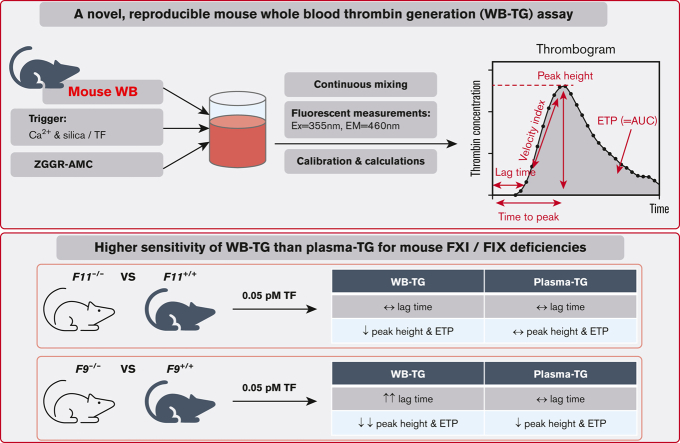
Key Points
-
•
A novel mouse WB-TG assay was developed.
-
•
Mouse WB-TG is highly sensitive to FXI- and FIX-mediated amplification of TF-initiated TG.
Visual Abstract
Abstract
Thrombin generation (TG) assays serve as a valuable tool to study the amplifying roles of intrinsic pathway factors in human coagulation and provide functional insights into the increased bleeding observed in individuals deficient in factors (F) XI, IX, or VIII. Mice are used extensively in hemostasis research owing to the availability of coagulation factor–deficient mice. However, phenotypic differences between mouse and human TG have become apparent. In this study, we describe a novel, calibrated mouse whole blood (WB) TG assay used to assess the amplifying roles of intrinsic pathway factors in mouse coagulation. WB- and plasma-TG was triggered with either silica or tissue factor (TF) in samples from wild-type mice and mice deficient for FXII, FXI, or FIX. Expectedly, silica-triggered WB-TG and platelet-poor plasma (PPP)-TG were significantly reduced by deficiencies for FXII, FXI, or FIX. FXII deficiency had no effect on WB-TG or PPP-TG when triggered with TF. However, FXI deficiency resulted in significantly reduced WB-TG triggered by low concentrations of TF but had no effect on TF-triggered PPP-TG. FIX deficiency profoundly reduced WB-TG when triggered by low or high concentrations of TF whereas TG in PPP or platelet-rich plasma was only moderately reduced under these conditions. In conclusion, we have developed a novel mouse WB-TG assay with enhanced sensitivity to FXI- and FIX-dependent amplification of coagulation compared with an established plasma-TG assay. The enhanced sensitivity of WB-TG to FXI and FIX-dependent amplification of coagulation suggests an important role of blood cells in this process.
Introduction
Activation of human coagulation can be initiated by either the extrinsic or intrinsic pathways. The extrinsic pathway is activated when factor (F) VIIa binds to tissue factor (TF) and forms the extrinsic tenase complex that activates FX into FXa.1, 2, 3 Exposure to negatively charged surfaces (eg, silica) or certain molecules (eg, RNA, DNA, collagen, and polyphosphate)4, 5, 6, 7 activates the intrinsic pathway through autoactivation of FXII into FXIIa, which is further enhanced when prekallikrein is converted by FXIIa to kallikrein. FXIIa activates FXI into FXIa which then activates FIX into FIXa. FIXa forms the intrinsic tenase complex with its cofactor FVIIIa and drives the activation of FX into FXa. FXa complexed with its cofactor FVa catalyzes the activation of prothrombin to the terminal coagulation protease thrombin (as reviewed elsewhere8, 9, 10). Recently, several groups have demonstrated that kallikrein can bypass FXII and FXI by directly activating FIX.11, 12, 13
Interactions between the extrinsic and intrinsic pathways have been described in various studies. Josso and others3 revealed that the TF:FVIIa complex activates FIX.2, 3 More recently, it was shown that the TF:FVIIa complex can activate FVIII.14,15 Further, Gailani and others16 revealed that thrombin can activate FXI leading to the amplification of thrombin generation (TG), a pathway only evident under conditions of low TF concentrations.17,18 However, the contribution of these pathways in more complex biological systems remains unclear.
TG assays, such as Calibrated Automated Thrombography (CAT),19 are widely used to study the molecular and cellular regulation of coagulation because they provide a global overview of this process.20 By tailoring the concentrations of extrinsic or intrinsic pathway triggers, they can be used to measure feedback loops and amplificatory pathways. For example, TG induced by a low concentration of TF, in the presence of the FXIIa inhibitor, corn trypsin inhibitor (CTI), is sensitive to FXI deficiency in human plasma.18 This is not detectable in a conventional prothrombin time assay in which nanomolar TF is used.
Genetically modified mouse models serve as valuable tools to study the contribution of a variety of clotting factors to hemostasis and thrombosis. In line with our understanding of contact activation in humans, mice deficient in prekallikrein, FXII, FXI, FIX, or FVIII have prolonged activated partial thromboplastin times.21, 22, 23 Consistent with the bleeding diathesis observed in humans with congenital deficiencies in FIX or FVIII, severe bleeding phenotypes have been observed in mice deficient in FIX or FVIII.24 No increased bleeding is observed for humans or mice deficient in FXII or prekallikrein.21,25 The situation is more complex regarding FXI deficiency. Some FXI-deficient humans exhibit bleeding after injury, primarily at sites of high fibrinolytic activity, whereas others do not have a bleeding phenotype.26 FXI-deficient mice do not show increased bleeding after tail nerve transection compared with wild-type mice.22,24 However, there is conflicting data regarding the saphenous vein bleeding model. Mohammed et al27 observed no difference in bleeding in FXI-deficient mice compared with wild-type mice, whereas we observed a mild hemostatic defect in FXI-deficient mice.24
Previously, we reported that platelet-poor plasma (PPP) from FIX-deficient mice supported reduced TG compared with controls when initiated with a low concentration of TF,28 suggesting that TF:FVIIa-mediated activation of FIX occurs in mouse PPP. However, when initiated with the same low concentration of TF, PPP-TG for FXI-deficient mice was not significantly different from controls. In mouse platelet-rich plasma (PRP), where thrombin-mediated FXI activation is thought to be accelerated by secreted polyphosphate from activated platelets,29 low TF–triggered TG was also not reduced by FXI deficiency.28 These observations suggested that the mouse plasma-TG assay was insensitive to thrombin-mediated activation of FXI.28
It has been proposed that mouse plasma has a strong intrinsic anticoagulant activity that requires predilution of plasma for TG assays.30,31 This dilution effect may hinder the ability to detect mild phenotypes associated with amplificatory pathways.32 Furthermore, previous studies assessing mouse TG were primarily performed in plasma omitting the influence of most circulating cells. Ninivaggi et al33 described a mouse whole blood (WB)-TG assay sensitive to hypercoagulability in mice. A filter paper was used in the assay to prevent light transmission distortion caused by red blood cell (RBC) sedimentation, but it induced markedly accelerated activation of coagulation.34
Here, we report the development of a novel calibrated mouse WB-TG assay based on a recently described human WB-TG assay that does not use filter paper.35 We determined the effect of FXII, FXI, and FIX deficiency on WB-TG and PPP-TG initiated with silica or TF. This WB-TG assay serves as a useful tool for assessing the influence of amplifying pathways on coagulation.
Methods
Additional information about reagents and mice has been provided in the Supplement Materials.
Blood sample collection and handling
WB was collected from the inferior vena cava of mice anesthetized with 3%-3.5% isoflurane in 2% oxygen into syringes containing sodium citrate (final concentration [f.c.] 0.38%; RICCA Chemical, Arlington, TX). CTI (f.c. in WB 50 μg/mL) was used during blood collection30,36,37 to prevent contact activation when indicated. Mouse WB was collected from the inferior vena cava to minimize TF contamination. Part of the WB was used in WB-TG measurements within 1 hour of the blood draw and the remaining blood was centrifuged at 4500g for 15 minutes to generate PPP, which was frozen and stored at −80°C. PPP was thawed at 37°C for 10 minutes before TG assessment. PRP was generated by centrifuging WB twice at 150g for 5 minutes. The preparation of reconstituted blood is described in the Supplement Materials.
Mouse WB-TG assay
The mouse WB-TG assay was developed based on a recently reported human WB-TG assay.35 TG reactions were simultaneously triggered by transferring 48 μL trigger solutions (ie, CaCl2 together with Innovin TF or silica) into a mixture of 72 μL WB and 24 μL of thrombin substrate Z-Gly-Gly-Arg-7-amino-4-methylcoumarin (ZGGR-AMC, Bachem, Bubendorf, Switzerland) using a multichannel pipette on a 96-well plate (#290-8117-01R, Caplugs Evergreen, Buffalo, NY) after preheating at 37°C for 10 minutes. After 8 times of gentle mixing, 60 μL aliquots of the final mixture were transferred to a 96-well plate (#2797, Corning, Corning, NY) in duplicate. The plate was immediately inserted into a Fluoroskan Ascent fluorometer (Thermo Fisher Scientific, Waltham, MA) and fluorescence signals were detected with λex = 355 nm and λem = 460 nm using the Ascent Software (version 2.6, Thermo Fisher Scientific) at 37°C. Thirty-six wells were always measured and the integration time was set as 6 seconds to ensure continuous mixing of the sample.35 In the final reaction, the volume ratios of the trigger solution, WB, and thrombin substrate were 2:3:1, respectively. The f.c. of calcium and thrombin substrate were 9 mM and 416.7 μM, respectively. Final concentrations of TF and silica were as indicated. Each WB sample was calibrated by running a α2-macroglobulin-thrombin complex calibrator in parallel with the TG trigger solution to correct for any influence of sample color.
WB-TG parameters were calculated as described.38 The highest transient thrombin concentration in a TG reaction is defined as peak height. The time when thrombin concentration reaches 1/6 of the peak height is defined as lag time, The time when thrombin concentration peaks is defined as time to peak, and the area under the TG curve is defined as endogenous thrombin potential (ETP).
To investigate the influence of WB predilutions, citrated mouse WB was prediluted to differing extents with buffer before WB-TG measurement. For comparisons between genotypes TG was assesssed in WB samples collected into citrate and CTI from both intrinsic factor–deficient mice and wild-type mice in the same run to reduce the influence of interassay variation. F12−/−, F11−/−, and F9−/− mice and wild-type control mice had similar counts of white blood cells, RBCs, and platelets (supplemental Table 1). The plasma levels of FX, prothrombin, and fibrinogen were not signficantly different between intrinsic factor–deficient mice and controls (supplemental Table 1).
Mouse plasma-TG assay
TG in mouse plasma was measured as described.19,28,30 Ten μL of trigger solution (4 μM synthetic phospholipids and 0.05, 0.1, or 0.5 pM TF, or 120× diluted silica, f.c.) or 100 nM thrombin calibrator were pipetted into wells of a 96-well plate (#290-8117-01R, Caplugs Evergreen), followed by 40 μL of prediluted mouse PPP or PRP (1 volume of plasma diluted in 3 volumes of buffer). Phospholipids were added when PPP was tested and were excluded when PRP was used. Plates were preheated at 37°C in a Fluoroskan Ascent fluorometer for 5 minutes before dispensing a 10 μL mixture of 16.7 mM calcium and 416.7 μM ZGGR-AMC thrombin substrate. The fluorescence signal was measured with λex = 390 nm and λem = 460 nm. Thrombinoscope software version 5.0 (Thrombinoscope BV, Maastricht, The Netherlands) was used to acquire the fluorescence signal, and plasma-TG parameters, including the lag time, peak height, and ETP, were calculated.
Statistics
Data were analyzed using the Prism software (version 9.1, GraphPad, San Diego, CA). The normality of the data was assessed using the Shapiro-Wilk normality test and comparisons between groups were conducted using parametric and nonparametric tests as appropriate. A 2-sided P value of <.05 was considered statistically significant.
Results
Establishing a mouse WB-TG assay
A continuous fluorogenic WB-TG assay is technically difficult because RBCs gradually sediment and cause variable quenching of the fluorescence signal during TG measurements.39 By mixing the WB sample continuously during measurement, RBC sedimentation is prevented.35 With the use of a wavelength that better excites the AMC fluorophore (355 nm instead of 390 nm used in the CAT assay), adequate fluorescence intensity was obtained by the fluorogenic thrombin substrate in WB-TG (data not shown).
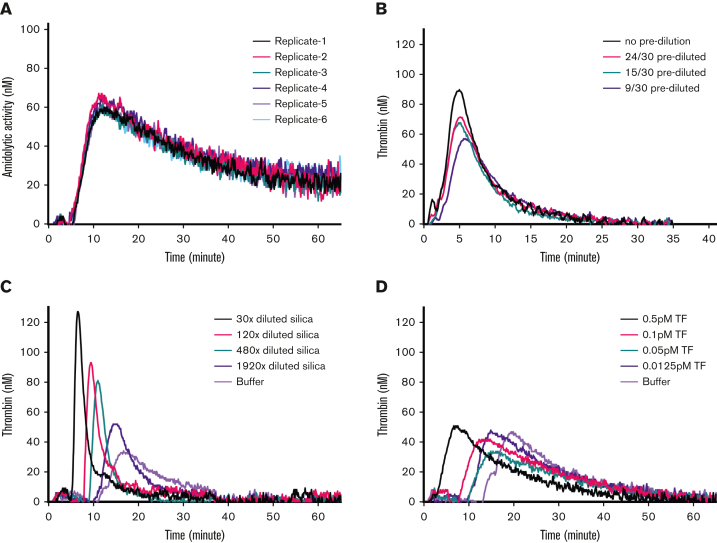
This approach was used to assess TG in mouse WB. In citrated mouse WB, 0.5 pM TF-induced TG was consistent between 6 replicates (Figure 1A). Next, we tested if mouse WB needed to be prediluted before TG measurement. Unlike results observed with plasma-TG,30,31 predilution of mouse WB did not enhance TG but rather led to reduced WB-TG (Figure 1B). A study found that TG in human PPP was dependent on the CaCl2 concentration used in the assay;40 therefore, we evaluated the influence of CaCl2 concentration on mouse WB-TG. Maximal WB-TG was observed with a f.c. of 9 mM CaCl2 (supplemental Figure 1). Using the above-described experimental conditions, mouse WB-TG was increased by ascending concentrations of extrinsic pathway trigger TF and intrinsic pathway trigger silica (Figure 1C-D), as shown by the concentration-dependent shortening of lag time and increased peak height.
Figure 1.
Evaluation of a new mouse WB-TG assay. (A) Citrated mouse WB was triggered by 0.5 pM TF and 16.7 mM CaCl2, in presence of 416.7 μM thrombin substrate ZGGR-AMC. Cleavage of the thrombin substrate was monitored with Ex = 355 nm and Em = 460 nm. The thrombin-like amidolytic activity was calculated from the first derivative of the fluorescence signal of TG reactions and calibration reactions. WB-TG curves of 6 replicates are shown. (B) The effect of predilution of mouse WB samples on WB-TG was tested by comparing 0.5 pM TF-triggered TG reactions in WB samples that were not prediluted (30 μL WB in 60 μL reaction mixture) against those prediluted (24, 15, or 9 μL WB in 60 μL mixture, indicated as 24/30 prediluted, 15/30 prediluted, and 9/30 prediluted, respectively). Averaged curves of 3 independent experiments are shown. (C-D) Mouse WB-TG in response to different concentrations of TF or silica. Representative curves of 3 independent experiments are shown.
Effect of CTI on mouse WB-TG
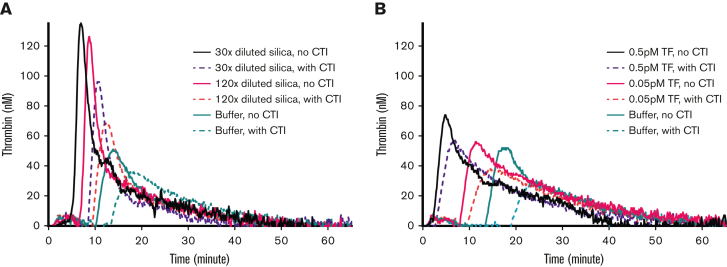
Previous studies found severe distortion of TG if contact activation was not prevented.30,37 Therefore, we evaluated the effect of the FXIIa inhibitor CTI on mouse WB-TG. As expected, WB-TG initiated by silica was markedly diminished when WB was collected in citrate with 50 μg/mL CTI, as evidenced by the prolonged lag time and reduced peak height (Figure 2A). However, even in the presence of CTI, a concentration-dependent increase in TG was observed when triggered with increasing silica concentrations. Notably, 120-fold diluted silica produced WB-TG curves similar to that triggered by 1.6 nM human FXIIa, with a lag time of ∼10 minutes and a thrombin peak height of ∼65 nM (supplemental Figure 2).
Figure 2.
Effect of CTI on mouse WB-TG. Citrated mouse WB samples were immediately mixed with 50 μg/mL CTI after blood collection or saline (no CTI). TG was then triggered with varying doses of (A) TF (0, 0.05, or 0.5 pM, f.c.) or (B) silica (0, 120× diluted, or 30× diluted, final dilutions). Representative curves of 3 independent experiments are shown.
The use of 50 μg/mL CTI also reduced TF-induced–WB-TG (Figure 2B), in line with a previous report in mouse plasma-TG.30 The lag time of WB-TG induced by recalcification only was >10 minutes and this was further prolonged to >13 minutes when CTI was used in blood collection. These results indicate that contact activation influences mouse WB-TG and inhibition of FXIIa generation is requried to eliminate nonspecific contact activation.
Reproducibility of the mouse WB-TG assay
The intraassay variation was determined by measuring TG in mouse WB collected into citrate and 50 μg/mL CTI triggered by 9 mM CaCl2 and either 0.05 pM TF or 120× diluted silica in the presence of 416.7 μM thrombin substrate ZGGR-AMC. As shown in Table 1, the intraassay coefficient of variation values were between 1.4% and 9.7% for mouse WB-TG parameters including lag time, time to peak, peak height, and ETP. The intermouse coefficient of variation of mouse WB-TG parameters were assessed in 6 mice in 2 independent runs and were between 4.8% and 13.7%.
Table 1.
Intraassay and intermouse variations of the mouse WB-TG assay
| Intraassay CV (n = 5 replicates) | ||||||
|---|---|---|---|---|---|---|
| Silica |
0.05 pM TF |
|||||
| Mean | SD | %CV | Mean | SD | %CV | |
| Lag time (min) | 10.7 | 0.4 | 3.7 | 9.3 | 0.1 | 1.4 |
| TtPeak (min) | 12.9 | 0.5 | 4.2 | 13.6 | 1.2 | 8.6 |
| Peak height (nM) | 71.8 | 6.8 | 9.4 | 40.6 | 3.9 | 9.7 |
| ETP (nM∗min) | 833.0 | 66.7 | 8.0 | 839.1 | 39.7 | 4.7 |
| Intermouse CV (n = 6 mice) | ||||||
|---|---|---|---|---|---|---|
| Silica |
0.05 pM TF |
|||||
| Mean | SD | %CV | Mean | SD | %CV | |
| Lag time (min) | 12.4 | 1.5 | 11.8 | 8.5 | 0.5 | 5.9 |
| TtPeak (min) | 15.7 | 1.4 | 8.9 | 12.6 | 0.7 | 5.5 |
| Peak height (nM) | 56.0 | 6.9 | 12.3 | 50.0 | 6.9 | 13.7 |
| ETP (nM∗min) | 810.3 | 55.2 | 6.8 | 850.0 | 40.9 | 4.8 |
The intraassay variation was determined by measuring TG in mouse WB collected in citrate and 50 μg/mL CTI triggered by 9 mM CaCl2 and either 0.05 pM TF or 120× diluted silica in the presence of 416.7 μM thrombin substrate ZGGR-AMC. The intermouse variations of WB-TG parameters were assessed in 6 mice done in 2 independent runs.
CV, coefficient of variation; SD, standard deviation; TtPeak, time to peak.
Effect of FXII deficiency on TG in mouse WB and PPP
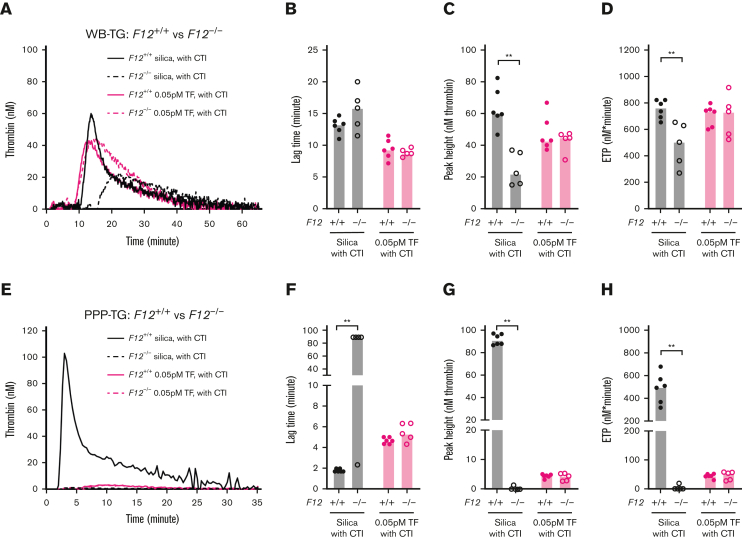
WB-TG and PPP-TG were evaluated in F12−/− mice and F12+/+ controls. As expected, WB collected in the presence of CTI from F12−/− mice had significantly reduced silica-initiated TG compared with F12+/+ controls, with reduced peak height and ETP (Figure 3A-D). However, interestingly, significant residual silica-initiated TG was observed in WB of F12−/− mice collected both with and without CTI (Figure 3A-D; supplemental Figure 3A-D).
Figure 3.
Effect of FXII deficiency on mouse TG in WB and PPP. TG in both WB and PPP of F12+/+ control mice and F12−/− mice were triggered by 120× diluted silica or 0.05/0.5 pM TF and recalcification (4 μM phospholipids were added in PPP). Representative curves of (A) WB- and (E) PPP-TG. Bar graphs of lag time (B,F), peak height (C,G), and ETP (D,H) are shown as median (n = 5 mice per group). Comparisons between groups were made using the Mann-Whitney test. ∗P < .05; ∗∗P < .01.
WB-TG induced by 0.05 pM TF, collected in the presence of CTI, was not significantly different between F12−/− mice and F12+/+ controls (Figure 3A-D). Similar results were obtained in WB collected in the absence of CTI with F12−/− mice showing an equivalent level of TF-initiated TG compared to F12+/+ controls (supplemental Figure 3A-D).
TG was also evaluated in PPP generated from WB collected in the presence of CTI. In PPP, silica-initiated TG was significantly reduced in F12−/− mice compared with F12+/+ controls (Figure 3E-H). However, unlike the observations in WB, silica-induced TG was almost completely abolished in the PPP of F12−/− mice. Complementary findings were made regarding FXII deficiency in PPP generated in the absence of CTI (supplemental Figure 3E-H). When initiated with 0.05 pM TF, no difference in TG between PPP from F12−/− mice or F12+/+ controls was observed (Figure 3E-H).
Effect of FXI deficiency on TG in mouse WB and PPP
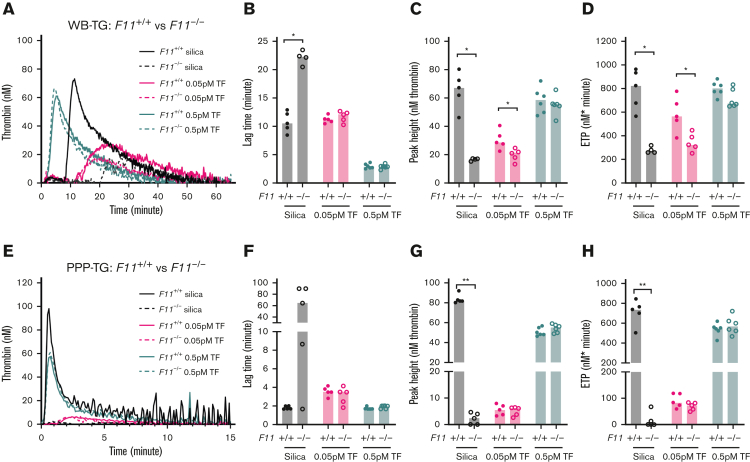
F11−/− mice and F11+/+ controls were used to evaluate the role of FXI on TG in WB and PPP. As expected, silica-initiated–WB-TG was significantly impaired in samples collected in the presence of CTI from F11−/− mice compared with F11+/+ controls (Figure 4A-D). Importantly, when WB-TG was initiated with a low concentration of 0.05 pM TF, although no significant difference in lag time was observed (Figure 4B), a significant reduction in peak height (35%) and ETP (40%) was observed in WB from F11−/− mice compared with F11+/+ controls (Figure 4C-D). In contrast, no significant difference between F11−/− mice and F11+/+ controls was observed when WB-TG was initiated with a high concentration of 0.5 pM TF (Figure 4B-D).
Figure 4.
Effect of FXI deficiency on mouse TG in WB and PPP. TG in both WB and PPP of F11+/+ control mice and F11−/− mice were triggered by 120× diluted silica or 0.05/0.5 pM TF and recalcification (4 μM phospholipids were added in PPP). Representative curves of WB- and PPP-TG are shown in (A) and (E), respectively. Bar graphs of lag time (B,F), peak height (C,G), and ETP (D,H) are shown as median (n = 4-6 mice per group). Comparisons between groups were made using the Mann-Whitney test. ∗P < .05; ∗∗P < .01.
Previous studies have shown that the contribution of FXI to human TG is TF-concentration dependent.18,41 Therefore, we initiated WB-TG with an intermediate concentration of 0.1 pM TF. Interestingly, using this trigger, the peak height and ETP were significantly reduced in the WB from F11−/− mice compared with F11+/+ controls (supplemental Figure 4A-D). However, it should be noted that the relative contribution of FXI to 0.1 pM TF-initiated–WB-TG was smaller than that observed with 0.05 pM TF (supplemental Figure 4C-D).
Silica-initiated TG was significantly reduced in PPP from F11−/− mice compared with F11+/+ controls (Figure 4E). In agreement with our previous study,28 TG triggered with either 0.05, 0.1, or 0.5 pM of TF was not different in PPP from F11−/− mice compared with F11+/+ controls (Figure 4E-H; supplemental Figure 4E-H).
The anti-FXI antibody 14E11, which inhibits FXI activation by FXIIa but not by thrombin,18 was used to determine if the low TF-triggered TG observed in F11−/− WB was a result of incomplete contact inhibition by CTI. 14E11 markedly reduced silica-induced TG in WB (supplemental Figure 5). However, importantly, 14E11 did not affect 0.05 pM TF-triggered TG in WB collected in the presence of CTI (supplemental Figure 5). This suggests that the TF-dependent phenotype observed in WB from F11−/− mice was not a result of incomplete inhibition of contact activation.
The presence of platelets and the use of CTI were previously shown to improve the sensitivity of TF-initiated TG to FXI deficiency in human samples.42 However, our previous report found no significant difference in 0.05 pM TF-initiated TG in PRP from F11−/− mice when compared with F11+/+ controls.28 This result was reproduced in this study (supplemental Figure 6). Furthermore, supplementation of autologous RBCs into PRP enhanced TG initiated with 0.05 pM TF to a similar extent in both F11−/− mice and F11+/+ controls. Interestingly, the peak height of 0.05 pM TF-initiated–WB-TG was not different between F11−/− mice and F11+/+ controls when WB samples were prediluted by fourfold (supplemental Figure 6).
Effect of FIX deficiency on TG in mouse WB, PPP, and PRP
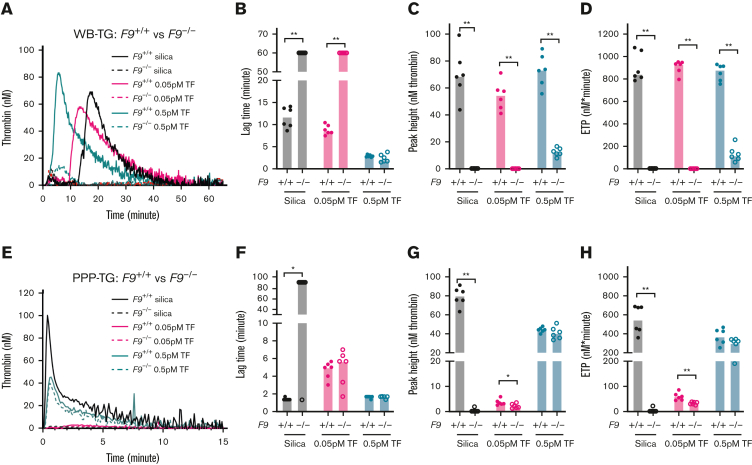
F9−/− mice and F9+/+ controls were used to evaluate the role of FIX on TG in WB and PPP. Silica-initiated–WB-TG was significantly reduced in WB collected in the presence of CTI from F9−/− mice compared with F9+/+ controls with virtually no silica-triggered TG observed in F9−/− WB (Figure 5A-D). Importantly, when WB from F9−/− mice was initiated with a low concentration of 0.05 pM TF, a profound reduction in TG was also observed (Figure 5A-D). When initiated with a higher concentration of 0.5 pM TF, although no difference in lag time was apparent (Figure 5B), peak height and ETP were still markedly reduced (>80%) in WB from F9−/− mice compared with F9+/+ controls (Figure 5C-D).
Figure 5.
Effect of FIX deficiency on mouse TG in WB and PPP. TG in both WB and PPP of F9+/+ control mice and F9−/− mice were triggered by 120× diluted silica or 0.05/0.5 pM TF and recalcification (4 μM phospholipids were added in PPP). Representative curves of WB- and PPP-TG are shown in (A) and (E), respectively. Bar graphs of lag time (B,F), peak height (C,G), and ETP (D,H) are shown as median (n = 6 mice per group). Comparisons between groups were made using the Mann-Whitney test. ∗P < .05; ∗∗P < .01.
As expected, silica-initiated TG was significantly reduced in PPP from F9−/− mice compared with F9+/+ controls (Figure 5E-H). Interestingly, when PPP-TG was initiated with a low concentration of 0.05 pM TF, a modest but significant reduction in peak height and ETP (40%) (Figure 5F-H) was observed. At the higher concentration of 0.5 pM TF, no significant difference in peak height or ETP was observed in PPP from F9−/− mice compared with F9+/+ controls (Figure 5G-H).
Activated platelets have been proposed to provide an important surface for FIXa activity.43 PRP from F9−/− mice and F9+/+ controls was used to evaluate the role of platelets. Similar to what was observed in WB and PPP, silica-induced TG was reduced in PRP from F9−/− mice compared with F9+/+ controls (supplemental Figure 7). Lag time was significantly prolonged while a small but significant reduction in peak height and ETP was observed in PRP from F9−/− mice compared with F9+/+ controls when triggered with 0.05 pM TF (supplemental Figure 7). No reduction in TG was observed in PRP from F9−/− mice compared with F9+/+ controls when initiated with 0.5 pM TF (supplemental Figure 7).
To determine if RBCs contribute to the increased sensitivity of WB-TG to FIX deficiency, TF-initiated TG was compared in PRP and PRP supplemented with washed autologous RBCs. RBC supplementation significantly enhanced TG initiated by 0.05pM TF in F9+/+ controls but not that in F9−/− mice (supplemental Figure 8A-C). The differences in 0.05 pM TF–initiated peak height between F9−/− mice and F9+/+ controls were larger in RBC reconstituted PRP than in PRP alone (12.2 vs 4.6-fold, supplemental Figure 8B).
Discussion
In this study, we have described a novel mouse WB-TG assay. Using this assay, we found that WB-TG was sensitive to the amplifying roles of FXI and FIX to coagulation in mice under conditions of low TF concentration (as summarized in Table 2). Importantly, low TF-initiated–WB-TG but not PPP-TG was reduced by FXI deficiency. In addition, low TF-initiated–WB-TG was profoundly impaired by FIX deficiency, whereas low TF-initiated PPP-TG was only modestly reduced by the same. The phenotypes observed in FXI- and FIX-deficient WB-TG triggered with TF are consistent with the contribution of thrombin-mediated activation of FXI18 and TF:FVIIa-mediated activation of FIX.2,3 Furthermore, the increased sensitivity of the WB-TG assay to TF-initiated TG seen in FXI and FIX-deficient samples suggests that cells present in WB contribute to intrinsic pathway–mediated amplification of coagulation in mice.
Table 2.
Summary of the comparisons of WB- and PPP-TG between intrinsic factors–deficient mice and wild-type mice
| Silica |
0.05 pM TF |
0.5 pM TF |
||||
|---|---|---|---|---|---|---|
| WB-TG | PPP-TG | WB-TG | PPP-TG | WB-TG | PPP-TG | |
| F12−/− vs F12+/+ | ||||||
| Lag time | ≈ | + + | ≈ | ≈ | ND | ND |
| Peak height & ETP | − | − − | ≈ | ≈ | ND | ND |
| F11−/− vs F11+/+ | ||||||
| Lag time | + | + + | ≈ | ≈ | ≈ | ≈ |
| Peak height & ETP | − | − − | − | ≈ | ≈ | ≈ |
| F9−/− vs F9+/+ | ||||||
| Lag time | + + | + + | + + | + | + | ≈ |
| Peak height & ETP | − − | − − | − − | − | − | ≈ |
Mouse WB was collected in citrate and 50 μg/mL CTI. TGs in WB and PPP were triggered by 9 mM CaCl2 and either 0.05/0.5 pM TF or 120× diluted silica (4 μM phospholipids were added in PPP).
≈, nonsignificantly different; − or +, modestly reduced or prolonged; − − or + +, severely reduced or prolonged; ND, not determined.
CAT is a useful tool to study the impact of coagulation factor deficiencies on TG in mouse models. Previous TG assays for mice have the shortcomings of either omitting the influence of most blood cells30,31 or using filter paper,33 which is a strong activator of the contact pathway. The WB-TG assay presented here has several advantages over previous approaches. In contrast to plasma-based TG,30,31 the WB-TG assay does not require predilution of blood. Furthermore, all circulating blood cells are present in the WB-TG assay allowing for consideration of the cellular contribution to TG.44 Compared with the filter paper-based WB-TG assay,33 this assay does not involve a foreign surface. In addition, compared with PPP-TG, this WB-TG assay does not require the introduction of exogenous phospholipids.
Thrombin-mediated activation of FXI has been proposed to facilitate physiologically relevant amplification of extrinsic pathway-initiated coagulation.18,26 In our previous study, we did not find evidence of thrombin-mediated activation of FXI in mouse PPP or PRP-TG,28 a finding confirmed in this study. These findings are in contrast with the low TF-triggered PPP-TG phenotype observed in human FXI-deficient plasma.18 Interestingly, our newly developed mouse WB-TG assay was sensitive to FXI deficiency when TG was triggered by a low concentration of TF. The addition of 14E11, an anti-FXI antibody that inhibits FXI activation by FXIIa but not thrombin, only inhibited TG triggered by silica but not by a low concentration of TF in FXI-deficient WB. This suggests that the increased sensitivity of WB-TG over PPP-TG to thrombin-mediated activation of FXI was not because of confounding effects of residual contact activation. Although the physiological implication of reduced TF-initiated WB-TG in FXI-deficient mice is currently unknown, it is encouraging that mouse WB-TG, rather than mouse plasma-TG, mimics the phenotype observed in human FXI–deficient PPP.
It is important to note that a major difference in FXI biology exists between humans and mice. In humans, FXI circulates freely in the blood. In contrast, in mice, the majority (∼80%) of FXI is bound to glycosaminoglycans on the endothelial surface via a cluster of basic residues on the apple 4 domain.45 The species-specific difference in FXI biology and resulting lower levels of FXI in wild-type mouse plasma may explain the reduced sensitivity of mouse PPP-TG to the FXI feedback loop when compared with human PPP-TG. FXI-deficient mice also fail to recapitulate the bleeding phenotype associated with FXI deficiency in humans. FXI-deficient mice do not show increased bleeding after tail vein transection compared with wild-type mice,22 and the bleeding phenotype after saphenous vein injury has been inconsistent.24,27 Bleeding in humans with FXI deficiency often occurs at sites with high fibrinolytic activity.46 The injury sites in mouse bleeding models may have different fibrinolytic activity than the bleeding sites associated with human FXI deficiency.47 Future studies should seek to compare the impact of FXI deficiency in mouse and human samples in a more direct manner.
In contrast to FXI-deficient mice, FIX-deficient mice have a severe bleeding phenotype after vascular injury.24,27 The phenotype of FIX- deficient mice complements the severe bleeding phenotype associated with FIX deficiency in humans. Importantly, a mutation in human FIX that limits activation by TF:FVIIa, but not FXIa, is associated with a mild form of hemophilia B,48 indicating a physiological role of FIX activation by the TF:FVIIa complex.2,3 In line with our previous report,28 PPP-TG was reduced in samples from FIX-deficient mice when triggered with low, but not high, concentrations of TF. A slightly more pronounced reduction in PRP-TG was observed in samples from FIX-deficient mice when triggered with low, but not high, concentrations of TF. WB-TG was profoundly reduced in samples from FIX-deficient mice when triggered with either low or high doses of TF. This indicates that WB is highly sensitive to TF:FVIIa-mediated activation of FIX and it is likely that platelets and other blood cells contribute to this phenotype.
In the “cell-based hemostasis” model, it is proposed that the platelet surface is an important determinant of coagulation factor interactions and kinetics.43 This is based on the ability of the activated platelet surface to provide negatively charged phospholipids and receptors that bind coagulation factors and modulate their interactions.4,29,49,50 Our findings in PRP from FIX and FXI-deficient mice suggested that although platelets potentiate activation of coagulation, their contribution may be relatively modest.28 Considering the comparative enhancement of TG in WB vs PPP and PRP, we hypothesized that other blood cells support activation and/or amplification of coagulation.
In this study, we assessed the potential of RBCs to modulate TG given the abundance of these cells in the blood. Reconstitution of PRP with RBCs markedly potentiated TG initiated with low levels of TF and enhanced the phenotypic difference associated with FIX deficiency, suggesting that RBCs play an important role in mouse TG. The ability of RBCs to enhance TG is consistent with findings in the human WB-TG assay.35 However, the mechanism by which RBCs support enhanced TG has yet to be determined. RBCs can externalize phosphatidylserine which could provide a surface for coagulation reactions.51 RBCs can also support the proteolytic activation of FIX by a surface-bound elastase-like enzyme.52 In addition, the RBC surface can support kallikrein-dependent activation of FIX.13 It is also possible that RBCs act cooperatively with platelets to enhance platelet activation and procoagulant activity.53,54 Noncanonical activation of FIX could also explain the presence of residual silica-initiated TG in WB from FXII and FXI-deficient mice that was not observed in FIX-deficient WB. Additional studies are required to determine the mechanism by which RBCs potentiate TG.
In addition to providing important insights into mechanisms of coagulation, the mouse WB-TG assay could also be used as a tool to evaluate novel therapies. In this study, the anti-FXI antibody 14E11 was effective in inhibiting silica-initiated TG. A recombinant humanized antibody called AB023, which is based on 14E11, is currently being evaluated as a novel anticoagulant in the setting of hemodialysis.55 The mouse WB-TG may also be particularly useful for evaluating novel therapies to treat hemophilia. Compared with plasma-TG, the mouse WB-TG provides a much larger window to assess the ability of therapies to support enhanced coagulation.
In conclusion, we have developed a novel mouse WB-TG assay with enhanced sensitivity to FXI- and FIX-mediated amplification of coagulation compared with plasma-TG. The enhanced sensitivity of WB-TG to FXI- and FIX-mediated amplification of coagulation indicates that blood cells likely play an important role in modulating coagulation.
Conflict-of-interest disclosure: M.R. is employed by Synapse Research Institute, a not-for-profit research unit of Diagnostica Stago. A.G. is a shareholder of Aronora, Inc. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank Ying Zhang for their technical support.
This work was supported by the National Heart Lung and Blood Institute of the National Institutes of Health (R01HL126974 to A.S.W, R01HL157441 to R.P., R35HL155657 to N.M.). S.P.G was supported by the American Society of Hematology (Scholar Award) and a National Institutes of Health training grant (T32HL007149). P.T. was supported by an International Society on Thrombosis and Haemostasis fellowship.
Authorship
Contribution: J.W., P.T., and S.P.G. conceptualized the study, performed experiments, and wrote the manuscript; M.R., A.G., R.P., and A.S.W. provided essential materials or animals and edited the manuscript; and N.M. conceptualized and supervised the study and wrote the manuscript.
Footnotes
∗S.P.G and N.M contributed equally to this study.
Explanation for mouse WB-TG assay and calculation template to transform fluorescence data into thrombin concentration is deposited online at: https://github.com/wanjunmax/WBTG/issues/1. Data are available on request from the corresponding author, Steven P. Grover (steven_grover@med.unc.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331–338. doi: 10.1161/ATVBAHA.118.312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josso F, Prou-Wartelle O. Interaction of tissue factor and factor VII at the earliest phase of coagulation. Thromb Diath Haemorrh Suppl. 1965;17:35–44. [PubMed] [Google Scholar]
- 4.Smith SA, Choi SH, Davis-Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116(20):4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noubouossie DF, Whelihan MF, Yu YB, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129(8):1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilner GD, Nossel HL, LeRoy EC. Activation of Hageman factor by collagen. J Clin Investig. 1968;47(12):2608–2615. doi: 10.1172/JCI105943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naudin C, Burillo E, Blankenberg S, Butler L, Renné T. Factor XII contact activation. Semin Thromb Hemost. 2017;43(8):814–826. doi: 10.1055/s-0036-1598003. [DOI] [PubMed] [Google Scholar]
- 9.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 10.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13(8):1383–1395. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearney KJ, Butler J, Posada OM, et al. Kallikrein directly interacts with and activates factor IX, resulting in thrombin generation and fibrin formation independent of factor XI. Proc Natl Acad Sci U S A. 2021;118(3) doi: 10.1073/pnas.2014810118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser M, van Oerle R, Ten Cate H, et al. Plasma kallikrein contributes to coagulation in the absence of factor XI by activating factor IX. Arterioscler Thromb Vasc Biol. 2020;40(1):103–111. doi: 10.1161/ATVBAHA.119.313503. [DOI] [PubMed] [Google Scholar]
- 13.Noubouossie DF, Henderson MW, Mooberry M, et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135(10):755–765. doi: 10.1182/blood.2019001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soeda T, Nogami K, Matsumoto T, Ogiwara K, Shima M. Mechanisms of factor VIIa-catalyzed activation of factor VIII. J Thromb Haemost. 2010;8(11):2494–2503. doi: 10.1111/j.1538-7836.2010.04042.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamikubo Y, Mendolicchio GL, Zampolli A, et al. Selective factor VIII activation by the tissue factor-factor VIIa-factor Xa complex. Blood. 2017;130(14):1661–1670. doi: 10.1182/blood-2017-02-767079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gailani D, Broze GJ., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253(5022):909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 17.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86(8):3035–3042. [PubMed] [Google Scholar]
- 18.Kravtsov DV, Matafonov A, Tucker EI, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114(2):452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 20.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(5):699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 21.Stavrou EX, Fang C, Merkulova A, et al. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125(4):710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gailani D, Lasky NM, Broze GJ., Jr. A murine model of factor XI deficiency. Blood Coagul Fibrinolysis. 1997;8(2):134–144. doi: 10.1097/00001721-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Pauer HU, Renné T, Hemmerlein B, et al. Targeted deletion of murine coagulation factor XII gene-a model for contact phase activation in vivo. Thromb Haemost. 2004;92(3):503–508. doi: 10.1160/TH04-04-0250. [DOI] [PubMed] [Google Scholar]
- 24.Ay C, Hisada Y, Cooley BC, Mackman N. Factor XI-deficient mice exhibit increased bleeding after injury to the saphenous vein. J Thromb Haemost. 2017;15(9):1829–1833. doi: 10.1111/jth.13766. [DOI] [PubMed] [Google Scholar]
- 25.Renne T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006;12(5):490–493. doi: 10.1111/j.1365-2516.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed BM, Cheng Q, Matafonov A, Monroe DM, Meijers JCM, Gailani D. Factor XI promotes hemostasis in factor IX-deficient mice. J Thromb Haemost. 2018;16(10):2044–2049. doi: 10.1111/jth.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grover SP, Schmedes CM, Auriemma AC, et al. Differential roles of factors IX and XI in murine placenta and hemostasis under conditions of low tissue factor. Blood Adv. 2020;4(1):207–216. doi: 10.1182/bloodadvances.2019000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118(26):6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dargaud Y, Spronk HM, Leenders P, Hemker HC, Ten Cate H. Monitoring platelet dependent thrombin generation in mice. Thromb Res. 2010;126(5):436–441. doi: 10.1016/j.thromres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Tchaikovski SN, VANV BJ, Rosing J, Tans G. Development of a calibrated automated thrombography based thrombin generation test in mouse plasma. J Thromb Haemost. 2007;5(10):2079–2086. doi: 10.1111/j.1538-7836.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 32.De Smedt E, Wagenvoord R, Hemker HC. The technique of measuring thrombin generation with fluorogenic substrates: 3. The effects of sample dilution. Thromb Haemost. 2009;101(1):165–170. [PubMed] [Google Scholar]
- 33.Ninivaggi M, Kelchtermans H, Kuijpers MJ, et al. Whole blood thrombin generation in Bmal1-deficient mice. Thromb Haemost. 2014;112(2):271–275. doi: 10.1160/TH13-11-0910. [DOI] [PubMed] [Google Scholar]
- 34.Ninivaggi M, Apitz-Castro R, Dargaud Y, de Laat B, Hemker HC, Lindhout T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin Chem. 2012;58(8):1252–1259. doi: 10.1373/clinchem.2012.184077. [DOI] [PubMed] [Google Scholar]
- 35.Wan J, Konings J, Yan Q, et al. A novel assay for studying the involvement of blood cells in whole blood thrombin generation. J Thromb Haemost. 2020;18(6):1291–1301. doi: 10.1111/jth.14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dargaud Y, Luddington R, Baglin TP. Elimination of contact factor activation improves measurement of platelet-dependent thrombin generation by calibrated automated thrombography at low-concentration tissue factor. J Thromb Haemost. 2006;4(5):1160–1161. doi: 10.1111/j.1538-7836.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- 37.Spronk HM, Dielis AW, Panova-Noeva M, et al. Monitoring thrombin generation: is addition of corn trypsin inhibitor needed? Thromb Haemost. 2009;101(6):1156–1162. [PubMed] [Google Scholar]
- 38.Hemker HC, Kremers R. Data management in thrombin generation. Thromb Res. 2013;131(1):3–11. doi: 10.1016/j.thromres.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Al Dieri R, Hemker CH. Thrombin generation in whole blood. Br J Haematol. 2008;141(6):895. doi: 10.1111/j.1365-2141.2008.07109.x. [DOI] [PubMed] [Google Scholar]
- 40.Parunov LA, Surov SS, Liang Y, Lee TK, Ovanesov MV. Can the diagnostic reliability of the thrombin generation test as a global haemostasis assay be improved? The impact of calcium chloride concentration. Haemophilia. 2017;23(3):466–475. doi: 10.1111/hae.13174. [DOI] [PubMed] [Google Scholar]
- 41.Lakshmanan HHS, Estonilo A, Reitsma SE, et al. Revised model of the tissue factor pathway of thrombin generation: role of the feedback activation of FXI. J Thromb Haemost. 2022;20(6):1350–1363. doi: 10.1111/jth.15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike GN, Cumming AM, Hay CRM, Bolton-Maggs PHB, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;126(3):397–405. doi: 10.1182/blood-2014-12-616565. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–965. [PubMed] [Google Scholar]
- 44.Wan J, Konings J, de Laat B, Hackeng TM, Roest M. Added value of blood cells in thrombin generation testing. Thromb Haemost. 2021;121(12):1574–1587. doi: 10.1055/a-1450-8300. [DOI] [PubMed] [Google Scholar]
- 45.Mohammed BM, Cheng Q, Matafonov A, et al. A non-circulating pool of factor XI associated with glycosaminoglycans in mice. J Thromb Haemost. 2019;17(9):1449–1460. doi: 10.1111/jth.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolton-Maggs PH. Factor XI deficiency and its management. Haemophilia. 2000;6(suppl 1):100–109. doi: 10.1046/j.1365-2516.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 47.Mohammed BM, Monroe DM, Gailani D. Mouse models of hemostasis. Platelets. 2020;31(4):417–422. doi: 10.1080/09537104.2020.1719056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor SA, Liddell MB, Peake IR, Bloom AL, Lillicrap DP. A mutation adjacent to the beta cleavage site of factor IX (valine 182 to leucine) results in mild haemophilia Bm. Br J Haematol. 1990;75(2):217–221. doi: 10.1111/j.1365-2141.1990.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 49.Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46:100733. doi: 10.1016/j.blre.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White-Adams TC, Berny MA, Tucker EI, et al. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2) Arterioscler Thromb Vasc Biol. 2009;29(10):1602–1607. doi: 10.1161/ATVBAHA.109.187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95(6):3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwata H, Kaibara M, Dohmae N, Takio K, Himeno R, Kawakami S. Purification, identification, and characterization of elastase on erythrocyte membrane as factor IX-activating enzyme. Biochem Biophys Res Commun. 2004;316(1):65–70. doi: 10.1016/j.bbrc.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Klatt C, Krüger I, Zey S, et al. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J Clin Invest. 2018;128(9):3906–3925. doi: 10.1172/JCI92077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du VX, Huskens D, Maas C, Al Dieri R, de Groot PG, de Laat B. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost. 2014;40(1):72–80. doi: 10.1055/s-0033-1363470. [DOI] [PubMed] [Google Scholar]
- 55.Lorentz CU, Tucker EI, Verbout NG, et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood. 2021;138(22):2173–2184. doi: 10.1182/blood.2021011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.