Abstract
Osteoarthritis (OA) is a prevalent progressive disease that frequently coexists with obesity. For several decades, OA was thought to be the result of ageing and mechanical stress on cartilage. Researchers’ perspective has been greatly transformed when cumulative findings emphasized the role of adipose tissue in the diseases. Nowadays, the metabolic effect of obesity on cartilage tissue has become an integral part of obesity research; hoping to discover a disease-modifying drug for OA. Recently, several adipokines have been reported to be associated with OA. Particularly, metrnl (meteorin-like) and retinol-binding protein 4 (RBP4) have been recognized as emerging adipokines that can mediate OA pathogenesis. Accordingly, in this review, we will summarize the latest findings concerned with the metabolic contribution of obesity in OA pathogenesis, with particular emphasis on dyslipidemia, insulin resistance and adipokines. Additionally, we will discuss the most recent adipokines that have been reported to play a role in this context. Careful consideration of these molecular mechanisms interrelated with obesity and OA will undoubtedly unveil new avenues for OA treatment.
Keywords: Osteoarthritis, Obesity, Dyslipidemia, Insulin resistance, Adipokines, Metrnl
Graphical abstract
Highlights
-
•
OA is a metabolic disease not just a tear and wear form of arthritis.
-
•
Dyslipidemia, IR, and adipokines are multiple key players mediating obesity-OA interplay.
-
•
Metrnl and RBP4 are novel adipokines that can mediate OA pathogenesis.
-
•
MetaBolic contribution of obesity in OA unveils new avenues for OA treatment.
1. Introduction
Osteoarthritis (OA) is one of the most common joint disorders currently affecting about 528 million people globally [1]. It typically affects knees, hips, hands, spine, and feet, with a profound impact on several health outcomes [2]. Actually, arthritis has a tremendous influence on individuals; the physical impairment resulting from pain and loss of functional capability reduces the quality of life and increases the incidence of further morbidity. Although there are several gadgets and palliative medications available that can alleviate pain and enhance the quality of life, there is no pharmaceutical product that can hinder or reverse the onset of OA [3]. These palliative medicines have a great cost over the long term besides their side effects such as nephrotoxicity and gastric complications [4]. Nowadays, scientists are focusing on using hyaluronic acid (HA), platelet-rich plasma (PRP), mesenchymal stem cells (MSC), vascular stromal fraction (VSF) and extracellular vesicles (EVs) as a promising treatment for OA [5]. These intra-articular injections have the advantages of high local bioavailability, fewer systemic side effects and lower cost compared to the surgery [6]. The majority of meta-analysis studies reported the superiority of PRP over HA [[7], [8], [9]]. However, Belk and co-workers have reported a short-term benefit after either HA or PRP treatment in their recent meta-analysis study [10]. Another recent study has reported no superiority of PRP when assessing joint structural changes [11]. Further high-quality multi-centre studies are warranted to prove clinical efficacy of HA and/or PRP, and to determine their long-term outcomes. On the other hand, MSC and VSF are still in the early phases of clinical trials to determine the optimum dose and their long-term side effects, while studies on EVs are still limited to in-vivo models [[12], [13], [14], [15]]. It’s noteworthy here that several meta-analysis studies reported the superiority of using MSC or VSF over PRP [5,16,17]. However, till now, the only option available to treat OA is total knee replacement surgery. This places OA among the most expensive conditions to treat [18,19]. Additionally, patients with comorbid conditions such as cardiovascular diseases are less suitable candidates for such intervention, and even if they undergo surgery, they will be at high risk of postoperative complications such as myocardial infarction and thrombosis [20,21]. Thus, it is crucial to elucidate pathogenesis, genetics as well as biomarkers of OA.
The increasing prevalence of obesity and a sedentary lifestyle nowadays, which are prominent risk factors for OA, resulted in a substantial increase in the number of people living with hip or knee OA [2]. Similarly to OA prevalence, obesity is dramatically increasing along with its accompanying consequences which places it at the top of public healthcare priorities. Unfortunately, the prevalence of obesity grew dramatically between 1975 and 2016 and this trend is still going on [22]. It is estimated that by the year 2030, about one billion people globally will be living with obesity [23]. As a matter of fact, the main burden of obesity lies in its interconnection with a plethora of other disorders. Obesity has always been distinguished as one of the most prominent and preventable risk factors for OA [22,24].
In this review, we aim to shed light on the different aspects of the obesity-OA relationship beyond the traditional theory of mechanical loading and to provide up-to-date information on the recently reported adipokines associated with OA in an attempt to highlight potential future therapeutic targets for OA. Before this, we will overview the difference between mechanical and metabolic theory for the obesity-OA relationship.
2. Mechanical load theory versus metabolic theory for explaining the obesity-OA relationship
Several authors showed a relationship between obesity and the risk of knee OA: for every 5-unit increase in body mass index (BMI), the associated increased risk of knee OA was 35% [25]. A cohort study in 2016 also showed that subjects with grade II obesity (BMI >35 kg/m2) were 4.7 times more likely to develop knee OA compared to subjects with normal weight [26]. Moreover, several reports showed that weight loss improved pain and function scores and decreased low-grade inflammation in patients with knee OA [[27], [28], [29], [30]]. For example, a study of overweight and obese adults with knee OA estimated that every pound (0.45 kg) of weight loss resulted in a four-fold reduction in the load exerted on the knee per step during daily activities, which appears to be clinically meaningful [31]. Similar findings were also reported in other studies [32,33]. The overloading on the joints could stimulate some inflammatory pathways such as (interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α) and nuclear factor-kappa B (NF-κB)) leading to irreversible matrix degradation and apoptosis. It is worth mentioning that physiological mechanical loading is pivotal for healthy cartilage by inducing anabolic factors [34,35].
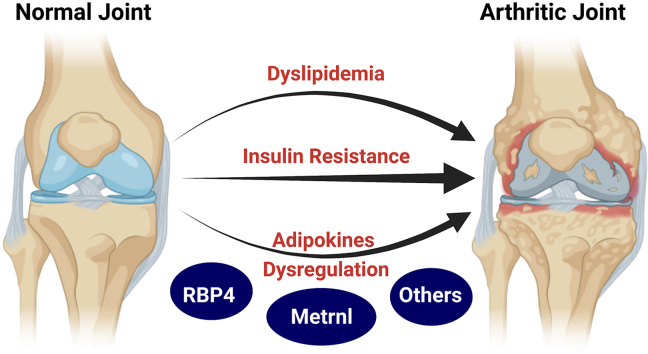
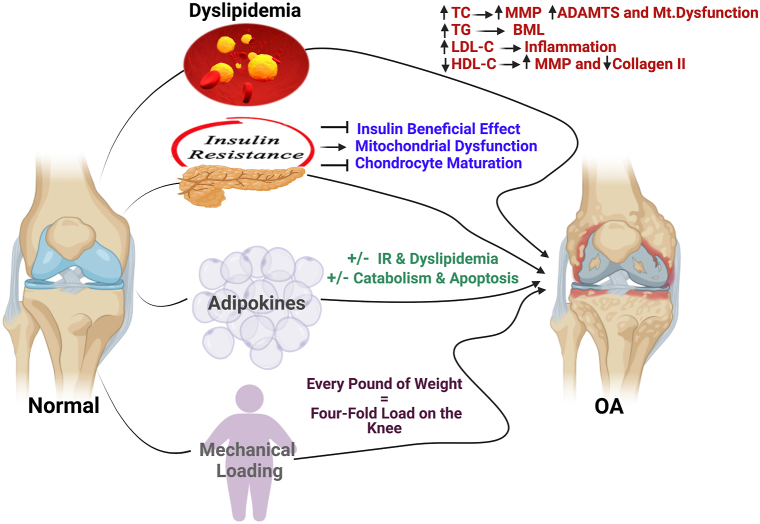
Surprisingly, obesity has also been found to be strongly associated with hand and wrist OA, despite the fact that they are non-weight-bearing joints. That is why the mechanical loading hypothesis fails to explain the relationship between obesity and OA in these non-weight-bearing joints, which led to the postulation that adipose tissues play a paramount role in this context [36,37]. In fact, adipose tissues are a major source of cytokines, chemokines, and metabolically-active mediators known as “adipokines”. Some of these mediators are responsible for systemic low-grade inflammation associated with obesity, which establishes an environment promoting catabolic factors that degrade the joint [37]. However, others were reported to preserve the cartilage integrity and minimize osteophytes development [38,39]. Furthermore, obesity-related dyslipidemia and insulin resistance (IR) contributes significantly to the degenerative process [[40], [41], [42], [43]]. We tried to simplify the complex multi-factorial interplay between obesity and OA in Fig. 1.
Fig. 1.
Multi-factorial interplay between obesity and OA. Dyslipidemia, insulin resistance, adipokines and mechanical loading are the four aspects by which obesity affects normal knee and induces OA. The arrows represent a summary of how each aspect leads to OA development. ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs, BML: bone marrow lesions, HDL-C: high-density lipoprotein cholesterol, IR: insulin resistance, LDL-C: low-density lipoprotein cholesterol, MMP: matrix metalloproteinases, Mt. dysfunction: mitochondrial dysfunction, OA: osteoarthritis, TC: total cholesterol and TAG: triglycerides. Created by Biorender.com.
3. Obesity-OA interplay beyond mechanical loading
3.1. Dyslipidemia and osteoarthritis
The relationship between dyslipidemia and OA is a controversial issue [44,45]. However, various studies revealed a significant relationship between lipotoxicity resulting from dyslipidemia and OA [41,43,46]. During OA, chondrocytes accumulate lipids, and their intracellular levels were reported to be correlated with the disease severity. Some scientists referred this to the change in the expression of genes involved in lipid efflux [47,48], while others highlighted some epigenetic modifications of cholesterol-related genes which are responsible for OA development [49]. Besides, several micro-RNAs were found to be associated with cholesterol dysregulation and hence OA [[50], [51], [52]]. Moreover, several animal studies unveiled a strong correlation between high-fat diet and the progression of OA via inducing synovial inflammation, formation of multiple osteophytes, systemic inflammation and metabolic changes [[53], [54], [55], [56]].
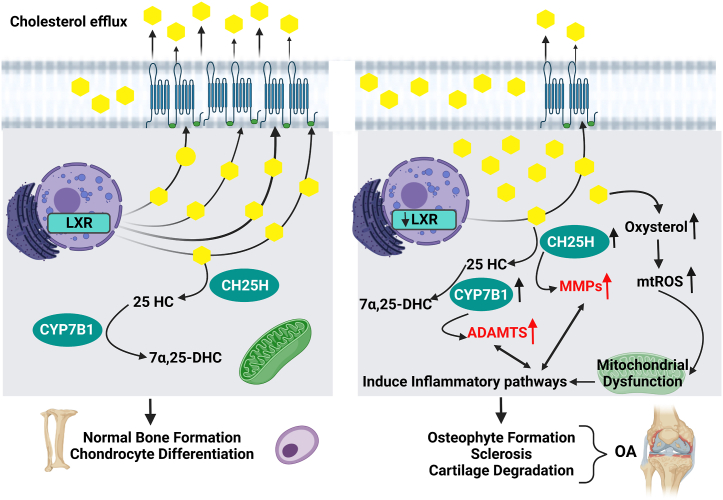
In fact, cholesterol is a vital constituent of the cell membrane and controls signalling pathways in conjunction with other intermediates. In this context, cholesterol is essential for bone formation and chondrocyte differentiation. Cholesterol diffusion is regulated through cholesterol-influx genes: apolipoprotein-B (Apo-B) and cholesterol-efflux genes: Apo-A1, adenosine triphosphate binding cassette transporter-1 (ABCA-1) and liver X receptor (LXR), namely LXRα and LXRβ [49,57]. The expression of these receptors which are responsible for cholesterol efflux was reported to be increased during chondrocytes differentiation and decreased during hypertrophic state and hence during OA [47,58], as represented in Fig. 2. Interestingly, an in-vivo study revealed stimulation in inflammatory responses upon increasing intracellular cholesterol [59], whereas LXR agonists were found to prevent cholesterol accumulation and subsequent cartilage degeneration [49]. Besides the increase in cholesterol uptake in osteoarthritic cartilage, Choi and co-workers discovered an upregulation of cholesterol hydroxylases (mainly cholesterol 25-hydroxylase (CH25H) and 25-hydroxycholesterol 7α-hydroxylase (CYP7B1)). The upregulation of these enzymes leads to a decrease in the anabolic factors and induces synovitis, osteophyte formation and sclerosis. Additionally, in the same study, the high expression levels of CH25H were followed by high expression levels of various matrix metalloproteinases (MMP), while the increase in the expression of CYP7B1 resulted in an upregulation of ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) [60], as shown in Fig. 2.
Fig. 2.
Hypercholesterolemia and OA interplay. During chondrocytes differentiation and bone formation, there is an increase in the expression of cholesterol efflux receptors. On the other hand, during OA, these receptors are downregulated that leads to cholesterol accumulation. Cholesterol hydroxylases are upregulated as well. As a result, degradative enzymes, catabolic factors and cholesterol byproducts increase leading to OA. ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs, CH25H: cholesterol 25-hydroxylase, CYP7B1: 25-hydroxycholesterol 7α-hydroxylase, 7α,25-DHC: 7α,25-dihydroxy cholesterol, 25-HC: 25-hydroxy cholesterol, LXR: liver X receptor, MMP: matrix metalloproteinases, mtROS: mitochondrial-reactive oxygen species, and OA: osteoarthritis. Created by Biorender.com.
Other studies referred this causal relationship between cholesterol and OA to mitochondrial dysfunction. The increase in cholesterol oxidation products such as oxysterols were found to be associated with mitochondrial-oxidative stress resulting in an increase in mitochondrial-reactive oxygen species (mtROS). Overproduction of these mtROS activates oxidative stress and inflammatory pathways, which contribute to permanent damage of joint tissue [57], as shown in Fig. 2. Other different studies referred cholesterol-OA relationship to osteophyte formation rather than aggravation of cartilage damage [61]. It is noteworthy that several studies reported a protective effect of statins in both in-vitro and in-vivo OA models while others observed a low incidence of developing OA in patients receiving statins for a long time [[62], [63], [64], [65], [66]].
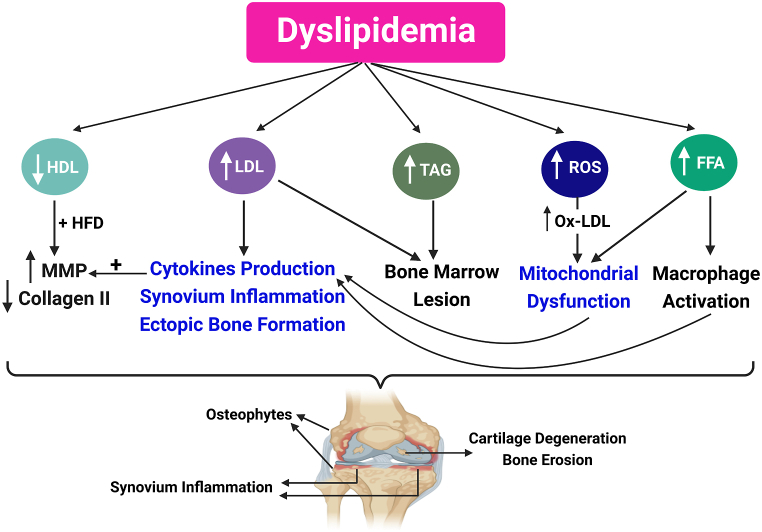
Moreover, in-vivo studies demonstrated a direct link between increased dietary cholesterol and OA development in Apo-A1 and LCAT (Lecithin–cholesterol acyltransferase) knockout mice [67]. In such models, the decrease in mature high-density lipoprotein (HDL) together with a high-fat diet was associated with an increase in MMP production and a decrease in collagen II and hence OA development [67], as shown in Fig. 3. It’s noteworthy here that this study was employed using LCAT(−/−) mice (having immature HDL and low HDL-cholesterol) and C57BL/6 mice (control mice having normal mature HDL). The authors in that study referred the lack of evidence of OA in the control group to the type and the duration of the high-fat diet administered. In contrast to other animal studies that unveiled a strong correlation between a high-fat diet and the progression of OA [53,54,68]; the energy from fats was very low (42% in Triantaphyllidou et al. study compared to 60% in other animal studies) and mice were placed on high-fat diet for a relatively shorter time (24 weeks in Triantaphyllidou et al. study, compared to 42 weeks in other animals studies) [67]. In addition, other in-vivo studies revealed an increase in cytokines production, synovium inflammation as well as ectopic bone formation arising from high low-density lipoprotein (LDL) levels [61,69]. It was also reported that hypercholesterolemia, hypertriglyceridemia and low HDL levels were associated with the occurrence of bone marrow lesions which is the source of pain during OA [46,48], represented in Fig. 3.
Fig. 3.
Multiple aspects of the contribution of dyslipidemia in the OA process. Dyslipidemia is characterized by high levels of LDL, TAG, FFA and ROS, and low levels of HDL. All these factors contribute to OA development and progression via several mechanisms. FFA: free fatty acids, HDL: high-density lipoprotein, HFD: high-fat diet, LDL: low-density lipoprotein, MMP: matrix metalloproteinases, Ox-LDL: oxidized low-density lipoprotein, ROS: reactive oxygen species, and TAG: triglycerides. Created by Biorender.com.
In fact, dyslipidemia is also characterized by an increase in free fatty acids (FFA) and reactive oxygen species (ROS) [70]. These ROS are responsible for the overproduction of oxidized-LDL (Ox-LDL) observed during dyslipidemia [71,72]. Both FFA and Ox-LDL participate in cartilage degeneration [[73], [74], [75], [76], [77]]. After binding to their receptors on chondrocytes, Ox-LDL either stimulate vascular endothelial growth factor (VEGF) release or affect the mitochondria. Consequently, the secretion of proteolytic enzymes and inflammatory cytokines increases leading to cartilage damage [75,78]. On the other hand, FFA activate macrophages and in turn macrophages start to secrete pro-inflammatory cytokines [48,57,79]. Besides, FFA induce mitochondrial dysfunction and IR which also participate in the degeneration process [42]. Fig. 3 summarizes these multiple aspects of the contribution of dyslipidaemia in the degenerative process in OA.
Recently, exploring and developing novel therapeutic avenues for the treatment of this lipotoxicity‐related OA has attracted many scientists [80,81]. A prime example of that is Sparstolonin B which has been identified as a novel therapy for lipotoxicity-induced OA. It prevents FFA from binding with their receptors; subsequently, it inhibits the NF-κB pathway and hence prevents cartilage degradation [81]. Another in-vitro study has reported that Firsocostat (ND-630) can attenuate lipid accumulation in chondrocytes by inhibiting acetyl co-A carboxylase (ACC; the key enzyme of de novo lipogenesis). Cartilage lipotoxicity was found to be associated with an up-regulation of ACC. Although Firsocostat is an investigational drug still under development, this study highlighted ACC inhibitors as a novel target for lipotoxicity-related OA [80].
3.2. Insulin resistance and osteoarthritis
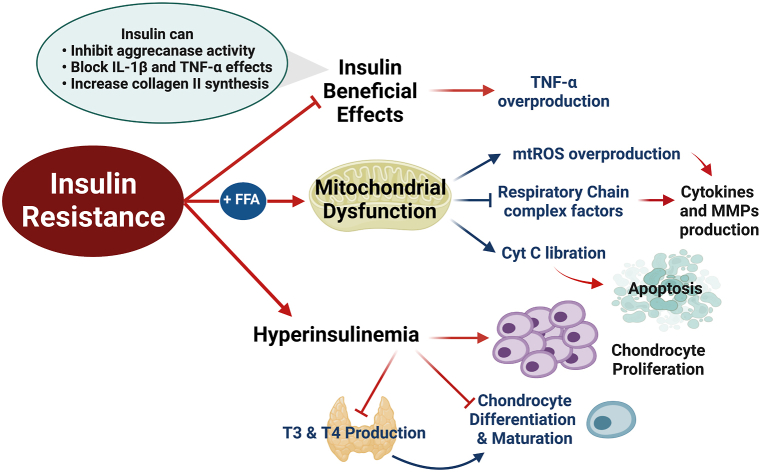
Several studies proved the role of insulin either as an anabolic or anti-catabolic factor. These studies reported that insulin can inhibit aggrecanase activity, block IL-1β and TNF-α effects as well as increase the synthesis of collagen II and other proteoglycans [[82], [83], [84], [85]]. Additionally, immunohistochemistry studies revealed that both synovium and chondrocytes possess insulin receptors [84,85]. Like other tissues, these receptors are also affected by the IR state in which they become less responsive to insulin’s beneficial effects [86]. In agreement with that, Hamada and co-workers reported that TNF-α expression is up-regulated in the synovium of diabetic OA patients when compared to non-diabetic OA patients [85]. They assigned these results to insulin-resistant state which rendered the synovium of diabetic patients more sensitive to overproducing local TNF-α in the absence of insulin regulatory effect [85], as shown in Fig. 4.
Fig. 4.
The role of insulin resistance in the OA degenerative process. During IR, the insulin receptors in chondrocytes and synovium become less responsive to insulin’s beneficial effects leading to the overproduction of cytokines. There is also an increase in FFA which leads to mitochondrial dysfunction which ultimately leads to cytokines release and apoptosis. Furthermore, hyperinsulinemia hinders the formation of mature chondrocytes by increasing the proliferation of chondrocytes, preventing their differentiation, and reducing the circulating levels of thyroid hormones. Red arrows with pointed ends imply the processes which are ultimately induced because of IR, while those with blunt ends imply processes which are ultimately inhibited as a consequence of IR. Cyt C: cytochrome C, FFA: free fatty acids, IL-1β: interleukin-1β, MMP: matrix metalloproteinases, mtROS: mitochondrial-reactive oxygen species, T3 and T4: thyroid hormones, and TNF-α: tumor necrosis factor α. Created by Biorender.com.
In fact, one of the hypotheses for the IR state is the increase of FFA [87]. Besides the aforementioned role of IR during OA, both FFA and IR can induce mitochondrial dysfunction leading to a reduction in the activity of complex factors of the respiratory chain, the liberation of cytochrome C as well as increased mtROS production [42,57,88]. As a consequence, the reduced activity of these complexes stimulates pro-inflammatory cytokine and MMP production inside chondrocytes, while cytochrome C initiates the apoptotic process [42,[88], [89], [90]], as discussed earlier. These concepts are presented in Fig. 4.
Moreover, another study investigated the effect of hyperinsulinemia, a characteristic feature in IR states, on the progression of OA. They reported that hyperinsulinemia increases the proliferation of chondrocytes and prevents their differentiation and maturation [91]. Furthermore, hyperinsulinemia reduces the circulating levels of thyroid hormones (T3 and T4) which are also necessary for chondrocytes maturation [91]. The role of hyperinsulinemia in the OA degenerative process is also presented in Fig. 4.
Another theory about how IR leads to OA is the hyperglycemic environment that results from IR. Glucose is necessary for articular chondrocyte to maintain cell homeostasis, produce energy and synthesize the cartilage matrix. However, hyperglycemia was found to increase inflammation, destruct subchondral bone and lead to chondrocyte dysfunction [92]. Previous studies revealed that ROS were found to be elevated in OA cartilage present in a hyperglycemic-like environment. As discussed before, ROS give rise to catabolic processes implicated in cell degradation and cell apoptosis [93]. Moreover, hyperglycemia is also known to favor the production of advanced glycation end products (AGE) and their accumulation in articular cartilage, which contribute to a toxic environment that might facilitate OA pathogenesis [93]. AGE then interact with the receptors of AGE (RAGE) and toll-like receptors (TLR), giving rise to a cascade of events that decrease the activation of peroxisome proliferator-activated receptor gamma (PPAR-γ), promote the release of pro-inflammatory factors such as TNF-α, and activate transcription factors such as NF-κB, which ultimately leads to inflammation, oxidative stress, and promotes cartilage degradation [93].
3.3. Adipokines and osteoarthritis
During the past years, adipokines have ignited much interest due to having either pro- or anti-inflammatory functions in various disorders [94]. Nowadays, adipokines are also believed to have significant implications in the pathogenesis and progression of obesity-induced OA [38,[95], [96], [97], [98]]. They can directly induce or inhibit catabolic or apoptotic pathways or affect bone metabolism [38,95,99]. For example, leptin, chemerin, resistin, visfatin and lipocalin-2 are now believed to have detrimental effects on joint tissue. However, others were reported to maintain cartilage integrity and reduce osteophyte formation, such as progranulin, vaspin and omentin-1 [38,39,95,[100], [101], [102], [103], [104], [105]]. Accordingly, adipokines have emerged as potential candidates to link obesity with OA which may also serve as putative targets for disease-modifying OA drugs, especially for obese patients [95,96,99,103,106]. Table 1 summarizes the reported adipokines interrelated with OA.
Table 1.
The role of different adipokines in the pathogenesis of OA.
| Adipokine | Role | References |
|---|---|---|
| Leptin | • Induces chondrocyte apoptosis by activating JAK2/STAT3 and mTOR pathways which stimulate ROS production. • Induces the secretion of MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5 and ADAMTS-9. • Activates JNK, MAPK, and NF-κB which Influence the synthesis of NO that induces inflammation via the expression of IL-6. IL-8 and PGE2. • Activates apoptosis process and inhibits autophagy via upregulation of LOXL3. |
Zhang et al. [107], Zhao et al. [108], Jiang et al. [109] Hui et al. [110], Yaykasli et al. [111] Vuolteenaho et al. [112] Wei et al. [38] |
| Chemerin | • Recruits macrophages in synovium as part of the inflammatory cascade. • Induces CCL2, MMP-1, MMP-3 and MMP-13 expression. • Decreases the proliferative capability of chondrocytes. • Activates TLR4 and Akt/ERK pathways. |
Eisinger et al. [113] Ma et al. [114] Wang et al. [104] |
| Resistin | • Binds to TLR4 and CAP1 receptors, which activate p38-MAPK and NF-κB, and C/EBP-β signalling pathways. • Promotes the release of pro-inflammatory cytokines/chemokines and degradative markers such as IL-6, IL-1α, IL-1β, TNF-α, CCL2, CCL3, CCL3L1, CCL4, CCL5, CCL8, CCL20, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8 (IL-8), CX3CL1, MMP-1, MMP-13, and ADAMTS-4. |
Zhang et al. [115] Lee et al. [116] Zhao et al. [105] |
| Visfatin | • Induces the production of IL-1β, IL-6, TNF-α, MMP-3, MMP-12, MMP-13, ADAMTS-4, and ADAMTS-5. • Induces osteophyte formation by inhibiting osteoclastogenesis. |
Gosset et al. [117] Laiguillon et al. [118] Yang et al. [101] Beak et al. [119] Han et al. [120] |
| Lipocalin-2 | • Reduces chondrocyte proliferation. • Forms a covalent complex with MMP-9 which blocks its auto-degradation. |
Gupta et al. [39] Carrión et al. [95] |
| Nesfatin | • Induces pro-inflammatory mediators such as COX-2, IL-8, IL-6, and CCL3. • Stimulates IL-1β production via activating PI3K, Akt, AP-1 and NF-κB pathways which suppress miR-204–5p synthesis. • Conversely, another study identified nesfatin as an anti-inflammatory mediator which attenuates NF-κB and MAPK inflammatory pathways and induces collagen II expression. |
Scotece et al. [121] Lee et al. [97] Jiang et al. [99] |
| Adiponectin | • Up-regulates TIMP-2 and inhibits IL-1β-mediated MMP13 production. • Induces autophagy by AMPK/mTOR activation. |
Chen et al. [122], Xie et al. [123] Hu et al. [124], Duan et al. [106] |
| Progranulin | • Maintains the cartilage integrity by inhibiting ADAMTS-7 and ADAMTS-12 and by blocking the effect of TNF-α. • Inhibits IL-1β and LPS-induced catabolic pathways in chondrocytes by blocking TNFR1. • Triggers the anabolic pathways by binding to TNFR2. • Inhibits Wnt/catenin signalling with reduction of osteophyte formation and cartilage degeneration. |
Guo et al. [125] Abella et al. [102] Zhao et al. [126] Zhao et al. [126], Carrión et al. [95] |
| Vaspin | • Reduces RANKL-induced expression of MMP-9. • Inhibits IL-1β and leptin-induced production of catabolic and pro-inflammatory mediators. • Its low level inhibits the expression of the cholesterol efflux pathway via miR155/LXRα led to cholesterol accumulation in cartilage. • Stimulates extracellular matrix anabolism and chondrocytes survival via activating Akt pathway. |
Carrión et al. [95] Bao et al. [100,127] He et al. [52] Wang et al. [128] |
| Omentin-1 | • Inhibits IL-1β-induced cartilage degradation via inhibition of the JAK2/STAT3 pathway which ameliorates MMP-1, MMP-3 and MMP-13 production. • Stimulates the mitochondrial biogenesis through PGC1α-AMPK signalling pathway. • Inhibits IL-1β-induced cellular senescence. |
Li et al. [129] Li et al. [130] Chai et al. [103] |
| Metrnl | • Its high serum level was associated with a low risk for OA. • Records high levels in SF which suggested a compensatory role for it in SF. • Negative association between its SF levels and MMP-13 was found. • Suppress the PI3K/Akt/NF-κB pathway which upregulates collagen II and inhibits MMP-13 and ADAMTS-5. • Reduces chondrocyte pyroptosis by blocking the NLRP-3/caspase-1/GSDMD cascade. |
Sobieh et al. [131] Liu et al. [132] |
| Retinol Binding Protein 4 (RBP4) | • Positive association was recorded between its level and MMP-1 and MMP-3. | Scotece et al. [133] |
JAK/STAT3: Janus kinase/signal transducer and activator of transcription protein-3, mTOR: the mammalian target of rapamycin, ROS: reactive oxygen species MMP: matrix metalloproteinase, ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs, JNK: c-Jun N-terminal kinases, MAPK: mitogen activated protein kinase, NF-κB: nuclear factor-kappa B, NO: nitric oxide, IL: interleukin, PGE2: prostaglandin E2, LOXL3: Lysyl oxidase-like 3, CCL: C–C motif chemokine ligand, TLR4: toll-like receptor 4, Akt: protein kinase B, ERK: extracellular signal-regulated kinase, CAP1: adenylyl cyclase-associated protein 1, C/EBP-β: C/enhancer binding protein β, TNF-α: tumor necrosis factor α, CCL3L1: CCL3 like 1, CXCL: C-X-C motif chemokine ligand, CX3CL1: C-X3-C motif chemokine ligand 1, COX-2: cyclooxygenase-2, PI3K: Phosphoinositide-3-kinases, AP-1: activator protein 1, miR: micro-RNA, TIMP-2: tissue inhibitor matrix metalloproteinase 2, AMPK: adenosine monophosphate-activated protein kinase, LPS: lipopolysaccharide, TNFR: tumor necrosis factor receptor, RANKL: receptor activator of NF-κB ligand, LXRα: liver X receptor α, PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1α, SF: synovial fluid, NLRP-3: nod-like receptor protein-3 and GSDMD: gasdermin D.
Surprisingly, adipose tissue is not the only source of adipokines production. Chondrocytes, synoviocytes as well as osteoblasts are also capable of producing certain adipokines, especially in arthritic patients [134,135]. Moreover, the infrapatellar fat pad, a loading force reducer which lies between the synovium and the joint capsule, has sparked a lot of curiosity because it is considered an intra-articular adipose tissue [19].
Since multiple reviews previously focused on the well-known adipokines and their role in OA in full details [95,96,98], currently this report will shed the light on only metrnl and retinol-binding protein 4 (RBP4) being emerging adipokines associated with OA, which are not only expressed by adipose tissue but also were found to be expressed by chondrocytes [131,133]. Few studies reported their association with OA with limited information about their exact molecular pathways. This review highlights the possible mechanism of action of metrnl and RBP4 in OA which will open the door for future experimental studies.
4. Emerging adipokines associated with OA
4.1. Metrnl
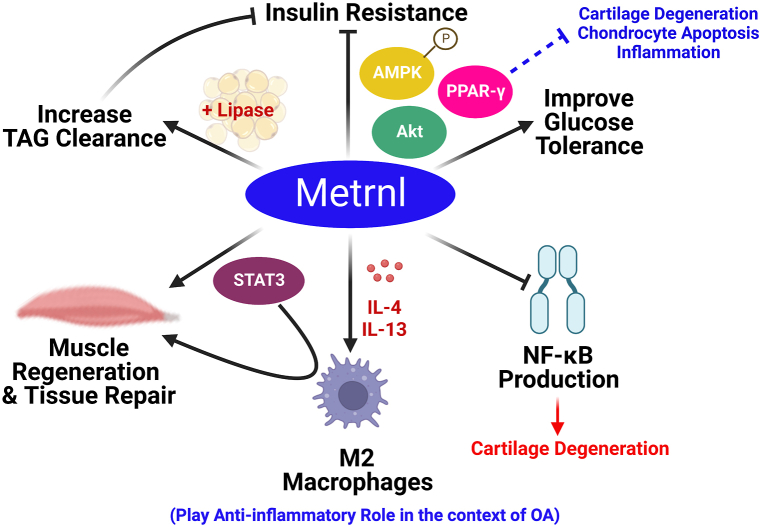
Metrnl was identified first as a protein homologous to the neurotrophin meteorin due to their sequence similarity, hence called meteorin-like (metrnl) [136]. However, unlike meteorin which is mainly expressed in the brain, this protein showed a relatively wider distribution in the body with higher expression levels in subcutaneous white adipose tissues and barrier tissues, and lower expression levels in the brain [136,137]. Metrnl is also called subfatin, IL-39 and cometin depending on its expression site and its possible effect as a cytokine [[136], [137], [138]]. Over the past years, several favourable actions for metrnl have been suggested. For instance, it can antagonize obesity-induced IR and improve glucose tolerance. This beneficial action is accomplished by the activation of PPAR-γ and by inducing protein kinase B (Akt) and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation [[139], [140], [141]]. It is noteworthy to mention that PPAR-γ plays a critical role in the pathophysiology of OA; Its downregulation causes cartilage degeneration, chondrocyte apoptosis, and an upsurge of inflammatory mediators [142].
Additionally, metrnl enhances serum triglyceride clearance and elevates the expression and activity of lipase in adipose tissue. Increasing triglycerides turnover may be a possible mechanism by which metrnl alleviates IR [139]. Furthermore, increasing circulating metrnl in mice produced remarkable upregulation in the expression of genes associated with anti-inflammatory cytokines and can attenuate NF-κB mediated signalling [141,143]. Several immune-inflammatory disorders were associated with up-regulation in metrnl expression. Thus, metrnl was initially called IL-39 and changed later to IL-41 [137,144]. Metrnl can induce the expression levels of IL-4 and IL-13 which in turn stimulate M2 macrophages (alternative activated macrophages; the anti-inflammatory phenotype) [140]. Recently, several studies have reported the involvement of metrnl in balancing the inflammatory responses which encourages tissue repair and remodelling [[145], [146], [147]]. Baht and co-corkers referred metrnl’s ability to induce skeletal muscle regeneration to its ability to activate STAT3 (signal transducer and activator of transcription protein-3) signalling in muscle which in turn activates M2 macrophage polarization [145]. Other studies also support the pivotal role of STAT3 as an inflammatory regulator in different tissues [148,149]. However, in chondrocytes, STAT3 activation was found to induce degradation and apoptosis [150,151]. Thus, the role of STAT3 in OA pathogenesis, and its interrelation with metrnl seems to be a bit complicated and far from complete elucidation. Nevertheless, the context-specific relationship between STAT3 and the inflammatory pathways in different tissues is strongly suggested [149]. Therefore, future mechanistic molecular studies are indeed warranted to further elucidate the interplay between metrnl and STAT3 in OA models. All the previously discussed effects of metrnl are represented in Fig. 5.
Fig. 5.
Metrnl beneficial effects as well as the reported/suggested pathways by which metrnl contributes to OA. Metrnl has several beneficial effects such as improving glucose tolerance and hence alleviating insulin resistance. This is mediated via the activation of PPAR-γ, Akt and AMPK. Metrnl has anti-inflammatory effects via inhibiting NF-κB signalling and activating M2 macrophages, the anti-inflammatory type. Additionally, Metrnl can regulate muscle regeneration and tissue repairs via the activation of STAT3 and M2 macrophages. Akt: protein kinase B, AMPK: adenosine monophosphate-activated protein kinase, IL: interleukin, NF-κB: nuclear factor-kappa B, PPAR-γ: peroxisome proliferator-activated receptor gamma, STAT3: signal transducer and activator of transcription protein-3, and TAG: triglycerides. Created by Biorender.com.
Interestingly, significant up-regulation of metrnl expression was observed in synovial membranes of patients with rheumatoid arthritis (RA) [137]. Furthermore, metrnl expression was found to be elevated in hypertrophic chondrocytes [152]. Taking into consideration that hypertrophic chondrocytes happen to be a characteristic feature during OA pathogenesis [153] and that all the aforementioned pathways for metrnl were also found to be interconnected with OA pathogenesis, this strongly suggests a possible role of metrnl in OA pathogenesis.
Recently, we observed lower serum metrnl levels in obese patients with OA compared to obese patients not suffering OA, and higher serum metrnl levels were shown to be substantially linked with a reduced risk of OA. Furthermore, serum metrnl levels were shown to be lower in participants with advanced grade OA compared to subjects with early grade OA [131]. Regarding its synovial fluid (SF) levels, in that same study, it showed higher levels in obese patients with OA compared to obese subjects without OA. This led to the suggestion that there may be some sort of interplay between SF-metrnl and compensatory mechanisms in OA based on the previously reported favourable actions for metrnl (anti-inflammatory, insulin-sensitizing and its tissue repair role) [141,145,146,154]. This postulation was also based on the observed negative association between SF-metrnl and MMP-13 which is a well-known degradative marker during OA pathogenesis [131,155]. In agreement with our results, a recently published article has reported an association between the increasing level of metrnl and the alleviation of OA severity [132]. Liu and co-workers referred metrnl’s anti-inflammatory effect to the suppression of the PI3K (Phosphoinositide-3-kinases)/Akt/NF-κB pathway. This was associated by increasing in the expression of collagen II and inhibiting the expression of MMP-13 and ADAMTS-5. In the same study, metrnl reduced chondrocyte pyroptosis (the inflammatory form of programmed cell death) by blocking the nod-like receptor protein-3/caspase-1/gasdermin D cascade [132]. Collectively, these findings portray metrnl as a potential novel therapeutic target for OA and open the door for future research to further elucidate its mechanism of action in obesity-OA interplay [131].
4.2. Retinol binding protein 4 (RBP4)
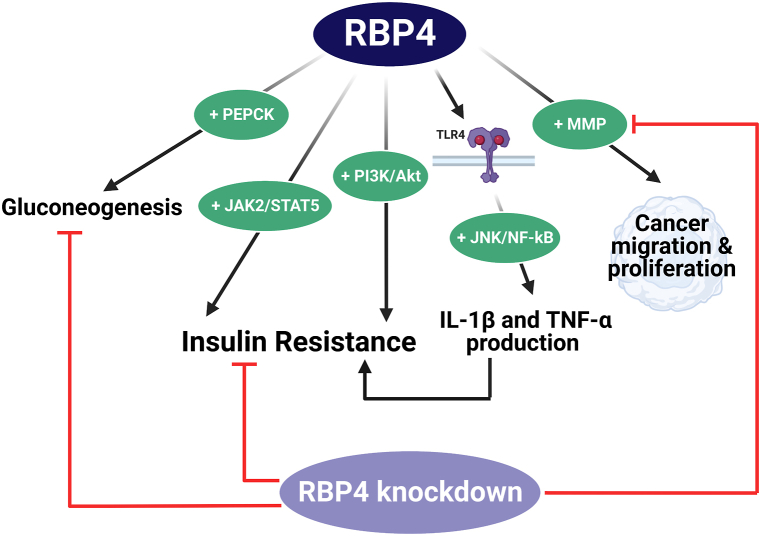
RBP4 is well-recognized as an adipose tissue-derived hormone that belongs to the lipocalin family and promotes IR. It has also been identified as a vitamin A carrier in the blood responsible for carrying retinol, being a member of vitamin A family, from liver to peripheral tissues. Although RBP4 is mainly expressed by hepatocytes, it is also expressed by adipose tissues, and elevated RBP4 levels have been reported in obese humans as well as animal models [156,157]. Moreover, several studies documented a strong correlation between RBP4 and both IR and dyslipidemia; as it impairs glucose tolerance and insulin sensitivity and enhances the production of the gluconeogenic enzyme, phosphoenolpyruvate carboxykinase. In addition, low RBP4 levels or deletion of its gene have been linked to the enhancement of insulin sensitivity [156,[158], [159], [160], [161]]. It was reported that RBP4 impairs insulin signalling pathways either via the activation of PI3K/Akt or the activation of JAK2 (Janus kinase 2)/STAT5 [157,162]. It is noteworthy that the activation of PI3K/Akt or JAK signalling stimulates inflammation, catabolism and apoptosis in OA [97,109,129,132].
Importantly, RBP4 binds to either retinoic acid gene homologous 6 receptor or TLR4 [157]. After binding to TLR4, RBP4 stimulate c-Jun N-terminal kinases (JNK) and NF-κB, which subsequently stimulate IL-1β and TNF-α production leading to insulin signalling impairment and hence IR state develops [157]. Interestingly, TLR activation leads to the production of pro-inflammatory cytokines in chondrocytes as well [163]. This partially explains the suggested contribution of RBP4 in OA pathogenesis, together with the latest investigations which have revealed that RBP4 can promote MMP release to promote cancerous cells migration and proliferation and that the knock-down of RBP4 suppresses the production of MMP [133,164,165]. The previously suggested mechanisms for the involvement of RBP4 in OA are presented in Fig. 6.
Fig. 6.
The reported effects of RBP4 by which it possibly contributes to OA pathogenesis. RBP4 induces IR by stimulating PI3K/Akt and JAK2/STAT5 pathways. In addition, RBP4 activates the inflammatory pathways (JNK and NF-κB) after binding with its receptor (TLR4). This leads to overproduction of inflammatory cytokines such as IL-1β and TNF-α. This also leads to IR development. RBP4 also induces gluconeogenesis by stimulating the gluconeogenic enzyme; PEPCK. RBP4 can promote MMP release in various tissues such as cancerous cells to enhance their migration and proliferation. Knockdown of RBP4 can suppress MMP production and gluconeogenesis and enhance insulin sensitivity. Akt: protein kinase B, IL-1β: interleukin-1β, IR: insulin resistance, JAK2/STAT5: Janus kinase 2/signal transducer and activator of transcription protein-5, JNK: c-Jun N-terminal kinases, MMP: matrix metalloproteinases, NF-κB: nuclear factor-kappa B, OA: osteoarthritis, PEPCK: phosphoenolpyruvate carboxykinase, PI3K: Phosphoinositide-3-kinases RBP4: retinol-binding protein 4, TLR4: toll-like receptor 4, and TNF-α: tumor necrosis factor α. Created by Biorender.com.
Recently, Scotece and co-workers have investigated the potential role of RBP4 in OA pathogenesis, and they found that RBP4 is expressed by osteoarthritic chondrocytes and at the same time its receptor was also detected [133]. In addition, a positive correlation was observed between RBP4 and the classic degradative markers MMP-1 and MMP-3. This study has exhibited RBP4 as a novel target in OA that connects IR, inflammation and cartilage degeneration [133].
5. The inter-relation between various metabolic aspects in OA reveals possible therapeutic targets
Dyslipidemia, insulin resistance and adipokines could affect the cartilage tissue by acting alone or in a synergistic way. Adipokines act as key players in the interplay between obesity and OA. Dysregulation of adipokines levels is responsible for developing IR and dyslipidemia [96], as shown in Fig. 1. Another possible key factor is the mitochondrial dysfunction resulting from hypercholesterolemia and IR. Mitochondrial dysfunction leads to stimulating inflammatory and catabolic pathways, as well as chondrocyte apoptosis [42,57,88,89], as shown in Figs. 2 and 4. In agreement with that, Coleman and co-workers support targeting mitochondrial responses in ameliorating OA. They proved that Amobarbital or N-acetylcysteine can inhibit oxidative stress after injury and protects from posttraumatic OA development [166]. Additionally, coenzyme Q10 (CoQ10) has been suggested to slow OA progression and enhance OA symptoms. CoQ10 is an anti-oxidant and an important factor in the mitochondrial respiratory chain. It could also modulate the inflammatory pathways such as NF-κB and TLR, and control the release of pro-inflammatory cytokines in different disorders [167,168]. In this context, a recent in-vivo study has reported the effect of CoQ10-micelles as oral supplements in ameliorating the inflammation associated with OA [169]. Similar findings were also reported about melatonin, dihydromyricetin, quercetin, taurine, resveratrol and diallyl disulfide. These anti-oxidants could be potential treatment for OA by protecting chondrocytes from mitochondrial oxidative stress [[170], [171], [172], [173], [174], [175], [176]]. However, clinical trials are still needed to prove such effects in OA patients.
Another interrelation between dyslipidemia, insulin resistance and adipokines is their abilities to induce inflammatory and catabolic pathways [42,60,85,94]. Thus, anti-cytokines and enzyme inhibitors are also suggested as potential therapy for OA such as TNF-inhibitors, IL-6 antibodies, IL-1β antibodies, MMP inhibitor and JAK inhibitors [177]. Some investigational studies on RA reported the role of anti-TNF therapy in enhancing IR and modulating adipokines levels such as resistin, chemerin and adiponectin [[178], [179], [180]]. However, these kinds of therapies are still in their early phases with severe adverse effects [177].
Till the discovery of a disease-modifying OA drug, a focus on prevention is valuable. Individuals' ability to self-monitor their weight, diet and physical activity is a complicated process that is influenced by their level of education and access to information. Moreover, it consumes time and effort to achieve change. Solutions that are focused on improved nutrition and enhancement of physical activity could be only successful if individuals receive personalized information that can be transformed into practical activities with a result that can be measured and related to their physiological status [181,182]. Over the last decade, technological applications and devices have aided in the maintenance of healthy behaviors. Web-based weight loss intervention programs helped many people to reduce their BMI scores [[182], [183], [184]]. For example, ArmOnIA is an online digital tool that incorporates dietary, anthropometric, and physical activity data and produces an individual’s estimation of energy balance [182]. Another personalized model is called personalized metabolic avatar (PMA). This model can predict the individuals’ responses to diet, physical activity and environmental and psychological factors. Thus, PMA could evaluate each diet plan and help to reach the ideal BMI [184]. Both ArmOnIA and PMA can raise public self-awareness and help people pursue a healthy life and observe long-lasting outcomes [182,184].
6. Conclusion
OA is no longer considered to be a tear and wear form of arthritis. To reduce its burden and improve patients’ life, discovering new biomarkers and therapeutic targets is crucial to improving diagnosis and treatment. This necessitates a more thorough understanding of the various molecular mechanisms governing the OA disease process from the very beginning and through the development of joint degenerative deterioration. Obesity is interrelated with OA pathogenesis and contributes to the disease process via multiple connection points, namely, dyslipidemia, IR, and adipokines. Several adipokines have been reported to be associated with OA. Particularly, metrnl and RBP4, not only have been reported to be associated with OA, but also, they are produced by chondrocytes. This sheds light on their strong potential to provide novel therapeutic targets for OA, and also highlights that further studies are warranted to further elucidate their molecular mechanisms in OA. Conclusively, several emerging findings establish OA as a metabolic disease and highlight the metabolic contribution of obesity in OA pathogenesis rather than the traditional mechanical-loading theory. Careful consideration for the various molecular and metabolic mechanisms interrelated with obesity-OA interplay will undoubtedly unveil a plethora of novel therapeutic targets and new avenues for OA treatment.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Authors confirm that all relevant data are included in the paper and/or supplementary information files.
Declaration of interest’s statement.
The authors declare no competing interests.
Contributor Information
Basma H. Sobieh, Email: basma.hassaneen@pharma.asu.edu.eg.
Hala O. El-Mesallamy, Email: hala_elmosalamy@hotmail.com.
Dina H. Kassem, Email: dina_kassem@pharma.asu.edu.eg.
Abbreviations
- OA
osteoarthritis
- HA
hyaluronic acid
- PRP
platelet-rich plasma
- MSC
mesenchymal stem cells
- VSF
vascular stromal fraction
- EVs
extracellular vesicles
- BMI
body mass index
- IL
interleukin
- TNF-α
tumor necrosis factor α
- NF-κB
nuclear factor-kappa B
- IR
insulin resistance
- LXR
liver X receptor
- CH25H
cholesterol 25-hydroxylase
- CYP7B1
25-hydroxycholesterol 7α-hydroxylase
- MMP
matrix metalloproteinase
- mtROS
mitochondrial-reactive oxygen species
- LCAT
lecithin–cholesterol acyltransferase
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- FFA
free fatty acids
- ROS
reactive oxygen species
- Ox-LDL
oxidized-LDL
- VEGF
vascular endothelial growth factor
- ACC
acetyl co-A carboxylase
- AGE
advanced glycation end product
- RAGE
receptors of AGE
- TLR
toll-like receptors
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- RBP4
retinol-binding protein 4
- metrnl
meteorin-like
- Akt
protein kinase B
- AMPK
adenosine monophosphate-activated protein kinase
- STAT3
signal transducer and activator of transcription protein-3
- RA
rheumatoid arthritis
- SF
synovial fluid
- PI3K
Phosphoinositide-3-kinases
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinases
- CoQ10
coenzyme Q10
- PMA
personalized metabolic avatar
References
- 1.Leifer V.P., Katz J.N., Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30(1):10–16. doi: 10.1016/j.joca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson G.P., Letson H.L., Grant A., McEwen P., et al. Defining the osteoarthritis patient: back to the future. Osteoarthritis Cartilage. 2018;26(8):1003–1007. doi: 10.1016/j.joca.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 3.OARSI, Osteoarthritis Research Society International Rsearch . Osteoarthritis: A Serious Disease. 2016. https://www.oarsi.org/research/oa-serious-disease [Google Scholar]
- 4.Bindu S., Mazumder S., Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anil U., Markus D.H., Hurley E.T., Manjunath A.K., et al. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee. 2021;32:173–182. doi: 10.1016/j.knee.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Emami A., Tepper J., Short B., Yaksh T.L., et al. Toxicology evaluation of drugs administered via uncommon routes: intranasal, intraocular, intrathecal/intraspinal, and intra-articular. Int. J. Toxicol. 2018;37(1):4–27. doi: 10.1177/1091581817741840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong M., Cheng C., Sun X., Yan Y., et al. Efficacy and safety of intra-articular platelet-rich plasma in osteoarthritis knee: a systematic review and meta-analysis. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/2191926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao D., Pan J.K., Yang W.Y., Han Y.H., et al. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy. 2021;37(7):2298–2314. doi: 10.1016/j.arthro.2021.02.045. e10. [DOI] [PubMed] [Google Scholar]
- 9.Singh H., Knapik D.M., Polce E.M., Eikani C.K., et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am. J. Sports Med. 2022;50(11):3140–3148. doi: 10.1177/03635465211029659. [DOI] [PubMed] [Google Scholar]
- 10.Belk J.W., Houck D.A., Littlefield C.P., Kraeutler M.J., et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of level I and II randomized controlled trials. Arthroscopy. 2022;38(6):2035–2046. doi: 10.1016/j.arthro.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Sax O.C., Chen Z., Mont M.A., Delanois R.E. The efficacy of platelet-rich plasma for the treatment of knee osteoarthritis symptoms and structural changes: a systematic review and meta-analysis. J. Arthroplasty. 2022;37(11):2282–2290. doi: 10.1016/j.arth.2022.05.014. e2. [DOI] [PubMed] [Google Scholar]
- 12.Matas J., Orrego M., Amenabar D., Infante C., et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Translat. Med. 2018;8(3):215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W.-S., Kim H.J., Kim K.-I., Kim G.B., et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Translat. Med. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaeili A., Hosseini S., Kamali A., Hosseinzadeh M., et al. Co-aggregation of MSC/chondrocyte in a dynamic 3D culture elevates the therapeutic effect of secreted extracellular vesicles on osteoarthritis in a rat model. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-22592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai M., Hotham W.E., Pattison L.A., Ma Q., et al. Role of human mesenchymal stem cells and derived extracellular vesicles in reducing sensory neuron hyperexcitability and pain behaviors in murine osteoarthritis. Arthritis Rheumatol. 2023 doi: 10.1002/art.42353. n/a(n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Z., Li Y., Gao F., Wu R., et al. Mesenchymal stem cells: a new choice for nonsurgical treatment of OA? Results from a bayesian network meta-analysis. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6663003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Yang H., He F., Zhu X. Intra-articular injection choice for osteoarthritis: making sense of cell source—an updated systematic review and dual network meta-analysis. Arthritis Res. Ther. 2022;24(1):260. doi: 10.1186/s13075-022-02953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC, Centers of Disease Control and Prevention 2020. https://www.cdc.gov/arthritis/data_statistics/cost.htm Data and Statistics.
- 19.Primorac D., Molnar V., Rod E., Jeleč Ž., et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020;11(8):854. doi: 10.3390/genes11080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oever I.A.M.v.d., Visman I.M., Rooij M.d., Roorda L.D., et al. Cardiovascular disease is associated with activity limitations in osteoarthritis patients. Int. J. Clin. Rheumatol. 2019;14:99. [Google Scholar]
- 21.Hall A.J., Stubbs B., Mamas M.A., Myint P.K., et al. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. European J. Prevent. Cardiol. 2020;23(9):938–946. doi: 10.1177/2047487315610663. [DOI] [PubMed] [Google Scholar]
- 22.WHO, World Health Organization 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Fact sheets.
- 23.World Obesity Atlas.https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2022.pdf. 2022.
- 24.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L., Tian W., Wang Y., Rong J., et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Reyes C., Leyland Kirsten M., Peat G., Cooper C., et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 2016;68(8):1869–1875. doi: 10.1002/art.39707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen R., Astrup A., Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13(1):20–27. doi: 10.1016/j.joca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Bliddal H., Leeds A.R., Stigsgaard L., Astrup A., et al. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann. Rheum. Dis. 2011;70(10):1798–1803. doi: 10.1136/ard.2010.142018. [DOI] [PubMed] [Google Scholar]
- 29.Richette P., Poitou C., Garnero P., Vicaut E., et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann. Rheum. Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 30.Gudbergsen H., Boesen M., Lohmander L.S., Christensen R., et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage. 2012;20(6):495–502. doi: 10.1016/j.joca.2012.02.639. [DOI] [PubMed] [Google Scholar]
- 31.Messier S.P., Gutekunst D.J., Davis C., DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 32.Aaboe J., Bliddal H., Messier S.P., Alkjaer T., et al. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):822–828. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Messier S.P., Legault C., Loeser R.F., Van Arsdale S.J., et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis Cartilage. 2011;19(3):272–280. doi: 10.1016/j.joca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang T., Zhou X., Jin M., Nie J., et al. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021;45(5):1125–1136. doi: 10.1007/s00264-021-04938-1. [DOI] [PubMed] [Google Scholar]
- 35.Felson D.T. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21(1):10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017;13(5):302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 37.Wang T., He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q. Apoptosis activation and autophagy inhibition of chondrocytes by leptin by the upregulation of LOXL3 in osteoarthritis pathogenesis. J. Healthc. Eng. 2022;2022 doi: 10.1155/2022/4026128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta K., Shukla M., Cowland J.B., Malemud C.J., et al. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56(10):3326–3335. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 40.Baudart P., Louati K., Marcelli C., Berenbaum F., et al. Association between osteoarthritis and dyslipidaemia: a systematic literature review and meta-analysis. RMD Open. 2017;3(2):e000442. doi: 10.1136/rmdopen-2017-000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pragasam S.S.J., Venkatesan V. Metabolic syndrome predisposes to osteoarthritis: lessons from model system. Cartilage. 2020;(1):1598S–1609S. doi: 10.1177/1947603520980161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchetina E.V., Markova G.A., Sharapova E.P. Insulin resistance in osteoarthritis: similar mechanisms to type 2 diabetes mellitus. J. Nutrit. Metabolism. 2020;2020 doi: 10.1155/2020/4143802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong J., Long J., Chen X., Li Y., et al. Dyslipidemia might Be associated with an increased risk of osteoarthritis. BioMed Res. Int. 2020;2020:3105248. doi: 10.1155/2020/3105248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y., Zhou W., Zhong Z., Zhao Z., et al. Metabolic syndrome, hypertension, and hyperglycemia were positively associated with knee osteoarthritis, while dyslipidemia showed no association with knee osteoarthritis. Clin. Rheumatol. 2021;40(2):711–724. doi: 10.1007/s10067-020-05216-y. [DOI] [PubMed] [Google Scholar]
- 45.Schwager J.L., Nevitt M.C., Torner J., Lewis C.E., et al. Association of serum low-density lipoprotein, high-density lipoprotein, and total cholesterol with development of knee osteoarthritis. Arthritis Care Res. 2022;74(2):274–280. doi: 10.1002/acr.24455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan F., Tian J., Mattap S.M., Cicuttini F., et al. Association between metabolic syndrome and knee structural change on MRI. Rheumatology. 2020;59(1):185–193. doi: 10.1093/rheumatology/kez266. [DOI] [PubMed] [Google Scholar]
- 47.Tsezou A., Iliopoulos D., Malizos K.N., Simopoulou T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J. Orthop. Res. 2010;28(8):1033–1039. doi: 10.1002/jor.21084. [DOI] [PubMed] [Google Scholar]
- 48.Thijssen E., van Caam A., van der Kraan P.M. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology. 2015;54(4):588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 49.Papathanasiou I., Anastasopoulou L., Tsezou A. Cholesterol metabolism related genes in osteoarthritis. Bone. 2021;152 doi: 10.1016/j.bone.2021.116076. [DOI] [PubMed] [Google Scholar]
- 50.Ntoumou E., Tzetis M., Braoudaki M., Lambrou G., et al. Serum microRNA array analysis identifies miR-140-3p, miR-33b-3p and miR-671-3p as potential osteoarthritis biomarkers involved in metabolic processes. Clin. Epigenet. 2017;9(1):127. doi: 10.1186/s13148-017-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S., Oh J., Kim Y.-I., Choe S.-K., et al. Suppression of ABCD2 dysregulates lipid metabolism via dysregulation of miR-141:ACSL4 in human osteoarthritis. Cell Biochem. Funct. 2018;36(7):366–376. doi: 10.1002/cbf.3356. [DOI] [PubMed] [Google Scholar]
- 52.He H., Lu M., Shi H., Yue G., et al. Vaspin regulated cartilage cholesterol metabolism through miR155/LXRα and participated in the occurrence of osteoarthritis in rats. Life Sci. 2021;269 doi: 10.1016/j.lfs.2021.119096. [DOI] [PubMed] [Google Scholar]
- 53.Griffin T.M., Fermor B., Huebner J.L., Kraus V.B., et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res. Ther. 2010;12(4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gierman L.M., Van Der Ham F., Koudijs A., Wielinga P.Y., et al. Metabolic stress–induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(4):1172–1181. doi: 10.1002/art.33443. [DOI] [PubMed] [Google Scholar]
- 55.Larrañaga-Vera A., Lamuedra A., Pérez-Baos S., Prieto-Potin I., et al. Increased synovial lipodystrophy induced by high fat diet aggravates synovitis in experimental osteoarthritis. Arthritis Res. Ther. 2017;19(1):264. doi: 10.1186/s13075-017-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Visser H.M., Mastbergen S.C., Kozijn A.E., Coeleveld K., et al. Metabolic dysregulation accelerates injury-induced joint degeneration, driven by local inflammation; an in vivo rat study. J. Orthop. Res. 2018;36(3):881–890. doi: 10.1002/jor.23712. [DOI] [PubMed] [Google Scholar]
- 57.Farnaghi S., Crawford R., Xiao Y., Prasadam I. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int. J. Rheumat. Diseases. 2017;20(2):131–140. doi: 10.1111/1756-185x.13061. [DOI] [PubMed] [Google Scholar]
- 58.Gentili C., Tutolo G., Pianezzi A., Cancedda R., et al. Cholesterol secretion and homeostasis in chondrocytes: a liver X receptor and retinoid X receptor heterodimer mediates apolipoprotein A1 expression. Matrix Biol. 2005;24(1):35–44. doi: 10.1016/j.matbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X., Owen J.S., Wilson M.D., Li H., et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51(11):3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi W.-S., Lee G., Song W.-H., Koh J.-T., et al. The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566(7743):254–258. doi: 10.1038/s41586-019-0920-1. [DOI] [PubMed] [Google Scholar]
- 61.De Munter W., Blom A.B., Helsen M.M., Walgreen B., et al. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res. Ther. 2013;15(6):R178. doi: 10.1186/ar4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farnaghi S., Prasadam I., Cai G., Friis T., et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterolinduced osteoarthritis. Faseb. J. 2017;31(1):356–367. doi: 10.1096/fj.201600600r. [DOI] [PubMed] [Google Scholar]
- 63.Haj-Mirzaian A., Mohajer B., Guermazi A., Conaghan P.G., et al. Statin use and knee osteoarthritis outcome measures according to the presence of heberden nodes: results from the osteoarthritis initiative. Radiology. 2019;293(2):396–404. doi: 10.1148/radiol.2019190557. [DOI] [PubMed] [Google Scholar]
- 64.Juybari K.B., Hosseinzadeh A., Sharifi A.M. Protective effects of atorvastatin against high glucose-induced nuclear factor-κB activation in cultured C28I2 chondrocytes. J. Recept. Signal Transduction. 2019;39(1):1–8. doi: 10.1080/10799893.2018.1557206. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka T., Matsushita T., Nishida K., Takayama K., et al. Attenuation of osteoarthritis progression in mice following intra-articular administration of simvastatin-conjugated gelatin hydrogel. J. Tissue Eng. Regenerat. Med. 2019;13(3):423–432. doi: 10.1002/term.2804. [DOI] [PubMed] [Google Scholar]
- 66.Veronese N., Koyanagi A., Stubbs B., Cooper C., et al. Statin use and knee osteoarthritis outcomes: a longitudinal cohort study. Arthritis Care Res. 2019;71(8):1052–1058. doi: 10.1002/acr.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Triantaphyllidou I.E., Kalyvioti E., Karavia E., Lilis I., et al. Perturbations in the HDL metabolic pathway predispose to the development of osteoarthritis in mice following long-term exposure to western-type diet. Osteoarthritis Cartilage. 2013;21(2):322–330. doi: 10.1016/j.joca.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Mooney R.A., Sampson E.R., Lerea J., Rosier R.N., et al. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res. Ther. 2011;13(6):R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Munter W., van den Bosch M.H., Slöetjes A.W., Croce K.J., et al. High LDL levels lead to increased synovial inflammation and accelerated ectopic bone formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2016;24(5):844–855. doi: 10.1016/j.joca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Engin A.B. What is lipotoxicity? Adv. Exp. Med. Biol. 2017;960:197–220. doi: 10.1007/978-3-319-48382-5_8. [DOI] [PubMed] [Google Scholar]
- 71.Wang R., Ding G., Liang W., Chen C., et al. Role of LOX-1 and ROS in oxidized low-density lipoprotein induced epithelial-mesenchymal transition of NRK52E. Lipids Health Dis. 2010;9:120. doi: 10.1186/1476-511x-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mollazadeh H., Carbone F., Montecucco F., Pirro M., et al. Oxidative burden in familial hypercholesterolemia. J. Cell. Physiol. 2018;233(8):5716–5725. doi: 10.1002/jcp.26466. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura S., Akagi M., Yoshida K., Hayakawa S., et al. Oxidized low-density lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1 (LOX-1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF-κB. Osteoarthritis Cartilage. 2004;12(7):568–576. doi: 10.1016/j.joca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Akagi M., Nishimura S., Yoshida K., Kakinuma T., et al. Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized ldl receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J. Orthop. Res. 2006;24(8):1782–1790. doi: 10.1002/jor.20211. [DOI] [PubMed] [Google Scholar]
- 75.Kanata S., Akagi M., Nishimura S., Hayakawa S., et al. Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-γ. Biochem. Biophys. Res. Commun. 2006;348(3):1003–1010. doi: 10.1016/j.bbrc.2006.07.133. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez-Garcia O., Rogers N.H., Smith R.G., Lotz M.K. Palmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 2014;66(7):1779–1788. doi: 10.1002/art.38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto K., Akagi M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: a narrative review. J. Int. Med. Res. 2020;48(6) doi: 10.1177/0300060520931609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen P., Jiao Z., Zheng J.S., Xu W.F., et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci. Rep. 2015;5 doi: 10.1038/srep16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen M.T., Favelyukis S., Nguyen A.K., Reichart D., et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 80.Liu H., Witzigreuter L., Sathiaseelan R., Agbaga M.P., et al. Obesity promotes lipid accumulation in mouse cartilage-A potential role of acetyl-CoA carboxylase (ACC) mediated chondrocyte de novo lipogenesis. J. Orthop. Res. 2022;12:2771–2779. doi: 10.1002/jor.25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma H., Xie C., He G., Chen Z., et al. Sparstolonin B suppresses free fatty acid palmitate-induced chondrocyte inflammation and mitigates post-traumatic arthritis in obese mice. J. Cell Mol. Med. 2022;26(3):725–735. doi: 10.1111/jcmm.17099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai L., Okumu F.W., Cleland J.L., Beresini M., et al. A slow release formulation of insulin as a treatment for osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):692–706. doi: 10.1053/joca.2002.0813. [DOI] [PubMed] [Google Scholar]
- 83.Kayal R.A., Alblowi J., McKenzie E., Krothapalli N., et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44(2):357–363. doi: 10.1016/j.bone.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosa S.C., Rufino A.T., Judas F., Tenreiro C., et al. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthritis Cartilage. 2011;19(6):719–727. doi: 10.1016/j.joca.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Hamada D., Maynard R., Schott E., Drinkwater C.J., et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol. 2016;68(6):1392–1402. doi: 10.1002/art.39561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Griffin T.M., Huffman K.M. Editorial: insulin resistance: releasing the brakes on synovial inflammation and osteoarthritis? Arthritis Rheumatol. 2016;68(6):1330–1333. doi: 10.1002/art.39586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sears B., Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oppenheim M.L.S., Hargreaves I.P., Pope S., Land J.M., et al. Mitochondrial cytochrome c release: a factor to consider in mitochondrial disease? J. Inherit. Metab. Dis. 2009;32(2):269–273. doi: 10.1007/s10545-009-1061-8. [DOI] [PubMed] [Google Scholar]
- 89.Cillero-Pastor B., Caramés B., Lires-Deán M., Vaamonde-García C., et al. Mitochondrial dysfunction activates cyclooxygenase 2 expression in cultured normal human chondrocytes. Arthritis Rheum. 2008;58(8):2409–2419. doi: 10.1002/art.23644. [DOI] [PubMed] [Google Scholar]
- 90.Kalpage H.A., Wan J., Morse P.T., Zurek M.P., et al. Cytochrome c phosphorylation: control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020;121 doi: 10.1016/j.biocel.2020.105704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Askari A., Ehrampoush E., Homayounfar R., Bahramali E., et al. Serum insulin in pathogenesis and treatment of osteoarthritis. Med. Hypotheses. 2017;99:45–46. doi: 10.1016/j.mehy.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 92.Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front. Endocrinol. 2012;3:153. doi: 10.3389/fendo.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piva S.R., Susko A.M., Khoja S.S., Josbeno D.A., et al. Links between osteoarthritis and diabetes:implications for management from a physical activity perspective. Clin. Geriatr. Med. 2015;31(1):67–87. doi: 10.1016/j.cger.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J.E., Kim J.S., Jo M.J., Cho E., et al. The roles and associated mechanisms of adipokines in development of metabolic syndrome. Molecules. 2022;27(2):334. doi: 10.3390/molecules27020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carrión M., Frommer K.W., Pérez-García S., Müller-Ladner U., et al. The adipokine network in rheumatic joint diseases. Int. J. Mol. Sci. 2019;20(17):4091. doi: 10.3390/ijms20174091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Francisco V., Ruiz-Fernández C., Pino J., Mera A., et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019;165:196–206. doi: 10.1016/j.bcp.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 97.Lee K.T., Chen B.C., Liu S.C., Lin Y.Y., et al. Nesfatin-1 facilitates IL-1β production in osteoarthritis synovial fibroblasts by suppressing miR-204-5p synthesis through the AP-1 and NF-κB pathways. Aging (Albany NY) 2021;13(18):22490–22501. doi: 10.18632/aging.203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zapata-Linares N., Eymard F., Berenbaum F., Houard X. Role of adipose tissues in osteoarthritis. Curr. Opin. Rheumatol. 2021;33(1):84–93. doi: 10.1097/bor.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 99.Jiang L., Xu K., Li J., Zhou X., et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging (Albany NY) 2020;12(2):1760–1777. doi: 10.18632/aging.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bao J.P., Jiang L.F., Li J., Chen W.P., et al. Visceral adipose tissue-derived serine protease inhibitor inhibits interleukin-1β-induced catabolic and inflammatory responses in murine chondrocytes. Mol. Med. Rep. 2014;10(4):2191–2197. doi: 10.3892/mmr.2014.2478. [DOI] [PubMed] [Google Scholar]
- 101.Yang S., Ryu J.H., Oh H., Jeon J., et al. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015;74(3):595–602. doi: 10.1136/annrheumdis-2013-204355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abella V., Scotece M., Conde J., López V., et al. The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Sci. Rep. 2016;6 doi: 10.1038/srep20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chai B., Zheng Z.H., Liao X., Li K.Y., et al. The protective role of omentin-1 in IL-1β-induced chondrocyte senescence. Artif. Cells, Nanomed. Biotechnol. 2020;48(1):8–14. doi: 10.1080/21691401.2019.1699803. [DOI] [PubMed] [Google Scholar]
- 104.Wang C., Zhang S., Huang L., Liu J., et al. Chemerin promotes MAPK/ERK activation to induce inflammatory factor production in rat synoviocytes. Exp. Ther. Med. 2022;24(5):684. doi: 10.3892/etm.2022.11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao C.W., Song W.X., Liu B., Gao Y.H., et al. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Clin. Exp. Rheumatol. 2022;40(3):501–513. doi: 10.55563/clinexprheumatol/avcj31. [DOI] [PubMed] [Google Scholar]
- 106.Duan Z.-x., Tu C., Liu Q., Li S.-q., et al. Adiponectin receptor agonist AdipoRon attenuates calcification of osteoarthritis chondrocytes by promoting autophagy. J. Cell. Biochem. 2020;121(5–6):3333–3344. doi: 10.1002/jcb.29605. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z.M., Shen C., Li H., Fan Q., et al. Leptin induces the apoptosis of chondrocytes in an in vitro model of osteoarthritis via the JAK2-STAT3 signaling pathway. Mol. Med. Rep. 2016;13(4):3684–3690. doi: 10.3892/mmr.2016.4970. [DOI] [PubMed] [Google Scholar]
- 108.Zhao X., Huang P., Li G., Lv Z., et al. Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis. Bone Joint Res. 2019;8(9):425–436. doi: 10.1302/2046-3758.89.bjr-2018-0325.r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang M., He J., Sun Y., Dong X., et al. Leptin induced TLR4 expression via the JAK2-STAT3 pathway in obesity-related osteoarthritis. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/7385160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hui W., Litherland G.J., Elias M.S., Kitson G.I., et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 2012;71(3):455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 111.Yaykasli K.O., Hatipoglu O.F., Yaykasli E., Yildirim K., et al. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-ĸB signaling pathways in human chondrocytes. Cell Biol. Int. 2015;39(1):104–112. doi: 10.1002/cbin.10336. [DOI] [PubMed] [Google Scholar]
- 112.Vuolteenaho K., Koskinen A., Kukkonen M., Nieminen R., et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediat. Inflamm. 2009;2009 doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eisinger K., Bauer S., Schäffler A., Walter R., et al. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp. Mol. Pathol. 2012;92(1):90–96. doi: 10.1016/j.yexmp.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Ma J., Ren L., Guo C.J., Wan N.J., et al. Chemerin affects the metabolic and proliferative capabilities of chondrocytes by increasing the phosphorylation of AKT/ERK. Eur. Rev. Med. Pharmacol. Sci. 2018;22(12):3656–3662. doi: 10.26355/eurrev_201806_15243. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z., Xing X., Hensley G., Chang L.W., et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62(7):1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee S., Lee H.C., Kwon Y.W., Lee S.E., et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metabol. 2014;19(3):484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gosset M., Berenbaum F., Salvat C., Sautet A., et al. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 2008;58(5):1399–1409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]
- 118.Laiguillon M.-C., Houard X., Bougault C., Gosset M., et al. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res. Ther. 2014;16(1):R38. doi: 10.1186/ar4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baek J.M., Ahn S.J., Cheon Y.H., Lee M.S., et al. Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation in vitro. Mol. Med. Rep. 2017;15(2):784–792. doi: 10.3892/mmr.2016.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han D.F., Li Y., Xu H.Y., Li R.H., et al. An update on the emerging role of visfatin in the pathogenesis of osteoarthritis and pharmacological intervention. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/8303570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scotece M., Conde J., Abella V., López V., et al. NUCB2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. J. Orthop. Res. 2014;32(5):653–660. doi: 10.1002/jor.22585. [DOI] [PubMed] [Google Scholar]
- 122.Chen T.H., Chen L., Hsieh M.S., Chang C.P., et al. Evidence for a protective role for adiponectin in osteoarthritis. Biochim. Biophys. Acta. 2006;1762(8):711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 123.Xie C., Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019;21(12):71. doi: 10.1007/s11926-019-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu J., Cui W., Ding W., Gu Y., et al. Globular adiponectin attenuated H2O2-induced apoptosis in rat chondrocytes by inducing autophagy through the AMPK/mTOR pathway. Cell. Physiol. Biochem. 2017;43(1):367–382. doi: 10.1159/000480416. [DOI] [PubMed] [Google Scholar]
- 125.Guo F., Lai Y., Tian Q., Lin E.A., et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62(7):2023–2036. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y.P., Liu B., Tian Q.Y., Wei J.L., et al. Progranulin protects against osteoarthritis through interacting with TNF-α and β-Catenin signalling. Ann. Rheum. Dis. 2015;74(12):2244–2253. doi: 10.1136/annrheumdis-2014-205779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bao J.P., Xu L.H., Ran J.S., Xiong Y., et al. Vaspin prevents leptin-induced inflammation and catabolism by inhibiting the activation of nuclear factor-κB in rat chondrocytes. Mol. Med. Rep. 2017;16(3):2925–2930. doi: 10.3892/mmr.2017.6911. [DOI] [PubMed] [Google Scholar]
- 128.Wang J., Zhang K., Zhang S., Guan Z. Vaspin promotes chondrogenic differentiation of BMSCs via Akt activation in osteoarthritis. BMC Muscoskel. Disord. 2022;23(1):344. doi: 10.1186/s12891-022-05295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Z., Liu B., Zhao D., Wang B., et al. Omentin-1 prevents cartilage matrix destruction by regulating matrix metalloproteinases. Biomed. Pharmacother. 2017;92:265–269. doi: 10.1016/j.biopha.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 130.Li Z., Zhang Y., Tian F., Wang Z., et al. Omentin-1 promotes mitochondrial biogenesis via PGC1α-AMPK pathway in chondrocytes. Arch. Physiol. Biochem. 2020:1–7. doi: 10.1080/13813455.2020.1819337. [DOI] [PubMed] [Google Scholar]
- 131.Sobieh B.H., Kassem D.H., Zakaria Z.M., El-Mesallamy H.O. Potential emerging roles of the novel adipokines adipolin/CTRP12 and meteorin-like/METRNL in obesity-osteoarthritis interplay. Cytokine. 2021;138 doi: 10.1016/j.cyto.2020.155368. [DOI] [PubMed] [Google Scholar]
- 132.Liu J., Jia S., Yang Y., Piao L., et al. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2022;158 doi: 10.1016/j.biopha.2022.114118. [DOI] [PubMed] [Google Scholar]
- 133.Scotece M., Koskinen-Kolasa A., Pemmari A., Leppänen T., et al. Novel adipokine associated with OA: retinol binding protein 4 (RBP4) is produced by cartilage and is correlated with MMPs in osteoarthritis patients. Inflamm. Res. 2020;69(4):415–421. doi: 10.1007/s00011-020-01326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conde J., Gomez R., Bianco G., Scotece M., et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 2011;70(3):551. doi: 10.1136/ard.2010.132399. [DOI] [PubMed] [Google Scholar]
- 135.Conde J., Scotece M., Abella V., Gómez R., et al. Identification of novel adipokines in the joint. Differential expression in healthy and osteoarthritis tissues. PLoS One. 2015;10(4):e0123601. doi: 10.1371/journal.pone.0123601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z.-Y., Zheng S.-L., Wang P., Xu T.-Y., et al. Subfatin is a novel adipokine and unlike meteorin in adipose and brain expression. CNS Neurosci. Ther. 2014;20(4):344–354. doi: 10.1111/cns.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ushach I., Burkhardt A.M., Martinez C., Hevezi P.A., et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin. Immunol. 2015;156(2):119–127. doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jørgensen J.R., Fransson A., Fjord-Larsen L., Thompson L.H., et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp. Neurol. 2012;233(1):172–181. doi: 10.1016/j.expneurol.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 139.Li Z.Y., Song J., Zheng S.L., Fan M.B., et al. Adipocyte metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes. 2015;64(12):4011. doi: 10.2337/db15-0274. [DOI] [PubMed] [Google Scholar]
- 140.Zheng S.-l., Li Z.-y., Song J., Liu J.-m., et al. Metrnl: a secreted protein with new emerging functions. Acta Pharmacol. Sin. 2016;37:571. doi: 10.1038/aps.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung T.W., Lee S.H., Kim H.-C., Bang J.S., et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018;50(9):122. doi: 10.1038/s12276-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fahmi H., Martel-Pelletier J Fau - Pelletier J.-P., Pelletier Jp Fau - Kapoor M., Kapoor M. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod. Rheumatol. 2011;21(1):1–9. doi: 10.1007/s10165-010-0347-x. [DOI] [PubMed] [Google Scholar]
- 143.Rao Rajesh R., Long Jonathan Z., James P. White, Svensson Katrin J., et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bridgewood C., Russell T., Weedon H., Baboolal T., et al. The novel cytokine Metrnl/IL-41 is elevated in Psoriatic Arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin. Immunol. 2019;208 doi: 10.1016/j.clim.2019.108253. [DOI] [PubMed] [Google Scholar]
- 145.Baht G.S., Bareja A., Lee D.E., Rao R.R., et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nature Metabolism. 2020;2(3):278–289. doi: 10.1038/s42255-020-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kerget B., Afşin D.E., Kerget F., Aşkın S., et al. Is metrnl an adipokine i?nvolved in the anti-inflammatory response to acute exacerbations of COPD? Lung. 2020;198(2):307–314. doi: 10.1007/s00408-020-00327-4. [DOI] [PubMed] [Google Scholar]
- 147.Gao X., Leung T.-F., Wong G.W.-K., Ko W.-H., et al. Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma. Cell. Mol. Immunol. 2022;19(2):245–259. doi: 10.1038/s41423-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fu X.-L., Duan W., Su C.-Y., Mao F.-Y., et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol. Immunother. 2017;66(12):1597–1608. doi: 10.1007/s00262-017-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Segatto I., Baldassarre G., Belletti B. STAT3 in breast cancer onset and progression: a matter of time and context. Int. J. Mol. Sci. 2018;19(9):2818. doi: 10.3390/ijms19092818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiao Z., Tang J., Wu W., Tang J., et al. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Compl. Alternative Med. 2019;19(1):264. doi: 10.1186/s12906-019-2673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang F., Guo Z., Yuan Y. STAT3 speeds up progression of osteoarthritis through NF-κB signaling pathway. Exp. Ther. Med. 2020;19(1):722–728. doi: 10.3892/etm.2019.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gong W., Liu Y., Wu Z., Wang S., et al. Meteorin-like shows unique expression pattern in bone and its overexpression inhibits osteoblast differentiation. PLoS One. 2016;11(10):e0164446. doi: 10.1371/journal.pone.0164446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Husa M., Liu-Bryan R., Terkeltaub R. Shifting HIFs in osteoarthritis. Nat. Med. 2010;16(6):641–644. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang R., Balu A.R., Molitoris K.H., White J.P., et al. The role of Meteorin-like in skeletal development and bone fracture healing. J. Orthop. Res. 2022;40(11):2510–2521. doi: 10.1002/jor.25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nguyen L.T., Sharma A.R., Chakraborty C., Saibaba B., et al. Review of prospects of biological fluid biomarkers in osteoarthritis. Int. J. Mol. Sci. 2017;18(3):601. doi: 10.3390/ijms18030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L., Cheng Y.-L., Xue S., Xu Z.-G. The role of circulating RBP4 in the type 2 diabetes patients with kidney diseases: a systematic review and meta-analysis. Dis. Markers. 2020:8830471. doi: 10.1155/2020/8830471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steinhoff J.S., Lass A., Schupp M. Biological functions of RBP4 and its relevance for human diseases. Front. Physiol. 2021;12:659977. doi: 10.3389/fphys.2021.659977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zemany L., Bhanot S., Peroni O.D., Murray S.F., et al. Transthyretin antisense oligonucleotides lower circulating RBP4 levels and improve insulin sensitivity in obese mice. Diabetes. 2015;64(5):1603–1614. doi: 10.2337/db14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boaghi A., Pop R.-M., Vasilache S.L., Banescu C., et al. Plasma RBP4 level in association with body composition, metabolic profile, STRA6 and RBP4 gene polymorphisms in obese Romanian children. Diabetes, Metab. Syndrome Obes. Targets Ther. 2020;13:4643–4650. doi: 10.2147/DMSO.S273146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gokulakrishnan K., Pandey G.K., Sathishkumar C., Sundararajan S., et al. Augmentation of RBP4/STRA6 signaling leads to insulin resistance and inflammation and the plausible therapeutic role of vildagliptin and metformin. Mol. Biol. Rep. 2021;48(5):4093–4106. doi: 10.1007/s11033-021-06420-y. [DOI] [PubMed] [Google Scholar]
- 161.Liu C., Zhou X.-R., Ye M.-Y., Xu X.-Q., et al. RBP4 is associated with insulin resistance in hyperuricemia-induced rats and patients with hyperuricemia. Front. Endocrinol. 2021;12:653819. doi: 10.3389/fendo.2021.653819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nono Nankam P.A., Blüher M. Retinol-binding protein 4 in obesity and metabolic dysfunctions. Mol. Cell. Endocrinol. 2021;531 doi: 10.1016/j.mce.2021.111312. [DOI] [PubMed] [Google Scholar]
- 163.Barreto G., Manninen M., K Eklund K. Osteoarthritis and toll-like receptors: when innate immunity meets chondrocyte apoptosis. Biology. 2020;9(4):65. doi: 10.3390/biology9040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li H., Cao G., Zhang N., Lou T., et al. RBP4 regulates trophoblastic cell proliferation and invasion via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2018;18(3):2873–2879. doi: 10.3892/mmr.2018.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y., Wang Y., Zhang Z. Adipokine RBP4 drives ovarian cancer cell migration. J. Ovarian Res. 2018;11(1):29. doi: 10.1186/s13048-018-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coleman M.C., Goetz J.E., Brouillette M.J., Seol D., et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci. Transl. Med. 2018;10(427):eaan5372. doi: 10.1126/scitranslmed.aan5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shimizu K., Kon M., Tanimura Y., Hanaoka Y., et al. Coenzyme Q10 supplementation downregulates the increase of monocytes expressing toll-like receptor 4 in response to 6-day intensive training in kendo athletes. Appl. Physiol. Nutr. Metabol. 2015;40(6):575–581. doi: 10.1139/apnm-2014-0556. [DOI] [PubMed] [Google Scholar]
- 168.Mantle D., Heaton R.A., Hargreaves I.P. Coenzyme Q10 and immune function: an overview. Antioxidants. 2021;10(5) doi: 10.3390/antiox10050759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Na H.S., Woo J.S., Kim J.H., Lee J.S., et al. Coenzyme Q10 encapsulated in micelles ameliorates osteoarthritis by inhibiting inflammatory cell death. PLoS One. 2022;17(6):e0270351. doi: 10.1371/journal.pone.0270351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cheleschi S., De Palma A., Pascarelli N.A., Giordano N., et al. Could oxidative stress regulate the expression of MicroRNA-146a and MicroRNA-34a in human osteoarthritic chondrocyte cultures? Int. J. Mol. Sci. 2017;18(12):2660. doi: 10.3390/ijms18122660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hosseinzadeh A., Jafari D., Kamarul T., Bagheri A., et al. Evaluating the protective effects and mechanisms of diallyl disulfide on interlukin-1β-induced oxidative stress and mitochondrial apoptotic signaling pathways in cultured chondrocytes. J. Cell. Biochem. 2017;118(7):1879–1888. doi: 10.1002/jcb.25907. [DOI] [PubMed] [Google Scholar]
- 172.Qiu L., Luo Y., Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018;103:1585–1591. doi: 10.1016/j.biopha.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Wang J., Wang K., Huang C., Lin D., et al. SIRT3 activation by dihydromyricetin suppresses chondrocytes degeneration via maintaining mitochondrial homeostasis. Int. J. Biol. Sci. 2018;14(13):1873–1882. doi: 10.7150/ijbs.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Z., Zhao C., Liu P., Huang H., et al. Anti-apoptosis and autophagy effects of melatonin protect rat chondrocytes against oxidative stress via regulation of AMPK/Foxo3 pathways. Cartilage. 2021;13(2_suppl):1041s–1053s. doi: 10.1177/19476035211038748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.El-Bidawy M.H., Omar Hussain A.B., Al-Ghamdi S., Aldossari K.K., et al. Resveratrol ameliorates type 2 diabetes mellitus-induced alterations to the knee joint articular cartilage ultrastructure in rats. Ultrastruct. Pathol. 2021;45(2):92–101. doi: 10.1080/01913123.2021.1882629. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Y., Hou M., Liu Y., Liu T., et al. Recharge of chondrocyte mitochondria by sustained release of melatonin protects cartilage matrix homeostasis in osteoarthritis. J. Pineal Res. 2022;73(2) doi: 10.1111/jpi.12815. [DOI] [PubMed] [Google Scholar]
- 177.Rodriguez-Merchan E.C. The current role of disease-modifying osteoarthritis drugs. Arch Bone Jt Surg. 2023;11(1):11–22. doi: 10.22038/abjs.2021.56530.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Corrado A., Colia R., Rotondo C., Sanpaolo E., et al. Changes in serum adipokines profile and insulin resistance in patients with rheumatoid arthritis treated with anti-TNF-α. Curr. Med. Res. Opin. 2019;35(12):2197–2205. doi: 10.1080/03007995.2019.1654988. [DOI] [PubMed] [Google Scholar]
- 179.Czókolyová M., Pusztai A., Végh E., Horváth Á., et al. Changes of metabolic biomarker levels upon one-year anti-TNF-α therapy in rheumatoid arthritis and ankylosing spondylitis: associations with vascular pathophysiology. Biomolecules. 2021;11(10):1535. doi: 10.3390/biom11101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.van den Oever I.A.M., Baniaamam M., Simsek S., Raterman H.G., et al. The effect of anti-TNF treatment on body composition and insulin resistance in patients with rheumatoid arthritis. Rheumatol. Int. 2021;41(2):319–328. doi: 10.1007/s00296-020-04666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Berry R., Kassavou A. Does self-monitoring diet and physical activity behaviors using digital technology support adults with obesity or overweight to lose weight? Systemat. Literat. Rev. Meta-Analysis. 2021;22(10) doi: 10.1111/obr.13306. [DOI] [PubMed] [Google Scholar]
- 182.Bianchetti G., Abeltino A., Serantoni C., Ardito F., et al. Personalized self-monitoring of energy balance through integration in a web-application of dietary, anthropometric, and physical activity data. J. Personalized Med. 2022;12(4):568. doi: 10.3390/jpm12040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Antwi F., Fazylova N., Garcon M.C., Lopez L., et al. The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: a systematic review. JBI Libr. Syst. Rev. 2012;10(42):1–14. doi: 10.11124/jbisrir-2012-248. [DOI] [PubMed] [Google Scholar]
- 184.Abeltino A., Bianchetti G., Serantoni C., Ardito C.F., et al. Personalized metabolic avatar: a data driven model of metabolism for weight variation forecasting and diet plan evaluation. Nutrients. 2022;14(17):3520. doi: 10.3390/nu14173520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors confirm that all relevant data are included in the paper and/or supplementary information files.