Abstract
This survey study assesses the health-related quality of life outcomes in adult patients with cutaneous manifestations of vasculitis.
Cutaneous manifestations of vasculitis can cause itching, pain, and ulceration, but their health-related quality of life (HRQOL) outcomes have not been systematically evaluated. This study assessed the HRQOL in patients with skin vasculitis using validated measures.
Methods
Through use of the Vasculitis Patient-Powered Research Network (VPPRN)1 and incorporation of feedback from patient research partners, an online survey was disseminated to adult patients (aged ≥18 years) with cutaneous manifestations of vasculitis on 4 occasions between January 2020 and August 2021. Primary outcomes were validated measures of skin-related HRQOL (Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]) and general health and well-being (36-Item Short Form Health Survey [SF-36]). Skindex-29 results were categorized using the Prinsen thresholds delineating severity,2 and SF-36 scores of 50 on a scale of 0 to 100 were considered average. This study was approved by the institutional review board of the University of Pennsylvania with a waiver of informed consent because the research presented no more than minimal risk of harm. Reporting follows the AAPOR reporting guideline. Analyses were done from March to August 2022 using Excel software version 365 (Microsoft).
Results
A total of 190 complete responses were received, a completion rate of 15.6% (190 of 1217) among patients in the VPPRN database who reported ever having had skin lesions of vasculitis. Of these, 107 patients had experienced active skin vasculitis within the preceding 4 weeks and were included for analysis. Ninety patients (84.1%) were female, and the mean (SD) age was 50.5 (16.1) years. Self-reported vasculitis disease activity was in remission (8.4% [9 of 107]), mildly active (42.1% [45 of 107]), moderately active (28.9% [31 of 107]), and very active (20.6% [22 of 107]). Most patients (63.6% [68 of 107]) reported having vasculitis for at least 5 years before data collection. Patients with a variety of vasculitides completed the survey (Figure 1).
Figure 1. Skindex-29 Scores With Prinsen Threshold Denoting Severity of Impairmenta.
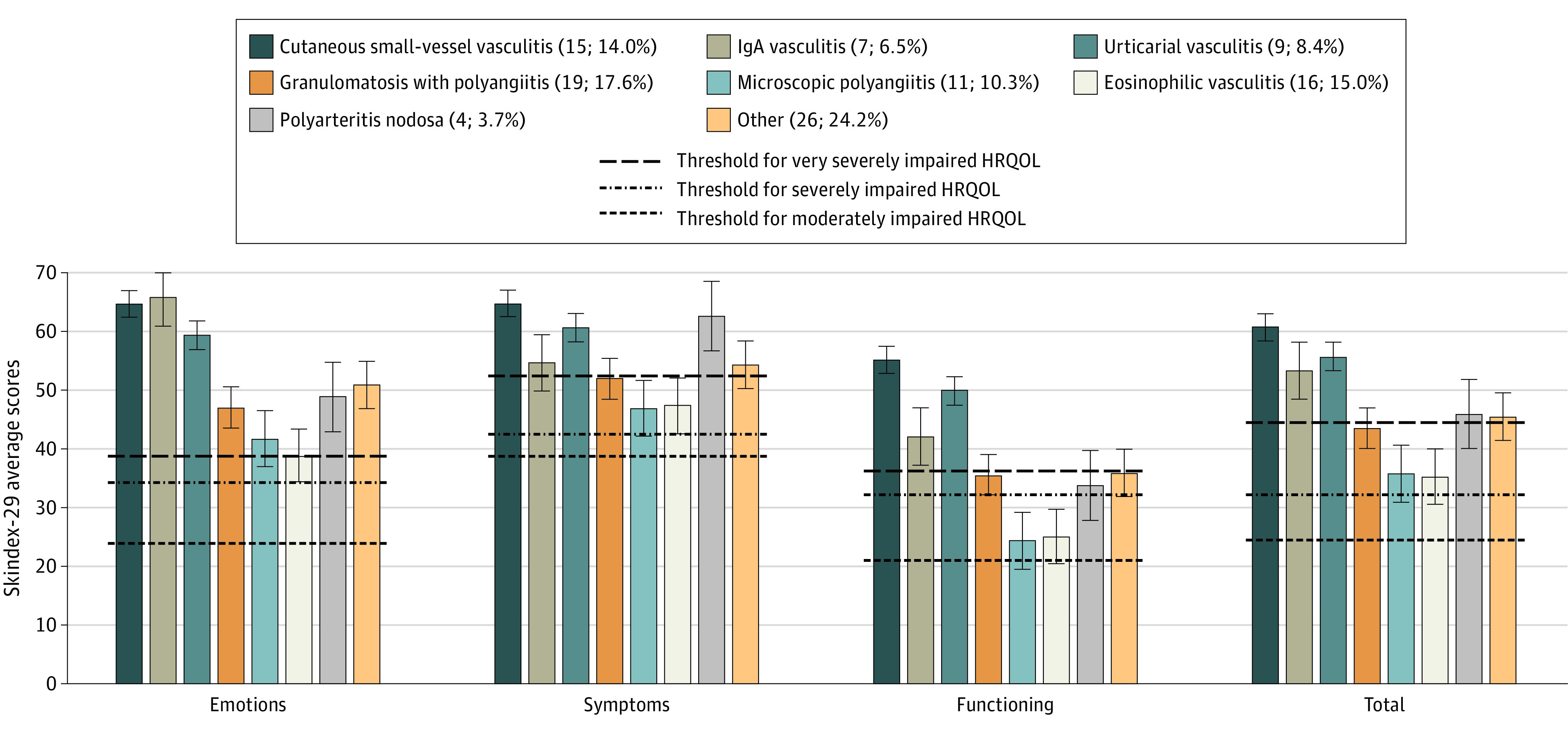
The Prinsen threshold cutoff points for severely impaired health-related quality of life (HRQOL) are ≥39 on emotions, ≥52 on symptoms, ≥37 on functioning, and ≥44 on the overall score.
aHigher scores indicate reduced HRQOL.
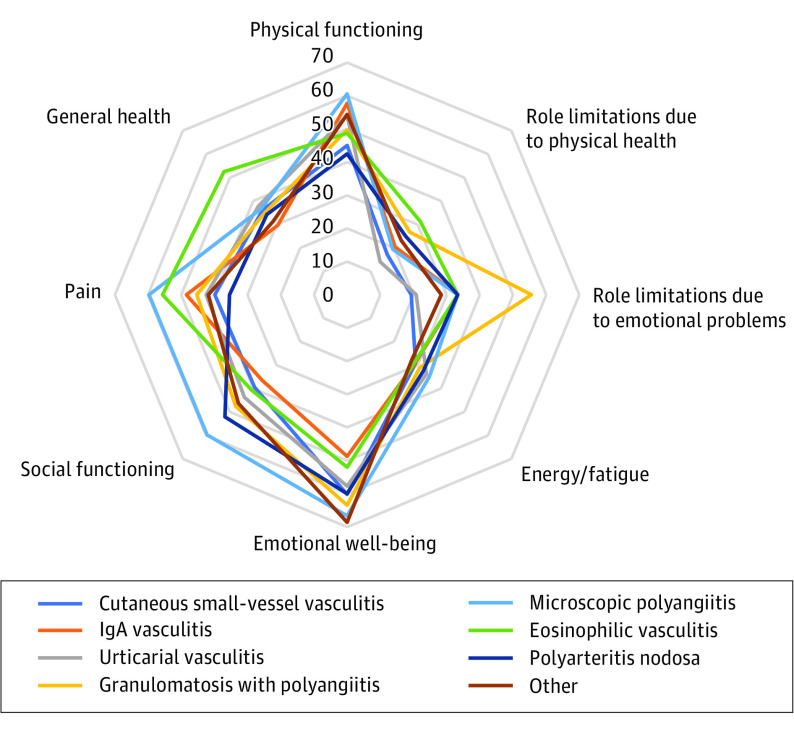
Analyzed using the Prinsen thresholds, most patients were found to have severely or very severely diminished HRQOL in each domain of the Skindex-29: emotions: 77.6% (83 of 107), symptoms: 78.5% (84 of 107), functioning: 60.7% (65 of 107), and total 75.7% (81 of 107) (Figure 1). The HRQOL was below average in 6 of the 8 SF-36 domains (Figure 2), and most patients had summative physical component scores (56% [60 of 107]) and mental component scores (52% [56 of 107]) below 50. Greater disease severity and more recent diagnosis showed a substantial negative association with HRQOL. Vasculitides in which skin disease predominated had an HRQOL outcome as bad as or worse than other types of vasculitis (Figure 1).
Figure 2. 36-Item Short Form Health Survey (SF-36) Domain Scores.
A mean score of 50 has been articulated as a normative value for all scales; lower scores indicate reduced health-related quality of life.
Discussion
Patients with cutaneous manifestations of vasculitis had markedly diminished HRQOL across multiple domains, suggesting that cutaneous vasculitis is an important factor in patient well-being, symptoms, and self-perception of health.
The HRQOL outcomes of skin vasculitis as measured by the Skindex, a skin-specific outcome measure, were substantially worse than as measured by the SF-36, a generic HRQOL instrument, particularly in the domains of emotions and emotional well-being. This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not.3
Potential limitations of this study include generalizability of VPPRN survey responses to the broader population of patients with vasculitis. The waxing and waning nature of vasculitis resulted in exclusion of survey recipients who previously had active skin vasculitis but not at the time of the survey, resulting in decreased response rate. Male patients and some types of vasculitis were underrepresented, reducing the ability to perform statistical comparisons. Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL. In addition, no disease-specific patient-reported measure exists for cutaneous vasculitis, so the instruments used in this study may not fully capture relevant disease burden. Development of a patient-reported outcome measure specific to cutaneous vasculitis may further improve understanding of disease from the patient perspective and provide valuable information for evaluating therapeutic interventions. Future work will assess the responsiveness of HRQOL measures to disease treatment and control.4
Data Sharing Statement
References
- 1.Young K, Kaminstein D, Olivos A, et al. ; Vasculitis Patient-Powered Research Network . Patient involvement in medical research: what patients and physicians learn from each other. Orphanet J Rare Dis. 2019;14(1):21. doi: 10.1186/s13023-018-0969-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinsen CA, Lindeboom R, de Korte J. Interpretation of Skindex-29 scores: cutoffs for mild, moderate, and severe impairment of health-related quality of life. J Invest Dermatol. 2011;131(9):1945-1947. doi: 10.1038/jid.2011.138 [DOI] [PubMed] [Google Scholar]
- 3.Robson JC, Tomasson G, Milman N, et al. OMERACT Endorsement of Patient-reported Outcome Instruments in Antineutrophil Cytoplasmic Antibody-associated Vasculitis. J Rheumatol. 2017;44(10):1529-1535. doi: 10.3899/jrheum.161139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micheletti RG, Pagnoux C, Tamura RN, et al. ; Vasculitis Clinical Research Consortium . Protocol for a randomized multicenter study for isolated skin vasculitis (ARAMIS) comparing the efficacy of three drugs: azathioprine, colchicine, and dapsone. Trials. 2020;21(1):362. doi: 10.1186/s13063-020-04285-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement