SUMMARY
How glia control axon regeneration remains incompletely understood. Here, we investigate glial regulation of regenerative ability differences of closely related Drosophila larval sensory neuron subtypes. Axotomy elicits Ca2+ signals in ensheathing glia, which activates regenerative neurons through the gliotransmitter adenosine and mounts axon regenerative programs. However, non-regenerative neurons do not respond to glial stimulation or adenosine. Such neuronal subtype-specific responses result from specific expression of adenosine receptors in regenerative neurons. Disrupting gliotransmission impedes axon regeneration of regenerative neurons, and ectopic adenosine receptor expression in non-regenerative neurons suffices to activate regenerative programs and induce axon regeneration. Furthermore, stimulating gliotransmission or activating the mammalian ortholog of Drosophila adenosine receptors in retinal ganglion cells promotes axon regrowth after optic nerve crush in adult mice. Altogether, our findings demonstrate that gliotransmission orchestrates neuronal subtype-specific axon regeneration in Drosophila and suggest targeting gliotransmission or adenosine signaling is a strategy for mammalian central nervous system repair.
Keywords: Neuronal subtype-specific axon regeneration, gliotransmission, purinergic signaling, mammalian CNS repair
eTOC
Sensory neuron subtypes in Drosophila show different axon regenerative abilities. Here, Wang et al. identify a mechanism by which a glia-neuron interaction through gliotransmission could promote axon regrowth after injury in Drosophila and show this may also be true in a mouse model.
Graphical Abstract

INTRODUCTION
Limited axon regeneration is a major hurdle for functional recovery after nervous system injury. Both neuronal intrinsic properties and neuron-glia interactions regulate axon regeneration.1 Available studies indicate that glia could limit axon regeneration by forming glial scar and providing myelin-associated inhibitory factors.2–4 However, a complete picture of glial regulation of axon regeneration has yet to emerge. Glia can rapidly modulate neuronal activity through gliotransmission, a process that is triggered by glial Ca2+ signals and subsequent release of small molecule transmitters known as gliotransmitters, including ATP and its metabolic product adenosine, glutamate, and D-serine.5,6 These gliotransmitters act on their neuronal receptors to regulate synaptic transmission and neuronal excitability.5,7–10 However, whether gliotransmission could play a role in axon regeneration remains unknown.
Activation of cell growth pathways promotes axon regeneration in the mammalian central nervous system (CNS).11–14 However, the effects on axon regeneration differ drastically among neuronal subtypes. For example, in the optic nerve crush model, only the alpha subtype retinal ganglion cells (RGCs) can regenerate their axons after activation of mammalian target of rapamycin (mTOR), whereas other RGC subtypes fail to regenerate.15 Such neuronal subtype-specific axon regeneration appears to be a general phenomenon as it is observed in both the CNS and the peripheral nervous system (PNS) and in other species including Drosophila and C. elegans.1,16–18 However, how closely related neuronal subtypes exhibit distinct regenerative abilities remains poorly understood. Deciphering the underlying mechanisms will likely shed light on a fundamental problem of how neurons respond to injury.
Stimulation of neuronal activity facilitates axon regeneration of mammalian CNS neurons.11,19,20 In these studies, the pattern of neuronal activity was not well controlled, as such, it is unclear whether increases of excitability or specific firing patterns are responsible for the observed pro-regenerative effect. Further, it is unknown whether the regenerative and non-regenerative neuron types could exhibit different neuronal activities, and if so, whether neuronal activities could be related to the different regenerative abilities.
Drosophila larval PNS sensory neurons have emerged as a powerful system for studying neuronal morphogenesis and regeneration.17,21,22 These closely related sensory neurons are divided into subtypes based on dendritic morphology and sensory functions.23,24 Like mouse RGCs, sensory neuron subtypes in Drosophila show different axon regenerative abilities.17 In the current study, we investigated in parallel the regeneration-competent, nociceptive neurons (aka class 4 dendritic arborization, or C4da) and the regeneration-incompetent, gentle touch-sensitive neurons (aka class 3 dendritic arborization, or C3da). We show that surrounding glia, which closely interact with sensory neurons by ensheathing the axon bundle,25 determine the different axon regenerative responses through gliotransmission. We further elucidate the axon regenerative programs that are activated by gliotransmission. Finally, we find that stimulation of gliotransmission and adenosine signaling could promote mammalian CNS axon regeneration.
RESULTS
Gliotransmission is essential for axon regeneration
Different subtypes of Drosophila larval sensory neurons reside in close proximity; their dendrites overlap, and their axons are bundled and project to ventral nerve cord (Figure S1A–S1C). The axon bundle is wrapped by three layers of ensheathing glia (Figure S1B–S1C),25 forming a non-myelinating structure like the mammalian Remak bundle. Unless specified, we performed in vivo axotomy by focusing two-photon laser to a single axon, to minimize damages to neighboring axons and ensheathing glia (Figure S1C). Distal axon undergoes Wallerian degeneration after axotomy and proximal axon started to regrow at ~24 hours post axotomy (hpa) (Figure S1C).17,26 We calculated axon regeneration index by measuring axonal growth between 24 and 48 hpa and normalizing axonal regrowth to larval body expansion during this period (see Star Methods). Consistent with a previous report,17 we found C4da neurons, which detect noxious heat, strong light, mechanical stimulation, and irritant chemicals and are labeled in the ppk-CD4-tdGFP, ppk-CD4-tdTomato, ppk-EGFP strains or by the ppk-Gal4/LexA drivers,27–32 showed robust axon regeneration (Figure 1A–1D). In contrast, C3da neurons, labeled either by the 19–12-Gal4 driver in conjunction with repo-Gal80, or by the NompC-Gal4/LexA drivers,27,33 failed to regenerate (Figure 1A–1D).
Figure 1. Gliotransmission is required for axon regeneration.
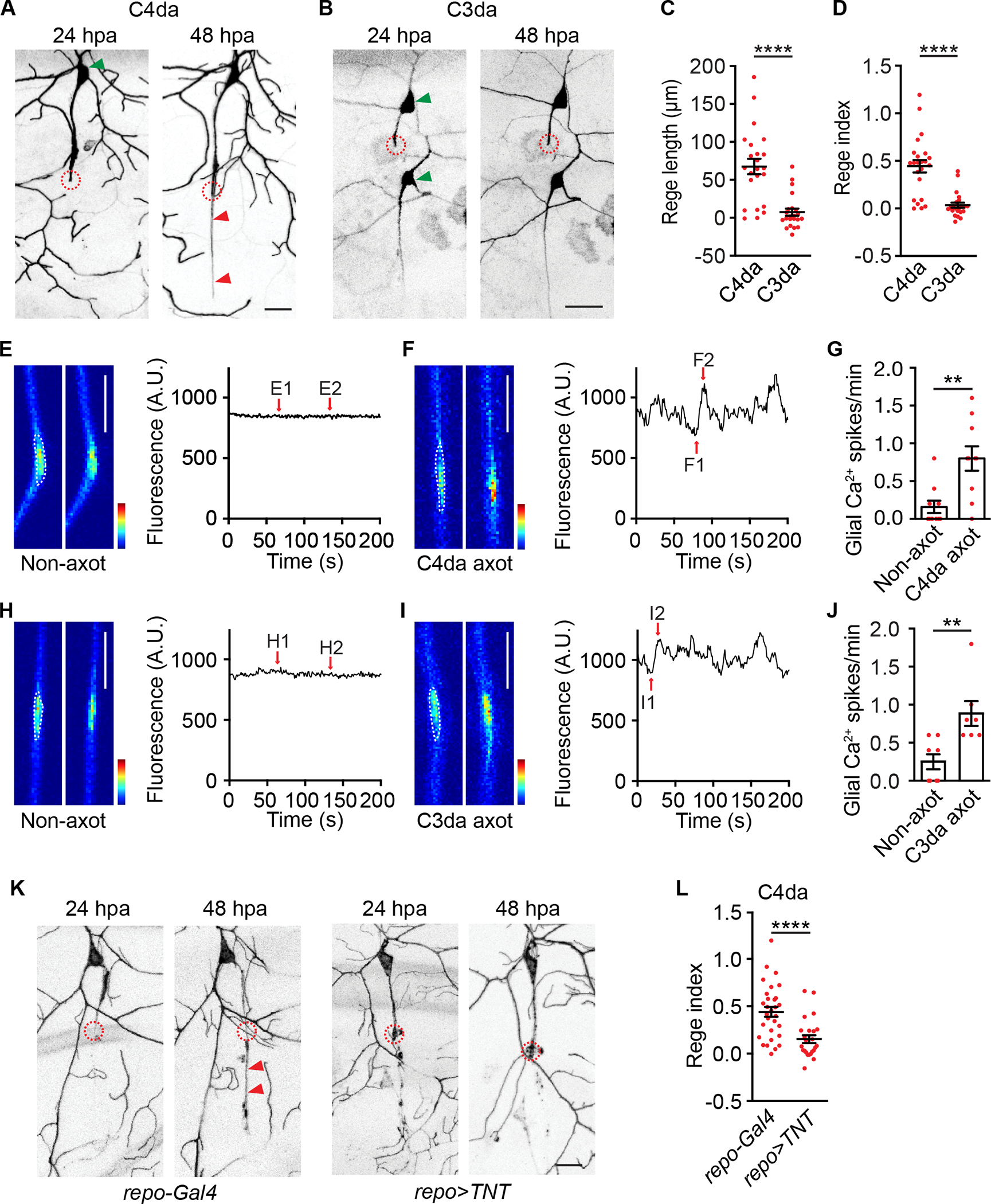
(A-B) Representative images of a C4da neuron (A) and a C3da neuron (B) at 24 and 48 hpa. Circles indicated axotomy sites. Red arrowheads in (A) indicated the regenerated axon, and green arrowheads indicated somata. ppk-CD4-tdGFP and repo-Gal80, 19-12-Gal4>UAS-EYFP labeled C4da and C3da neurons, respectively. Throughout this work, repo-Gal80 was used to suppress non-specific expression of 19-12-Gal4 in glia. Scale bar, 20 μm. Of the two C3da neurons in (B), axotomy was performed on the dorsal neuron ddaF (upper).
(C-D) Quantifications of axon regeneration length (C) and regeneration index (D). n=23 for C4da and n=22 for C3da neurons, respectively.
(E-G) Ensheathing glial Ca2+ spikes after axotomy of C4da neurons. Larvae bearing repo-Gal4>UAS-GCaMP6s, ppk-CD4-tdTomato were used. (E-F) Representative GCaMP6s images and traces showed glial Ca2+ signals without axotomy (E, non-axot) and at 24 hours after axotomy of C4da neurons (F, C4da axot). (G) Quantifications of glial Ca2+ spikes. Panels E1-E2 and F1-F2 showed individual frames. Glial Ca2+ spikes were quantified from regions indicated by white dashed lines. Scale bar, 20 μm. GCaMP6s color scale: 0–2,500. n=10 and 10.
(H-J) Analogous to (E-G), axotomy of C3da neurons induced Ca2+ spikes in ensheathing glia. Larvae bearing repo-Gal4>UAS-GCaMP6s, NompC-LexA>LexAop2-mCherry were used. Scale bar, 20 μm. GCaMP6s color scale: 0–3,000. n=8 and 7.
(K-L) Representative images (K) and axon regeneration index (L) of C4da neurons from control larvae (repo-Gal4 group: repo-Gal4, ppk-CD4-tdGFP) and larvae expressing TNT in glia (repo>TNT group: repo-Gal4>UAS-TNT, ppk-CD4-tdGFP). Circles indicated axotomy sites and arrowheads indicated the regenerated axon. Scale bar, 20 μm. n=30 and 24.
Mann-Whitney test (C, D, G, J and L). **P<0.01, ****P<0.0001.
To assess the role of ensheathing glia, we performed ‘glial bundle cut’ by increasing the laser scanning area. Unlike standard axotomy, glial bundle cut disrupted ensheathing glial integrity and abrogated axon regeneration of C4da neurons (Figure S2A–S2B). Therefore, ensheathing glia critically control axon regeneration, consistent with a previous report.17 Next, we determined how glial cells respond to axotomy by GCaMP imaging.34 Without axotomy, the glial Ca2+ level was stable. In contrast, axotomy of C4da neurons elicited Ca2+ oscillations in ensheathing glia, a response that we termed as glial Ca2+ spikes (Figure 1E–1G). Similar glial Ca2+ spikes were observed after axotomy of C3da neurons (Figure 1H–1J). Further analysis revealed that the frequency of glial Ca2+ spikes gradually increased over time and became apparent as early as 6 hours after axotomy (Figure S2D–S2F). A major effector of glial Ca2+ signals is gliotransmitter release,5 a process that depends on soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE).10,35–40 To disrupt gliotransmitter release, we expressed tetanus toxin (TNT) in glia to cleave synaptobrevin,41 a SNARE complex component. Glial TNT expression, driven by either the Gal4-UAS or QF-QUAS binary expression systems,42,43 markedly decreased axon regeneration of C4da neurons (Figure 1K–1L and S2G–S2H). To corroborate these results, we expressed temperature-sensitive shibirets1 in glia to interfere with vesicle recycling by raising larvae at restricted temperature post axotomy,44 and found that axon regeneration of C4da neurons was markedly reduced (Figure S2I–S2J). Ensheathing glia are comprised of three glial subtypes that include wrapping, subperineural, and perineural glia.25 Axon regeneration of C4da neurons was impaired after TNT expression in subperineural or perineural, but not wrapping, glia (Figure S2K–S2L), implicating a role of select glial subtypes in axon regeneration.
Neuronal subtype-specific responses to glial stimulation
To directly assess the effects of gliotransmission on C4da and C3da neurons, we elevated Ca2+ in ensheathing glia by thermogenetics to stimulate gliotransmitter release. TrpA1-B (i.e., TrpA1 splice variant B) is a heat-sensitive Ca2+ channel.28 Here, we expressed TrpA1-B in glia and heated the dissected larvae to ~33°C, which is above the TrpA1-B threshold but does not activate C4da neurons.27,28 As expected, heating increased Ca2+ signals in ensheathing glia that expressed TrpA1-B (Figure S3A–S3B). Next, we expressed GCaMP6s in C4da or C3da neurons and found that glial stimulation activated C4da, but not C3da, neurons (Figure 2A–2B, Video S1 and S2). Moreover, the response of C4da neurons was abolished after glial expression of TNT (Figure 2B), demonstrating that ensheathing glia activate C4da neurons through gliotransmitter release. To corroborate these findings, we expressed CsChrimson and stimulated ensheathing glia with light. When compared to thermogenetics, optogenetics offers better temporal control. We noted that light stimulation of CsChrimson-expressing glia was sufficient to activate C4da neurons (Figure S3C–S3D). The response latency appeared to be short (Figure S3E–S3F), consistent with the notion that gliotransmission quickly modulates neuronal excitability.
Figure 2. Gliotransmission controls regenerative differences of C4da versus C3da neurons.
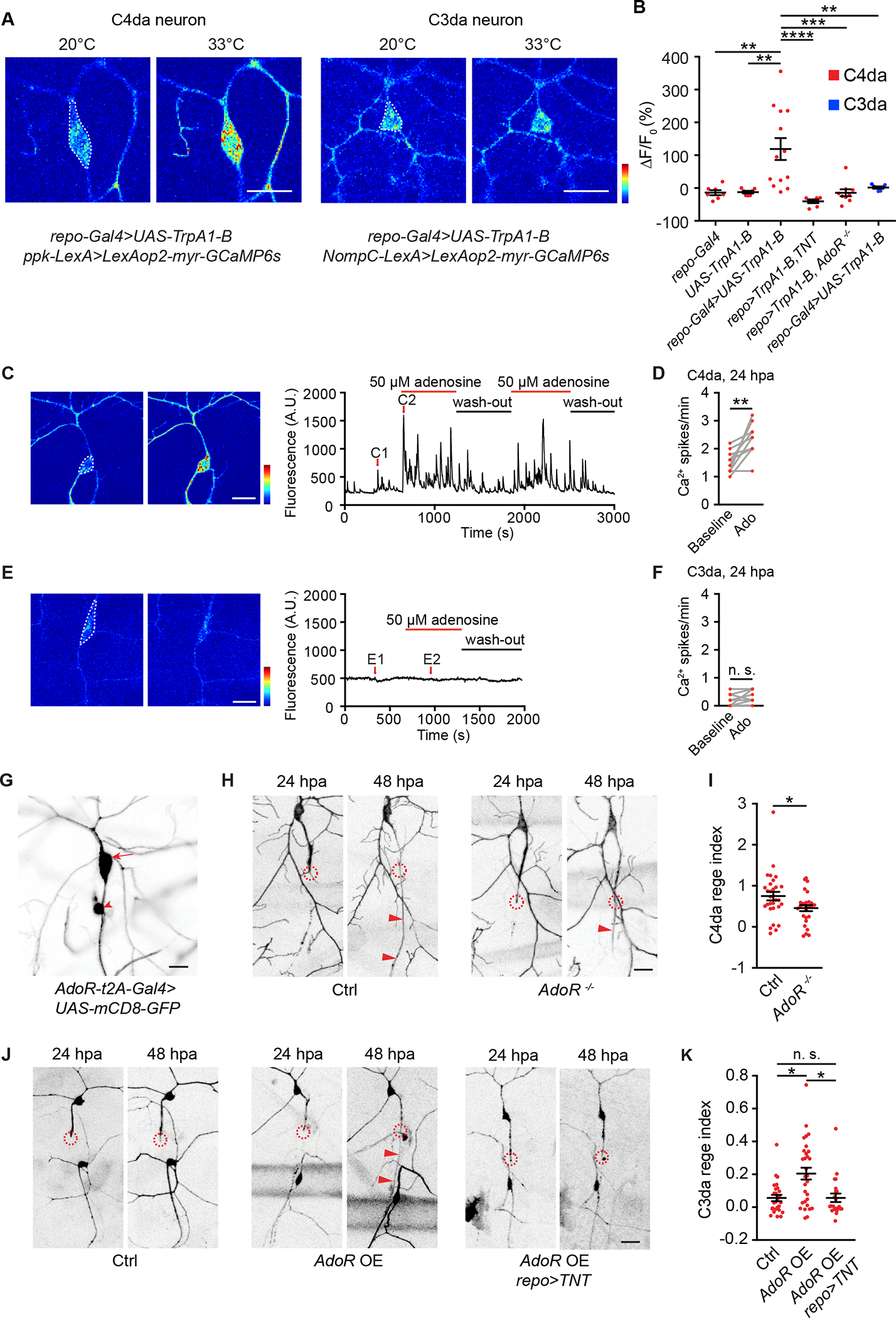
(A) Representative GCaMP6s responses of C4da neurons (labeled by ppk-LexA>LexAop2-myr-GCaMP6s) and C3da neurons (labeled by NompC-LexA>LexAop2-myr-GCaMP6s) in response to heating to 33°C. Larvae also expressed TrpA1-B in glia (repo-Gal4>UAS-TrpA1-B). GCaMP6s responses were quantified from neuron somata as indicated by white dashed lines. Scale bar, 20 μm. GCaMP6s color scale: 0–2,500.
(B) Quantifications of neuronal GCaMP6s signals in response to heating to 33°C. Two groups in (A) were presented in the 3rd and 6th column, respectively. C4da neurons were labeled by ppk-LexA>LexAop2-myr-GCaMP6s and C3da neurons by NompC-LexA>LexAop2-myr-GCaMP6s. Additionally, following transgenes were included: 1st column, repo-Gal4. 2nd column, UAS-TrpA1-B. 3rd column, repo-Gal4>UAS-TrpA1-B. 4th column, repo-Gal4>UAS-TrpA1-B, UAS-TNT. 5th column, repo-Gal4>UAS-TrpA1-B; AdoR−/−. 6th column, repo-Gal4>UAS-TrpA1-B. n=7, 7, 13, 7, 9, 7. One-way ANOVA followed by Tukey’s test.
(C-F) Representative GCaMP6s responses and quantifications of axotomized C4da neurons (C-D, ppk-LexA>LexAop2-myr-GCaMP6s) and C3da neurons (E-F, NompC-LexA>LexAop2-myr-GCaMP6s), in response to adenosine. C1-C2 and E1-E2 showed individual frames before and during adenosine application. Scale bar, 20 μm. GCaMP6s color scale: 0–3,000. Adenosine perfusion was followed by washout. Ca2+ spike frequency of axotomized C4da and C3da neurons was quantified from the somata (white dashed lines in (C) and (E)) before and during adenosine application. n=10 for C4da and n=8 for C3da neurons. Two-tailed paired t-test.
(G) GFP signals from a 3rd instar larva bearing AdoR-t2A-Gal4>UAS-mCD8-GFP showed AdoR expression in the C4da neuron (arrow) and another sensory neuron (arrowhead, likely dmd). No GFP signals were found in C3da neurons or ensheathing glia. Scale bar, 20 μm.
(H-I) Representative images (H) and regeneration index (I) of C4da neurons from wildtype larvae (Ctrl group: ppk-CD4-tdGFP) and larvae bearing AdoR deletion mutation (AdoR−/− group: ppk-CD4-tdGFP; AdoR−/−). Scale bar, 20 μm. n=30 and 27. Mann-Whitney test.
(J-K) Representative images (J) and regeneration index (K) of C3da neurons from control larvae (Ctrl group: repo-Gal80, 19-12-Gal4>UAS-EGFP), larvae with AdoR overexpression in C3da neurons (AdoR OE group: repo-Gal80, 19-12-Gal4>UAS-EGFP, UAS-AdoR), and larvae with AdoR overexpression in C3da neurons and TNT expression in glia (AdoR OE, repo>TNT group: repo-Gal80, 19-12-Gal4>UAS-EGFP, UAS-AdoR, repo-QF>QUAS-TNT). n=26, 30 and 22. Scale bar, 20 μm. Kruskal-Wallis test followed by Dunn’s test.
In (H) and (J), circles indicated axotomy sites and arrowheads indicated the regenerated axon. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. n. s., not significant.
ATP is a gliotransmitter in both mammals and Drosophila.8–10,40 Once released to the extracellular space, ATP is quickly converted to adenosine.45,46 Drosophila genome encodes no ATP receptors but a single adenosine receptor (AdoR), which is a member of G protein-coupled receptors (GPCRs) that signal through Gαs to increase intracellular Ca2+.9,47,48 In AdoR−/− mutant larvae,49 thermogenetic stimulation of ensheathing glia induced no Ca2+ responses in C4da neurons (Figure 2B). These data suggest that adenosine is the gliotransmitter that acts on AdoR to activate C4da neurons. Moreover, in AdoR−/− mutant larvae, C4da neurons responded normally to the irritant chemical allyl isothiocyanate (AITC) (Figure S3G–S3H).
Next, we acutely applied adenosine and found this increased the Ca2+ spike frequency in axotomized C4da neurons (Figure 2C–2D, see below for neuronal Ca2+ spikes). In contrast, adenosine had no effects on Ca2+ signals in axotomized C3da neurons (Figure 2E–2F). Moreover, application of two other gliotransmitters glutamate and D-serine had no effect on Ca2+ spikes in axotomized C4da neurons (Figure S4A–S4D). Taken together, our data indicate that ensheathing glia could differentially modulate activity of C4da versus C3da neurons through the gliotransmitter adenosine.
Ectopic AdoR expression in C3da neurons suffices to induce axon regeneration
Could AdoR expression underlie neuronal type-specific responses to adenosine? To test this, we employed AdoR-t2A-Gal4, a strain in which t2A-Gal4 is knocked into the AdoR locus immediately before the stop codon so that AdoR and Gal4 are produced as a single peptide during translation but are later separated via t2A-mediated cleavage.49 Hence, Gal4 activity in AdoR-t2A-Gal4 animals should report endogenous AdoR expression. A similar strategy has been successfully used to report in vivo expression of ion channels and receptors in Drosophila.28,49 By crossing AdoR-t2A-Gal4 to a GFP reporter line, we found AdoR expression in C4da neurons, but not in C3da neurons or ensheathing glia (Figure 2G). This expression pattern provides a molecular explanation of neuronal type-specific responses to glial stimulation and adenosine.
Next, we found that axon regeneration of C4da neurons was impaired in AdoR−/− mutant larvae (Figure 2H–2I). Therefore, disruption of gliotransmission, either by glial TNT expression or by AdoR mutation could impair axon regeneration.
Axotomy of either C4da or C3da neurons evoked glial Ca2+ spikes (Figure 1E–1J), suggesting that gliotransmitters are released regardless of the injured neuronal types. This raised a possibility that different regenerative abilities of C4da and C3da neurons could be related to AdoR expression. We tested this idea by ectopic expression of AdoR in C3da neurons. Adenosine activated AdoR-expressing, but not control, C3da neurons (Figure S4E–S4F), validating functional AdoR expression in C3da neurons. Moreover, C3da neurons that expressed AdoR robustly regenerated their axons, in contrast to control C3da neurons that failed to regenerate (Figure 2J–2K). Further, gliotransmission appears to be essential as AdoR-induced axon regeneration of C3da neurons was abrogated after glial expression of TNT (Figure 2J–2K). Given that gliotransmission is necessary for axon regeneration of C4da neurons and that engineering C3da neurons to respond to adenosine is sufficient to induce their axon regeneration, we propose an instructive role of gliotransmission to specify the axon regenerative differences of C4da versus C3da neurons.
Gliotransmission elicits Ca2+ spikes and burst firing in C4da, but not C3da, neurons
What are the axon regenerative programs that are activated by gliotransmission? These programs should meet the following criteria: i) they are active in axotomized C4da neurons and require gliotransmission, ii) they are inactive in axotomized C3da neurons but can be reactivated after ectopic AdoR expression, and iii) disrupting them should impair axon regeneration of C4da neurons and reactivating them in C3da neurons should induce axon regeneration. We hypothesized that neuronal Ca2+ signals could be one such program, because glial stimulation and adenosine activated Ca2+ responses in C4da, but not C3da, neurons (Figure 2A–2B and 2C–2F). We tested this hypothesis by GCaMP imaging of sensory neurons. Intriguingly, axotomized C4da neurons exhibited Ca2+ spikes that were synchronized across the cell body, axon and dendrites, in contrast, Ca2+ spikes were seldom detected in non-axotomized C4da neurons, axotomized C3da neurons, and non-axotomized C3da neurons (Figure 3A–3F, Video S3 and S4). Ca2+ influx is required as Ca2+ spikes of axotomized C4da neurons were abolished after removing extracellular Ca2+ (Figure S5A). Moreover, no Ca2+ spikes were found after severing dendrites of C4da neurons or after induction of systemic inflammatory responses by exposing larvae to UV-C light (Figure 3E).31,50 These results indicate that Ca2+ spikes are not a general injury response. Therefore, Ca2+ spikes are neuronal type-specific and axon injury-specific. Next, we imaged C4da neurons at different time points after axotomy and found that Ca2+ spikes had delayed onset and gradually increased; their frequency was low in the first few hours, reached a plateau at ~18 hpa and sustained afterwards (Figure 3G). This time window indicates that Ca2+ spikes precede and persist throughout axon regeneration, which started at ~24 hpa (Figure S1C). The temporal correlation between neuronal Ca2+ spikes and axon regeneration implies a role of Ca2+ spikes in axon regeneration.
Figure 3. Axotomy elicits Ca2+ spikes and burst firing in C4da, but not C3da, neurons.
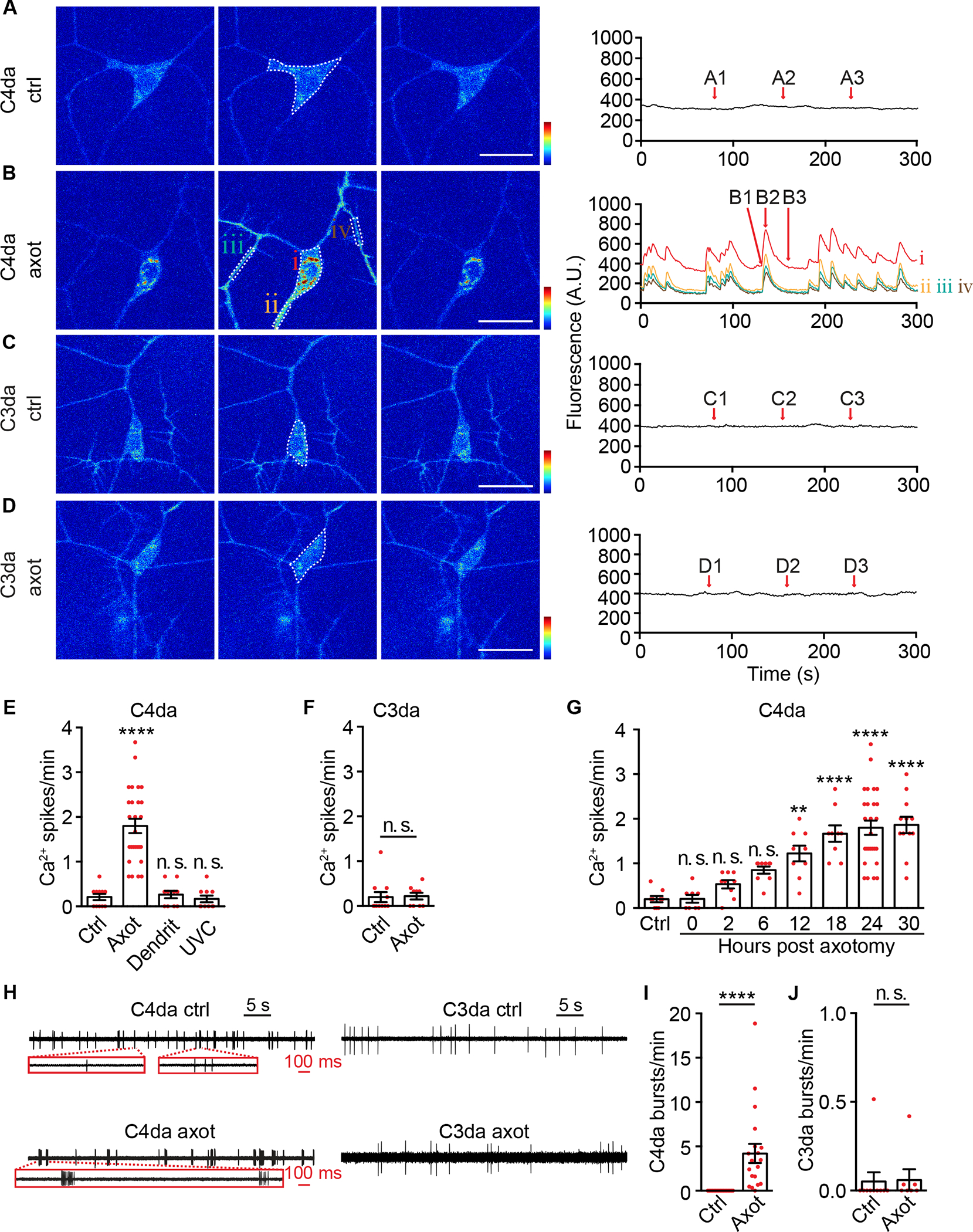
(A-D) Representative GCaMP6s imaging from control (ctrl, non-axotomized) and axotomized (axot) C4da neurons (A-B, ppk-LexA>LexAop2-myr-GCaMP6s) and C3da neurons (C-D, NompC-LexA>LexAop2-myr-GCaMP6s). Left three panels (e.g., A1-A3) showed individual frames from associated time-lapse traces. For traces in (A), (C), and (D), GCaMP6s signals from the cell body as indicated by dashed lines were shown. For traces in (B), GCaMP6s signals from the cell body (i), axon (ii) and dendrites (iii and iv) as indicated by dashed lines were shown. Scale bar, 20 μm. GCaMP6s color scale: 0–1,800.
(E) Quantifications of Ca2+ spike frequency of C4da neurons at 24 hpa (Axot), dendritomy (Dendrit), or exposure to 256 nm UVC light (UVC). n=11, 27, 10 and 10. C4da neurons from non-injured larvae served as control (Ctrl). One-way ANOVA followed by Bonferroni’s test.
(F) Quantifications of Ca2+ spike frequency of control (Ctrl, non-axotomized) and axotomized (Axot) C3da neurons at 24 hpa. n=11 and 9. Mann-Whitney test.
(G) Ca2+ spike frequency of C4da neurons at different time points after axotomy. n=9, 8, 9, 9, 9, 9, 27 and 12 for each time point. One-way ANOVA followed by Dunnett’s test between control (Ctrl, non-axotomized) and axotomized groups.
(H) Representative extracellular recording traces showing spontaneous firing of control (ctrl, non-axotomized) and axotomized (axot) C4da and C3da neurons. Zoom-in showed representative burst firing in axotomized, but not non-axotomized, C4da neurons. Each vertical tick mark represented an action potential.
(I-J) Average frequency of burst firing events (bursts/min) of C4da neurons (I) and C3da neurons (J). n=17 and 19 for non-axotomized (Ctrl) and axotomized (Axot) C4da neurons. n=10 and 7 for non-axotomized (Ctrl) and axotomized (Axot) C3da neurons, respectively. Mann-Whitney test.
In (H-J), C4da neurons are labeled by ppk-CD4-tdGFP and C3da neurons are labeled by repo-Gal80, 19-12-Gal4>UAS-EYFP.
**P<0.01, ****P<0.0001. n. s., not significant.
In addition to theses Ca2+ spikes with delayed onset, axotomy elicits immediate Ca2+ transients that last only a few minutes after axotomy.51,52 Immediate Ca2+ transients regulate early axon regeneration events such as membrane resealing and growth cone formation.51,53,54 We found that immediate Ca2+ transients were elicited after axotomy of either C3da or C4da neurons (Figure S5B–S5C), in contrast to Ca2+ spikes that were C4da neuron-specific. Hence, immediate Ca2+ transients are mechanistically different from Ca2+ spikes and are unlikely to underlie axon regeneration differences of C4da and C3da neurons.
We next performed extracellular recordings and found that axotomized C4da neurons exhibited spontaneous burst firing, in which rapid action potential sequences were discharged intermittently (Figure 3H, see Star Methods for definition of burst firing events). Bursting firing was frequently observed in axotomized C4da neurons (18 out of 19), but barely in non-axotomized C4da neurons (1 out of 17) (Figure S5F and S5H). Moreover, the frequency of burst firing events, and the percentage of total action potentials found in the burst firing events, were significantly increased in axotomized C4da neurons when compared to non-axotomized C4da neurons (Figure 3I and S5I). On the other hand, overall firing frequency was similar between axotomized and non-axotomized C4da neurons (Figure S5D). Hence, axotomy does not alter excitability of C4da neurons; rather, it transforms neuronal activity patterns by evoking burst firing. In contrast to C4da neurons, axotomy did not evoke burst firing or otherwise alter excitability of C3da neurons (Figure 3H, 3J, S5E, S5G–S5H and S5J). By simultaneous GCaMP imaging and extracellular recording on the same axotomized C4da neurons, we found a high correlation between Ca2+ spikes and burst firing (Figure S5K and Video S5). These findings indicate that Ca2+ spikes are a surrogate for burst firing and suggest that Ca2+ spikes are evoked by depolarization associated with burst firing.
After axotomy, glial Ca2+ spikes developed with a similar time window as neuronal Ca2+ spikes (Figure 3G and S2D–S2F), suggesting a role of gliotransmission in neuronal Ca2+ spikes. Indeed, Ca2+ spikes of axotomized C4da neurons were markedly reduced after glial TNT expression (Figure 4A–4C) and in AdoR−/− mutants (Figure 4D–4F). Consistently, burst firing events in axotomized C4da neurons were greatly reduced after glial bundle cut (Figure S2C), indicating that glia-neuron interactions are essential for burst firing. Finally, we tested whether ectopic AdoR expression could be sufficient to induce Ca2+ spikes in axotomized C3da neurons. Indeed, AdoR-expressing, axotomized C3da neurons exhibited robust Ca2+ spikes (Figure 4G–4I), which resemble those found in axotomized C4da neurons. Axotomy is required, as no Ca2+ spikes were found in non-axotomized, AdoR-expressing C3da neurons (Figure S4G–S4H). Taken together, we propose neuronal Ca2+ spikes as a downstream effector of gliotransmission.
Figure 4. Neuronal Ca2+ spikes are an effector of gliotransmission.

(A-C) Representative GCaMP6s images (A, B) and quantifications (C) of Ca2+ spikes of axotomized C4da neurons (labeled by ppk-LexA>LexAop2-myr-GCaMP6s) at 24 hpa, from control larvae bearing repo-Gal4 (panel A) and larvae bearing glial TNT expression (repo>TNT group: repo-Gal4>UAS-TNT, panel B). GCaMP6s color scale: 0–2,500. n=11 and 9.
(D-F) Representative (D, E) and quantifications (F) of Ca2+ spikes of axotomized C4da neurons (labeled by ppk-LexA>LexAop2-myr-GCaMP6s) at 24 hpa, from wildtype larvae (Ctrl, panel D) and larvae bearing AdoR deletion mutation (AdoR−/−, panel E). GCaMP6s color scale: 0–2,500. n=12 and 10.
(G-I) Representative (G, H) and quantifications (I) of Ca2+ spikes of axotomized C3da neurons at 24 hpa, from control larvae (repo-Gal80, 19-12-Gal4>UAS-GCaMP6s, panel G) and larvae bearing AdoR overexpression in C3da neurons (AdoR OE group: repo-Gal80, 19-12-Gal4>UAS-AdoR, UAS-GCaMP6s, panel H). GCaMP6s color scale: 50–1,400. n=11 and 12.
(A1-A2), (B1-B2), (D1-D2), (E1-E2), (G1-G2), and (H1-H2) showed individual frames from the time-lapse GCaMP6s traces. Ca2+ spikes were quantified from somata indicated by white dashed lines. Scale bar, 20 μm.
Two-tailed unpaired t-test (C, F, and I). **P<0.01, ***P<0.001.
See also Table S1.
The firing pattern determines axon regeneration outcome
To determine the role of neuronal activity in axon regeneration, we expressed the inward rectifying potassium channel Kir2.1 in C4da neurons.55 As expected, Kir2.1 expression reduced Ca2+ spikes of axotomized C4da neurons (Figure 5A). Moreover, Kir2.1 greatly reduced axon regeneration of C4da neurons (Figure 5B–5C). Similarly, C4da neurons with expression of the Ca2+ binding protein parvalbumin, which sequesters Ca2+ in Drosophila tissues,56 also showed impaired Ca2+ spikes and axon regeneration (Figure 5A–5C). These data indicate that neuronal activity is essential for axon regeneration.
Figure 5. Action potential firing patterns determine axon regeneration outcome.
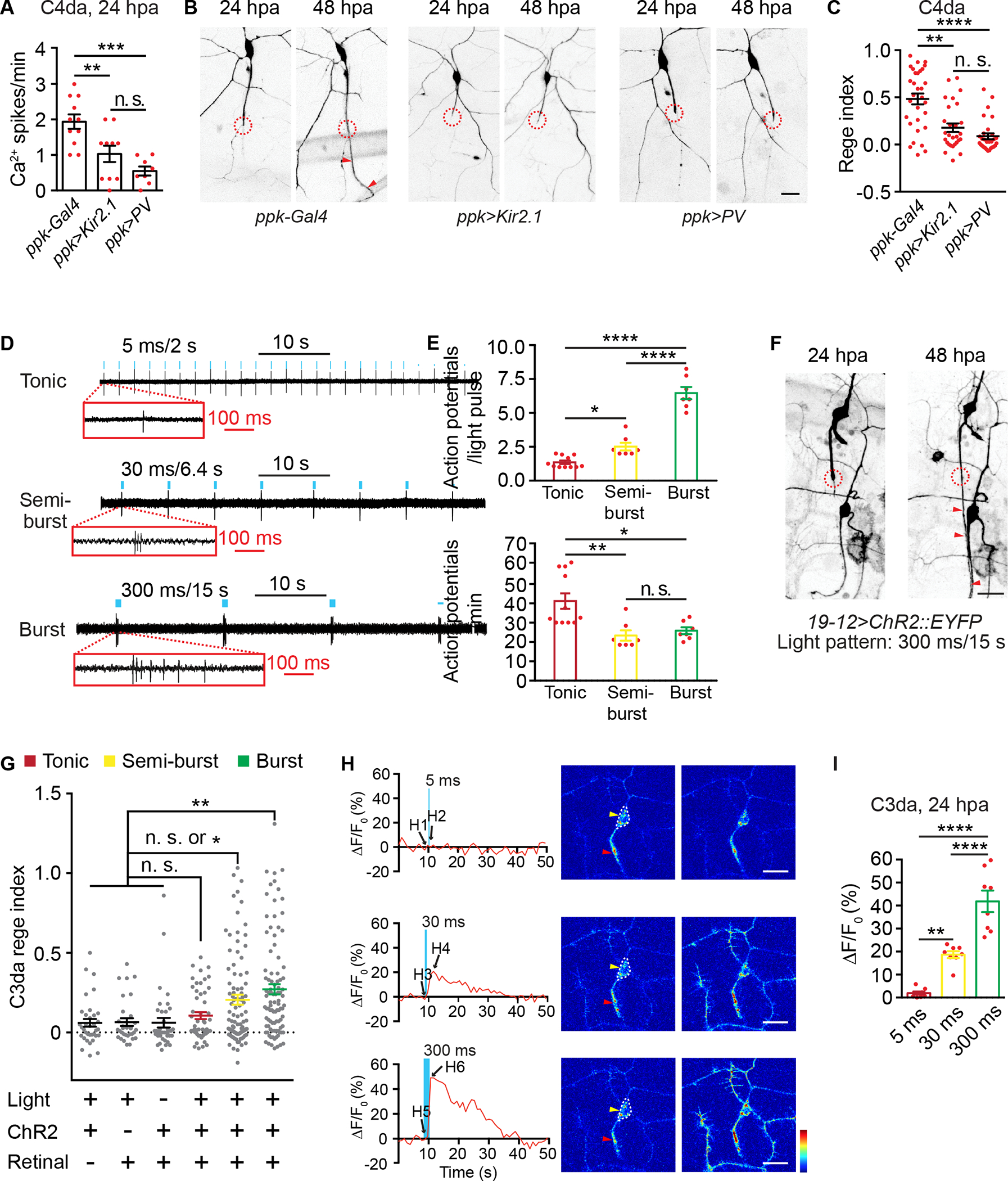
(A) Quantifications of Ca2+ spike frequency of axotomized C4da neurons at 24 hpa, from control larvae (ppk-Gal4 group: ppk-Gal4, ppk-LexA>LexAop2-myr-GCaMP6s), larvae with Kir2.1 expression in C4da neurons (ppk>Kir2.1 group: ppk-Gal4>UAS-Kir2.1, ppk-LexA>LexAop2-myr-GCaMP6s), or larvae with parvalbumin (PV) expression in C4da neurons (ppk>PV group: ppk-Gal4>UAS-PV, ppk-LexA>LexAop2-myr-GCaMP6s). n=11, 10 and 8.
(B-C) Representative (B) and regeneration index (C) of C4da neurons from control larvae (ppk-Gal4 group: ppk-Gal4, ppk-EGFP), larvae expressing Kir2.1 in C4da neurons (ppk>Kir2.1 group: ppk-Gal4>UAS-Kir2.1, ppk-EGFP), and larvae expressing parvalbumin (PV) in C4da neurons (ppk>PV group: ppk-Gal4>UAS-PV, ppk-EGFP). Scale bar, 20 μm. n=31, 28 and 29. (D) Representative extracellular recordings of C3da neurons (labeled by repo-Gal80, 19-12-Gal4>UAS-ChR2::EYFP) showing three firing patterns in responses to optogenetics. 470 nm, 1.4 mW/mm2 light pulses were marked by blue dots (not to scale). Scale bar, 10 s. Zoom-in traces show spike patterns. Scale bar, 100 ms.
(E) Summary of (D). Upper, quantifications of action potential numbers in response to a single optogenetic light pulse. Lower, quantifications of average action potential numbers per minute in response to optogenetic stimulation. n=11, 7 and 7.
(F) Representative showing axon regeneration of C3da neurons (labeled by repo-Gal80, 19-12-Gal4>UAS-ChR2::EYFP) in response to optogenetics-induced burst firing. Scale bar, 20 μm. (G) Axon regeneration index of C3da neurons in three control groups (no retinal, n=35; no ChR2 expression, n=32; no light, n=39) and under three optogenetic stimulation conditions (tonic firing, n=51; semi-burst firing, n=99; burst firing, n=97).
(H) Ca2+ signals of axotomized C3da neurons (labeled by NompC-LexA>LexAop2-myr-GCaMP6s, repo-Gal80, 19-12-Gal4>UAS-ChR2) at 24 hpa, in response to a single light pulse that induced tonic, semi-burst, and burst firing. Left panels showed time-lapse GCaMP6s imaging traces from somata. Blue bars marked delivery of a single light pulses (470 nm, 1.4 mW/mm2) with 5 ms, 30 ms and 300 ms duration. Right panels showed GCaMP6s imaging frames before and immediately after light stimulation. Red and yellow arrowheads marked axons and somata, respectively. Scale bar, 20 μm. GCaMP6s color scale: 0–1,500.
(I) Summary of (H) by quantifying peak GCaMP6s responses from somata of axotomized C3da neurons. n=8, 8 and 8.
One-way ANOVA followed by Bonferroni’s test (A, E, and I). Kruskal-Wallis test followed by Dunn’s test (C and G).
In (B) and (F), circles indicated axotomy sites and arrowheads marked the regenerated axon. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. n. s., not significant.
Previous studies report that elevating neuronal activity promotes axon regeneration.11,19,20 However, it is yet unknown whether neuronal excitability per se or a specific firing pattern is responsible for the observed effect. To address this, we optogenetically stimulated axotomized C3da neurons in free moving larvae with three different firing patterns, including burst, semi-burst, and tonic, firing, while maintaining the total action potential numbers comparable (Figure 5D–5E). To ‘replay’ the burst firing pattern of C4da neurons onto C3da neurons, we first determined the firing characteristics of axotomized C4da neurons (7.3 action potential/burst, 4.2 bursts/minute and 40.8 ms inter-spike interval, Figure 3I and Figure S5L–S5M). Next, we expressed channelrhodopsin-2 (ChR2) in C3da neurons.57 and recorded their responses to 470 nm blue light. By varying intensity, duration, and frequency of light pulses, we found a specific stimulation paradigm that elicited burst firing events resembling those of axotomized C4da neurons (1.4 mW/mm2, 300 ms duration for every 15 s, see ‘burst’ in Figure 5D–5E and S5L–S5M). Because Ca2+ spikes appeared in C4da neurons at ~6 hpa (Figure 3G), we stimulated C3da neurons starting at ~6 hpa and lasting throughout the experiment (Figure S5N–S5O). Burst firing stimulation was able to induce robust axon regeneration of C3da neurons (Figure 5F and ‘burst’ in Figure 5G), when compared to control groups in which ChR2, light, or retinal was not supplied (Figure 5G). The magnitude of axon regeneration induced by burst firing was comparable to that after AdoR expression (regeneration index: 0.271±0.0319, n=97, optogenetics versus 0.205±0.0361, n=30, AdoR expression, Mann-Whitney test, P=0.779). We next stimulated C3da neurons with tonic and semi-burst firing, which elicited less action potentials per light pulse than burst firing, but nevertheless higher (tonic) or comparable (semi-burst) number of total action potentials per minute (Figure 5D–5E). We found that tonic firing was the least effective, whereas semi-burst firing was intermediate in promoting axon regeneration of C3da neurons (Figure 5G). Finally, we tested whether different firing patterns could evoke different Ca2+ responses in axotomized C3da neurons. Indeed, burst firing evoked strong Ca2+ responses, whereas semi-burst and tonic firing evoked moderate and no overt Ca2+ responses, respectively (Figure 5H–5I). In summary, these studies provide direct evidence that specific neuronal activity patterns, rather than overall excitability, determine the axon regenerative strength. Moreover, axon regenerative strength correlates with the amplitude of Ca2+ responses.
Ras is an effector of neuronal activity
Data presented so far suggest that gliotransmission activates burst firing and Ca2+ spikes in C4da neurons to promote their axon regeneration. One effector of neuronal Ca2+ is Ras.58 Moreover, the mitogen-activated protein kinase (MAPK) pathway has been shown to regulate axon regeneration in mammals and flies.58–62 We found that C4da neuron-specific expression of either the Drosophila or mouse version of dominant negative Ras (RasDN) impaired axon regeneration (Figure 6A–6B). To test sufficiency, we expressed constitutively active Ras (RasCA) in C3da neurons and found that this manipulation induced robust axon regeneration (Figure 6C–6D). Ras activity is reported by diphospho-extracellular signal–regulated kinase (dpERK).48,63 Immunostaining revealed that the dpERK level was low in both C4da and C3da neurons, but was significantly increased in C4da, but not C3da, neurons after axotomy (Figure 6E–6H), indicating that axotomy activates Ras in a neuronal type-specific fashion. Next, we assessed genetic interactions between neuronal activity and Ras. Burst firing-induced axon regeneration of C3da neurons was abolished after RasDN expression in C3da neurons (Figure S6A–S6B). Moreover, inhibition of axon regeneration of C4da neurons by parvalbumin was reversed after RasCA expression in C4da neurons (Figure S6C–S6D). Finally, we directly tested whether neuronal activity could activate Ras. Acute AITC application, which potently increases neuronal activity and Ca2+ levels of C4da neurons,27,28 increased the dpERK level in C4da neurons (Figure S6E–S6F). Similarly, burst firing stimulation by optogenetics increased the dpERK level in C3da neurons (Figure S6G–S6H). Together, our results indicate that Ras acts downstream of neuronal activity to determine axon regenerative differences of C3da and C4da neurons.
Figure 6. Neuronal-type specific Ras activity determines axon regeneration outcome.

(A-B) Representative images (A) and regeneration index (B) of C4da neurons from control larvae (ppk-Gal4 group: ppk-Gal4, ppk-CD4-tdGFP) and larvae expressing dominant negative Ras in C4da neurons (ppk>Ras85D.N17 group: ppk-Gal4>UAS-Ras85D.N17, ppk-CD4-tdGFP. ppk>ras.N17 group: ppk-Gal4>UAS-ras.N17, ppk-CD4-tdGFP). Scale bar, 20 μm. n=25, 27 and 24.
(C-D) Representative images (C) and regeneration index (D) of C3da neurons from control larvae (19-12-Gal4 group: repo-Gal80, 19-12-Gal4>UAS-EGFP) and larvae expressing constitutively active Ras in C3da neurons (19-12-Gal4>Ras85D.V121 group: repo-Gal80, 19-12-Gal4>UAS-Ras85D.V121, UAS-EGFP. 19-12-Gal4>Ras85D.V122 group: repo-Gal80, 19-12-Gal4>UAS-Ras85D.V122, UAS-EGFP). Scale bar, 20 μm. n=26, 25 and 27.
(E-H) Representative images and quantifications of dpERK immunostaining signals from non-axotomized (non-axot) and axotomized (axot) C4da neurons at 24 hpa (E-F, n=11 and 11), and from non-axotomized (non-axot) and axotomized (axot) C3da neurons at 24 hpa (G-H, n=15 and 15). dpERK signals were quantified from somata indicated by white dashed lines. Scale bar, 10 μm. C4da neurons were labeled by ppk-EGFP. C3da neurons were labeled by repo-Gal80, 19-12-Gal4>UAS-EGFP.
In (A) and (C), circles indicated axotomy sites and arrowheads marked the regenerated axon. Kruskal-Wallis test followed by Dunn’s test (B and D) and Two-tailed unpaired t-test (F and H). *P<0.05, **P<0.01, ***P<0.001.
Stimulation of gliotransmission induces RGC axon regeneration after Adora2b expression
Inspired by our Drosophila studies, we set out to determine whether stimulation of gliotransmission could have an effect on mammalian CNS axon regeneration, using the optic nerve crush model in adult mice.12 Gq-coupled designer receptors exclusively activated by designer drugs (DREADDs) has been widely used to stimulate glial Ca2+ and gliotransmitter release.64–67 Here, we intravitreally injected the adeno-associated virus expressing Gq-coupled hM3D under control of the glia-specific promoter GFAP (AAV2-GFAP-hM3D(Gq)-mCherry) (Figure 7A).67 We validated by immunostaining that most mCherry-positive cells also expressed GFAP (Figure S7A). To activate DREADDs, its agonist clozapine-N-oxide (CNO) was intraperitoneally injected.67 However, DREADD activation failed to increase RGC axon regeneration (Figure 7B–7C), indicating that stimulation of gliotransmitter release alone was ineffective.
Figure 7. Glial stimulation and Adora2b activation promote RGC axon regeneration.
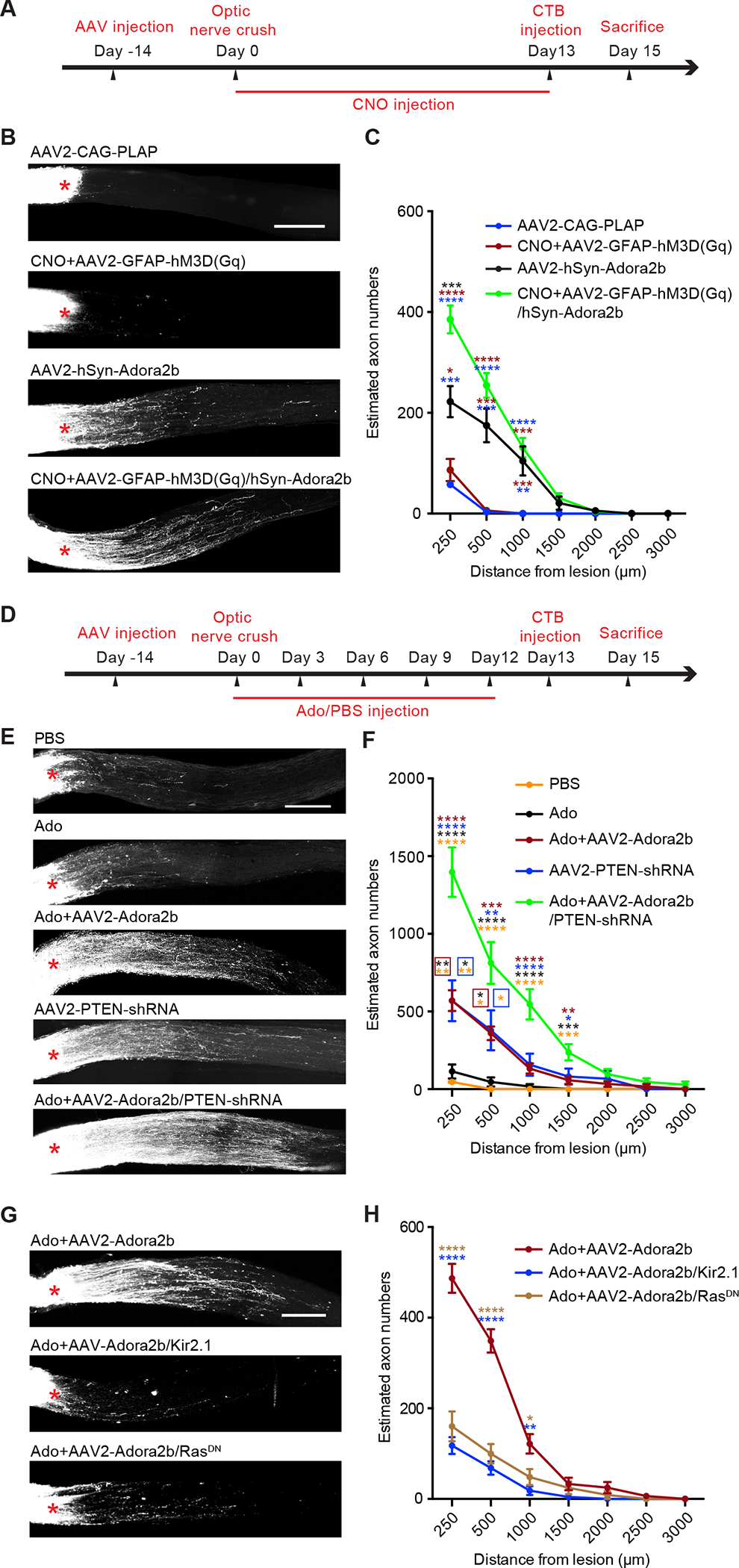
(A) Experimental procedure for studying RGC axon regeneration after optic nerve crush in adult mice. The following viruses AAV2-hSyn-Adora2b, AAV2-GFAP-hM3D(Gq), and AAV2-CAG-PLAP were intravitreally injected. 1mg/kg CNO was intraperitoneally administrated twice daily.
(B) Representative confocal images of optic nerve cryosections in various treatment groups. RGC axons were labeled by CTB. Crush sites were marked by asterisks. Scale bar, 250 μm.
(C) Quantifications of RGC axon regeneration in different treatment groups. Y-axis showed the number of axons and X-axis indicated the distance to the crush sites. n = 12, 9, 12 and 20 nerves from n=6, 6, 9 and 12 mice in AAV2-CAG-PLAP, CNO+AAV2-GFAP-hM3D(Gq), AAV2-hSyn-Adora2b, and CNO+AAV2-GFAP-hM3D(Gq)+AAV2-hSyn-Adora2b groups.
(D) Experimental procedure. The following viruses AAV2-hSyn-Adora2b, AAV2-hSyn-PTEN-shRNA, AAV2-EF1a-RasDN, and AAV2-CAG-Kir2.1 were intravitreally delivered. 2 μL 10 μM adenosine (Ado) or PBS was administrated by intravitreal injection for a total of 5 times.
(E) Representative confocal images of optic nerve cryosections in various treatment groups. RGC axons were labeled by CTB. Crush sites were indicated by asterisks. Scale bar, 250 μm.
(F) Quantifications of RGC axon regeneration in different treatment groups. n = 9, 13, 17, 11 and 11 nerves from n=6, 8, 12, 8 and 8 mice in PBS, Ado, Ado+AAV2-hSyn-Adora2b, AAV2-hSyn-PTEN-shRNA, Ado+AAV2-hSyn-Adora2b+AAV2-hSyn-PTEN-shRNA groups.
(G) Representative confocal images of optic nerve cryosections in various treatment groups. RGC axons were labeled by CTB. Crush sites were indicated by asterisks. Scale bar, 250 μm.
(H) Quantifications of RGC axon regeneration in different treatment groups. n = 17, 21 and 10 nerves from n=13, 14 and 8 mice in Ado+AAV2-hSyn-Adora2b, Ado+AAV2-hSyn-Adora2b+AAV2-EF1a-RasDN and Ado+AAV2-hSyn-Adora2b+AAV2-CAG-Kir2.1 groups.
*P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001. One-way ANOVA followed by Tukey’s test (C, F and H). The color of asterisks indicated the group that the P value was significant against (e.g., blue asterisks tested against AAV2-CAG-PLAP group in (C)).
Drosophila larval C3da neurons do not express AdoR hence they fail to respond to the gliotransmitter adenosine (Figure 2B and 2G). Similarly, ineffectiveness of glial DREADD stimulation on axon regeneration could be due to lack of gliotransmitter receptor expression on RGCs. One gliotransmitter in mouse retina is ATP,68,69 which can be converted to adenosine.45,46 All four mammalian adenosine receptors (i.e., Adora1, Adora2a, Adora2b and Adora3) are GPCRs, however, they differentially modulate neuronal excitability; Adora2a and Adora2b activate Gαs to excite neurons,8 whereas Adora1 and Adora3 activate Gαi to inhibit neuronal activity.70,71 Because Drosophila AdoR couples to Gαs and its activation increased neuronal activity (Figure 2C–2D),47 and because Drosophila AdoR shared the highest sequence similarity with Adora2a and Adora2b (54% and 50% respectively), Drosophila AdoR and mammalian Adora2a and Adora2b are close functional orthologs. By analyzing adenosine receptor expression in mouse RGCs using a published single-cell RNA-seq dataset,72 we found that Adora1 was broadly expressed, whereas Adora2a, Adora2b, and Adora3 were sparsely expressed at low levels. Next, we assessed the effect of ectopic expression of adenosine receptors in RGCs. As Adora2a and Adora2b are functionally similar, we chose to study Adora2b. We found that neuronal Adora2b expression by viral transfection (AAV2-hSyn-AdoRa2b) moderately increased the number of regenerated axons of RGCs, however, combining Adora2b expression and glial DREADD stimulation strongly promoted RGC axon regeneration (Figure 7B–7C). Therefore, stimulation of gliotransmitter release is capable of promoting RGC axon regeneration only if Adora2b was expressed. This is consistent with the notion that glial DREADD stimulation triggers release of adenosine (or ATP that is converted to adenosine), which is normally ineffective due to lack of Adora2b expression but could otherwise activate ectopically expressed Adora2b to promote RGC axon regeneration. Together, our findings in Drosophila and mice suggest a conserved role of gliotransmission in promoting axon regeneration.
Adora2b activation promotes RGC axon regeneration and survival
Could pharmacological activation of Adora2b promote axon regeneration? To test this, we expressed Adora2b in RGCs by intravitreal injection of AAV2-hSyn-Adora2b. 14 days later, we crushed the optic nerve, and intravitreally injected adenosine to activate Adora2b for another two weeks (Figure 7D). We found this treatment strongly promoted RGC axon regeneration (Figure 7E–7F). In contrast, adenosine injection alone was ineffective (Figure 7E–7F). Therefore, Adora2b expression is prerequisite for axon regeneration induced by either glial DREADD stimulation or adenosine. Inhibition of phosphatase and tensin homolog (PTEN), a negative regulator of mTOR, robustly promotes axon regeneration and cell survival of RGCs.12,19 Notably, we found that RGC axon regeneration induced by Adora2b activation was as strong as AAV2-hSyn-PTEN-shRNA-mediated PTEN knockdown (Figure 7E–7F). Furthermore, combining Adora2b activation and PTEN knockdown led to even stronger axon regeneration when compared to singular treatment (Figure 7E–7F), suggesting a synergistic effect. Optic nerve crush is followed by loss of ~80% RGCs.12 We found that Adora2b activation, but not adenosine alone, increased RGC survival (Figure S7B–S7C). This neuronal protection is further enhanced after combining Adora2b activation and PTEN knockdown, when compared to singular treatment (Figure S7B–S7C). Together, these results indicate that direct activation of Adora2b promotes axon regeneration and increases RGC survival.
Gαs-coupled adenosine receptors excite neurons,8 prompting us to determine whether neuronal activity could underlie RGC axon regeneration. We found that RGC axon regeneration induced by Adora2b activation was markedly impaired after viral transfection of inward rectifier potassium channel Kir2.1 (Figure 7G–7H). Similar effect was obtained after viral transfection of RasDN (Figure 7G–7H). Conversely, expression of RasCA facilitated RGC axon regeneration (Figure S7D–S7E). Therefore, like Drosophila, activation of Adora2b appears to promote RGC axon regeneration through neuronal activity and Ras.
DISCUSSION
Axon regeneration failure is a major hurdle for functional recovery after central nervous system lesion.1 In addition, Wallerian axon degeneration is associated with neurodegenerative diseases,73 hence, promoting axon regeneration in these diseases is of great interest. By parallel investigation of closely related Drosophila neuronal subtypes, we propose gliotransmission as a previously unknown mechanism by which glia could control axon regeneration. These findings expand the existing glial roles in axon regeneration. In a working model that summarizes our Drosophila studies (Figure S6I–S6K), ensheathing glia actively respond to axotomy and the resulting glial Ca2+ spikes lead to release of ATP/adenosine. Although adenosine could interact with all sensory neurons, the AdoR expression pattern ensures that C4da, but not C3da, neurons respond to adenosine. This glia-to-neuron signaling then mounts pro-regenerative programs in C4da neurons, including burst firing, Ca2+ spikes, and Ras activity, to promote axon regrowth of C4da neurons. Moreover, ectopic AdoR expression or activation of those regenerative programs in C3da neurons is sufficient to induce their axon regeneration.
How could axotomy elicit glial Ca2+ signals in Drosophila larvae? Glia are known to express neurotransmitter receptors, allowing them to ‘listen to’ neuronal activity and provide feedback modulation of neuronal activity through gliotransmitters.5 To assess whether neuronal activity could have an effect on glial Ca2+ signals, we applied AITC to activate C4da neurons.27,28 However, AITC did not evoke Ca2+ signals in ensheathing glia (Figure S3I–S3J), arguing against neuronal activity as a trigger of glial Ca2+ signals. These results support unidirectional signaling from glia to sensory neurons (Figure S6I–S6K). They also suggest that glial Ca2+ spikes are produced either cell-autonomously, or alternatively through a cell-non-autonomous mechanism that involves glial interactions with non-neuronal cells.
Burst firing observed in Drosophila larval C4da neurons is reminiscent of ectopic discharges of injured dorsal root ganglion (DRG) neurons, the mammalian counterpart of Drosophila larval PNS sensory neurons. As a hallmark of neuropathic pain,74 ectopic discharges of DRG neurons are also regulated by gliotransmission.75 This suggests that burst firing of C4da neurons could reflect nociceptive hypersensitivity after axotomy. Hence, one possible role of gliotransmission could be to sensitize injured C4da neurons. Taken together, gliotransmission appears to be related to both axon regeneration and nociceptive hypersensitivity. Future work is needed to determine whether gliotransmission could regulate DRG axon regeneration.
By stimulating Drosophila larval C3da neurons with different firing patterns, we provide direct evidence that firing patterns, instead of excitability, dictate the axon regenerative strength. A possible mechanism is that neuronal activity patterns are associated with different Ca2+ signals. For example, burst, but not tonic, firing evokes strong Ca2+ signals in C3da neurons (Figure 5H–5I), likely due to prolonged depolarization associated with burst firing. Previous studies indicate that stimulation of neuronal activity promotes mammalian axon regeneration,11,19,20 but the reported effects are variable.76 One possibility could be that the activity patterns are not controlled in these studies. In the future, it will be of interest to deliver precisely controlled neuronal activity patterns to determine their effects on axon regeneration in other experimental models.
We propose the Ca2+ spikes in Drosophila larval C4da neurons as a pro-regenerative signal with the following characteristics: they are neuronal type-specific, correlate with burst firing, exhibit delayed onset, persist throughout axon regeneration, and are evoked by axonal but not dendritic injury. Optogenetic stimulation of C3da neurons starting at ~6 hpa (i.e., after immediate Ca2+ transients) further indicates that Ca2+ spikes are sufficient to induce axon regeneration. Because Ca2+ signals with different spatiotemporal patterns activate diverse neuronal pathways,77 it is likely that Ca2+ spikes and immediately Ca2+ transients regulate different aspects of axon regeneration. While immediately Ca2+ transients could regulate growth cone formation,54 persistent Ca2+ spikes could maintain Ras activity at a high level for sustained axon regrowth. It will be interesting to determine whether axotomy could trigger Ca2+ spikes in other systems, and if so, their roles in axon regeneration.
Unlike CNS, mammalian PNS neurons such as DRG can regenerate their axons.78 Therefore, mammalian CNS and PNS neurons could be broadly defined as two neuronal groups bearing axon regenerative differences, although each group is highly heterogeneous and contains multiple neuronal subtypes. In this regard, differences between mammalian CNS and PNS are much larger than those between Drosophila C4da and C3da neurons. To explore whether Adora2b signaling could underlie regenerative differences of mammalian CNS and PNS neurons, we searched the published single-cell RNAseq dataset but found Adora2b was expressed at low levels in both CNS and DRG neurons.72,79,80 Hence, other mechanisms must exist to explain their regenerative differences. One such mechanism could be related to intrinsic growth abilities. For example, injury of mouse RGCs causes reduction of mTOR activity,12 in contrast, no reduction of mTOR activity is found after injury of DRG neurons.81,82
Glial cells in mammalian retina could release ATP, glutamate, and D-serine.83 Our mouse study indicates that stimulation of gliotransmitter release alone by DREADDs is ineffective but combining glial DREADD stimulation and neuronal Adora2b expression greatly enhanced axon regeneration (Figure 7B–7C). Because Adora2b is barely expressed in mouse retina, these results suggest that engineering RGCs to respond to the gliotransmitter adenosine is a prerequisite for the observed effect. Similarly, Drosophila C3da neurons do not express AdoR and engineering them to respond to adenosine induces axon regeneration (Figure 2J–2K). These analyses suggest that stimulation of gliotransmission could promote axon regeneration across species if Adora2b orthologs are expressed in neurons. Notably, Adora2b overexpression in RGCs alone moderately increased axon regeneration (Figure 7B–7C), suggesting that extracellular adenosine exists in injured retina. These results further suggest that in the absence of glial DREADD stimulation, axotomy could trigger basal adenosine release.
Adenosine exerts protective functions in different mammalian tissues including the nervous system,70 by triggering diverse signaling events. Extracellular adenosine is normally low but is rapidly increased after injury.70 As a classic neuromodulator, adenosine controls essential homeostatic functions such as sleep.84 However, whether adenosine could play a role in axon regeneration is largely unknown. Pharmacological Adora3 activation is shown to promote neurite outgrowth of cultured RGCs.85 In the current study, we pharmacologically activated Adora2b (Figure 7E–7F) and found this promotes axon regeneration and survival of RGCs in adult mice. The magnitude of RGC axon regeneration induced by Adora2b activation was comparable to that after PTEN knockdown and combining Adora2b activation with PTEN knockdown further enhanced axon regeneration. These findings suggest that targeting Adora2b signaling, either alone or in combination with other pro-regenerative pathways, is likely a strategy for mammalian CNS repair.
Limitations of the study
In our mouse study, we used DREADDs to stimulate gliotransmitter release.64–67 However, the spatiotemporal dynamics of Ca2+ elevations and gliotransmitter release are yet unknown and will be the focus of future studies. Moreover, the current study has focused on mouse retinal glial cells that are positive for GFAP, which is normally found in astrocytes.86 Future studies are needed to determine whether stimulation of gliotransmission from other retinal glial types such as Müller glia or microglia could have a similar role in RGC axon regeneration.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yang Xiang (yang.xiang@umassmed.edu).
Materials availability
Requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yang Xiang (yang.xiang@umassmed.edu).
Data and code availability
This paper does not report original code. All data used for analysis and figure generation are available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly strains
All fly stocks were raised in low yeast brown food, which was prepared by the University of Massachusetts Chan Medical School Drosophila Resource Facility. Flies were incubated at 25 °C and 60 % humidity with a 12 h: 12 h light dark cycle unless otherwise stated. ppk-Gal4 88, ppk-LexA,89 ppk-CD4-tdGFP,30 ppk-CD4-tdTomato,30 ppk-EGFP,24 19–12-Gal4,27 UAS-CD4-tdGFP,30 UAS-CD4-tdTomota,30 UAS-mCD8-GFP,27 UAS-Kir2.1,55 UAS-ChR2::EYFP,29 UAS-TrpA1-B,28 UAS-AdoR47 (a gift from Drs. Norbert Perrimon and Michal Zurovec) were described previously. repo-Gal4 (BL#7415), repo-QF (BL#66477), nrv2-Gal4 (BL#6800), NompC-Gal4 (BL#36361), NompC-LexA (BL#52241), LexAop2-mCherry(BL#52271), QUASTNT (BL#91808), UAS-EYFP (BL#6659), UAS-EGFP (BL#5430, BL#5431), UAS-GCaMP6s (BL#42746, BL#42749), UAS-ChR2 (BL#52258), UAS-CsChrimson::mCherry (#BL82181), UAS-Parvalbumin (BL#25030), UAS-Ras85D.N17 (BL#4845), UAS-ras.N17 (BL#4846), UAS-Ras85D.V12 (BL#4847, BL#64195), UAS-shibirets1 (BL#66000), UAS-TNT (BL#28838), AdoR deletion mutant (AdoR−/−, BL#84447), AdoR-t2A-Gal4 (BL#86138) were from Bloomington Drosophila Stock Center. LexAop2-myr-GCaMP6s, moody-Gal4, NP6293-Gal4 were gifts from Dr. Marc Freeman (Oregon Health and Science University). A complete list of fly stocks used can be found in the key resource table. All detailed cross information was provided in Supplementary Data.
KEY RESOURCE TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling | #4370, RRID:AB_2315112 |
| Goat anti-rabbit Alexa Fluor Plus 647 | Jackson Immunoresearch | #111-605-144, RRID:AB_2338078 |
| Mouse anti-Tuj1 (tubulin beta 3) | BioLegend | #801202, RRID:AB_10063408 |
| Donkey anti-mouse Alexa Fluor Plus 488 | Abcam | #ab150109, RRID:AB_2571721 |
| Rat anti-GFAP | Thermo Fisher | #13-0300, RRID:AB_2532994 |
| Donkey anti rat Dylight™ 650 | Thermo Fisher | # A5-10029, RRID:AB_2556609 |
| Rabbit anti-RFP | Abcam | #ab34771, RRID:AB_777699 |
| Donkey anti-rabbit Alexa Fluor Plus 555 | Thermo Fisher | #A-31572, RRID:AB_162543 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Paraformaldehyde | Sigma | P6148 |
| Mounting media | Thermo Fisher | P36961 |
| Triton X-100 | Fluka | BP151 |
| Normal donkey serum | Sigma | D9663 |
| Phosphate buffered saline (powder) | Sigma | P3813 |
| Bovine serum albumin | Sigma | A2058 |
| All-trans retinal | Sigma | R2500 |
| Agarose | Thermo Fisher | BP160-500 |
| Sevoflurane | Patterson Veterinary | NDC-14043-110-25 |
| Adenosine | Sigma | A9251 |
| L-glutamate | Sigma | G1251 |
| D-serine | Sigma | S4250 |
| Cholera Toxin Subunit B, Alexa Fluor 555 Conjugate (CTB-555) | Thermo Fisher | C34776 |
| Tetrahydrofuran | Sigma | 186562 |
| Dichloromethane | Sigma | 270997 |
| DiBenzy Ether | Sigma | 108014 |
| Optimal cutting temperature compound | Sakura Finetek | 4583 |
| Recombinant DNA | ||
| pAAV-CAG-Kir2.1-T2A-tdTomato | CEBSIT Viral core | Addgene #60598 |
| pAAV-CAG-PLAP | CEBSIT Viral core | N/A |
| pAAV-EF1a-RasCA | CEBSIT Viral core | N/A |
| pAAV-EF1a-RasDN | CEBSIT Viral core | N/A |
| pAAV-GFAP-hM3D(Gq)-mCherry | CEBSIT Viral core | Addgene #50478 |
| pAAV-hSyn-Adora2b | CEBSIT Viral core | N/A |
| pAAV-hSyn-PTEN-shRNA | CEBSIT Viral core from Zukor et al., 2013 87 | N/A |
| Experimental Models: Organisms/Strains | ||
| Drosophila, ppk-Gal4 | Han et al., 2012 88 | N/A |
| Drosophila, ppk-LexA | Gou et al., 2014 89 | N/A |
| Drosophila, NompC-Gal4 | Bloomington Drosophila Stock Center | #BL36361 |
| Drosophila, ppk-CD4-tdGFP | Han et al., 2011 30 | N/A |
| Drosophila, ppk-CD4-tdTomato | Han et al., 2011 30 | N/A |
| Drosophila, UAS-CD4-tdGFP | Han et al., 2011 30 | N/A |
| Drosophila, UAS-CD4-tdTomato | Han et al., 2011 30 | N/A |
| Drosophila, UAS-mCD8-GFP | Xiang et al., 2010 27 | N/A |
| Drosophila, ppk-EGFP | Grueber et al., 2003 24 | N/A |
| Drosophila, LexAop2-myr-GCaMP6s | Laboratory of Marc Freeman | N/A |
| Drosophila, 19-12-Gal4 | Xiang et al., 2010 27 | N/A |
| Drosophila, repo-Gal80 | Awasaki et al., 2008 90 | N/A |
| Drosophila, QUAS-TNT | Bloomington Drosophila Stock Center | #B91808 |
| Drosophila, NP6293-Gal4 | Awasaki et al., 2008 90 from Laboratory of Marc Freeman | N/A |
| Drosophila, moody-Gal4 | Bainton et al., 2005 91 from Laboratory of Marc Freeman | N.A |
| Drosophila, nrv2-Gal4 | Bloomington Drosophila Stock Center | #B6800 |
| Drosophila, UAS-shibire ts1 | Bloomington Drosophila Stock Center | #B66000 |
| Drosophila, repo-QF | Bloomington Drosophila Stock Center | #B66477 |
| Drosophila, repo-Gal4 | Bloomington Drosophila Stock Center | #B7415 |
| Drosophila, NompC-LexA | Bloomington Drosophila Stock Center | #BL52241 |
| Drosophila, LexAop2-mCherry | Bloomington Drosophila Stock Center | #BL52271 |
| Drosophila, UAS-2×EYFP | Bloomington Drosophila Stock Center | #BL6659 |
| Drosophila, UAS-2×EGFP | Bloomington Drosophila Stock Center | #BL5430, #BL5431 |
| Drosophila, UAS-GCaMP6s | Bloomington Drosophila Stock Center | #BL42746, #BL 42749 |
| Drosophila, UAS-TrpA1-B | Gu et al., 2019 28 | N/A |
| Drosophila, UAS-AdoR | Dolezelova et al., 2007 47 | N/A |
| Drosophila, UAS-Kir2.1 | Yang et al., 2014 55 | N/A |
| Drosophila, UAS-ChR2::EYFP | Hwang et al., 2007 29 | N/A |
| Drosophila, UAS-ChR2 | Bloomington Drosophila Stock Center | #BL52258 |
| Drosophila, UAS-CsChrimson::mCherry | Bloomington Drosophila Stock Center | #BL82181 |
| Drosophila, UAS-Parvalbumin | Bloomington Drosophila Stock Center | #BL25030 |
| Drosophila, UAS-Ras85D.N17 | Bloomington Drosophila Stock Center | #BL4845 |
| Drosophila, UAS-ras.N17 | Bloomington Drosophila Stock Center | #BL4846 |
| Drosophila, UAS-Ras85D.V12 | Bloomington Drosophila Stock Center | #BL4847, #BL64195 |
| Drosophila, UAS-TNT | Bloomington Drosophila Stock Center | #BL28838 |
| Drosophila, AdoR-t2A-Gal4 | Bloomington Drosophila Stock Center | #BL86138 |
| Drosophila, AdoR−/− (deletion mutant) | Bloomington Drosophila Stock Center | #BL84447 |
| Mouse: C57BL/6J | Shanghai Laboratory Animal Center, Chinese Academy of Sciences | N/A |
| Software and Algorithms | ||
| MATLAB 2016b | Mathworks | https://www.mathworks.com/ |
| pClamp 10 | Molecular Devices | https://www.moleculardevices.com/ |
| GraphPad Prism 9 | GraphPad | https://www.graphpad.com |
| Zen | Zeiss | N/A |
| ImageJ | https://imagej.nih.gov/ij/ | N/A |
| BASIC Stamp editor v2.5 | www.parallax.com | N/A |
| Adobe Illustrator | www.adobe.com | N/A |
| Adobe Premiere | www.adobe.com | N/A |
| Other | ||
| Drosophila low yeast brown food | Drosophila Resource Facility at UMassMed | https://www.umassmed.edu/research/cores/drosophila-resource-facility/media-recipes/ |
| DigiTherm 38-liter Heating/Cooling Incubator (for optogenetic experiment) | Tritech research | DT2-MP-47L |
| Dissection scope | Zeiss | Discovery.V8 |
| UVC crosslinker | Spectroline | Spectorlinker, 254 nm, XL-1000 |
| Hand-held UV spectrophotometer | Spectroline | AccuMAX XS-254 |
| Two-photon microscope | Zeiss | LSM 7 MP |
| Confocal fluorescence microscope | Zeiss | LSM 700 |
| Fluorescence microscope | Zeiss | Axio Examiner.D1 |
| Objective W Plan-Apochromat 20x/1.0 DIC M27 75mm parfocal length 75mm (FWD=1.8mm) | Zeiss | 421452-9800 |
| Objective W N-Achroplan 40x/0.75 M27 (FWD=2.1mm) | Zeiss | 420967-9900 |
| Compact light source (Mercury lamp) | Zeiss | HXP-120 V |
| Microelectrode puller | Sutter | P-97 |
| Borosilicate glass capillary | World Precision Instrument | TW150-4 |
| 470 nm blue LED, mounted on a 10mm square coolbase | LUXEON Rebel LED | SR-05-B0040 |
| 700mA, buckpuck DC driver | LUXEON Rebel LED | 3023-D-E-700 |
| Carclo 29.8° frosted 10mm circular beam optic-integrated legs | LUXEON Rebel LED | 10413 |
| 50mm square × 25mm high alpha heat sink | LUXEON Rebel LED | N50-25B |
| Hand-held optical meter | Newport | 1918-R |
| BASIC Stamp2.0 microcontroller | Parallax | N/A |
| Digidata 1440 | Molecular Devices | https://www.moleculardevices.com |
| Multiclamp 700A Amplifier | Molecular Devices | https://www.moleculardevices.com |
| T-type thermal coupler | Omega | 5SRTC-TT-T-36-36 |
| Thermometer | Physitemp | BAT-10 |
Constructs
The AAV-CAG-PLAP vector was cloned by Vigene Sciences Inc. The AAV-CAG-Kir2.1-T2A-tdTomato (from Addgene #60598), AAV-EF1a-RasCA, AAV-EF1a-RasDN, AAV-GFAP-hM3D(Gq)-mCherry (from Addgene #50478), AAV-hSyn-Adora2b, AAV-hSyn-PTEN-shRNA vectors were cloned by Vectorbuilder Inc.
METHOD DETAILS
In vivo two-photon laser axotomy
Axotomy was performed at 80–86 hours after egg laying (AEL). We measured the axon regrowth between 24 hpa and 48 hpa time points as regeneration. We followed the previous protocol with several modifications.17 Briefly, a larva was anesthetized with sevofluorane (Patterson Veterinary, NDC-14043–110-25) vapor for 3 min with dorsal side up. Axotomy was achieved using the bleaching function of a Zeiss LSM 7 MP two-photon microscope by localized axon injury (laser spot is ~1.5 μm in diameter). We found 910 nm wavelength worked well for axotomy. Axons were severed at a point around half of the distance between the soma of C3da or C4da neurons and the soma of bipolar dendrite (BD) neurons (around 40–70 μm to the soma of C3da or C4da neurons). Following axotomy, larvae were recovered on damp Kimwipe paper and then were transferred to recovery vials containing regular brown food (for axon regeneration, Ca2+ imaging and electrophysiology) or white grape juice agar plates (for optogenetic induction of axon regeneration). For larvae expressing shibirets in glia cells, larvae were raised at 29 °C between 24 hpa to 48 hpa to inhibit gliotransmission.
In vivo axon regeneration analysis
Quantitative analysis was performed as described previously.17,22 Briefly, the proximal axon length of C3da or C4da neurons were measured as L1 at 24 hpa and L2 at 48 hpa. Regeneration Length (L2-L1) is the increase of proximal axon length. The distances between the soma of C3da or C4da neurons and the soma of BD neurons were also measured as BD1 at 24 hpa and BD2 at 48 hpa to account for larval body size expansion during this period. Regeneration Index is calculated as: L2/BD2-L1/BD1.
Electrophysiology
Extracellular recording of C4da or C3da neurons was performed as described previously.27 The ex vivo fillet preparation from 3rd instar larvae was prepared at 24 hpa in external saline composed of (in mM): NaCl 120, KCl 3, MgCl2 4, CaCl2 1.5, NaHCO3 10, trehalose 10, glucose 10, TES 5, sucrose 10, HEPES 10. Osmolality was 305 mOsm/kg. pH was adjusted to 7.25 by NaOH. The larval fillet preparation was mounted on a Zeiss Axio Examiner.D1 microscope with a water immersion objective lens (Objective W N-Achroplan 40x/0.75). Gentle negative pressure was applied to trap the neuronal cell body in a recording pipette (5 μm tip opening; 1.5–2.0 MΩ resistance) filled with the external saline. Recordings were performed with a Molecular Devices 700A amplifier, and the data were acquired with a Molecular Devices 1440A Digidata and Clampex 10.6 software (Molecular Devices). Extracellular recordings were obtained in voltage clamp mode with a holding potential of 0 mV, a 2 kHz low-pass filter and a sampling frequency of 20 kHz. Burst firing events were detected by the ‘Burst Analysis’ function in Clampfit 10.6 and were defined as a cluster of 5 or more consecutive action potentials, with an inter-spike interval of adjacent two action potentials less than 100 ms. The number of burst firing events divided by total recording duration (minute) was quantified as the frequency of burst firing events (bursts/min).
GCaMP imaging
Ca2+ signals of sensory neurons and ensheathing glia were measured by GCaMP6s imaging in larval fillet preparation as described previously28 and in the same external saline used for electrophysiology. Time-lapse imaging was performed using the Zeiss LSM 700 confocal microscope, under a water immersion objective lens (Objective W Plan-Apochromat, 20x/1.0). Frame scan rate is 0.97 Hz. GCaMP signals were obtained from regions of interest (ROI) from soma, dendrite or axon for neurons or from glial branches. They are then corrected for horizontal drifting with ImageJ slice alignment plugin before analysis. Non-axotomized C3da or C4da neurons imaged from the same larvae were used as control for axotomized C3da or C4da neurons. Glia that ensheath the non-axotomized neurons from the same larvae were used as control for glia that ensheath the axotomized neurons. For imaging Ca2+ signals of C4da neurons after dendrite severing, a primary dendrite branch of C4da neuron was severed by two-photon microscope laser, similar to axotomy, and GCaMP signals of C4da neurons were measured at 24 hours after dendrite injury. For imaging Ca2+ signals of C4da neurons after UV-C treatment,50 larvae were mounted dorsal side up with double-sided tape on a cover slide and mounted in an ultraviolet crosslinker (Spectroline, XL-1000). 20 mJ/cm2 254 nm UV-C light was delivered. After treatment, larvae were recovered in regular food and GCaMP signals of C4da neurons were measured at 24 hours after UV-C illumination. For imaging ChR2-expressing C3da neuron responses to blue light stimulation, the average fluorescence signals of soma from 10 s before light stimulation was set as F0. The peak fluorescence signal during light stimulation was set as F. The response was quantified as ΔF/F0=(F-F0)/F0*100%. For imaging TrpA1-B-expressing ensheathing glial responses to thermogenetic stimulation, 3rd instar larval fillet was prepared in a customized silicone elastomer perfusion chamber. Pre-heated hot water (46 °C) was perfused through the inner cavity of the stainless-steel tube embedded in the perfusion chamber to apply heat stimulation. The saline temperature in the chamber was monitored by a T-type thermal coupler (Omega 5SRTC-TT-T-36–36) connected with a thermometer (Physitemp BAT-10) and recorded by the Clampex software (Molecular Devices). It took 40 to 60 s to elevate from room temperature (~20 °C) to 30 °C. Temperature was then held at a value between 30 °C and 34°C for 2 min. Peak GCaMP signals of glial branch during this 2-min time-window were calculated as F. Average GCaMP signals in a 1-minute time-window before heating were calculated as F0. Glial responses were quantified as ΔF/F0 =(F-F0)/F0*100%. Larvae were raised at 21°C, a temperature point below the TrpA1-B activation threshold.28 For imaging C4da and C3da neuronal responses to thermogenetic activation of ensheathing glia, heating process was performed similarly to imaging TrpA1-B-expressing ensheathing glial responses. Temperature was held at a value between 30 °C and 34°C for 2 min. Peak somatic GCaMP signals during this 2-min time-window were calculated as F. Average GCaMP signals in a 1-min time-window before heating were calculated as F0. Neuronal responses were quantified as ΔF/F0=(F-F0)/F0*100%. For imaging C4da neuron responses to optogenetic stimulation of ensheathing glia, average somatic GCaMP6s signals from 10 s before light stimulation were set as F0, and peak somatic GCaMP6s signals during light stimulation were set as F. The response was quantified as ΔF/F0=(F-F0)/F0*100%. 555 nm green light pulses (500 ms on, 500 ms off, 5.0 mW/mm2) were repeated 10 times to stimulate glia. Larvae were raised in food containing 400 μM all-trans retinal (ATR, Sigma, R2500) at 25 °C in constant dark. For testing the latency between light stimulation of glia and neuronal response, 1s, 2s or 3s 555 nm 50.8 mW/mm2 green light pulses were used to stimulate glia. For imaging responses of C4da and C3da neurons to adenosine, L-glutamate and D-serine, a 5-min time-window before and after drug perfusion was used to determine the frequency of neuronal Ca2+ spikes. For imaging ensheathing glial responses to AITC, average GCaMP signals of glial branches from 1min before AITC application was set as F0, and average GCaMP signals of glial branches from 2 min to 3 min after AITC application was set as F. The response was quantified as ΔF/F0=(F-F0)/F0*100%.
Analysis of Ca2+ spike frequency
Time-lapse GCaMP signals (F) from C4da or C3da somatic regions were normalized to average intensity (F0) of each sample by (F-F0)/F0*100%. After normalization, we used the Findpeaks function in Matlab to find local maxima to isolate Ca2+ spike peaks from background. GCaMP signals from non-axotomized C4da neurons also show weak Ca2+ spikes with small amplitude. To rule out these weak spikes and isolate strong spikes, we calculated standard deviation (σ) of GCaMP signals in each non-axotomized C4da neuron somatic regions, then calculated the mean value of σ (defined as σ’). We set minimum peak amplitude parameter in Findpeaks as three folds of σ’ (3σ’), and only amplitude larger than 3σ’ is considered as a Ca2+ spike. After acquiring the position, number and amplitude of each spike, we divided the number of spikes by the time duration to calculate Ca2+ spike frequency (Ca2+ spikes/min).
Optogenetic stimulation of axon regeneration in vivo
The setup for optogenetic stimulation of free moving larvae was modified from the previous report.92 Larvae were raised in food containing 400 μM ATR at 25 °C in constant dark in the fly incubator (Tritech research, DT2-MP-47L). At 80–82 hours AEL, early 3rd instar larvae were subject to laser axotomy. Larvae were allowed to recover for 6h in darkness. Next, we transferred larvae to a 35 mm petri dish plate containing 1 mL solidified transparent grape agar. The plate was covered with transparent film. The formula of the agar plate was: 2.4 g agar, 2.6 g sucrose, 20 mL white grape juice, 2 ml 95% ethanol, then add ddH2O to 100 mL final volume. White grape juice was chosen to facilitate light penetration. To supplement ATR, grape agar was melted by microwave, and ATR was added to a final concentration of 400 μM after cooling down to 55 °C. The ATR-containing grape agar plates were freshly prepared, stored at 4 °C in dark, and used within 1–2 days. 470 nm blue LED (LUXEON Rebel LED, SR-05-B0040) was mounted on a 10 mm square coolbase and 50 mm square ×25 mm high alpha heat sink (LUXEON Rebel LED, N50–25B), set under circular beam optic with integrated legs (LUXEON Rebel LED, 10413) for even light illumination, and finally placed over the grape juice agar plate for optogenetic stimulation. The light pattern was programmed with BASIC Stamp 2.0 microcontroller and buckpuck DC driver (LUXEON, 700 mA, externally dimmable, 3023-D-E-700). Light intensity (1.4 mW/mm2 470 nm blue light) was measured by a power meter (Newport, 1918-R). Free moving larvae in the grape juice agar plate were continuously stimulated with three different parameters that elicited burst, semi-burst, and tonic firing in C3da neurons. At 24 and 48 hpa, axon lengths were imaged and measured by confocal microscope and regeneration index was calculated.
Immunostaining of Drosophila larvae
For immunostaining with the antibody against dpERK, 3rd instar larvae were dissected in ice-cold external saline used for electrophysiology. Between each of the following treatment, fillets were washed 4 times of 15 min, each with washing buffer containing 0.3% Triton X-100 (Fluka, BP151) in phosphate buffered saline (PBS, Sigma). Larvae were firstly fixed with PBS containing 4% paraformaldehyde (PFA, Sigma) for 30 min. Then larvae were treated with blocking buffer for 1h at room temperature. The blocking buffer was PBS containing 0.3% Triton X-100, 5% donkey serum (Sigma, D9663), and 0.1% bovine serum albumin (Sigma). Next, larvae were incubated in rabbit anti-Phospho-p44/42 Erk1/2 (Thr202/Tyr204) primary antibody (Cell signaling, rabbit, #4370S, RRID:AB_2315112, 1:100 in blocking buffer) for 18 hours at 4°C, and then in the goat anti-rabbit Alexa 647-conjugated secondary antibody (Jackson immunoresearch, 111–605-144, RRID:AB_2338078, 1:500 in blocking buffer) for 2 hours at room temperature. After final wash, larvae were mounted using the anti-fade mountant (Thermo Fisher, P36961) prior to confocal imaging. For dpERK immunostaining after AITC stimulation, larvae were treated with external saline containing 100 μM AITC for 15 min before proceeding with the above immunostaining protocol. For dpERK immunostaining of C3da neurons after burst firing stimulation via optogenetics, dissected larvae were illuminated by 300ms/15s, 1.4mW/mm2 470nm blue light stimulation for 20 minutes immediately before PFA fixation.
Intravitreal injection and optic nerve crush
Intravitreal viral or drug injection, optic nerve crush and RGC axon labeling were performed as previously described.12 Briefly, under anesthesia, rAAV2 (2 μL), adenosine (10 μM in 2 μL PBS, Sigma), PBS (2 μL) or Alexa 555-conjugated cholera toxin beta subunit (CTB-555, 1 mg/ml; 2 μL; Thermo Fisher) was injected intravitreally with a fine glass micropipette connected to the Hamilton syringe using plastic tubing. The position and direction of the injection were well controlled to avoid lens damage. Clozapine-N-oxide (CNO) was administrated twice daily by intraperitoneal injection (1 mg/kg for the hM3D(Gq) group) starting from the optic nerve crush. For optic nerve crush in anesthetized animals, the optic nerve was exposed intraorbitally and crushed using a pair of Dumont #5 forceps (Fine Science Tools) for 5 s at approximately 1 mm behind the optic disc at 14 days after AAV2 injection. Adenosine or PBS was injected intravitreally for 5 times every 3 days after optic nerve crush. CTB-555 injection was performed 2 days before euthanasia to trace regenerating RGC axons. At the final step of the experiment procedure, both retina and the optic nerve were dissected out and post-fixed in 4% PFA overnight at 4°C for further axon regeneration and neuronal survival quantifications.
Virus production
All viruses were packaged by the virus core in CEBSIT (Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China). All virus used had titers > 1 × 1013.
Optic nerve dehydration and clearing
Dehydration and clearing of optic nerves were performed following previous reports.93,94 Briefly, fixed optic nerves were first dehydrated in incremental concentrations of tetrahydrofuran (TFH, 50%, overnight; 80%, 1 hour and 100%, 2 hours, v/v % in distilled water, Sigma) in amber glass bottles with a silicon-coated cap (Thermo Fisher). Incubations were performed on a shaker at room temperature. Then the nerves were incubated in Dichloromethane (DCM, Sigma) until the nerves sinks at the bottom (5 min to 1 hour max), then in DiBenzyl Ether (Sigma) until the sample is clear (20 min to 2 hours). The nerves were protected from light during the whole process to avoid potential photo bleaching of the fluorescence.
Analysis of RGC axon regeneration
The cleared whole-mount optic nerves were imaged with a 10x objective lens on a Nikon C2 confocal microscope equipped with a motorized stage and Nikon software. For each optic nerve, we used the Z-stack function to acquire a stack of 8.17-μm-thick slices, with 60% overlap between adjacent slices. The slices were then stitched to obtain an image of the whole-mount nerve. Quantification of regenerating axons in the optic nerve was performed as previous described.12,95 Specifically, every 10 consecutive slices were Z-projected with maximum intensity to generate a series of Z-projection images of 81.7-μm-thick optical sections. At every 250-μm interval from the crush site, we counted the number of individual CTB-labeled axons and measured the width of the nerve at each optical section site. Both numbers were used to calculate the number of axons per micrometer of nerve width, which was then averaged over all optical sections as reported previously.95 Σad, the total number of axons extending distance d in a nerve with a radius of r, was estimated by summing over all optical sections with a thickness of t (81.7 μm): Σad = πr2 × (average axons/μm)/t.
Immunostaining of mouse retina
Following overnight incubation in 30% sucrose, the whole retina was first incubated in optimal cutting temperature (OCT) compound for cryosection and then was sectioned for staining. The cryosections were washed with PBS for 3 times of 15 min, and then blocked in PBS containing 2% bovine serum albumin and 0.1% Triton X-100 for 1 hour. We immunostained the retina neurons with mouse anti-Tuj1 (tubulin beta 3) primary antibody (BioLegend, #801202, RRID: AB_10063408) and donkey anti-mouse Alexa 488-conjugated secondary antibody (abcam, #150109, RRID: AB_2571721) to examine the number of surviving RGCs after injury. Fluorescent images were acquired with a 20x objective on a Zeiss Imager M2 microscope. RGC numbers were counted from at least three 20 μm thick retinal sections per retina (approximately 500–800 μm per section) using the cell counter plugin from ImageJ software. We calculated the linear density of Tuj1 positive cells in the ganglion cell layer (GCL) and normalized these counts to a standard intact control non-injured retina, as reported previously.12,95 For the efficiency of AAV2-GFAP-hM3D(Gq)-mCherry infection, we immunostained the retina with rat anti-GFAP primary antibody (Thermo Fisher, #13–0300, RRID:AB_2532994) and donkey anti-rat Dylight™ 650-conjugated secondary antibody (Thermo Fisher, #A5–10029, RRID:AB_2556609) as well as rabbit anti-RFP primary antibody (Abcam, #ab34771, RRID: AB_777699) and donkey anti-rabbit Alexa 555-conjugated secondary antibody (Thermo Fisher, A-31572, RRID: AB_162543). Fluorescent images were acquired with a 20x objective on a Zeiss Imager M2 microscope.
Quantification and statistics
Data were represented as mean ± S.E.M. in point graphs. All statistical analyses and graph making were performed using GraphPad Prism 9 (GraphPad Software). We did not pre-determine sample sizes and our sample sizes are like those reported in previous publications.17,22 Mann-Whitney rank-sum test or Student’s t-test was performed for comparisons between two groups of samples. Kruskal-Wallis statistic test followed by Dunn’s multiple comparison tests or one-way ANOVA followed by Bonferroni’s, Dunnett’s or Tukey’s multiple comparison tests were performed for comparisons among three or more groups of samples. Two-tailed unpaired t-tests were performed with Welch’s correction to adjust for unequal variance. Two-sided Wilcoxon matched-pairs signed-rank test was used to analyze data that did not follow normal distribution. The sample sizes of ‘n’ are provided in the figure legends. Error bar are mean±SEM across all graph panels. All detailed statistical analyses are provided in Table S1.
Supplementary Material
Table S1. Detailed fly crossing information, sample size number and statistics in each main figure panel and supplemental figure panel, related to Figure 1–7 and Figure S1–S7.
Video S1. GCaMP6s imaging showing that glial thermogenetic stimulation activates non-axotomized C4da neurons, related to Figure 2. A single C4da neuron was labeled by ppk-LexA>LexAop2-myr-GCaMP6s. TrpA1-B was expressed in glia. Scale bar, 20 μm.
Video S2. GCaMP6s imaging showing that glial thermogenetic stimulation did not activate non-axotomized C3da neurons, related to Figure 2. A single C3da neuron was labeled by NompC-LexA>LexAop2-myr-GCaMP6s. TrpA1-B was expressed in glia. Scale bar, 20 μm.
Video S3. GCaMP6s imaging of Ca2+ signals of non-axotomized C4da neurons, related to Figure 3. A single C4da neuron was labeled by ppk-LexA>LexAop2-myr-GCaMP6s. Scale bar, 20 μm.
Video S4. GCaMP6s imaging of Ca2+ signals of axotomized C4da neurons at 24 hpa, related to Figure 3. A single axotomized C4da neuron is labeled by ppk-LexA>LexAop2-myr-GCaMP6s. Each Ca2+ spike was highlighted by beep sound. Scale bar, 20 μm.
Video S5. Simultaneous extracellular recording and GCaMP6s imaging of an axotomized C4da neuron at 24 hpa, related to Figure 3. A single axotomized C4da neuron is labeled by ppk-LexA>LexAop2-myr-GCaMP6s. The soma region is defined as ROI for GCaMP6s signals. Scale bar, 20 μm.
HIGHLIGHTS.
Axotomy elicits Ca2+ signals in Drosophila ensheathing glia
Gliotransmission elicits neuron subtype-specific regenerative programs in Drosophila
Firing patterns but not overall excitability dictates the axon regeneration outcome
Stimulation of gliotransmission or Adora2b activation promotes RGC axon regrowth
ACKNOWLEDGEMENTS
Authors are grateful to Dr. B. Ye for help with in vivo optogenetic stimulation, Drs. M. Zurovec, N. Perrimon, C. Collins, C. Wu and A. Bergmann for reagents; Q. Wen and G. Dai for help on Matlab coding; Y. Peng for RNAseq data analysis. Authors also thank Drs. B. Ye, W. Ge, A. Byrne, M. Francis, and K. Thompson-Peer for critical comments on the manuscript; Y. Song for advice on laser axotomy; Bloomington and Vienna Drosophila Resource Center for fly stocks. S.Z. is supported by National Major Project of Research and Development (2022YFA1105500). J.W. is supported by National Natural Science Foundation of China (32200794). Y.L. is supported by National Natural Science Foundation of China (92168105) and Shanghai Municipal Science and Technology Major Project No. 2018SHZDZX05. Y.X. is supported by National Institutes of Health grants R01NS126821, Whitehall Foundation, Martina Stern Memorial Fund, and Dan and Diane Riccio Fund for Neuroscience.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He Z, and Jin Y (2016). Intrinsic Control of Axon Regeneration. Neuron 90, 437–451. 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Yiu G, and He Z (2006). Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7, 617–627. 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filbin MT (2003). Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature reviews. Neuroscience 4, 703–713. 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett JW, and Asher RA (1999). The glial scar and central nervous system repair. Brain Res Bull 49, 377–391. 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Haydon PG (2001). GLIA: listening and talking to the synapse. Nat Rev Neurosci 2, 185–193. 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 6.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, and Volterra A (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazargani N, and Attwell D (2016). Astrocyte calcium signaling: the third wave. Nat Neurosci 19, 182–189. 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 8.Lezmy J, Arancibia-Carcamo IL, Quintela-Lopez T, Sherman DL, Brophy PJ, and Attwell D (2021). Astrocyte Ca(2+)-evoked ATP release regulates myelinated axon excitability and conduction speed. Science 374, eabh2858. 10.1126/science.abh2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Stork T, Bergles DE, and Freeman MR (2016). Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432. 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, and Haydon PG (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science (New York, N.Y.) 310, 113–116. 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, Brecha NC, Kerschensteiner D, and He Z (2019). Elevating Growth Factor Responsiveness and Axon Regeneration by Modulating Presynaptic Inputs. Neuron 103, 39–51 e35. 10.1016/j.neuron.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, and He Z (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, and He Z (2011). Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375. 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang Z, Zhu J, Chen B, Williams PR, Zhang Y, et al. (2017). A Sensitized IGF1 Treatment Restores Corticospinal Axon-Dependent Functions. Neuron 95, 817–833 e814. 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan X, Qiao M, Bei F, Kim IJ, He Z, and Sanes JR (2015). Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256. 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, and Chisholm AD (2007). Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci U S A 104, 15132–15137. 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, and Jan YN (2012). Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes & development 26, 1612–1625. 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canty AJ, Huang L, Jackson JS, Little GE, Knott G, Maco B, and De Paola V (2013). In-vivo single neuron axotomy triggers axon regeneration to restore synaptic density in specific cortical circuits. Nat Commun 4, 2038. 10.1038/ncomms3038. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Yang C, Zhang L, Gao X, Wang X, Liu W, Wang Y, Jiang S, Wong YH, Zhang Y, and Liu K (2016). Promoting axon regeneration in the adult CNS by modulation of the melanopsin/GPCR signaling. Proc Natl Acad Sci U S A 113, 1937–1942. 10.1073/pnas.1523645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, and Huberman AD (2016). Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci 19, 1073–1084. 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson-Peer KL, DeVault L, Li T, Jan LY, and Jan YN (2016). In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev 30, 1776–1789. 10.1101/gad.282848.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL, et al. (2019). The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 102, 373–389 e376. 10.1016/j.neuron.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jan YN, and Jan LY (2001). Dendrites. Genes Dev 15, 2627–2641. 10.1101/gad.916501. [DOI] [PubMed] [Google Scholar]
- 24.Grueber WB, Ye B, Moore AW, Jan LY, and Jan YN (2003). Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Current biology : CB 13, 618–626. 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 25.Yadav S, Younger SH, Zhang L, Thompson-Peer KL, Li T, Jan LY, and Jan YN (2019). Glial ensheathment of the somatodendritic compartment regulates sensory neuron structure and activity. Proceedings of the National Academy of Sciences of the United States of America 116, 5126–5134. 10.1073/pnas.1814456116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney TM, and Freeman MR (2014). Drosophila models of neuronal injury. ILAR J 54, 291–295. 10.1093/ilar/ilt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, and Jan YN (2010). Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926. 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu P, Gong J, Shang Y, Wang F, Ruppell KT, Ma Z, Sheehan AE, Freeman MR, and Xiang Y (2019). Polymodal Nociception in Drosophila Requires Alternative Splicing of TrpA1. Curr Biol 29, 3961–3973 e3966. 10.1016/j.cub.2019.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, and Tracey WD (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Current biology : CB 17, 2105–2116. 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han C, Jan LY, and Jan YN (2011). Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci U S A 108, 9673–9678. 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu P, Wang F, Shang Y, Liu J, Gong J, Xie W, Han J, and Xiang Y (2022). Nociception and hypersensitivity involve distinct neurons and molecular transducers in Drosophila. Proc Natl Acad Sci U S A 119, e2113645119. 10.1073/pnas.2113645119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong J, Chen J, Gu P, Shang Y, Ruppell KT, Yang Y, Wang F, Wen Q, and Xiang Y (2022). Shear stress activates nociceptors to drive Drosophila mechanical nociception. Neuron 110, 3727–3742 e3728. 10.1016/j.neuron.2022.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, and Jan YN (2013). Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, and McCarthy KD (2008). What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946. 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, and Volterra A (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature neuroscience 7, 613–620. 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 37.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, and Volterra A (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nature neuroscience 10, 331–339. 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 38.Perea G, and Araque A (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science (New York, N.Y.) 317, 1083–1086. 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 39.Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, and Robitaille R (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Park A, Croset V, Otto N, Agarwal D, Treiber CD, Meschi E, Sims D, and Waddell S (2022). Gliotransmission of D-serine promotes thirst-directed behaviors in Drosophila. Curr Biol 32, 3952–3970 e3958. 10.1016/j.cub.2022.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney ST, Broadie K, Keane J, Niemann H, and O’Kane CJ (1995). Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351. 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 42.Potter CJ, Tasic B, Russler EV, Liang L, and Luo L (2010). The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141, 536–548. 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand AH, and Perrimon N (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 44.Kitamoto T (2001). Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol 47, 81–92. 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 45.Boison D, Chen JF, and Fredholm BB (2010). Adenosine signaling and function in glial cells. Cell Death Differ 17, 1071–1082. 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, and Duan S (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 47.Dolezelova E, Nothacker HP, Civelli O, Bryant PJ, and Zurovec M (2007). A Drosophila adenosine receptor activates cAMP and calcium signaling. Insect Biochem Mol Biol 37, 318–329. 10.1016/j.ibmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Xu C, Franklin B, Tang HW, Regimbald-Dumas Y, Hu Y, Ramos J, Bosch JA, Villalta C, He X, and Perrimon N (2020). An in vivo RNAi screen uncovers the role of AdoR signaling and adenosine deaminase in controlling intestinal stem cell activity. Proc Natl Acad Sci U S A 117, 464–471. 10.1073/pnas.1900103117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng B, Li Q, Liu X, Cao Y, Li B, Qian Y, Xu R, Mao R, Zhou E, Zhang W, et al. (2019). Chemoconnectomics: Mapping Chemical Transmission in Drosophila. Neuron 101, 876–893 e874. 10.1016/j.neuron.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 50.Babcock DT, Landry C, and Galko MJ (2009). Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol 19, 799–806. 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho Y, Sloutsky R, Naegle KM, and Cavalli V (2013). Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 155, 894–908. 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun L, Shay J, McLoed M, Roodhouse K, Chung SH, Clark CM, Pirri JK, Alkema MJ, and Gabel CV (2014). Neuronal regeneration in C. elegans requires subcellular calcium release by ryanodine receptor channels and can be enhanced by optogenetic stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 15947–15956. 10.1523/JNEUROSCI.4238-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, and Chisholm AD (2010). Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30, 3175–3183. 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahar M, and Cavalli V (2018). Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19, 323–337. 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Li R, Kaneko T, Takle K, Morikawa RK, Essex L, Wang X, Zhou J, Emoto K, Xiang Y, and Ye B (2014). Trim9 regulates activity-dependent fine-scale topography in Drosophila. Current biology : CB 24, 1024–1030. 10.1016/j.cub.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weavers H, Evans IR, Martin P, and Wood W (2016). Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 165, 1658–1671. 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenno L, Yizhar O, and Deisseroth K (2011). The development and application of optogenetics. Annual review of neuroscience 34, 389–412. 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen LB, Ginty DD, Weber MJ, and Greenberg ME (1994). Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12, 1207–1221. 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Fan H, Li F, Skeeters SS, Krishnamurthy VV, Song Y, and Zhang K (2020). Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila. eLife 9. 10.7554/eLife.57395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Donovan KJ, Ma K, Guo H, Wang C, Sun F, Han SB, Kim H, Wong JK, Charron J, Zou H, et al. (2014). B-RAF kinase drives developmental axon growth and promotes axon regeneration in the injured mature CNS. J Exp Med 211, 801–814. 10.1084/jem.20131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, and Fainzilber M (2005). Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45, 715–726. 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Hollis ER 2nd, Jamshidi P, Low K, Blesch A, and Tuszynski MH (2009). Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci U S A 106, 7215–7220. 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gabay L, Seger R, and Shilo BZ (1997). In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277, 1103–1106. 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- 64.Erickson EK, DaCosta AJ, Mason SC, Blednov YA, Mayfield RD, and Harris RA (2021). Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology 46, 500–508. 10.1038/s41386-020-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Qi Y, and Yang Y (2015). Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell reports 11, 798–807. 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, and Kalivas PW (2015). Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry 78, 441–451. 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, and Han W (2016). Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife 5. 10.7554/eLife.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newman EA (2015). Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci 370. 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newman EA (2003). Glial cell inhibition of neurons by release of ATP. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonioli L, Blandizzi C, Pacher P, and Hasko G (2013). Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13, 842–857. 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson KA, and Gao ZG (2006). Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5, 247–264. 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, et al. (2019). Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 104, 1039–1055 e1012. 10.1016/j.neuron.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman MP, and Freeman MR (2010). Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci 33, 245–267. 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costigan M, Scholz J, and Woolf CJ (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annual review of neuroscience 32, 1–32. 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang LY, Gu Y, and Chen Y (2013). Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 61, 1571–1581. 10.1002/glia.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, and Bradke F (2016). The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron 92, 419–434. 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Berridge MJ (1998). Neuronal calcium signaling. Neuron 21, 13–26. 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 78.Huebner EA, and Strittmatter SM (2009). Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 48, 339–351. 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience 18, 145–153. 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 80.Kupari J, Usoskin D, Parisien M, Lou D, Hu Y, Fatt M, Lonnerberg P, Spangberg M, Eriksson B, Barkas N, et al. (2021). Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nature communications 12, 1510. 10.1038/s41467-021-21725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abe N, Borson SH, Gambello MJ, Wang F, and Cavalli V (2010). Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem 285, 28034–28043. 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, and Steen JA (2015). Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 86, 1000–1014. 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, and Sharma SC (2016). Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51, 1–40. 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Peng W, Wu Z, Song K, Zhang S, Li Y, and Xu M (2020). Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science (New York, N.Y.) 369. 10.1126/science.abb0556. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima KI, Iwao K, Inoue T, Haga A, Tsutsumi T, Mochita MI, Fujimoto T, and Tanihara H (2018). Stimulation of the adenosine A3 receptor, not the A1 or A2 receptors, promote neurite outgrowth of retinal ganglion cells. Exp Eye Res 170, 160–168. 10.1016/j.exer.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 86.Eisenfeld AJ, Bunt-Milam AH, and Sarthy PV (1984). Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci 25, 1321–1328. [PubMed] [Google Scholar]
- 87.Zukor K, Belin S, Wang C, Keelan N, Wang X, and He Z (2013). Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 15350–15361. 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, and Jan YN (2012). Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron 73, 64–78. 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gou B, Liu Y, Guntur AR, Stern U, and Yang CH (2014). Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell reports 9, 522–530. 10.1016/j.celrep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Awasaki T, Lai SL, Ito K, and Lee T (2008). Organization and postembryonic development of glial cells in the adult central brain of Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 13742–13753. 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, and Heberlein U (2005). moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 123, 145–156. 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 92.Kaneko T, Macara AM, Li R, Hu Y, Iwasaki K, Dunnings Z, Firestone E, Horvatic S, Guntur A, Shafer OT, et al. (2017). Serotonergic Modulation Enables Pathway-Specific Plasticity in a Developing Sensory Circuit in Drosophila. Neuron 95, 722. 10.1016/j.neuron.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 93.Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, and Chedotal A (2017). Tridimensional Visualization and Analysis of Early Human Development. Cell 169, 161–173 e112. 10.1016/j.cell.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, and Dodt HU (2012). Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7, 1983–1995. 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- 95.Wang XW, Li Q, Liu CM, Hall PA, Jiang JJ, Katchis CD, Kang S, Dong BC, Li S, and Zhou FQ (2018). Lin28 Signaling Supports Mammalian PNS and CNS Axon Regeneration. Cell reports 24, 2540–2552 e2546. 10.1016/j.celrep.2018.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed fly crossing information, sample size number and statistics in each main figure panel and supplemental figure panel, related to Figure 1–7 and Figure S1–S7.
Video S1. GCaMP6s imaging showing that glial thermogenetic stimulation activates non-axotomized C4da neurons, related to Figure 2. A single C4da neuron was labeled by ppk-LexA>LexAop2-myr-GCaMP6s. TrpA1-B was expressed in glia. Scale bar, 20 μm.
Video S2. GCaMP6s imaging showing that glial thermogenetic stimulation did not activate non-axotomized C3da neurons, related to Figure 2. A single C3da neuron was labeled by NompC-LexA>LexAop2-myr-GCaMP6s. TrpA1-B was expressed in glia. Scale bar, 20 μm.
Video S3. GCaMP6s imaging of Ca2+ signals of non-axotomized C4da neurons, related to Figure 3. A single C4da neuron was labeled by ppk-LexA>LexAop2-myr-GCaMP6s. Scale bar, 20 μm.
Video S4. GCaMP6s imaging of Ca2+ signals of axotomized C4da neurons at 24 hpa, related to Figure 3. A single axotomized C4da neuron is labeled by ppk-LexA>LexAop2-myr-GCaMP6s. Each Ca2+ spike was highlighted by beep sound. Scale bar, 20 μm.
Video S5. Simultaneous extracellular recording and GCaMP6s imaging of an axotomized C4da neuron at 24 hpa, related to Figure 3. A single axotomized C4da neuron is labeled by ppk-LexA>LexAop2-myr-GCaMP6s. The soma region is defined as ROI for GCaMP6s signals. Scale bar, 20 μm.
Data Availability Statement
This paper does not report original code. All data used for analysis and figure generation are available from the lead contact upon request.


