Abstract
Although recent decades have witnessed incremental improvements in the treatment of gastroesophageal junction (GEJ) carcinoma, outcomes remain modest. For locally advanced esophageal cancer, the addition of chemotherapy and/or radiation to surgery is considered the standard of care. Chemotherapy remains the primary treatment for metastatic disease and improves survival over best supportive care. However, the prognosis for patients with GEJ cancers, which are treated along the same paradigms as esophageal and gastric carcinomas, remain poor because of the emergence of chemoresistance and limited targeted therapeutic approaches, which include agents that target the HER2 and vascular endothelial growth factor pathways. Evaluation of immune checkpoint inhibitors in the chemorefractory setting have confirmed the activity of immunotherapy in esophagogastric cancer. Ongoing immunotherapeutic strategies are being evaluated in both the locally advanced and metastatic settings. This review focuses on the treatment of locally advanced and metastatic GEJ carcinomas, which encompass all tumors that have an epicenter within 5 cm proximal or distal to the anatomical Z-line (Siewert classification). Because the vast majority of GEJ tumors are adenocarcinoma, the management of adenocarcinoma is the focus of this review. Evolving approaches and areas of clinical equipoise are discussed.
Keywords: adenocarcinoma, chemoradiation, chemotherapy, gastroesophageal junction cancer, immunotherapy, (18F)2-fluorodeoxy-D-glucose positron emission tomography (FDG-PET), targeted therapy
INTRODUCTION
Esophagogastric cancer (EGC) is a global disease and accounts for 1.6 million cases a year, making it the third most common cancer worldwide.1 However, in the United States, it is relatively uncommon. Adenocarcinomas, predominantly located at the distal esophagus and the gastroesophageal junction (GEJ), represent 75% of esophageal cancers in the United States after a significant rise in incidence since the 1970s.2,3 Because squamous cell carcinomas (SCCs) rarely occur at the GEJ, this review will focus on the management of GEJ adenocarcinoma.
SURGERY FOR EARLY-STAGE DISEASE
Current guidelines from the National Comprehensive Cancer Network (NCCN) recommend upfront surgery for patients with clinical T1N0 (cT1N0) tumors. For those with low-risk T2 lesions (measuring <2 cm and well differentiated), surgery alone can also be considered. One study has addressed whether patients with early-stage disease should receive additional therapy. The French Francophone de Cancérologie Digestive (FFCD) 9901 trial randomized 195 patients with cT1-cT2Nany or cT3N0 tumors to 2 cycles of preoperative 5-fluorouracil (5-FU)/cisplatin and radiation (45 grays [Gy]) followed by surgery versus surgery alone.4 Only 28% of patients had adenocarcinoma; 24% and 74% had cT1 and cN0 tumors, respectively. There was no improvement in the complete resection (R0) rate (93% in the surgery-alone arm), disease-free survival, or overall survival (OS) with the addition of preoperative chemoradiation to surgery. However, in-hospital, postoperative mortality was significantly increased in the chemoradiation arm (11.1% vs 3.4%; P = .049). It is possible that the unexpectedly high postoperative mortality rate in the chemoradiation arm (compared with 4% in both arms of the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study [CROSS] study) may have obscured a small survival benefit from chemoradiation.
Management of Locally Advanced GEJ Adenocarcinoma
Given their anatomic location, the management of GEJ cancers is reflective of the treatment approach for esophageal and gastric adenocarcinomas. Five-year OS rates for patients with locally advanced esophageal cancer who undergo surgery alone range from 23% to 33% in contemporary studies.5–7 The risk of incomplete (R1) resection, local recurrence, and systemic dissemination is significant, and multimodal treatment has become standard. Numerous studies have demonstrated that preoperative and postoperative strategies, including chemotherapy or chemoradiation, improve outcomes when added to surgery.
Preoperative and perioperative chemotherapy
Preoperative and perioperative chemotherapy in esophageal/GEJ adenocarcinoma have been evaluated mostly in patients with T3/lymph node–positive disease, yielding mixed results. The Intergroup 113 trial was a phase 3 study in which approximately one-half of the 440 patients enrolled had adenocarcinoma.8 Patients were randomized to preoperative 5-FU and cisplatin or immediate surgery. There was no significant difference in local recurrence or OS between groups. The larger UK Medical Research Council (MRC) OEO2 trial randomized patients (two-thirds had adenocarcinoma, and 75% were distal esophageal/gastric cardia tumors) to preoperative cisplatin/5-FU or surgery alone. Long-term follow-up reported a modest improvement in 5-year OS from 17% to 23% (P = .03).9 The European Organization for Research and Treatment of Cancer (EORTC) 40954 study, which also investigated the addition of preoperative cisplatin/5-FU to surgery in patients with GEJ/gastric adenocarcinoma (52.8% had GEJ or proximal stomach tumors), was closed early because of poor accrual, and a benefit for preoperative therapy was not demonstrated.10
The seminal phase 3 MAGIC trial evaluated 3 cycles each of epirubicin, cisplatin, and 5-FU (ECF) before and after surgery versus surgery alone.5 Fifteen percent and 11% of patients had GEJ and lower esophageal tumors, respectively. Perioperative chemotherapy significantly improved 5-year OS (36% vs 23%; P = .009), establishing perioperative chemotherapy as a new standard of care in Europe and the United States. In subgroup analysis, there was no evidence of a difference in treatment effect by primary tumor site. However, results of more contemporary studies raise questions regarding the benefit of anthracyclines. The FFCD 9703 trial showed a significant improvement in 5-year OS (38% vs 24%; P = .02) in patients who received 6 cycles of perioperative 5-FU and cisplatin with surgery versus surgery alone.6 Sixty-four percent of patients in that study had GEJ tumors, and there was no significant variation in chemotherapy effect by tumor site observed. Although cross-trial comparisons should be made cautiously, the magnitude of OS benefit was similar between the FFCD and MAGIC studies. The UK MRC OEO-5 study randomized patients with lower esophageal/GEJ adenocarcinomas (67% with GEJ tumors) to 6 weeks of preoperative cisplatin/5-FU or 12 weeks of epirubicin, cisplatin, and capecitabine (ECX).11 Despite an improvement in pathologic complete response (pCR) among ECX-treated patients (7% vs 1%), there was no significant difference in OS between groups. These studies suggest that an anthracycline does not provide additional benefit over a platinum and 5-FU doublet.
Preliminary results of the FLOT4-AIO phase 3 trial have established 5-FU, oxaliplatin, and docetaxel (FLOT) as a new standard of care.12 This study randomized 716 patients with resectable gastric/GEJ adenocarcinoma to perioperative FLOT or ECF/ECX. Fifty-six percent of patients had GEJ tumors. FLOT was superior to ECF/ECX in all efficacy endpoints, including the complete resection (R0) rate (85% vs 78%; P = .016), progression-free survival (PFS), and OS (median OS, 50 vs 35 months; 3-year OS, 57% vs 48%; hazard ratio [HR], 0.77; P = .012). In subgroup analysis, GEJ tumors also benefitted (HR, 0.76). The rate of adverse events was similar between groups. Only one-half of patients completed all planned chemotherapy, similar to completion rates in the MAGIC and FFCD studies, highlighting the difficulty in administering adjuvant therapy and suggesting that patients benefit from short durations of chemotherapy.
Preoperative chemoradiation
Of contemporary trials13–17 that have evaluated preoperative chemoradiation in esophageal cancer, 3 have shown a survival benefit. The landmark phase 3 Dutch CROSS trial evaluated preoperative radiation (41.4 Gy) with weekly carboplatin and paclitaxel for 5 weeks in 366 patients with mostly locally advanced esophageal tumors; 75% of patients had adenocarcinoma, and 24% had GEJ tumors.13 The addition of chemoradiation resulted in higher R0 resection and pCR rates (29%: adenocarcinoma, 23%; SCC, 49%), and improved 5-year OS (47% vs 33%; HR, 0.067).7 There was no increased postoperative mortality associated with chemoradiation. This regimen became a standard of care. Whereas patients with SCC derived greater benefit than those with adenocarcinoma, long-term follow-up confirmed an OS benefit for both histologies.7 Long-term follow-up reported a 9% reduction in distant metastases in the chemoradiation arm, with similar OS to that seen in preoperative chemotherapy studies, mitigating concern regarding the short duration of chemotherapy administered.
Despite good tolerability and a favorable pCR rate, it remains unclear whether carboplatin and paclitaxel is the optimal regimen to combine with preoperative radiation. In the Cancer and Leukemia Group B (CALGB) 80803 study, 257 patients with esophageal/GEJ adenocarcinoma (57% had GEJ tumors) were randomized to induction 5-FU, leucovorin, and oxaliplatin (FOLFOX6) or carboplatin and paclitaxel followed by a (18F)2-fluorodeoxy-D-glucose–positron emission tomography (FDG-PET) scan.18 PET responders continued with the same regimen during concurrent chemoradiation, and nonresponders crossed to the alternate chemotherapy with radiation before surgery. The pCR rate in patients who were PET responders to induction FOLFOX and continued that regimen during radiation was 37.5%, compared with 12.5% in PET responders to induction carboplatin and paclitaxel who received this regimen with radiation. Both treatments were well tolerated. A 4-year OS of 52.7% was seen in PET responders to FOLFOX versus 44.7% in PET responders to carboplatin and paclitaxel.19 Although the study was not powered to evaluate survival differences between regimens, these results are hypothesis-generating.
Conversely, in the phase 2 NEOSCOPE study, patients received induction capecitabine and oxaliplatin and then underwent randomization to capecitabine and oxaliplatin with radiation or carboplatin and paclitaxel with radiation. Carboplatin and paclitaxel was associated with higher pCR rates (29.3% vs 11.1%),20 although the study was not designed to detect a difference in this endpoint.
The German POET study enrolled 119 patients with GEJ adenocarcinoma to preoperative chemotherapy with 5-FU, leucovorin, and cisplatin or 5-FU, leucovorin, and cisplatin followed by chemoradiation with cisplatin and etoposide before surgery.21 The study’s power to detect a difference between groups was restricted by the small numbers accrued. Patients who received chemoradiation had a higher pCR rate (15.6% vs 2%; P = .03) and lymph node–negative status, and a trend toward improved local control and 5-year OS was observed.22
A benefit of preoperative chemoradiation over chemotherapy is the signal of an improvement in R0 rates for GEJ tumors. In contemporary preoperative chemotherapy studies, R0 rates were <70%, whereas patients who received chemoradiation in the CROSS trial had R0 resection rates >90%.5,9,11,13,23 In the FLOT4-AIO study, the R0 rate was 84% in patients who received FLOT versus 77% in those who received ECF/ECX.12 Subgroup analysis is awaited to establish the R0 rate in GEJ tumors. An added value of chemoradiation over chemotherapy is also suggested by improvement in pCR rates and pathologic lymph node–negative rates. In chemoradiation studies, pCR rates from 16% to 23% have been reported versus 2% to 9% in trials of chemotherapy alone.13,21,23 In the phase 2 part of the FLOT4-AIO study, the pCR rate was 17%, whereas, in the phase 3 study, 25% of patients had ≤T1 disease at surgery versus 15% in the ECF/ECX group (P = .001).12,24 In a retrospective analysis of the MAGIC trial, lymph node status was the only independent predictor of survival (HR, 3.36; P < .001).25 The OEO-5 and FLOT4-AIO trials reported N0 rates of approximately 30% to 40% for cisplatin/5-FU and ECX and a 49% N0 rate in patients who received FLOT.11,12 In contrast, the CROSS and POET studies reported N0 rates of 64% and 68%, respectively, in chemoradiation-treated patients, further validating the role of chemoradiation in patients with GEJ tumors.13,21 The addition of radiation did not appear to increase postoperative mortality in contemporary studies, which evaluated differences in surgical morbidity and mortality between surgery only and preoperative chemoradiation plus surgery. Whereas the POET study reported a numerical increase in in-hospital mortality among patients who received preoperative radiation therapy (10.2% vs 3.8%), this did not reach statistical significance (P = .26).21 The median length of hospital stay did not differ between groups. Furthermore, 2 studies, including the landmark CROSS study, did not report an increase in morbidity or mortality in the chemoradiation group compared with the surgery group.13,14 Table 15,7,11–13,21–23 outlines contemporary trials of preoperative and perioperative chemotherapy and preoperative chemoradiation.
TABLE 1.
Contemporary Trials: Preoperative/Perioperative Therapy in Locally Advanced Gastroesophageal Junction Cancer
| Trial (Reference) | Treatment | No. of Patients | GEJ, % | Complete Resection Rate, % | Pathologic No Status, % | Pathologic Complete Response Rate, % | Survival |
Local Failure, % | |
|---|---|---|---|---|---|---|---|---|---|
| Median, mo | Overall, % | ||||||||
| MAGIC (Cunningham 20065)a | Peri-op ECF + surgery | 250 | 11.2 | 69 | 31 | 0 | 24 | 5-y, 36 | 14 |
| Surgery | 253 | 11.9 | 66 | 27 | NA | 20 | 5-y, 23 | 21 | |
| STO3 (Cunningham 201723)a | Peri-op ECX + Bev + surgery | 530 | 51 | 61b | NR | 11 | NR | 3-y, 48 | NR |
| Peri-op ECX + surgery | 533 | 50 | 64 | NR | 8 | NR | 3-y, 50 | NR | |
| OEO5 (Alderson 201711) | Pre-op ECX (×4) + surgery | 446 | 67 | 66 | 39 | 7 | 26.1 | 3-y, 42 | 10 |
| Pre-op 5FU/Cis (×2) + surgery | 451 | 67 | 59 | 30 | 1 | 23.4 | 3-y, 39 | 13 | |
| FLOT4-AIO (Al-Batran 201712)a | Peri-op FLOT + surgery | 356 | 56 | 84 | 49 | 16c | 50 | 3-y, 57 | NR |
| Peri-op ECF/ECX + surgery | 360 | 56 | 77 | 41 | 6c | 35 | 3-y, 48 | NR | |
| CROSS (Shapiro 2015,7 van Hagen 201213)d | Pre-op CRT with Carbo/paclitaxel + surgery | 178 | 22 | 92 | 69 | 29 | 49 | 5-y, 47 | 22 |
| Surgery | 188 | 26 | 69 | 26 | N/A | 24 | 5-y, 33 | 38 | |
| POET (Stahl 2009,21 201722) | Pre-op 5FU/Cis → Pre-op CRT with Cis/Etop + surgery | 60 | 100 | 72 | 64 | 16 | 31 | 5-y, 40 | 21 |
| Pre-op 5FU/Cis + surgery | 59 | 100 | 70 | 37 | 2 | 21 | 5-y, 24 | 38 | |
Abbreviations: 5FU, 5-fluorouracil; Bev, bevacizumab; Carbo, carboplatin; Cis, cisplatin; pre-op CRT, preoperative chemoradiation. ECF, epirubicin, cisplatin, 5FU; ECX, epirubicin, cisplatin, capecitabine; Etop, etoposide; FLOT, 5-fluorouracil, oxaliplatin, docetaxel; GEJ, gastroesophageal junction; NA, not applicable; NR, not reported; Pre-op, preoperative; peri-op, peri-operative.
The study included gastric and esophageal/GEJ cancers.
The rate varied by tumor location (69% of patients with esophageal/GEJ cancers vs 87% of those with gastric cancers achieved R0).
Results from the phase II trial.
The study included adenocarcinoma and squamous cell carcinoma.
Numbers in bold indicate statistically significant values.
Metabolic Imaging to Guide Preoperative Therapy
FDG-PET imaging is emerging as a tool to assess response to treatment in GEJ and esophageal adenocarcinoma. Over a decade ago, the MUNICON investigators showed that, in patients with locally advanced GEJ adenocarcinomas who underwent FDGPET after 2 weeks of induction 5-FU and cisplatin, PET responders (defined as a ≥35% reduction in the standard uptake value [SUV] between baseline and repeat PET) had an improved prognosis versus nonresponders.26 In that study, PET responders continued with 12 more weeks of chemotherapy before surgery, whereas PET nonresponders underwent upfront surgery. The outcomes in PET nonresponders were similar to outcomes of PET nonresponders in an earlier trial, in which patients completed 3 months of planned preoperative chemotherapy despite nonresponse, suggesting that outcomes were not affected by immediate surgery.27 These results also suggest that discontinuing a likely inactive chemotherapy regimen and proceeding to surgery did not compromise outcome. Studies have also explored whether a suboptimal metabolic response can be overcome by a change in treatment. In MUNICON 2, PET nonresponders to 5-FU and cisplatin were treated with “salvage” chemoradiation plus cisplatin before surgery.28 These patients had inferior 2-year PFS and a trend toward poorer 2-year OS, suggesting underlying unfavorable biology. Yet, “salvage” therapy was likely suboptimal, because cisplatin was administered with low-dose radiation (32 Gy) despite an initial nonresponse to 5-FU and cisplatin induction therapy. The previously described CALGB 80803 study evaluated whether changing to alternative chemotherapy during chemoradiation based on response to induction chemotherapy, as assessed by PET, may lead to improved pCR rates.18,19 In preliminary reporting, patients who were PET nonresponders and changed chemotherapy regimens had pCR rates of 17% to 19%, meeting the primary endpoint of improving the pCR rate from a historic control rate of 3%. The median OS was 47.3 months in PET responders versus 28.9 months in PET nonresponders (P = .09). When examined in the context of historic controls, the strategy of leveraging PET nonresponse to optimize the chemotherapy regimen during radiation appears to improve outcomes.
Definitive Chemoradiation and Postoperative Strategies
Patients with adenocarcinoma have lower rates of pCR than those with SCC after chemoradiation, and there are no randomized data supporting definitive chemoradiation in these patients. However, in patients with substantial operative risk who obtain a clinical complete response to chemoradiation, close surveillance may be considered.
Although most patients with locally advanced disease receive preoperative therapy in the United States, postoperative chemoradiation remains a standard of care for GEJ/gastric cancers, based on the Intergroup 116 study. This study randomized patients (20% with GEJ tumors) who had resected, stage ≥IB disease to either adjuvant chemoradiation with bolus 5-FU and leucovorin or observation.29 Chemoradiation was associated with significantly improved 3-year OS (51% vs 40%; P = .005). The largest impact of chemoradiation was a reduction in local recurrence, which potentially compensated for suboptimal surgery (54% did not undergo D1/D2 resections). The CALGB 80101 study evaluated more intensive chemotherapy in 546 patients with gastric cancer (30% had GEJ or proximal tumors).30 Patients were randomized to bolus 5-FU and leucovorin or ECF before and after postoperative chemoradiation with infusional 5-FU. There was no improvement in 5-year disease-free survival or OS with ECF versus 5-FU and leucovorin.
The role of adding postoperative radiotherapy to perioperative chemotherapy was recently addressed in the CRITICS trial, which compared perioperative ECX/EOX (epirubicin, oxaliplatin, capecitabine) versus preoperative ECX/EOX and adjuvant chemoradiation with capecitabine in 788 patients with gastric and GEJ (17% of patients) adenocarcinoma. Preliminary results demonstrated no difference in PFS or 5-year OS, suggesting that adjuvant chemoradiation is not warranted in patients who have received preoperative chemotherapy.31
Postoperative chemotherapy is a standard of care in East Asia based on large phase 3 trials in patients with gastric carcinoma. The Japanese ACTS-GC study evaluated 1 year of adjuvant S-1 versus observation in patients with stage II/III gastric cancer who had undergone D2 resection.32 The CLASSIC trial randomized patients to either postoperative capecitabine and oxaliplatin for 6 months or observation.33 These studies enrolled >2000 patients combined and found an OS benefit of 10% and 9%, respectively. However, the studies enrolled a minority of patients with GEJ tumors, because most tumors in East Asia occur in the distal stomach. It is unclear whether the data from these studies can be extrapolated to patients with GEJ tumors. The NCCN guidelines include adjuvant capecitabine and oxaliplatin as an option for patients with lymph node–positive esophageal/GEJ tumors not treated with preoperative therapy. Recently, preliminary results of the Japanese JACCRO GC-07 trial were presented.34 Patients with stage III gastric cancer (n = 925; 23% with upper gastric tumors) who underwent D2 gastrectomy were randomized to receive either adjuvant S-1 or S-1 plus docetaxel. Three-year relapse-free survival was significantly improved in patients treated with S-1/docetaxel (65.9% vs 49.5%; HR, 0.63). In subgroup analysis, patients with upper gastric tumors also benefitted from the addition of docetaxel to S-1. Again, the applicability of these results to the US population is unclear. On the basis of the potential benefit of combination versus single-agent adjuvant chemotherapy, particularly in lymph node–positive disease, in some centers, adjuvant monotherapy with S-1 is reserved for earlier stage or lymph node–negative patients.
Future Directions in the Treatment of Locally Advanced Disease
Targeted therapies have yet to be incorporated into the management of locoregional GEJ cancers. The antivascular endothelial growth factor-A (anti-VEGF-A) antibody bevacizumab and the anti-epidermal growth factor receptor (anti-EGFR) antibody cetuximab did not improve outcomes when added to perioperative chemotherapy or definitive chemoradiation, respectively.23,35 The Radiation Therapy Oncology Group 1010 study (clinicaltrials.gov identifier NCT01196390) evaluated the addition of human epidermal growth factor receptor 2 (HER2)-directed therapy to preoperative chemoradiation, and for 9 months after surgery, in patients with esophageal/GEJ adenocarcinoma; results are awaited. Immune checkpoint inhibitors (antiprogrammed death [PD]-1 and anti–PD ligand-1 (anti–PD-L1) antibodies are also being evaluated. The CheckMate-577 study (NCT02743494) is randomizing patients with stage II/III esophageal/GEJ carcinoma who have residual pathologic disease after preoperative chemoradiation, to adjuvant nivolumab or placebo. KEYNOTE-585 (NCT03221426) is evaluating pembrolizumab plus perioperative chemotherapy in patients with gastric/GEJ adenocarcinoma.
Ongoing comparative studies of perioperative chemotherapy versus chemoradiation may clarify the optimal multimodal treatment strategy. ESOPEC (NCT92509286) and Neo-AEGIS (NCT01726452) are comparing chemoradiation as per the CROSS study versus FLOT and ECF/ECX, respectively, in patients with locally advanced esophageal/GEJ adenocarcinoma. TOPGEAR (NCT01924819) is randomizing patients to receive either perioperative ECF or 2 cycles of ECF followed by fluoropyrimidine-sensitized radiation followed by surgery and 3 cycles of ECF. Both Neo-AEGIS and TOPGEAR were modified to allow the substitution of FLOT for ECF/ECX. Finally, the PROTECT study (NCT02359968) is comparing chemoradiation with carboplatin and paclitaxel versus chemoradiation with FOLFOX.
TREATMENT OF METASTATIC DISEASE
As a single agent, 5-FU results in response rates (RRs) of 15% to 20% and has been the cornerstone of chemotherapy in EGC.36 When combined with other cytotoxics, RR and time to progression improve, although with increased toxicity.37 Although there are global variations, the standard doublet in the first-line setting is a fluoropyrimidine and platinum doublet.38 In the United States, the most widely accepted regimen is modified FOLFOX, which has shown similar outcomes and improved toxicity compared with 5-FU and cisplatin in a phase 3 study in patients with advanced EGC.39 FOLFOX has an RR of approximately 40% and median PFS and OS of about 6 and 11 months, respectively.39–41
Intensifying treatment by adding a third drug is controversial. In the United Kingdom, the addition of an anthracycline to platinum and fluoropyrimidine is a standard of care.42 The CALGB 80403 phase 2 study demonstrated similar response rates and OS, with less toxicity, using FOLFOX compared with ECF.41 Patients received cetuximab in that study, and it was not designed to assess the noninferiority of FOLFOX versus ECF. In a French study, FOLFIRI (5-FU, leucovorin, irinotecan) was comparable to ECX, with similar RR, PFS, and OS in both groups.43 The only 3-drug regimen that has demonstrated superiority in a phase 3 study is DCF (docetaxel, cisplatin, and 5-FU).44 DCF resulted in modestly increased RR and OS over cisplatin/5-FU but was associated with significant toxicity. An alternative dose and schedule was associated with reduced toxicity, and this may be an option in patients with excellent performance status.45 Despite the absence of phase 3 data, the triplet FLOT regimen used in preoperative chemotherapy is also frequently used in Europe to treat metastatic disease.
The only US Food and Drug Administration (FDA)–approved additional therapy in the first-line setting is trastuzumab, which led to improved outcomes in the phase 3 ToGA study when combined with a fluoropyrimidine and platinum doublet in patients with HER2-positive, advanced EGC.46 Studies of other anti-HER2 agents, including lapatinib and pertuzumab, in the first-line setting did not show benefit when added to combination chemotherapy or chemotherapy plus trastuzumab, respectively.47,48 Recent results from the phase 3 RAINFALL study found that the addition of ramucirumab to chemotherapy modestly improved the primary endpoint of PFS (P = .011), with no improvement in RR or OS.40 On the basis of these results, this agent will not move forward in first-line therapy. Bevacizumab also failed to improve outcomes when added to first-line therapy in the phase 3 AVAGAST trial.49 Moreover, the anti-EGFR therapies cetuximab and panitumumab have not improved outcomes when combined with first-line chemotherapy in phase 3 trials.50,51 Recently, rilotumumab, which selectively targets the ligand of the MET receptor tyrosine kinase, hepatocyte growth factor (HGF), did not demonstrate a survival benefit when added to ECX in patients with advanced, MET-positive gastric/GEJ adenocarcinoma.52
Several phase 3 studies have demonstrated a benefit for second-line chemotherapy. Single-agent irinotecan or taxane (docetaxel or paclitaxel) are associated with a modest improvement in median OS over BSC alone,53,54 with no apparent difference in efficacy between irinotecan versus taxane.53,55 Two phase 3 studies have demonstrated an OS benefit for ramucirumab, either as monotherapy (REGARD)56 or in combination with paclitaxel (RAINBOW).57 Ramucirumab combined with paclitaxel improved RR (28% vs 16%; P = .0001), PFS, and OS (9.6 vs 7.4 months; P = .017) versus paclitaxel alone. On the basis of these studies, ramucirumab was FDA approved as monotherapy or in combination with paclitaxel in this setting. In HER2-positive disease, second-line Ado-trastuzumab emtansine (TDM-1) versus taxane and lapatinib plus paclitaxel versus paclitaxel alone both failed to improve OS.58,59
Third-Line Setting and Beyond
The phase 3 TAGS study evaluated trifluridine and tipiracil (TAS-102) versus placebo in patients with refractory gastric cancer who had received ≥2 prior regimens.60 TAS-102 improved median OS (5.7 vs 3.6 months; P = .003) and 12-month OS (21% vs 13%) versus placebo and was recently approved by the FDA in this setting. VEGF tyrosine kinase inhibitors have also been evaluated in phase 2 and 3 trials in the refractory disease setting. Apatinib improved OS modestly versus BSC in a Chinese phase 3 trial,61 regorafenib demonstrated a modest improvement PFS versus placebo in a randomized phase 2 study,62 and phase 3 trials of these therapies are ongoing in patients with refractory GEJ/gastric carcinoma (NCT03042611 and NCT02773524).
Immune checkpoint inhibitors have been evaluated at rapid pace in EGC. The ATTRACTION-2 and KEYNOTE-059 studies confirmed the activity of the anti–PD-1 antibodies, nivolumab and pembrolizumab, respectively, in the chemorefractory setting.63,64 The ATTRACTION-2 study was a phase 3 study that randomized East Asian patients to nivolumab or placebo. The phase 2 KEYNOTE-059 study evaluated pembrolizumab in Western patients. Both studies reported almost identical RRs (11.2% and 11.6%, respectively), and modest improvements in PFS and OS were observed in patients treated with nivolumab in ATTRACTION-2. Patients with GEJ tumors obtained similar benefit to patients with gastric cancer in these studies. The 12-month OS was 26.2% and 23.4% in ATTRACTION-2 and KEYNOTE-059, respectively. Pembrolizumab is approved in the United States for patients with advanced gastric/GEJ adenocarcinoma whose tumors express PD-L1 and who have received ≥2 chemotherapy regimens. Nivolumab is approved in Japan, regardless of PD-L1 status.
The phase 1/2 CheckMate-032 study evaluated the safety and efficacy of nivolumab alone or in combination with ipilimumab in 2 dosing schedules for patients with chemorefractory esophagogastric adenocarcinoma.65 Almost one-half of patients enrolled had GEJ adenocarcinoma. The highest RR, 24%, was observed in patients treated with 1 mg/kg of nivolumab plus 3mg/kg of ipilimumab (NIVO1 + IPI3). In comparison, the RR was 12% in patients treated with nivolumab 3 mg/kg (NIVO3) and 8% with 3 mg/kg nivolumab plus 1 mg/kg of ipilimumab (NIVO3 + IPI1). The RR was 40% in PD-L1–positive patients in the NIVO1 + IPI3 cohort, which is the highest RR reported with immune checkpoint inhibitors in EGC. The 12-month OS was 39%, 35%, and 24% in the NIVO3, NIVO1 + IPI3, and NIVO3 + IPI1 arms, respectively. The highest rate of grade ≥3 toxicity occurred in the NIVO1 + IPI3 group (47%). Similar 12-month and 18-month OS rates between the NIVO3 and the NIVO1 + IPI3 arms, despite a numerically higher RR with combination therapy, may be partly explained by a high rate of microsatellite instability (MSI-high) tumors in the NIVO3 group.
Taken together, these studies confirm modest activity for immunotherapy in esophagogastric adenocarcinoma. Three phase 3 trials have compared anti–PD-1/PD-L1 antibodies with chemotherapy. KEYNOTE-061 did not show a greater benefit for pembrolizumab versus paclitaxel in the second-line setting in patients who had a PD-L1 combined positive score (CPS) >1.66 However, there was a suggestion of a potential greater benefit in patients with a CPS ≥5 and ≥10 in a post hoc analysis. Furthermore, patients with GEJ tumors also appeared to benefit (HR, 0.61), whereas patients with gastric tumors did not. The JAVELIN 300 study evaluated avelumab versus physician’s-choice paclitaxel or irinotecan in patients who had received ≥2 lines of chemotherapy.67 Avelumab did not improve OS versus chemotherapy, and no benefit was seen in a subgroup analysis of PD-L1–positive patients (≥1% of tumor cells). Finally, preliminary results of the phase 3 KEYNOTE-181 study evaluating second-line pembrolizumab versus physician’s-choice docetaxel or irinotecan in patients with advanced esophageal/GEJ carcinoma (64% SCC) show that pembrolizumab significantly improved RR, median OS (9.3 vs 6.7 months; HR, 0.69; P = .0074), and 12-month OS (43% vs 20%) in patients whose tumors expressed PD-L1 CPS ≥10 (n = 222).68 There was no difference in PFS or OS in the intention-to-treat population. Combined, these studies suggest that pembrolizumab is not superior to chemotherapy in an unselected population. Table 264–69 outlines the studies evaluating immune checkpoint inhibitors in the metastatic setting that have been completed to date.
TABLE 2.
Completed Immunotherapy Trials for Metastatic Esophagogastric Cancer
| Trial: Phase (Reference) | Treatment | Prior Lines | No. of Patientsa | ORR, % | Median PFS, mo | Median OS, mo | 1-Year OS, % |
|---|---|---|---|---|---|---|---|
| ATTRACTION-2 (Kang 201763) | |||||||
| Phase 3 | Nivo 3 mg/kg | ≥2 | 330 (9% GEJ) | 11.2 | 1.6 | 5.3 | 26 |
| KEYNOTE-059 (Fuchs 201864) | |||||||
| Phase 2 | Pembro | ≥2 | 259 (51% GEJ) | 11.6 | 2.0 | 5.6 | 23 |
| PD-L1–positive, 148 | 15.5 | 5.8 | |||||
| PD-L1–negative, 109 | 6.4 | 4.9 | |||||
| CheckMate-032 (Janjigian 201865) | |||||||
| Phase 1/2 | Nivo 3 mg/kg | ≥2 | 59 (53% GEJ) | 12.0 | 1.4 | 6.2 | 39b |
| PD-L1–positive, 16 | 19.0 | ||||||
| PD-L1–negative, 26 | 12.0 | ||||||
| Nivo 1 mg/kg + Ipi 3 mg/kg | ≥2 | 49 (39% GEJ) | 24.0 | 1.4 | 6.9 | 35 | |
| PD-L1–positive, 10 | 40.0 | ||||||
| PD-L1–negative, 32 | 22.0 | ||||||
| Nivo 3 mg/kg + Ipi 1 mg/kg | ≥2 | 52 (48% GEJ) | 8.0 | 1.6 | 4.8 | 24 | |
| PD-L1–positive, 13 | 23.0 | ||||||
| PD-L1–negative, 30 | 0.0 | ||||||
| KEYNOTE-061: Phase 3 (Shitara 201866) |
Pembro | 1 | 296 (30% GEJ) | 16.0 | 1.5 | 9.1 | 40 |
| [PD-L1 CPS ≥1, 196] | |||||||
| Paclitaxel | 296 (32% GEJ) | 14.0 | 4.1 | 8.3 | 27 | ||
| [PD-L1 CPS ≥1, 199] | |||||||
| JAVELIN Gastric 300: Phase 3 (Bang 201867) |
Avelumab | ≥2 | 185 (34% GEJ) | 2.2 | 1.4 | 4.6 | NR |
| PD-L1–positive, 46 | 4.3 | 1.4 | 4.0 | ||||
| PD-L1–negative, 111 | 1.8 | 1.4 | 4.6 | ||||
| Chemo | ≥2 | 186 (26% GEJ) | 4.3 | 2.7 | 5.0 | NR | |
| PD-L1–positive, 39 | 5.1 | 2.8 | 4.5 | ||||
| PD-L1–negative, 121 | 5.0 | 2.7 | 5.3 | ||||
| KEYNOTE-181: Phase 3 (Kojima 201968)c |
Pembro | 1 | 222 | 21.5 | 3.0 | 9.3 | 43 |
| PD-L1 CPS ≥10, 107 | |||||||
| Chemo | PD-L1 CPS ≥10, 115 | 6.1 | 2.6 | 6.7 | 20 |
Abbreviations: Chemo, physician’s-choice chemotherapy; CPS, combined positive score; GEJ, gastroesophageal junction; Ipi, ipilimumab; Nivo, nivolumab; NR, not reported; ORR, overall response rate; PD-L1, programmed-death ligand 1; Pembro, pembrolizumab.
Percentages of patients who had GEJ tumors are highlighted in bold where values were available.
Seven patients had microsatellite instability-high tumors.
The trial included adenocarcinoma and squamous cell carcinoma.
Along with HER2 and PD-L1 testing, MSI or mismatch-repair deficiency testing is now standard in patients with newly diagnosed, locally advanced or metastatic EGC to identify those who are candidates for PD-1 inhibitors. In the first tissue-site–agnostic FDA approval, pembrolizumab is approved for patients with unresectable/metastatic MSI-high solid tumors that have progressed on 1 prior standard therapy. MSI status was reported in KEYNOTE-059, KEYNOTE-061, and the CHECKMATE-032 study. In KEYNOTE-059, 4% of patients who underwent MSI testing had MSI-high tumors, and the ORR in this group was 57.1%.64 In CHECKMATE-032, 11 patients had MSI-high tumors. Four patients (36%) had an objective response. In KEYNOTE-061, 25 patients (4%) had MSI-high tumors, and the ORR to pembrolizumab in these patients was 47%.66 In an exploratory post hoc analysis from ATTRACTION-2, 28% (n = 136) of patients had their tumors tested for MSI, and 4 (3%) were MSI-high.69 Given the small number of patients, no correlation with MSI and outcomes to nivolumab were identified. None of these studies reported the primary location of MSI-high tumors (gastric vs GEJ); however, in The Cancer Genome Atlas (TCGA) analyses of gastric adenocarcinomas and esophageal/GEJ adenocarcinomas, very few GEJ tumors were MSI-high, and it is expected that most patients with MSI-high status across these 3 studies had primary gastric tumors.70,71
Future Directions in the Treatment of Metastatic Disease
Greater understanding of molecular networks and their interactions may lead to the emergence of new therapeutic strategies in GEJ cancers. TCGA analyzed 295 primary gastric adenocarcinomas and identified 4 molecular subtypes. The chromosomal instability (CIN) subtype accounted for almost 95% of esophageal and GEJ adenocarcinomas.70 CIN tumors frequently have amplification of receptor tyrosine kinases, such as EGFR, HER2, FGFR, and MET, and have frequent mutation of tumor suppressor genes, such as TP53, ARID1A, and SMAD4, and cell cycle pathways, including CDKN2A. More recent data show that esophageal and GEJ adenocarcinomas resemble the CIN variant of gastric adenocarcinoma but display a higher frequency of DNA hypermethylation.71
Novel treatments that target specific molecular alterations are undergoing evaluation. Zolbetuxiumab is a CLDN 18.2-specific antibody. CLDN 18.2 is a major structural component of tight junctions and is expressed in 80% to 90% of gastric cancers, but not in most healthy tissue, and this agent may stimulate antibody-dependent and complement-dependent cellular cytotoxicity. On the basis of promising data from the randomized phase 2 FAST study of EOX chemotherapy with or without zolbetuximab,72 a randomized phase 3 study of FOLFOX with or without zolbetuximab (NCT03504397) is recruiting patients with metastatic GEJ/gastric adenocarcinoma with CLDN 18.2 immunohistochemistry scores of 2+/3+ in ≥75% of cells (expected in 30%−40% of patients).
Currently, there is no approved HER2-directed therapy beyond first-line trastuzumab. DS-8201a is a novel antibody-drug conjugate of an HER2 antibody bound to a topoisomerase inhibitor. A phase 1 study enrolling multiple HER2-expressing solid tumors included 44 patients with GEJ/gastric adenocarcinoma who previously received treatment with trastuzumab. The RR was 43%, with a disease control rate of 80%. The median PFS was 5.6 months.73 The DESTINY-Gastric 01 study (NCT03329690) is an East Asian phase 2 study evaluating DS-8201a versus physician’s-choice chemotherapy in patients with HER2-expressing, advanced gastric/GEJ adenocarcinoma who have received 2 prior therapies.
Studies evaluating first-line chemotherapy in combination with immunotherapy are at an advanced stage. The KEYNOTE-062 study of fluoropyrimidine and cisplatin with or without pembrolizumab (NCT02494583) has completed accrual, and Checkmate 649 (NCT02872116) is evaluating FOLFOX chemotherapy versus FOLFOX and nivolumab. An ipilimumab plus nivolumab arm closed after an interim analysis. Examples of other combination strategies include the FRACTION-Gastric Cancer (NCT02935634) adaptive-design, phase 2 study and the MORPHEUS-Gastric Cancer study (NCT03281369).
Debate continues regarding the role of PD-L1 as a biomarker, and novel biomarkers are undergoing evaluation. In KEYNOTE-059, an 18-gene, T-cell–inflamed, gene expression signature was associated with improved response to pembrolizumab.64 Elevated tumor mutation burden has been shown to predict response to checkpoint inhibitors across several tumor types.74 Prospective studies should evaluate its role as a biomarker in EGC. The Epstein-Barr virus (EBV) and MSI-high TCGA subtypes have elevated tumor mutation burden and, in a recent study, the RR to pembrolizumab was 100% and 86% in the EBV and MSI-high groups, respectively, and the RR was only 12% and 5%, respectively, in those who had the genomically stable and chromosomally unstable subtypes.75 Patients should be stratified by MSI/EBV status to better understand the efficacy of immunotherapy in microsatellite-stable patients.
Conclusions
The management of GEJ cancer is complex. The appropriate workup and treatment by stage are outlined in Figure 1. Preoperative chemotherapy or chemoradiation improves outcomes compared with surgery alone. Perioperative FLOT is a new standard of care in the treatment of locally advanced GEJ adenocarcinoma. However, chemoradiation results in higher R0 resection rates, pCR rates, and lymph node–negative status. We await subgroup analysis from the FLOT study regarding the complete resection rate in patients with GEJ adenocarcinoma. In advanced disease, combination fluoropyrimidine and platinum remains standard in the first-line setting. Incremental improvements in outcomes have occurred with the use of first-line trastuzumab in HER2-positive disease and the anti-VEGF antibody, ramucirumab, in the second-line setting. Immune checkpoint inhibitors are approved in the chemorefractory setting in PD-L1–positive patients and have an emerging role in earlier lines of therapy in patients with high PD-L1 CPS scores. Recent advances in PET-adapted therapy, molecular targeting and immuno-oncology may usher in a new wave of multimodality approaches to improve outcomes.
Figure 1.
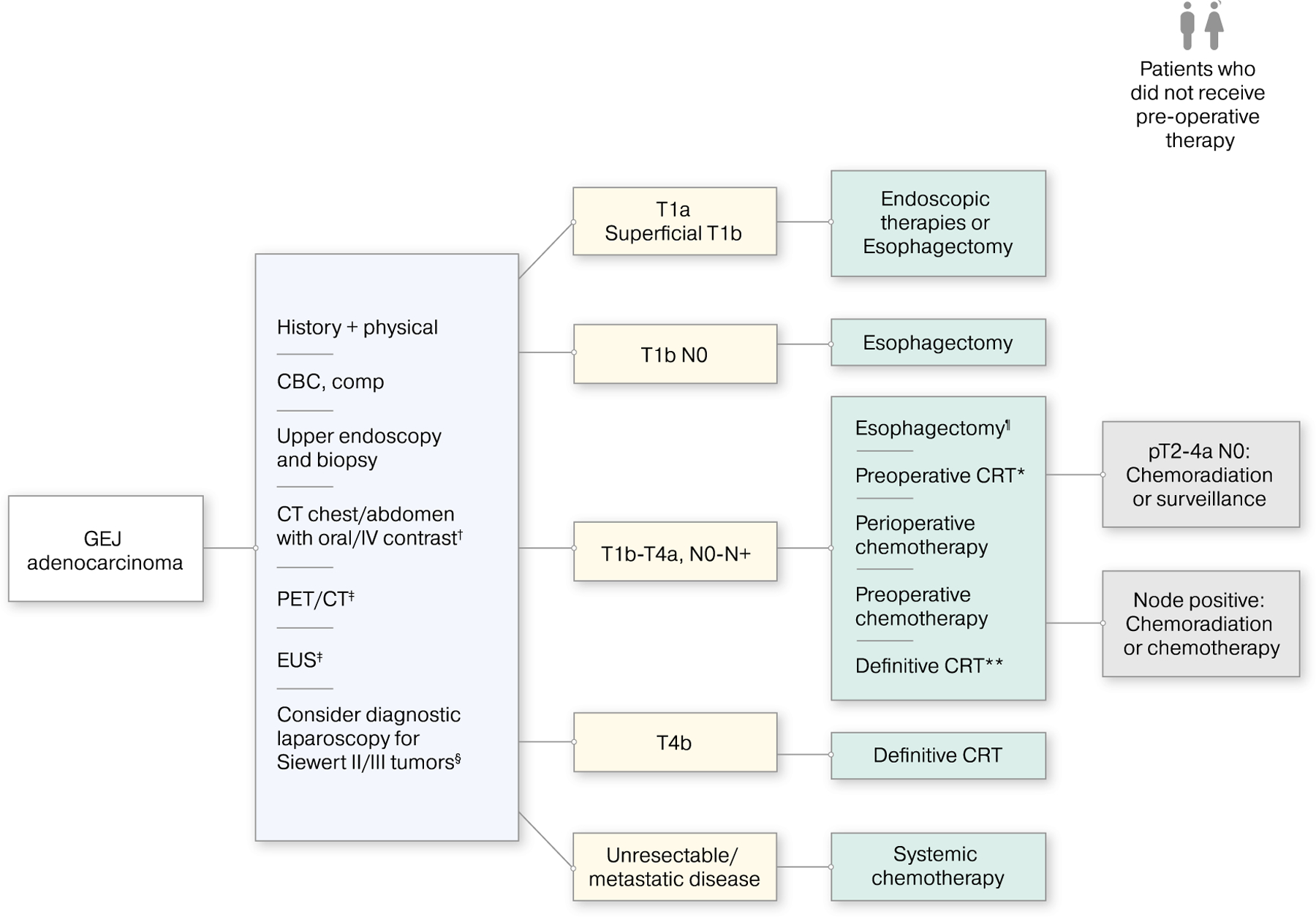
The appropriate workup and treatment by disease stage are illustrated for patients with gastroesophageal junction (GEJ) cancer. †Patients may undergo pelvis computed tomography (CT) with contrast as clinically indicated. ‡If the initial workup demonstrates no evidence of metastatic disease, then patients undergo positron-emission tomography (PET)/CT and endoscopic ultrasound (EUS) studies. §Additional evaluations may be required to assess the patient’s ability to tolerate major surgery, including pulmonary function testing, cardiac testing, and nutritional assessment. Among patients who have T1B-T4a, N0-N+ disease, possible treatments include ¶esophagectomy (for those with T1b-T2, N0, low-risk lesions [<2 cm, well differentiated]), *preoperative chemoradiation (CRT) (preferred option), and **definitive CRT (for those who decline or are not candidates for surgery). CBC indicates complete blood count; comp, comprehensive chemistry profile; pT, pathologic tumor classification.
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
David. H. Ilson is a consultant/advisory board member for Taiho, Pieris, Roche, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Merck, and Astellas. The remaining other authors report no conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol 2017;112:1247–1255. [DOI] [PubMed] [Google Scholar]
- 4.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416–2422. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 6.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–1721. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 8.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979–1984. [DOI] [PubMed] [Google Scholar]
- 9.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27: 5062–5067. [DOI] [PubMed] [Google Scholar]
- 10.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Batran SE, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and f luorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and f luorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial [abstract]. J Clin Oncol 2017;35(15 suppl):4004. [Google Scholar]
- 13.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 14.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659–668. [DOI] [PubMed] [Google Scholar]
- 15.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 16.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 17.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer [abstract]. J Clin Oncol 2017;35(4 suppl):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman KA, Hall N, Bekaii-Saab TS, et al. Survival outcomes from CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer [abstract]. J Clin Oncol 2018;36(4 suppl):4012. [Google Scholar]
- 20.Mukherjee S, Hurt CN, Gwynne S, et al. NEOSCOPE: a randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based preoperative chemoradiation for resectable oesophageal adenocarcinoma. Eur J Cancer 2017;74:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851–856. [DOI] [PubMed] [Google Scholar]
- 22.Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183–190. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol 2017;18:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homann N, Pauligk C, Luley K, et al. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer 2012;130:1706–1713. [DOI] [PubMed] [Google Scholar]
- 25.Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council adjuvant gastric infusional chemotherapy trial. J Clin Oncol 2016;34:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797–805. [DOI] [PubMed] [Google Scholar]
- 27.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2008;24:4692–4698. [DOI] [PubMed] [Google Scholar]
- 28.zum Buschenfelde CM, Herrmann K, Schuster T, et al. F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011;52:1189–1196. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725–730. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (Alliance). J Clin Oncol 2017;35:3671–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheij M, Jansen EP, Cats A, et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: first results from the CRITICS Study [abstract]. J Clin Oncol 2016;34(15 suppl):4000.27646943 [Google Scholar]
- 32.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387–4393. [DOI] [PubMed] [Google Scholar]
- 33.Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389–1396. [DOI] [PubMed] [Google Scholar]
- 34.Kodera Y, Yoshida K, Kochi M, et al. A randomized phase III study comparing S-1 plus docetaxel with S-1 alone as a postoperative adjuvant chemotherapy for curatively resected stage III gastric cancer (JACCRO GC-07 trial) [abstract]. J Clin Oncol 2018;36(4 suppl):4007. [Google Scholar]
- 35.Suntharalingam M, Winter K, Ilson D, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG Oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 2017;3:1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cocconi G, DeLisi V, Di Blasio B. Randomized comparison of 5-FU alone or combined with mitomycin and cytarabine (MFC) in the treatment of advanced gastric cancer. Cancer Treat Rep 1982; 66:1263–1266. [PubMed] [Google Scholar]
- 37.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric Cancer. Crit Rev Oncol Hematol 2009;71:127–164. [DOI] [PubMed] [Google Scholar]
- 38.Ku GY, Ilson DH. Management of gastric cancer. Curr Opin Gastroenterol 2014;30:596–602. [DOI] [PubMed] [Google Scholar]
- 39.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435–1442. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs CS, Shitara K, Bartolomeo MD, et al. RAINFALL: a randomized, double-blind, placebo-controlled phase III study of cisplatin (Cis) plus capecitabine (Cape) or 5FU with or without ramucirumab (RAM) as first-line therapy in patients with metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(4 suppl):5. [Google Scholar]
- 41.Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: a randomized phase II study of 3 chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol 2016;34:2736–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358: 36–46. [DOI] [PubMed] [Google Scholar]
- 43.Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–3526. [DOI] [PubMed] [Google Scholar]
- 44.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–4997. [DOI] [PubMed] [Google Scholar]
- 45.Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol 2015;33:3874–3879. [DOI] [PubMed] [Google Scholar]
- 46.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 47.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol 2016;34:443–451. [DOI] [PubMed] [Google Scholar]
- 48.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastrooesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19:1372–1384. [DOI] [PubMed] [Google Scholar]
- 49.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–3976. [DOI] [PubMed] [Google Scholar]
- 50.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010;102:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okines AF, Ashley SE, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol 2010;28:3945–3950. [DOI] [PubMed] [Google Scholar]
- 52.Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–1518. [DOI] [PubMed] [Google Scholar]
- 54.Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- 55.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–4444. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–39. [DOI] [PubMed] [Google Scholar]
- 57.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 58.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–653. [DOI] [PubMed] [Google Scholar]
- 59.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol 2014;32:2039–2049. [DOI] [PubMed] [Google Scholar]
- 60.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:1437–1448. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–1454. [DOI] [PubMed] [Google Scholar]
- 62.Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol 2016;34:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least 2 previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–2471. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018;36:2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123–133. [DOI] [PubMed] [Google Scholar]
- 67.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29:2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kojima T, Muro K, Francois E, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study [abstract]. J Clin Oncol 2019;37(suppl 4):2. [Google Scholar]
- 69.Kang YK, Satoh T, Chao Y, et al. Evaluation of efficacy of nivolumab by baseline factors from ATTRACTION-2 [abstract]. J Clin Oncol 2019;37(4 suppl):8. [Google Scholar]
- 70.The Cancer Genome Atlas Research Network, Bass AJ, Thorsson V, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Naturexs 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The Cancer Genome Atlas Research Network, Asan University, BC Cancer Agency, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;41:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Batran SE, Schuler MH, Zvirbule Z, et al. FAST: an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2016;34(18 suppl):LBA4001. [Google Scholar]
- 73.Iwata H, Tamura K, Doi T, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: long-term results of a large phase 1 study with multiple expansion cohorts [abstract]. J Clin Oncol 2018;36(15 suppl):2501. [Google Scholar]
- 74.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–1458. [DOI] [PubMed] [Google Scholar]


