Graphical abstract
Keywords: Vaccine, Nasal administration, Immunization, Nasal-associated lymphoid tissue, Nanoparticles, Advanced delivery system
Abstract
The importance of vaccination has been proven particularly significant the last three years, as it is revealed to be the most efficient weapon for the prevention of several infections including SARS-COV-2. Parenteral vaccination is the most applicable method of immunization, for the prevention of systematic and respiratory infections, or central nervous system disorders, involving T and B cells to a whole-body immune response. However, the mucosal vaccines, such as nasal vaccines, can additionally activate the immune cells localized on the mucosal tissue of the upper and lower respiratory tract. This dual stimulation of the immune system, along with their needle-free administration favors the development of novel nasal vaccines to produce long-lasting immunity. In recent years, the nanoparticulate systems have been extensively involved in the formulation of nasal vaccines as polymeric, polysaccharide and lipid ones, as well as in the form of proteosomes, lipopeptides and virosomes. Advanced delivery nanosystems have been designed and evaluated as carriers or adjuvants for nasal vaccination. To this end, several nanoparticulate vaccines are undergone clinical trials as promising candidates for nasal immunization, while nasal vaccines against influenza type A and B and hepatitis B have been approved by health authorities. This comprehensive literature review aims to summarize the critical aspects of these formulations and highlight their potential for the future establishment of nasal vaccination. Both preclinical (in vitro and in vivo) and clinical studies are incorporated, summarized, and critically discussed, as well as the limitations of nasal immunization.
1. Introduction
Vaccination is an effective weapon for disease prevention and has been proven to significantly reduce the transmission of infections and the number of deaths worldwide. Almost all the approved vaccines are injectables and consequently parenteral vaccination is the most used method for their administration. The continuous emergence of new pathogens and the resistance of microorganisms increase the trend towards the development of new strategies to produce long-lasting immunity. Furthermore, the interest of the scientific community on the non-invasive methods of vaccine administration is risen constantly the last decade. It is quite known that the nasal cavity is the first-contact area when the antigens are entered in the human body. Hence, the development of nasal vaccines may be a feasible alternative for a more effective immunization [1], [2].
Many studies have been performed to prove the efficiency of intranasal (IN) vaccination, and several have also undergone clinical trials. These vaccines may not be applied only to respiratory infections, but also to systematic ones. Nasal cavity is rich in lymphatic tissue, commonly called as nasal-associated lymphoid tissue (NALT), and it combines humoral and cellular immune responses inducing not only the systemic, but also the mucosal immunity. In addition, immunoglobulin A (IgA), which constitutes more than 15 % of the total immunoglobulins, is presented in higher percentage at mucosal secretions, than in serum (secretory IgA: sIgA). The predominance of IgA in nasal mucosa contributes to the defense of the mucosa against the antigens, preventing their attachment and/or permeation across the nasal epithelium [3]. Published studies [4], [5] have shown that the IgA induction, along with that of serum IgG can increase the effectiveness of IN immunization, producing cross-reactive antibodies. The cross-reactivity enables the reduction of vaccination frequency as the antibodies can be activated by more than one antigen [3].
The practical and functional advantages of nasal vaccines over the conventional ones cannot be ignored. The improper disposal and/or reuse of needles contaminated with blood-borne pathogens raises critical safety issues [6]. Needle-free nasal vaccination systems can reduce the risk of injury and cross-contamination with pathogens, such as the Human Immunodeficiency Virus (HIV) and hepatitis B virus [7]. Moreover, the compliance of patients is improved, especially in the case of children, avoiding issues of non-compliance associated with needle-fear or pain [8]. In addition, the self-administration of these vaccines would be feasible, facilitating the quick immunization of the community, especially in the case of pandemics, where rapid and mass vaccination is required [1], [2].
It is worth mentioning, that the cost of nasal vaccination is reduced compared to parenteral. The requirements of cold chain for maintaining low temperatures during the production, transport and storage of certain vaccines constitute essential additional cost. Moreover, the risk of temperature fluctuations is considered adequately high [7]. The nasal route allows the administration of both liquid and dry vaccine formulations [1] also ensuring the avoidance of exposure in extreme pH values and digestive enzymes [7]. Furthermore, the slower aging of NALT, compared to other mucosal immune sites, such as the intestine lymphatic tissue, renders nasal vaccination an advantageous application for elderly patients [2].
At the moment, five different nasal vaccines have been approved by the regulatory authorities for the markets of USA, Europe, Asia and Cuba (Table 1 ). FluMist® [9] and Fluenz Tetra® [10] are quadrivalent vaccines approved by FDA and EMA, respectively, and ensure immunization against the subtypes of influenza A and B. These vaccines have been formulated in the conventional dosage form of nasal spray. Accordingly, Nasovac S® [11] is a trivalent nasal flu vaccine marketed in Asia in lyophilized form able to be reconstituted with sterile water in a homogeneous suspension for inhalation and administered with a syringe. In 2016, the pandemic influenza vaccine Η5Ν1 [12], formulated in suspension, was licensed in Europe for the prevention against a single strain of influenza virus. HeberNasvac [13] is the only approved nasal vaccine, licensed in Cuba, for immunization against hepatitis B and it can be administered in the form of nasal drops.
Table 1.
Approved nasal vaccines.
| Vaccine name | Disease | Type | Antigen |
|---|---|---|---|
| Fluenz Tetra | Influenza type A, B | Live attenuated | A/H1N1 strain, A/H3N2 strain, two B strains (B/Washington/02/2019 and B/Phuket/3073/2013 lineages) |
| FluMist Quadrivalent | Influenza type A, B | Live attenuated | A/H1N1 strain, A/H3N2 strain, two B strains (B/Yamagata/16/88 and B/Victoria/2/87 lineages) |
| HeberNasvac | Chronic hepatitis B | Recombinant Virus-Like Particles (VLP) | Surface antigen (HBsAg) and nucleocapside antigen (HBcAg) from HBV |
| Nasovac-S | Influenza type A, B | Live attenuated | A/H1N1 strain, A/H3N2 strain, one B strain (B/56/Brisbane/60/08) |
| Pandemic influenza vaccine Η5Ν1 – AstraZeneca | Influenza type A | Live attenuated | A/H5N1 strain |
The aim of this comprehensive review is to summarize and critically discuss the advances in the field of nasal vaccines to reveal their prospect as an alternative mode of immunization. We attempt to assess the clinical applicability (protective and/or therapeutic) of IN vaccination is various diseases, such as influenza [14], pertussis [15], [16], meningitis [17], [18], hepatitis [19], [20], based on in vitro, preclinical, and clinical studies.
2. Methods – literature search
The main literature search for this review concerns references found both in online and book resources. These include online databases such as PubMed and Google Scholar from 2000 to November 2022, the EMA, FDA, and clinical trials websites, as well as anatomy and physiology books. The research was focused on the research articles of the last 20 years, including preclinical and clinical studies of intranasally administered vaccines. The reliability of the clinical studies was verified through the official website (https://clinicaltrials.gov/), where the actual dates of each phase are reported using the unique NTC number of each trial. Α summary of the conclusions of the included studies are presented in Table 1, Table 2, Table 3 . Our search included the titles, abstracts, and Medical Subject Headings (MeSH) and we used the following search terms: nasal administration, nasal mucosa, nanotechnology, influenza virus, antigens, and mucosal adjuvants. In our search we included preclinical experimental studies, both preclinical (in vitro and in vivo) and clinical studies. To draw firm conclusions, we excluded the following studies: full-text unavailable, publication language other than English, and conference abstracts.
Table 2.
Preclinical studies of nasal vaccines against influenza subtypes and other viruses.
| Indication | Influenza type/Antigen | Adjuvant/Excipient | Key outcome |
|---|---|---|---|
| Acquired Immune Deficiency Syndrome (AIDS) from HIV-1 |
HIV-1 DNA plasmids encoding gagp37 of subtype B [122] | N3 (cationic lipid-based adjuvant) [81] | ↑ of HIV-1-specific humoral immune response without causing damage to the nasal epithelium and interfering with the CNS |
| Recombinant multiepitopic protein from HIV-1 (CR3) [123] | HBsAg (Hepatitis B virus surface antigen), HBcAg (Hepatitis B virus core antigen) | High titers of Th1 cells in spleen and IFNγ secreting cells in the intestine, less anti-CR3 antibodies at the vagina, simultaneously immunity against HBV | |
| Acute otitis media from non-typeable Haemophilus influenzae (NTHi) |
Outer membrane protein (OMP) from NTHi (strain 76) [116] | Monophosphoryl lipid A (MPL: TLR-4 agonist) | MPL augmented OMP-specific IgA titers in the nasopharynx |
| Anthrax | AdVAV (replication-deficient adenovirus type 5-vectored vaccine) encoding the protective antigen (PA83) from B. anthracis[124] | Faster induction of immune response and higher levels of anti-PA IgG and toxin neutralized antibodies compared to intramuscular immunization against B. anthracis | |
| Anti-protective antigen (PA) against Bacillus anthracis toxin alone or conjugated with a 10-mer peptide of capsule of B. anthracis[68] | Monophosphoryl Lipid A (MPL) with or without Chitosan (ChiSys™) | Protection against B. anthracis infection through high levels of anti-PA IgG in serum, when the vaccine combines PA protein with capsule epitopes and chitosan | |
| Recombinant Bacillus anthracis protective antigen (rPA) [110] |
Aluminum hydroxide or unmethylated, phosphorothioate-linked, CpG-containing oligonucleotides | Powder formulations achieved higher protection against anthrax challenge compared to liquid, unit-dose disposable devices |
|
| rPA alone or conjugated with a 10-mer peptide of capsule of B. anthracis[66] | MPL, ChiSys ® | Dry powder anthrax vaccine induces immunization | |
| Cutaneous leishmaniasis |
Whole lyophilized strains from Leishmania amazonensis (LaAg) [118] | ↑ of the Τh1, CD4 + and CD8 + T cells levels, ↓ of the Treg subpopulations, protection against cutaneous leishmaniasis and visceral leishmaniasis | |
| Ebola virus disease |
Ebola virus envelope glycoprotein (GP) [117] | CTA1-DD (subunit of cholera toxin combined with two Ig-binding domains of staphylococcal protein A) |
The CTA1-DD adjuvant enhanced the humoral, cellular and mucosal immune response through IgG, Th1, IFN-c/IL-4 secreting cells and IgA in vaginal lavages |
| Hepatitis B |
HBsAg, HBcAg [113] | Influenza surface protein haemagglutinin (HA) from H1N1 | HA complexed liposomes Influenza surface protein haemagglutinin |
| Influenza | H7N9 avian influenza A virus/Recombinant H7 protein [80] | PEG-b-PLACL (PELC: squalene-based oil-in-water emulsion) and CpG compared to poly(I:C) and flagellin (plasmid-encoded TLR5 agonist) | ↑ of IgG and IgA titers in serum and spleen even in low doses |
| Influenza A virus (H1N1)/Whole inactivated virus [81] | N3 (cationic lipid-based adjuvant), pFliC(-gly) (flagellin derived adjuvant) | ↑ of humoral and cellular influenza specific immune response | |
| Influenza A virus (H3N1, H3N2, H1N1)/Pam2Cys [125] | Pegylated Pam2Cys (PEG-Pam2Cys: TLR2 agonist) | Induction of pulmonary innate immune response and adaptive immunity | |
| Influenza A virus (H5N1)/Recombinant HA protein [88] | Thermal-sensitive hydrogel from HTCC (chitosan derivative) and α, β-GP (α, β-glycerophosphate) | ↑ of the residence time in nasal cavity and extended transepithelial transport, antigen-specific systemic and mucosal immunity through cellular and humoral response | |
| Swine influenza virus/Inactivated antigen (H1N2-OH10) [14] | Chitosan, poly(I:C) | ↑ of HI serum titers and antigen-specific IFN-γ response, memory cells, Th1, Th2 and γ, δ T-cells in pulmonary and distant lymphoid sites | |
| Norovirus infections |
Bivalent formulation with GI and GII.4 VLPs [72] | GelSite ™ | Systemic and mucosal immunogenicity occurs after two doses of the VLPs |
| Self-assembling Norwalk virus-like particles (NV VLPs) [73] | GelSite ™ (inert in-situ gelling polysaccharide), gardiquimod (TLR7 agonist) | GelSite-based dry powder formulations induced systemic and mucosal antibody titers equal to those achieved by liquid formulations | |
| Pertussis |
Acellular pertussis vaccine consisting of 2 or 3B. pertussis antigens (including recombinant pertussis toxin, TLR2 lipoprotein ligands from B. pertussis) [15], [16] | c-di-GM (intracellular receptor stimulator of interferon genes agonist), LP1569 (TLR2 agonist from B. pertussis) | Induction of Th1, Th17 and IgG2 antibody response, ↑of IL-17-secreting respiratory tissue memory cells |
| Respiratory syncytial virus (RSV) infections |
Plasmid DNA encoding the CTL epitope from the M2 protein of RSV [78] | Chitosan | ↑ the induction of CTL response comparable to those induced via intradermal route |
| SARS-CoV-2 |
Chimpanzee adenovirus-vectored vaccine encoding a prefusion stabilized spike (S) protein modifying it with two proline substitutions in the S2 subunit [89] | Protection of upper and lower respiratory tract from SARS-CoV-2 infection, ↑of the levels of serum and mucosal IgA after a single dose compared to intramuscular immunization | |
| Lipid nanoparticle‑based SARS‑CoV‑2 proteins and mRNA vaccines [96] |
NP-monosodium urate adjuvant | Induction of Th1 and Th2 immune responses and of antibodies secretion | |
| Tetanus |
Tetanus toxoid [67] | Alginate microspheres, cross-linked dextran microspheres, quillaja saponins |
↑ of sIgA and serum IgG titers, ↑ of mucoadhesion |
| Tuberculosis | BCG vaccine (different Mycobacterium bovis strains) [126], [127] | ↑ of the CD8 T cells, interferons and interleukins levels in spleen compared to subcutaneous immunization, ↔ pulmonary protection | |
| Messenger RNA of Hsp65 protein from Mycobacterium leprae[107] | Production of IL-10 and TNF-α against Hsp65 in spleen, nitric oxide production from TLR7 cells |
Table 3.
Ongoing and completed clinical trials concerning nasal vaccines.
| Ongoing clinical trials | ||
|---|---|---|
| Disease/Clinical trial number | Study characteristics | Stage |
| Anthrax/NCT04148118 [111] | Evaluation of the safety and immunogenicity of intranasally administrated BW-1010 vaccine to healthy adults. The vaccine comprises of adjuvanted rPA in nanoemulsion formulation. It will be administered through nasal sprayers and nasal drops. Nasal samples and serum will be assessed for total IgA, anti-rPA IgA and IgG and toxin neutralization antibodies | Phase 1 |
| Influenza type A, Respiratory syncytial virus (RSV) infections/NCT02755948 [128] | Vaccination of healthy adult volunteers with Influenza and RSV antigens through nasal drops. Cell mediated immunity against these viruses will be examined | Recruiting |
| Meningitis/NCT04135053 [18] | Nasal inoculation of healthy adults with reconstituted and lyophilized Neisseria lactamica. The study aims to examine safety and immunogenicity, and determine the required dose for the induction of colonization in 80 % of the volunteers | Recruiting |
| Meningitis/NCT04665791 [129] | Dose-ranging nasal inoculation of healthy Malian adults with reconstituted and lyophilized Neisseria lactamica. The optimal dose and the immunization ability will be examined | Recruiting |
| RSV infections/NCT01893554 [130] | Evaluation of safety and immune responses against RSV infections. Healthy RSV-seropositive and RSV-seronegative infants and children will be vaccinated with nasal drops | Phase 1 |
| Completed clinical trials | ||
| Indication/Registration number | Study main characteristics | Stage |
| Chronic Hepatitis B/NCT01374308 [19], [20] | Comparison between therapeutic vaccination with HBsAg and HBcAg (NASVAC) and the pegylated interferon alfa 2b (Peg-IFN). Higher reduction of HBV DNA levels in serum and increase of alanine aminotransferases levels compared to Peg-IFN recipients. | Phase 3 |
| Diphtheria/[119] | Immunization with the genetically inactivated mutant diphtheria toxoid CRM197 in a polycationic polysaccharide chitosan nanoformulation. Antitoxin neutralizing activity was observed, equivalent to intramuscular vaccination. The presence of chitosan enhanced the immunogenicity. SIgA induction was not as high as expected with the first inoculation. | Phase 1 |
| HIV-1 infection/NCT00122564 [120] | Transmucosal (nasal or vaginal) administration of HIV-1 vaccine with the recombinant gp-160, with or without the adjuvant DC-chol (cationic lipid). | Phase 1 |
| HIV-1 infection/NCT01084343 [104] | In administration of HIV-1 vaccine (MYM-V101) after two intramuscular doses to female volunteers. MYM-V101 is a virosomal vaccine that carries on its surface the lipid peptide P1, derived from the gp41 of HIV-1. Evaluation of the adverse events and immune responses. Induction of the vaginal and rectal P1-specific IgGs, vaginal P1-IgAs and, specific IgG and IgA antibodies in serum | Phase 1 |
| HIV infection/NCT01473810 [105] | IN administration of Vacc-4x cause dose-dependent immunogenicity. Induction of T-cell (CD8+, CD4+) and mucosal and systemic humoral responses. | Phase 1,2 |
| HIV/NCT00369031 [131] | Estimation of safety and immunogenicity of nasal vaccine, containing HIV-glycoprotein 140 mixed with a toxoid or a liquid adjuvant (Labile Toxin mutant: LTK63) | Phase 1 |
| Influenza type A, B/NCT03023553 [132] | Comparison of the immunogenicity against seasonal influenza, between intramuscular administration of trivalent inactivated Fluzone® vaccine and IN delivery of live attenuated FluMist® vaccine. | Phase 4 |
| Influenza type A, B/[133] | Administration of inactivated, trivalent influenza virus vaccine using chitosan as delivery system. | Phase 1 |
| Influenza type A (GamFluVac)/[134] | Comparative assessment of immunity responses derived from two different methods of IN administration of GamFluVac (nasal drops and nasal spray) | Phase 2 |
| Influenza type A (H1N1, H3N2) and B/[135] | Virosomal vaccine adjuvanted with Escherichia coli HLT elicits humoral and cell-mediated responses and high titers of IgA neutralizing antibodies at the mucosa, comparable to parenteral vaccination | Phase 2 |
| Influenza type A (H1N1, H3N2) and B (Fluzone)/[136] | IN administration of the quadrivalent influenza vaccine Fluzone® and comparison of the immunogenicity after intramuscular and IN vaccination | Phase 1 |
| Influenza type A (H1N1, H3N2) and B/[137] | Virosomal vaccines administered through intranasal sprays, Escherichia coli heat-labile toxin (HLT) adjuvanted vaccines provoked comparable humoral immune responses with parenteral vaccination, higher IgA titers in the saliva after two nasal doses | Phase 1 |
| Influenza type A (H1N1, H3N2) and B/[57], [58] | Trivalent subunit influenza vaccine using Proteosome™ as delivery system elicits serum and mucosal immune responses, without severe adverse reactions. | Phase 1,2 |
| Influenza type A (H1N1)/[56] | Evaluation of the monovalent influenza vaccine connected with outer membrane proteins (OMP) extracted from N. meningitidis, employing Proteosome™ as delivery system. Well-tolerated vaccine, causing serum HAI antibodies and HA-specific sIgA. | Phase 1 |
| Influenza type A (H5N1)/NCT01258062 [138] | GelVac™ nasal powder H5N1 influenza vaccine, evaluation of frequency and severity of the adverse effects | Phase 1 |
| Μeningitis, Diphtheria/[17] | Comparison of immunogenicity between intramuscular injection and nasal insufflations of Neisseria meningitidis serogroup C polysaccharide (MCP) with diphtheria toxoid (CRM197) conjugated vaccine. Induction of MCP-specific and CRM197-specific IgG, MCP-specific sIgA, serum bactericidal antibodies and diphtheria toxin-neutralizing activity. | Phase 1 |
| Norovirus infections/NCT00806962 [71], [112] |
Norwalk VLP vaccine with GI.1 genotype adjuvanted with MPL and chitosan. Two studies examining the effect of different antigen dosages. No serious vaccine-related adverse events were reported. High immunogenicity with Norwalk VLP-specific IgG and IgA titers related to the dose. | Phase 1 |
| Pertussis/NCT01188512 [98], [99] | IN delivery of BPZE1, a live attenuated nasal pertussis vaccine with a genetically modified Bordetella pertussis strain. Induction of immune responses to all adult volunteers can lead to the use of this vaccine as THE first vaccination of young infants, before the injectable one. | Phase 1 |
| Pertussis/NCT02453048 [100], [101] | Single IN administration of BPZE1. Induction of B. pertussis–specific antibody responses, Th1-type cellular responses, and killing mechanisms. | Phase 1 (Ib) |
| Pertussis/NCT03541499 [102] | Single IN administration of BPZE1. Evaluation of the optimal dose, humoral and mucosal immunogenicity. | Phase 2 |
| Pertussis/NCT04036526 [139] | Comparison of doses and application methods of GamLPV, a live IN Bordetella pertussis vaccine | Phase 1,2 |
| Respiratory illness from human Parainfluenza virus type 2 (HPIV2)/NCT01139437 [121] | Safety of and immune response of live attenuated intranasally administered HPIV2 vaccine. This approach activates antibody-mediated, the cell-mediated and mucosal immune responses. | Phase 1 |
| Shigellosis/[59] | Shigella flexneri 2a vaccine connected to meningococcal outer membrane proteins of Proteosomes. Dose-escalated clinical trial. Dose-related immune reactions, containing S. flexneri 2a lipopolysaccharide-specific IgA, IgG and IgM antibody-secreting cells. | Phase 1 |
| Shigellosis/NCT00485134 [115], [114] | IN administration of Shigella flexneri 2a Invaplex 50 vaccine (subunit vaccine), via the Dolphin™ spray device lead to more effective immune responses than the droplet method, by the induction of the antibody secreting cells (ASC) and the antigen-specific mucosal and intestinal IgA | Phase 1,2 |
| Tuberculosis/[108] | Subunit tuberculosis vaccine with a toxoid adjuvant. Αssessment of safety and immunogenicity. | Phase 1 |
| Tuberculosis/NCT03017378 [109] | IN and sublingual tuberculosis vaccine delivery (TB/FLU-01L) to healthy adults, already vaccinated with the common tuberculosis vaccine (BCG) | Phase 1 |
Using the above-mentioned terms, we initially found 1256 hints. After abstracts’ screening, we removed 96 duplicated studies, and 1046 studies as irrelevant. 114 full-text studies were screened for eligibility. After removing studies with wrong design, irrelevant outcomes, unavailable full-text, we ended-up in a total of 28 experimental studies, 14 clinical studies and 27 additional clinical trials which were not include those studies.
3. Anatomy and main characteristics of nasal cavity
The anatomical position and physiology of the nasal cavity render it important for vital functions, such as breathing and olfaction. The human nasal cavity is divided into three distinct areas: the nasal vestibule, the respiratory, and the olfactory region. The respiratory region can be further divided by bone conchae structures into three turbinates [21]. Although the epithelial features between these regions differ, all the three are lined with a rich layer of mucosal tissue, of 10–15 μm thickness. Furthermore, the nasal epithelium is composed by epithelial cells, covered with microvilli with anatomical projections called cilia. The role of the cilia is significant, since with their coordinated movement the mucociliary clearance (MCC) is achieved. MCC contributes to the trapping and removing of inhaled particles and pathogens, as part of the digestive system [22]. Τhe nasal mucosa also filters, heats and humidifies the inhaled air before it reaches the lower respiratory tract [23]. Its protective activity is enhanced by the components of the mucus, which are 95 % water, 2.5–3 % the glycoprotein mucin and 2 % electrolytes, proteins, lipids, enzymes, and antibodies. IgA and IgG are presented in high titers in nasal mucus, providing a first-line immunological response to both inhaled and administered antigens [24].
The anatomical position of the olfactory region, at the top of the nasal cavity, and the pathways of both olfactory and trigeminal nerve are directly connected with the central nervous system (CNS). More precisely, this feature has been extensively studied by researchers, as a targeting area for nose-to-brain delivery (NBD). The development of formulations for NBD of drugs aims to the improvement of treatment strategies, for neurodegenerative or other diseases. Τhe drug can easily penetrates the olfactory epithelium, through intracellular and paracellular pathways, ending in the olfactory bulb and subarachnoid space of brain, respectively [25]. The nose-to-brain strategy gives the opportunity of bypassing the tight junctions of Blood–Brain–Barrier (BBB), avoiding the gastrointestinal and hepatic metabolism, as well. Thus, the bioavailability of the administered substances is enhanced, and the targeting becomes more effective and achievable, through a non-invasive way [23].
3.1. Nasopharynx-associated lymphoid tissue (NALT)
Mucosal tissue covers a large area in human adults (approximately 400 m2) and it is the gateway for many pathogens. Almost 80 % of the total immune cells are located in mucosal areas [24]. Moreover, in mucosal regions inductive sites are found, where the immune response to pathogenic molecules and administered antigens can be initiated. These regions comprise the mucosal associated lymphoid tissue (MALT), which is found in specific compartments of human body such as nose (NALT), lungs (Bronchus-Associated Lymphoid Tissue: BALT), gastrointestinal tract (Gut-associated lymphoid tissue: GALT), as well as on vaginal/rectal surfaces [3]. All these tissues contain immunity inducive sites, such as lamina propria and antigen-presenting cells (APCs), which are at the same effectively connected with effector sites or distant mucosal tissues of the human body. That system is known as the common mucosal immune system (CMIS) and has the ability either to initiate cell-mediated immune responses or to potentially provide immunological memory [2], [26].
An intranasally administrated vaccine first adheres to the mucosal membrane and then interacts with the lymphatic tissue of the nasal cavity. NALT in humans and rodents contains aggregates of organized lymphoid follicles and is described as the Waldeyer’s ring. It is a group of tonsils and adenoids, that includes a pair of tubal and palatine tonsils; one nasopharyngeal (adenoid) and one lingual [27]. NALT is located below the epithelium of the nasal cavity. Antigen-sampling M cells (microfold cells) are responsible for transporting antigens to NALT, while immunocompetent cells are required for the induction of an immune response [26].
3.2. Mucosal immunity and IN immunization
Nasal mucosa is the first tissue of contact for the administered antigen. The predominant immunoglobulin in the nasal cavity is the secretory IgA (sIgA), which is secreted in large quantities after antigen administration. Antigen secretion starts from nasal mucosa, which is the main inducive site, and then is enhanced by other mucosal tissues of the CMIS, as previously reported. The mechanism governing IgA secretion is directly related to the presence of the highly vascularized lamina propria layer. Plasma cells secrete polymeric IgA molecules (pIgA), which interact with specialized pIgA receptors found on the surface of the mucosal epithelial cells. Subsequently, proteolysis of pIgA takes place and quantities of immunoglobulin are secreted [3].
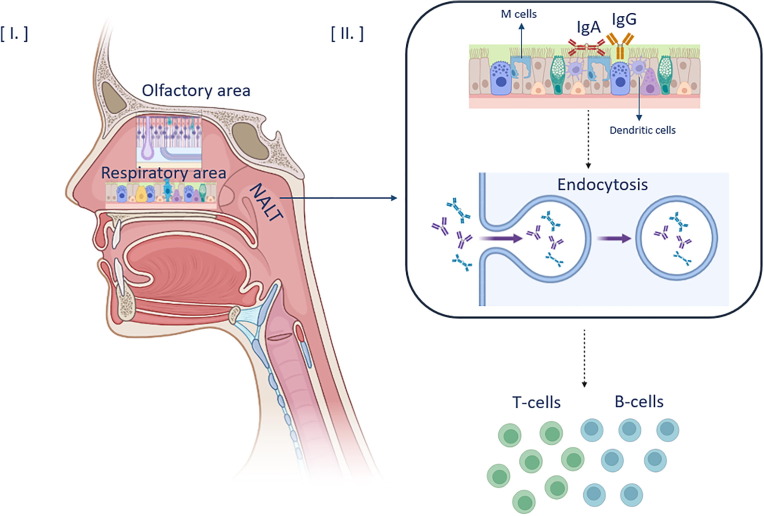
Concerning the transport of the antigen, it initially attaches to the epithelial cells, where several microfold cells (M cells) are located. M cells are responsible for the uptake of the particulate antigens and their transport to the underlying lymph nodes that constitute the NALT. Soluble antigens can passively penetrate the whole nasal epithelium and reach the superficial lymph nodes through interactions with toll-like receptors (TLRs) [28]. The type of interactions taking place in NALT are highly dependent on the antigen’s characteristics. In the NALT, the immunocompetent cells required for the generation of the immune response, are mainly the APCs, such as dendritic cells (DCs) and macrophages. These cells are responsible for the endocytosis of antigens (Fig. 1 ). More precisely, their epitopes interact with the Major Histocompatibility Complex (MHC) class I, II molecules and efficiently activate CD8+ and CD4+ T-helper (Th) cells, as well as the cytotoxic T lymphocytes [29]. The most common types of T-cells involved in this process are Th1, Th2 and Th17, which secrete cytokines, including the interleukins (4, 5, 9, 12, 13 and 17), the interferon γ (IFN-γ) and the tumor necrosis factor β (TNF-β). These factors combined with the functions of Th cells describe the innate immunity system and lead to the apoptosis of pathogens and development of immunological memory. To conclude, regulatory T-cells (Tregs) have a modulatory role in terms of innate immunity, as they help to control and terminate further the Th responses [3].
Fig. 1.
Nasal cavity anatomy and generation of the mucosal immune response. I) The anatomy of the nasal cavity. II) The M and dendritic cells are responsible for the endocytosis of the antigens into the NALT to interact with T and B cells and generate the immune response.
4. Innovative delivery systems for nasal vaccines
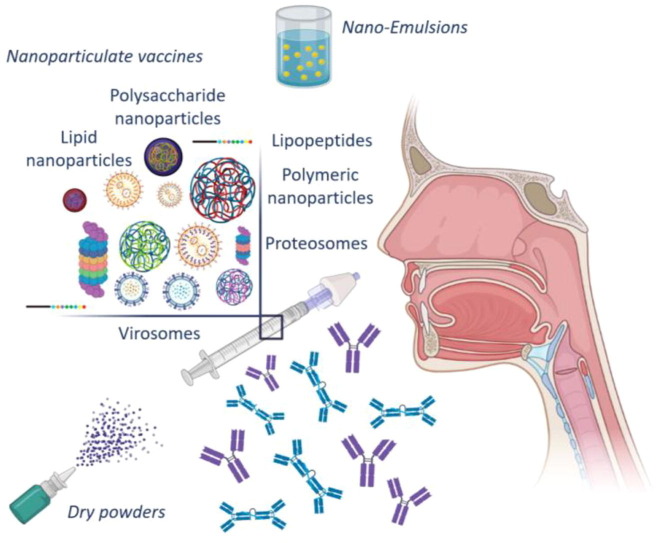
4.1. Nanoparticulate nasal vaccines
Several preclinical studies have focused on using nanoparticles as delivery systems and adjuvants either for intramuscular or nasal vaccination employing rodent or pig animal models [30], [31], [32], [33], [34]. Particularly, the recent review of Nian et al. (2022) [35] efficiently summarizes the available types of mucosal adjuvants for nasal vaccines. Interestingly, through the nasal passage, nanoparticles can pass across mucus and interact directly with NALT cells. The stimulation of mucosal immune responses leads to the production of persistent immunological memory. The main reason for the use of nanoparticulate vaccines is their ability to protect antigens from the proteolytic degradation and improve the cellular distribution [36]. The sustained release of the antigen in the mucosa can also be achieved by its formulation into nanoparticles. Thus, the probability of antigen uptake by the mucosal and lymphatic cells is increased. Modifications in physicochemical properties increase the stability of nanoparticles in biological fluids, allowing the prolonged presentation of the antigen in the body. Furthermore, various types of antigens can be entrapped and higher loading capacities, not only for the antigens but also for the adjuvants, can be achieved [37]. It is also feasible to modify the physicochemical properties of the particles, such as their charge, shape and size, rendering them ideal carriers for protein delivery, as well as for mimicking the viruses’ properties [36]. Specifically, it has been shown that the spherical shape, size around 100 nm, cationic charge and hydrophobic character favors the uptake of nasally administrated antigens from APCs. Another useful feature of nanoparticles is the ability to integrate TLR ligands in their surface, leading to a prolonged TLR signaling and reducing the number of antigens and adjuvants needed for an efficient immune response. Τhis cascade of events can also reduce the incidences of toxicity at nonimmune cells [37].
4.1.1. Nanoparticle types used in nanoparticulate nasal vaccines
4.1.1.1. Polymeric nanoparticles
Nanoparticles from natural and synthetic polymers have been extensively studied as antigen delivery systems. Chitosan and PLGA molecules are the main representatives [28]. Particularly, their mucoadhesive properties have been shown to enhance the immune response at both mucosal and systemic level. This is also verified in preclinical studies in which nasal immunizations were performed involving surface antigens of the hepatitis B virus (HBsAg) [32], [38], ovalbumin molecules [39], [40] and strains of the influenza A virus [41]. In the study of Zaman et al. (2011) [42] the self-assembling of amphiphilic dendrimers from polyacrylate molecules is described. These polymers could form IN subunit vaccines, giving a self-adjuvating effect against systemic group A streptococcus infection [42]. In the case of influenza A several chitosan-based mucosal vaccines have been tested in vivo, in poultry and pigs, having been delivered either orally or intranasally, triggering both mucosal and cellular immune responses [43].
4.1.1.2. Polysaccharide nanoparticles
Polysaccharide nanoparticles are among the most well-established carriers of intranasally administrated antigens. Due to their biocompatibility, polysaccharides such as starch and dextran, have been used to formulate nanoparticulate nasal vaccines. Cationic nanoparticles consist of maltodextrins (Supramolecular biovectors: SMBV™) were evaluated as delivery systems of two recombinant proteins, either of a particulate (Hepatitis B surface antigen: HBsAg) or of a soluble (β-galactosidase) one, for their immunogenicity to mice. This type of vector comprises a polysaccharide core, that can be further surrounded by a phospholipid layer, mimicking the size and structure of viruses. Thus, it can be used for protein and peptide delivery, especially through nasal sprays [44]. The results showed high levels of serum IgG, mucosal IgA and Cytotoxic T lymphocytes (CTL)-mediated responses [45]. Similar outcomes were observed after the IN immunization of rabbits, with nanoparticles consisting of starch and Carbopol 974P, which served as influenza virus antigens’ carriers [46]. Furthermore, dextran-based nanovaccines induced dendritic cells (DCs)-mediated responses after IN administration of tetanus toxoid in rabbits [47]. In another study, sweet corn-derived cationic alpha-D-glucan nanoparticles were combined with a synthetic double RNA molecule and a toll-like receptor (TLR)-3 ligand to be delivered intranasally in pigs. This vaccine was found to induce the production of antibodies and the cytokine response more efficiently than the inactivated influenza A vaccine given either intranasally or intramuscularly [48].
4.1.1.3. Lipid nanoparticles
Another commonly used type of nanoparticles are the lipid nanoparticles, such as liposomes, micelles, lipid nanocapsules and immunostimulating complexes (ISCOMs). Their components can be phospholipids, triglycerides, or cholesterol molecules, classified into unilamellar or multilamellar spherical vesicles [36]. IN inoculation with multilamellar phospholipid-based liposomes, containing influenza A viral nucleoprotein, found to activate mucosal CTLs and DCs, reducing virus replication in the lung [49]. Moreover, the performed experiments prove that the positively charged liposomes interact with the negatively charged groups of sialic acid in nasal mucin glycoprotein, allowing a more effective interaction with NALT. Furthermore, IN immunization of mice with influenza HA antigen encapsulated in polycationic liposomes, known as VaxiSome™, manages to improve humoral and cellular immune responses, as follows by the high levels of IFN-γ and IL-2 cytokines [50]. Accordingly, when cationic liposomes, containing ovalbumin as antigenic factor, were intranasally administered, they showed an adjuvant effect on the activation of antigen-specific mucosal and systemic immune responses [51]. Regarding the ISCOMs, their main characteristic is the presence of the natural adjuvant Quil A in their open spherical configurations. Ovalbumin ISCOMs were able to induce both systemic and mucosal immune responses after being twice administrated in mice, via IN route. Cibulski et al. (2016) [52] and Coulter et al. (2003) [53] in their study, found that ISCOM structures (ISCOMatrix™) can trigger higher antibody titers compared to a Heat-labile toxin, after IN vaccination of mice with influenza antigens. Concerning the lipid nanocapsules, they have been identified as an ideal carrier for mucosal inoculation, showing higher stability than liposomes. However, there are no published studies about their use in nasal vaccination [36].
4.1.1.4. Proteosomes
Proteosomes are protein-based multilayered structures, including hydrophobic outer membrane proteins. They are mainly used for the administration of subunit mucosal vaccines, such as influenza hemagglutinin glycoproteins. These glycoproteins include hydrophobic domains in their structure and thus, they can non-covalently associate with the macromolecules of proteosomes, and successfully present these antigens to the mucosal immune system [54], [55]. In addition, the proteosomes have been successfully tested in phases 1 and 2 of clinical trials, as delivery and adjuvant systems (Table 3) [56], [57], [58], [59].
4.1.1.5. Lipopeptides
Lipopeptides are a category of nanoparticles, consist of one or more lipid moieties with antigenic and/or immunomodulatory peptides, forming amphiphilic liposomal or micellar structures. They can self-assemble in aquatic environment, forming both nano- and micro-particle structures, due to the interactions between peptides and lipids [28]. Lipopeptides were tested as vaccine delivery systems and evaluated for their immunogenicity against Group A Streptococcus. Their IN administration demonstrated that the appropriate size of vector and epitopes can induce epitope-specific antibody responses, as well as high IgG serum titers [60]. Moreover, the entrapment of lipopeptides in cationic liposomes can induce both mucosal and systemic immunity, increasing IgA and IgG titers in mice. High levels of antibodies were also detected five months after the inoculation [61].
4.1.1.6. Virosomes
Virosomes are proteoliposomes, consisted of a single or double layered phospholipid vesicle, incorporating glycoproteins, extracted from viruses, inside or on their surface. They can increase NALT binding with APCs, enabling the induction of Th1, Th2 and CTLs immune responses [2]. Preclinical studies have demonstrated that virosomes can be ideal carriers for a trivalent influenza subunit vaccine [62], as well as for DNA-based vaccines [63] when administered intranasally. It is worth to mention the NasalFlu, a trivalent virosomal inactivated influenza vaccine, which was launched in Switzerland in 2001. However, it was soon discontinued due to its association with partial facial paralysis cases.
4.2. Nano-Emulsions for nasal vaccines
Emulsions are stable dispersions of two or more immiscible phases (oily and aqueous), containing small amounts of surfactants as stabilizing agents. These formulations are considered ideal for the development of nasal vaccines, as they can produce particles smaller than 200 nm. Nano-emulsified antigens are controlled release formulations, showing an increased resistance to enzymatic degradation. Moreover, the properties of the oils and surfactants used in their composition can increase the interaction with the nasal mucus. The total charge of the nanoemulsion, as well as the degree of their induction can vary, depending on the components of the formulation and the type of the immune response [28]. Nanoemulsion-based nasal vaccine was developed and tested for its protective effects against hepatitis B. More precisely, surface antigens of the hepatitis B virus (HBsAg) were trapped into a soybean oil-based emulsion and administered intranasally to animal models. The nanoemulsion was able to cross the epithelial membrane intracellularly. Therefore, the opening of nasal mucosa tight junctions or the involvement of M cells were not required to achieve effective permeation. The results demonstrate that needle-free nasal immunization with emulsion formulation can induce an effective Th1 associated cellular immunity, providing therapeutic benefit to patients with chronic hepatitis B infection, comparable to that derived by parenteral vaccination [64].
4.3. Dry powders for nasal vaccines
Dry powders have also been tested as candidates for the formulation of nasal vaccines. They are considered as advantageous forms being less susceptible to physical, chemical, and thermal degradation, than the liquid ones. Dry powder nasal vaccines have been widely tested in vivo (Table 3) for immunization against various diseases, such as diphtheria [65], influenza [46], [66], tetanus [67], anthrax [68], [69], meningitis [17] and gastroenteritis [70], [71], [72], [73]. Moreover, recently a nano-adjuvanted dry powder vaccine was assessed for its ability to induce mucosal immunization against the Mycoplasma hyopneumoniae, producing higher IgA and IgG levels than the conventional intramuscular vaccine [74] . To administer the Dry Powders Vaccines (DPVs) intranasally, appropriate devices are needed to ensure the stability and protection of the product by humidity. In addition, these devices should aerosolize and improve the nasal deposition of DPVs, eliminating the lung deposition of vaccine particles. The most used devices are Dry Powder Inhalers (DPIs) and Monopowder single-dose disposable device Powder-Jet® is their main representative [1].
5. Antigen form in nasal vaccines
5.1. Live attenuated nasal vaccines (LAIV)
Live attenuated vaccines are produced by genetically attenuated version of the virus, which is reproduced to a limited extent, without causing disease but inducing immune responses such those triggered by natural infection. Attenuation can be achieved by subjecting the virus to adverse conditions, such as low temperatures and growth in cells outside the human body, or by modifying the virus through the optimization of nucleotide sequences, or the inactivation of the genes which are responsible for virus’ innate immune recognition [75], [76]. The virus replicates within the vaccinated person, and then the immune response is mediated by humoral and cellular reactions. LAIVs stimulate the production of a complete set of influenza antigens in their natural form, imitating the immune response induced by natural infection and allowing cross-reactive immunity. Many live attenuated cold strains of seasonal and pandemic influenza vaccines are under research, while LAIV nasal vaccines are already on the market (Table 1). However, the disadvantages of these vaccines are associated with safety issues and the need for frequent modification of the virus [2], [77].
5.2. DNA vaccines
DNA vaccines have been developed to solve the problem of continuous genetic mutation of viruses. Antibodies are produced against specific proteins, determined by the DNA sequence. However, the over-accumulated form and negative charge of DNA impede the effectiveness and stability of the vaccine. The addition of chitosan in nanoparticles can lead to more stable DNA vaccine formulations [2]. The vaccination of BALB/c mice with a chitosan-based IN vaccine expressing epitopes of Respiratory Syncytial Virus (RSV) plasmid resulted in protective responses mediated by cytotoxic T-lymphocytes [78]. In addition, DNA sequences can also be introduced as adjuvants in nasal vaccines. In this category unmethylated oligodeoxynucleotide (CpG), circular dinucleotides (CDNs) and Flagellin, which is a bacterial component encoded by plasmid DNA, can be found [79]. All these adjuvants have been tested and proven to be effective in preclinical studies [15], [80], [81].
5.3. mRNA vaccines
An alternative approach to tackling the viral diversity and the continuous mutations is the development of nasal vaccines based on mRNA sequences. Conversely to DNA vaccines, RNA ones can infect either by dividing or non-dividing cells, without entering the nucleus, resulting in higher and faster gene expression. In the study of Li et al. (2016) [82], an RNA nasal vaccine was administered at BALB/c mice to evaluate its immunogenicity against HIV-1 infection. The antigen, consisted of the mRNA sequence which expresses the glycoprotein 120 of the virus, was encapsulated into cationic polymeric nanoparticles. After its IN administration, mucosal and systemic immune responses, as well as cytokine production, were achieved [82].
Polyinosine-polycytidylic acid (polyI:C), a synthetic analog of double-stranded RNA (dsRNA), induces type I IFN production and innate immune responses via specific pathways [79]. The success of these molecules has been verified by preclinical studies of IN immunization against the influenza virus [14], [83].
5.4. Virus like particles (VLPs)
A recent promising approach for IN immunization is the use of VLPs, consisted of specific viral proteins that spontaneously assemble into oval shape. Thanks to their size, they can mimic the natural structure of viruses, without containing the viral genes, being safer than the previously mentioned categories [2]. The small size of the particles (less than 100 nm) enables their permeation across the mucosa barrier increasing the interaction with the NALT cells [36]. IN immunization with VLPs containing the structural proteins of the H1N1 (1918) virus, found to protect mice against replication-competent homologous virus, as well as against the lethal challenge, inducing high levels of cross-reactive IgG and IgA antibodies [84]. This aspect was verified in a preclinical study where nasal administration of this system aimed to ensure immunization against HIV-1, after the incorporation of glycoprotein-expressing DNA sequences into the VLPs [85].
6. Nasal vaccines approach against SARS-CoV-2
The outbreak of severe coronavirus syndrome caused by the SARS-CoV-2 rendered the development of an effective vaccine the priority for health scientists globally. The SARS-CoV-2 is a single-stranded RNA virus comprises four main structural proteins as follows: S (spike), E (envelope), M (membrane) and N (nucleocapsid). The S glycoprotein forms trimeric spikes on the virion, enabling virus attachment on the membrane of the host cells after binding with the surface receptor of the angiotensin-converting enzyme 2 (ACE2). It has been found that most of ACE2 receptors are located on the nasal epithelium and salivary gland ducts, while less are found in the alveoli. The virus replication takes places on the nasal mucosa before reaching the systematic circulation. Thus, the infection of the individual with the virus elicits the immune response of both upper and lower respiratory tract, mediated by the sIgA and serum IgG antibodies, located on the nasal mucosa and lungs, respectively [86].
Intramuscular vaccination results to the intense production of serum IgG, but not of the mucosal IgA. Although adequate amounts of IgG can be found on the mucus membranes of the upper respiratory tract, sIgA deficiency may leave a person vulnerable to covid-19 infection. IN vaccination can induce effective mucosal antibody response, providing sterile immunity in the upper respiratory tract [86].
The attempts of research groups to produce a nasal vaccine for SARS-CoV-2 were as follows:
-
1.
King et al. (2020) developed a nasally administered, single dose, covid-19 vaccine, named AdCovid™. In this vaccine, the sequence expressing the Receptor Binding Domain (RBD) was entrapped into a type 5 adenovirus. RBD is one of the two functional regions of the S protein that binds the virus with the ACE2 receptor. The vaccine was intranasally administrated to mice and found to induce intense IgG serum neutralizing activity, along with high titers of mucosal IgA in the respiratory tract. Cell mediated immunity was also observed via CD4+ and CD8+ T and Th1 cells [87]. The vaccine is now further tested in phase 1 of clinical trials for the evaluation of its safety and immunogenicity in healthy volunteers [88].
-
2.
An alternative adenovirus-based vaccine was developed by Washington University School of Medicine. The vaccine candidate encodes the stabilized S protein of the virus, and it was evaluated after IN administration to mice and non-human primates. It was found that a single dose of IN inoculation ensures complete protection of upper and lower airways, in contrast to the intramuscular injection of the same vaccine, which elicits minimal mucosal immune response [89].
-
3.
Codagenix Inc. developed a nasal vaccine containing a whole inactivated virus, with inactivated immunogenic regions. The vaccine, named COVI-VAC, is now under phase 1 of clinical trials since 12th January 2021 [86].
-
4.
Scientists from AstraZeneca/Oxford Jenner Inst. administered intranasally to rhesus macaques the approved vaccine for intramuscular injection. As is well known, it is a monovalent vaccine with the full-length SARS-CoV-2 spike glycoprotein gene into a recombinant replication-deficient chimpanzee adenovirus vector [90]. Neutralized antibodies were significantly higher and viral RNA was significantly reduced in oropharyngeal swabs of IN vaccinated animals compared to the intramuscular vaccination [91].
-
5.
An alternative approach was the development of the recombinant vectored Newcastle Disease Virus (NDV), the wild type of NDV, in its live attenuated form. The virus encodes the S glycoprotein gene of SARS-CoV-2 and can be administered intranasally. The safety and immunogenicity of this type of vaccine carrier were proven in preclinical studies, supporting also that the humoral and cell-mediated immune responses in both lungs and serum were amplified. In addition, the viral presence into nasal cavity and lungs, as well as the transmission of the disease found to be totally inhibited four days after the vaccination [92], [93].
-
6.
A team from the University of Houston developed an IN-subunit vaccine, containing a trimeric or monomeric S protein from the SARS-CoV-2 virus as antigen, and a stimulator of interferon genes (STING) as adjuvant. The vaccine was encapsulated in liposomal formulations and intranasally inoculated in BALB/c mice in single dose. Serum and fluid collection 7- and 15-days post-immunization showed that the trimeric-STING-liposomal form of the vaccine achieved higher titers of secretory and S-specific IgA antibodies, not only in NALT and lungs, but also in spleen [94].
-
7.
In the study of Ku et al. (2021) [95] the interest was focused on a lentiviral vector, which was able to induce neutralized antibodies against the Spike glycoprotein of SARS-CoV-2. IN inoculation of the vector to mice with induced ACE2 receptors, decreased more effectively the lung viral loads, as well as the local inflammation, compared to intramuscular injection [95].
-
8.
The most recently developed intranasal vaccine against SARS-CoV-2 was lipid nanoparticle (NP)-based containing virus proteins or their mRNAs combined with NP-monosodium urate adjuvant. The in vitro and in vivo evaluation of both formulations revealed that the lipid nanoparticles with virus proteins were more effective in respiratory tract disinfection, upregulating the mRNA expression of proinflammatory cytokines (IFNα, MCP1 and IL-4) in lungs enhancing the Th1 and Th2 immune responses. In the case of IN administration of lipid nanoparticles with virus mRNAs high levels of antibodies were noted. These outcomes demonstrated the better performance of NP-based vaccine containing virus proteins when administered intranasally in ferrets animal model [96].
7. Preclinical and clinical studies on nasal vaccines
The last decade, the field of nasal vaccines is rapidly developed, because of the advantages accompanying the nasal route as vaccine delivery site. The nanocarriers used for antigen administration have been tested at both preclinical and clinical levels, for multiple human diseases.
7.1. Influenza
Most of the nasal vaccine candidates have been developed for influenza. These vaccines aim at immunization against the H1N1 and H3N2 strains of influenza A and specific subtypes of influenza B. In a preclinical study performed in 2019 against the influenza A virus (H1N1), the tested antigenic part was a whole inactivated virus. The adjuvants used in this study enhanced the ability of the antigen to induce, not only mucosal, but also the systemic immunity. Cationic lipid-based molecules, combined with plasmid-encoded DNA, increased the amount of antigen linked with TLR5 cells, and consequently the efficacy of the immune response [81]. Another interesting case concerns the IN administration of a flu vaccine, using Proteosome™ as delivery system. That novel formulation comprises proteins from Neisseria meningitidis, combined with three monovalent influenza antigens containing hemagglutinin (HA) strains from influenza A (H1N1, H3N2) and influenza B (Guangdong lineage). The vaccine was tested in healthy populations in phase 1 of clinical trials and was found to be safe and immunogenic without any side effects related to the antigenic vector. Alongside, serum and mucosal anti-HA antibodies, against all three types of virus vaccines, were effectively detected after one or two vaccine doses [57], [58]. Similar studies concerning IN immunization against influenza subtypes have been done, including different antigen forms, such as inactivated swine antigens [14], recombinant hemagglutinin protein [80], and the already licensed influenza vaccines Fluzone® (NCT01385215) and GamFluVac (NCT04034290). The adjuvants included in these studies were either chitosan derivatives [14], [97] and emulsions, or other molecules [76].
7.2. Acellular pertussis
Acellular pertussis is a disease affecting the upper respiratory tract after its infection by the bacterium Bordetella pertussis. In a preclinical study, B. pertussis antigens were administered intranasally to mice as recombinant toxins or lipoprotein ligands [15], [16]. C-di-GM and LP1569 were the main selected adjuvants for this study, characterized as interferon receptor (STING) agonist and a TLR2 agonist, respectively. This combination leads to higher Th1, Th17 and IgG2a antibody induction and cellular immunity than that derived by the same vaccine without these immunostimulatory molecules. It was also observed that IN immunization, induces the secretion of the respiratory IL-17 memory cells, which are responsible for the impediment of nasal colonization and lung infection [15], [16]. Furthermore, IN BPZE1 vaccine, a live attenuated nasal pertussis vaccine containing a genetically modified Bordetella pertussis strain, shown satisfactory immune response, reaching the phase 2 of clinical trials. It can be used as the first vaccination of young infants, before the injectable one, according to the vaccination plan [98], [99], [100], [101], [102].
7.3. HIV
MYM-V101 is another interesting case of nasal vaccine that has been developed and tested in phase 1 for the prophylaxis of HIV-1 infection. It is a virosomal vaccine that carries on its surface the lipid peptide P1, derived from the gp41 of HIV-1. After been tested at two different doses, it was found to be able to produce serum and mucosal P1 specific IgG and IgA antibodies in dose-dependent way [103], [104]. Vacc-4x is a peptide vaccine that has also been developed to treat AIDS. The vaccine provokes dose-dependent immune response, which is mainly mediated by IL-10, TGF-β, CD8+ and CD4+ T cells. This vaccine has undergone phase 2 clinical trials [105], [106].
7.4. RSV
The RSV usually enters the body via mucosal surfaces. Therefore, several efforts have been made to prevent the infection by this virus, using nasal vaccines. In a preclinical study, a chitosan-based vaccine formulation containing the plasmid DNA which encodes the M2 protein of the virus was intranasally administered in mice. The vaccine elicited peptide- and virus-specific CTL responses equivalent to those induced via the intradermal route of immunization [78].
7.5. Tuberculosis
Tuberculosis is another disease for which a nasal vaccine has been developed. An interesting case which was studied in 2010 included the IN administration of the virulent mRNA strains that encode the Hsp65 protein from Mycobacterium leprae. This protein is the causative pathogen of the disease. In this study, it was observed that APCs found in lungs were able to capture the mRNA and induce the cellular immune response [107]. Other tuberculosis vaccine formulations have been tested for their safety and immunogenicity after given intranasally to healthy adults [108], [109].
7.6. Inhalational anthrax
Anthrax vaccine has been extensively evaluated in preclinical studies concerning nasal immunization against the inhalational anthrax. The main antigen of all these studies is the recombinant Bacillus anthracis toxin (rPA) and can be found either alone or combined with peptides from the capsule of the pathogen. The used adjuvants vary and can be CpG oligonucleotides, MPL or chitosan. They have been found to enhance the immunogenicity when the vaccine is administered in certain dose and form. Τhe key indicator for the success of the immunity was the release of anti-rPA titers and anthrax lethal toxin-neutralizing antibodies [68], [69], [110]. Moreover, the BW-1010 vaccine, which is an adjuvanted nanoemulsion with the same antigen, has been developed for the same disease and is currently undergoing clinical trials to evaluate its immunological effect when administered intranasally [111].
7.7. Norwalk infection
A different formulation studied in terms of its immunogenicity after IN administration concerns the self-assembled virus like particles (VLPs). This type of antigen carrier has been tested in both preclinical and clinical studies for the development of a nasal vaccine against Norwalk infection. Preclinical studies showed that the integration of a natural polysaccharide with in-situ-gelling properties (GelSite) automatically increases the immunogenicity. This phenomenon occurs as the VLPs remain longer on nasal mucosal surfaces and resist at mucociliary clearance, extending the residence time of the antigen on the immune effector sites [72], [73]. Norwalk VLP vaccine with GI.1 genotype was given intranasally in different antigen doses to study the extent of antibody expression. Chitosan and MPL were the main adjuvants employed. The main outcome of the study was that Norwalk VLP-specific IgG and IgA titers are secreted in a dose-dependent way [71], [112].
7.8. Hepatitis B
Another application of nanotechnology on nasal vaccines concerns the treatment of chronic hepatitis B. In this case, the antigens used in the study were HBsAg (Hepatitis B virus surface antigen) and HBcAg (Hepatitis B virus core antigen). These antigens were formed in liposomes that were complexed with the influenza surface protein haemagglutinin (HA). That combination significantly enhances immune response, particularly by the secretion of IgA from the mucosal sites [113]. A series of preclinical and clinical studies led to the approval of HeberNasvac, a therapeutic nasal vaccine against chronic hepatitis B. More precisely, healthy adults were recruited for phase 1 and 2 of the clinical trials to test the safety of intranasally administrated Shigella flexneri 2a Invaplex 50 vaccine. It was a subunit vaccine delivered via the Dolphin™ spray device. This trial aimed to compare the spray to droplet administration. It was found that the spray device induced higher antibody titers, highlighting the importance of the chosen delivery device for an effective treatment [114], [115].
A series of additional studies confirmed the importance and effectiveness of nasal vaccination, as well as the need to incorporate molecules that manage to enhance the immune response. Preclinical studies of the last decade ensure the validity of IN vaccination in several diseases, such as acute otitis [116], ebola virus disease [117], cutaneous leishmaniasis [118] and tetanus [67] (Table 2). Moreover, as mentioned above, clinical trials have assured the safety of vaccine formulations for meningitis [17], diphtheria [119], HIV [120] and respiratory disease from parainfluenza [121] (Table 3).
8. Limitations of nasal vaccines
Nasal vaccination is an advantageous mode of immunization, as the active agents of vaccines are not exposed in extreme pH values and/or digestive enzymes of the gastrointestinal tract. However, the presence of specific physiology features of the nasal cavity constitutes an important limitation of vaccines’ effectiveness. Mucociliary clearance occurs continuously, impeding the attachment of the antigens on the epithelium. The soluble antigens when administered alone can be diffused through TLR receptors. Nevertheless, their uptake is often inefficient, revealing the need of particulate formulations [2]. In addition, the small capacity of the nasal cavity, the protein efflux systems, and the possible pathological conditions can reduce the extent of the antigen absorption [23].
The anatomical position of the nasal cavity requires special care during the nasal vaccine delivery, as the excipients used can often lead either to the irritation of the nasal cavity, or to respiratory infections and neurotoxicity. Specifically, in the case of the NasalFlu vaccine, which was approved in Switzerland in 2001, it was quickly withdrawn due to reports of several Bell's palsy cases attributed to the adjuvant contained in the formulation. Τhe presence of the Escherichia coli heat-labile toxin as mucosal adjuvant and its direct transfer in the CNS, through the olfactory epithelium, led to this severe adverse effect [140]. To avoid this event, accurate targeting of antigens is required, via the proper design of specialized nasal vaccine delivery devices [35], [141].
9. Conclusions
Nasal vaccination ensures effective immunization based on three critical actions; the simultaneous activation of humoral and cellular immune responses involving both T and B cells, along with the mucosal immunity expressed by the extensive secretion of IgA in significantly higher levels than in serum. The beginning of nasal vaccines in the form of LAIV and their successful application established the conditions for the development of other forms such as the DNA and mRNA vaccines, as well as the VLPs. These novels forms are able to resolve safety issues ascribed to the frequent modification of the viruses. Furthermore, the outbreak of SARS-CoV-2 has increased interest towards the IN immunization, resulting in the development of six vaccine candidates for nasal administration. In addition, more than 30 clinical trials concerning the nasal vaccines have been registered for respiratory or non-respiratory infections. Although several limitations accompany the existed products, the optimization of the antigen form in the nasal vaccines using advanced delivery systems can increase its absorption and diffusion, as well as reduce the possible adverse effects and the events of toxicity. Nasal vaccination tends to gain ground against the conventional intramuscular administration, as a non-invasive method and thus, a promising alternative to increase patients’ compliance. Certainly, critical steps towards the improvement of delivery devices and added adjuvants are required to bend the last doubts against nasal vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
This is a review paper and thus no experimental or clinical data have not been gemerated
References
- 1.Bahamondez-Canas T.F., Cui Z. Intranasal immunization with dry powder vaccines. Eur J Pharm Biopharm. 2018;22:167–175. doi: 10.1016/j.ejpb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18(10):771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf H., Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccines Immunother. 2017;13(1):34–45. doi: 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lijek R.S., Luque S.L., Liu Q., Parker D., Bae T., Weiser J.N. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. PNAS. 2012;109(34):13823–13828. doi: 10.1073/pnas.1208075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang Y.H., Byun Y.H., Lee Y.J., Lee Y.H., Lee K.H., Seong B.L. Cold-adapted pandemic 2009 H1N1 influenza virus live vaccine elicits cross-reactive immune responses against seasonal and H5 influenza A viruses. J Virol. 2012;86(10):5953–5958. doi: 10.1128/JVI.07149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z., Diaz-Arévalo D., Guan H., Zeng M. Noninvasive vaccination against infectious diseases. Hum Vaccines Immunother. 2018;14(7):1717–1733. doi: 10.1080/21645515.2018.1461296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nir Y., Paz A., Sabo E., Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68(3):341–344. [PubMed] [Google Scholar]
- 9.https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/flumist-qlaiv-product-monograph-en.pdf.
- 10.https://www.ema.europa.eu/en/medicines/human/EPAR/fluenz-tetra.
- 11.https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadSmPC/5serum.pdf.
- 12.https://www.ema.europa.eu/en/documents/product-information/pandemic-influenza-vaccine-h5n1-astrazeneca-epar-product-information_en.pdf.
- 13.https://www.cecmed.cu/sites/default/files/adjuntos/rcp/biologicos/rcp_hebernasvac_0.pdf.
- 14.Renu S., Feliciano-Ruiz N., Ghimire S., Han Y., Schrock J., Dhakal S., et al. Poly(I:C) augments inactivated influenza virus-chitosan nanovaccine induced cell mediated immune response in pigs vaccinated intranasally. Vet Microbiol. 2020;242 doi: 10.1016/j.vetmic.2020.108611. [DOI] [PubMed] [Google Scholar]
- 15.Allen A.C., Wilk M.M., Misiak A., Borkner L., Murphy D., Mills K. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 2018;11(6):1763–1776. doi: 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 16.Dunne A., Mielke L.A., Allen A.C., Sutton C.E., Higgs R., Cunningham C.C., et al. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015;8(3):607–617. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- 17.Huo Z., Sinha R., McNeela E.A., Borrow R., Giemza R., Cosgrove C., et al. Induction of protective serum meningococcal bactericidal and diphtheria-neutralizing antibodies and mucosal immunoglobulin A in volunteers by nasal insufflations of the Neisseria meningitidis serogroup C polysaccharide-CRM197 conjugate vaccine mixed with chitosan. Infect Immun. 2005;73(12):8256–8265. doi: 10.1128/IAI.73.12.8256-8265.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCT04135053/A controlled study to assess safety, colonisation and immunogenicity of reconstituted lyophilised neisseria lactamica (Lac5-Nasal).
- 19.Al Mahtab M., Akbar S., Aguilar J.C., Guillen G., Penton E., Tuero A., et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial) PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCT01374308/NASVAC Phase-III Trial in Chronic Hepatitis B (CHB) Patients (NASVAC).
- 21.Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. 2012;9(6):566–582. doi: 10.2174/156720112803529828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistry A., Stolnik S., Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Pires A., Fortun A., Alves G., Falcão A. Intranasal drug delivery: how, why and what for? JPPS. 2009;12(3):288–311. doi: 10.18433/j3nc79. [DOI] [PubMed] [Google Scholar]
- 24.Lobaina M.Y. Nasal route for vaccine and drug delivery: features and current opportunities. Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118813. [DOI] [PubMed] [Google Scholar]
- 25.Crowe T.P., Greenlee M., Kanthasamy A.G., Hsu W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Kiyono H., Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellings P., Jorissen M., Ceuppens J.L. The Waldeyer's ring. Acta Otorhinolaryngol Belg. 2000;54(3):237–241. [PubMed] [Google Scholar]
- 28.Marasini N., Skwarczynski M., Toth I. Intranasal delivery of nanoparticle-based vaccines. Ther Deliv. 2017;8(3):151–167. doi: 10.4155/tde-2016-0068. [DOI] [PubMed] [Google Scholar]
- 29.Diebold S.S., Cotten M., Koch N., Zenke M. MHC class II presentation of endogenously expressed antigens by transfected dendritic cells. Gene Ther. 2001;8(6):487–493. doi: 10.1038/sj.gt.3301433. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Christopher M.E., Nagata L.P., Zabielski M.A., Li H., Wong J.P., et al. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J Clin Virol. 2004;31(Suppl 1):S99–S106. doi: 10.1016/j.jcv.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Subbiah R., Ramalingam P., Ramasundaram S., Kim D.Y., Park K., Ramasamy M.K., et al. N, N, N-Trimethyl chitosan nanoparticles for controlled intranasal delivery of HBV surface antigen. Carbohydr Polym. 2012;89(4):1289–1297. doi: 10.1016/j.carbpol.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 32.Pawar D., Jaganathan K.S. Mucoadhesive glycol chitosan nanoparticles for intranasal delivery of hepatitis B vaccine: enhancement of mucosal and systemic immune response. Drug Deliv. 2016;23(1):185–194. doi: 10.3109/10717544.2014.908427. [DOI] [PubMed] [Google Scholar]
- 33.Noh Y.W., Hong J.H., Shim S.M., Park H.S., Bae H.H., Ryu E.K., et al. Polymer nanomicelles for efficient mucus delivery and antigen-specific high mucosal immunity. Angew Chem. 2013;52(30):7684–7689. doi: 10.1002/anie.201302881. [DOI] [PubMed] [Google Scholar]
- 34.Dhakal S., Renukaradhya G.J. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet Res. 2019;50:90. doi: 10.1186/s13567-019-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nian X., Zhang J., Huang S., Duan K., Li X., Yang X. Development of nasal vaccines and the associated challenges. Pharmaceutics. 2022;14:1983. doi: 10.3390/pharmaceutics14101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernocchi B., Carpentier R., Betbeder D. Nasal nanovaccines. Int J Pharm. 2017;530(1–2):128–138. doi: 10.1016/j.ijpharm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Lambricht L., Peres C., Florindo H., Préat V., Vandermeulen G. Polymer-based nanoparticles as modern vaccine delivery systems. Micro Nanotechnol Vacc Dev. 2017:185–203. doi: 10.1016/B978-0-323-39981-4.00010-5. [DOI] [Google Scholar]
- 38.Pawar D., Mangal S., Goswami R., Jaganathan K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm. 2013;85:550–559. doi: 10.1016/j.ejpb.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Scherließ R., Mönckedieck M., Young K., Trows S., Buske S., Hook S. First in vivo evaluation of particulate nasal dry powder vaccine formulations containing ovalbumin in mice. Int J Pharm. 2015;479(2):408–415. doi: 10.1016/j.ijpharm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Primard C., Poecheim J., Heuking S., Sublet E., Esmaeili F., Borchard G. Multifunctional PLGA-based nanoparticles encapsulating simultaneously hydrophilic antigen and hydrophobic immunomodulator for mucosal immunization. Mol Pharm. 2013;10(8):2996–3004. doi: 10.1021/mp400092y. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q., Zheng X., Zhang C., Shao X., Zhang X., Zhang Q., et al. Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J Med Virol. 2015;87(11):1807–1815. doi: 10.1002/jmv.24253. [DOI] [PubMed] [Google Scholar]
- 42.Zaman M., Skwarczynski M., Malcolm J.M., Urbani C.N., Jia Z., Batzloff M.R., et al. Self-adjuvanting polyacrylic nanoparticulate delivery system for group A streptococcus (GAS) vaccine. Nanomed: Nanotechnol Biol Med. 2011;7(2):168–173. doi: 10.1016/j.nano.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Renu S., Renukaradhya G.J. Chitosan nanoparticle based mucosal vaccines delivered against infectious diseases of poultry and pigs. Front Bioeng Biotechnol. 2020;13(8) doi: 10.3389/fbioe.2020.558349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Hoegen P. Synthetic biomimetic supra molecular Biovector (SMBV) particles for nasal vaccine delivery. Adv Drug Deliv Rev. 2001;51(1–3):113–125. doi: 10.1016/s0169-409x(01)00175-2. [DOI] [PubMed] [Google Scholar]
- 45.Debin A., Kravtzoff R., Santiago J.V., Cazales L., Sperandio S., Melber K., et al. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine. 2002;20(21–22):2752–2763. doi: 10.1016/s0264-410x(02)00191-3. [DOI] [PubMed] [Google Scholar]
- 46.Coucke D., Schotsaert M., Libert C., Pringels E., Vervaet C., Foreman P., et al. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine. 2009;27(8):1279–1286. doi: 10.1016/j.vaccine.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Sajadi Tabassi S.A., Tafaghod M., Jaafari M.R. Induction of high antitoxin titers against tetanus toxoid in rabbits by intranasal immunization with dextran microspheres. Int J Pharm. 2008;360(1–2):12–17. doi: 10.1016/j.ijpharm.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 48.Renu S., Feliciano-Ruiz N., Lu F., Ghimire S., Han Y., Schrock J., et al. Combination adjuvant enhances the breadth of the immune response to inactivated influenza virus vaccine in pigs. Vaccines. 2020;18:8(2): 229. doi: 10.3390/vaccines8020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ninomiya A., Ogasawara K., Kajino K., Takada A., Kida H. Intranasal administration of a synthetic peptide vaccine encapsulated in liposome together with an anti-CD40 antibody induces protective immunity against influenza A virus in mice. Vaccine. 2002;20(25–26):3123–3129. doi: 10.1016/s0264-410x(02)00261-x. [DOI] [PubMed] [Google Scholar]
- 50.Even-Or O., Joseph A., Itskovitz-Cooper N., Samira S., Rochli E., Eliyahu H., et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine. 2011;29(13):2474–2486. doi: 10.1016/j.vaccine.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Tada R., Hidaka A., Iwase N., Takahashi S., Yamakita Y., Iwata T., et al. Intranasal immunization with DOTAP cationic liposomes combined with DC-cholesterol induces potent antigen-specific mucosal and systemic immune responses in mice. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cibulski S.P., Mourglia-Ettlin G., Teixeira T.F., Quirici L., Roehe P.M., Ferreira F., et al. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine. 2016;34(9):1162–1171. doi: 10.1016/j.vaccine.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Coulter A., Harris R., Davis R., Drane D., Cox J., Ryan D., et al. Intranasal vaccination with ISCOMATRIX adjuvanted influenza vaccine. Vaccine. 2003;21(9–10):946–949. doi: 10.1016/s0264-410x(02)00545-5. [DOI] [PubMed] [Google Scholar]
- 54.Plante M., Jones T., Allard F., Torossian K., Gauthier J., St-Félix N., et al. Nasal immunization with subunit proteosome influenza vaccines induces serum HAI, mucosal IgA and protection against influenza challenge. Vaccine. 2001;20(1–2):218–225. doi: 10.1016/s0264-410x(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 55.Jones T., Allard F., Cyr S.L., Tran S.P., Plante M., Gauthier J., et al. A nasal Proteosome influenza vaccine containing baculovirus-derived hemagglutinin induces protective mucosal and systemic immunity. Vaccine. 2003;21(25–26):3706–3712. doi: 10.1016/s0264-410x(03)00387-6. [DOI] [PubMed] [Google Scholar]
- 56.Treanor J., Nolan C., O'Brien D., Burt D., Lowell G., Linden J., et al. Intranasal administration of a proteosome-influenza vaccine is well-tolerated and induces serum and nasal secretion influenza antibodies in healthy human subjects. Vaccine. 2006;24(3):254–262. doi: 10.1016/j.vaccine.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 57.Langley J.M., Halperin S.A., McNeil S., Smith B., Jones T., Burt D., et al. Safety and immunogenicity of a Proteosome-trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine. 2006;24(10):1601–1608. doi: 10.1016/j.vaccine.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 58.Munoz F.M. Technology evaluation: FluINsure, ID biomedical. Curr Opin Mol Ther. 2004;6(2):212–220. [PubMed] [Google Scholar]
- 59.Fries L.F., Montemarano A.D., Mallett C.P., Taylor D.N., Hale T.L., Lowell G.H. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect Immun. 2001;69(7):4545–4553. doi: 10.1128/IAI.69.7.4545-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaman M., Chandrudu S., Giddam A.K., Reiman J., Skwarczynski M., McPhun V., et al. Group A Streptococcal vaccine candidate: contribution of epitope to size, antigen presenting cell interaction and immunogenicity. Nanomedicine. 2014;9(17):2613–2624. doi: 10.2217/nnm.14.190. [DOI] [PubMed] [Google Scholar]
- 61.Ghaffa K.A., Marasini N., Giddam A.K., Batzloff M.R., Good M.F., Skwarczynski M., et al. Liposome-based intranasal delivery of lipopeptide vaccine candidates against group A streptococcus. Acta Biomater. 2016;41:161–168. doi: 10.1016/j.actbio.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Lambkin R., Oxford J.S., Bossuyt S., Mann A., Metcalfe I.C., Herzog C., et al. Strong local and systemic protective immunity induced in the ferret model by an intranasal virosome-formulated influenza subunit vaccine. Vaccine. 2004;22(31–32):4390–4396. doi: 10.1016/j.vaccine.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 63.Cusi M.G., Terrosi C., Savellini G.G., Di Genova G., Zurbriggen R., Correale P. Efficient delivery of DNA to dendritic cells mediated by influenza virosomes. Vaccine. 2004;22(5–6):735–739. doi: 10.1016/j.vaccine.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 64.Makidon P.E., Bielinska A.U., Nigavekar S.S., Janczak K.W., Knowlton J., Scott A.J., et al. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeela E.A., Jabbal-Gill I., Illum L., Pizza M., Rappuoli R., Podda A., et al. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine. 2004;22(8):909–914. doi: 10.1016/j.vaccine.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Dehghan S., Tafaghodi M., Bolourieh T., Mazaheri V., Torabi A., Abnous K., et al. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int J Pharm. 2014;475(1–2):1–8. doi: 10.1016/j.ijpharm.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 67.Tafaghodi M., Rastegar S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 2010;18(3):235–242. doi: 10.3109/10611860903434035. [DOI] [PubMed] [Google Scholar]
- 68.Wimer-Mackin S., Hinchcliffe M., Petrie C.R., Warwood S.J., Tino W.T., Williams M.S., et al. An intranasal vaccine targeting both the Bacillus anthracis toxin and bacterium provides protection against aerosol spore challenge in rabbits. Vaccine. 2006;24(18):3953–3963. doi: 10.1016/j.vaccine.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 69.Klas S.D., Petrie C.R., Warwood S.J., Williams M.S., Olds C.L., Stenz J.P., et al. A single immunization with a dry powder anthrax vaccine protects rabbits against lethal aerosol challenge. Vaccine. 2008;26(43):5494–5502. doi: 10.1016/j.vaccine.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Springer M.J., Ni Y., Finger-Baker I., Ball J.P., Hahn J., DiMarco A.V., et al. Preclinical dose-ranging studies of a novel dry powder norovirus vaccine formulation. Vaccine. 2016;34(12):1452–1458. doi: 10.1016/j.vaccine.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J., et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202(11):1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ball J.P., Springer M.J., Ni Y., Finger-Baker I., Martinez J., Hahn J., et al. Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Velasquez L.S., Shira S., Berta A.N., Kilbourne J., Medi B.M., Tizard I., et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29(32):5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canelli E., Ferrari L., Borghetti P., Candela F., Abiakam N.S., Bianchera A., et al. Nano-adjuvanted dry powder vaccine for the mucosal immunization against airways pathogens. Front Vet Sci. 2023;10:1116722. doi: 10.3389/fvets.2023.1116722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talon J., Salvatore M., O’Neill R.E., Nakaya Y., Zheng H., Muster T., et al. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. PNAS. 2000;97(8):4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broadbent A.J., Santos C.P., Anafu A., Wimmer E., Mueller S., Subbarao K. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets. Vaccine. 2016;34(4):563–570. doi: 10.1016/j.vaccine.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 78.Iqbal M., Lin W., Jabbal-Gill I., Davis S.S., Steward M.W., Illum L. Nasal delivery of chitosan-DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) induces protective CTL responses in BALB/c mice. Vaccine. 2003;21(13–14):1478–1485. doi: 10.1016/s0264-410x(02)00662-x. [DOI] [PubMed] [Google Scholar]
- 79.Takaki H., Ichimiya S., Matsumoto M., Seya T. Mucosal immune response in nasal-associated lymphoid tissue upon intranasal administration by adjuvants. J Innate Immun. 2018;10(5–6):515–521. doi: 10.1159/000489405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen T.H., Chen C.C., Huang M.H., Huang C.H., Jan J.T., Wu S.C. Use of PELC/CpG adjuvant for intranasal immunization with recombinant hemagglutinin to develop H7N9 mucosal vaccine. Vaccines. 2020;8(2):240. doi: 10.3390/vaccines8020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinkula J., Nyström S., Devito C., Bråve A., Applequist S.E. Long-lasting mucosal and systemic immunity against influenza A virus is significantly prolonged and protective by nasal whole influenza immunization with mucosal adjuvant N3 and DNA-plasmid expressing flagellin in aging in- and outbred mice. Vaccines. 2019;7(3):64. doi: 10.3390/vaccines7030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M., Zhao M., Fu Y., Li Y., Gong T., Zhang Z., et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J Control Release. 2016;228:9–19. doi: 10.1016/j.jconrel.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 83.Ainai A., Suzuki T., Tamura S.I., Hasegawa H. Intranasal administration of whole inactivated influenza virus vaccine as a promising influenza vaccine candidate. Viral Immunol. 2017;30(6):451–462. doi: 10.1089/vim.2017.0022. [DOI] [PubMed] [Google Scholar]
- 84.Perrone L.A., Ahmad A., Veguilla V., Lu X., Smith G., Katz J.M., et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83(11):5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buonaguro L., Devito C., Tornesello M.L., Schröder U., Wahren B., Hinkula J., et al. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine. 2007;25(32):5968–5977. doi: 10.1016/j.vaccine.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 86.Tiboni M., Casettari L., Illum L. Nasal vaccination against SARS-CoV-2: synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603 doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.10.1101/2020.10.10.331348. [DOI]
- 88.Altimmune Commences Enrollment in Phase 1 Clinical Trial of AdCOVID™ – a Needle-Free, Single-Dose Intranasal COVID-19 Vaccine Candidate – Altimmune [WWW Document]. URL https://ir.altimmune.com/news-releases/news-release-details/altimmune-commences-enrollment-phase-1-clinical-trial-adcovidtm (accessed 3.22.21).
- 89.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf.
- 91.10.1101/2021.01.09.426058. [DOI]
- 92.Park J.G., Oladunni F.S., Rohaim M.A., Whittingham-Dowd J., Tollitt J., Hodges M., et al. Immunogenicity and protective efficacy of an intranasal live-attenuated vaccine against SARS-CoV-2. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun W., Liu Y., Amanat F., González-Domínguez I., McCroskery S., Slamanig S., et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat Commun. 2021;12(1):6197. doi: 10.1038/s41467-021-26499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.An X., Martinez-Paniagua M., Rezvan A., et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ku M.W., Bourgine M., Authié P., Lopez J., Nemirov K., Moncoq F., et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29(2):236–249.e6. doi: 10.1016/j.chom.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boley P.A., Lee C.M., Schrock J., Yadav K.K., Patil V., Suresh R., et al. Enhanced mucosal immune responses and reduced viral load in the respiratory tract of ferrets to intranasal lipid nanoparticle-based SARS-CoV-2 proteins and mRNA vaccines. J Nanobiotechnol. 2023;21:60. doi: 10.1186/s12951-023-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y., Wei W., Zhou M., Wang Y., Wu J., Ma G., et al. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33(7):2351–2360. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 98.NCT01188512/First Adult safety trial on nasal live attenuated B. Pertussis vaccine.
- 99.Thorstensson R., Trollfors B., Al-Tawil N., Jahnmatz M., Bergström J., Ljungman M., et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine–BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.NCT02453048/Study of BPZE1 (high dose) nasal live attenuated B. Pertussis vaccine.
- 101.Lin A., Apostolovic D., Jahnmatz M., Liang F., Ols S., Tecleab T., et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J Clin Invest. 2020;130(5):2332–2346. doi: 10.1172/JCI135020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.NCT03541499/safety and immunogenicity of intranasal BPZE1 vaccination in healthy adults.
- 103.Leroux-Roels G., Maes C., Clement F., van Engelenburg F., van den Dobbelsteen M., Adler M., et al. Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 Gp41 P1 peptide on virosomes. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.NCT01084343/Investigation of the safety of an HIV-1 vaccine given intra-muscularly and intra-nasally to healthy female subjects.
- 105.Brekke K., Lind A., Holm-Hansen C., Haugen I.L., Sørensen B., Sommerfelt M., et al. Intranasal administration of a therapeutic HIV vaccine (Vacc-4x) induces dose-dependent systemic and mucosal immune responses in a randomized controlled trial. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NCT01473810/Intranasal modified Vacc-4x gag peptides with endocine as adjuvant.
- 107.Lorenzi J.C., Trombone A.P., Rocha C.D., Almeida L.P., Lousada R.L., Malardo T., et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis. BMC Biotechnol. 2010;10:77. doi: 10.1186/1472-6750-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.NCT00440544/A phase I trial of a LTK63 adjuvated tuberculosis nasal subunit vaccine (Ag85B-ESAT6) (™UVA-01).
- 109.NCT03017378/reactogenicity, safety and immunogenicity of a TB/FLU-01L tuberculosis vaccine.
- 110.Mikszta J.A., Sullivan V.J., Dean C., Waterston A.M., Alarcon J.B., Dekker J.P., 3rd, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191(2):278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 111.NCT04148118/A safety and immunogenicity of intranasal nanoemulsion adjuvanted recombinant anthrax vaccine in healthy adults (IN NE-rPA).
- 112.NCT00806962/Phase 1 Norwalk vaccine study.
- 113.Tiwari S., Verma S.K., Agrawal G.P., Vyas S.P. Viral protein complexed liposomes for intranasal delivery of hepatitis B surface antigen. Int J Pharm. 2011;413(1–2):211–219. doi: 10.1016/j.ijpharm.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Riddle M.S., Kaminski R.W., Williams C., Porter C., Baqar S., Kordis A., et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine. 2011;29(40):7009–7019. doi: 10.1016/j.vaccine.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 115.NCT00485134/Shigella flexneri 2a invaplex 50 vaccine dose finding and assessment of protection.
- 116.Iwasaki T., Hirano T., Kodama S., Kadowaki Y., Moriyama M., Kawano T., et al. Monophosphoryl lipid A enhances nontypeable Haemophilus influenzae-specific mucosal and systemic immune responses by intranasal immunization. Int J Pediatr Otorhinolaryngol. 2017;97:5–12. doi: 10.1016/j.ijporl.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 117.Su Q.D., He S.H., Yi Y., Qiu F., Lu X.X., Jia Z.Y., et al. Intranasal vaccination with ebola virus GP amino acids 258–601 protects mice against lethal challenge. Vaccine. 2018;36(41):6053–6060. doi: 10.1016/j.vaccine.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 118.Pereira Silva Bezerra I., Amaral Abib M., Rossi-Bergmann B. Intranasal but not subcutaneous vaccination with LaAg allows rapid expansion of protective immunity against cutaneous leishmaniasis. Vaccine. 2018;36(18):2480–2486. doi: 10.1016/j.vaccine.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 119.Mills K.H., Cosgrove C., McNeela E.A., Sexton A., Giemza R., Jabbal-Gill I., et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect Immun. 2003;71(2):726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.NCT00122564/Study of HIV-1 Rgp-160 administered by mucosal routes in healthy volunteers.
- 121.NCT01139437/Safety of a live attenuated human parainfluenza virus type 2 (HPIV2) vaccine for adults, children, and infants.
- 122.Hinkula J., Hagbom M., Wahren B., Schroder U. Safety and immunogenicity, after nasal application of HIV-1 DNA gagp37 plasmid vaccine in young mice. Vaccine. 2008;26(40):5101–5106. doi: 10.1016/j.vaccine.2008.03.098. [DOI] [PubMed] [Google Scholar]
- 123.Iglesias E., García D., Carrazana Y., Aguilar J.C., Sánchez A., Gorobaya L., et al. Anti-HIV-1 and anti-HBV immune responses in mice after parenteral and nasal co-administration of a multiantigenic formulation. Curr HIV Res. 2008;6(5):452–460. doi: 10.2174/157016208785861186. [DOI] [PubMed] [Google Scholar]
- 124.Krishnan V., Andersen B.H., Shoemaker C., Sivko G.S., Tordoff K.P., Stark G.V., et al. Efficacy and immunogenicity of single-dose AdVAV intranasal anthrax vaccine compared to anthrax vaccine absorbed in an aerosolized spore rabbit challenge model. Clin Vaccine Immunol. 2015;22(4):430–439. doi: 10.1128/CVI.00690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan A.C., Mifsud E.J., Zeng W., Edenborough K., McVernon J., Brown L.E., et al. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012;9(9):2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 126.
- 127.Derrick S.C., Kolibab K., Yang A., Morris S.L. Intranasal administration of Mycobacterium bovis BCG induces superior protection against aerosol infection with Mycobacterium tuberculosis in mice. Clin Vaccine Immunol. 2014;21(10):1443–1451. doi: 10.1128/CVI.00394-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.NCT02755948/A study of protective immunity against RSV and influenza in experimental human challenge of volunteers.
- 129.NCT04665791/A human controlled infection study with neisseria lactamica in Malian adults.
- 130.NCT01893554/Evaluating the safety and immune response to a single dose of a respiratory syncytial virus (RSV) vaccine in infants and children.
- 131.NCT00369031/Safety study of nasal HIV vaccine adjuvanted with LTK63.
- 132.NCT03023553/The human mucosal immune responses to influenza virus (SLVP026).
- 133.Read R.C., Naylor S.C., Potter C.W., Bond J., Jabbal-Gill I., Fisher A., et al. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23(35):4367–4374. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 134.NCT04034290/A Double-blind randomized placebo-controlled study study of the safety, reactogenicity and immunogenicity of the GamFluVac.
- 135.Durrer P., Glück U., Spyr C., Lang A.B., Zurbriggen R., Herzog C., et al. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine. 2003;21(27–30):4328–4334. doi: 10.1016/s0264-410x(03)00457-2. [DOI] [PubMed] [Google Scholar]
- 136.NCT01385215/strategies for enhancing mucosal immunity to influenza vaccine.
- 137.Glück U., Gebbers J.O., Glück R. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J Virol. 1999;73(9):7780–7786. doi: 10.1128/JVI.73.9.7780-7786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.NCT01258062/Study to assess safety of an inactivated H5N1 influenza vaccine administered in GelVac nasal powder to healthy young adults.
- 139.NCT04036526/A phase 1/2 clinical trial of a GamLPV, a live intranasal bordetella pertussis vaccine.
- 140.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 141.Ghori M.U., Mahdi M.H., Smith A.M., Conway B.R. Nasal drug delivery systems: an overview. Am J Pharmacol Sci. 2015;3:110–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review paper and thus no experimental or clinical data have not been gemerated