α and GalN, a liver-specific inhibitor of transcription (9). In this model, mice are extremely sensitized to TNF-α, and lethality appears to be the result of apoptosis and necrosis of the liver (22, 42). This model resembles viral hepatitis (11, 20).
We believe that, in mammals, several endogenous feedback mechanisms exist that are capable of reducing or preventing (TNF-α-induced) inflammation and lethality. Because of the extremely sensitizing effect of GalN (13, 21, 27) and of partial hepatectomy (14), we hypothesize that at least part of these feedback systems are located in the liver.
We are studying the molecular basis of two independent protective feedback systems: desensitization and tolerance. Desensitization is a short period of resistance to TNF-α or TNF-α–GalN by a single injection of either TNF-α itself or IL-1β (28, 43). We found that, very likely, IL-1β-induced desensitization is the result of induction of one or more proteins in the liver (28). In the liver, IL-1β is a strong inducer of the acute-phase reaction, both by inducing IL-6 and by inducing glucocorticoids (16, 26, 31, 40, 45). IL-6 is a cytokine that directly provokes an acute-phase reaction and that induces glucocorticoids (34). But the latter also induce an acute-phase response (1). Induction, by IL-1, of both IL-6 and glucocorticoids simultaneously leads to a synergistic effect on the induction of the acute-phase response (7). We previously determined that indeed two of the acute-phase proteins, viz., α1-acid glycoprotein and α1-antitrypsin, protect against both models of TNF-α-induced lethality (25, 29, 42). Tolerance is induced by repetitive treatment with TNF-α itself, given twice per day, for 5 days. After this treatment, mice are resistant to TNF-α- but not to TNF-α–GalN-induced lethality. An involvement of the liver has not been described (37, 38).
We describe here our efforts to further characterize the factors that mediate the induction of desensitization as well as tolerization. We have focused our attention on SAP because the latter is, together with serum amyloid A protein, the major acute-phase protein in the mouse. SAP belongs to the family of pentraxins, which have been conserved throughout vertebrate evolution. The homology to C-reactive protein, the classical acute-phase protein in humans, is about 51%. In the mouse, SAP is an acute-phase reactant, while it is constitutively present in humans, with a maximal twofold increase during sepsis (12). SAP shows calcium-dependent binding to DNA (35), chromatin (18), and glycosaminoglycans (17). SAP has also been described to play a role in the complement cascade (4, 6, 15, 44). SAP0/0 mice were found to be protected against induction of amyloidosis by injection of casein (3).
Using SAP0/0 mice, we found that the presence of SAP is irrelevant to the sensitivity of the animals to a lethal challenge of TNF-α–GalN or TNF-α. These data do not exclude a role of SAP in the induction of protection by desensitization or tolerization. In fact, we found that after injection of a desensitizing dose of IL-1β, as well as after tolerizing, at the moment the challenge is given, serum SAP levels are significantly increased (4- and 3.5-fold, respectively) in SAP+/+ mice only. However, like SAP+/+ mice, SAP0/0 mice could very well be desensitized and tolerized, despite the absence of SAP. These data illustrate that SAP, though a major acute-phase reactant in the mouse and a potential candidate as a mediator for desensitization and tolerance, is not involved in both protective mechanisms.
We were also interested in studying the effect of SAP deficiency in a model of TNF-α-induced skin hemorrhagic necrosis since it has been reported that several strains of mice (BALB/c, DBA/2, and A/J) had >10-fold-higher basal serum levels of SAP compared to C57BL/6 mice (32) and that these strains failed to develop typical necrosis (C. Libert, unpublished data). However, a further decrease in SAP levels to zero in C57BL/6 mice by gene targeting and subsequent backcross in a C57BL/6 background did not lead to increased sensitivity to this TNF-α effect as previously described.
In conclusion, we found no evidence for a protective role of SAP in several models of TNF-α-induced inflammation or lethal shock. Also, the induction of endogenous protection by the processes of desensitization and tolerization appears to occur in the absence of SAP.
ACKNOWLEDGMENTS
We thank M. Botto and M. B. Pepys for providing the SAP0/0 mice and J. Vanden Berghe for technical assistance.
W.V.M. is a research assistant and P.B. is a research associate with the Fonds voor Wetenschappelijk Onderzoek–Vlaanderen. T.H. is a fellow with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-technologisch Onderzoek in de Industrie. This research was supported by the Fonds voor Wetenschappelijk Onderzoek–Vlaanderen (grant G023698N) and the Interuniversitaire Attractiepolen.
REFERENCES
- 1.Baumann H, Prowse K R, Marinkovi S, Won K A, Jahreis G P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann NY Acad Sci. 1989;557:280–295. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- 2.Beyaert R, Fiers W. Tumor necrosis factor and lymphotoxin. In: Mire-Sluis A R, Thorpe R, editors. Cytokines. San Diego, Calif: Academic Press, Inc.; 1998. pp. 335–360. [Google Scholar]
- 3.Botto M, Hawkins P N, Bickerstaff M C M, Herbert J, Bygrave A E, McBride A, Hutchinson W L, Tennent G A, Walport M J, Pepys M B. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 4.Bristow C L, Boackle R J. Evidence for the binding of human serum amyloid P component to C1q and Fab gamma. Mol Immunol. 1986;10:1045–1052. doi: 10.1016/0161-5890(86)90003-9. [DOI] [PubMed] [Google Scholar]
- 5.Brouckaert P, Fiers W. Tumor necrosis factor and the systemic inflammatory response syndrome. Curr Top Microbiol Immunol. 1996;216:167–187. doi: 10.1007/978-3-642-80186-0_8. [DOI] [PubMed] [Google Scholar]
- 6.Brown M R, Anderson B E. Receptor-ligand interactions between serum amyloid P component and model soluble immune complexes. J Immunol. 1993;151:2087–2095. [PubMed] [Google Scholar]
- 7.Castell J V, Gómez-Lechón M J, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich P C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 8.Creaven P J, Brenner D E, Cowens J W, Huben R P, Wolf R M, Takita H, Arbuck S G, Razak M S, Proefrock A D. A phase I clinical trial of recombinant human tumor necrosis factor given daily for five days. Cancer Chemother Pharmacol. 1989;23:186–191. doi: 10.1007/BF00267953. [DOI] [PubMed] [Google Scholar]
- 9.Decker K, Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont A M. TNF-α in isolated perfusion systems: success in the limb, developments for the liver credits, debits and future perspectives. Anticancer Res. 1998;18:3899–3905. [PubMed] [Google Scholar]
- 11.El-Mofty S K, Scrutton M C, Serroni A, Nicolini C, Farber J L. Early, reversible plasma membrane injury in galactosamine-induced liver cell death. Am J Pathol. 1975;3:579–595. [PMC free article] [PubMed] [Google Scholar]
- 12.Emsley J, White H E, O'Hara B P, Oliva G, Srinivasan N, Tickle I J, Blundell T L, Pepys M B, Wood S P. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberg M A, Galanos C. Tumor necrosis factor α mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukushima H, Ikeuchi J, Tohkin M, Matsubara T, Harada M. Lethal shock in partially hepatectomized rats administered tumor necrosis serum. Circ Shock. 1988;26:1–14. [PubMed] [Google Scholar]
- 15.García de Frutos P, Härdig T, Dahlbäck B. Serum amyloid P component binding to C4b-binding protein. J Biol Chem. 1995;270:26950–26955. doi: 10.1074/jbc.270.45.26950. [DOI] [PubMed] [Google Scholar]
- 16.Gelin J L, Moldawer L L, Iresjo B M, Lundholm K G. The role of the adrenals in the acute phase response to interleukin-1 and tumor necrosis factor-α. J Surg Res. 1993;54:70–78. doi: 10.1006/jsre.1993.1012. [DOI] [PubMed] [Google Scholar]
- 17.Heegaard N H, Mortensen H D, Roepstorff P. Demonstration of a heparin-binding site in serum amyloid P component using affinity capillary electrophoresis as an adjunct technique. J Chromatogr A. 1995;717:83–90. doi: 10.1016/0021-9673(95)00644-3. [DOI] [PubMed] [Google Scholar]
- 18.Hicks P S, Saunero-Nava L, Du Clos T W, Mold C. Serum amyloid P component binds to histones and activates the classical complement pathway. J Immunol. 1992;149:3689–3694. [PubMed] [Google Scholar]
- 19.Jones A L, Selby P. Tumour necrosis factor: clinical relevance. Cancer Surveys. 1989;8:817–836. [PubMed] [Google Scholar]
- 20.Keppler D, Lesch R, Reutter W, Decker K. Experimental hepatitis induced by d-galactosamine. Exp Mol Pathol. 1968;2:279–290. doi: 10.1016/0014-4800(68)90042-7. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leist M, Gantner F, Bohlinger I, Germann P G, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 23.Lejeune F, Liénard D, Eggermont A, Schraffordt Koops H, Kroon B, Gérain J, Rosenkaimer F, Schmitz P. Clinical experience with high-dose tumor necrosis factor alpha in regional therapy of advanced melanoma. Circ Shock. 1994;43:191–197. [PubMed] [Google Scholar]
- 24.Lejeune F J, Ruegg C, Liénard D. Clinical applications of TNF-α in cancer. Curr Opin Immunol. 1998;10:573–580. doi: 10.1016/s0952-7915(98)80226-4. [DOI] [PubMed] [Google Scholar]
- 25.Libert C, Brouckaert P, Fiers W. Protection by α1-acid glycoprotein against tumor necrosis factor-induced lethality. J Exp Med. 1994;180:1571–1575. doi: 10.1084/jem.180.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libert C, Takahashi N, Cauwels A, Brouckaert P, Bluethmann H, Fiers W. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality. Eur J Immunol. 1994;24:2237–2242. doi: 10.1002/eji.1830240945. [DOI] [PubMed] [Google Scholar]
- 27.Libert C, Van Bladel S, Brouckaert P, Fiers W. The influence of modulating substances on tumor necrosis factor and interleukin-6 levels after injection of murine tumor necrosis factor or lipopolysaccharide in mice. J Immunother. 1991;10:227–235. doi: 10.1097/00002371-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Libert C, Van Bladel S, Brouckaert P, Shaw A, Fiers W. Involvement of the liver, but not of IL-6, in IL-1-induced desensitization to the lethal effects of tumor necrosis factor. J Immunol. 1991;146:2625–2632. [PubMed] [Google Scholar]
- 29.Libert C, Van Molle W, Brouckaert P, Fiers W. α1-Antitrypsin inhibits the lethal response to TNF in mice. J Immunol. 1996;157:5126–5129. [PubMed] [Google Scholar]
- 30.Liénard D, Ewalenko P, Delmotte J-J, Renard N, Lejeune F. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 31.Lin B, Ku N, Zahedi K, Whitehead A S, Mortensen R F. IL-1 and IL-6 mediate increased production and synthesis by hepatocytes of acute-phase reactant mouse serum amyloid P-component (SAP) Inflammation. 1990;14:297–313. doi: 10.1007/BF00915814. [DOI] [PubMed] [Google Scholar]
- 32.Pepys M B, Baltz M, Gomer K, Davies A J S, Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278:259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 33.Rothstein J L, Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci USA. 1988;85:607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schöbitz B, Pezeshki G, Pohl T, Hemmann U, Heinrich P C, Holsboer F, Reul J M. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in vivo. FASEB J. 1995;9:659–664. doi: 10.1096/fasebj.9.8.7768358. [DOI] [PubMed] [Google Scholar]
- 35.Serban D, Rordorf-Adam C. Binding characteristics of human serum amyloid P component. Scand J Immunol. 1987;25:275–281. doi: 10.1111/j.1365-3083.1987.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 36.Spriggs D R, Yates S W. Clinical studies of tumor necrosis factor in the USA. In: Osawa T, Bonavida B, editors. Tumor necrosis factor: structure-function relationship and clinical application. Basel, Switzerland: Karger; 1992. pp. 275–284. [Google Scholar]
- 37.Takahashi N, Brouckaert P, Fiers W. Induction of tolerance allows separation of lethal and antitumor activities of tumor necrosis factor in mice. Cancer Res. 1991;51:2366–2372. [PubMed] [Google Scholar]
- 38.Takahashi N, Fiers W, Brouckaert P. Anti-tumor activity of tumor necrosis factor in combination with interferon-γ is not affected by prior tolerization. Int J Cancer. 1995;63:846–854. doi: 10.1002/ijc.2910630616. [DOI] [PubMed] [Google Scholar]
- 39.Taktak Y S, Stenning B. Solid-phase enzyme immunoassays for the quantification of serum amyloid P (SAP) and complement component 3 (C3) proteins in acute-phase mouse sera. Horm Metab Res. 1992;24:371–374. doi: 10.1055/s-2007-1003338. [DOI] [PubMed] [Google Scholar]
- 40.Taylor A W, Ku N O, Mortensen R F. Regulation of cytokine-induced human C-reactive protein production by transforming growth factor-β. J Immunol. 1990;145:2507–2513. [PubMed] [Google Scholar]
- 41.Tracey K J, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–344. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 42.Van Molle W, Libert C, Fiers W, Brouckaert P. α1-Acid glycoprotein and α1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J Immunol. 1997;159:3555–3564. [PubMed] [Google Scholar]
- 43.Wallach D, Holtmann H, Engelmann H, Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988;140:2994–2999. [PubMed] [Google Scholar]
- 44.Ying S C, Gewurz A T, Jiang H, Gewurz H. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14-26 and 76-92 of the A chain collagen-like region of C1q. J Immunol. 1993;150:169–176. [PubMed] [Google Scholar]
- 45.Zahedi K, Whitehead A S. Regulation of mouse serum amyloid P gene expression by cytokines in vitro. Biochim Biophys Acta. 1993;1176:162–168. doi: 10.1016/0167-4889(93)90192-r. [DOI] [PubMed] [Google Scholar]