Abstract
The world is in need of next-generation COVID-19 vaccines. Although first-generation injectable COVID-19 vaccines continue to be critical tools in controlling the current global health crisis, continuous emergence of SARS-CoV-2 variants of concern has eroded the efficacy of these vaccines, leading to staggering breakthrough infections and posing threats to poor vaccine responders. This is partly because the humoral and T-cell responses generated following intramuscular injection of spike-centric monovalent vaccines are mostly confined to the periphery, failing to either access or be maintained at the portal of infection, the respiratory mucosa (RM). In contrast, respiratory mucosal-delivered vaccine can induce immunity encompassing humoral, cellular, and trained innate immunity positioned at the respiratory mucosa that may act quickly to prevent the establishment of an infection. Viral vectors, especially adenoviruses, represent the most promising platform for RM delivery that can be designed to express both structural and nonstructural antigens of SARS-CoV-2. Boosting RM immunity via the respiratory route using multivalent adenoviral-vectored vaccines would be a viable next-generation vaccine strategy.
Current Opinion in Virology 2023, 61:101334
This review comes from a themed issue on Mucosal Immunology
Edited by Hiroshi Kiyono and Xiaoping Zhu
https://doi.org/10.1016/j.coviro.2023.101334
1879–6257/© 2023 Elsevier B.V. All rights reserved.
Introduction
Despite the continued rollout of vaccines even with updated formulations, SARS-CoV-2 remains a global health concern. To-date, 11 first-generation COVID-19 vaccines have received Emergency Use Listing by the World Health Organization, with approximately 70% of the global population receiving at least a single dose of any COVID-19 vaccine [1]. Following their initial rollout in 2020, first-generation mRNA COVID-19 vaccines were designed to induce neutralizing antibodies against the spike protein of ancestral SARS-CoV-2, which showed remarkable protection against infection, conferring greater than 90% effectiveness 2, 3. However, waning immunity and the emergence of variants of concern (VOC) harboring numerous mutations in the spike protein have eroded the efficacy of these vaccines. As such, breakthrough infections are commonplace, especially since the emergence of the Delta and Omicron VOC [4].
Nonetheless, vaccine-induced T-cell immunity continues to provide protection against severe disease, hospitalization, and mortality 5••, 6. Although it is known that T-cell immunity lasts longer than humoral immunity, the real longevity of COVID-19 vaccine-induced protective T-cell immunity remains to be seen. As the COVID-19 pandemic transitions to a state of endemicity owing to an accumulation of population-wide immunity from natural infection and vaccination, it is anticipated that there remains a need to maintain the optimal immunity via boost vaccination in general populations. This is particularly relevant to individuals with immune deficiencies, transplant recipients, and the elderly who responded poorly to the first-generation vaccines. The lessons learned from first-generation vaccines indicate that chasing after the evolving SARS-CoV-2 by updating the spike antigen in the vaccines is an unsustainable strategy; rather, next-generation vaccine strategies that aim to boost multilayered immunity, encompassing trained innate, humoral, and cellular T-cell immunity (tripartite) at the respiratory mucosa may provide hope for fortifying the immune system against future emerging variants.
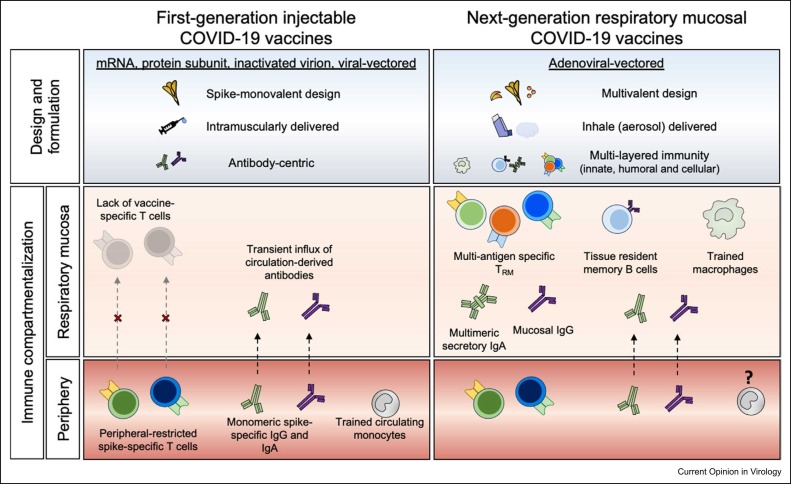
The robust humoral and T-cell responses induced following intramuscular injection are constrained to the periphery, failing to either access or be maintained at the portal of infection, the respiratory mucosa 7, 8, 9••. The significance of positioning immunity at the mucosa for effective protection against pathogens that enter the host via mucosal sites has been well acknowledged. Presently, strategies to develop next-generation SARS-CoV-2 vaccines amenable for respiratory mucosal (RM) delivery are garnering collective interest ( Figure 1) 10, 11, 12, 13. In this article, we provide the framework for the development of next-generation COVID-19 vaccination strategies, with specific consideration given to respiratory mucosal-delivered adenoviral-vectored (AdV) vaccines that can induce tripartite respiratory mucosal immunity capable of not only protecting against SARS-CoV-2 VOC but also reducing viral transmission and mitigating the development of post-acute COVID-19 sequalae (PACS/long COVID).
Figure 1.
Differential geographical localization and types of immune responses following first- and next-generation COVID-19 vaccines. Current first-generation injectable COVID-19 vaccines generate robust systemic humoral and T-cell responses but suboptimal magnitude of antibody responses and no T-cell responses at the respiratory mucosa compared with natural infection. Importantly, while structural and nonstructural SARS-CoV-2 protein-specific immune responses are induced by natural infection, injectable vaccines are designed to generate only spike-specific immune responses. This is reflected by relatively less efficiency in protecting against infection by first-generation COVID-19 vaccines compared with prior natural infection. In contrast, AdV multivalent COVID-19 vaccines, which are safe and amenable for respiratory mucosal delivery, can induce long-lived tripartite immunity at the respiratory mucosa capable of not only protecting against SARS-CoV-2 VOC but also reducing viral transmission and mitigating the development of PACS/long COVID.
Limited induction of respiratory mucosal immunity by first-generation injectable COVID-19 vaccination
First-generation injectable COVID-19 vaccines continue to be critical tools in combatting the current global health crisis. Indeed, first-generation COVID-19 vaccines induce robust serological IgM, IgG, and IgA antibodies and long-lasting memory B- and T-cell responses (Figure 1) [14]. Given the robust efficacy of these vaccines in the early stages of the pandemic, studies began to define and establish vaccine-induced humoral immunity as the protective correlates 15, 16. This largely steered the field to adopt the notion that serological-neutralizing antibody titers were central in defining vaccine efficacy against SARS-CoV-2. However, realizing that vaccines developed to target SARS-CoV-2 should aim at inducing immunity at geographical locations that match immunity induced by natural infection [17], studies have eventually assessed the ability of first-generation vaccines to induce mucosal immunity. Salivary anti-RBD and anti-spike IgG responses, most likely derived from the circulation, were detected and correlated with serological IgG levels after mRNA vaccination [18]. However, secretory salivary IgA (SIgA) responses, which were shown to have potent neutralizing activity against SARS-CoV-2, were detected only in a minority of vaccinees and at much lower levels compared with SIgA levels in convalescent saliva [19••]. Indeed, durable vaccine-induced IgA responses have been correlated with reduced incidence of breakthrough infection [19]. Nevertheless, whether the presence of salivary antibody responses would predict induction of anti-SARS-CoV-2 immunity at the portal of infection, the respiratory mucosa, remains unknown. A limited number of studies have assessed the injectable (mRNA) vaccine-induced humoral and cellular responses in the nasal mucosa. However, whether injectable COVID-19 vaccines can induce meaningful neutralizing antibody and tissue-resident T and B cells in the nasal tissue is still debatable because of contradicting observations 20, 21. Inherent factors such as variable baseline nasal immunoglobulins and limited sampling by nasal swabs and nasal washes and sample processing make assessment of immune responses at the nasal mucosa difficult.
Currently, little is known about the magnitude, quality, and kinetics of RM immunity induced by first-generation injectable COVID-19 vaccines in humans. To-date, only one clinical study has examined mRNA COVID-19 vaccine-induced humoral and cellular responses at the respiratory mucosa through analyzing bronchoalveolar lavage fluid [9]. Compared with COVID-19 convalescent individuals, vaccinated individuals had significantly lower levels of neutralizing antibodies in the airways against SARS-CoV-2 VOC, including Omicron (BA1.1). Importantly, in contrast to natural infection, mRNA vaccination failed to induce notable lung-resident spike-specific memory B- and T-cell responses. This may explain why mRNA vaccine-induced immunity is relatively less efficient in protecting against infection compared with prior infection-induced immunity as seen during the SARS-CoV-2 Delta wave [22]. The inability of injectable COVID-19 vaccines in inducing RM immunity resonates with the findings from the clinical trials of novel tuberculosis (TB) vaccines wherein parenteral injection with a viral-vectored vaccine failed to induce airway tissue-resident memory (TRM) T cells despite robust peripheral blood T-cell immunity 23, 24••.
Recruitment of immune cells to the lung is highly regulated to avoid unnecessary inflammation and preserve the vital function of the lung, gas exchange [25]. As such, local respiratory mucosal immune responses are mounted only in the event of local insults. This notion signifies that respiratory mucosal booster vaccination rather than repeated intramuscular injections can induce local mucosal immunity against SARS-CoV-2. In fact, RM booster vaccination with viral-vectored vaccines in mRNA-primed animals showed promising outcomes against SARS-CoV-2 VOC [26••]. Furthermore, real-world evidence that current vaccines are highly effective in preventing severe disease and hospitalization against VOC indicates a critical role for other immune cells, including circulating memory T cells induced by vaccination. Even though VOC can evade mucosal-neutralizing antibodies to cause infection, circulating memory T cells recruited to the lung enable protection by restraining viral replication and spread, resulting in milder disease outcomes [27]. Notably, current first-generation COVID-19 vaccines may also induce changes in circulating monocytes suggestive of ‘trained innate immunity’ (TII) 28, 29••. Recruitment of these cells to the lung may also contribute to antiviral immunity. Thus, evidence so far suggests that positioning anti-SARS-CoV-2 immunity at the respiratory mucosa that is ready-to-go can quickly act upon and prevent establishment of an infection, supporting boosting RM immunity via the respiratory route to be a viable next-generation vaccine strategy (Figure 1).
Respiratory mucosal vaccination and anti-COVID-19 immunity
Conceptually, inducing adaptive immunity at the respiratory mucosa, involving secretory antibody responses and tissue-resident B and T cells (TRM), and TII has the capacity to prevent SARS-CoV-2 from establishing infection (Figure 1) 11, 12, 30. Indeed, following natural infection, SARS-CoV-2-specific memory B- and T-cell responses were found in many tissue sites with lung and lung-associated lymph nodes (LN) being the most prevalent sites [17]. Furthermore, SARS-CoV-2-specific germinal centers and follicular helper T cells were also found in the lung and lung-associated LNs. Such local tissue-associated immune mechanisms against non-Omicron SARS-CoV-2 appeared linked with reduced risk (50% reduction) for Omicron infection [31]. Interestingly, protection reached 94% in the presence of prior infection and vaccination (hybrid immunity). These observations highlight the importance of focusing next-generation vaccines to boost RM tripartite immunity that can protect against future emerging VOC without a need for regularly updating the vaccines. A multitude of factors must be taken into consideration to ensure the safety, amenability, and efficacy of RM-delivered vaccines. These considerations span vector selection, antigenic design, and formulation.
Vector and antigen selection
Viral-vectored vaccines, in particular AdV vaccines, represent the most promising platform for RM delivery owing to their excellent safety profile, amenability, and robust intrinsic immunogenicity [32]. To-date, multiple human and nonhuman primate adenoviruses have been utilized for vaccine development. Numerous preclinical studies support the potential of RM-delivered AdV vaccines to stimulate robust and long-lasting antibody, cell-mediated, and TII immune responses in the lung. Their potential for RM delivery has been recently shown in clinical trials 24••, 33, 34. To circumvent the effect of anti-Ad backbone antibodies, different serotypes or origins of AdV platforms could be used for repeated heterologous booster immunization if needed.
To meet the challenges arising from emerging VOC compounded with the limited durability of first-generation vaccine-induced immunity, there is an intensifying interest to develop multivalent vaccines that express additional SARS-CoV-2 internal antigens that show high levels of sequence conservation among coronaviruses. These conserved antigens include nucleocapsid (N), RNA-dependent RNA polymerase (RdRp/NSP12), and other structural/nonstructural proteins, and are selected to broaden T-cell immunity. Adenoviral vectors are highly plastic, capable of accommodating large transgenes, thereby making them ideal multivalent vaccine platforms [32]. Indeed, robust T-cell responses to N are found in COVID-19, SARS, and uninfected individuals [35]. Furthermore, NSP12-specific T cells can recognize and kill target cells expressing NSP12 and have been associated with abortive seronegative SARS-CoV-2 in a cohort of healthcare workers 36••, 37••. We have recently provided preclinical evidence that a monovalent spike-expressing AdV vaccine failed to protect against B.1.351, whereas a multivalent AdV expressing N and NSP12 together with spike protein provided complete protection [38••].
RM vaccination and humoral immunity
Foundational studies utilizing human adenoviruses expressing spike from ancestral SARS-CoV-2 laid the groundwork in vaccine-induced respiratory mucosal immunity against COVID-19. RM but not intramuscular immunization with AdV COVID-19 vaccines induced robust IgG and IgA responses within the airways, which was associated with enhanced protection and reduced transmission of infection 39, 40, 41. Murine studies utilizing spike-expressing chimpanzee adenoviruses have further expanded the immunological superiority of RM-delivered AdV against SARS-CoV-2 38••, 42. In addition to inducing airway nAb responses against VOC, RM but not intramuscular vaccination induces systemic antibody responses with enhanced Fc effector function against immune-evasive (beta) VOC 42, 43. In line with these observations, induction of lung vaccine-specific memory B cells and bone-marrow long-lived plasma cells has been observed following RM but not intramuscular vaccination (Figure 1) 38••, 42.
The above evidence supports the relevance of inducing humoral anti-spike-neutralizing antibody responses at the respiratory mucosa. However, since continued viral evolution will progressively erode nAb efficacy, additional respiratory mucosal humoral correlates for protection against VOC need to be investigated with next-generation vaccines. Indeed, non-neutralizing antibodies have been clinically correlated with immunity against VOC through Fc-mediated effector functions even considering reducing neutralizing activity [44]. Preclinical studies have recently shown that first-generation mRNA vaccines induce antibodies that protect against Omicron BA.5 through Fc-mediated effector functions, further highlighting the protective capacity of vaccine-induced humoral immunity against antigenically divergent VOC even considering reduced neutralizing potentials [45]. Additionally, recent work has shown that improved outcomes by convalescent serum therapy were associated with non-neutralizing antibodies against the N of SARS-CoV-2 46, 47, which supports the inclusion of additional structural SARS-CoV-2 antigens into next-generation vaccines.
RM vaccination and T-cell immunity
Mounting human clinical data suggest a critical role for T-cell immunity in the observed protection 27, 48, including protection against Alpha-to-Omicron VOC [49]. To this end, differential T-cell responses have been detected between vaccine-naive individuals who have had mild and severe disease outcomes. While robust antibody responses with almost undetectable circulating SARS-CoV-2-specific T cells were found in individuals with prolonged severe COVID-19, rapid expansion of structural and nonstructural SARS-CoV-2 protein-specific T-cell responses was detected in individuals who rapidly controlled SARS-CoV-2 replication 50, 51. Importantly, memory T-cell responses against NSP12 and N have been associated with abortive SARS-CoV-2 infection without seroconversion 37••, 52. Furthermore, depleting CD8 T cells in convalescent or vaccinated macaques resulted in partial abrogation of protective efficacy of natural and vaccine-induced immunity 53, 54.
Despite clinical data supporting the significance of T-cell immunity against SARS-CoV-2, important questions remain regarding the location, longevity, quantity, and quality of T cells needed for optimal protection. This is partly because most of those observations are confined to the circulatory compartment, and as such, clear understanding of T-cell responses in the upper and lower respiratory tract is still lacking following natural infection and vaccination 9••, 55. In fact, in contrast to convalescent individuals, infection-naive individuals who have received injectable first-generation COVID-19 vaccines lacked vaccine-specific lung TRM T-cell immunity [9], which is localized to nonlymphoid tissues and plays critical roles in tissue-localized immunity to infections 11, 12, 13. Preclinical studies with AdV COVID-19 vaccines have shown that RM vaccination induces long-lasting TRM cells within the lung tissue and airways that played a critical role in protection through elimination of virally infected cells 26••, 38••, 42, 43. On the other hand, although parenteral AdV COVID-19 vaccine (ChAdOx1) prevented pneumonia in macaques, it failed to prevent viral replication in the upper respiratory tract (URT) [56]. Indeed, oral or intranasal delivery of an AdV vaccine not only protected hamsters against infection but also prevented transmission to unvaccinated naive hamsters. Recent studies have shown that AdV vaccines against TB and COVID-19 are safe and well-tolerated in humans when delivered via inhaled aerosol 24••, 57, 58. Inhaled AdV COVID-19 booster vaccination in previously inactivated CoronaVac-primed individuals resulted in induction of 18–24-times higher circulating cross-neutralizing antibodies compared with homologous boost with CoronaVac [33]. However, the detail of local respiratory mucosal immune responses following AdV COVID-19 vaccination remains unknown. Alternatively, an aerosol AdV TB vaccine clinical trial found long-lasting TRM cells to be induced in the lung [24]. Such mucosal T-cell immunity is possibly maintained via self-renewal of T cells in an antigen-dependent manner [59]. This trial offers the proof of concept for the capability of RM-delivered AdV COVID-19 vaccine to elicit long-lasting immunity in hosts with complex immunological history and genetic backgrounds.
RM vaccination and trained innate immunity
Beside the mucosal-adaptive immune pathways, the innate immune system, which functions as the first line of defense against incoming pathogens including SARS-CoV-2, plays a critical role in orchestrating mucosal immunity. Importantly, given that ‘TII’, a state of innate hyperresponsiveness induced by prior local immunologic exposure at the respiratory mucosa, has the potential to fight against heterologous infections, next-generation vaccine strategies can exploit this third arm of the immune system 12, 60, 61. A growing body of evidence now indicates that immunization with some vaccines can induce TII in circulating monocytes and alveolar macrophages 29••, 62, 63, 64, 65. For instance, BCG-induced TII has been associated with reduced all-cause mortality in low-birth-weight infants and reduced the incidence of respiratory tract infections in the elderly 66, 67. In fact, a recent study carried out in Greece demonstrated that BCG vaccination reduced the occurrence of new infections and reduced the risk of COVID-19 [68]. However, preclinical studies have been at odds with clinical studies in that BCG vaccination does not provide protection against SARS-CoV-2 [69]. Although recent studies have shed much-needed light on induction of TII following mRNA or ChAdOx1 COVID-19 vaccination, these responses are likely restricted to the systemic compartments and their contribution to respiratory mucosal immunity remains unknown 28, 29••. In contrast, TII induced in alveolar macrophages following RM vaccination with AdV vaccines renders protection against respiratory infections, including SARS-CoV-2 38••, 62, 63. In humans, RM immunization with AdV vaccine induced persisting transcriptional changes in alveolar macrophages [24]. These observations provide the rationale to harness TII in the design of next-generation COVID-19 vaccines.
Clinical landscape of current respiratory mucosal COVID-19 vaccine strategies
The most explored respiratory vaccine strategy against COVID-19 is the intranasal route of delivery. Currently, several COVID-19 mucosal vaccines are under development and 14 are in clinical trials, of which three — in India, Iran, and Russia have received emergency approval and are being administered as a nasal spray ( Table 1). Although efficacy data for many of these trials have not yet been published, the trial results for the intranasally delivered AstraZeneca ChAdOx1-S vaccine have recently been released. Unfortunately, the findings indicated poor immunogenicity in previously mRNA-vaccinated participants [34]. This is in stark contrast to the excellent results obtained for this vaccine in preclinical animal models [70]. Such discrepancy may be owed to anatomical differences in the URT between humans and animals and the delivery methods. For instance, it is known that volumes between 25 and 50 µL of an agent delivered intranasally to mice also have the potential to reach the lower respiratory tract, hence inducing immunity not only in the nasal passage but also deep in the lung [71]. Optimally formulating vaccines for intranasal delivery is challenging and critical to overcoming the natural nasal defense barriers.
Table 1.
Vaccines in the clinical pipeline evaluated for respiratory mucosal delivery.
| Vaccine vector | Vaccine ID | Developer | Route | Delivery device | Phase | Status | Clinical trial ID |
|---|---|---|---|---|---|---|---|
| Adenovirus | BBV154 | Bharat Biotech International Limited | I.N. | Droppers | III | Active, not recruiting | NCT05522335 |
| SC-Ad6-1 | Tetherex Pharmaceuticals Corporation | I.N. or I.M. | N/A | I | Recruiting | NCT04839042 | |
| Ad5-triCoV/Mac, ChAd-triCoV/Mac | McMaster University | Aerosol inhalation | Aeroneb Solo nebulizer | I | Recruiting | NCT05094609 | |
| AZD1222/ChAdOx1 nCov-19 | Imperial College London/University of Oxford/AstraZeneca | Aerosol inhalation | MAD NasalTM intranasal mucosal atomization device | I | Recruiting | NCT05007275 | |
| Ad5-nCoV | Jiangsu Province Centers for Disease Control and Prevention | Aerosol inhalation | Aerogen Ultra device | III | Active, not recruiting | NCT05204589 | |
| Ad5-nCoV | CanSino Biologics Inc. | Aerosol inhalation and/or I.M. | Aerogen Solo | I/II | Active, not recruiting | NCT04840992 | |
| Parainfluenza virus | CVXGA1 | CyanVac LLC | I.N. | Spray devices | I | Recruiting | NCT04954287 |
| Respiratory syncytial virus | MV-014-212 | Meissa Vaccines, Inc. | I.N. | Droppers/spray devices | I | Recruiting | NCT04798001 |
| Live-attenuated influenza virus | DelNS1-2019-nCoV–RBD–OPT1 | The University of Hong Kong | I.N. | Spray devices | II | Recruiting | NCT05200741 |
| III | Active, not recruiting | ChiCTR2100051391 | |||||
| Newcastle disease virus | NDV–HXP-S | Sean Liu, Icahn School of Medicine at Mount Sinai | I.N. and/or I.M. | N/A | I | Recruiting | NCT05181709 |
| AVX/COVID-12 | Laboratorio Avi-Mex, S.A. de C.V. | I.N. or I.M. | Automatic syringe (prima mist sprayer) | II | Active, not recruiting | NCT05205746 | |
| Combined vector vaccine | Gam–COVID–Vac | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | I.N. | Spray devices | I | Not yet recruiting | NCT05248373 |
| Protein subunit | Avacc 10 | Intravacc B.V. | I.N. | N/A | I | Not yet recruiting | NCT05604690 |
| Live-attenuated SARS-CoV-2 | COVI–VAC | Codagenix, Inc | I.N. | Droppers | I | Active, not recruiting | NCT05233826 |
Inhaled aerosol delivery has been recently developed and assessed as an alternative respiratory mucosal vaccination strategy. Aerosol vaccination has been utilized to deliver measles, and numerous AdV vaccines against TB and COVID-19 23, 24••, 58, 72. Importantly, compared with intranasal delivery, the deepened and widened biodistribution in the lung following endotracheal delivery (akin inhalation) is associated with much improved vaccine-mediated immunogenicity and protection against the target pathogen in mice [71]. Inhaled aerosol vaccination of viral-vectored TB vaccines deposited 2–5-µm vaccine droplets in major airways [24], inducing robust and sustainable antigen-specific cellular immunity in the respiratory tract as measured by the responses in bronchoalveolar lavage fluid 23, 24••. Furthermore, persisting alterations in the transcriptional profile of alveolar macrophages poised for defense responses in aerosol-vaccinated participants indicate the potential of aerosol vaccines to induce TII. The availability of well-characterized inhaled aerosol technology (used in TB vaccine trials) and its superiority to induce immunity in the URT offers a foundation for developing inhaled next-generation COVID-19 vaccine strategies. Indeed, Chinese regulators have recently approved the world’s first inhaled aerosol first-generation AdV COVID-19 vaccine (Convidecia Air). In clinical trials, this vaccine was well-tolerated and induced robust neutralizing antibodies and T-cell immunity in the circulation, and importantly, qualitatively enhanced neutralizing antibody responses against the Delta SARS-CoV-2 VOC, compared with homologous intramuscular boost 39, 58. However, to what extent Convidecia Air can induce mucosal immunity remains to be investigated. To this end, a phase-I trial currently undergoing in Canada to evaluate the safety and immunogenicity of aerosolized viral-vectored trivalent vaccines, Ad5-triCoV/Mac and ChAd-triCoV/Mac, will carry out a comprehensive analysis of immune responses within the airways (NCT05094609). Although clinical respiratory mucosal vaccine trials will conduct efficacy studies comparing the vaccinated against placebo, the waning immunity in previously vaccinated and infected individuals will make assessing next-generation vaccines more difficult than investigating first-generation vaccines carried out in an infection-naive population [73]. Nonetheless, these studies may provide the foundation to establish correlates of protection following inhaled immunization. Apart from the benefit of being able to induce mucosal immunity, inhaled immunization holds many other advantages. Since the vaccine is directly delivered to the lung, a much smaller dose than that used for injection can generate an effective immune response [24]. The potential to develop a thermostable spray-dried form of viral-vectored vaccines for inhalation will allow transport and storage less challenging and could be a solution for vaccine inequity [74]. Notably, dry powder vaccine formulation of mRNA encoding spike-loaded exosome when administered via jet nebulization to nonhuman primates elicited stronger IgG and SIgA responses compared with their synthetic counterparts [75].
Outstanding questions and future perspectives
The COVID-19 pandemic continues to expedite our understanding of innovative and novel vaccination strategies. Decades of research have firmly established the intricate link between vaccination route and immune geography, with experimental and clinical evidence showing the unique capacity of RM-delivered AdV vaccines in establishing humoral, cellular, and TII at the respiratory mucosa. Of relevance, preclinical COVID-19 vaccine studies have shown the superiority of RM delivery in inducing local, long-lasting immunological memory capable of providing broad protection against both ancestral and variants of SARS-CoV-2. As the clinical landscape of RM-delivered AdV vaccines continues to expand through and beyond SARS-CoV-2 to other respiratory pathogens, numerous outstanding questions remain to be addressed before their wide application in humans.
Extremely rare incidences of vaccine-induced immune thrombotic thrombocytopenia (VITT) have been characterized following initial intramuscular (IM) vaccination with AdV COVID-19 vaccines (case rate of 3–15 per million vaccinations) 76, 77, 78. Clinically, VITT is associated with multiple diagnostic criteria, including seropositivity for antiplatelet factor 4 (PF4) IgG autoantibodies, elevated D-dimer levels, and disseminated signs of thrombosis, with unique presentation of cerebral venous sinus thrombosis and cerebral venous thrombosis [79]. Although the mechanisms remain to be fully understood, the unintentional administration of AdV vaccines via the intravenous route, or the ensuing microvascular injury and leakage of the adenovirus into the bloodstream due to vaccine constituents, has been empirically demonstrated to potentially contribute to the development of VITT 80, 81, 82. Since anti-PF4 antibody-mediated platelet activation is one of the major mechanisms for VITT, leaked adenoviral particles are believed to directly interact with platelets and/or PF4, which may result in B-cell engagement and subsequent production and maturation anti-PF4 IgG antibodies 82, 83. While the potential for VITT following RM administration of AdV vaccines remains to be investigated, it is our belief that RM delivery of AdV vaccines will not lead to VITT via circumventing viral vector leakage into the circulation.
Moreover, our knowledge of RM vaccines in populations with pulmonary comorbidities remains limited. As research interest continues to grow, clinical trials should be designed to assess the immunogenicity and safety of RM vaccines both in the elderly and in individuals with pulmonary comorbidities. These studies will be crucial in determining the necessary adjustments to vaccine formulations, doses, and delivery methods, as well as in developing a comprehensive framework to ensure that the best strategies are available for providing sufficient coverage in terms of both vaccine immunogenicity and safety following RM delivery in a population-wide setting.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Z.X. is one of the inventors on a patent application PCT/CA2022/051107, entitled “Novel COVID vaccine and method for delivery”. All other authors declare no competing interests.
Acknowledgements
The work was supported by the Canadian Institutes of Health Research (CIHR) COVID-19 Rapid Research Project, CIHR Foundation Program, and the Innovative Research Program of National Sanitarium Association of Canada. The authors are grateful to support from other members of McMaster COVID-19 vaccine project team.
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.WHO: Registry Data of COVID-19 Vaccine Candidates; 2022. Available at: 〈https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list〉 (Accessed 21 November 2022).
- 2.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchan S.A., Chung H., Brown K.A., Austin P.C., Fell D.B., Gubbay J.B., Nasreen S., Schwartz K.L., Sundaram M.E., Tadrous M., et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Liu J., Chandrashekar A., Sellers D., Barrett J., Jacob-Dolan C., Lifton M., McMahan K., Sciacca M., VanWyk H., Wu C., et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Spike-specific T cell responses induced by first-generation COVID-19 vaccines are conserved in their ability to recognize the omicron spike protein.
- 6.Jung M.K., Jeong S.D., Noh J.Y., Kim D.U., Jung S., Song J.Y., Jeong H.W., Park S.H., Shin E.C. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat Microbiol. 2022;7:909–917. doi: 10.1038/s41564-022-01123-x. [DOI] [PubMed] [Google Scholar]
- 7.Azzi L., Dalla Gasperina D., Veronesi G., Shallak M., Ietto G., Iovino D., Baj A., Gianfagna F., Maurino V., Focosi D., et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J.I., Dropulic L., Wang K., Gangler K., Morgan K., Liepshutz K., Krogmann T., Ali M.A., Qin J., Wang J., et al. Comparison of levels of nasal, salivary, and plasma antibody to SARS-CoV-2 during natural infection and after vaccination. Clin Infect Dis. 2022;76:1391–1399. doi: 10.1093/CID/CIAC934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Tang J., Zeng C., Cox T.M., Li C., Son Y.M., Cheon I.S., Wu Y., Behl S., Taylor J.J., Chakaraborty R., et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7:eadd4853. doi: 10.1126/sciimmunol.add4853. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study that comprehensively compared the respiratory mucosal responses between mRNA vaccinated participants and convalescent people.
- 10.Alu A., Chen L., Lei H., Wei Y., Tian X., Wei X. Intranasal COVID-19 vaccines: from bench to bed. EBioMedicine. 2022;76 doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavelle E.C., Ward R.W. Mucosal vaccines — fortifying the frontiers. Nat Rev Immunol. 2022;22:236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone F.R. Unique properties of tissue-resident memory T cells in the lungs: implications for COVID-19 and other respiratory diseases. Nat Rev Immunol. 2022;23:329–335. doi: 10.1038/s41577-022-00815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon M.M.L., Rybkina K., Kato Y., Kubota M., Matsumoto R., Bloom N.I., Zhang Z., Hastie K.M., Grifoni A., Weiskopf D., et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6:eabl9105. doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., Li Z., Chao G., Rojas O.L., Bang Y.M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Sheikh-Mohamed S., Isho B., Chao G.Y.C., Zuo M., Cohen C., Lustig Y., Nahass G.R., Salomon-Shulman R.E., Blacker G., Fazel-Zarandi M., et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15:799–808. doi: 10.1038/s41385-022-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Spike-specific salivary antibodies are poorly induced following intramuscular vaccination but are readily induced following breakthrough infection.
- 20.Ssemaganda A., Nguyen H.M., Nuhu F., Jahan N., Card C.M., Kiazyk S., Severini G., Keynan Y., Su R.C., Ji H., et al. Expansion of cytotoxic tissue-resident CD8+ T cells and CCR6+CD161+ CD4+ T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat Commun. 2022;13:3357. doi: 10.1038/s41467-022-30913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim J.M.E., Tan A.T., Bert N., le, Hang S.K., Low J.G.H., Bertoletti A. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. 2022;219 doi: 10.1084/jem.20220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L.S., Ash N., Alroy-Preis S., Huppert A., Milo R. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satti I., Meyer J., Harris S.A., Thomas Z.-R.M., Griffiths K., Antrobus R.D., Rowland R., Ramon R.L., Smith M., Sheehan S., et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:939–946. doi: 10.1016/S1473-3099(14)70845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Jeyanathan M., Fritz D.K., Afkhami S., Aguirre E., Howie K.J., Zganiacz A., Dvorkin-Gheva A., Thompson M.R., Silver R.F., Cusack R.P., et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight. 2022;7 doi: 10.1172/jci.insight.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]; First in-human clinical trial that evaluates the immunogenicity following inhaled aerosol vaccination with an AdVTB vaccine. This study has demonstrated that the aerosol, but not intramuscular vaccination induces immunity at the respiratory mucosa as measured in the bronchoalveolar fluids.
- 25.Holt P.G., Strickland D.H., Wikström M.E., Jahnsen F.L. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 26••.Lapuente D., Fuchs J., Willar J., Vieira Antão A., Eberlein V., Uhlig N., Issmail L., Schmidt A., Oltmanns F., Peter A.S., et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12:1–14. doi: 10.1038/s41467-021-27063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vaccine-specific mucosal immune responses are strongly induced following respiratory mucosal boosting of intramuscular-primed animals.
- 27.Wherry E.J., Barouch D.H. T cell immunity to COVID-19 vaccines. Science. 2022;377:821–822. doi: 10.1126/science.add2897. [DOI] [PubMed] [Google Scholar]
- 28.Saresella M., Piancone F., Marventano I., Hernis A., Trabattoni D., Invernizzi M., La Rosa F., Clerici M. Innate immune responses to three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.947320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Murphy D.M., Cox D.J., Connolly S.A., Breen E.P., Brugman A.A.I., Phelan J.J., Keane J., Basdeo S.A. Trained immunity is induced in humans after immunization with an adenoviral vector COVID-19 vaccine. J Clin Investig. 2022 doi: 10.1172/JCI162581. [DOI] [PMC free article] [PubMed] [Google Scholar]; First human study to characterize induction of TII in circulating monocytes following intramuscular immunization with a first-generation AdV COVID-19 vaccine.
- 30.Singh R., Kang A., Luo X., Jeyanathan M., Gillgrass A., Afkhami S., Xing Z. COVID-19: current knowledge in clinical features, immunological responses, and vaccine development. FASEB J. 2021;35 doi: 10.1096/fj.202002662R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carazo S., Skowronski D.M., Brisson M., Sauvageau C., Brousseau N., Gilca R., Ouakki M., Barkati S., Fafard J., Talbot D., et al. Estimated protection of prior SARS-CoV-2 infection against reinfection with the Omicron variant among messenger RNA-vaccinated and nonvaccinated individuals in Quebec, Canada. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afkhami S., Yao Y., Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev. 2016;3 doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., Zhu T., Zhang J., Luo L., Fan P., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhavan M., Ritchie A.J., Aboagye J., Jenkin D., Provstgaad-Morys S., Tarbet I., Woods D., Davies S., Baker M., Platt A., et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. EBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 36••.Nesterenko P.A., McLaughlin J., Tsai B.L., Burton Sojo G., Cheng D., Zhao D., Mao Z., Bangayan N.J., Obusan M.B., Su Y., et al. HLA-A∗02:01 restricted T cell receptors against the highly conserved SARS-CoV-2 polymerase cross-react with human coronaviruses. Cell Rep. 2021;37:110167. doi: 10.1016/j.celrep.2021.110167. [DOI] [PMC free article] [PubMed] [Google Scholar]; RdRp-specific T cells are highly conserved among human coronaviruses and are cross-reactive against SARS-CoV-2 RdRp.
- 37••.Swadling L., Diniz M.O., Schmidt N.M., Amin O.E., Chandran A., Shaw E., Pade C., Gibbons J.M., Le Bert N., Tan A.T., et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2022;601:110–117. doi: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highly exposed but seronegative healthcare workers possess reactive RdRp-specific T cells, which may contribute to rapid clearance/abortive infection with SARS-CoV-2.
- 38••.Afkhami S., D’Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., Singh R., Bavananthasivam J., Ye G., Luo X., et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896–915. doi: 10.1016/j.cell.2022.02.005. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; First pre-clinical study assessing a respiratory-mucosal delivered multivalent vaccine expressing spike, nucleocapsid, and polymerase (NSP12) COVID-19 vaccine capable of generating tripartite immunity against SARS-CoV-2 VOC.
- 39.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., Wang B., Zhao Z., Song X., Chen Y., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11:1–7. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langel S.N., Johnson S., Martinez C.I., Tedjakusuma S.N., Peinovich N., Dora E.G., Kuehl P.J., Irshad H., Barrett E.G., Werts A.D., et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abn6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu F., Wu S., Yi L., Peng S., Wang F., Si W., Hou L., Zhu T. Safety, mucosal and systemic immunopotency of an aerosolized adenovirus-vectored vaccine against SARS-CoV-2 in rhesus macaques. Emerg Microbes Infect. 2022;11:438–441. doi: 10.1080/22221751.2022.2030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184. doi: 10.1016/j.cell.2020.08.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassan A.O., Shrihari S., Gorman M.J., Curiel D.T. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. CellReports. 2021;36 doi: 10.1016/j.celrep.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zohar T., Loos C., Fischinger S., Atyeo C., Wang C., Slein M.D., Burke J., Yu J., Feldman J., Hauser B.M., et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183:1508–1519. doi: 10.1016/j.cell.2020.10.052. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackin S.R., Desai P., Whitener B.M., Karl C.E., Liu M., Baric R.S., Edwards D.K., Chicz T.M., McNamara R.P., Alter G., et al. Fcγ receptor-dependent antibody effector functions are required for vaccine protection against infection by antigenic variants of SARS-CoV-2. bioRxiv. 2022 doi: 10.1101/2022.11.27.518117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman J.D., Wang C., Burke J.S., Zur Y., Compere H., Kang J., Macvicar R., Taylor S., Shin S., Frank I., et al. Nucleocapsid-specific antibody function is associated with therapeutic benefits from COVID-19 convalescent plasma therapy. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang A., Stacey H.D., D’Agostino M.R., Tugg Y., Marzok A., Miller M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat Rev Immunol. 2022;23:381–396. doi: 10.1038/s41577-022-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muik A., Lui B.G., Diao H., Fu Y., Bacher M., Toker A., Grosser J., Ozhelvaci O., Grikscheit K., Hoehl S., et al. Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T-cell recognition epitopes. bioRxiv. 2022 doi: 10.1101/2022.12.15.520569. [DOI] [PubMed] [Google Scholar]
- 49.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., Bloom N.I., Goodwin B., Phillips E., Mallal S., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859. doi: 10.1016/j.cell.2022.01.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandran A., Rosenheim J., Nageswaran G., Swadling L., Pollara G., Gupta R.K., Burton A.R., Guerra-Assunção J.A., Woolston A., Ronel T., et al. Rapid synchronous type 1 IFN and virus-specific T cell responses characterize first wave non-severe SARS-CoV-2 infections. Cell Rep Med. 2022;3:100557. doi: 10.1016/j.xcrm.2022.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., Zhuang Y., Tham C.Y.L., Chia A., Smith G.J.D., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kundu R., Narean J.S., Wang L., Fenn J., Pillay T., Fernandez N.D., Conibear E., Koycheva A., Davies M., Tolosa-Wright M., et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat Commun. 2022;13:80. doi: 10.1038/s41467-021-27674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Yu J., McMahan K., Jacob-Dolan C., He X., Giffin V., Wu C., Sciacca M., Powers O., Nampanya F., et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abq7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grau-Expósito J., Sánchez-Gaona N., Massana N., Suppi M., Astorga-Gamaza A., Perea D., Rosado J., Falcó A., Kirkegaard C., Torrella A., et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. 2021;12:3010. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Doremalen N., Purushotham J.N., Schulz J.E., Holbrook M.G., Bushmaker T., Carmody A., Port J.R., Yinda C.K., Okumura A., Saturday G., et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., Zhu T., Zhang J., Luo L., Fan P., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J.-X., Wu S.-P., Guo X.-L., Tang R., Huang B.-Y., Chen X.-Q., Chen Y., Hou L.-H., Liu J.-X., Zhong J., et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10:739–748. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeyanathan M., Mu J., McCormick S., Damjanovic D., Small C.-L., Shaler C.R., Kugathasan K., Xing Z. Murine airway luminal antituberculosis memory CD8 T cells by mucosal immunization are maintained via antigen-driven in situ proliferation, independent of peripheral T cell recruitment. Am J Respir Crit Care Med. 2010;181 doi: 10.1164/rccm.200910-1583OC. [DOI] [PubMed] [Google Scholar]
- 60.O’Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing Z., Afkhami S., Bavananthasivam J., Fritz D.K., D’Agostino M.R., Vaseghi-Shanjani M., Yao Y., Jeyanathan M. Innate immune memory of tissue-resident macrophages and trained innate immunity: re-vamping vaccine concept and strategies. J Leukoc Biol. 2020;108:825–834. doi: 10.1002/JLB.4MR0220-446R. [DOI] [PubMed] [Google Scholar]
- 62.D’Agostino M.R., Lai R., Afkhami S., Khera A., Yao Y., Vaseghi-Shanjani M., Zganiacz A., Jeyanathan M., Xing Z. Airway macrophages mediate mucosal vaccine-induced trained innate immunity against Mycobacterium tuberculosis in early stages of infection. J Immunol. 2020;205:2750–2762. doi: 10.4049/jimmunol.2000532. [DOI] [PubMed] [Google Scholar]
- 63.Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., Lai R., Afkhami S., Chen Y., Dvorkin-Gheva A., et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634–1650. doi: 10.1016/j.cell.2018.09.042. e17. [DOI] [PubMed] [Google Scholar]
- 64.Bannister S., Kim B., Domínguez-Andrés J., Kilic G., Ansell B.R.E., Neeland M.R., Moorlag S.J.C.F.M., Matzaraki V., Vlahos A., Shepherd R., et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci Adv. 2022;8 doi: 10.1126/sciadv.abn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeyanathan M., Vaseghi-Shanjani M., Afkhami S., Grondin J.A., Kang A., D’Agostino M.R., Yao Y., Jain S., Zganiacz A., Kroezen Z., et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat Immunol. 2022;23:1687–1702. doi: 10.1038/s41590-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N., et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period. J Infect Dis. 2011:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 67.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–323. doi: 10.1016/j.cell.2020.08.051. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., Kostoula M., Paneta M., Adamis G., Papanikolaou I., et al. ACTIVATE-2: A Double-blind Randomized Trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaufmann E., Khan N., Tran K.A., Ulndreaj A., Pernet E., Fontes G., Lupien A., Desmeules P., McIntosh F., Abow A., et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Doremalen N., Purushotham J.N., Schulz J.E., Holbrook M.G., Bushmaker T., Carmody A., Port J.R., Yinda C.K., Okumura A., Saturday G., et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021;13:eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeyananthan V., Afkhami S., D’Agostino M.R., Zganiacz A., Feng X., Miller M.S., Jeyanathan M., Thompson M.R., Xing Z. Differential biodistribution of adenoviral-vectored vaccine following intranasal and endotracheal deliveries leads to different immune outcomes. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.860399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Low N., Bavdekar A., Jeyaseelan L., Hirve S., Ramanathan K., Andrews N.J., Shaikh N., Jadi R.S., Rajagopal A., Brown K.E., et al. A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med. 2015;372:1519–1529. doi: 10.1056/NEJMoa1407417. [DOI] [PubMed] [Google Scholar]
- 73.Waltz E. How nasal-spray vaccines could change the pandemic. Nature. 2022;609:240–242. doi: 10.1038/d41586-022-02824-3. [DOI] [PubMed] [Google Scholar]
- 74.Toniolo S.P., Afkhami S., D’Agostino M.R., Lichty B.D., Cranston E.D., Xing Z., Thompson M.R. Spray dried VSV-vectored vaccine is thermally stable and immunologically active in vivo. Sci Rep. 2020;10 doi: 10.1038/s41598-020-70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Popowski K.D., Moatti A., Scull G., Silkstone D., Lutz H., López de Juan Abad B., George A., Belcher E., Zhu D., Mei X., et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5:2960–2974. doi: 10.1016/j.matt.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muir K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrera-Comoglio R., Lane S. Vaccine-induced immune thrombocytopenia and thrombosis after the Sputnik V vaccine. N Engl J Med. 2022;387:1431–1432. doi: 10.1056/NEJMc2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., Rampotas A., Ambler G., Makris M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cines D.B., Greinacher A. Vaccine-induced immune thrombotic thrombocytopenia. Blood. 2023;141:1659–1665. doi: 10.1182/blood.2022017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicolai L., Leunig A., Pekayvaz K., Esefeld M., Anjum A., Rath J., Riedlinger E., Ehreiser V., Mader M., Eivers L., et al. Thrombocytopenia and splenic platelet-directed immune responses after IV ChAdOx1 nCov-19 administration. Blood. 2022;140:478–490. doi: 10.1182/blood.2021014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., Waraich K., Vant J., Wilson E., Truong C.D., et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv. 2021;7 doi: 10.1126/sciadv.abl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greinacher A., Schönborn L., Siegerist F., Steil L., Palankar R., Handtke S., Reder A., Thiele T., Aurich K., Methling K., et al. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT) Semin Hematol. 2022;59:97–107. doi: 10.1053/j.seminhematol.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J.J., Armour B., Chataway T., Troelnikov A., Colella A., Yacoub O., Hockley S., Tan C.W., Gordon T.P. Vaccine-induced immune thrombotic thrombocytopenia is mediated by a stereotyped clonotypic antibody. Blood. 2022;140:1738–1742. doi: 10.1182/blood.2022016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.