Abstract
BACKGROUND:
Daily application of mechanical insufflation-exsufflation (MI-E) is used increasingly in patients with neuromuscular diseases (NMDs) to prevent pulmonary congestion and thereby respiratory tract infections, although its beneficial effect remains uncertain. We, therefore, conducted a systematic review, registered in PROSPERO (CRD42020158278), to compile available evidence for daily MI-E use in subjects with NMDs and stable respiratory condition.
METHODS:
We performed a systematic comprehensive search of MEDLINE, Embase, CINAHL, and Web of Science up to December 23, 2021. We excluded articles studying the effect of MI-E in case of acute respiratory failure or infections and studies comparing different MI-E devices and settings. Studied outcomes were prevalence and severity of respiratory infections, lung function, respiratory characteristics, and patient satisfaction. We performed a meta-analysis using DerSimonian-Laird random effects model and assessed methodological quality by using the Alberta Heritage Foundation for Medical Research tool.
RESULTS:
A total of 3,374 records were screened, of which 25 were included, studying 608 subjects. One randomized controlled trial (RCT) found a trend toward reduced duration of respiratory infections compared to air stacking (AS) that was not statistically significant. Long-term effects on pulmonary function tests (PFT) results were reported in one RCT and one retrospective study, with mixed results regarding vital capacity. Most studies compared PFT results before and immediately after MI-E use. Meta-analysis showed an overall beneficial effect of MI-E on cough peak flow (CPF) compared to unassisted CPF (mean difference 91.6 L/min [95% CI 28.3–155.0], P < .001). Subject satisfaction was high, though possibly influenced by major bias.
CONCLUSIONS:
There is limited evidence available to support beneficial effects of daily use of MI-E in clinically stable subjects with NMDs, with the possible exception of increased CPF immediately after MI-E application. Lack of longitudinal studies preclude conclusions regarding long-term effects. The very limited data comparing MI-E to AS preclude comparisons.
Keywords: neuromuscular diseases, airway clearance, medical devices, mechanical insufflation-exsufflation, home care, cough, adult, pediatrics
Introduction
The primary cause of morbidity and mortality in patients with neuromuscular diseases (NMDs) is respiratory failure due to progressive respiratory muscle weakness. 1 Respiratory muscle weakness causes insufficient cough, thereby increasing the risk of recurrent respiratory tract infections (RTIs), resulting in hospital admissions and further lung function decline. 1-4
To prevent pulmonary congestion, several consensus statements of respiratory care for children and adults with NMD recommend initiation of airway clearance techniques when cough is weak, that is, when cough peak flow (CPF) is < 270 L/min. 5 - 7 Airway clearance employs expiratory support (manually assisted cough) or inspiratory support (air stacking [AS] or glossopharyngeal breathing) or both (mechanical insufflation-exsufflation [MI-E]). MI-E uses positive pressure to promote maximal lung inflation followed by an abrupt switch to negative pressure to the upper airway. The rapid change from positive to negative pressure attempts to simulate the flow changes that occur during a cough, thereby assisting sputum clearance. 8 MI-E does not require active cooperation and can, therefore, also be performed in patient groups that are more difficult to instruct, in particular young children or patients with intellectual impairment; however, it is also more expensive. 2 , 7 , 9
Although expert opinion has facilitated the introduction of MI-E in individual patients or for specific indications, 2 , 7 , 10 , 11 reimbursement of MI-E may be complicated by the perceived scarcity of evidence for its efficacy. For this reason, we conducted a systematic literature review using a comprehensive search strategy to document evidence for regular, daily MI-E use in subjects with NMDs with stable respiratory status (ie, absence of RTIs). We were aware that studies on the most important outcome, that is, prevalence and severity of RTIs, were limited. For this reason, the studied outcome was the overall efficacy, including prevalence and severity of RTIs, pulmonary function tests (PFT) results, respiratory characteristics, and patient comfort and satisfaction.
Methods
For this systematic literature review, we followed the PRISMA guidelines. 12 The protocol was registered on PROSPERO (ID: CRD42020158278).
Search Strategy
We performed a systematic comprehensive electronic search of MEDLINE, Embase, CINAHL, and Web of Science from inception to December 23, 2021, using a detailed search. We used the following search items: “(mechanical insufflation exsufflation) OR (mechanical insufflation-exsufflation) OR (mechanical in-exsufflation) OR (mechanical in-exsufflator) OR (mechanical cough assistance) OR (cough assist) OR (cough assist therapy).” We purposely did not include outcome or NMD in the search, as this narrowed the search with risk of missing studies on this topic. No filters were applied to the search. We conducted handsearching of the reference lists of included articles.
Inclusion and Exclusion Criteria
We used the following criteria for inclusion: original research, English-language studies with data pertaining to clinically stable subjects (children or adults) with documented NMD. Clinically stable was defined as the absence of an RTI or acute respiratory failure at study enrollment. We excluded studies that used artificial lung or animal models, compared different MI-E devices or settings without an unassisted or other airway clearance comparator, or examined the effect of MI-E in acute respiratory failure or infection. We also excluded conference abstracts, reviews, editorials, letters, case reports, duplicate reports, and studies of which we could not access full text (even after contacting the authors).
Selection of Studies and Data Extraction
Two authors (EV and RW) independently screened titles and abstracts of all studies identified by the literature search. Studies for which at least one reviewer concluded that it possibly met the inclusion criteria were selected for full-text screening. Finally, all references of included research were checked for missing studies. Next, both authors independently extracted data from included studies to a standard form. Discrepancies in data interpretation were discussed until consensus. If necessary, we asked a third assessor (LVO) to resolve the discrepancy. We extracted the following data from each study for final analysis: study design, study objectives, years of study conduct, setting, subjects’ age, underlying NMD, MI-E settings, and outcome.
Assessment of Quality
We assessed methodological quality of each study by using the tools developed by the Alberta Heritage Foundation for Medical Research: standard quality assessment criteria for evaluating primary research papers from a variety of fields (https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e. Accessed April 26, 2022). For the quantitative studies, 14 items were scored depending on the degree to which the specific criteria were met (yes = 2, partial = 1, no = 0). Items not applicable to a particular study design were excluded from the calculation of the summary score. For the qualitative studies, 10 items were scored 0–2 points. A summary score was calculated for each paper by summing the total score obtained across relevant items and dividing by the total possible score.
Data Analysis
In case of multiple studies using the same comparison and outcome parameters, we performed a meta-analysis using DerSimonian-Laird random effects model to obtain overall pooled effect with 95% CIs. The mean (SD) in individual studies was estimated from those that were reported as median and interquartile range (IQR) by using the method described by Wan et al. 13 Because in amyotrophic lateral sclerosis (ALS) upper-airway closure may be present in the absence of or before the onset of bulbar symptoms, 14 , 15 we performed a subgroup analysis on studies without subjects with ALS. Heterogeneity of pooled data were assessed by using I2 statistic. The I2 statistic describes the percentage of total variation across studies due to true heterogeneity rather than chance. All analyses were performed using OpenMeta[Analyst] (http://www.cebm.brown.edu/openmeta). Data are not publicly available but available upon reasonable request.
Results
Study Selection
Totally, titles and abstracts of 3,374 records were screened (Fig. 1). After title and abstract screening, 50 articles were considered for full-text analysis. Twenty-five were excluded because they did not meet inclusion criteria. The remaining 25 studies were included in our review. 8,14 - 37
Fig. 1.
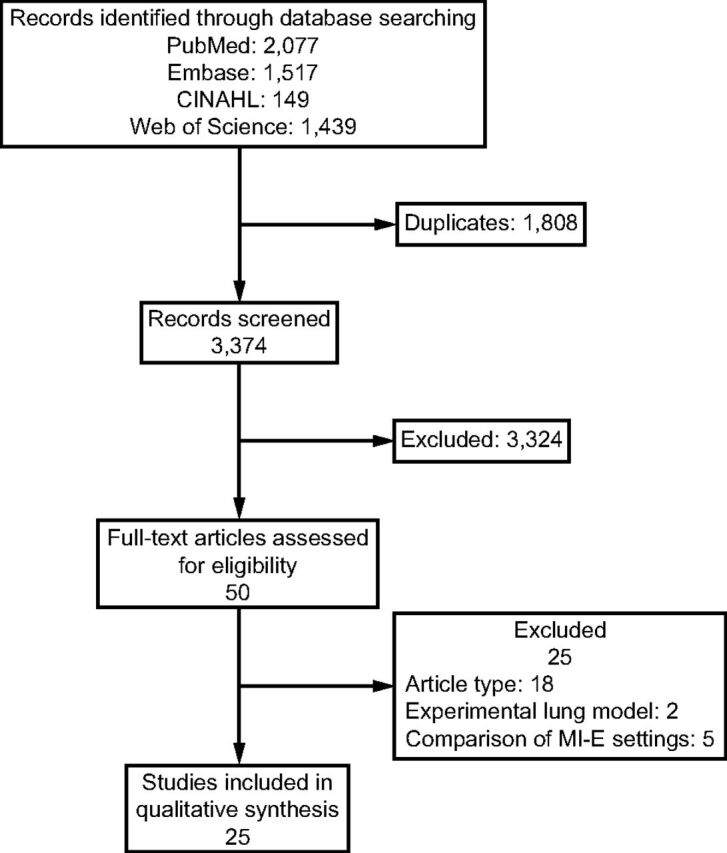
Flow chart. MI-E = mechanical insufflation-exsufflation.
Description of Included Studies
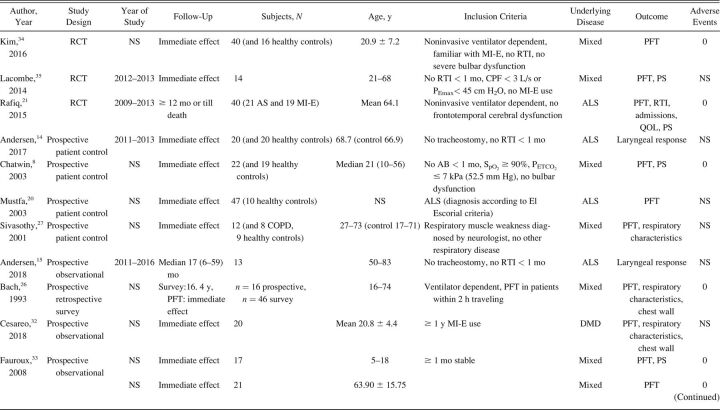
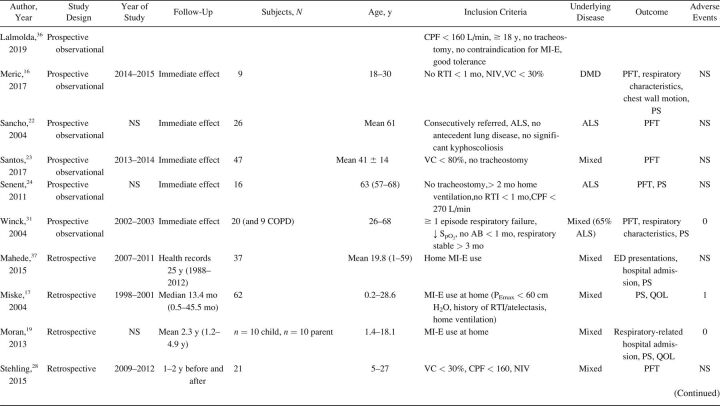
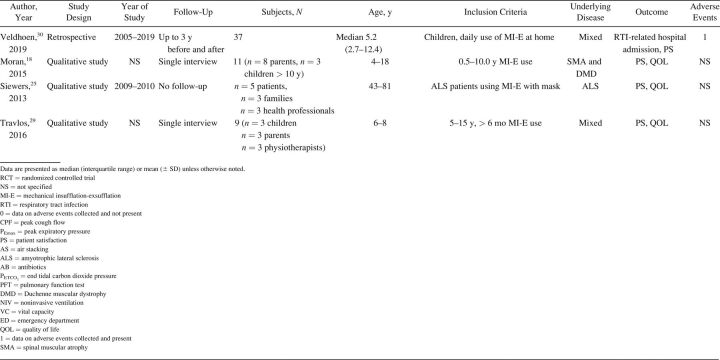
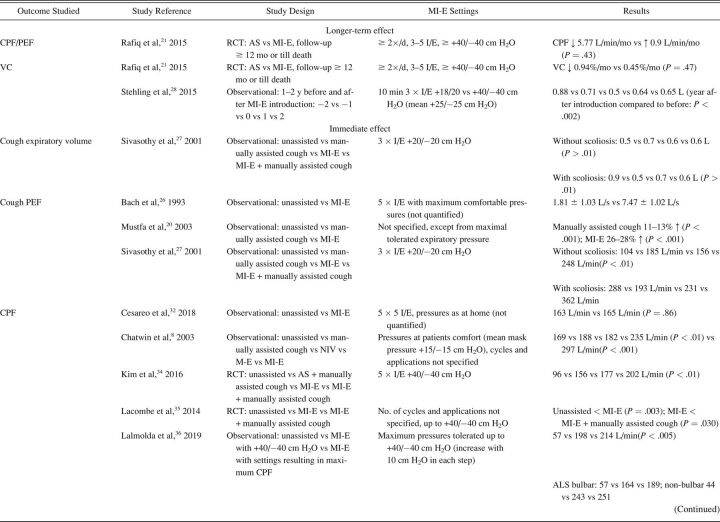
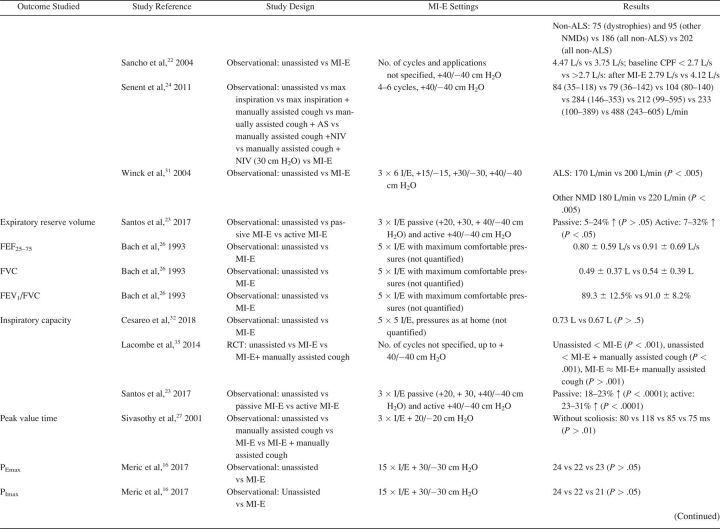
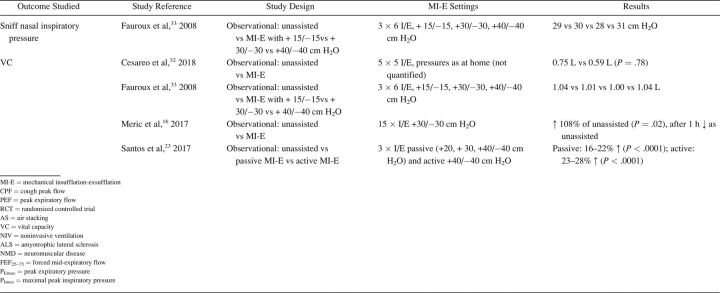
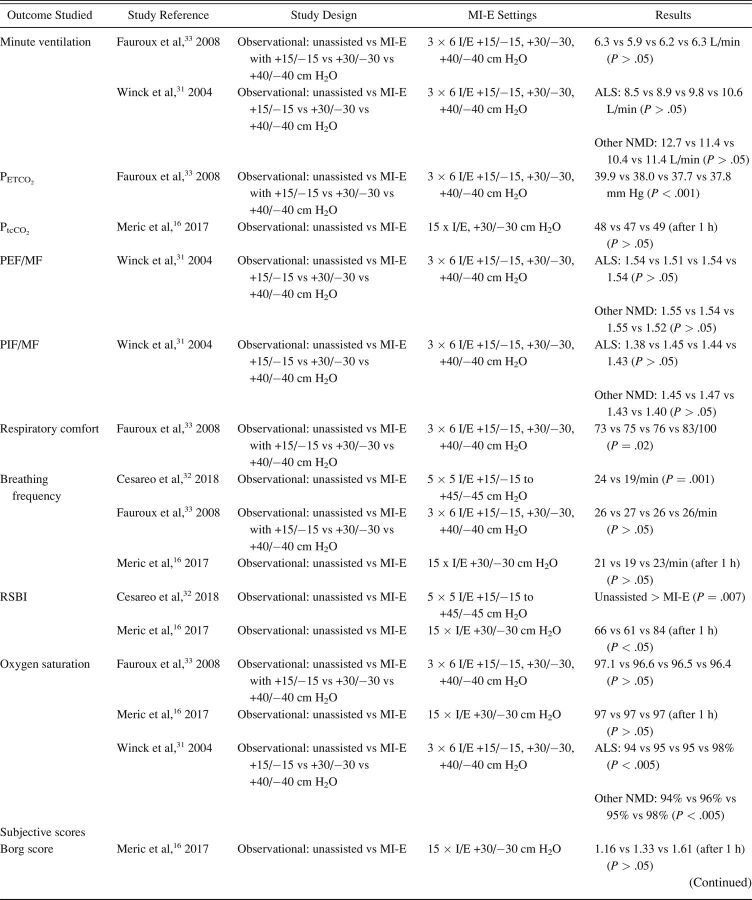
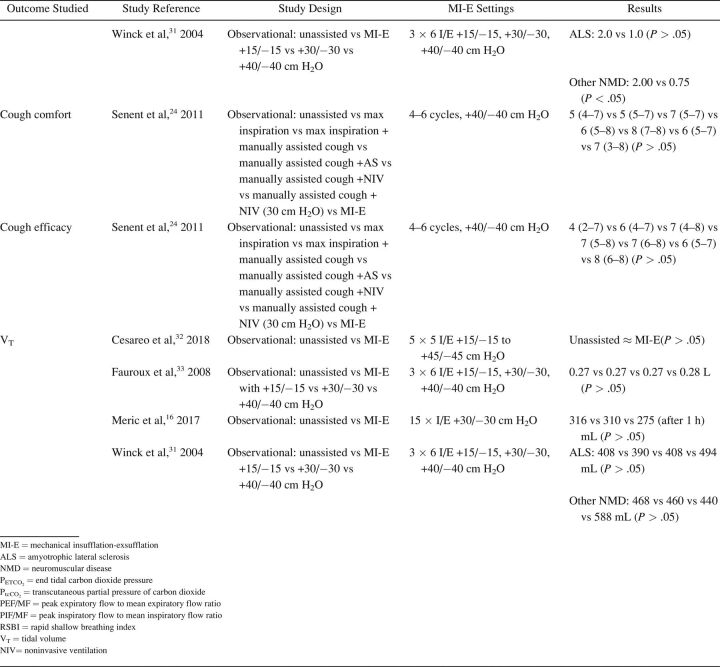
The characteristics of included studies are summarized in Table 1. Most studies were single-center cohort studies. A total of 608 subjects were studied, with a sample size range from 5–62 participants. Inclusion and exclusion criteria and study population varied significantly between studies. Fifteen studies included subjects with different NMD diagnoses. 17,20,24,27-32,33 - 37 Ten studies included a more homogeneous group of diagnoses, such as ALS (no. = 7), 14 , 15 , 20-22 , 24 , 25 Duchenne muscular dystrophy (no. = 2), 16 , 32 and both Duchenne muscular dystrophy and spinal muscular atrophy (no. = 1). 18 Study outcomes included respiratory-related events such as RTIs or hospital admissions (Table 2), PFT results (Table 3), respiratory characteristics (Table 4), laryngeal response (Supplementary Table 1, see related supplementary materials at http://www.rcjournal.com), and quality of life (Supplementary Table 2, see related supplementary materials at http://www.rcjournal.com).
Table 1.
Study Characteristics
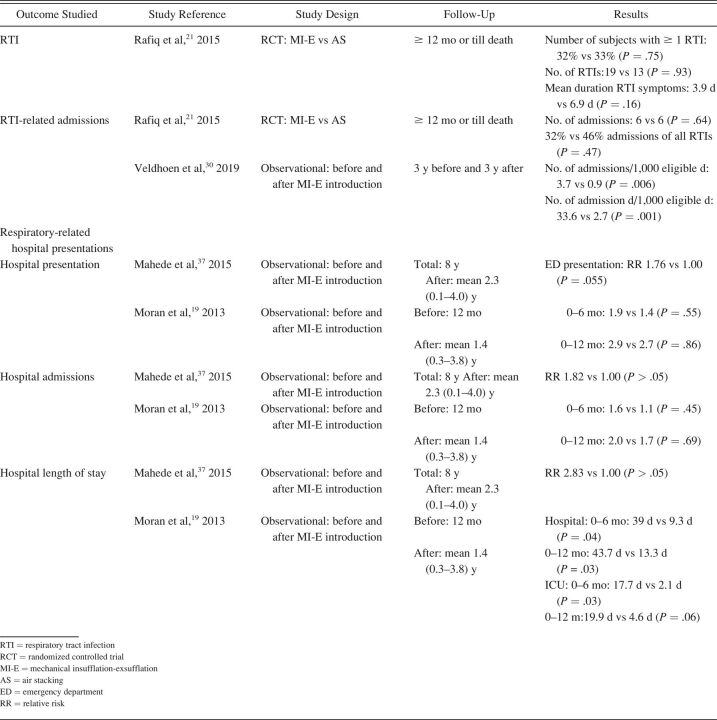
Table 2.
Outcome: Respiratory Tract Infections and Hospital Admissions
Table 3.
Outcome: Pulmonary Function Test Results
Table 4.
Outcome: Respiratory Characteristics
Methodological Quality and Risk of Bias
Summary of quality assessment of the included studies is provided in the Supplementary Table 3 (see related supplementary materials at http://www.rcjournal.com). Median summary score was 0.85 (IQR 0.79–0.87). Blinding of investigator was only done in one study. 24 Control of confounding was limited in most studies. Sample size was limited in many studies and method of subject selection not always clearly described. 19 , 20 , 26 , 27 Supplementary Table 4 (See related supplementary materials at http://www.rcjournal.com) shows PRISMA 2020 checklists of this systematic review.
Respiratory-Related Outcomes
Respiratory-related outcomes were studied in 4 studies. 19 , 21 , 30 , 37 One randomized controlled trial (RCT) studied number and duration of RTIs and related hospital admissions because of RTIs in subjects with ALS, comparing AS and MI-E. This study reported a trend toward reduced duration of RTIs in the MI-E group but this was not statristically significant. 21 The other 3 were observational, pediatric studies comparing number of RTIs and respiratory-related hospital presentations years before and after introduction of MI-E 19 , 30 , 37 (Table 2). Veldhoen et al 30 (n = 37) showed a significant effect on admissions because of RTIs, with MI-E use. Moran et al 19 and Veldhoen et al 30 (n = 47) showed reduced hospital stay. However, meta-analysis was not possible due to the limited number of studies and different outcome measures.
Pulmonary Function Test Results
PFT results were reported in 16 studies (Table 3), with 2 investigating long-term effects. 21 , 28 Rafiq et al 21 conducted an RCT comparing AS and MI-E in subjects with ALS. The researchers found no between-group differences in vital capacity decline per month (P = .47) or CPF (P = .43). 21 In a retrospective observational study, Stehling et al 28 showed a significant beneficial effect of MI-E comparing vital capacity one year before and one year after MI-E introduction in a group with mixed NMDs.
Fourteen studies examined the effect of MI-E on PFT results before and immediately after application of MI-E. 8,17,21,23-25,27,28,33 - 37 Two RCTs compared MI-E to unassisted maneuvers, including MI-E with and without the addition of manually assisted cough. 34 , 35 Both studies showed increased CPF after MI-E application. 34 , 35 Kim et al 34 also compared MI-E with and without manually assisted cough to AS with manually assisted cough (n = 40) and found that MI-E alone improved CPF significantly more than AS with manually assisted cough. MI-E used in conjunction with manually assisted cough improved CPF even further. 34
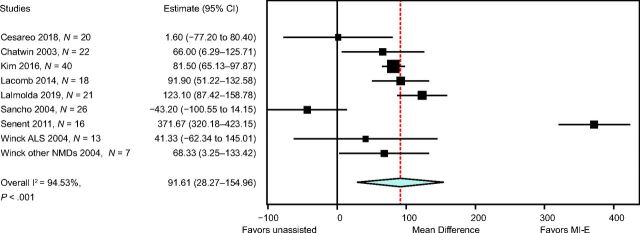
Lung function outcomes varied in 12 observational studies. 8,17,21,23-25,27,28,32-34 Five studies were unique in studying a specific outcome. 16 , 23 , 26 , 27 , 33 Three studies explored inspiratory capacity comparing unassisted maneuvers to MI-E with and without manually assisted cough. 23 , 32 , 35 The immediate effect on CPF was studied in 8 studies: CPF after MI-E was compared to unassisted CPF, 8,22 , 24 , 31,34 - 36 CPF after manually assisted cough, 8,24 CPF after AS with manually assisted cough, 24,34 and CPF after MI-E with manually assisted cough. 34 , 35 We contacted the corresponding author of the study by Lacombe et al 35 to obtain the exact values of means and range of CPF, allowing us to include this study in the meta-analysis. Meta-analysis showed an overall beneficial effect of MI-E on CPF compared to unassisted CPF (mean difference 91.61 [95% CI 28.3–155.0] L/min, P < .001) (Fig. 2). There was considerable heterogeneity regarding the effects on CPF across 8 studies (I2 = 0 95%, P < .001). Subgroup analysis on 6 studies after the exclusion of studies on ALS showed similar effects on CPF (mean difference 83.1 [95% CI 59.4–106.7] L/min, P < .001) (Supplementary Fig. 1, see related supplementary materials at http://www.rcjournal.com). Moderate heterogeneity was observed across these 6 studies (I2 = 48%, P = .09).
Fig. 2.
Meta-analysis. Cough peak flow (CPF) after mechanical insufflation-exsufflation (MI-E) versus unassisted CPF. ALS = amyotrophic lateral sclerosis, NMD = neuromuscular disease.
Respiratory Parameters
There were mixed results for respiratory parameter outcomes between studies (Table 4). There was no significant effect on oxygen saturation in all 16 , 33 but one study. 31 Meric et al 16 found no changes in transcutaneous CO2 levels, whereas Fauroux et al 33 found improved end-tidal CO2. Data on breathing frequency after MI-E application were also conflicting. 16 , 32 , 33 Only one study observed a statistically significant reduction in breathing frequency. 32 None of the studies showed a significant effect on tidal volume comparing unassisted maneuvers to MI-E. 16 , 31 , 32 , 33 The rapid shallow breathing index, which is the ratio of breathing frequency and tidal volume, increased significantly immediately after MI-E application in one study 32 and 1 h after MI-E use in another study. 33
Laryngeal Response
Andersen et al 14,15 found upper-airway closure from a variety of mechanisms during MI-E treatment in subjects with ALS and recommended customized MI-E settings to prevent this problem. (Supplementary Table 1).
Patient Satisfaction
Satisfaction of subjects and caregivers was reported in 7 studies 17-19 , 21 , 25 , 29 , 30 (Supplementary Table 2). Subject satisfaction was generally high. In nearly all cases, treatment with MI-E was perceived as a valuable improvement of subjects’ health by managing the disease at home, preventing hospital admissions, and maintaining social participation.
Discussion
This systematic review shows that there is limited evidence for efficacy of daily use of MI-E in clinically stable subjects with NMDs, with the possible exception of increased CPF immediately after MI-E application. Little research has shown the superiority of MI-E compared to other airway clearance therapy. Only one RCT showed a superior effect of MI-E on CPF compared to AS and manually assisted cough. Unfortunately, there is no evidence on long-term efficacy, implying that additional studies are needed.
Since RTIs are the primary cause of acute respiratory deterioration and hospital admission in patients with NMDs, the most clinically relevant, long-term outcome of MI-E is a reduction in the number, duration, and severity of these infections. 4 Reducing respiratory-related infections would most likely have an important impact on patient/caregiver quality of life and may reduce health care costs. 38 - 40 However, there is only one RCT that compared AS and MI-E using the number of RTIs as an outcome measure. This study included subjects with ALS and reported a possible trend toward a reduction of these events but this was not statistically significant. 21 However, the study had a small sample size and may not be generalizable to other NMDs. The reported effect may have been more pronounced if this RCT included subjects with other NMDs. Previous studies cautioned about possible counterproductive effects of MI-E in subject with ALS if high pressures and no individualized settings were used. 14 , 15 The difference in effect between study populations with and without inclusion of subjects with ALS was not supported by the forest plots in our results (Fig. 2 and Supplementary Fig. 1). This may be explained by the sample size, which was 43% more if all studies were included in the meta-analysis (n = 183) compared to study analyses without subjects with ALS (n = 128). Observational studies were of limited quality, and confounding factors preclude a conclusion regarding the effect of MI-E on RTIs.
PFT results are an important surrogate outcome measure, as studies in subjects with Duchenne muscular dystrophy suggest that vital capacity is a valuable predictor of susceptibility to RTIs, need for respiratory support, and survival. 7 Long-term effects on lung-function were reported in 2 studies. An RCT 21 found no significant improvement in vital capacity after MI-E introduction, whereas a retrospective observational study 28 showed significant vital capacity improvement. The studies that reported PFT results immediately after application of MI-E suggest direct improvement of lung volumes 17 , 24 , 27 , 36 but no change in respiratory muscle strength. 16 , 33 It is unclear how long these immediate, mainly beneficial, effects of MI-E application last.
Most studies showed no significant immediate effect on respiratory parameters.
Although, probably at least partly influenced by selection and study bias, the qualitative studies reported high subject satisfaction of MI-E. This is important, especially when evidence for beneficial effect is limited. Qualitative research involving studies with NMDs is very important, including the subject’s choice of technique and health-related quality of life. 10
The included studies had clear limitations. We identified and included only 3 RCTs in our analysis. 22 , 35 , 36 Results from other included studies should be interpreted with caution due to the retrospective nature of these studies on respiratory-related hospital admissions and RTIs, which render them susceptible to potential flaws and bias. Most prospective observational studies only described the immediate effect on PFT results but did not assess longer-term outcome. Forced maneuvers during lung function testing prior to MI-E application may have resulted in lung volume recruitment, thereby underestimating the effect of MI-E. 41 On the other hand, respiratory muscle fatigue may underestimate the effect of MI-E. 42 In our meta-analysis on the effect of MI-E on CPF, we combined absolute CPF measurements obtained with different devices. Different measurement devices perform variably, leading to potentially substantial inaccuracy in CPF measurements. 43
NMDs are a large and heterogeneous group of diseases. This heterogeneity, the variation in clinical characteristics (type and severity of disease, affected respiratory muscle groups, age, scoliosis deformity), the small sample sizes, and varying MI-E settings preclude definite conclusions. Inclusion of predominantly subjects with ALS limits generalizability and may even have resulted in underestimation of beneficial effects. In addition to the 2 studies on laryngeal response, 14 , 15 5 other studies exclusively included subjects with ALS, 20-22 , 24 , 25 and in 3 studies the majority of included subjects with NMDs consisted of those with ALS. 27 , 31 , 36 The RCT by Kim et al 21 that did not show a statistically significant effect on the number and duration of RTIs only included subjects with ALS.
Rarity of NMDs complicates the inclusion of larger numbers of subjects, particularly single-center studies. Future research on MI-E should ensure increased statistical power. Due to the heterogeneity of subjects, interventions, and outcome measures, we could only perform a meta-analysis on effect on CPF.
Adherence to treatment was not described in any study, whereas it is possible to check MI-E use for the preceding months in most, if not all, MI-E devices. Blinding of the researcher, although possible, was very uncommon. Blinding of the subjects was not possible but may have caused a placebo effect in some studies. Subjects naïve to MI-E treatment may have a different response to treatment than subjects who regularly use MI-E. Being an experienced or naïve user of MI-E was not specified in many studies. In addition, the technical and methodological information was often very limited. In studies comparing different airway clearance techniques or different settings of MI-E, the order of treatments was not always specified nor the use or length of pauses between treatments. Some qualitative studies did not describe the selection process of subjects for inclusion, which complicates the interpretation of subject satisfaction and the possibility of bias. On the other hand, patient-reported outcome measures are important. The reported subject satisfaction because of prevention of hospital admissions was confirmed by observational studies. 19 , 30 , 37 Only a limited number of studies reported adverse events (Table 1). Evaluation of the benefits alone, without evaluation of harm, is likely to bias conclusions about the net efficacy or effectiveness of the intervention. 44 The current review was restricted to English-language articles. Publication bias cannot be excluded as beneficial effects of MI-E are more likely to be published. No studies looked at the total costs of different airway clearance therapies, including the purchase and maintenance of the device, hospital visits, and admissions.
Reproducibility and transparency of reported results are ensured by our methodology. The broad search strategy has reduced the chance of incomplete overview of studies. We included 25 studies, considerably more than recent reviews. A Cochrane review on cough-augmenting therapy included 11 trials, with minority including data on MI-E. 10 A systematic review on MI-E use in subjects with NMDs published a few years ago included 12 studies published before 2015. 45 We cannot draw conclusions on the longer-term effects of MI-E. The results of this systematic review help to identify knowledge gaps regarding the use of MI-E. High-quality controlled studies, preferably RCTs, are required not only to study the longer-term effects of daily MI-E use on the most clinically relevant outcomes measures including RTIs or hospital admissions but also to compare different airway clearance techniques. Long-term RCTs provide the best evidence, as observational studies are prone to potential confounders, such as concomitant use of ventilatory support, disease progression, and introduction of (gene modifying) treatments. We would advise a follow-up of at least 2 years to reduce seasonal influence on results and to be able to study the longer-term effects on lung function. RTIs are difficult to define, and the decision to start antibiotic treatment and to admit a patient are prone to subjectivity. Also, the decision and ability to admit a patient are highly variable between different countries and health systems. PFT results may be an alternative outcome because it may predict susceptibility to RTIs, need for respiratory support, and survival. Because some countries do not reimburse MI-E, we also suggest studying total cost of care as an outcome measure. Future studies should focus on NMDs such as Duchenne muscular dystrophy, other muscular dystrophies, congenital myopathies, and spinal muscular atrophy. These studies should be performed separately from studies in subjects with ALS given the fact that in this disease upper-airway closure may be present in the absence of (or before the onset of) bulbar symptoms. 14 , 15 Also, ALS is a more rapidly progressive condition in most patients, making long-term trials more difficult. Additional studies or subgroup analyses are needed to identify patient subgroups in whom MI-E has superior effect compared to other airway clearance therapy, allowing future patient selection for MI-E treatment. Optimal MI-E settings need to be investigated and most likely should be individualized to obtain maximal beneficial effects and avoid adverse effects.
Conclusions
At this moment, there are very limited data available to analyze the effect of MI-E on RTIs or respiratory-related admissions. Although MI-E has an immediate beneficial effect on CPF, evidence on longer-term lung function improvement is lacking. In subjects with ALS, upper-airway closure is described after MI-E application, even prior to the onset of bulbar symptoms, necessitating individualized approach to therapy. Qualitative studies describe high subject satisfaction.
Supplementary Material
Footnotes
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1. Boitano LJ. Management of airway clearance in neuromuscular disease. Respir Care 2006;51(8):913-922. [PubMed] [Google Scholar]
- 2. Chatwin M, Toussaint M, Gonçalves MR, Sheers N, Mellies U, Gonzales-Bermejo J, et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med 2018;136:98-110. [DOI] [PubMed] [Google Scholar]
- 3. Andrews JG, Soim A, Pandya S, Westfield CP, Ciafaloni E, Fox DJ, et al. ; on behalf of Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet). Respiratory care received by individuals with Duchenne muscular dystrophy from 2000 to 2011. Respir Care 2016;61(10):1349-1359. [DOI] [PubMed] [Google Scholar]
- 4. Tzeng AC, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest 2000;118(5):1390-1396. [DOI] [PubMed] [Google Scholar]
- 5. Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, et al. ; on behalf of DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018;17(4):347-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al. ; on behalf of SMA Care group. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements, and immunizations; other organ systems; and ethics. Neuromuscul Disord 2018;28(3):197-207. [DOI] [PubMed] [Google Scholar]
- 7. Hull J, Aniapravan R, Chan E, Chatwin M, Forton J, Gallagher J, et al. British Thoracic Society guideline for respiratory management of children with neuromuscular disease. Thorax 2012;67(Suppl 1):i1-40. [DOI] [PubMed] [Google Scholar]
- 8. Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation-exsufflation in patients with neuromuscular weakness. Eur Respir J 2003;21(3):502-508. [DOI] [PubMed] [Google Scholar]
- 9. Sato T, Murakami T, Ishiguro K, Shichiji M, Saito K, Osawa M, et al. Respiratory management of patients with Fukuyama congenital muscular dystrophy. Brain Dev 2016;38(3):324-330. [DOI] [PubMed] [Google Scholar]
- 10. Morrow B, Argent A, Zampoli M, Human A, Corten L, Toussaint M. Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database Syst Rev 2021;4(4):CD013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toussaint M, Chatwin M, Gonzales J, Berlowitz DJ, Toussaint M, Chatwin M, et al. ; ENMC Respiratory Therapy Consortium. 228th ENMC International Workshop: airway clearance techniques in neuromuscular disorders Naarden, the Netherlands, 3–5 Mar, 2017. Neuromuscul Disord 2018;28(3):289-298. [DOI] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range, and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersen T, Sandnes A, Brekka AK, Hilland M, Clemm H, Fondenes O, et al. Laryngeal response patterns influence the efficacy of mechanical assisted cough in amyotrophic lateral sclerosis. Thorax 2017;72(3):221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen TM, Sandnes A, Fondenes O, Nilsen RM, Tysnes O-B, Heimdal J-H, et al. Laryngeal responses to mechanically assisted cough in progressing amyotrophic lateral sclerosis. Respir Care 2018;63(5):538-549. [DOI] [PubMed] [Google Scholar]
- 16. Meric H, Falaize L, Pradon D, Lacombe M, Petitjean M, Orlikowski D, et al. Short-term effect of volume recruitment-derecruitment maneuvers on chest-wall motion in Duchenne muscular dystrophy. Chron Respir Dis 2017;14(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miske LJ, Hickey EM, Kolb SM, Weiner DJ, Panitch HB. Use of the mechanical in-exsufflator in pediatric patients with neuromuscular disease and impaired cough. Chest 2004;125(4):1406-1412. [DOI] [PubMed] [Google Scholar]
- 18. Moran FC, Spittle AJ, Delany C. Lifestyle implications of home mechanical insufflation-exsufflation for children with neuromuscular disease and their families. Respir Care 2015;60(7):967-974. [DOI] [PubMed] [Google Scholar]
- 19. Moran FCE, Spittle A, Delany C, Robertson CF, Massie J. Effect of home mechanical in-exsufflation on hospitalization and lifestyle in neuromuscular disease: a pilot study. J Paediatr Child Health 2013;49(3):233-237. [DOI] [PubMed] [Google Scholar]
- 20. Mustfa N, Aiello M, Lyall RA, Nikoletou D, Olivieri D, Leigh PN, et al. Cough augmentation in amyotrophic lateral sclerosis. Neurology 2003;61(9):1285-1287. [DOI] [PubMed] [Google Scholar]
- 21. Rafiq MK, Bradburn M, Proctor AR, Billings CG, Bianchi S, Mcdermott CJ, Shaw PJ. A preliminary randomized trial of the mechanical insufflator-exsufflator versus breath-stacking technique in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2015;16(7-8):448-455. [DOI] [PubMed] [Google Scholar]
- 22. Sancho J, Servera E, Díaz J, Marín J. Efficacy of mechanical insufflation-exsufflation in medically stable patients with amyotrophic lateral sclerosis. Chest 2004;125(4):1400-1405. [DOI] [PubMed] [Google Scholar]
- 23. Santos DB, Boré A, Castrillo LDA, Lacombe M, Falaize L, Orlikowski D, et al. Assisted vital capacity to assess recruitment level in neuromuscular diseases. Respir Physiol Neurobiol 2017;243:32-38. [DOI] [PubMed] [Google Scholar]
- 24. Senent C, Golmard JL, Salachas F, Chiner E, Morelot-Panzini C, Meninger V, et al. A comparison of assisted cough techniques in stable patients with severe respiratory insufficiency due to amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2011;12(1):26-32. [DOI] [PubMed] [Google Scholar]
- 25. Siewers V, Holmoy T, Frich JC. Experiences with using mechanical in-exsufflation in amyotrophic lateral sclerosis. Eur J Physiother 2013;15(4):201-207. [Google Scholar]
- 26. Bach JR. Mechanical insufflation-exsufflation: comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest 1993;104(5):1553-1562. [DOI] [PubMed] [Google Scholar]
- 27. Sivasothy P, Brown L, Smith IE, Shneerson JM. Effect of manually assisted cough and mechanical insufflation on cough flow of normal subjects, patients with chronic obstructive pulmonary disease (COPD), and patients with respiratory muscle weakness. Thorax 2001;56(6):438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stehling F, Bouikidis A, Schara U, Mellies U. Mechanical insufflation-exsufflation improves vital capacity in neuromuscular disorders. Chron Respir Dis 2015;12(1):31-35. [DOI] [PubMed] [Google Scholar]
- 29. Travlos V, Drew K, Patman S. The value of the CoughAssist in the daily lives of children with neuromuscular disorders: experiences of families, children, and physiotherapists. Dev Neurorehabil 2016;19(5):321-326. [DOI] [PubMed] [Google Scholar]
- 30. Veldhoen ES, Verweij-van den Oudenrijn LP, Ros LA, Hulzebos EH, Papazova DA, van der Ent CK, et al. Effect of mechanical insufflation-exsufflation in children with neuromuscular weakness. Pediatr Pulmonol 2020;55(2):510-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winck JC, Gonçalves MR, Lourenço C, Viana P, Almeida J, Bach JR. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest 2004;126(3):774-780. [DOI] [PubMed] [Google Scholar]
- 32. Cesareo A, LoMauro A, Santi M, Biffi E, D’Angelo MG, Aliverti A. Acute effects of mechanical insufflation-exsufflation on the breathing pattern in stable subjects with Duchenne muscular dystrophy. Respir Care 2018;63(8):955-965. [DOI] [PubMed] [Google Scholar]
- 33. Fauroux B, Guillemot N, Aubertin G, Nathan N, Labit A, Clément A, Lofaso F. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest 2008;133(1):161-168. [DOI] [PubMed] [Google Scholar]
- 34. Kim SM, Choi WA, Won YH, Kang SW. A comparison of cough assistance techniques in patients with respiratory muscle weakness. Yonsei Med J 2016;57(6):1488-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lacombe M, Del Amo Castrillo L, Boré A, Chapeau D, Horvat E, Vaugier I, et al. Comparison of three cough-augmentation techniques in neuromuscular patients: mechanical insufflation combined with manually assisted cough, insufflation-exsufflation alone, and insufflation-exsufflation combined with manually assisted cough. Respiration 2014;88(3):215-222. [DOI] [PubMed] [Google Scholar]
- 36. Lalmolda C, Prados H, Mateu G, Noray M, Pomares X, Luján M. Titration of mechanical insufflation-exsufflation optimal pressure combinations in neuromuscular diseases by flow/pressure waveform analysis. Arch Bronconeumol (Engl Ed) 2019;55(5):246-251. [DOI] [PubMed] [Google Scholar]
- 37. Mahede T, Davis G, Rutkay A, Baxendale S, Sun W, Dawkins HJS, et al. Use of mechanical airway clearance devices in the home by people with neuromuscular disorders: effects on health service use and lifestyle benefits. Orphanet J Rare Dis 2015;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dobkin C, Finkelstein A, Kluender R, Notowidigdo MJ. The economic consequences of hospital admissions. Am Econ Rev 2018;102(2):308-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rennick JE, Dougherty G, Chambers C, Stremler R, Childerhose JE, Stack DM, et al. Children’s psychological and behavioral responses following pediatric intensive care unit hospitalization: the caring intensively study. BMC Pediatr 2014;14(276). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rokach A. Psychological, emotional, and physical experiences of hospitalized children. Clin Case Reports Rev 2016;2(4):399-401. [Google Scholar]
- 41. Veldhoen ES, Vercoelen F, Ros L, Verweij-van den Oudenrijn LP, Wösten-van Asperen RM, Hulzebos EHJ, et al. Short-term effect of air stacking and mechanical insufflation-exsufflation on lung function in patients with neuromuscular diseases. Chron Respir Dis 2022;19:14799731221094620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartels B, de Groot JF, Habets LE, Wadman RI, Asselman FL, Nieuwenhuis EES, et al. Correlates of fatigability in patients with spinal muscular atrophy. Neurology 2021;96(6):e845-52-e852. [DOI] [PubMed] [Google Scholar]
- 43. Kulnik ST, Lewko A, Macbean V, Spinou A. Accuracy in the assessment of cough peak flow: good progress for a “work in progress.” Respir Care 2020;65(1):133-134. [DOI] [PubMed] [Google Scholar]
- 44. Viswanathan M, Ansari MT, Berkman NDN, Chang S, Hartling L, McPheeters M, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In: Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 45. Auger C, Hernando V, Galmiche H. Use of mechanical insufflation-exsufflation devices for airway clearance in subjects with neuromuscular disease. Respir Care 2017;62(2):236-245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.