Abstract
Enteropathogenic Escherichia coli (EPEC) produces a characteristic attaching and effacing (A/E) lesion in the small intestines of infected children. The immune response to EPEC infection remains poorly characterized. The molecular targets that elicit protective immunity against EPEC disease are unknown. In this study protein antigens from EPEC were identified using secretory immunoglobulin A (sIgA) antibodies isolated from milk from Mexican women by Western blot analysis. Purified sIgA antibodies, which inhibit the adherence of EPEC to cells, reacted to many EPEC proteins, the most prominent of which were intimin (a 94-kDa outer membrane protein) and two unknown proteins with apparent molecular masses of 80 and 70 kDa. A culture supernatant protein of 110 kDa also reacted strongly with the sIgA antibodies. The molecular size of this protein and its reactivity with specific anti-EspC antiserum suggest that it is EPEC-secreted protein C (EspC). These EPEC surface protein antigens were consistently recognized by all the different sIgA samples obtained from 15 women. Screening of clinical isolates of various O serogroups from cases of severe infantile diarrhea revealed that all EPEC strains able to produce the A/E lesion showed expression of intimin and the 80- and 70-kDa proteins. Such proteins reacted strongly with the purified sIgA pool. Moreover, nonvirulent E. coli strains were unable to generate a sIgA response. The immunogenic capacities of the 80- and 70-kDa proteins as virulence antigens have not been previously reported. The strong sIgA response to intimin and the 80- and 70-kDa proteins obtained in this study indicates that such antigens stimulate intestinal immune responses and may elicit protective immunity against EPEC disease.
Enteropathogenic Escherichia coli (EPEC) causes acute and persistent diarrhea in infants and young children mainly in underdeveloped countries (19, 37). During infection, EPEC forms small microcolonies on the surfaces of jejunal epithelial cells followed by intimate contact and localized degeneration of the epithelial brush border microvilli, resulting in an attaching and effacing (A/E) lesion (32, 50). The A/E lesion (or pedestal) is associated with the assembly of highly organized cytoskeletal structures in epithelial cells immediately beneath the adherent bacteria that include the cytoskeletal components actin, myosin light chain (17, 39), and tyrosine-phosphorylated proteins (45).
Initial adherence is associated with the production of type IV fimbriae, the bundle-forming pili (18), which are encoded on the large EPEC adherence factor (EAF) plasmid (48). The intimate adherence of the bacteria to epithelial cells is mediated by a 94-kDa outer membrane protein called intimin (the product of the eae gene), which participates in the reorganization of the underlying host cytoskeleton after other bacterial factors stimulate epithelial signal transduction (26). Intimin binding to host cells also stimulates a second wave of signal transduction inside the mammalian cell, including tyrosine phosphorylation of phospholipase C (30). Intimin was required for full virulence in volunteers in a previous study (14). Recently, Kenny et al. (31) reported that EPEC produces a protein that is transferred from the bacteria to the eukaryotic cell, where it then serves as a cell surface receptor for intimin. This protein is named Tir (translocated intimin receptor). Transfer of Tir into host cells has been shown to be dependent on the type III secretion system and at least two other proteins secreted by this system, Esp/A and Esp/B (28, 29).
One of the most striking clinical features of EPEC infections is the propensity of these enteropathogenic strains to cause disease in infants (6, 10, 19), with few reports of cases of EPEC associated with diarrhea in older children and adults (52). Infants are more likely to develop diarrhea during the first episode of colonization with EPEC than they are during subsequent encounters (11). It is not known whether the low incidence of EPEC diarrhea in older children and adults is due to acquired immunity. However, previous investigations have demonstrated that volunteers convalescing from experimental EPEC infection develop immunoglobulin G (IgG) antibodies to the O-antigen component of the lipopolysaccharide of the infecting strain, to intimin, and to type 1-like fimbriae (14, 27, 36).
Breast feeding has been found to be an important protective factor against intestinal and respiratory infections in infants (1, 23, 25, 51). Epidemiological studies indicate that breast feeding protects infants against the most important enteric pathogens (2, 21), including diarrheagenic E. coli (8, 25, 54). Reports supporting an important role for milk-derived antibodies in protection against gastrointestinal infectious diseases in humans and animals have also been published (43, 53). We have previously shown that human secretory IgA (sIgA) from breast milk inhibited localized adherence of an EPEC strain to cultured cells and that sIgA responded to a 94-kDa outer membrane protein (12). Camara et al. (4) confirmed the participation of colostrum sIgA in the inhibition of adhesion of EPEC to cells and its response to a 94-kDa protein. The aim in the present study was to search for virulence antigens expressed by EPEC strains that showed a marked ability to adhere to cells and to produce the A/E lesion. To do so, sIgA antibodies isolated from milk from Mexican women were used.
MATERIALS AND METHODS
Strains.
Some E. coli strains of various O serogroups have been described previously, and others are recent isolates from stool samples from Mexican infants with or without diarrhea. All strains were serotyped by agglutination in 96-well microtiter plates with antisera raised in rabbits against 170 somatic and 56 flagellar antigens (44).
Antibodies and antisera.
Commercially purified serum IgA was purchased from Caltag Laboratories (Omnichem S.A. de C.V. Mexico). Anti-intimin and anti-Pet (the plasmid-encoded toxin) polyclonal antisera were generously provided by Gad Frankel (34) and F. Navarro-Garcia (16), respectively. For Western blot assays the anti-intimin antiserum was diluted 1:200 in phosphate-buffered saline (PBS) and the anti-Pet antiserum was diluted 1:100 in PBS.
Milk samples.
Milk was collected in the Hospital General of Mexico City, after informed consent, from healthy, well-nourished Mexican mothers who were not receiving any medications that might interfere with lactation. For this study we selected mothers of low socioeconomic status who lived in poor sanitation conditions. The primary reason was because, in a preliminary study, we found that milk samples from these women gave stronger sIgA reactions to protein extracts from EPEC than milk samples from women who lived in good sanitation conditions (H. A. Manjarrez-Hernandez, S. Gavilanes-Parra, M. E. Chavez-Berrocal, and A. Cravioto, unpublished data). In addition, it is more likely that women living in poor sanitation conditions have been infected with EPEC. Samples were collected by manual expression into sterile glass flasks 10 to 30 days after delivery. Samples were immediately frozen and kept at −20°C until use.
Purification of sIgA antibodies from milk.
sIgA antibodies were purified as described by Mestecky and Kilian (42). Briefly, each milk sample was defatted by centrifugation at 15,000 × g at 4°C for 1 h. The clear middle layer was collected by a Pasteur pipette, which was carefully inserted through the top lipid layer. Clarified milk was then acidified with 2% acetic acid to pH 4.2, and the precipitated casein was removed by centrifugation at 15,000 × g at 4°C for 1 h. The pH of the clear supernatant was adjusted to neutrality with 0.1 M NaOH, and Igs were precipitated with saturated (NH4)2SO4 to 50% final saturation. The precipitate was collected by centrifugation, dissolved in distilled water, and dialyzed overnight at 4°C against PBS. The sample was then fractionated by chromatography in a HiLoad 16/60 Sephacryl S-200 column (Pharmacia LKB Biotechnology, Uppsala, Sweden) equilibrated in PBS. sIgA-containing fractions were pooled and concentrated by positive pressure on an Amicon YM 100 membrane. Total sIgA was measured by radial immunodiffusion (38) using commercial IgA as the standard. The purity of the sIgA preparations was analyzed on sodium dodecyl sulfate (SDS)-polyacrylamide gels.
Cell culture.
HEp-2 cells were routinely grown in plastic tissue culture flasks (Nunc, Inc.) at 37°C under 5% CO2 in Dulbecco modified Eagle medium (DMEM) containing 10% (vol/vol) fetal calf serum.
Intestinal samples.
Human duodenal and jejunal sections from the small intestines of infants and adults were obtained from the relatives of the patients. Fresh samples were flushed with cold PBS to remove intestinal contents. Segments of the small intestine were stored in cold PBS on ice until they were processed, which was within 3 h of collection.
Brush border preparation.
Human brush borders were prepared by the method described by Saxon et al. (46). For preparation of epithelial cell scrapings, which consist predominantly of villus cells, the small intestine was gently flushed with normal saline containing 1 mM dithiothreitol (DTT) at 4°C to remove the contents and slit open with scissors to expose the epithelium and cells were scraped into PBS (containing 25 μg of leupeptin, 25 μg of aprotinin, and 10 μg of pepstatin/ml; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 0.5 mM benzamidine; and 1 mM EDTA) using a glass slide and washed twice with PBS. All procedures were performed at 4°C. Epithelial cell scrapings were suspended in buffer A (5 mM EDTA, 1 mM HEPES-Tris [pH 7.5] containing PMSF, 1 mM DTT) and homogenized at 450 × g for 15 min. The pellet was resuspended in buffer A and centrifuged again at 800 × g for 15 min. The resulting brush border pellet was resuspended in a small volume of buffer B (0.09 M NaCl, 0.8 mM EDTA, 1 mM HEPES-Tris [pH 7.5] containing PMSF, 1 mM DTT) and filtered through a nylon mesh to remove aggregates. Brush borders were recovered by centrifugation for 10 min at 800 × g and washed once with fresh buffer B. The purity of the preparation and the presence of intact brush borders were confirmed by light microscopy.
Adherence assays.
Assays for adherence to HEp-2 cells were performed in the presence of 1% d-mannose as described by Cravioto et al. (9). HEp-2 cells were grown to near confluence in 24-well chamber slides in 1.0 ml of DMEM. A cell monolayer was infected with 30 ml of bacterial culture, which had been grown overnight at 37°C. After 3 h of incubation at 37°C, the cells were washed six times with PBS, fixed with methanol, stained with Giemsa, and examined by light microscopy under oil immersion. The assay for adherence of EPEC to brush borders isolated from biopsies of human intestinal epithelial cells was carried out according to the method described by Cheney et al. (7) with some modifications. The number of bacteria bound to brush borders was expressed as counts of [35S]methionine per minute (mean ± standard error of the mean) as measured by liquid scintillation counting at the end of the assay as previously described (40).
Fluorescent actin stain (FAS) test.
Actin accumulation in tissue culture cells was examined essentially by the method described by Knutton et al. (33).
Inhibition assay.
Bacterial strains were cultivated in L broth (∼109 CFU/ml), diluted 1:100 in DMEM (cell culture medium), and grown with shaking at 37°C for an additional 3 h to obtain an exponential-phase culture. An inoculum of 2 × 108 bacteria/ml was used in all experiments. To study the effect of sIgA antibodies on EPEC adherence, the assays were performed in the presence of purified sIgA antibodies at 0.5 and 1.0 mg/ml in cell culture medium with 2% fetal calf serum and 1% d-mannose. The suspension containing bacteria and sIgA antibodies was incubated in well chamber slides that contained the HEp-2 cells for 30 min (infection period) and washed with PBS after which new medium was added. After an incubation period of 3 h (multiplication period) the cells were fixed, stained, and mounted on glass slides. The degree of inhibition was determined by counting all bacteria that adhered to 200 tissue culture cells selected at random and comparing this number with that for control preparations done simultaneously in the same plates after incubation of the bacteria with PBS instead of the purified sIgA antibodies. Preparations were coded and read in the blind by two independent observers, previously standardized to a <1% difference. Each experiment was carried out in duplicate and repeated three times. Results were expressed as the percentages of adherence compared with that from binding assays run in the absence of putative inhibitors. The relation between percentages of adherence inhibition and levels of anti-EPEC sIgA was analyzed by the Spearman correlation coefficient.
Statistical analysis.
Results were expressed as means ± standard errors. Differences between two groups were determined by using the two-tailed, unpaired Student t test. A critical P value of 0.05 was used for all analysis.
SDS-PAGE.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed in 7.5% polyacrylamide slab gels, essentially as described by Laemmli (35).
Immunoblotting.
Bacterial strains were grown overnight in L broth (∼109 CFU/ml), diluted 1:100 in DMEM, and grown with shaking at 37°C for an additional 3 h. The replication of bacteria was determined by measuring culture turbidity at 600 nm. Following electrophoresis on SDS–7.5% polyacrylamide gels, the whole-cell lysates were transferred to nitrocellulose membranes (0.45-μm pore size), according to the method described by Towbin et al. (49) with some modifications. Membranes were blocked with 5% nonfat dry milk in PBS, pH 7.2, for 2 h under agitation, washed with PBS containing 0.2% Tween 20 (PBST), and incubated overnight with 1:10 or 1:200 dilutions of the pooled milk sample or 0.3 mg of the purified sIgA antibodies/ml. Following three washes with PBST, the membranes were incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated anti-human IgA and the reaction was visualized with 4-chloro-1-naphthol.
RESULTS
sIgA from human milk inhibits EPEC adhesion.
The mean number of bacteria adherent to cells in a control experiment conducted without sIgA antibodies was 812 ± 48 organisms per 200 epithelial cells (adherence, 100%). sIgA purified from milk samples obtained from 15 women markedly inhibited bacterial adhesion (strain E2348/69) to HEp-2 cells. Inhibition values produced by 0.5 and 1.0 mg of sIgA/ml varied from 51.1% ± 7.1% to 65.2% ± 5.2% and from 68.3% ± 6.9% to 87.1% ± 6.3%, respectively. The difference between the adherence of EPEC cells exposed to sIgA and that for the PBS control was statistically significant (P < 0.001). Commercially obtained serum IgA at the same concentration as the sIgA from milk (1.0 mg/ml) had no effect on the adherence of the bacteria to tissue culture cells.
Inhibition of E2348/69 adhesion by a pool of purified sIgA in human intestinal brush borders was also examined. The degree of binding of the radiolabeled EPEC strain to 25 μg of brush borders after a 1-h incubation at 37°C was expressed as counts of [35S]methionine per minute at the end of the assay. From these data, the mean number of adherent organisms was approximately 1.0 × 106 ± 6.0 × 104 per 20 μg of brush borders (control adherence, 100%). A significant percentage of inhibition of bacterial adhesion (mean, 76.8% ± 3.4%) was observed, comparable to that produced in the cultured human epithelial cell line HEp-2.
Human milk contains sIgA antibodies, which are reactive to EPEC antigens.
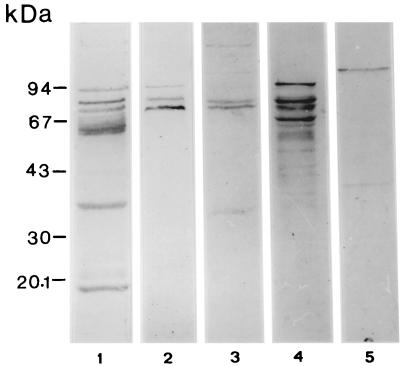
Western blot analysis shows that milk from Mexican women contains significant amounts of sIgA antibodies against proteins from the EPEC strain E2348/69. Many immunostained bands appeared when a 1:10 dilution of the pooled milk sample was used against whole-cell extracts (Fig. 1). However, diluting the milk sample 1:200 resulted in a strong reaction to only three proteins with apparent molecular masses of 94, 80, and 70 kDa (Fig. 1). Additional new bands were observed when the amounts of some bacterial antigens were increased in the Western blot assay (milk sample diluted 1:200) due to cell fractionation of the bacteria, indicating a large quantity of sIgA antibodies against a large variety of proteins from the E2348/69 strain (Fig. 1). The sIgA antibodies also reacted with a 110-kDa culture supernatant protein of E2348/69 (Fig. 1). The supernatant protein had been concentrated 20-fold by ultrafiltration.
FIG. 1.
Western blot of sIgA antibodies to EPEC (strain E2348/69) in human milk. Lanes 1 and 2, whole-cell extracts probed with pooled human milk diluted at a ratio of 1:10 (lane 1) and 1:200 (lane 2); lanes 3 and 4, bacterial surface and outer-membrane extracts probed with human milk (1:200); lane 5, culture supernatant proteins probed with human milk (1:200).
Only EPEC strains with the ability to produce the A/E lesion express the 94-, 80-, and 70-kDa proteins.
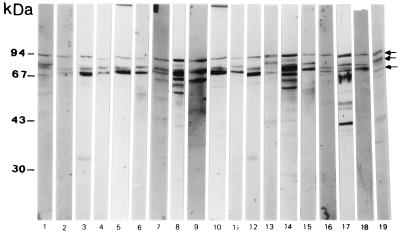
Nineteen EPEC isolates of various O serogroups from cases of severe infantile diarrhea were selected. Selection was based on their ability to adhere abundantly to HEp-2 cells with a localized pattern and to produce an A/E lesion, as indicated by a positive FAS reaction. All the selected EPEC strains tested expressed the 94-, 80-, and 70-kDa proteins, which were detected by Western blotting assay using a purified sIgA pool (Fig. 2). In contrast, 20 nonvirulent E. coli strains isolated from children without diarrhea were unable to generate an sIgA response (Table 1). These strains of diverse O serogroups showed less than 10% adherence to HEp-2 cells (strains and serotypes in Tables 1 and 2).
FIG. 2.
Screening of EPEC isolates for the ability to express the 94-, 80-, and 70-kDa proteins (arrows). A Western blot of whole-cell extracts probed with purified sIgA antibodies (obtained from a milk pool) is shown. Nineteen EPEC isolates of various O serotypes from cases of infantile diarrhea were used. Lanes 1 to 19, strains from Table 2 from top to bottom starting with strain 88255.
TABLE 1.
Nonvirulent E. coli strains unable to generate an sIgA responsea
| Strain | Serotype | sIgA response to protein of:
|
Adhesion to HEp-2 cells | ||
|---|---|---|---|---|---|
| 94 kDa | 80 kDa | 70 kDa | |||
| 89519 | O?:NM | − | − | − | None |
| 65551 | O114:H2 | − | − | − | None |
| 88303 | O26:H11 | − | − | − | None |
| 89583 | O102:NM | − | − | − | None |
| 91626 | O141:H4 | − | − | − | None |
| 89515 | O20:H16 | − | − | − | None |
| 32544 | O41:H10 | − | − | − | None |
| 65570/1 | O126:H14 | − | − | − | None |
| 66289 | O126:NM | − | − | − | None |
| 91627 | O141:H4 | − | − | − | None |
| 89541 | O126:H51 | − | − | − | None |
| 92321 | O28:NM | − | − | − | None |
| 89246 | O20:H− | − | − | − | None |
| 89468 | O16:H? | − | − | − | None |
| 89469 | O?:H10 | − | − | − | None |
| 89232 | OR:NM | − | − | − | None |
| 35307 | O77:H18 | − | ± | ± | Aggregative |
| 35479 | O77:H18 | − | − | − | Aggregative |
| 35367 | OR:H33 | − | − | − | Aggregative |
| 92294 | O111ab:H12 | − | − | − | Diffuse |
All strains were isolated from stool samples from children without diarrhea. FAS test results were negative for all samples.
Question marks, existing but unidentified O or H antigens. NM, nonmotile strain.
±, less than 10% of HEp-2 cells showed adhering bacteria.
TABLE 2.
EPEC strains with the ability to produce the A/E lesion express the 94-, 80-, and 70-kDa proteins detected with a purified sIgA poola
| Strainc | Serotype | sIgA response to protein of:
|
||
|---|---|---|---|---|
| 94 kDa | 80 kDa | 70 kDa | ||
| E2348/69b | O127:H6 | + | + | + |
| 88255 | OR:H6 | + | + | + |
| B171 | O111:NM | + | + | + |
| 88259 | O55:H6 | + | + | + |
| 29358 | O111ab:NM | ±d | + | + |
| 65570/0 | O55:H6 | + | + | + |
| E851/71b | O142:H6 | + | − | + |
| Sc80b | O86:H34 | + | ± | + |
| 47151 | O111:NM | + | + | + |
| 41141 | O111ac:NM | + | + | + |
| 65553 | O114:H2 | + | + | + |
| 65893 | O114:H2 | + | + | + |
| 65900 | O114:H2 | + | + | + |
| 47146 | O111ab:NM | + | + | + |
| 203680b | O119:H6 | + | + | + |
| 94645 | O119:H6 | + | + | + |
| E380/69b | O114:H2 | + | + | + |
| 88263 | O55:H6 | + | + | + |
| 66295 | O126:NM | + | + | − |
| 63961 | O145:H45 | + | + | + |
For all strains, the adhesion to HEp-2 cells was localized and the FAS test was positive.
From B. Rowe, Division of Enteric Pathogens, Central Public Health Laboratory, London, England.
Strains were isolated from human stool samples. Unless otherwise indicated, strains were from our collection.
±, weak reaction (faint bands).
Purified sIgA from individual milk samples recognizes proteins expressed by EPEC.
To identify which bacterial antigens were recognized more frequently by individual samples of purified sIgA, Western immunoblot assays were carried out using a protein preparation obtained from the whole E2348/69 bacteria or from the culture supernatant. Purified sIgA samples showed a response to several proteins. Analysis of these data revealed that the 94-, 80-, and 70-kDa proteins were recognized by all 15 sIgA samples. The 110-kDa protein from the culture medium in which the bacteria had been grown also reacted with all the sIgA samples. No bands were recognized by commercially obtained serum IgA. Serum IgA from three women in the areas of EPEC endemicity reacted strongly with many protein bands from EPEC in the Western blot analysis, including bands between 70 and 94 kDa (data not shown).
Conditions that affect the expression of the 94-, 80-, and 70-kDa proteins by EPEC.
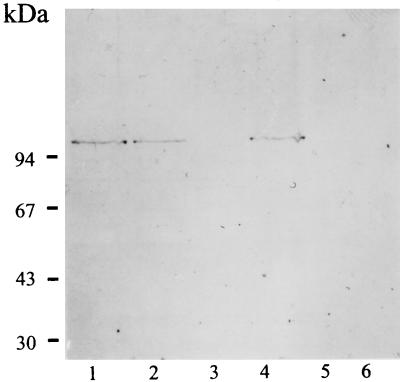
Bacterial expression of the 94-, 80-, and 70-kDa proteins was diminished significantly when strain E2348/69 was grown at room temperature (25°C) in DMEM (Fig. 3). This effect was recorded by immunostaining analysis. Growing the bacteria at this temperature also diminished the ability of E2348/69 to adhere to HEp-2 cells. A reduction of bacterial adhesion of 81.5% was observed.
FIG. 3.
Western blots of bacterial extracts. Lanes 1 and 2, whole-cell extracts, probed with an anti-intimin polyclonal antiserum, from strains E2348/69 (lane 1) and the eae gene deletion mutant CVD206 (lane 3); lanes 2 and 4, whole-cell extracts, probed with purified sIgA antibodies (obtained from a milk pool), from strain E2348/69 (lane 2) and CVD206 (lane 4); lane 5, outer-membrane extract (E2348/69) probed with anti-intimin antiserum. Arrow, intimin band.
No protein bands were detected when sIgA was incubated with preparations of EAF plasmid-cured derivative strains JPN15 and MAR20.
Anti-intimin polyclonal antiserum and sIgA antibodies reacted strongly with the same 94-kDa protein band expressed by strain E2348/69 (Fig. 3). In contrast, this antiserum and sIgA did not react with any 94-kDa protein when the eaeA (intimin) gene deletion mutant strain CVD206 was used (Fig. 3). The identity of the 94-kDa protein, recognized by the sIgA as intimin, was supported by detection of this protein in an outer-membrane preparation from E2348/69. An 80-kDa band was found also in the outer-membrane fraction (Fig. 1). The 70-kDa protein seems to be anchored on the outer leaflet of the outer membrane since this protein was easily detached from the bacterium by mechanical agitation (data not shown).
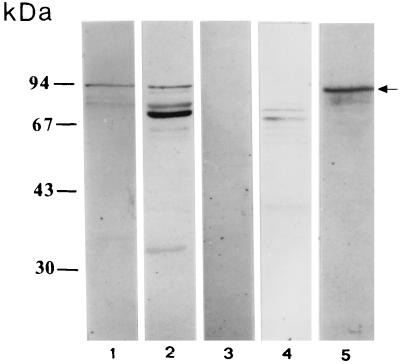
The molecular size (110 kDa) of our culture supernatant protein is very similar to that of a known secreted protein called EspC (EPEC-secreted protein C) (47); therefore we probed the 110-kDa protein with anti-EspC antiserum in a Western blot assay. The specific anti-EspC antiserum, Pet (the plasmid-encoded toxin) (16), and sIgA antibodies reacted with the same 110-kDa protein band secreted by EPEC (Fig. 4). The toxin Pet is secreted by enteroaggregative E. coli strains and shares amino acid sequences with EspC and members of the IgA protease family. Finally, the 110-kDa protein was not detected in nonvirulent E. coli strains by any of the specific antisera (anti-EspC and anti-Pet) or sIgA antibodies (Fig. 4).
FIG. 4.
Western blot of concentrated supernatant from strain E2348/69 probed with anti-EspC polyclonal antiserum (lane 1), anti-Pet polyclonal antiserum (lane 2), commercially obtained serum IgA (lane 3), and sIgA antibodies (obtained from a milk pool) (lane 4). Also shown is concentrated supernatant from nonvirulent E. coli strain 89515 (O20:H16) probed with anti-EspC antiserum (lane 5) and sIgA antibodies (obtained from a milk pool) (lane 6).
DISCUSSION
Although inhibition of EPEC adherence by sIgA milk antibodies has been previously demonstrated (4, 5, 12), identification of the molecular target of protective immunity has remained elusive. The present study showed that EPEC strains express many surface proteins that are recognized by sIgA antibodies obtained from the milk from Mexican women. However, only three proteins were recognized strongly by the sIgA antibodies (as indicated by the immunostaining intensity), the 94-kDa outer membrane protein called intimin and two unknown proteins of 80 and 70 kDa, suggesting that these proteins are potent antigens able to stimulate significantly a sIgA immune response. A 94-kDa EPEC protein has been reported previously because of its reaction with sIgA antibodies (4, 12). Another EPEC protein of 110 kDa found in the bacterial culture supernatant also reacted strongly with the sIgA antibodies. Based on the molecular mass of the secreted protein and its reactivity with antisera, we think that the 110-kDa protein is EspC, one of the five proteins reported to be secreted by EPEC (47). However, the identity of this protein needs to be confirmed. These proteins (110, 94, 80, and 70 kDa) were consistently recognized by all 15 sIgA samples. Interestingly, nonvirulent E. coli strains that showed poor adhering ability did not express any of these proteins.
Intimin is essential for A/E lesion formation induced by EPEC both in vivo and in vitro and was also essential for full expression of EPEC virulence in volunteers in previous studies (13, 14). The intimate adherence of EPEC to host epithelia is mediated by the binding of intimin to its receptor, Tir (translocated intimin receptor), in the host cell membrane (31). Tir is actually of bacterial, not mammalian, host origin. Preliminary evidence from volunteer studies suggests that antibodies (of the IgG isotype) to intimin correlate with protection (15, 36). The strong sIgA response to intimin and to the 80- and 70-kDa proteins obtained in this study indicates that these proteins stimulate intestinal immune responses and that this effect may elicit protective immunity against EPEC disease.
EspC is a highly immunogenic protein; human serum collected from a volunteer 28 days after infection with EPEC strongly recognized EspC, while serum collected prior to infection did not (24). EspC belongs to the autotransporter family of proteins and has amino acid homology with members of the IgA protease family (47). The toxin Pet shares several characteristics with EspC, which include amino acid homology, a conserved serine protease motif, molecular weight, the mechanism by which they are secreted (the autotransporter secretion system) (22), and the capacity to induce a strong immune response. These similarities suggest that EspC plays a role as a virulence factor, probably as an enterotoxin.
Expression of the 80- and 70-kDa proteins by EPEC strains could be important for the generation of A/E lesions on the intestinal mucosa. This is suggested because such proteins were produced only by EPEC strains which promoted gross cytoskeletal changes in HEp-2 cells, detectable as actin accretion at the point of bacterial contact by the FAS test. Moreover, EPEC strains that are deficient in the ability to elicit the accumulation of filaments of actin were found, and these strains apparently express the 80- and 70-kDa proteins to a lesser degree. These strains were detected as faint immunostained bands (data not shown). Interestingly, diarrheagenic E. coli strains that showed diffuse or aggregative adherence phenotypes but that were unable to induce the A/E lesion were also unable to produce the 94-, 80-, and 70-kDa proteins (data not shown). Although in this study the numbers of EPEC strains and milk samples were small, it is possible to speculate that these proteins are expressed only by FAS-positive EPEC strains.
The fact that 1.0 mg of purified sIgA antibodies/ml blocked the adherence of EPEC to epithelial cells in vitro indicates that such antibodies are directed against surface proteins of the EPEC bacteria. This idea was supported by cell fractionation experiments which indicate that the 94-, 80-, and 70-kDa proteins are located on the surfaces of the bacteria, probably anchored to the outer membrane. The overall results suggest that breast milk from Mexican women contains an abundance of specific sIgA antibodies directed to a large variety of EPEC proteins. We think that this broad sIgA reaction to EPEC is due to exposure of women donors to EPEC strains, which induced an antibody response in their milk. This explanation is supported by a preliminary study which showed that milk samples from women living in poor sanitation conditions gave a stronger sIgA reaction to EPEC proteins than milk samples from women living in good sanitation conditions (Manjarrez-Hernandez et al., unpublished data). We assume that for the same reason the commercial sIgA antibodies did not react to EPEC proteins.
Most, if not all, of the sIgA antibodies must participate in the inhibition of bacterial adherence to cells. Given the strong sIgA response observed in the immunoblots, the abundance of antibodies in the pooled milk sample against the 94-, 80-, and 70-kDa proteins suggests that these antibodies are the most important. It must be noted that for the Western blot assays a total protein extract from strain E2348/69 was used in the proportion that these proteins are present in the bacteria, in an attempt to avoid a concentration-dependent favoring of certain antigens in the immunoassays.
It is known that the immune systems of animals, through bacterial adherence to the intestinal mucosa, are able to discriminate between pathogenic and nonpathogenic (commensal) bacteria (20). The capacities of EPEC strains to adhere to intestinal mucosa must be very important for the induction of intestinal immune responses; in contrast, nonvirulent E. coli strains in this study showed both very low capacities for adherence to cells and negative responses to sIgA antibodies.
It is well established that to induce an antibody response in secretions the antigens must adhere to the follicle-associated epithelium on the intestine and invade the Peyer's patches, where the antigens are captured by specialized endocytic cells (M cells or macrophages) and presented to T and B lymphocytes. The antigen-sensitized IgA precursor B cells subsequently migrate via lymph fluid and blood to exocrine glands and mucosal membranes, where the specialized B cells (called plasma cells) produce IgA antibodies (3, 41). The 94-, 80-, and 70-kDa proteins appeared to be the most immunogenic produced by EPEC strains since they gave the strongest sIgA response after the concentration of antibodies in the immunoassay mixture decreased.
The fact that the detected antigens were not expressed by the EAF plasmid-cured EPEC strains leads us to speculate that the EAF plasmid may control the expression of the genes that encode such proteins. It has been reported that the bfp TVW per (ABC) operon activates transcription of the eae gene, which is located on the chromosome and which encodes the protein intimin. The complete nucleotide sequence of the EAF plasmid revealed that this plasmid not only contains potential virulence-associated genes but also may control the expression of chromosomally located genes.
The present study indicated that expression of the 94-kDa protein (intimin) and of the 80- and 70-kDa proteins is regulated by temperature during bacterial growth, since faint bands in the immunoblots were detected when the EPEC strains were grown at 25°C. At this temperature EPEC lost its ability to adhere to cells. Knutton et al. (34) reported that intimin expression is regulated by environmental factors during bacterial growth and that the A/E activity of EPEC depends on the bacterial growth phase. An increased expression of intimin and of 80- and 70-kDa proteins was also observed during the exponential-growth phase of EPEC (data not shown). It is believed that the 80- and 70-kDa proteins form part of a set of virulence determinants of EPEC pathogenicity, similarly to intimin. These experiments show which protein antigens from EPEC induced an intestinal immune response, since the elicited sIgA antibodies were probably produced by the women's immune systems after prolonged contact with these organisms. The information in the present study may be useful in the development of future vaccines.
ACKNOWLEDGMENTS
We thank Claudio Quinzaños, Ignacio Del Rio, Luis A. Leon, and Gabriel Perez for expert technical assistance.
This study was supported by CONACyT (grant 31051M) and by Direccion General de Asuntos del Personal Académico, UNAM, through its PAPIT Program (no. IN224399).
REFERENCES
- 1.Anderson B, Porras O, Hanson L A, Lagergard T, Svanborg-Edén C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153:232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 2.Blake P A, Ramos S, MacDonald L, Rassi V, Gomes T A T, Ivey C, Bean N H, Trabulsi L R. Pathogen-specific risk factors and protective factors for acute diarrheal disease in urban Brazilian infants. J Infect Dis. 1993;167:627–632. doi: 10.1093/infdis/167.3.627. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. Acta Pathol Microbiol Immunol Scand. 1995;103:1–19. doi: 10.1111/j.1699-0463.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 4.Camara L M, Carbonare S B, Silva L M L, Carneiro-Sampaio M M S. Inhibition of enteropathogenic Escherichia coli (EPEC) adhesion to HeLa cells by human colostrum: detection of specific sIgA related to EPEC outer-membrane proteins. Int Arch Allergy Immunol. 1994;103:307–310. doi: 10.1159/000236645. [DOI] [PubMed] [Google Scholar]
- 5.Carbonare S B, Palmeira P, Silva M L M, Carneiro-Sampaio M M S. Effect of microwave radiation, pasteurization and lyophilization on the ability of human milk to inhibit Escherichia coli adherence to HEp-2 cells. J Diarrhoeal Dis Res. 1996;14:90–94. [PubMed] [Google Scholar]
- 6.Chatkaeomorakot A, Echeverria P, Taylor D N, Bettelheim K A, Blacklow N R, Sethabutr O, Seriwatana J, Kaper J B. HeLa cell-adherent Escherichia coli in children with diarrhea in Thailand. J Infect Dis. 1987;156:669–672. doi: 10.1093/infdis/156.4.669. [DOI] [PubMed] [Google Scholar]
- 7.Cheney C P, Boedeker E C, Formal S B. Quantitation of the adherence of an enteropathogenic Escherichia coli to isolated rabbit intestinal brush borders. Infect Immun. 1979;26:736–743. doi: 10.1128/iai.26.2.736-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens J D, Rao M R, Chakraborty J, Yunus M, Ali M, Kay B, van Loon F P L, Naficy A, Sack D A. Breastfeeding and the risk of life-threatening enterotoxigenic Escherichia coli diarrhea in Bangladesh infants and children. Pediatrics. 1997;100:E2. doi: 10.1542/peds.100.6.e2. [DOI] [PubMed] [Google Scholar]
- 9.Cravioto A, Gross R J, Scotland S M, Rowe B. An adhesive factor found in strains of Escherichia coli belonging to traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979;3:95–99. [Google Scholar]
- 10.Cravioto A, Reyes R, Ortega R, Fernandez G, Hernandez R, Lopez D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988;101:123–134. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravioto A, Reyes R E, Trujillo F, Uribe F, Navarro A, De La Roca J M, Hernandez J M, Perez G, Vazquez V. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990;131:886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- 12.Cravioto A, Tello A, Villafan H, Ruiz J, del Vedovo S, Neeser J R. Inhibition of localised adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Tacket C O, Losonsky G, Frankel G, Nataro J P, Dougan G, Levine M M. Effect of prior experimental human enteropathogenic Escherichia coli infection on illness following homologous and heterologous rechallenge. Infect Immun. 1998;66:52–58. doi: 10.1128/iai.66.1.52-58.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay B B, Rosenshine Y, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girón J A, Ho A S Y, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 19.Gomes T A T, Rassi V, Macdonald K L, Ramos S R T S, Trabulsi L R, Vieira M A M, Guth B E C, Candeias J A N, Ivey C, Toledo M R F, Blake P A. Enteropathogens associated with acute diarrheal disease in urban infants in Sao Paulo, Brazil. J Infect Dis. 1991;164:331–337. doi: 10.1093/infdis/164.2.331. [DOI] [PubMed] [Google Scholar]
- 20.Hanson L A, Telemo E, Wiedermann U, Dahlman-Hoglund A, Lundin S, Friman V, Dahlgren U. Immunological mechanisms of the gut. Pediatr Allergy Immunol. 1995;6(Suppl. 8):7–12. [PubMed] [Google Scholar]
- 21.Hanson L A. Human milk and host defence: immediate and long-term effects. Acta Paediatr Suppl. 1999;88:42–46. doi: 10.1111/j.1651-2227.1999.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 22.Henderson I H, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren J, Svennerholm A M, Ahrén C. Nonimmunoglobulin fraction of human milk inhibits bacterial adhesion (hemagglutination) and enterotoxin binding of Escherichia coli and Vibrio cholerae. Infect Immun. 1981;33:136–141. doi: 10.1128/iai.33.1.136-141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jason J M, Nieburg P, Marks J S. Mortality and infectious disease associated with infant-feeding practices in developing countries. Pediatrics. 1984;74:702–727. [PubMed] [Google Scholar]
- 26.Jerse A E, Yu J, Tall B D, Kapper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karch H, Heesemann J, Laufs R, Kroll H P, Kaper J B, Levine M M. Serological response to type 1-like somatic fimbriae in diarrheal infection due to classical enteropathogenic Escherichia coli. Microb Pathog. 1987;2:425–434. doi: 10.1016/0882-4010(87)90049-0. [DOI] [PubMed] [Google Scholar]
- 28.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 30.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 32.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutton S, Adu-Bobie J, Christopher B, Phillips A D, Dougan G, Frankel G. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect Immun. 1997;65:1644–1652. doi: 10.1128/iai.65.5.1644-1652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 37.Levine M M. Escherichia coli that cause diarrhoea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohaemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 38.Mancini G, Carbonara O A, Heremans J F. Immunochemical quantification of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 39.Manjarrez-Hernandez H A, Baldwin T J, Aitken A, Knutton S, Williams P. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoea. Lancet. 1992;339:521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- 40.Manjarrez-Hernandez H A, Gavilanes-Parra S, Chavez-Berrocal M E, Molina-Lopez J, Cravioto A. Binding of diarrheagenic Escherichia coli to 32- to 33-kilodalton human intestinal brush border proteins. Infect Immun. 1997;65:4494–4501. doi: 10.1128/iai.65.11.4494-4501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcotte H, Lavoie M C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 43.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1768–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orsokov F, Orsokov I. Escherichia coli O:H serotypes isolated from human blood. APMIS. 1975;83B:565–600. [PubMed] [Google Scholar]
- 45.Rosenshine I S, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1992;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 46.Saxon M L, Zhao X, Black J D. Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ. J Cell Biol. 1994;126:747–763. doi: 10.1083/jcb.126.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli, which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone K D, Zhang H Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 49.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulshen M H, Rollo J L. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 51.Victora C G, Vaughan J P, Lombardi C, Teixeira A M, Fuchs S M, Moreira L B, Gigante L P, Barros F C. Evidence for protection by breast-feeding against infant deaths from infectious diseases in Brazil. Lancet. 1987;ii:319–322. doi: 10.1016/s0140-6736(87)90902-0. [DOI] [PubMed] [Google Scholar]
- 52.Viljanen M K, Peltola T, Junnila S Y, Olkkonen L, Jarvinen H, Kuistila M, Huovinen P. Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet. 1990;336:831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]
- 53.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 54.Young H B, Buckley A E, Hamza M, Mandarano C. Milk and lactation: some social and developmental correlates among 1000 infants. Pediatrics. 1982;69:169–175. [PubMed] [Google Scholar]