Highlights
-
•
Extended criteria donor organs in heart-lung transplant do not worsen mortality.
-
•
Advanced donor age and P/F ratio remain predictive of mortality.
-
•
Extended criteria donor organ use is safe in appropriately chosen patients.
Keywords: Organ donor management, Heart-lung transplantation, Heart transplantation, Lung transplantation
Abstract
Background
Demand for donor hearts and lungs exceeds their supply. Extended Criteria Donor (ECD) organs are used to help meet this demand, but their impact on heart-lung transplantation outcomes is poorly characterized.
Methods and results
: The United Network for Organ Sharing was queried for data on adult heart-lung transplantation recipients (n = 447) from 2005‒2021. Recipients were stratified based on whether they received ECD hearts and/or lungs. Morbidity was analyzed using Kruskal-Wallis, chi-square, and Fisher's exact tests. Mortality was analyzed using Kaplan-Meier estimation, log-rank tests and Cox regression. Sixty-five (14.5%) patients received two ECD organs, 134 (30.0%) received only an ECD lung, and 65 (14.5%) only an ECD heart. Recipients of two ECD organs were older, more likely to have diabetes, and more likely transplanted from 2015‒2021 (p < 0.05). Groups did not differ by pre-transplant diagnosis, intensive care unit disposition, life support use, or hemodynamics. Group five-year survival rates ranged from 54.5% to 63.2% (p = 0.428). Groups did not differ by 30-day mortality, strokes, graft rejection, or hospital length of stay.
Conclusions
: Using ECD hearts and/or lungs for heart-lung transplantation is not associated with increased mortality and is a safe strategy for increasing donor organ supply in this complex patient population.
Introduction
Demand for donor hearts and lungs continues to exceed their supply. One strategy to increase supply is the use of Extended Criteria Donor (ECD) organs. While there is no consensus on what the extended criteria for hearts or lungs are, previously published lists have considerable overlap and tend to include increased donor age, ischemic time, and other risk factors for end-organ dysfunction [1,2]. Several transplant centers have found comparable outcomes between recipients of carefully selected ECD hearts and recipients of Standard Criteria Donor (SCD) hearts [1]. The extended heart criteria found to minimally impact morbidity and mortality include age over 50 [1], norepinephrine requirement [3], hepatitis C positivity [1,4], left ventricular ejection fraction < 50% [5], and donor/recipient weight ratio less than 0.80 [1]. Extended lung criteria found to minimally impact these outcomes include age over 50 [6], tobacco use history [7], and hepatitis C positivity [8].
Simultaneous Heart-Lung Transplantation (HLTx) remains the preferred treatment for concomitant end-stage cardiac and pulmonary dysfunction. Yet, finding organs for these very ill patients can be challenging. While the benefit of using carefully selected ECD organs for single-organ heart and lung transplantations has been shown, it is unclear what effect ECD organs have on HLTx outcomes. Furthermore, the complexities of this patient subset, including the presence of Eisenmenger syndrome with an unrepairable cardiac defect, differ from those of single-organ heart or lung transplantation [9]. It remains to be determined whether transplant recipients with these unique pathologies tolerate ECD organs as well as recipients of single-organ thoracic transplantations. Further, no study has assessed which ECD criteria, if any, contribute to mortality in the setting of HLTx. Lastly, it is unknown how often ECD organs are used in the setting of HLTx and whether their use has increased in response to accumulating data on the tolerability of ECD organs in single-organ thoracic transplants.
The authors undertook this study to 1) Assess the effect of ECD organs on morbidity and mortality in the setting of HLTx and 2) Assess the prevalence of ECD heart and lung use in HLTx over time.
Materials and methods
The United Network for Organ Sharing (UNOS) thoracic database was queried for all patients aged 18 or older who received HLTx from January 1, 2005, through July 19, 2021. 2005 was selected to exclude transplantations prior to the implementation of lung allocation scores. Patients with unknown mortality status at the last follow-up were excluded. Each HLTx recipient was assigned to one of four groups based on donor organ status. The four groups were recipients of either two Standard Criteria Donor (SCD) organs, two Extended Criteria Donor (ECD) organs, an ECD heart and SCD lung, or an ECD lung and SCD heart. Donor hearts and lungs were determined to be ECD organs if they satisfied two ECD criteria (Table 1). ECD criteria were adapted from the literature describing extended criteria in hearts [1,3, 4, 5,10, 11, 12, 13, 14, 15, 16, 17] and lungs [2,6, 7, 8,17].
Table 1.
List of extended donor criteria for hearts and lungs.
| Extended heart criteria | Extended lung criteria |
|---|---|
| Age ≥ 55 [1,14,16,17] | Age ≥ 55 [6,9,17] |
| Cold ischemic time > 360 min [1,13,14] | Infiltrates on chest radiograph [2,9,17] |
| Donor/recipient weight ratio 〈0.80 or〉 ≥ 1.30 [1,13,17,20] | P/F ratio < 300 [2,9,17] |
| Hepatitis C positivity [1,4,8,16] | Purulent secretions on bronchoscopy [2,9,17] |
| Drug use history [16] | Cigarette use history (> 20 pack-years) [2,7,9,17] |
| Renal insufficiency (serum creatinine > 2.0 mg/dL) [11] | |
| Left ventricular ejection fraction < 50% [5,13,17] | |
| Donation after circulatory death [12,13] |
P/F ratio, Ratio of arterial oxygen partial pressure to a fraction of inspired oxygen.
Baseline characteristics, morbidity, and mortality data are reported for all donor recipients and compared by donor organ status. Predicted total lung capacity was calculated from height using previously described methods [18]. Categorical variables are expressed as frequency (%) and continuous variables are presented as median [interquartile range]. Comparisons between donor organ status groups were performed using chi-square for categorical variables with group sample sizes greater than 5, Fisher's exact test for categorical variables with sample sizes less than or equal to 5, and Kruskal-Wallis for continuous variables that are non-parametrically distributed. Parametricity was assessed for each continuous variable using the Shapiro-Wilk test. Mortality was censored at five years and analyzed using Kaplan-Meier estimation. Mortality comparisons between donor organ status groups were performed using a log-rank test. A Cox proportional hazards regression model was used to determine predictors of five-year mortality. Variables used in the Cox regression model included each extended donor criterion, as well as each recipient and donor characteristic that differed significantly between donor organ groups on univariate analysis. All statistical analysis was performed using STATA/MP 17.0 software (StataCorp LLC, College Station, TX).
The University of Pennsylvania Institutional Review Board deemed the study to be not human subjects research and waived the need for patients’ written informed consent (protocol #: 850952, approval date: March 10, 2022). The study was completed in conformity with STROBE Statement guidelines.
Results
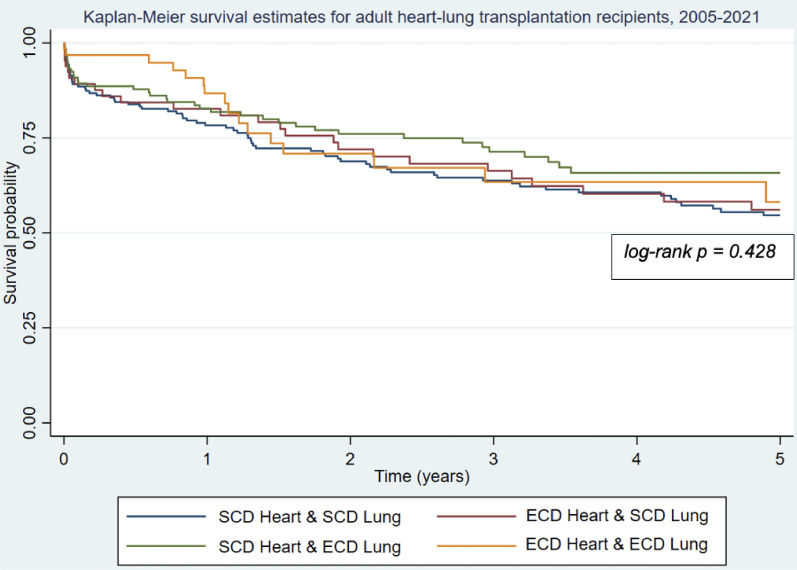
447 adults undergoing simultaneous HLTx from 2005 to 2021 were analyzed (Fig. 1). 232 (51.9%) were female. The median recipient age was 45. The most common recipient diagnosis was primary pulmonary hypertension (n = 111, 24.9%). The largest cohort of recipients (n = 178, 39.8%) received two SCD organs. 134 (30.0%) received only an ECD lung, 70 (15.7%) received only an ECD heart, and 65 (14.5%) received both an ECD lung and an ECD heart.
Fig. 1.
Kaplan-Meier survival estimates for adult heart-lung transplantation recipients from 2005‒2021, stratified by donor organ status. ECD, Extended Criteria Donor; SCD, Standard Criteria Donor.
The four donor organ status groups differed by recipient characteristics including age, diabetes, cytomegalovirus positivity, and year of transplant, as well as donor characteristics including sex, age, body mass index, hepatitis C status, drug use, cigarette use history (> 20 pack-years), inotrope need, serum creatinine, infiltrates on chest radiographs, purulent secretions on bronchoscopy, a ratio of arterial oxygen partial pressure to the fraction of inspired oxygen (P/F ratio), and circulatory death (p < 0.05) (Table 2, Table 3, Table 4). Recipients of two ECD organs were older, more likely to have diabetes, and more likely transplanted in the recent era (2015‒2021). Recipients of ECD hearts but SCD lungs were more likely than other groups to be transplanted from 2005‒2009. Organ donor status groups did not differ by pre-operative recipient diagnosis, intensive care unit disposition, hemodynamics, waiting list time, ischemic time, or need for life support devices including dialysis, mechanical ventilation, Extracorporeal Membrane Oxygenation (ECMO), ventricular assist devices, and intra-aortic balloon bumps.
Table 2.
Pre-transplant demographics of adult heart-lung transplantation recipients from 2005‒2021, stratified by donor organ status.
| All patients (n = 447) | SCD heart and lung recipients (n = 178) | ECD heart only recipients (n = 70) | ECD lung only recipients (n = 134) | ECD heart and lung recipients (n = 65) | p-value | |
|---|---|---|---|---|---|---|
| Age, years | 45 [34‒54] | 43 [33‒53] | 41 [33‒49] | 47 [35‒56] | 50 [39‒54] | 0.019 |
| Female | 232 (51.9%) | 102 (57.3%) | 33 (47.1%) | 68 (50.8%) | 29 (44.6%) | 0.244 |
| Ethnicity | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| White | 263 (58.8%) | 102 (57.3%) | 43 (61.4%) | 83 (61.9%) | 35 (53.9%) | 0.667 |
| Black | 88 (19.7%) | 34 (19.1%) | 15 (21.4%) | 24 (17.9%) | 15 (23.1) | 0.822 |
| Hispanic | 64 (14.3%) | 27 (15.2%) | 5 (7.1%) | 18 (13.4%) | 14 (21.5%) | 0.117 |
| Body mass index, kg/m2 | 23.5 [20.8‒27.2] | 23.1 [20.1‒26.4] | 22.0 [19.5‒25.8] | 23.3 [20.9‒27.5] | 24.2 [20.9‒28.1] | 0.120 |
| Diagnosis | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Primary pulmonary hypertension | 111 (24.9%) | 47 (26.4%) | 20 (29.4%) | 30 (22.4%) | 14 (21.5%) | 0.615 |
| Interstitial lung disease | 86 (19.3%) | 33 (18.5%) | 10 (14.7%) | 29 (21.6%) | 14 (21.5%) | 0.598 |
| Cardiomyopathy | 58 (13.3%) | 25 (14.0%) | 7 (10.3%) | 15 (11.2%) | 11 (16.9%) | 0.572 |
| Eisenmenger's syndrome | 45 (10.1%) | 24 (13.5%) | 7 (10.3%) | 9 (6.7%) | 5 (7.7%) | 0.245 |
| Diabetes | 65 (14.6%) | 27 (15.2%) | 3 (4.4%) | 21 (15.8%) | 14 (21.5%) | 0.040 |
| Dialysis since listing | 22 (5.0%) | 9 (5.1%) | 3 (4.7%) | 6 (4.5%) | 4 (6.2%) | 0.966 |
| Mechanical ventilation since listing | 95 (21.3%) | 35 (19.7%) | 18 (25.7%) | 29 (21.6%) | 13 (20.0%) | 0.760 |
| ECMO since listing | 76 (17.0%) | 24 (13.5%) | 13 (18.6%) | 24 (17.9%) | 15 (23.1%) | 0.327 |
| IABP since listing | 19 (4.3%) | 8 (4.5%) | 3 (4.3%) | 3 (2.2%) | 5 (7.6%) | 0.356 |
| VAD since listing | 17 (3.8%) | 5 (1.7%) | 4 (5.9%) | 8 (6.0%) | 2 (3.1%) | 0.190 |
| ICU disposition at time of transplant | 180 (40.5%) | 67 (37.6%) | 28 (41.8%) | 53 (39.6%) | 32 (49.2%) | 0.432 |
| Waiting list time, days | 75 [22‒209] | 96.5 [26‒260] | 66 [21‒165] | 87.5 [16‒186] | 68 [20‒182] | 0.363 |
| CMV positive | 269 (63.2%) | 119 (69.6%) | 32 (50.8%) | 83 (64.3%) | 35 (55.6%) | 0.032 |
| Previous heart surgery | 139 (31.2%) | 46 (25.9%) | 23 (33.8%) | 49 (36.6%) | 21 (32.3%) | 0.220 |
| Previous lung surgery | 14 (3.2%) | 3 (1.7%) | 3 (4.5%) | 5 (3.7%) | 3 (4.6%) | 0.524 |
| Cigarette use history | 133 (29.9%) | 53 (29.8%) | 14 (20.6%) | 45 (33.6%) | 21 (32.3%) | 0.278 |
| Serum creatinine > 2.0 mg/dL | 26 (6.0%) | 7 (3.9%) | 9 (12.9%) | 7 (5.2%) | 4 (6.2%) | 0.063 |
| Total bilirubin > 2.0 mg/dL | 67 (15.0%) | 22 (12.4%) | 14 (20.0%) | 22 (16.4%) | 9 (13.9%) | 0.454 |
| Cardiac output, L/min | 4.0 [3.1‒5.0] | 3.8 [3.0‒4.9] | 3.8 [3.0‒4.8] | 4.1 [3.4‒5.1] | 4.4 [3.2‒5.3] | 0.230 |
| Mean PA pressure, mmHg | 48 [34‒63] | 50 [34‒63] | 52 [38‒70] | 44.5 [34‒65] | 45 [27‒60] | 0.141 |
| Systolic PA pressure, mmHg | 74.5 [52‒96] | 79.5 [55‒96] | 77 [60‒98] | 68 [50‒99] | 69 [45‒89] | 0.239 |
| Functional deficits | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Severe | 238 (54.3%) | 94 (54.0%) | 33 (50.0%) | 77 (57.9%) | 34 (52.3%) | 0.564 |
| Moderate | 145 (33.1%) | 50 (28.7%) | 25 (37.9%) | 45 (33.8%) | 25 (38.5%) | 0.386 |
| Mild to none | 55 (12.6%) | 30 (17.2%) | 8 (12.1%) | 11 (8.3%) | 6 (9.2%) | 0.206 |
| Private payor | 255 (57.1%) | 105 (59.0%) | 41 (58.6%) | 74 (55.2%) | 35 (53.9%) | 0.851 |
| Transplant era | ||||||
| 2005‒2009 | 128 (28.6%) | 66 (37.1%) | 29 (41.4%) | 26 (19.4%) | 7 (10.8%) | <0.001 |
| 2010‒2014 | 122 (27.3%) | 53 (29.8%) | 14 (20.0%) | 41 (30.6%) | 14 (21.5%) | 0.236 |
| 2015‒2021 | 197 (44.1%) | 59 (33.2%) | 27 (38.6%) | 67 (50.0%) | 44 (67.7%) | <0.001 |
All values excluding p-values are medians with IQR in brackets or frequencies with prevalence in parentheses. Each p-value is derived from Chi-Squared, Fisher's exact, or Kruskal-Wallis tests.
CMV, Cytomegalovirus; ECD, Extended criteria donor; ECMO, Extracorporeal membrane oxygenation; IABP, Intra-Aortic Balloon Pump; ICU, Intensive Care Unit; LV EF, Left Ventricular Ejection Fraction; PA, Pulmonary Artery; SCD; Standard Criteria Donor; VAD, Ventricular Assist Device.
Table 3.
Pre-transplant donor demographics of adult heart-lung transplantation recipients from 2005‒2021, stratified by donor organ status.
| All patients | SCD heart and lung recipients | ECD heart only recipients | ECD lung only recipients | ECD heart and lung recipients | p-value | |
|---|---|---|---|---|---|---|
| Female | 228 (51.0%) | 107 (60.1%) | 26 (37.1%) | 69 (51.5%) | 26 (40.0%) | 0.002 |
| Age, years | 31 [21‒43] | 29 [20‒43] | 24 [19‒33] | 35.5 [23‒44] | 31 [23‒42] | 0.002 |
| Body mass index, m/kg2 | 24.4 [21.7‒27.6] | 23.6 [21.4‒26.8] | 24.2 [20.9‒28.5] | 24.7 [22.0‒27.5] | 25.6 [23.3‒28.3] | 0.015 |
| Ethnicity | ‒ | ‒ | ‒ | ‒ | ‒ | |
| White | 252 (56.4%) | 100 (56.2%) | 43 (61.4%) | 70 (52.2%) | 39 (60.0%) | 0.571 |
| Black | 68 (15.2%) | 23 (12.9%) | 15 (21.4%) | 22 (16.4%) | 8 (12.3%) | 0.334 |
| Hispanic | 98 (21.9%) | 47 (26.4%) | 10 (14.3%) | 28 (20.9%) | 13 (20.0%) | 0.195 |
| Hypertension | 73 (16.5%) | 30 (17.1%) | 9 (12.9%) | 22 (16.7%) | 12 (18.5%) | 0.827 |
| Diabetes | 20 (4.5%) | 10 (5.7%) | 1 (1.4%) | 9 (6.8%) | 0 (0.0%) | 0.080 |
| Heavy alcohol use | 47 (10.7%) | 18 (10.2%) | 10 (14.3%) | 9 (7.0%) | 10 (15.6%) | 0.215 |
| On inotropes at procurement | 217 (48.8%) | 102 (57.3%) | 30 (43.5%) | 59 (44.0%) | 26 (40.6%) | 0.031 |
| LV EF | 60% [55‒65%] | 61% [56‒65%] | 60% [55‒65%] | 60.5% [55‒65%] | 60% [55‒65%] | 0.570 |
| P/F ratio | 450 [369‒509] | 461 [390‒517] | 471 [393‒520] | 437 [344‒488] | 423 [334‒497] | 0.001 |
| Cold ischemic time, hours | 3.8 [3.1‒4.4] | 3.8 [3.0‒4.4] | 3.4 [2.9‒4.3] | 3.9 [3.2‒4.4] | 3.8 [3.1‒4.2] | 0.539 |
| Donor/recipient weight ratio | 1.1 [0.9‒1.3] | 1.1 [0.9‒1.2] | 1.2 [0.8‒1.5] | 1.0 [0.9‒1.2] | 1.0 [0.8‒1.4] | 0.198 |
| Donor/recipient predicted total lung capacity ratio | 1.0 [0.9‒1.1] | 1.0 [0.9‒1.1] | 1.0 [0.9‒1.2] | 1.0 [0.9‒1.1] | 1.0 [0.9‒1.1] | 0.230 |
| HLA mismatches | 5 [4‒5] | 5 [4‒5] | 5 [4‒6] | 5 [4‒5] | 5 [4‒5] | 0.947 |
All values excluding p-values are medians with IQR in brackets or frequencies with prevalence in parentheses. Each p-value is derived from Chi-Squared, Fisher's exact, or Kruskal-Wallis tests.
ECD, Extended Criteria Donor; HLA, Human Leukocyte Antigen; LV EF, Left Ventricular Ejection Fraction; P/F ratio, Ratio of arterial oxygen partial pressure to fraction of inspired oxygen; SCD, Standard Criteria Donor.
Table 4.
Pre-transplant extended donor criteria of adult heart-lung transplantations from 2005‒2021, stratified by donor organ status.
| All patients | SCD heart and lung recipients | ECD heart only recipients | ECD lung only recipients | ECD heart and lung recipients | p-value | |
|---|---|---|---|---|---|---|
| Age ≥ 55 years | 19 (4.3%) | 2 (1.1%) | 2 (2.9%) | 5 (3.7%) | 10 (15.4%) | <0.001 |
| Cold ischemic time > 6-hours | 30 (6.7%) | 9 (5.1%) | 15 (21.4%) | 3 (2.2%) | 3 (4.6%) | <0.001 |
| Donor/recipient weight ratio 〈0.80 or〉≥ 1.30 | 156 (34.9%) | 36 (20.2%) | 50 (71.4%) | 30 (22.4%) | 40 (61.5%) | <0.001 |
| Hepatitis C positivity | 9 (2.0%) | 1 (0.6%) | 1 (1.4%) | 0 (0.0%) | 7 (10.8%) | <0.001 |
| Drug use history | 199 (45.0%) | 51 (29.0%) | 50 (72.5%) | 48 (36.4%) | 50 (76.9%) | <0.001 |
| Serum creatinine > 2.0 mg/dL | 55 (12.3%) | 6 (3.4%) | 21 (30.0%) | 6 (4.5%) | 22 (33.9%) | <0.001 |
| LV EF < 50% | 6 (1.3%) | 0 (0.0%) | 2 (2.9%) | 1 (0.8%) | 3 (4.6%) | 0.026 |
| Donation after circulatory death | 27 (6.1%) | 5 (2.8%) | 11 (15.9%) | 0 (0.0%) | 11 (16.9%) | <0.001 |
| Infiltrate on chest radiograph | 220 (49.8%) | 39 (22.4%) | 13 (18.8%) | 113 (84.3%) | 55 (84.6%) | <0.001 |
| P/F ratio < 300 | 36 (8.1%) | 3 (1.7%) | 2 (2.9%) | 20 (14.9%) | 11 (16.9%) | <0.001 |
| Purulent secretions on bronchoscopy | 72 (16.4%) | 9 (5.1%) | 3 (4.5%) | 43 (32.1%) | 17 (27.0%) | <0.001 |
| Cigarette use history (> 20 pack-years) | 45 (10.3%) | 6 (3.4%) | 9 (13.0%) | 21 (16.3%) | 9 (14.1%) | 0.001 |
All values excluding p-values are medians with IQR in brackets or frequencies with prevalence in parentheses. Each p-value is derived from chi squared, Fisher's exact, or Kruskal-Wallis tests.
CMV, Cytomegalovirus; ECD, Extended Criteria Donor; LV EF, Left Ventricular Ejection Fraction; P/F ratio, Ratio of arterial oxygen partial pressure to Fraction of inspired oxygen; SCD, Standard Criteria Donor.
For all adults undergoing HLTx from 2005‒2015, one-year survival was 81.6% and five-year survival was 58.5% (Table 5). 120 (27.2%) recipients were diagnosed with a rejection of at least one organ within one year of transplant. 105 patients (25.3%) had a new dialysis requirement post-HLTx and 24 (5.5%) suffered strokes. At 72 h post-HLTx, 103 patients (53.9%) were intubated and 27 (14.1%) were on ECMO. The median hospital length of stay was 28 days (interquartile range 16‒54).
Table 5.
Morbidity and mortality of adult heart-lung transplantation recipients from 2005‒2021, stratified by donor organ status.
| Mortality | All patients | SCD heart and lung recipients | ECD heart only recipients | ECD lung only recipients | ECD heart and lung recipients | p-value |
|---|---|---|---|---|---|---|
| 30-day survival function | 90.8% [87.7‒93.2] | 89.1% [83.5‒92.9] | 89.2% [78.7‒94.7] | 90.9% [84.5‒94.7] | 96.8% [87.9‒99.2] | 0.339 |
| One-year survival function | 81.6% [77.5‒85.0] | 78.3% [71.4‒83.8] | 82.7% [70.8‒90.0] | 82.8% [75.0‒88.3] | 86.7% [74.1‒93.5] | 0.394 |
| Five-year survival function | 58.5% [52.9‒63.7] | 54.6% [46.1‒62.4] | 56.1% [41.9‒68.0] | 65.8% [55.5‒74.3] | 58.1% [38.9‒73.2] | 0.428 |
| Morbidity | ||||||
| Rejection within one year | 120 (27.2%) | 51 (28.7%) | 20 (29.9%) | 32 (24.1%) | 17 (26.6%) | 0.777 |
| New dialysis requirement | 105 (25.3%) | 44 (26.2%) | 9 (15.0%) | 34 (26.8%) | 18 (30.0%) | 0.233 |
| Stroke | 24 (5.5%) | 8 (4.6%) | 8 (12.3%) | 5 (3.8%) | 3 (4.7%) | 0.113 |
| PPM placement | 8 (1.8%) | 0 (0.0%) | 2 (3.1%) | 3 (2.3%) | 3 (4.7%) | 0.019 |
| Airway dehiscence | 6 (1.4%) | 4 (2.3%) | 1 (1.6%) | 0 (0.0%) | 1 (1.5%) | 0.281 |
| Intubated at 72 h | 103 (53.9%) | 29 (49.2%) | 14 (66.7%) | 37 (55.2%) | 23 (52.3%) | 0.571 |
| On ECMO at 72 h | 27 (14.1%) | 8 (13.6%) | 3 (14.3%) | 8 (11.9%) | 8 (18.2%) | 0.804 |
| Reintubated | 119 (27.3%) | 49 (28.0%) | 18 (28.6%) | 32 (24.1%) | 20 (30.1%) | 0.755 |
| Length of stay, days | 28 [16‒54] | 25 [16‒51] | 33 [14‒63] | 28 [18‒52] | 36.5 [17‒63.5] | 0.562 |
All values excluding p-values are mortality estimates with 95% confidence intervals in brackets or frequencies with incidence in parentheses, except length of stay which is a median with interquartile range in brackets. Each p-value is derived from Chi-Squared, Fisher's exact, Kruskal-Wallis tests, or log-rank tests.
ECMO, Extracorporeal Membrane Oxygenation; PPM, Permanent Pacemaker.
The four donor organ status groups did not differ in terms of 30-day survival (ranging from 89.1% to 96.8%, p = 0.339), one-year survival (which ranged from 78.3% to 86.7%, p = 0.394), or five-year survival (which ranged between groups from 54.6% to 65.8%, p = 0.428) (Fig. 1). When limiting the analysis to patients who were transplanted from 2011‒2021, the donor organ status groups still did not differ in survival at 30-days (which ranged from 88.5% to 96.1%, p = 0.529), 1-year (which ranged from 75.3% to 85.4%, p = 0.260), or 5-years (which ranged from 59.1% to 71.1%, p = 0.509). These groups also did not differ in terms of early postoperative outcomes including rates of intubation (ranging from 49.2% to 52.3%, p = 0.571) and ECMO at 72 h (11.9% to 18.2%, p = 0.804), rates of reintubation (24.1% to 30.1%, p = 0.755), and median hospital length of stay (25 to 36.5 days, p = 0.562). Additionally, the donor organ status groups had similar incidences of complications including rejection within one year of transplant (ranging from 24.1% to 29.9%, p = 0.777), new dialysis requirement (ranging from 15.0% to 30.0%, p = 0.233), and postoperative stroke (ranging from 3.8% to 12.3%, p = 0.113). The groups did however differ in terms of postoperative permanent pacemaker placement with recipients of two ECD organs having the highest rate (4.7%) and recipients of two SCD organs having the lowest (0.0%, p = 0.019).
A Cox regression found that donor organ status was not a significant contributor to five-year mortality. It did however identify two predictors of mortality among the extended donor criteria: age ≥ 55 years (HR = 5.52 [2.13‒14.28], p < 0.001) and P/F ratio < 300 (HR = 2.38 [1.20‒4.71], p = 0.013) (Table 6). No other extended criteria were predictive of five-year mortality on Cox regression.
Table 6.
Cox regression for five-year mortality of adult heart-lung transplantation recipients from 2005‒2021.
| Hazard ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Donor group | |||
| ECD heart only | 0.87 | 0.43‒1.79 | 0.715 |
| ECD lung only | 1.08 | 0.58‒2.01 | 0.801 |
| ECD heart and lung | 0.40 | 0.14‒1.17 | 0.093 |
| Recipient characteristics | |||
| Age | 1.01 | 0.99‒1.02 | 0.350 |
| Diabetes | 1.28 | 0.78‒2.07 | 0.326 |
| CMV positive | 0.70 | 0.48‒1.02 | 0.060 |
| Donor characteristics | |||
| Male sex | 0.67 | 0.47‒0.97 | 0.034 |
| Body mass index, m/kg2 | 1.01 | 0.98‒1.05 | 0.459 |
| On inotropes at procurement | 1.05 | 0.73‒1.50 | 0.809 |
| Transplant year 2005‒2009 | 1.42 | 0.91‒2.21 | 0.119 |
| Transplant year 2015‒2021 | 0.91 | 0.58‒1.43 | 0.690 |
| Extended donor criteria | |||
| Age ≥ 55 years | 5.52 | 2.13‒14.28 | <0.001 |
| Ischemic time > 360 min | 0.54 | 0.23‒1.30 | 0.171 |
| Donor/recipient weight ratio 〈 0.80 or 〉 1.30 | 1.13 | 0.69‒1.83 | 0.629 |
| Hepatitis C positive | 1.78 | 0.20‒15.79 | 0.604 |
| Drug use history | 1.10 | 0.70‒1.72 | 0.682 |
| Serum creatinine > 2.0 mg/dL | 1.10 | 0.59‒2.05 | 0.769 |
| LV EF < 50% | 0.55 | 0.07‒4.19 | 0.565 |
| Donation after circulatory death | 1.32 | 0.62‒2.79 | 0.470 |
| Infiltrates on chest radiograph | 0.50 | 0.30‒0.82 | 0.006 |
| P/F ratio < 300 | 2.38 | 1.20‒4.71 | 0.013 |
| Purulent secretions on bronchoscopy | 1.00 | 0.59‒1.68 | 0.985 |
| Cigarette use history (> 20 pack-years) | 1.10 | 0.62‒1.94 | 0.740 |
CMV, Cytomegalovirus; ECD, Extended Criteria Donor; LV EF, Left Ventricular Ejection Fraction; P/F ratio, Ratio of arterial oxygen partial pressure to fraction of inspired oxygen.
Discussion
Among adults undergoing simultaneous HLTx from 2005 to 2021, the use of an ECD heart and/or lung is not associated with increased mortality at five years, one year, or 30 days. Further, the use of one or more ECD organs is not associated with increased graft rejection, postoperative stroke, or hospital length of stay. The only measure of morbidity positively associated with ECD organ use is postoperative permanent pacemaker placement, which was more likely in recipients of both an ECD heart and an ECD lung. Of the 12 extended donor criteria identified in this study, only two – age ≥ 55 and P/F ratio < 300 – were associated with mortality at 5 years.
The comparable mortality and morbidity between recipients of SCD and ECD organs are not easily explained by recipient characteristics. In fact, recipients of two ECD donor organs were less healthy than other recipients with respect to increased age and diabetes prevalence. The only reported characteristics that may have biased outcomes in favor of recipients of ECD organs were the relative increase in transplantation of ECD organs from 2015‒2021, the higher proportion of SCD organ donors on inotropes at the time of procurement, and the lower rates of cytomegalovirus positivity among recipients of ECD organs. Yet on Cox regression, year of transplant, donor inotrope use, and recipient cytomegalovirus positivity were not significantly associated with five-year mortality. Further, the donor organ status group itself was not associated with five-year survival. It is unclear why recipients of two ECD donor organs had increased pacemaker need, though this may be attributable to these recipients being older [19] and/or have higher rates of donor conduction abnormalities not captured in the UNOS database.
The relative safety of using ECD hearts and ECD lungs is consistent with existing literature showing that the use of ECD hearts or lungs in single-organ transplantation yields comparable outcomes to using SCD organs 1, 2, 3, 4, 5, 6. The present study is unique in demonstrating the safety of ECD organs in the setting of simultaneous HLTx, which is consistent with the literature on single-organ thoracic transplants. Interestingly, the authors found an increase in the relative amount of ECD organs being transplanted in 2015‒2021 relative to previous eras, suggesting that clinical practice is shifting to reflect literature on the tolerability of ECD organs. The present study is also unique in showing the associations between various ECD criteria and heart-lung transplantation outcomes. On Cox regression, the only ECD heart criteria found to significantly contribute to five-year mortality was the age of 55 or greater. The only additional ECD lung criterion found to significantly decrease mortality was P/F ratio < 300. These findings add to the literature suggesting a need to revise ECD heart and lung criteria based on modern outcomes, or otherwise to develop a donor risk score as has been done for single-organ heart transplantation [20,21]. Using a less restrictive set of criteria in the setting of HLTx would likely increase the supply of donor organs given that, at least in the setting of single-organ heart transplantation, donors are often turned down due to ejection fraction less than or equal to 50% or ischemic time greater than four hours [21]. While the present study confirmed that advanced donor age and low P/F ratio are significant risk factors for mortality after HLTx, the authors, nonetheless, advocate that donor selection decisions be made on a holistic basis considering all relevant donor and recipient characteristics.
Limitations of this study are a function of its retrospective design and reliance on the UNOS database. While the UNOS database is invaluable for understanding trends in transplantation, it does not report all pre-transplant variables that are useful for assessing donor organ health nor does it investigate every significant post-transplant outcome. It fails, for example, to report the dose of donor inotrope support at the time of procurement, which is notable given that high-dose inotrope support has been identified as an extended donor criterion. It is thus possible that the present study underestimated the prevalence of ECD organs being used and/or mischaracterized some ECD organ recipients and SCD organ recipients. Additionally, the UNOS database does not report significant post-operative outcomes including primary graft dysfunction, pulmonary function test findings, or echocardiographic measures. Future studies could better characterize the impact of ECD organs on morbidity and mortality by using a prospective design and reporting these additional pre- and post-transplant variables.
Conclusion
With appropriate patient selection, using one or two ECD organs in HLTx represents a viable strategy for increasing the supply of limited donor hearts and lungs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Noah Weingarten: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Amit Iyengar: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. David Alan Herbst: Writing – original draft, Writing – review & editing. Mark Helmers: Writing – original draft, Writing – review & editing. Danika Meldrum: Writing – original draft, Writing – review & editing. Sara Guevara-Plunkett: Writing – original draft, Writing – review & editing. Jessica Dominic: Writing – original draft, Writing – review & editing. Pavan Atluri: Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge the United Network for Organ Sharing for kindly supplying us with the data that was used in this analysis.
References
- 1.Wang Y., Cai J., Sun Y., Zhang J., Xie F., Alshirbini M.H., Shi J., Dong N. Extended donor criteria in heart transplantation: a retrospective study from a single Chinese institution. J Thorac Dis. 2018;10(4):2153–2165. doi: 10.21037/jtd.2018.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka S., Campo-Cañaveral de la Cruz J.L., Barturen M.G., Carrasco S.C., Román A.R., et al. Post-transplant outcomes of standard and extended criteria donation after circulatory death donor lungs categorized by donation after brain death lung criteria. Eur J Cardiothorac Surg. 2021;60(3):590–597. doi: 10.1093/ejcts/ezab121. [DOI] [PubMed] [Google Scholar]
- 3.Angleitner P., Kaider A., Gökler J., Moayedifar R., Osorio-Jaramillo E., Zuckermann A., et al. High-dose catecholamine donor support and outcomes after heart transplantation. J Heart Lung Transplant. 2018;37(5):596–603. doi: 10.1016/j.healun.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Reyentovich A., Gidea C.G., Smith D., Lonze B., Kon Z., Fargnoli A., et al. Outcomes of the Treatment with Glecaprevir/Pibrentasvir following heart transplantation utilizing hepatitis C viremic donors. Clin Transplant. 2020;34(9):e13989. doi: 10.1111/ctr.13989. [DOI] [PubMed] [Google Scholar]
- 5.Sugimura Y., Immohr M.B., Aubin H., Mehdiani A., Rellecke P., Tudorache I., et al. Impact of reported donor ejection fraction on outcome after heart transplantation. Thorac Cardiovasc Surg. 2021;69(6):490–496. doi: 10.1055/s-0041-1725179. [DOI] [PubMed] [Google Scholar]
- 6.Fischer S., Gohrbandt B., Struckmeier P., Niedermeyer J., Simon A., Hagl C., et al. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg. 2005;129(4):919–925. doi: 10.1016/j.jtcvs.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Oto T., Griffiths A.P., Levvey B., Pilcher D.V., Whitford H., Kotsimbos T.C., et al. A donor history of smoking affects early but not late outcome in lung transplantation. Transplantation. 2004;78(4):599–606. doi: 10.1097/01.tp.0000131975.98323.13. [DOI] [PubMed] [Google Scholar]
- 8.Woolley A.E., Singh S.K., Goldberg H.J., Mallidi H.R., Givertz M.M., Mehra M.R., et al. DONATE HCV Trial Team. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019;380(17):1606–1617. doi: 10.1056/NEJMoa1812406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadre S., Turowski J., Budev M. Overview of lung transplantation, heart-lung transplantation, liver-lung transplantation, and combined hematopoietic stem cell transplantation and lung transplantation. Clin Chest Med. 2017;38(4):623–640. doi: 10.1016/j.ccm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Fiore A., Grande A.M., Gatti G., Youssari A., Piscitelli M., Bergoend E., et al. Valvular surgery in donor hearts before orthotopic heart transplantation. Arch Cardiovasc Dis. 2020;113(11):674–678. doi: 10.1016/j.acvd.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Samsky M.D., Patel C.B., Owen A., Schulte P.J., Jentzer J., Rosenberg P.B., et al. Ten-year experience with extended criteria cardiac transplantation. Circ Heart Fail. 2013;6(6):1230–1238. doi: 10.1161/CIRCHEARTFAILURE.113.000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederberger P., Farine E., Raillard M., Dornbierer M., Freed D.H., Large S.R., et al. Heart transplantation with donation after circulatory death. Circ Heart Fail. 2019;12(4) doi: 10.1161/CIRCHEARTFAILURE.118.005517. [DOI] [PubMed] [Google Scholar]
- 13.Kitagaki K., Ono R., Shimada Y., Yanagi H., Konishi H., Nakanishi M. Cardiac rehabilitation program improves exercise capacity in heart transplantation recipients regardless of marginal donor factors. Heart Vessels. 2021;36(5):659–666. doi: 10.1007/s00380-020-01735-5. [DOI] [PubMed] [Google Scholar]
- 14.Galeone A., Lebreton G., Coutance G., Demondion P., Schmidt M., Amour J., et al. A single-center long-term experience with marginal donor utilization for heart transplantation. Clin Transplant. 2020;34(11):e14057. doi: 10.1111/ctr.14057. [DOI] [PubMed] [Google Scholar]
- 15.Kakuta T., Fukushima S., Shimahara Y., Yajima S., Kawamoto N., Tadokoro N., et al. Benefits of the modified bicaval anastomosis technique for orthotopic heart transplantation from a size-mismatched marginal donor. Circ J. 2019;84(1):61–68. doi: 10.1253/circj.CJ-19-0441. [DOI] [PubMed] [Google Scholar]
- 16.Schumer E.M., Ising M.S., Trivedi J.R., Slaughter M.S., Cheng A. Early outcomes with marginal donor hearts compared with left ventricular assist device support in patients with advanced heart failure. Ann Thorac Surg. 2015;100(2):522–527. doi: 10.1016/j.athoracsur.2015.02.089. [DOI] [PubMed] [Google Scholar]
- 17.Copeland H., Hayanga J.W.A., Neyrinck A., MacDonald P., Dellgren G., Bertolotti A., et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 2020;39(6):501–517. doi: 10.1016/j.healun.2020.03.020. Erratum in: J Heart Lung Transplant. 2020;39(7):734. [DOI] [PubMed] [Google Scholar]
- 18.Stocks J., Quanjer P.H. Reference values for residual volume, functional residual capacity, and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 19.Cantillon D.J., Tarakji K.G., Hu T., Hsu A., Smedira N.G., Starling R.C., et al. Long-term outcomes and clinical predictors for pacemaker-requiring bradyarrhythmias after cardiac transplantation: analysis of the UNOS/OPTN cardiac transplant database. Heart Rhythm. 2010;7(11):1567–1571. doi: 10.1016/j.hrthm.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Smits J.M., De Pauw M., de Vries E., Rahmel A., Meiser B., Laufer G., et al. Donor scoring system for heart transplantation and the impact on patient survival. J Heart Lung Transplant. 2012;31(4):387–397. doi: 10.1016/j.healun.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Whited W.M., Trivedi J.R., van Berkel V.H., Fox M.P. Objective donor scoring system for lung transplantation. Ann Thorac Surg. 2019;107(2):425–429. doi: 10.1016/j.athoracsur.2018.08.034. [DOI] [PubMed] [Google Scholar]