Highlights
-
•
Atypical femoral fractures (AFFs) are suggested to have association with bone resorption inhibitors for osteoporosis.
-
•
Bone modifying agents (BMAs) for bone metastasis are also bone resorption inhibitors with more dosage.
-
•
It is difficult to cease BMA because of AFF occurrence.
-
•
Bone union time of AFF in BMA using patients was longer than ordinary AFF.
-
•
It would be important to prevent complete AFF via prophylactic internal fixation.
Keywords: Atypical femoral fracture, Bone metastasis, Zoledronic acid, Denosumab, Bone-modifying agent, Bisphosphonate
Abstract
Bone-modifying agents (BMAs), with bone-resorptive inhibitory effects, such as zoledronic acid and denosumab, are widely used at higher doses for bone-related events caused by bone metastasis of malignant tumors. These drugs have been suggested to be associated with atypical femoral fractures (AFFs), and the relationship between BMAs and AFFs has attracted attention. To investigate the clinical features including bone union time of AFFs in patients administered BMA for bone metastasis, we conducted a retrospective multicenter study. Thirty AFFs from 19 patients were enrolled in this study. Thirteen patients had bilateral AFFs, and nineteen AFFs had prodromal symptoms. Eighteen AFFs underwent surgery after complete fracture, three failed to achieve bone union and required nonunion surgery, and 11 AFFs that achieved bone union had an average period until bone union of 16.2 months, which was much longer than that previously reported for ordinary AFFs. Seven patients discontinued the BMAs, but not due to AFFs. Stopping BMAs in patients with bone metastasis would make it difficult to secure their performance of activities of daily living, and AFF with BMA administration might require a longer time for union. Therefore, it would be important to prevent incomplete AFF from becoming complete AFF via prophylactic internal fixation.
1. Introduction
Malignant tumors can metastasize to any part of the body, and metastasis accounts for more than 90% of malignant tumor-related deaths [1]. Various tumor types have preferential sites of metastases; however, the bone is a common site for distant metastasis [2], [3].
It is feared that recent improvements in the prognosis of primary malignant tumors could lead to an increasing prevalence of skeletal-related events (SREs), including pathologic fractures, spinal cord compression, surgery of metastatic bone, and hypercalcemia following bone metastasis. Zoledronic acid (a type of bisphosphonate) and denosumab (an antibody against the receptor activator of nuclear factor-kappa β ligand) suppress bone resorption. They are widely used for the treatment of bone metastasis and are called bone-modifying agents (BMAs), whose efficacy in reducing SREs has been demonstrated in many studies [4], [5], [6]. Moreover, these drugs are regarded as effective in improving quality of life and decreasing bone pain [7], [8], [9].
These bone resorption inhibitors are also considered standard medications for osteoporosis, but the number of reports indicating a causative relationship between these drugs and the occurrence of atypical femoral fractures (AFFs) has been increasing [10], [11], [12], [13], [14]. It has been reported that long-term use of bisphosphonates results in an increased risk of AFFs [15], [16]. In addition, there are reports on the relationship between denosumab and AFF [17], [18], [19], [20]. As the doses of zoledronic acid and denosumab for bone metastasis are notably higher than those for osteoporosis, it is predicted that the abnormal clinical features of AFF might be more difficult to resolve in patients with bone metastasis than in those with osteoporosis. However, AFF has not been well studied in patients with bone metastasis treated with bone resorption inhibitors. The purpose of this study was to retrospectively investigate clinical features including bone union period of AFF cases in patients from several institutions who had malignant tumors and were receiving these drugs.
2. Materials and methods
This study was approved by the institutional review boards of all nine participating medical institutions. The participants were patients who were diagnosed with AFF, surgically treated in any participating institution from 2010 to 2019, and who had been treated with BMAs for bone metastases before being diagnosed with AFF. We included patients 1) who underwent surgery for AFF between 2010 and 2019 at the participating institutions, 2) who had a history of BMA (zoledronate acid or denosumab) administration before the diagnosis of AFF, and 3) whose treatment information could be obtained from the medical records. We excluded patients who 1) did not participate in the current study based on the opt-out and 2) were deemed inappropriate for the current study by the principal investigator (Table 1). AFF was diagnosed according to the revised major criteria of the American Society for Bone and Mineral Research (ASBMR) Taskforce [21]. Thirty AFFs in 19 patients were enrolled, and age, sex, primary malignant tumor, administered BMAs (zoledronic acid and/or denosumab), BMA administration period, presence of prodromal symptoms, fracture type, implants for surgeries, bone union, and cessation of BMA administration were studied based on medical records.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
2.1. Radiographic assessment
The fracture type (complete or incomplete) was judged in each participating institution where surgery was performed and confirmed by three orthopedic trauma surgeons. Three orthopedic surgeons assessed the radiographs of each patient and independently determined the time of bone union. The bone union time of the patient was the time bone fusion was considered to have occurred based on the judgment of two or more orthopedic surgeons. The bone union period was calculated as the number of months between the surgery for AFF and the patient’s bone union time. The inter-observer reliability was assessed with intraclass correlation coefficients (ICCs) [22] using IBM SPSS Statistics 26 (IBM Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics (Table 2)
Table 2.
Patient characteristics.
| Patient | Right or Left | Age at AFF surgery (years) |
Sex | Primary malignant tumor |
|---|---|---|---|---|
| 1 | Right | 71 | Male | Prostate cancer |
| Left | 73 | |||
| 2 | Left | 56 | Female | Breast cancer |
| Right | 56 | |||
| 3 | Right | 71 | Female | Breast cancer |
| Left | 71 | |||
| 4 | Right | 69 | Female | Lung cancer |
| 5 | Left | 60 | Female | Multiple myeloma |
| 6 | Right | 56 | Female | Renal cancer |
| 7 | Right | 53 | Female | Breast cancer |
| Left | 52 | |||
| 8 | Left | 65 | Female | Breast cancer |
| Right | 62 | |||
| 9 | Right | 53 | Female | Breast cancer |
| Left | 53 | |||
| 10 | Right | 74 | Female | Breast cancer |
| Left | 74 | |||
| 11 | Right | 45 | Female | Breast cancer |
| 12 | Right | 76 | Female | Breast cancer |
| 13 | Right | 71 | Female | Breast cancer |
| 14 | Left | 59 | Female | Breast cancer |
| 15 | Right | 46 | Female | Breast cancer |
| Left | 47 | |||
| 16 | Right | 53 | Female | Breast cancer |
| Left | 53 | |||
| 17 | Left | 77 | Female | Breast cancer |
| Right | 70 | |||
| 18 | Left | 70 | Female | Breast cancer |
| 19 | Right | 63 | Female | Breast cancer |
| Left | 66 |
No patient was excluded for opting out or for being deemed as inappropriate for the study by the principal investigator. The mean age of the patients at AFF surgery was 62.2 (range 45–77) years. Eighteen women and one man were included in the study. The primary malignant tumors were lung cancer, prostate cancer, renal cancer, multiple myeloma in one case each, and breast cancer in 15 cases. The primary malignant tumor in the male patient was prostate cancer.
3.2. Administered bone-modifying agents (Table 3)
Table 3.
Administered BMA and administration period.
| Patient | Right or Left | Administered BMA prior to AFF diagnosis | BMA administration period before AFF surgery (months) |
|---|---|---|---|
| 1 | Right | Zoledronic acid | 39.0 |
| Left | Zoledronic acid → Denosumab | 73.0 | |
| 2 | Left | Zoledronic acid | 41.0 |
| Right | Zoledronic acid | 41.0 | |
| 3 | Right | Zoledronic acid → Denosumab | 76.0 |
| Left | Zoledronic acid → Denosumab | 76.0 | |
| 4 | Right | Zoledronic acid | 84.5 |
| 5 | Left | Zoledronic acid | 17.2 |
| 6 | Right | Zoledronic acid | 30.0 |
| 7 | Right | Zoledronic acid | 57.1 |
| Left | Denosumab | 45.1 | |
| 8 | Left | Zoledronic acid → Denosumab | 91.4 |
| Right | Zoledronic acid → Denosumab | 61.9 | |
| 9 | Right | Denosumab → Zoledronic acid | 71.7 |
| Left | Denosumab → Zoledronic acid | 65.4 | |
| 10 | Right | Zoledronic acid → Denosumab | 150.6 |
| Left | Zoledronic acid → Denosumab | 156.9 | |
| 11 | Right | Zoledronic acid | 75.8 |
| 12 | Right | Zoledronic acid → Denosumab | 25.9 |
| 13 | Right | Zoledronic acid | 49.4 |
| 14 | Left | Zoledronic acid → Denosumab | 124.7 |
| 15 | Right | Zoledronic acid | 75.6 |
| Left | Zoledronic acid | 85.7 | |
| 16 | Right | Zoledronic acid → Denosumab | 108.9 |
| Left | Zoledronic acid → Denosumab | 108.9 | |
| 17 | Left | Zoledronic acid | 181.0 |
| Right | Zoledronic acid | 90.0 | |
| 18 | Left | Zoledronic acid → Denosumab | 28.8 |
| 19 | Right | Zoledronic acid → Denosumab → Zoledronic acid → Denosumab | 47.6 |
| Left | Zoledronic acid → Denosumab → Zoledronic acid → Denosumab | 77.4 |
In 30 AFFs, BMAs prior to AFF diagnosis were zoledronic acid and denosumab in 16 AFFs, zoledronic acid in 12 AFFs, and denosumab in 2 AFFs. Of the 16 AFFs with both BMAs, 12 AFFs occurred after the administration of zoledronic acid followed by denosumab and two AFFs occurred after the administration of denosumab followed by zoledronic acid. In the remaining two AFFs, zoledronic acid was changed to denosumab, but the patient had a recurrence of bone pain and was re-prescribed zoledronic acid. However, the patient was placed again on denosumab because of decreased renal function. The average administration period of BMAs before AFF surgery was 75.3 (range 17–181) months.
3.3. Fracture-related items (Table 4)
Table 4.
Fracture-related information.
| Patient | Right or Left | Bilateral AFF | Prodromal symptom | Fracture level | Fracture type | Intraoperative complete fracture |
|---|---|---|---|---|---|---|
| 1 | Right | ✓ | Subtrochanter | Complete | ||
| Left | Subtrochanter | Complete | ||||
| 2 | Left | ✓ | ✓ | Subtrochanter | Complete | |
| Right | ✓ | Subtrochanter | Incomplete | |||
| 3 | Right | ✓ | ✓ | Subtrochanter/Diaphysis | Incomplete | |
| Left | ✓ | Subtrochanter | Incomplete | |||
| 4 | Right | ✓ | Subtrochanter | Complete | ||
| 5 | Left | ✓ | Diaphysis | Complete | ||
| 6 | Right | ✓ | Subtrochanter | Incomplete | ✓ | |
| 7 | Right | ✓ | ✓ | Subtrochanter | Incomplete | |
| Left | Subtrochanter | Complete | ||||
| 8 | Left | ✓ | ✓ | Subtrochanter | Incomplete | ✓ |
| Right | ✓ | Subtrochanter | Complete | |||
| 9 | Right | ✓ | ✓ | Subtrochanter | Incomplete | |
| Left | ✓ | Subtrochanter | Complete | |||
| 10 | Right | ✓ | ✓ | Subtrochanter | Complete | |
| Left | ✓ | Subtrochanter | Incomplete | |||
| 11 | Right | Subtrochanter | Complete | |||
| 12 | Right | ✓ | Subtrochanter | Incomplete | ||
| 13 | Right | ✓ | Subtrochanter | Complete | ||
| 14 | Left | ✓ | Subtrochanter | Complete | ||
| 15 | Right | ✓ | ✓ | Subtrochanter | Complete | |
| Left | ✓ | Subtrochanter | Complete | |||
| 16 | Right | ✓ | Subtrochanter | Incomplete | ||
| Left | Subtrochanter | Incomplete | ✓ | |||
| 17 | Left | ✓ | ✓ | Subtrochanter | Incomplete | |
| Right | Subtrochanter | Complete | ||||
| 18 | Left | ✓ | Subtrochanter | Complete | ||
| 19 | Right | ✓ | Subtrochanter | Complete | ||
| Left | Diaphysis | Complete |
Prodromal symptoms were observed in 19 of 30 AFFs (63%), and bilateral AFF were observed in 13 of 19 cases (68%). In two of the cases of bilateral AFFs, one side was treated surgically, while the other side was managed conservatively without surgery; these cases were not included in the analyses. The fracture sites were the subtrochanteric area in 27 AFFs (90%), diaphysis in 2 AFFs, and both the subtrochanteric area and diaphysis in 1 AFF. Eighteen AFFs were complete fractures during the surgeries, whereas prophylactic surgeries were performed for 12 incomplete AFFs. Three of these incomplete AFFs resulted in a complete fracture during surgery.
3.4. Treatment for AFF (Table 5)
Table 5.
Treatment related information.
| Patient | Right or Left | Implant type | Follow-up period after surgery (months) | Bone fusion | Period to bone fusion (months) | Reason for BMA discontinuation | Occurrence of ONJ |
|---|---|---|---|---|---|---|---|
| 1 | Right | Nail | 43.3 | ○ | 29.6 | ✓ | |
| Left | Nail | 13.3 | × | ||||
| 2 | Left | Nail | 77.9 | ○ | 24.8 | ||
| Right | Nail | 77.7 | N.A. * | ||||
| 3 | Right | Nail | 4.4 | N.A. | |||
| Left | Nail | 4.4 | N.A. | ||||
| 4 | Right | Nail | 33.3 | × | Unknown | ||
| 5 | Left | Nail | 8.0 | N.A. | Risk of ONJ | ||
| 6 | Right | Nail | 57.6 | ○ | 6.1§ | ||
| 7 | Right | SHS * | 52.5 | N.A. | Unknown | ||
| Left | SHS | 64.6 | × | Unknown | |||
| 8 | Left | Nail | 4.7 | × | |||
| Right | Nail | 34.2 | ○ | 23.3 | |||
| 9 | Right | Nail | 15.6 | N.A. | Unknown | ||
| Left | Nail | 21.9 | ○ | 4.6 | Unknown | ||
| 10 | Right | Nail | 5.5 | ○ | 21.0 | ||
| Left | Nail | 34.0 | N.A. | ||||
| 11 | Right | Nail | 24.1 | ○ | 7.6 | Deterioration of the general condition | |
| 12 | Right | Nail | 33.1 | N.A. | |||
| 13 | Right | Nail | 2.1 | × | Deterioration of the general condition | ||
| 14 | Left | Nail | 1.2 | × | Deterioration of the general condition | ✓ | |
| 15 | Right | Nail | 29.3 | ○ | 16.1 | ✓ | |
| Left | Nail | 18.2 | ○ | 15.4 | |||
| 16 | Right | Nail | 67.4 | N.A. | |||
| Left | Nail | 67.4 | ○ | 3.1§ | |||
| 17 | Left | Nail | 13.5 | N.A. | |||
| Right | Nail | 120.1 | ○ | 12.2 | |||
| 18 | Left | Nail | 12.4 | ○ | 5.5 | ||
| 19 | Right | Nail | 51.3 | × | |||
| Left | Nail | 21.5 | ○ | 6.4 |
§: bone union time after intraoperative fracture of incomplete AFF.
N.A.: not applicable since surgeries were performed for incomplete AFFs.
SHS: sliding hip screw, ONJ: osteonecrosis of jaw.
○: bone union achieved.
×: bone union not confirmed in follow up.
Intramedullary nails were used for 28 AFFs, while sliding hip screws were used for 2 AFFs (due to narrow medullary canals of the femurs). These 2 AFFs were actually fractures in the same patient; one was a complete fracture and the other was an incomplete fracture. The mean follow-up period after surgeries was 32.3 (range 1.2–120.1) months. Bone fusion was observed in 11 of the 18 complete AFFs. The average fracture healing period of the 11 AFFs assessed by the three orthopedic trauma surgeons was 15.1 (range 4.6–29.6) months. The inter-observer ICC for the fracture healing period was excellent (0.769). Regarding three incomplete AFFs resulting in complete AFFs intraoperatively, two fractures achieved bone fusion at 6.1 and 3.1 months postoperatively, and the other patient died 4 months after AFF surgery without bone fusion.
The treatment courses of 7 AFFs where surgery was performed for complete fracture, but bony union was not confirmed are outlined subsequently. In four of these AFFs, the patients died before bone union was achieved. The follow-up periods at the time of death were 1, 2, 8, and 13 months postoperatively. For the other 3 AFFs, nonunion surgeries were performed 9, 9, and 35 months, respectively, after the AFF surgeries. The initial surgeries for these three AFFs were performed using short femoral nails, sliding hip screws, and intramedullary nails. The fractures fixed with intramedullary nails were fixed following an inappropriate reduction. Insufficient stability and inappropriate reduction at the fracture sites were considered the main factors that induced nonunion. Two of these three nonunions following AFFs achieved bony union, and the other AFF nonunion did not achieve complete bony union at the latest follow-up of 16 months after nonunion surgery.
There were 7 cases of discontinuation of BMAs during follow-up. The reasons for discontinuation were deterioration of the general condition in 3 cases, risk of osteonecrosis of the jaw (ONJ) in 1 case, and unknown in 3 cases. Multiple myeloma was the primary malignant tumor in the case where BMA was discontinued owing to the risk of ONJ.
Three patients were diagnosed with ONJ and were treated during follow-up after AFF surgery; however, BMAs were not discontinued in all three cases.
3.5. Case presentation
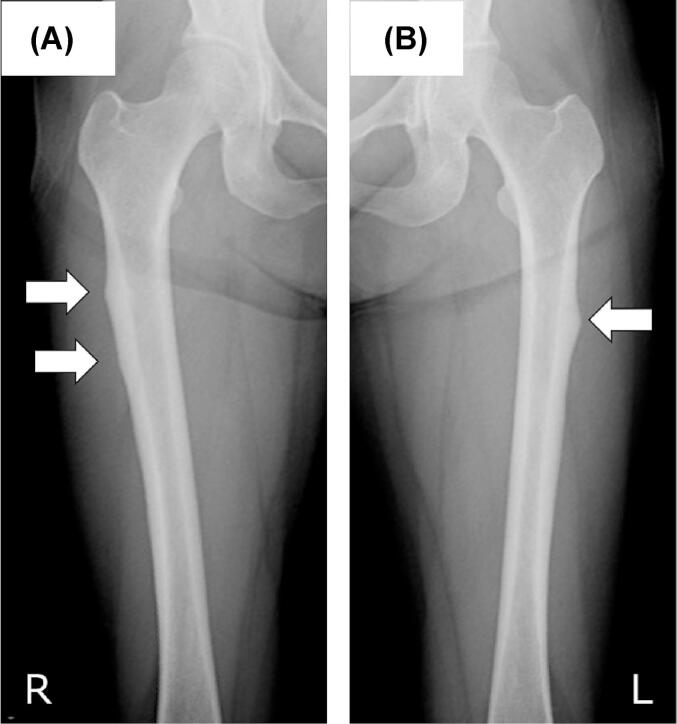
A female patient was diagnosed with breast cancer with bone metastasis at the age of 52 years, after which zoledronic acid was used as BMA for 3 years and 5 months. At the age of 56, she noticed bilateral thigh pain, which was later identified as a prodromal symptom. She was subsequently diagnosed with bilateral subtrochanteric AFFs and referred to our institution for further treatment. Radiographs showed a thickened lateral cortex at both subtrochanteric levels (Fig. 1).
Fig. 1.
Radiographs taken immediately after referral to our institution. (A) Right femur. Two lateral beaks with thickened cortices at the subtrochanteric level were shown (white arrows) (B) Left femoral shaft showing one lateral beak at the proximal diaphyseal level (white arrow).
Two parts of the thickened cortex were observed on the right femur. Although prophylactic surgery with an intramedullary nail was recommended considering the fracture risk, she refused surgery. During observation in the outpatient clinic, she developed a sudden onset of severe left femoral pain when loading the left lower limb while riding a motorcycle, and a complete fracture was diagnosed (Fig. 2).
Fig. 2.
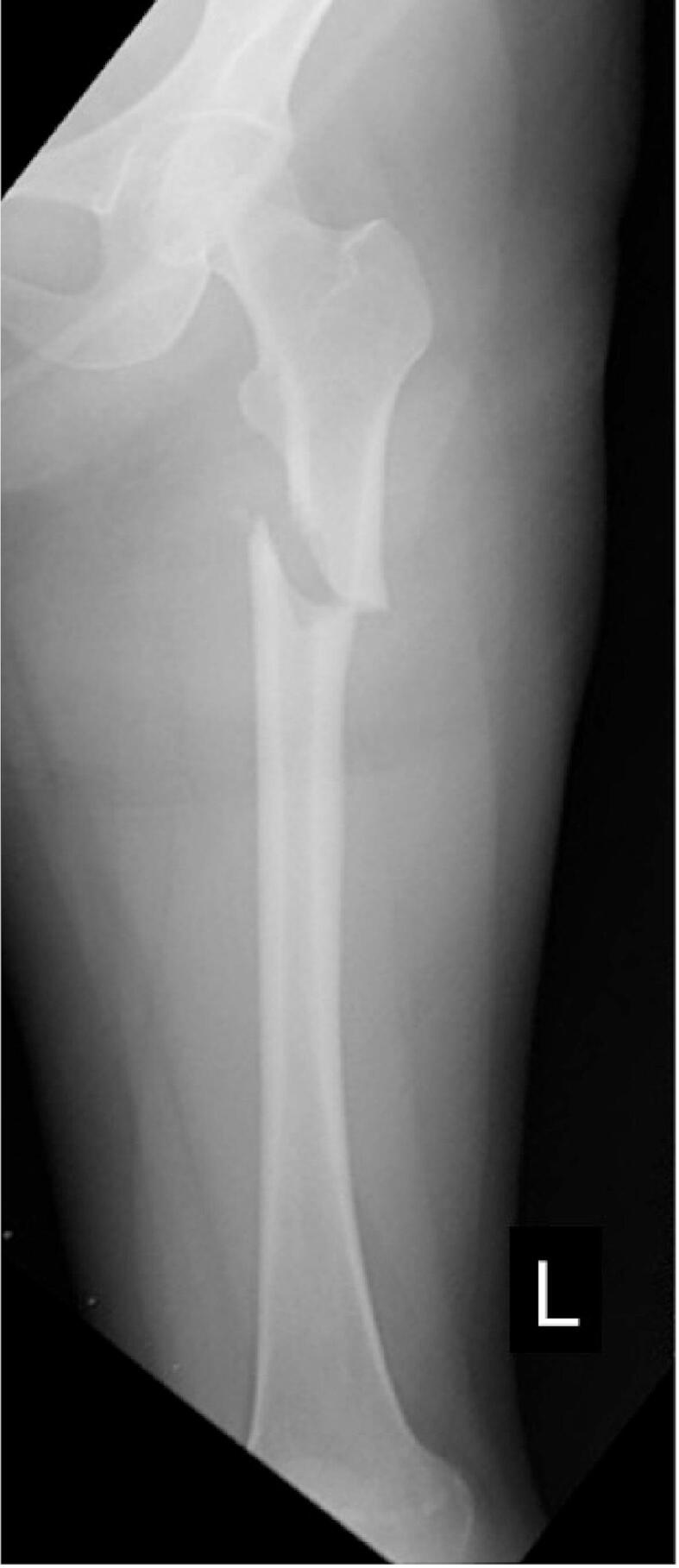
Radiograph of the left femur with a complete atypical femoral fracture at the level of the beak demonstrated in Fig. 1.
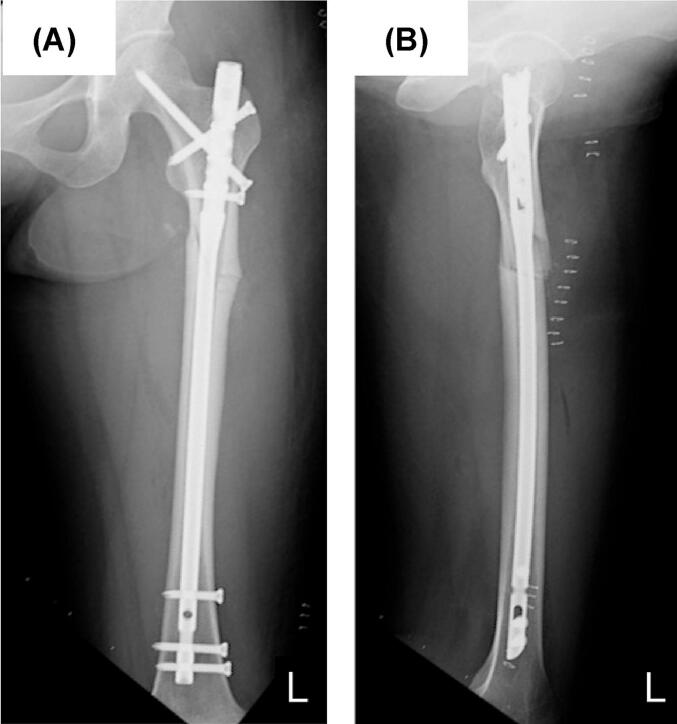
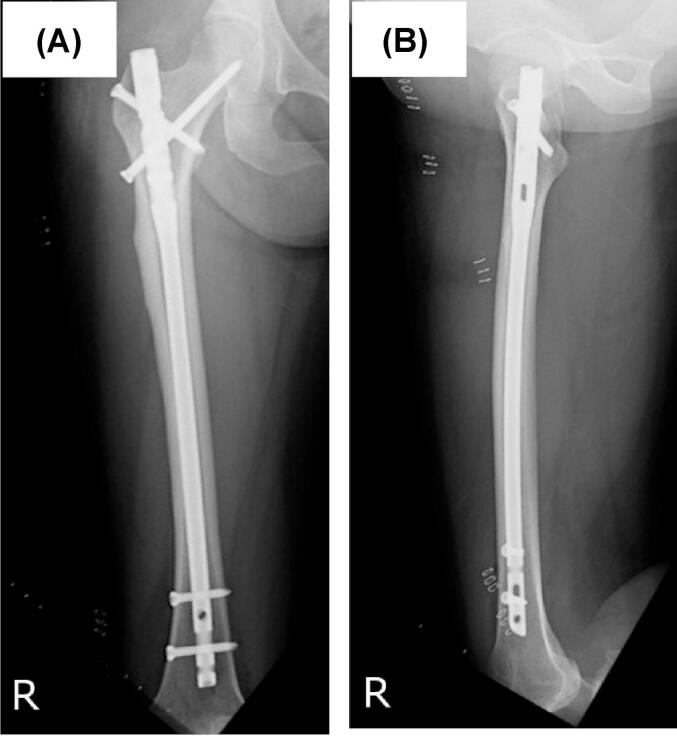
The left femoral fracture was fixed with an intramedullary nail (Fig. 3), and 1 week later, she underwent prophylactic surgery with an intramedullary nail for an incomplete fracture on the contralateral side (Fig. 4).
Fig. 3.
Radiographs of the left femur just after osteosynthesis with intramedullary nailing. (A) Anterior-posterior view, (B) Lateral view.
Fig. 4.
Radiographs of the right femur after prophylactic surgery for incomplete atypical femoral fracture with intramedullary nailing. (A) Anterior-posterior view, (B) Lateral view.
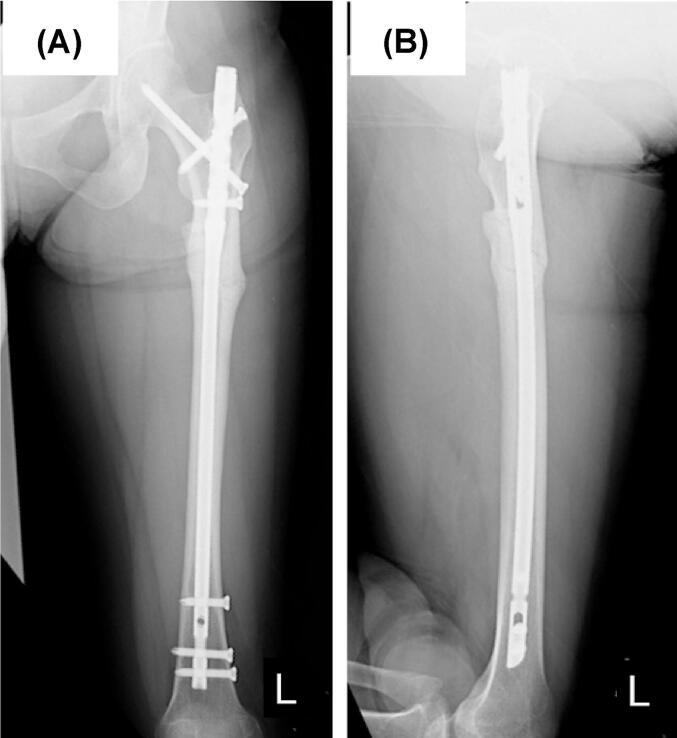
Full weight-bearing on the left leg was allowed 3 weeks postoperatively. She was able to walk with a cane one month after the initial surgery. Thirty-three months after the osteosynthesis, bony union was observed radiographically (Fig. 5), and there was no limit on activities of daily living (ADLs) at the final follow-up 36 months postoperatively.
Fig. 5.
Radiograph of the left femur 2 years and 9 months after the surgery showing bone union. (A) Anterior-posterior view, (B) Lateral view.
4. Discussion
We reported a retrospective series of patients with AFFs who received treatment with BMAs for bone metastases. The number of reports of AFF following the administration of bisphosphonates to patients with malignant tumors has increased in recent years [23], [24], [25]. In a report by Puhaindran et al., 4 in 327 patients with skeletal malignant involvement who received intravenous bisphosphonates were noted as having AFF [25]. Chang et al. reported 6 cases of AFF in 62 femoral fractures in patients with breast cancer and multiple myeloma using bisphosphonates for bone metastasis [23]. In the study of Edwards et al., 23 cases of AFF were radiographically identified in 10,587 patients with malignant tumor using bisphosphonates [24]. Occurrences of AFF have also been reported in patients with malignant tumors being treated with denosumab, although they have been fewer than in those receiving bisphosphonates [26], [27]. A retrospective study conducted by Yang et al. demonstrated that the incidence of AFF in patients receiving denosumab for metastatic bone disease was 0.4% (1 of 253 cases), and the incidence based on radiological review was 4.5% (3 of 66 cases) [27]. However, the incidence of AFF in patients receiving bisphosphonate for osteoporosis was reported as 5 cases per 10,000 patient-years by Schilcher et al [14], and a recent study reported an incidence of 1.74 cases per 10,000 patient-years [10]. For patients receiving denosumab treatment for osteoporosis, the incidence of AFF is 0.8 cases per 10,000 patient-years [28]. The reported incidence of AFF in patients receiving for bone metastasis, although they differ among studies, seem to be higher than that in patients receiving BMA for osteoporosis. The current study contained as many as 30 AFFs in 19 cases of BMA administration for bone metastasis. To the best of our knowledge, our study included the largest number of cases compared to previous reports; therefore, the number of cases seems valuable for determining the clinical features of such rare fractures.
Prodromal symptoms, such as femoral and groin pain, are present in approximately 70% of AFFs [29]. In the current study, prodromal symptoms were recognized in 19 of 30 fractures (63%); this rate is supposed to be similar to that of ordinary AFFs. Bilateral occurrence is a characteristic of AFF and is described as a minor feature in the ASBMR definition [21], [29]. Bilateral fractures were reported in 28% of patients and bilateral radiologic changes were recognized in 28% of patients with AFF, not limited to those with bone metastases [21], [29]. In the current study, 13 of 19 patients (68%) had bilateral AFFs. Although the sample size was small, patients with AFF who were taking denosumab and/or zoledronic acid for bone metastasis might have had bilateral fractures more frequently than those without BMA administration.
In the future, as the number of cancer patients increases, those taking BMAs will increase, and AFFs related to these drugs will also increase owing to improvements in the prognosis of patients with malignant tumors. Three problems that do not need to be considered when treating ordinary AFF without bone metastasis could exist when treating AFF with bone metastasis. First, alternative drugs for SREs that are currently available in clinical settings are limited; therefore, it is difficult to stop the administration of BMAs. Although the discontinuation of bisphosphonates has been reported to decrease the risk of AFF [11], [14], BMA cannot be discontinued in AFF patients with bone metastasis as it may lead to decreased ADL performance in the patient. In fact, there were no cases where BMA was discontinued because of AFF. However, some clinical trials investigated the effects of a de-escalation of the administrated BMA amount; they revealed that SRE prevention achieved by a 12-week therapy was non-inferior to that achieved by a 4-week therapy [30], [31], [32], [33]. Therefore, in case BMAs are difficult to discontinue, de-escalation of BMAs could be a reasonable option for patients with AFFs and bone metastasis. Second, teriparatide can be used as an anabolic agent for the treatment of osteoporosis patients with AFF [34], [35]. However, it is contraindicated in the presence of bone metastases; therefore, BMAs cannot be replaced with teriparatide for AFF with bone metastases. Conversely, the prolonged fracture-healing period in the current study would be a severe problem for patients with bone metastases whose life prognosis may be limited as compared to that of otherwise healthy people with these fractures. The possibility of off-label use of teriparatide, which has been reported to promote fracture healing [36], [37], [38], might be reconsidered. Third, higher doses of bone resorption inhibitors are used for bone metastases than for osteoporosis; therefore, the influence of these drugs on AFF are expected to increase. Zoledronic acid at 4 mg/3 weeks has been reported to reduce SRE in prostate cancer patients with bone metastases [5], while the clinical dosage of zoledronic acid for osteoporosis is 5 mg once a year. This discrepancy in dosage between osteoporosis and bone metastasis as the target disease has also been applied to denosumab [39], [40]. Based on these points, patients with AFF taking zoledronic acid and/or denosumab for bone metastases are likely to be more difficult to treat than those without a malignant tumor. Delayed union is a characteristic of AFF and is listed as a minor feature in the ASBMR definition. The average healing time for AFFs was reported as 5.2 months in a study of 179 patients by Bogdan et al [41]. According to a meta-analysis of 833 AFFs, the mean time to healing was 7.3 (range, 2–31) months postoperatively [42]. Compared to in these reports, the healing time of complete AFFs in the current study (average 15.1 months) was longer, although our patients differed from the patients in these previous studies in terms of several factors (including sex, age, ethnicity, and so on). The reason for the longer healing time is unknown; however, it may be related to the higher drug dosage used for bone metastasis. Considering the prolonged healing time, it is important to prevent incomplete AFFs from proceeding to complete fracture. Thus, imaging examination should be performed quickly when prodromal symptoms appear, and attention should be paid to bilateral occurrence.
The current study had some limitations. First, it was a retrospective study involving a single cohort. Second, the sample size was relatively small. To investigate the characteristics of AFF in patients with metastatic bone tumors who were administered BMAs, including the period of fracture healing, further studies with a larger sample size and AFF without bone metastasis are required.
5. Conclusions
It is necessary to consider the occurrence of AFF in patients with bone metastases receiving BMAs. Since AFF with bone metastasis might require a longer time to achieve union, it would be important to prevent incomplete AFF from proceeding to complete AFF via prophylactic internal fixation, taking care of prodromal symptoms and bilateral occurrence.
6. Institutional review board statement
The Institutional Review Board of Kobe University Hospital approved this study (approval number: B190034). The institutional review boards of all other participating institutions approved the study.
7. Informed consent statement
Patient consent was waived because this is a retrospective study.
CRediT authorship contribution statement
Tomoaki Fukui: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Keisuke Oe: Investigation, Writing – review & editing, Visualization. Teruya Kawamoto: Writing – review & editing, Supervision. Masayuki Morishita: Investigation. Ikuo Fujita: Investigation. Shunsuke Takahara: Investigation. Atsushi Sakurai: Investigation. Takashi Iwakura: Investigation. Keiji Yoshida: Investigation. Kenjiro Ito: Investigation. Etsuo Shoda: Investigation. Takafumi Hiranaka: Investigation. Masaya Tsunoda: Investigation. Ryosuke Kuroda: Supervision, Project administration. Takahiro Niikura: Conceptualization, Methodology, Investigation, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank Editage (www.editage.jp) for the English language editing.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E., Lipton A., Roodman G.D., Guise T.A., Boyce B.F., Brufsky A.M., Clezardin P., Croucher P.I., Gralow J.R., Hadji P., Holen I., Mundy G.R., Smith M.R., Suva L.J. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat. Rev. 2010;36(8):615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 4.Rosen L.S., Gordon D.H., Dugan W., Jr., Major P., Eisenberg P.D., Provencher L., Kaminski M., Simeone J., Seaman J., Chen B.L., Coleman R.E. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100(1):36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 5.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Qiao D., Lu Y., Curtis D., Wen X., Yao Y., Zhao H. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist. 2015;20(4):440–449. doi: 10.1634/theoncologist.2014-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn S.G., Kim S.H., Lee H.M., Lee S.A., Jeong J. Survival benefit of zoledronic Acid in postmenopausal breast cancer patients receiving aromatase inhibitors. J. Breast Cancer. 2014;17(4):350–355. doi: 10.4048/jbc.2014.17.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin M., Bell R., Bourgeois H., Brufsky A., Diel I., Eniu A., Fallowfield L., Fujiwara Y., Jassem J., Paterson A.H., Ritchie D., Steger G.G., Stopeck A., Vogel C., Fan M., Jiang Q., Chung K., Dansey R., Braun A. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012;18(17):4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 9.Wong M.H., Stockler M.R., Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2012;2:CD003474. doi: 10.1002/14651858.CD003474.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Black D.M., Eastell R., Adams A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. Reply. N. Engl. J. Med. 2020;383(22):2189–2190. doi: 10.1056/NEJMc2029828. [DOI] [PubMed] [Google Scholar]
- 11.Dell R.M., Adams A.L., Greene D.F., Funahashi T.T., Silverman S.L., Eisemon E.O., Zhou H., Burchette R.J., Ott S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012;27(12):2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 12.Gani L., Anthony N., Dacay L., Tan P., Chong L.R., King T.F. Incidence of Atypical Femoral Fracture and Its Mortality in a Single Center in Singapore. JBMR Plus. 2021;5(8):e10515. doi: 10.1002/jbm4.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenart B.A., Lorich D.G., Lane J.M. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N. Engl. J. Med. 2008;358(12):1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 14.Schilcher J., Michaelsson K., Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 15.Lenart B.A., Neviaser A.S., Lyman S., Chang C.C., Edobor-Osula F., Steele B., van der Meulen M.C., Lorich D.G., Lane J.M. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20(8):1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier R.P., Perneger T.V., Stern R., Rizzoli R., Peter R.E. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch. Intern. Med. 2012;172(12):930–936. doi: 10.1001/archinternmed.2012.1796. [DOI] [PubMed] [Google Scholar]
- 17.Khow K.S., Yong T.Y. Atypical femoral fracture in a patient treated with denosumab. J. Bone Miner. Metab. 2015;33(3):355–358. doi: 10.1007/s00774-014-0606-6. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi M., Gokita T., Toda K. Impending Atypical Femoral Fracture in Patients With Medullary Thyroid Cancer With Skeletal Metastasis Treated With Long-term Bisphosphonate and Denosumab. Clin. Nucl. Med. 2017;42(6):463–464. doi: 10.1097/RLU.0000000000001592. [DOI] [PubMed] [Google Scholar]
- 19.Paparodis R., Buehring B., Pelley E.M., Binkley N. A case of an unusual subtrochanteric fracture in a patient receiving denosumab. Endocr. Pract. 2013;19(3):e64–e68. doi: 10.4158/EP12367.CR. [DOI] [PubMed] [Google Scholar]
- 20.Thompson R.N., Armstrong C.L., Heyburn G. Bilateral atypical femoral fractures in a patient prescribed denosumab - a case report. Bone. 2014;61:44–47. doi: 10.1016/j.bone.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D.W., Ebeling P.R., Einhorn T.A., Genant H.K., Geusens P., Klaushofer K., Lane J.M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Howe T.S., van der Meulen M.C., Weinstein R.S., Whyte M.P. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 22.Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 23.Chang S.T., Tenforde A.S., Grimsrud C.D., O'Ryan F.S., Gonzalez J.R., Baer D.M., Chandra M., Lo J.C. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone. 2012;51(3):524–527. doi: 10.1016/j.bone.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Edwards B.J., Sun M., West D.P., Guindani M., Lin Y.H., Lu H., Hu M., Barcenas C., Bird J., Feng C., Saraykar S., Tripathy D., Hortobagyi G.N., Gagel R., Murphy W.A., Jr. Incidence of Atypical Femur Fractures in Cancer Patients: The MD Anderson Cancer Center Experience. J. Bone Miner. Res. 2016;31(8):1569–1576. doi: 10.1002/jbmr.2818. [DOI] [PubMed] [Google Scholar]
- 25.Puhaindran M.E., Farooki A., Steensma M.R., Hameed M., Healey J.H., Boland P.J. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J. Bone Joint Surg. Am. 2011;93(13):1235–1242. doi: 10.2106/JBJS.J.01199. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M., Ozaki Y., Kizawa R., Masuda J., Sakamaki K., Kinowaki K., Umezu T., Kondoh C., Tanabe Y., Tamura N., Miura Y., Shigekawa T., Kawabata H., Baba N., Iguchi H., Takano T. Atypical femoral fracture in patients with bone metastasis receiving denosumab therapy: a retrospective study and systematic review. BMC Cancer. 2019;19(1):980. doi: 10.1186/s12885-019-6236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S.P., Kim T.W., Boland P.J., Farooki A. Retrospective Review of Atypical Femoral Fracture in Metastatic Bone Disease Patients Receiving Denosumab Therapy. Oncologist. 2017;22(4):438–444. doi: 10.1634/theoncologist.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwinski E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., Reginster J.Y., Roux C., Malouf J., Bradley M.N., Daizadeh N.S., Wang A., Dakin P., Pannacciulli N., Dempster D.W., Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 29.Shane E., Burr D., Ebeling P.R., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D., Einhorn T.A., Genant H.K., Geusens P., Klaushofer K., Koval K., Lane J.M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Sen H.T., van der Meulen M.C., Weinstein R.S., Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 30.Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G., Gaion F., Bertoldo F., Santini D., Rondena R., Bogani P., Ripamonti C.I. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 31.Clemons M., Ong M., Stober C., Ernst S., Booth C., Canil C., Mates M., Robinson A., Blanchette P., Joy A.A., Hilton J., Aseyev O., Pond G., Jeong A., Hutton B., Mazzarello S., Vandermeer L., Kushnir I., Fergusson D. REaCT investigators, A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer. 2021;142:132–140. doi: 10.1016/j.ejca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Himelstein A.L., Foster J.C., Khatcheressian J.L., Roberts J.D., Seisler D.K., Novotny P.J., Qin R., Go R.S., Grubbs S.S., O’Connor T., Velasco M.R., Jr., Weckstein D., O’Mara A., Loprinzi C.L., Shapiro C.L. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. J. Am. Med. Assoc. 2017;317:48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hortobagyi G.N., Van Poznak C., Harker W.G., Gradishar W.J., Chew H., Dakhil S.R., Haley B.B., Sauter N., Mohanlal R., Zheng M., Lipton A. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol. 2017;3:906–912. doi: 10.1001/jamaoncol.2016.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang C.Y., Zebaze R.M., Ghasem-Zadeh A., Iuliano-Burns S., Hardidge A., Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52(1):360–365. doi: 10.1016/j.bone.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Lin T.L., Wang S.J., Fong Y.C., Hsu C.J., Hsu H.C., Tsai C.H. Discontinuation of alendronate and administration of bone-forming agents after surgical nailing may promote union of atypical femoral fractures in patients on long-term alendronate therapy. BMC Res. Notes. 2013;6:11. doi: 10.1186/1756-0500-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K., García-Hernández P.A., Recknor C.P., Einhorn T.A., Dalsky G.P., Mitlak B.H., Fierlinger A., Lakshmanan M.C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 37.Kim S.J., Park H.S., Lee D.W., Lee J.W. Short-term daily teriparatide improve postoperative functional outcome and fracture healing in unstable intertrochanteric fractures. Injury. 2019;50:1364–1370. doi: 10.1016/j.injury.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1–84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Joint Surg. Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 39.Cadieux B., Coleman R., Jafarinasabian P., Lipton A., Orlowski R.Z., Saad F., Scagliotti G.V., Shimizu K., Stopeck A. Experience with denosumab (XGEVA(R)) for prevention of skeletal-related events in the 10 years after approval. J. Bone Oncol. 2022;33 doi: 10.1016/j.jbo.2022.100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A., Kutilek S., Adami S., Zanchetta J., Libanati C., Siddhanti S., Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan Y., Tornetta P., 3rd, Einhorn T.A., Guy P., Leveille L., Robinson J., Bosse M.J., Haines N., Horwitz D., Jones C., Schemitsch E., Sagi C., Thomas B., Stahl D., Ricci W., Brady M., Sanders D., Kain M., Higgins T.F., Collinge C., Kottmeier S., Friess D. Healing Time and Complications in Operatively Treated Atypical Femur Fractures Associated With Bisphosphonate Use: A Multicenter Retrospective Cohort. J. Orthop. Trauma. 2016;30(4):177–181. doi: 10.1097/BOT.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 42.Koh A., Guerado E., Giannoudis P.V. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J. 2017;99-B(3):295–302. doi: 10.1302/0301-620X.99B3.BJJ-2016-0276.R2. [DOI] [PubMed] [Google Scholar]