Abstract
In recent years commensal microorganisms are not just “passive occupants”, but important element of homeostasis. There are numerous reports documenting the composition and role of the gut, skin or vagina microbiome but the role of commensal organisms living in the lungs is relatively unknown. Pulmonary microbiome impact on the immune response of the host organism and may indicate new therapeutic directions. Lung microbiome, by modulating the expression of innate immunity genes, causes an increase in the concentration of interleukin (IL)-5, IL-10, interferon γ and C-C motif chemokine ligand 11, affects the toll-like receptor-4-dependent response of pulmonary macrophages and modulate the production of antibacterial peptides contained in the mucus. It is documented that disorders of the lung microbiome contribute to asthma or chronic obstructive pulmonary disease. However it is known that pulmonary dysbiosis also occurs in critically ill patients. It is possible, therefore, that microbiota-targeted therapy may constitute the future therapeutic direction in ICU.
Keywords: ICU, lung microbiome, lung-gut interaction
The human body is colonized by millions of microorganisms, which are not only ‘passive residents’ but also play an extremely important role in maintaining homeostasis [1]. The term ‘microbiota’ refers to all the microorganisms living in a particular environment. The commonly used term ‘microbiome’, on the other hand, is defined as the ensemble of genomes of the microbiota considered in terms of the modulatory factors and interactions among them. The above term was coined microbiome by Joshua Lederberg in 2001. Another important term is ‘dysbiosis’, which means alterations in the number, composition and function of the microflora [2]. Variably intensified imbalance of commensal flora is observed virtually in every pathology and is always accompanied by dysfunction of the immune system [3–5]. Therefore, a detailed understanding of the relationships between the microbiome and functioning of individual systems allows implementing the most appropriate treatment, both pharmacological and dietary.
THE GUT MICROBIOME
The human gastrointestinal (GI) tract is colonized by more than 100 trillion bacteria, both commensal and pathogenic, affecting homeostasis. The gut microbiome consists of four main phyla, which include Bacteroides (23%), Firmicutes (64%), Actinobacteria (3%) and Proteobacteria (8%) [6, 7]. It is worth noting that the composition of microflora varies depending on the GI segment and age of an individual [6]. The lowest number of bacteria is found in the stomach due to its low pH, mostly different species of the genera Lactobacillus, Veillonella and Helicobacter [7]. The major bacterial families found in the small intestine include Bacilli, Actinobacteria, Streptococcaceae, Actinomycinaeae and Corynebacteriaceae while Bacteroidetes predominate in the large intestine [7, 8]. The composition and activity of the gut microbiome is affected by diet, lifestyle, environment, and genetic conditions [9]. The gut microbiome plays an important role in nutrient absorption, synthesis of vitamins (K, B1, B6, B12, folic acid), amino acids, enzymes, and production of short-chain fatty acids (SCFA). For instance, cellulose and pectin are converted into SCFA and simple sugars. By-products, including acetate, propionate and butyrate, are involved in the production of energy in cells, strengthen the integrity of the epithelial barrier and play an essential role in immunomodulation and protection against pathogens. Scientific studies have shown that up to 10% of the energy supplied can come from the above metabolic processes, e.g. butyrate is an important energy source for cells in the large intestine. Furthermore, butyrate stimulates the production of cathelicidin, which shows antibacterial properties [10]. The GI microflora plays an equally important role in maintaining homeostasis of the immune system. The immune system of the GI tract has an exceptional ability to create immune tolerance to the ever-changing and huge microbiome. Moreover, it is capable of producing an effective immune response to pathogens. Therefore, the largest number of immune cells is found in the places colonised by commensal organisms, such as the GI tract or skin. Furthermore, the microbiome strengthens the intestinal epithelial integrity, stimulates the proliferation of enterocytes and mucin, affecting ‘the barrier immunity’ [11, 12]. The barrier formed by the epithelium, mucus, immunoglobulin A (IgA), antimicrobial peptides and immune cells is organized around hyperglycosylated mucin 2 (MUC2), which provides static protection and constrains the immunogenicity of gut antigens via the dendritic cells. Moreover, the intercellular junctions and the mucus produced by the goblet cells form an important barrier limiting the translocation of microbes [13]. Scientific studies have shown that the gut microbiome affects the production of secretory IgA antibodies and cationic antimicrobial peptides (CAMPs) by the epithelial cells, which additionally maintain the function of the mucosal barrier [14, 15]. The production of regenerating islet-derived protein 3-gamma (Reg III-γ), the expression of which begins immediately after birth, is controlled by the flora in a myeloid differentiation primary response gene 88 (MyD88)-dependent manner and has the direct bactericidal effect on Gram-positive pathogens [16, 17]. Moreover, antimicrobial peptides have the potential to maintain the microbiome-intestine barrier [18]. Commensal bacterial antigen-specific IgA is produced by the dendritic cells in the GI tract [19]. IgA interacts with B and T lymphocytes in Peyer’s patches [19]. Of note, IgA responses lack classical memory characteristics and can address the changes in the microflora composition [20].
THE LUNG MICROBIOME
In recent years, the belief that the lungs are “sterile” has been denied, although the role of commensal organisms inhibiting the lungs has not been explicitly documented. It is known, however, that during micro-aspiration of nasopharyngeal content or in cases of gastro-oesophageal reflux to the alveoli, microorganisms are constantly “supplied” [21, 22]. Studies in animals and healthy volunteers have demonstrated that the composition of the lung microbiome is different from that of the oral and gut microbiome [23, 24]. The exact composition of the lung microbiome is difficult to be determined, as difficulties in ideal sampling of materials significantly limit its reliability. The key factor affecting the reliability of bronchoscopic samples is their contamination with the nasopharyngeal bacterial flora. The difficulties in collecting the microbial material, representing a reliable composition of the lower respiratory tract microbiome, are lesser in intubated patients [24]. According to some reports, only highly invasive methods, such as an open lung biopsy, allow the examiner to obtain a reliable testing material [25]. The lung microbiome is a dynamic ecosystem that consists of a variety of commensal microorganisms, mostly bacteria whose distribution depends on the part of the respiratory system. Moreover, there is a complex integrity between the upper and lower respiratory tract microbiomes, which may be confirmed by the fact that Firmicutes, Bacteriodetes, Proteobacteria, Fusobacteria and Actinobacteria predominate in healthy lungs [26–30]. It is worth stressing that the density of the pulmonary microbiome is low, ranging from 103–105 CFU g−1 tissue under physiological conditions [31, 32]. Animal studies have revealed that the airway flora formed during the first weeks of life is crucial for the development of a properly functioning immune system. This flora affects the Helios (−) regulatory T cells (Tregs) and reduces the susceptibility to allergic respiratory diseases [33]. Early formation of the microbiome also affects the stability of microflora in the upper respiratory tract and lower susceptibility to infectious diseases [34]. Numerous observations have shown a close relationship between the upper and lower respiratory tract microbiomes, especially in acute and chronic inflammations, such as obstructive pulmonary disease or cystic fibrosis [35–38]. The above correlation is associated with microaspiration, especially in cases of gastro-oesophageal reflux disease or disorders of airway cleansing [35]. The respiratory microflora induces the differentiation of peripheral Tregs, which are essential for controlling type 2 immune responses. Experimental studies have revealed a significant relationship between the natural killer T cells (NKT cells) and the microbiome [39, 40]. In the absence of airway microflora, increased numbers of eosinophils and type 2 T helper (TH2) lymphocytes have been observed in animal lungs [39, 40]. The lung microbiome is believed to modulate the expression of immune cell genes by affecting an increase in interleukin-5 (IL-5), interleukin-10 (IL-10), interferon γ (IFN-γ), C-C motif chemokine ligand 11 (CCL11) and toll-like receptor-4 (TLR4)-dependent responses of the lung macrophages [41] (Figure 1).
FIGURE 1.
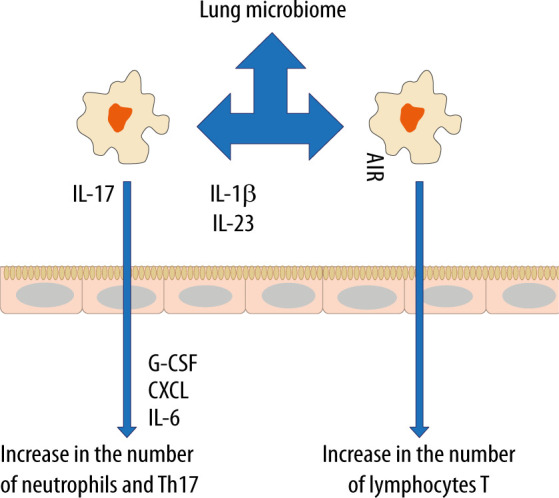
Changes in the lung microbiome alter the allergic response in the airways
The microbiome also affects the production of antibacterial peptides and proteins (AMPs) in the mucus, which are responsible for inhibiting the multiplication of pathogenic bacteria and for protecting the epithelium [42]. When the lung microbiome is absent or impaired, the production of these proteins is reduced and the susceptibility to infections caused by Pseudomonas aeruginosa, Streptococcus pneumoniae or Klebsiella pneumoniae is increased [43–45]. Of note, in the lungs of animals with the normal microbiome, the number of alveoli was found to be increased [31]. Moreover, it has been revealed that the presence of Proteobacteria increases the risk of developing asthma, partly due to an increased risk of viral infections, especially in the lower airways. Viral infections induce the release of thymic stromal lymphopoietin (TSLP), an epithelial cytokine (alarmin), IL-33 and IL-25 from the airway epithelium, causing type 2 inflammation [46].
THE GUT MICROBIOME AND THE RESPIRATORY SYSTEM
The gut microbiome harbours the largest and most diverse array of commensal bacteria, which shapes the host immune response [47]. It is stressed that imbalance of the gut microflora can affect distant organs, e.g. the brain, liver, skin or heart [48]. Such an imbalance can also contribute to various types of subsequent diseases such as post-traumatic stress disorder in patients with multiple organ injuries [49, 50]. Moreover, a highly significant association has been demonstrated between the gut microbiome and respiratory diseases [21, 51]. It is worth stressing, however, that not only the gut microbiome itself, but also its metabolites can stimulate the immune system within the lungs, forming the gut-lung axis [51, 52]. Metabolites, such as SCFA, have been shown to exert a systemic effect by stimulating the formation of antigen-presenting cells (APCs) filling the airway that induce the type 2 response to a lesser extent [53].
Pro-inflammatory metabolites of the dysbiotic gut microflora may also play an important role in inducing abnormal immune responses in the airways. Experimental studies have shown that the administration of lipopolysaccharide into the bronchial tree causes dysbiosis in the lungs, which leads to disorders of homeostasis within the gut microbiome [54]. Moreover, it has been documented that pneumonia caused by multiple drug resistance (MDR) pathogens, Staphylococcus aureus or Pseudomonas aeruginosa, contributes to endothelial damage and alterations in the gut microbiome [55]. In turn, gastrointestinal dysbiosis, especially in childhood, contributes to the development of asthma [56]. In children with a confirmed risk of asthma, a decreased amount of Rothia, Faecalibacterium, Lachnospira and Veillonella was observed in the GI tract [57]. Experiments in mice have demonstrated that colonization with the above microorganisms and with their metabolites, contributes to a milder course of allergic airway inflammation [58]. Dietary fibre is fermented by commensal bacteria in the colon to SCFAs that have anti-inflammatory effects, maintain gut homeostasis and epithelial integrity, regulate the Treg pool [58–60]. Animals receiving a high-fibre diet have been found to increase the production of SCFAs, including acetate, by the gut microflora, associated with the inhibition of inflammation in the airway. Based on the study results, acetate has been demonstrated to increase the acetylation of forkhead box P3 (FOXP3) by inhibiting histone deacetylase 9 (HDAC9) [61]. This relationship is most likely related to the activation of signallers of acetate-binding GPR 43 as an increased level of acetate in the intestinal loops, e.g. due to the diet used, induces the differentiation of Treg cells and inhibits histone deacetylase [61]. It should be emphasised that the number and functionality of Tregs in asthma is reduced [62]. Subsequent studies have revealed that diets rich in fibre, acting on the dendritic cells and macrophage precursors, stimulate the production of propionate by the microbiome, which reduces the inflammatory response induced by Th2 lymphocytes. In turn, butyrate inhibits the activation of IL-5, IL-13, and group 2 innate lymphoid cells (ILC2s), alleviating the symptoms of allergic pneumonia [63]. Thus, SCFAs produced by bacterial fermentation of dietary fibre modulate oxidative phosphorylation, glycolytic pathways in pulmonary ILC2s and GATA binding protein 3 (GATA3), affecting the production of IL-17a and recruitment of neutrophils into the airway [61–63] (Figure 2).
FIGURE 2.

Disorders of the lung microbiome, particularly the expansion of Bacteroides ovatus, B. stercoris, Prevotella melaninogenica, Sphingomonadaceae, Herbaspirillum, contribute to chronic inflammation, which favours the advancement of lung diseases, including fibrosis and cancers
THE LUNG MICROBIOME IN CRITICALLY ILL PATIENTS
Recently, considerable attention has been devoted to microbiome imbalance in the context of intensive care. In critically ill patients, the gut microbiome and the lung microbiome undergo profound changes. Dysbiosis in critically ill patients has a complex background. In order to explain impaired homeostasis of the lung microbiome, Dickson et al. have suggested three mechanisms influencing the composition and homeostasis of the microbiome [64]. The first is associated with the effects of migration, elimination, and reproduction of commensal microorganisms on the composition of the lung microflora. In this case, the factors determining microbiome reproduction are oxygen pressure, pH, blood flow, alveolar ventilation, temperature, and immune cells [30, 64]. In sepsis and acute respiratory distress syndrome (ARDS), the ongoing inflammatory process changes the physicochemical environment (pH, oxygen pressure, presence of free radicals) and alveolar metabolism. Areas of lung atelectasis and oedema promote an increase in the number of pathogenic microbes and ultimately lead to the development of pneumonia [65]. Furthermore, the oxygen concentration used during therapy has been shown to affect the community of bacteria in the lungs of animals and humans. Recent studies have revealed that hyperoxia causes a selective increase in Staphylococcus aureus in critically ill patients and an altered composition of the microbiome contributes to the development of pneumonia and organ damage [66]. The second mechanism assumes the influence of dietary factors; in healthy individuals, their airways are mainly filled with air and the availability of nutrients for bacteria is relatively limited [67]. Note, however, that in patients with chronic lung diseases, such as cystic fibrosis, chronic bronchitis or asthma, the airways contain dense, protein-rich mucus. Moreover, in ARDS or pneumonia, the alveoli are “flooded” with protein-rich fluid due to damage to the alveolar-capillary barrier, which seems to affect the lung microbiome [68]. The third mechanism is most likely related to intercellular signalling at the molecular level, which can be influenced by glucocorticoids, oestrogens, androgens, neurotransmitters (catecholamine, endogenous opioids) and cytokines such as TNF, IL-1, IL-6 and IL-8 [69, 70]. Imbalance in the lung microbiome is observed in asthma and chronic obstructive pulmonary diseases, as well as in critically ill patients without prior diseases [9, 71, 72] (Figure 3).
FIGURE 3.
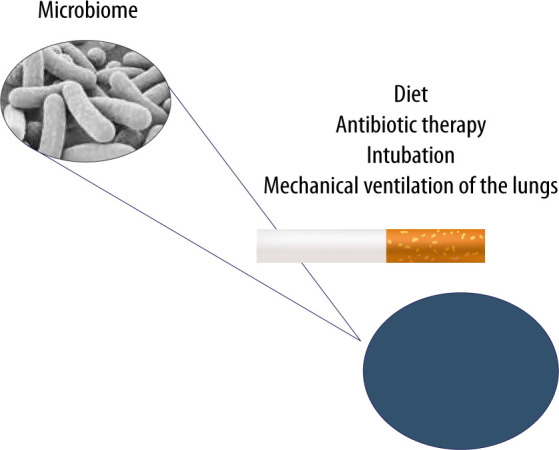
The lung microbiome can undergo modifications resulting from improper diet, ongoing infection, antibiotic therapy, cigarette smoking and mechanical ventilation of the lungs in cases of airway pathologies
The lung microbiome imbalance is believed to be affected by antibiotic therapy, endotracheal intubation itself and mechanical ventilation of the lungs [73–75]. The available studies have shown that the lung microbiome is enriched with gut microbes through bacterial translocation facilitated by increased intestinal and alveolar permeability in ARDS and sepsis. The presence of Bacteroidetes and Enterobacteriaceae in the lungs of seriously ill patients, which are characteristic of the gut microbiome, correlates with an increased inflammatory response and may affect the development of ARDS [3, 76, 77]. In cases of increased intestinal and alveolar endothelial permeability, bacteria migrate through the lymphatic system, systemic or portal circulation. The translocation of intestinal bacteria into the lungs is a mechanism potentially affecting the development of lung dysbiosis, inflammation and ultimately lung damage, e.g. associated with mechanical ventilation (called vaping-associated lung injury – VALI) [35]. Early lung dysbiosis in mechanically ventilated patients has been associated with an increase in inflammatory markers – IL-6 and IL-8 – and is strongly related to the development of late ARDS [77, 78]. The observed changes are also responsible for clinically relevant systemic disorders [32, 76]. The presence of artificial airways contributes to continuous micro-aspiration of the oropharyngeal flora and impaired mechanisms of purification of the respiratory tract. It should also be noted that the intubation procedure and mechanical ventilation promote micro-aspiration to the lungs, and the presence of an endotracheal tube significantly impairs the removal of bronchial tree secretions [75, 76]. In critically ill patients undergoing mechanical ventilation, the diversity of bacteria decreases, and opportunistic pathogens can become dominant. Therefore, VALI, considered according to traditional physiopathology, should be modified. Ventilator-associated pneumonia (VAP) should be addressed in terms of the presence of a microbiome and dysbiosis associated with broad-spectrum antibiotic therapy, as well as the need to include molecular techniques to diagnose and accelerate the use of immunomodulatory drugs or probiotics for prevention and treatment [74, 79]. Another important factor influencing the lung microbiome in critically ill patients is antibiotic therapy. Broad-spectrum antibiotic therapy disrupts the homeostasis of the microbiome and affects the development of mechanical ventilation-induced lung injury (VILI) [74, 80]. It is worth noting that impaired homeostasis of the lower airway microbiome is associated with an increased risk of pneumonia [35]. In HIV-infected patients, a close link has been observed between dysbiosis, versus increased Prevotella–Veillonella populations and the risk of severe pneumonia [81]. Moreover, the use of antibiotics, low diversity of the intestinal microbiome and enrichment of the gut microbe with Gammaproteobacteria increase the risk of pulmonary complications in patients after hematopoietic stem-cell transplantation (HSCT) [82]. Commensal microorganisms can also cause pneumonia, as in the case of Staphylococcus epidermidis [83]. This fact seems to prove a close link between the upper and lower airway microbiome and interactions with the population of the entire microbiome [35]. Clinical studies have demonstrated a significant correlation between the severity of ischaemic-reperfusion syndrome, versus impaired homeostasis of the microbiome, activation of the immune system, and epithelial damage [58]. The multiplication of Enterobacteriaceae, accompanied by a persistent inflammatory process, seems of particular importance [84]. Following ischaemic-reperfusion injury, the bacteria characteristic of the intestinal flora are detected in the lungs, serum and mesenteric cells [85, 86]. These bacteria use the activation of TLR 2, TLR 4 and MyD88, inducible nitric oxide synthase (iNOS) and reactive oxygen species to damage the cells. However, it should be stressed that IL-22 signalling and signal transducer and activator of transcription 3 (STAT3) protect the epithelial barrier of the intestines and can prevent translocation of Enterobacteriaceae following burn trauma. Additionally, IL-22 reduces inflammation in the lungs [87]. The impact of nutrition on the microbiome in ICU patients has been an important issue dealt with in many reports over recent years. Diet, quantity and type of individual nutrients affect the species composition of the gut microflora, modulate the number of individual species and their functions as a microbiome [88, 89]. Diets high in animal protein and fats has been found to lead to an increase in the number of Bacteroides in the composition of the gut microbiome, as compared to carbohydrate-high diets, leading to the dominance of Prevotella [90]. Moreover, dietary fibre affects the composition and metabolism of the microbiome. Low fibre intake endangers the mucous layer in the GI tract, causing microbiological instability accompanied by an increase in pathogenic strains and production of potentially harmful metabolites [91]. It should be stressed that in mice with a phenotype associated with high fat content and obesity, an increased number of Firmicutes and Proteobacteria as well as a reduced number of Bacteroidetes have been observed in the microbiome. Weight loss restores the original configuration by limiting fats and carbohydrates [92]. Recent studies have shown that the way nutritional therapy is conducted also diversely affects the microbiome [93]. Total parenteral nutrition changes the gut microbiome by increasing the number of Proteobacteria [94]. In animals receiving total parenteral nutrition, the altered composition of the intestinal lumen has favoured dysbiosis and dysfunction of the epithelial barrier [94]. Parenteral nutrition and fasting are associated with a loss of bacterial diversity that can change the interactions of the microbiome with the host immune system and the ability to control increases in the number of potentially more pathogenic bacteria, e.g. Escherichia coli, Salmonella, Yersinia and Helicobacter, fostering increased expression of pro-inflammatory cytokines in the intestinal mucosa [95]. Such disorders can lead to an increased risk of pneumonia caused by Streptococcus pneumoniae [80]. The mechanisms responsible for the above changes include lower expression of alkaline phosphatase in the enterocytes or activation of TLR receptors leading to the expression of tumour necrosis factor receptor (TNFR) in the epithelial cells, lower expression of cytoskeleton proteins and increased bacterial translocation [96]. Moreover, animals receiving total parenteral nutrition have demonstrated increased expression of interferon-γ in the intestinal epithelium [97]. On the other hand, enteral nutrition has been found to exert anti-inflammatory effects, as reflected in decreased concentrations of pro-inflammatory cytokines, TNF-α and IL-6, in serum and increased concentrations of anti-inflammatory cytokine IL-10, which is associated with lower mortality rates [93, 98].
THERAPEUTIC DIRECTIONS
Numerous studies have demonstrated that maintaining the microbiome balance can be another important therapeutic direction in critically ill patients treated in intensive care units. Intestinal microflora-targeted dietary interventions can potentially improve clinical outcomes, taking into account the connection between the GI tract and the respiratory system. In medical practice, the effects of nutrients on the microflora are neglected. Therefore, the purpose of nutritional therapy, especially in critically ill patients, should be broadened. Fibre-rich diets alter the microbiome in the GI tract or respiratory system and increase the concentration of SCFAs in the blood, reducing the inflammatory process associated with allergies and mortality rates in lung diseases [14, 99–101], as dietary fibre is a product consumed by commensal bacteria for the biosynthesis of SCFAs [102]. In patients with asthma, the diet has been shown to affect the systemic inflammatory response and the diet rich in fruit and vegetables exerts positive effects on the ongoing diseases [103, 104]. Analysis of the reports available in literature has revealed that the use of probiotics in ICUs reduces the incidence of ventilator-associated pneumonia without affecting the mortality and length of hospitalization [105]. Supplementation with probiotic bacteria such as Lactobacillus rhamnosus, Bifidobacterium lactis and B. breve, affects the inflammatory response to allergic lung diseases [106, 107]. Moreover, the use of Lactobacillus rhamnosus and Bifidobacterium breve in smokers with COPD inhibits the release of pro-inflammatory mediators by macrophages in response to cigarette smoke [108]. Moreover, it should be highlighted that probiotics have immunomodulatory effects, stimulating the natural smoking-suppressed activity of NK cells [109]. When analysing the treatment administered in critically ill patients, the effect of antibiotic therapy and steroid therapy on the lung microbiome should be considered. Inadequate antibiotic therapy in patients treated for COPD exacerbations has significantly impaired homeostasis of the lung microbiome [110–112]. Furthermore, it has been documented that the antibiotics reduce the number and diversity of microflora, while steroid therapy increases the number of Moraxellaceae, Pasteurellaceae, Pseudomonadaceae and Enterobacteriaceae [113]. Combined steroid and antibiotic therapies have resulted in a significant increase in Proteobacteria in the lungs [114]. However, the above disorders seem to occur only in critically ill patients, as in patients with mild to moderate asthma, the Proteobacteria family dominates, suggesting that dysbiosis is independent of steroid therapy [113]. Nevertheless, the observations in question come from the studies carried out in a small population of patients and have to be confirmed in multicentre studies.
CONCLUSIONS
The lung microbiome plays an important role in lung diseases. The interactions between impaired lung and gut microbiomes seem to be essential for the management of patients in intensive care units. Demonstrating such interactions should also elucidate the mechanism of the gut–lung axis and suggest a new therapeutic direction, fundamentally changing the treatment of critically ill patients.
ACKNOWLEDGEMENTS
Financial support and sponsorship
none.
Conflicts of interest
none.
REFERENCES
- 1.Schirmer M, Smeekens SP, Vlamakis H, et al. . Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016; 167: 1125-1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen C, Round JL.. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014; 16: 1024-1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4: 59-72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojima M, Motooka D, Shimizu K, et al. . Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci 2016; 61: 1628-1634. doi: 10.1007/s10620-015-4011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC.. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock 2016; 45: 475-482. doi: 10.1097/SHK.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belizário JE, Napolitano M.. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol 2015; 6: 1050. doi: 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yatsunenko T, Rey FE, Manary MJ, et al. . Human gut microbiome viewed across age and geography. Nature 2012; 486: 222-227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI.. Host-bacterial mutualism in the human intestine. Science 2005; 307: 1915-1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 9.Hooks KB, O’Malley MA.. Dysbiosis and its discontents. mBio 2017; 8: e01492-17. doi: 10.1128/mBio.01492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattori M, Taylor TD.. The human intestinal microbiome: a new frontier of human biology. DNA Res 2009; 16: 1-12. doi: 10.1093/dnares/dsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mowat AMI. To respond or not to respond – a personal perspective of intestinal tolerance. Nat Rev Immunol 2018; 18: 405-415. doi: 10.1038/s41577-018-0002-x. [DOI] [PubMed] [Google Scholar]
- 12.MacPherson AJ, Slack E, Geuking MB, McCoy KD.. The mucosal firewalls against commensal intestinal microbes. Semin Immunopathol 2009; 31: 145-149. doi: 10.1007/s00281-009-0174-3. [DOI] [PubMed] [Google Scholar]
- 13.McGuckin MA, Lindén SK, Sutton P, Florin TH.. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 2011; 9: 265-278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, Laszczyńska M.. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol 2019; 66: 1-12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, MacPherson AJ.. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10: 159-169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 16.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG.. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 2007; 204: 1891-1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cash HL, Whitham CV, Behrendt CL, Hooper LV.. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006; 313: 1126-1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishnava S, Yamamoto M, Severson KM, et al. . The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 2011; 334: 255-258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macpherson AJ, Uhr T.. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303: 1662-1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 20.Peterson DA, McNulty NP, Guruge JL, Gordon JI.. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007; 2: 328-339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Budden KF, Gellatly SL, Wood DLA, et al. . Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 2017; 15: 55-63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 22.Cui L, Morris A, Huang L, et al. . The microbiome and the lung. Ann Am Thorac Soc 2014; 11 Suppl 4: S227-232. doi: 10.1513/AnnalsATS.201402-052PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budden KF, Gellatly SL, Wood DLA, et al. . Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 2017; 15: 55-63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 24.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB.. The microbiome and the respiratory tract. Ann Rev Physiol 2016; 78: 481-504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G, Gail MH, Consonni D, et al. . Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol 2016; 17: 163. doi: 10.1186/s13059-016-1021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ES, Bittinger K, Haas AR, et al. . Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011; 184: 957-963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson RP, Erb-Downward JR, Prescott HC, et al. . Intraalveolar catecholamines and the human lung microbiome. Am J Respir Crit Care Med 2015; 192: 257-259. doi: 10.1164/rccm.201502-0326LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly BJ, Imai I, Bittinger K, et al. . Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome 2016; 4: 7. doi: 10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla SD, Budden KF, Neal R, Hansbro PM.. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunol 2017; 6: e133. doi: 10.1038/cti.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbrizzi A, Amedei A, Lavorini F, Renda T, Fontana G.. The lung microbiome: clinical and therapeutic implications. Int Emerg Med 2019; 14: 1241-1250. doi: 10.1007/s11739-019-02208-y. [DOI] [PubMed] [Google Scholar]
- 31.Remot A, Descamps D, Noordine ML, et al. . Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J 2017; 11: 1061-1074. doi: 10.1038/ismej.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson RP, Martinez FJ, Huffnagle GB.. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014; 384: 691-702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gollwitzer ES, Saglani S, Trompette A, et al. . Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med 2014; 20: 642-647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 34.Biesbroek G, Tsivtsivadze E, Sanders EAM, et al. . Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 2014; 190: 1283-1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 35.Wu BG, Segal LN.. The lung microbiome and its role in pneumonia. Clin Chest Med 2018; 39: 677-689. doi: 10.1016/j.ccm.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C.. The microbiome of the upper respiratory tract in health and disease. BMC Biol 2019; 17: 87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morinaga Y, Take Y, Sasaki D, et al. . Exploring the microbiota of upper respiratory tract during the development of pneumonia in a mouse model. PLoS One 2019; 14: e0222589. doi: 10.1371/journal.pone.0222589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuthbertson L, Walker AW, Oliver AE, et al. . Lung function and microbiota diversity in cystic fibrosis. Microbiome 2020; 8: 45. doi: 10.1186/s40168-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olszak T, An D, Zeissig S, et al. . Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336: 489-493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbst T, Sichelstiel A, Schär C, et al. . Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011; 184: 198-205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 41.Segal LN, Rom WN, Weiden MD.. Lung microbiome for clinicians. New discoveries about bugs in healthy and diseased lungs. Ann Am Thorac Soc 2014; 11: 108-116. doi: 10.1513/AnnalsATS.201310-339FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo RL, Hooper LV.. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012; 12: 503-516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun Y, Srinivas G, Kuenzel S, et al. . Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS One 2014; 9: e113466. doi: 10.1371/journal.pone.0113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown RL, Sequeira RP, Clarke TB.. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun 2017; 8: 1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox AC, McConnell KW, Yoseph BP, et al. . The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock 2012; 38: 508-514. doi: 10.1097/SHK.0b013e31826e47e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han M, Rajput C, Hong JY, et al. . The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J Immunol 2017; 199: 1308-1318. doi: 10.4049/jimmunol.1700216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gensollen T, Iyer SS, Kasper DL, Blumberg RS.. How colonization by microbiota in early life shapes the immune system. Science 2016; 352: 539-544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shreiner AB, Kao JY, Young VB.. The gut microbiome in health and in disease. Curr Opin Gastroenterol 2015; 31: 69-75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemmings SMJ, Malan-Müller S, Van Den Heuvel LL, et al. . The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 2017; 79: 936-946. doi: 10.1097/PSY.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bingula R, Filaire M, Radosevic-Robin N, et al. . Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol 2017; 2017: 5035371. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauptmann M, Schaible UE.. Linking microbiota and respiratory disease. FEBS Lett 2016; 590: 3721-3738. doi: 10.1002/1873-3468.12421. [DOI] [PubMed] [Google Scholar]
- 52.He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J.. Gut–lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol 2017; 43: 81-95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 53.Bachem A, Makhlouf C, Binger KJ, et al. . Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 2019; 51: 285-297.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Sze MA, Tsuruta M, Yang SWJ, et al. . Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One 2014; 9: e111228. doi: 10.1371/journal.pone.0111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrone EE, Jung E, Breed E, et al. . Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock 2012; 38: 68-75. doi: 10.1097/SHK.0b013e318259abdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimura KE, Sitarik AR, Havstad S, et al. . Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016; 22: 1187-1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. . Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7: 307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 58.Maslowski KM, Vieira AT, Ng A, et al. . Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461: 1282-1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith PM, Howitt MR, Panikov N, et al. . The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 2013; 341: 569-573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arpaia N, Campbell C, Fan X, et al. . Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504: 451-455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorburn AN, McKenzie CI, Shen S, et al. . Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6: 7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 62.Lloyd CM, Hawrylowicz CM.. Regulatory T cells in asthma. Immunity 2009; 31: 438-449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis G, Wang B, Shafiei Jahani P, et al. . Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front Immunol 2019; 10: 2051. doi: 10.3389/fimmu.2019.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickson RP, Erb-Downward JR, Huffnagle GB.. Homeostasis and its disruption in the lung microbiome. Am J Physiol Cell Mol Physiol 2015; 309: L1047-1055. doi: 10.1152/ajplung.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huffnagle GB, Dickson RP, Lukacs NW.. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 2017; 10: 299-306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashley SL, Sjoding MW, Popova AP, et al. . Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci Transl Med 2020; 12: eaau9959. doi: 10.1126/scitranslmed.aau9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I.. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015; 7: 2839-2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Günther A, Siebert C, Schmidt R, et al. . Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 1996; 153: 176-184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 69.Hume ME. Historic perspective: prebiotics, probiotics, and other alternatives to antibiotics. Poult Sci 2011; 90: 2663-2669. doi: 10.3382/ps.2010-01030. [DOI] [PubMed] [Google Scholar]
- 70.Zaborina O, Lepine F, Xiao G, et al. . Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog 2007; 3: e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickson RP, Huffnagle GB.. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 2015; 11: e1004923. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE.. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 2012; 7: e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zarb P, Coignard B, Griskeviciene J, et al. . The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill 2012; 17: 20316. doi: 10.2807/ese.17.46.20316-en. [DOI] [PubMed] [Google Scholar]
- 74.Wienhold SM, Macrì M, Nouailles G, et al. . Ventilator-induced lung injury is aggravated by antibiotic mediated microbiota depletion in mice. Crit Care 2018; 22: 282. doi: 10.1186/s13054-018-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nseir S, Zerimech F, Jaillette E, Artru F, Balduyck M.. Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets 2011; 11: 413-423. doi: 10.2174/187152611796504827. [DOI] [PubMed] [Google Scholar]
- 76.Dickson RP, Singer BH, Newstead MW, et al. . Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 2016; 1: 16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panzer AR, Lynch SV, Langelier C, et al. . Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. Am J Respir Crit Care Med 2018; 197: 621-631. doi: 10.1164/rccm.201702-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickson RP. The lung microbiome and ARDS. It is time to broaden the model. Am J Respir Crit Care Med 2018; 197: 549-551. doi: 10.1164/rccm.201710-2096ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernández-Barat L, López-Aladid R, Torres A.. Reconsidering ventilator-associated pneumonia from a new dimension of the lung microbiome. EBioMedicine 2020; 60: 102995. doi: 10.1016/j.ebiom.2020.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuijt TJ, Lankelma JM, Scicluna BP, et al. . The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65: 575-583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Twigg HL, Knox KS, Zhou J, Crothers KA, Nelson DE, Toh E, et al. . Effect of advanced HIV infection on the respiratory microbiome. Am J Respir Crit Care Med 2016; 194: 226-235. doi: 10.1164/rccm.201509-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris B, Morjaria SM, Littmann ER, et al. . Gut microbiota predict pulmonary infiltrates after allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med 2016; 194: 450-463. doi: 10.1164/rccm.201507-1491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otto M. Staphylococcus epidermidis – the “accidental” pathogen. Nat Rev Microbiol 2009; 7: 555-567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lupp C, Robertson M, Wickham M, et al. . Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007; 2: 119-129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Prakash A, Sundar SV, Zhu YG, et al. . Lung ischemia-reperfusion is a sterile inflammatory process influenced by commensal microbiota in mice. Shock 2015; 44: 272-279. doi: 10.1097/SHK.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kinross J, Warren O, Basson S, et al. . Intestinal ischemia/reperfusion injury: defining the role of the gut microbiome. Biomark Med 2009; 3: 175-192. doi: 10.2217/bmm.09.11. [DOI] [PubMed] [Google Scholar]
- 87.Hammer AM, Morris NL, Cannon AR, et al. . Interleukin-22 prevents microbial dysbiosis and promotes intestinal barrier regeneration following acute injury. Shock 2017; 48: 657-665. doi: 10.1097/SHK.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flint HJ. The impact of nutrition on the human microbiome. Nutr Rev 2012; 70 Suppl 1: S10-13. doi: 10.1111/j.1753-4887.2012.00499.x. [DOI] [PubMed] [Google Scholar]
- 89.David LA, Maurice CF, Carmody RN, et al. . Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559-563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu GD, Chen J, Hoffmann C, et al. . Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334: 105-108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valdes AM, Walter J, Segal E, Spector TD.. Role of the gut microbiota in nutrition and health. BMJ 2018; 361: 36-44. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. . High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137: 1716-1724.e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schörghuber M, Fruhwald S.. Effects of enteral nutrition on gastrointestinal function in patients who are critically ill. Lancet Gastroenterol Hepatol 2018; 3: 281-287. doi: 10.1016/S2468-1253(18)30036-0. [DOI] [PubMed] [Google Scholar]
- 94.Ralls MW, Demehri FR, Feng Y, et al. . Bacterial nutrient foraging in a mouse model of enteral nutrient deprivation: insight into the gut origin of sepsis. Am J Physiol Liver Physiol 2016; 311: G734-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyasaka EA, Feng Y, Poroyko V, et al. . Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol 2013; 190: 6607-6615. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demehri FR, Barrett M, Teitelbaum DH.. Changes to the intestinal microbiome with parenteral nutrition: review of a murine model and potential clinical implications. Nutr Clin Pract 2015; 30: 798-806. doi: 10.1177/0884533615609904. [DOI] [PubMed] [Google Scholar]
- 97.Lubbers T, Kox M, De Haan JJ, et al. . Continuous administration of enteral lipid-and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit Care Med 2013; 41: 1258-1265. doi: 10.1097/CCM.0b013e31827c0a17. [DOI] [PubMed] [Google Scholar]
- 98.Briassoulis G, Venkataraman S, Thompson A.. Cytokines and metabolic patterns in pediatric patients with critical illness. Clin Dev Immunol 2010; 2010: 354047. doi: 10.1155/2010/354047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R.. Regulation of inflammation by short-chain fatty acids. Nutrients 2011; 3: 858-876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohira H, Tsutsui W, Fujioka Y.. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb 2017; 24: 660-672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF.. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int 2016; 99: 110-132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 102.Makki K, Deehan EC, Walter J, Bäckhed F.. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018; 23: 705-715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Larsen V, Del Giacco SR, Moreira A, et al. . Asthma and dietary intake: an overview of systematic reviews. Allergy 2016; 71: 433-442. doi: 10.1111/all.12800. [DOI] [PubMed] [Google Scholar]
- 104.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E.. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107: 129-134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 105.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE.. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care 2016; 20: 262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zuccotti G, Meneghin F, Aceti A, et al. . Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy 2015; 70: 1356-1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 107.Kukkonen K, Savilahti E, Haahtela T, et al. . Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007; 119: 192-198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 108.Mortaz E, Adcock IM, Ricciardolo FLM, et al. . Anti-inflammatory effects of Lactobacillus rahmnosus and Bifidobacterium breve on cigarette smoke activated human macrophages. PLoS One 2015; 10: e0136455. doi: 10.1371/journal.pone.0136455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS.. Beneficial properties of probiotics. Trop Life Sci Res 2016; 27: 73-90. doi: 10.21315/tlsr2016.27.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dickson RP, Erb-Downward JR, Huffnagle GB.. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 2013; 7: 245-257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Noci V, Guglielmetti S, Arioli S, et al. . Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep 2018; 24: 3528-3538. doi: 10.1016/j.celrep.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 112.Hufnagl K, Pali-Schöll I, Roth-Walter F, Jensen-Jarolim E.. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol 2020; 42: 75-93. doi: 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Denner DR, Sangwan N, Becker JB, et al. . Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol 2016; 137: 1398-1405.e3. doi: 10.1016/j.jaci.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV.. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52: 2813-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]


