Graphical abstract
Keywords: HHLA2, KIR3DL3, TMIGD2, Pathway, Cancer, Immunotherapy
Highlights
-
•
This review elucidated the KIR3DL3-HHLA2 and TMIGD2-HHLA2 interaction pathways, including intrinsic and extrinsic signal cascades. We also presented a possible mechanism by which KIR3DL3 and HHLA2 prevent immune cell overreaction by negative feedback in normal immune cells based on past studies.
-
•
In the tumour microenvironment, the expression of HHLA2, TMIGD2, and KIR3DL3 are all affected. TMIGD2 on tumour cells may act as an adhesion molecule to further promote tumour progressions, such as cell-cell interactions, cell migration, and angiogenesis.
-
•
We addressed the relationship between HHLA2 expression and the clinical prognosis of cancer patients.
-
•
KIR3DL3/TMIGD2-HHLA2 may represent novel pathways within the tumour microenvironment and serve as crucial immune checkpoints for developing novel therapeutic drugs against human cancer.
Abstract
Background
T cells and natural killer (NK) cells are essential components of the immune system and are regulated by coinhibitory and costimulatory molecules in which the B7 family and CD28 family play significant roles. Previous immune checkpoint studies on B7/CD28 family members, such as PD-1, have led to remarkable success in cancer immunotherapy. However, there is still a need to find new immune checkpoint molecules. Recent studies have demonstrated that HHLA2 exerts inhibitory and stimulatory functions on the immune system by binding to different receptors on different sites. However, the pathways between HHLA2 and its two receptors on T cells and NK cells remain controversial.
Aim of Review
Here, we reviewed recent studies about HHLA2 ligand interactions with KIR3DL3 and TMIGD2. We focused on elucidating the pathways between KIR3DL3/TMIGD2 and HHLA2 as well as their function in tumour progression. We also addressed the relationship between HHLA2 expression and the clinical prognosis of cancer patients.
Key Scientific Concepts of Review
KIR3DL3/TMIGD2-HHLA2 may represent novel pathways within the tumour microenvironment and serve as crucial immune checkpoints for developing novel therapeutic drugs against human cancer.
Introduction
Immune checkpoints reveal the complexity of immune system signal transduction. A ligand may have two receptors playing paradoxical roles. Both stimulatory and inhibitory signals from ligand–receptor interactions regulate T cell and NK cell functions [1], [2]. Accordingly, the potential roles of the CD28 family and B7 family in cancer pathogenesis have gained significant interest. Immune checkpoint blockade of PD-1 and CLTA-4 has gained considerable advancement in many cancer patients due to its robust and durable function in many types of tumours [3]. Nevertheless, many patients cannot obtain adequate treatment to date. Therefore, there is still a need to target new inhibitory receptors for immunotherapy.
HHLA2 protein expression is limited in normal tissues but diffusely expressed in human cancers; furthermore, its high expression is related to worse prognosis in most tumours [4], [5], [6], [7], [8]. HHLA2 is located in the cell membrane and cytoplasm of tumour cells without significant intratumoural heterogeneity in some tumours, suggesting that HHLA2 expression in the tumour may be a primary change during tumour evolution and/or may be influenced by the tumour microenvironment [4]. HHLA2 is expressed more frequently than PD-L1 and does not overlap with PD-L1 in some cancers [9], [10]. Therefore, HHLA2 may be a potential target for cancer immunotherapy and is worth exploring.
HHLA2 interacts with KIR3DL3 and TMIGD2 on different sites to present inhibitory and stimulatory functions, respectively, and the KIR3DL3-HHLA2 inhibitory function plays a prominent role, even in the presence of TMIDG2. Therefore, tumours may escape immune surveillance through the KIR3DL3-HHLA2 pathway, and HHLA2 may act as a new target for immunotherapy [4], [10], [11], [12].
Structure, expression, and function of HHLA2, KIR3DL3, and TMIGD2
HHLA2
HHLA2 (also known as B7H5, B7H7, or B7y) is a member of the B7 family, sharing 10–18 % homology and 23–33 % similarity in amino acid level with other B7 family members, and it phylogenetically forms the B7 family group III with B7x and B7H3 [13], [14]. HHLA2 was first reported as a new member of the immunoglobulin superfamily in 1999 [15]. HHLA2 is encoded on human chromosome 3q13.13, and it is expressed in various species but not in mice and rats, differing from other members of the B7 family [13].
HHLA2 is a type I transmembrane protein containing 414 amino acids, consisting of an extracellular part with tandem IgV1-IgC-IgV2 domains, a transmembrane region, and a cytoplasmic tail [13]. Interestingly, the IgV1 domain of HHLA2 has the highest homology with other B7 family members [5].
HHLA2 is mainly expressed on APCs, such as monocytes and stimulated B cells [11], [13]. Interestingly, a recent study has reported that cross-linking CD3 and CD28 allows HHLA2 to be expressed on exhausted Th1 and Tc1 cells. About 15–20 % of T cells express HHLA2 after anti-CD3/28 activation, and Expression is higher on exhausted activated T cells with 55 % of PD-1+LAG-3+ T cells expressing HHLA2. Thus, HHLA2 can be used to identify exhausted T cells [16].
HHLA2 protein is limited in normal tissues, except placental trophoblastic cells, the epithelium of the bronchus, pancreatic duct, intestine, kidney, gallbladder, and breast. In contrast, HHLA2 is diffusely expressed in human cancer tissue [4], [5], [6], [7].
KIR3DL3
Killer Cell Immunoglobulin Like Receptor, Three Ig Domains And Long Cytoplasmic Tail 3 (KIR3DL3) is a member of the KIR family. The KIR3DL3 gene encodes an extracellular part with tandem D0-D1-D2 domains and a cytoplasmic tail containing an ITIM. However, KIR3DL3 lacks the exon to encode the stem between D0-D1-D2 domains and the transmembrane [10], [11], [17], [18].
As a framework gene, KIR3DL3 is inherited in all haplotypes of humans [17]. Early analysis suggested KIR3DL3 is undetectable in most normal human tissue except in CD56bright NK cells of healthy blood samples at the mRNA level, especially in women's samples [17], [19]; however, more recent studies show KIR3DL3 is mainly expressed on CD8+ TEMRA cells and CD56dim NK cells [11]. Although the low KIR3DL3 expression in peripheral blood is caused by consistent methylation of the promoter, it is functional, and KIR3DL3 can be induced by demethylation in certain pathological or developmental situations [19], [20], [21]. Anti-CD3 and CD28 mAbs can induce CD4+ and CD8+ T cells to express KIR3DL3. However, unlike early PD1 expression following T cell activation, KIR3DL3 is expressed later and peaks around Day 21 [10].
HHLA2 has been shown to be the ligand of KIR3DL3, and KIR3DL3 binds HHLA2 through the D0 domain. The interaction between KIR3DL3 and HHLA2 plays an inhibitory role on T cells and NK cells [10], [11]. The KIR3DL3 monomers interact with downstream signalling proteins [18], and tyrosine 381 in the ITIM of the intracellular tail of KIR3DL3 is essential for KIR3DL3-mediated NK cell suppression. KIR3DL3 inhibits both TCR-dependent and TCR-independent pathways in CD8+ T cells. Moreover, the KIR3DL3-HHLA2 inhibitory function plays a prominent role, even in TMIGD2 presentation [10], [11].
TMIGD2
TMIGD2 (also known as CD28H/IGPR-1), sharing around 10 % amino acid sequences with CD28, PD-1, ICOS, and CTLA, is a single transmembrane protein consisting of an extracellular region with a single IgV domain, a transmembrane region and a cytoplasmic tail. The TMIGD2 gene of humans is located on chromosome 19q13.3 [4], [5], [22]. As an adhesion molecule, previous studies have shown that TMIGD2 also plays a vital role in cell–cell interactions, cell migration, and angiogenesis [22].
TMIGD2 is expressed on naïve T cells, memory T lymphocytes, tissue-resident T cells (TRMs), NK cells, plasmacytoid dendritic cells (PDCs), and innate lymphoid cells (ILCs). However, constant antigen stimulation causes many T cells, such as naïve T cells, to lose the capacity to express TMIGD2 [5], [6], [23], [24].
TMIGD2 is identified as a stimulatory receptor of the HHLA2 ligand by binding to the IgV1 domains of HHLA2, and their interaction promotes T cell proliferation, T cell differentiation, and NK cell activation. As the receptor of HHLA2, TMIGD2 is also absent in mice or rats [5], [11], [16].
HHLA2, KIR3DL3, and TMIGD2 in the immune microenvironment
Phenotypical characteristics of T cells expressing KIR3DL3 and TMIGD2
TMIGD2 expression is higher in naïve T cells than in memory T cells, in which TMIGD2+ naïve T cells account for 85–94 % of total naïve T cells, while TMIGD2+ memory T cells account for 20 % of all memory T cells [23]. Few spleen CD8+ T cells exhibited TRM phenotype, while TMIGD2+ T cells account for 33 % of splenic TRM [6]. Although the percent of TMIGD2+ in TRM still low (33 %), is higher than the percent in all memory T cells (20 %). Furthermore, TMIGD2+ TILs are young and less-differentiated previously activated T cells [6] In contrast, KIR3DL3 expression is rare in naïve T cells [10], but it is relatively abundant in CD8+ TEMRA cells [11].
TMIGD2+ naïve T cells have increased naïve features, and TMIGD2+ memory T cells have a less effective functional phenotype [23]. TMIGD2+ memory T cells produce higher levels of IL-2 but lower levels of IFN-γ, TMIGD2+ TILs produce less CD107a, perforin, and Granzyme B, while TMIGD2- cells express higher levels of CD57, T-bet, IFN-γ, and TNF-α. [6]. TMIGD2- T cells show terminal differentiation and senescence phenotypes [5]. Furthermore, sestrins also induce a NK-like function in senescent CD8+ T cells. CD8+ TEMRA cells with terminal differentiation and senescence phenotypes have higher expression of NK adaptors, NKRs, and cytotoxic mediators. Human CD8+ TEMRA cells also express fewer TCR signalling molecules [25]. KIR3DL3+CD8+ TEMRA cells acquire a NK-like phenotype; for example, KIR3DL3+CD8+ TEMRA cells express more NK cell-related regulatory receptors and fewer T cell regulatory receptors than KIR3DL3−CD8+ TEMRA cells [11].
Phenotypical characteristics of NK cells expressing KIR3DL3 and TMIGD2
TMIGD2 is primarily expressed on most freshly isolated human resting NK cells, and the CD56birghtCD16− NK subset has higher TMIGD2 expression. In contrast, KIR3DL3 is mainly expressed on CD56dim NK at the protein level [11], [24], [26]. The CD56dim NK cell subset expresses higher NKRs and CD16, and it has more potent natural cytotoxicity. Furthermore, the CD56dimCD16+ NK subset is more differentiated and predominant in peripheral blood [26], [27]. The CD56dim NK cell subset can be further divided into two groups by CD57 expression, in which CD57+ NK cells are more mature and terminally differentiated. In contrast, CD57- NK cells produce IFN-γ and lytic activity when stimulated by CD16 [14]. The TMIGD2 expression level is lower in CD56dimCD57+ cells than in the CD56dimCD57– NK subset [24].
In summary, TMIGD2+ T/NK cells present more naïve factors, resulting in less activation and differentiation, while KIR3DL3+ T/NK cells present more differentiated characteristics.
Intrinsic regulation of HHLA2, KIR3DL3, and TMIGD2 expression
Methylation Low expression of KIR3DL3 in peripheral blood is caused by consistent methylation of the promoter, and KIR3DL3 is induced after treatment with a methyltransferase inhibitor [20]. The high methylation level of the promoter is also related to B7 family low expression at the mRNA level in gastrointestinal cancer and oral squamous cell carcinoma (OSCC) [28], [29]. Moreover, B7 member dysregulation may involve both DNA methylation and gene alteration in gastric cancer [30].
miRNA miR-26a-5p, miR-26b-5p, and miR-185-5p may impact expression level of KIR3DL3, and knockdown of the same miRNAs induce KIR3DL3 expression [21]. miR-146a-5p is also involved in downregulating KIR expression [31]; and hsa-miR-6870–5p and hsa-miR-3116 may bind to HHLA2 mRNA to play a role in this modulatory mechanism [32].
HHLA2 copy number It has been reported that copy number have little impact on B7 family expression in oral squamous cell carcinoma [28]. However, HHLA2 copy number gains are identified in 29 % of breast cancers, revealing that it may be a potential mechanism by which HHLA2 expression is upregulated in breast cancer [4].
Extrinsic regulation of HHLA2, KIR3DL3, and TMIGD2 expression
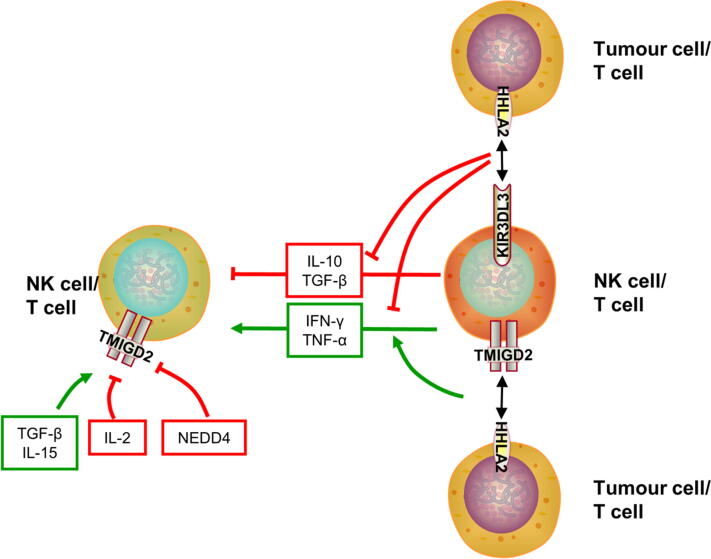
Cytokines and antigens IL-2 stimulation causes T cells to lose TMIGD2 expression during division. TGF-β induces high TMIGD2 expression regardless of stimulation by CD3 mAb in CD8+ T cells. Moreover, TGF-β increases the TMIGD2 expression level, and the percentage of TMIGD2+ cells. IL-15 and TGF-β induce the population of TRMs and sustain TMIGD2 expression to manage constant antigenic stimulation (Fig. 1) [6]. However, TMIGD2 expression is downregulated during repetitive antigenic exposure on T cells [5]. In contrast, anti-CD3 and CD28 mAbs induce KIR3DL3 to express on T cells [10].
Fig. 1.
The bidirectional arrow represents the interaction between the receptor and ligand. The thickness of the arrow represents the strength of action. The KIR3DL3/TMIGD2-HHLA2-related extrinsic relationship is described as follows: 1) T cells secrete IL-10 and TGF-β to inhibit T/NK cell function; 2) T cells secrete IFN-γ and TNF-α to activate T/NK cell function; 3 and 4) HHLA2 inhibits TCR-induced IL-10, IFN-γ, and TNF-αproduction; 5) the TMIGD2-HHLA2 interaction induces IFN-γ and TNF-αproduction; and 6) TGF-β and IL-15 sustain TMIGD2 expression, while NEDD4 and IL-2 downregulate TMIGD2 expression.
NEDD4 NEDD4 is a member of the E3 ubiquitin ligase family. Moreover, NEDD4 comprises the HECT domain, C2 domain, and WW domains. NEDD4 is expressed in various human organs [33]. The WW domain 4 on NEDD4 and the PPR motif on TMIGD2 form a complex to ubiquitinate TMIGD2. Furthermore, NEDD4-mediated polyubiquitination regulates TMIGD2 expression in lysosomal-dependent pathway [34].
EGFR Epidermal growth factor receptor (EGFR) is a growth factor receptor involved in cell differentiation and proliferation [35]. B7 family members are affected by EGFR tyrosine kinase inhibitor resistance in gastric cancer [30], and HHLA2 expression is higher in the EGFR mutant group in lung cancer [36], [37], [38]. There is a negative correlation between EGFR and HHLA2 expression in cell lines and non-small-cell lung carcinoma [39].
KIR3DL3-HHLA2 pathway in immune responses
Previous studies have shown opposite outcomes regarding the stimulatory function of HHLA2. The expression of HHLA2 on activated T cells acts as an inhibitory ligand to regulate T cell activation [16]. HHLA2 acts as an inhibitory signal on TCR and CD28-mediated activation [40], and blockage of HHLA2 enhances T cell activation and proliferation [40]. HHLA2 inhibits TCR-mediated proliferation and cytokines production of T cells [13]. However, the discovery of the inhibitory receptor of HHLA2 elucidates how HHLA2 exerts an inhibitory function by binding to KIR3DL3 [11].
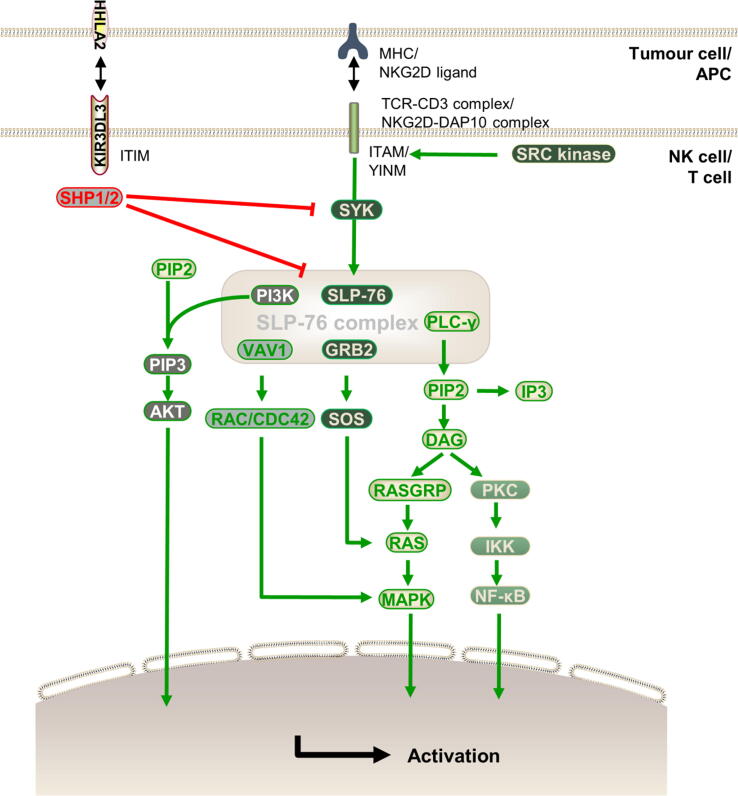
The intrinsic signalling cascades of KIR3DL3 are described below.(Fig. 2) The CD3 cytoplasmic tail contains ITAM, which is phosphorylated by SRC family kinases. SYK binds to phosphorylated tyrosine, activating the signalling cascade by phosphorylating and activating the SLP-76 complex, including SLP-76, PLC-γ, VAV1, GRB2, and PI3K [41], [42], [43], [44]. DAP10 does not have the ITAM but has the YINM in its cytoplasmic region, which is also phosphorylated by SRC family kinases. YINM couples to VAV1 and PLC-γ by a SYK-independent pathway [45], [46]. GRB2 recruits and phosphorylates VAV1, SLP-76, and PLC-γ [47] followed by activation of RAC and CDC42 by VAV1 [48]. Furthermore, PLC-γ hydrolyses PIP2 into DAG and IP3 [49], and DAG activates several downstream signals, such as the RAS/MAPK pathway, through RASGRP and activated PKC [48], [49]. SOS is recruited via GRB2, RASGRP, and SOS, leading to rapid, bistable amplification of RAS activation [50]. PI3K phosphorylates PIP2 to generate PIP3, which then activates AKT and downstream pathways [47], [51]. These costimulatory receptors and cytokine receptor signals integrate to tune T cell and NK cell responses, including cytokine production, proliferation, migration, and effector functions [48].
Fig. 2.
The bidirectional arrow represents the interaction between the receptor and ligand. The thickness of the arrow represents the strength of action. The intrinsic signal cascades of TCR or NKG2D that activate cells are described below. ITAM and YINM are phosphorylated by SRC kinase. SYK is recruited to phosphorylated ITAM, which causes the SLP-76 complex to be phosphorylated, while YINM couples to VAV1 and PLC-γ in the SYK-independent pathway. Downstream signals, including PLC-γ, PI3K, and VAV1, are then activated. However, KIR3DL3 contains an ITIM, which can also be phosphorylated, and phosphorylated ITIM recruits SHP1/2 to dephosphorylate SYK, and the SLP-76 complex to block the downstream stimulatory signal in T cells and NK cells.
ITIM of KIR3DL3 can also be phosphorylated. Phosphorylated ITIM recruits SHP1/2, resulting in dephosphorylation of SLP-76 complex components and SYK, which in turn inhibits downstream signals, such as PI3K and RAS [43], [44], [46], [52], [53], [54]. Moreover, downstream signals, including NF-κB, VAV1, ERK1/2, and AKT, are constricted [11]. Therefore, KIR3DL3 and HHLA2 interactions inhibit T cell and NK cell functions in these pathways.
TMIGD2-HHLA2 pathway in immune responses
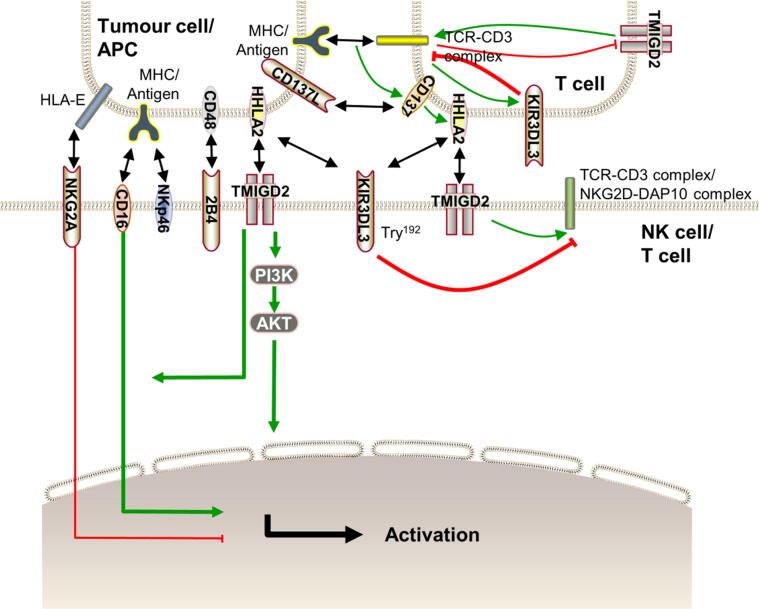
HHLA2 interacts with TMIGD2 to enhance proliferation and cytokine production of T cell by acting as a costimulatory signal [12] (Fig. 3). TMIGD2-mediated T cell and NK cell activation is primarily related to Tyr192 on the TMIGD2 cytoplasmic tail [24].
Fig. 3.
The bidirectional arrow represents the interaction between the receptor and ligand. The arrow thickness represents the strength of action. Activation of T cells and NK cells is primarily related to Tyr192 on the TMIGD2 cytoplasmic tail, and the activation occurs via the AKT pathway. The TMIGD2-HHLA2 interaction does not provide an efficacious stimulatory signal alone, but TMIGD2 synergizes with 2B4 and NKp46 to activate T cells and NK cells. Moreover, the TMIGD2-HHLA2 interaction promotes ADCC caused by CD16. Long-term stimulation causes T cells to lose TMIGD2 expression but increases KIR3DL3 expression. By cross-linking CD3 and CD28, HHLA2 is expressed on T cells, which can be used to identify exhausted T cells. TCR signalling induces transient expression of CD137 on T cells. Furthermore, CD137 increases the expression of HHLA2 on T cells. Thus, by upregulating KIR3DL3 and downregulating TMIGD2, HHLA2 may interact with KIR3DL3 to avoid T cell overaction in exhausted T cells.
The HHLA2 and TMIGD2 interaction does not provide an efficacious stimulatory signal alone [40]. However, TMIGD2 synergizes with 2B4 and NKp46 to cause degranulation and cell lysis as well as cytokines secretion. Moreover, the HHLA2-TMIGD2 interaction strengthens NK cell degranulation, cytotoxicity, and ADCC caused by CD16, and it promotes NK cell activation in ADCC. Furthermore, NK cell degranulation and cytokine production only partially overlap [24]. The HHLA2 and TMIGD2 interaction activates T cells via the AKT pathway [5]. NKG2A, acting as an inhibitory receptor, binds with the nonclassical MHC-I antigen, HLA-E, to suppress NK cell activation [55]. The interaction between TMIGD2 and HHLA2 overcomes the inhibitory function mediated by NKG2A [24].
The KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways in cancer immunology
By cross-linking CD3 and CD28, HHLA2 is expressed on activated T cells [16], and TCR stimulation and subsequent CD3 signalling induce transient expression of CD137 on T cells. Moreover, CD28 costimulation results in markedly enhanced CD137 surface expression [56], and CD137 costimulation induces HHLA2 continuous demethylation in CD8+ T cells and chromatin reprogramming [57]. Therefore, CD137 increases HHLA2 expression on T cells, allowing HHLA2 to be used for identification of exhausted T cells [16]. Anti-CD3 and CD28 mAbs induce KIR3DL3 to be expressed on T cells [10]. At the same time, stimulation by antigen or IL-2 causes many T cells to lose the capacity to express TMIGD2 [5]. Therefore, a possible mechanism by which T cells express HHLA2 and KIR3DL3 may be used to negatively regulate T cell function to prevent overreaction or provide immune privilege in some processes, such as pregnancy. However, tumour cells may utilize negative feedback to escape immune surveillance. On the one hand, tumour cells highly express HHLA2 or may induce HHLA2 expression on T/NK cells in the tumour microenvironment. On the other hand, KIR3DL3 expression on T/NK cells is regulated in the tumour microenvironment. Thus, tumours inhibit function of immune cells, and then achieve tumour cells existence.
The potent inhibitory function of KIR3DL3 is dominant over the stimulatory function of TMIGD2 when KIR3DL3 and TMIGD2 are coexpressed [11]. TMIGD2 is also altered in tumour tissue, which has been demonstrated by several studies. TMIGD2 is higher in gastric cancer than in adjacent tissues [58]. However, TMIGD2 is expressed at low levels in OSCC and dysplasia [59]. Moreover, TMIGD2 expression on immune cells in the tumour microenvironment is influenced. The expression level of TMIGD2 on ILCs is increased in specific human cancer tissues [23]; TMIGD2+ TILs present a comparable percentage in tumours, such as melanoma, glioma, and pancreatic ductal adenocarcinoma [6]. CD8 + TILs are recruited to the site where HHLA2 is highly expressed in ovarian cancer [60].
In addition, TMIGD2 promotes tumour multicellular aggregation, and TMIGD2 regulates the sensitivity of tumour cells to the doxorubicin/adriamycin chemotherapeutic agent in a mechanism that involves Ser220 phosphorylation and AKT activation in colon cancer [61]. As an adhesion molecule, TMIGD2 plays a vital role in cell–cell interactions, cell migration, and angiogenesis [22]. Therefore, further investigation of TMIGD2 expression in tumour tissues is still needed. Because the expression of TMIGD2 may be influenced in the tumour microenvironment, TMIGD2 may act as an adhesion molecule that exerts many functions related to tumour survival. Although the stimulatory function of TMIGD2 does not exist, TMIGD2 may play a role in tumour progression as an adhesion molecule. Furthermore, TMIGD2 may also present a target to treat cancer.
HHLA2 expression is more abundant in metastatic tumours than in primary tumours without metastasis and is also higher in metastatic tissue and primary tumours with metastasis than in primary tumours without metastasis in osteosarcoma [62]. HHLA2 deficiency inhibits NSCLC cell proliferation, migration, and invasion, and it inhibits EGFR/MAPK/ERK, resulting in partial G0/G1 phase arrest. Knockdown of HHLA2 results in downregulation of IL-10 followed by inhibition of TAM M2 polarization. In addition, knockdown of HHLA2 also inhibits tumour growth in vivo [63]. Therefore, HHLA2 may also participate in cell–cell adhesion, which is vital in tumour cell survival. Further investigations are needed to confirm the HHLA2 function in tumour metastasis and whether the function is related to the HHLA2-TMIGD2 interaction.
HHLA2 expression in solid tumours and its relationship with cancer prognosis
HHLA2 is aberrantly expressed in various solid tumours, and HHLA2 expression levels are related to the prognosis of patients (Table 1). The relationship between HHLA2 expression and the prognosis of patients is controversial in oral squamous cell carcinoma and clear cell renal cell carcinoma. High HHLA2 expression is related to better prognosis in pancreatic cancer, epithelial ovarian cancer, cervical adenocarcinoma, ampullary tumour, and glioma. In comparison, high HHLA2 expression is related to a worse prognosis in prostate cancer, lung cancer, osteosarcoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, bladder urothelial carcinoma, gastric cancer, breast cancer, colorectal carcinoma, and oesophageal cancer.
Table 1.
The relationship between HHLA2 expression in tumour tissue and cancer patient prognosis.
| Human cancer type | cancer cases | Results: Expression in tumour cells and relationship with prognosis | Analysis method | antibody | Refs |
|---|---|---|---|---|---|
| Prostate cancer | 239 | HHLA2 higher expression was related to worse prognosis because of higher tumour grade, tumour stage, and lymphatic metastasis. | IHC | clone 566.1 | [64] |
| Hepatocellular carcinoma (HCC) | 462 | HHLA2 expression was higher, and the high level of HHLA2 was related to a worse prognosis. | qRT-PCR IHC IHC |
– Cell Signaling Technology, Danvers, MA, USA ab214327, Abcam, Cambridge, UK |
[65], [67], [66] |
| Oral squamous cells carcinoma (OSCC) | 201 | Increased HHLA2 expression in oral squamous cells carcinoma and higher HHLA2 expression levels indicate poor prognosis. | IHC | Abcam, Cambridge, UK | [59] |
| 241 | Approximately 23.1 % of patients showed B7 high expression, and HHLA2 expression at a high level was related to good clinical outcomes. | – | – | [28] | |
| Pancreatic cancer |
365 | Higher HHLA2 expression was related to a better prognosis, such as prolonged OS, delayed cancer recurrence, improved post-operative cancer-specific survival, and less immune evasion. | IHC and RT-qPCR IHC IHC IHC |
AP10650a, Abgent, San Diego, CA, USA B7H5 mAb HPA055478, Sigma-Aldrich clone 566.1 |
[12], [7], [68], [69] |
| Osteosarcoma tumours |
62 | HHLA2 was expressed in a large proportion of osteosarcoma tumours, and its expression was related to poorer survival. | IHC | clone 566.1 | [62] |
| Lung cancer | 1344 | High HHLA2 expression was related to metastasis higher stage and poor progression-free survival in lung cancer. | IHC IHC IHC |
ab214327, Abcam, Cambridge, UK clone 566.1 A13262, IgG, Abclonal, Inc |
[36], [37], [38] |
| Ovarian cancer | 64 | HHLA2 overexpression was related to inhibiting the proliferation of ovarian cancer cells. | IHC and qRT–PCR | clone 566.1 | [60] |
| Gastric cancer |
633 | HHLA2 was overexpressed in gastric cancer tissue, and the high level of HHLA2 was associated with deep tumour invasion, advanced clinical stage, metastasis, and short OS. | IHC and qRT–PCR IHC |
Abcam, Cambridge, UK B7H5 mAb |
[70], [58] |
| Glioma | 669 | High expression of HHLA2 was associated with a better prognosis for patients with glioma. | IHC | cat. no. HPA055478, Sigma-Aldrich, Merck KGaA | [71] |
| Intrahepatic cholangiocarcinoma (ICC) | 153 | High HHLA2 expression level was significantly associated with poor OS. | IHC | Atlas, Stockholm, Sweden | [9] |
| Ampullary tumour | 72 | High HHLA2 expression levels were related to delayed cancer recurrence and improved post-operative cancer-specific survival. | IHC and RT-PCR | clone 566.1 | [69] |
| Bladder urothelial carcinoma | 212 | HHLA2 was overexpressed in tumour tissue, and high HHLA2 expression significantly correlated with poor prognosis. | IHC and qRT-PCR | ab214327, Abcam, Cambridge, UK | [72] |
| Breast cancer | 50 | HHLA2 expression was aberrant in tumour tissue. In addition, overexpression of HHLA2 was related to worse consequences | IHC | clone 566.1 | [4] |
| Colorectal carcinoma | 63 | High HHLA2 expression was related the invasion depth. | IHC | ab214327, Abcam, Cambridge, UK | [73] |
| Clear cell renal cell carcinoma (ccRCC) |
534 | High HHLA2 expression was present in tumours, and higher HHLA2 expression indicates better prognosis of the patient with kidney clear cell carcinoma. | IHC | clone 566.1 | [74] |
| 585 | High HHLA2 mRNA expression level was present in tumour tissues, and higher expression of HHLA2 was related to poorer OS, larger tumour size, and advanced TNM stage. | IHC IHC and RT-PCR IHC and qRT-PCR |
Sigma- Aldrich, HPA055478 LSC321945, Lifespan Biosciences Dako Corporation, USA |
[75], [76], [77] | |
| Cervical adenocarcinoma | 76 | HHLA2 expression was negatively related to lymph node metastasis, and high HHLA2 expression was associated with more prolonged disease-free survival. | IHC | Thermo Fisher Scientific, IL | [78] |
| Oesophageal cancer | 185 | High HHLA2 expression in oesophageal cancer was related to worse OS. | – | – | [29] |
IHC immunohistochemistry.
qRT-PCR quantitative reverse transcription-polymerase chain reactio.
Prostate cancer
Zhou et al. conducted a retrospective study in patients with prostate cancer treated with radical prostatectomy with 126 cases in the training cohort and 113 cases in the validation cohort, and immunohistochemistry demonstrated that HHLA2 expression was higher than PD-L1. Furthermore, high HHLA2 expression was related to a higher Gleason score, poorer overall survival (OS), shorter cancer-specific survival (CSS), more advanced tumour stage, and more likely lymph node metastasis. At the same time, HHLA2 expression was negatively associated with CD8+ TILs. Moreover, HHLA2 was an independent prognostic predictor for prostate cancer [64].
Lung cancer
Farrag et al. conducted a retrospective study, in which they collected 62 biopsy specimens from patients diagnosed with lung cancer by either bronchoscopic or CT-guided biopsies. Moreover, they reported that HHLA2 expression was high in lung cancer and that patients with positive HHLA2 expression were associated with metastasis and lower progression-free Survival (PFS) compared to patients with negative HHLA2 expression. HHLA2 expression was positively related to EGFR markers [38]. Chen et al. used samples from two independent retrospective collections of lung cancer, including 372 adenocarcinoma patients with lung cancer who underwent lung cancer resection, which formed a discovery cohort, and 231 patients with lung adenocarcinoma who underwent surgical treatment, which formed a validation cohort. In the EGFR mutant samples, HHLA2 expression was significantly higher, while PD-L1 expression was lower. The increased HHLA2 and decreased CD8 expression levels were related to short disease-free survival in lung adenocarcinoma [36].
Sun et al. downloaded corresponding RNA-seq data from the UCSC Xena database; they reported that HHLA2 expression was widespread in non-small-cell lung carcinoma (NSCLC) and that HHLA2 promoted proliferation, invasion, and migration. They also demonstrated that HHLA2 expression was upregulated in some human NSCLC cell lines, such as A549 and H1299 [63]. Cheng et al. also collected corresponding pan-cancer data by downloading data from the UCSC Xena database, and they reported a similar outcome with HHLA2 expression was widely identified in lung cancer. HHLA2 expression was also associated with clinical characteristics and clinical outcome in patients with lung cancer. HHLA2 expression was higher in non-Hispanic White patients than that in Hispanic patients. Moreover, Cheng and colleagues reported that HHLA2 high expression was related to EGFR mutational status, HHLA2 expression was higher in EGFR-mutated NSCLC samples, and they demonstrated that EGFR mutation status and high TIL density were independently related to HHLA2 expression in lung adenocarcinoma [37].
Hepatocellular carcinoma (HCC)
Luo et al. collected data from the GEO database and then compared HHLA2 expression in HCC tissues with paracarcinoma liver tissues from 55 patients. Luo and colleagues reported high HHLA2 expression in HCC, and they suggested that high HHLA2 expression was related to tumour differentiation, advanced cancer stage, and tumour invasion to adjacent structures [65]. Luo et al. also used the LinkFinder module to collect data from TCGA database, including 202 samples from patients who underwent surgical resection [66], and the outcome was consistent with previous research [65]. These researchers concluded that high HHLA2 expression in HCC was related to poorer tumour differentiation, advanced clinical cancer stage, microvascular invasion, hepatic capsule invasion, and multiple tumours. In addition, they reported that HHLA2 expression was associated with the density of TILs but not with PD-L1 expression and that high HHLA2 expression was related to poor prognosis. In addition, intermediate and high TIL densities were related to a better prognosis [66]. Xu et al. studied 205 HCC patients who had received tumour resection, and they reported that HHLA2 expression was positively related to CD204+ and CD11b+ cell infiltration in the peritumour region and negatively related to the antitumour immune response. High expression of HHLA2 in peritumoural tissue was related to poor patient OS. HHLA2 expression was negatively related to PD-L1. Moreover, patients with HHLA2 and PD-L1 coexpression had the poorest prognosis [67].
Intrahepatic cholangiocarcinoma (ICC)
Jing et al. divided 218 patients who underwent resection for ICC into training and validation cohorts, comprising 153 and 65 patients, respectively; they reported that high HHLA2 expression was significantly associated with poor OS and that HHLA2 expression was more frequent than PD-L1 expression in ICC. HHLA2 and PD-L1 coexpression was infrequent, and PD-L1-negative samples had elevated HHLA2 expression. High expression of HHLA2 was related to fewer CD3+ TILs and CD8+ TILs [9].
Oral squamous cells carcinoma (OSCC)
Xiao et al. collected 201 samples and demonstrated that HHLA2 was highly expressed but that TMIGD2 was expressed at low levels in OSCC and dysplasia. Moreover, they reported that higher HHLA2 or TMIGD2 expression levels in OSCC revealed a poorer prognosis. Furthermore, HHLA2 expression levels were positively related to TIM3, LAG3, B7H3, B7H4, and VISTA, but TMIGD2 expression levels were negatively related to TIM3, LAG3, and B7H3 levels in OSCC, dysplasia, and mucosa [59].
However, a different conclusion has been reported regarding the relationship between HHLA2 expression and OSCC prognosis. Ren et al. collected detailed clinical information from TCGA database and the MD Anderson Cancer Centre. Approximately 23.1 % of patients showed upregulation of B7 expression in OSCC tissue compared to normal oral epithelial tissues, and high HHLA2 expression was related to good clinical outcomes. Mutations and copy number alterations had little effect on B7 expression. At the same time, methylation levels were negatively related to HHLA2 expression at the mRNA level. Furthermore, the prognosis of OSCC patients was negatively related to PD-L1 but positively related to HHLA2 [28].
Pancreatic cancer (PC)
Chen et al. enrolled 136 pancreatic ductal adenocarcinoma patients whose diagnoses were confirmed by pathological examination of tumour tissue after surgery. They found that when cocultured with T cells, PC cells with high HHLA2 expression can exert a more potent immune reaction than PC cells with low HHLA2 expression. Furthermore, in the xenograft model, HHLA2 high expression PC cell growth was inhibited compared to HHLA2 low expression after transfusion with T lymphocytes in immune-deficient mice. Patients with higher HHLA2 expression had more prolonged OS than those with lower expression [12]. Yan et al. collected 92 cases of surgically resected pancreatic ductal adenocarcinoma (PDAC) tumour tissues and 91 cases of matched peritumoural tissues; they demonstrated that HHLA2 was widely expressed in PDAC. HHLA2 expression was detected in 77.17 % of PDAC cases and was significantly related to a better prognosis [68]. Boor et al. studied tumour tissues from 122 patients who underwent resection of pancreatic cancer, and they reported HHLA2 expression in 67 % of tumour cells in pancreatic tumours. The high expression of HHLA2 was related to improved cancer-specific survival, and they suggested that HHLA2 can act as an independent predictor of cancer-specific survival [69]. Byer et al. obtained pancreatic tissue from patients after surgical resection, and they reported that HHLA2 was on normal ductal epithelium within the pancreas. However, HHLA2 expression was decreased in adenocarcinoma, which disagreed with the results reported in another study. Byer and colleagues reported that HHLA2 expression in intraductal papillary mucinous neoplasms can be different with grade and that loss of HHLA2 may be associated with immune evasion [7].
Osteosarcoma
Koirala et al. collected a total of 62 primary tumour specimens and metastatic disease samples to evaluate HHLA2 expression. HHLA2 expression was upregulated in primary tumour specimens and metastatic disease. HHLA2 expression was identified in 68 % of osteosarcoma samples. Almost all metastatic disease specimens presented HHLA2 expression, which was more prevalent than in primary samples without known metastases. They also reported that TILs were identified in 75 % of all osteosarcoma specimens and that high HHLA2 expression was associated with poorer survival [62].
Ovarian cancer
Xu et al. examined HHLA2 expression levels in 64 ovarian cancer tissues and 16 normal ovarian tissues by immunohistochemistry, and they obtained relative mRNA data from the CCLE database. HHLA2 overexpression was related to inhibiting the proliferation of ovarian cancer cells. HHLA2 was identified in only 17.2 % of ovarian cancer patients. At the same time, HHLA2 was positively associated with the differentiation of ovarian cancer cells and the density and count of CD8+ TILs. Moreover, they suggested that HHLA2 expression can act as an independent prognostic factor for predicted improved survival and that overexpression of HHLA2 can inhibit ovarian cancer cell proliferation [60].
Gastric cancer (GC)
Wei et al. collected 154 gastric cancer tissue specimens, 61 adjacent normal stomach tissue specimens, and they also collected data for 408 gastric cancer tissue specimens and 211 normal stomach tissue specimens from TCGA and GTEx databases. Wei and colleagues concluded that HHLA2 expression was upregulated in tumour tissue. Moreover, high HHLA2 expression was related to advanced clinical stage, deep tumour invasion, distant metastasis, lymph node metastasis, and short OS in gastric cancer [70]. Hu et al. collected GC tissues and corresponding adjacent noncancerous tissues from 71 patients diagnosed with GC to examine the expression levels of HHLA2 and TMIGD2. They demonstrated that HHLA2 and TMIGD2 were higher in tumour tissue than in adjacent tissues. However, there was no relationship between the expression of HHLA2 and TMIGD2. The 5-year OS in the HHLA2+TMIGD2+ group was lower than that in the HHLA2+TMIGD2− group (4.5 % vs 33.5 %). In summary, they suggested that both CH28H and HHLA2 positivity can predict the poorest prognosis [58].
Glioma
Yangzhi Qi et al. downloaded RNA sequencing data for human glioma samples from TCGA and GlioVis databases, and they concluded that high expression of HHLA2 was associated with a better prognosis for patients with glioma. HHLA2 was negatively related to tumour-associated macrophages. HHLA2 was negatively related to PD-L1, LAG3, and B7H3. Moreover, they suggested that HHLA2-associated genes can play roles in inhibiting neoplasia-associated processes and that HHLA2 upregulation indicates a favourable outcome for patients with glioma [71].
Ampullary tumour
Patrick P. C. Boor et al. collected 72 surgical resection samples of ampullary tumour tissues. High HHLA2 expression levels were related to delayed cancer recurrence and improved post-operative cancer-specific survival. HHLA2 was present in 93 % of ampullary tumours. The association between HHLA2 expression and cancer-specific survival and recurrence was similar in ampullary cancer [69].
Bladder urothelial carcinoma (BUC)
Guobing Lin et al. collected BUC tissue specimens and adjacent normal bladder mucosal tissues from 212 patients and 36 pairs of normal bladder urethral tissues and lymph nodes of BUC specimens. HHLA2 expression was higher in BUC tissues than in normal bladder tissues. HHLA2 expression was primarily related to tumour size, tumour grade, tumour stage, and lymph node metastasis in BUC tissues. Lin and colleagues suggested that HHLA2 expression can act as an independent prognostic factor to predict tumour metastasis. High HHLA2 expression was significantly related to poor 5-year recurrence-free survival (RFS) and OS in BUC patients [72].
Breast cancer
Murali Janakiram et al. selected 50 cases diagnosed with local or locally advanced breast cancer followed by chemotherapy, radiotherapy, or both to form a triple-negative breast cancer (TNBC) cohort and performed a retrospective study. These researchers reported that approximately 56 % of patients with TNBC had aberrant HHLA2 expression in tumour tissue. Furthermore, high HHLA2 expression was significantly related to regional lymph node metastasis and higher tumour stage. HHLA2 copy number gains were present in 29 % of the samples, revealing a possible mechanism for high HHLA2 expression in breast cancer, which was verified by the cBioPortal for Cancer Genomics database and the Cancer Genome Atlas [4].
Colorectal carcinoma (CRC)
Ziwen Zhu et al. enrolled 63 patients diagnosed with CRC and used their resected specimens to examine the expression of HHLA2 and CD8. HHLA2 expression was high in CRC tumour tissues, and the HHLA2 expression level was significantly associated with the depth of invasion, predicted high mortality rate, and CD8 + T cell infiltration status [73].
Clear cell renal cell carcinoma (ccRCC)
Zhang et al. downloaded data from TCGA database and GEO datasets to demonstrate that HHLA2 was highly expressed in tumours. Higher expression of HHLA2 indicated a better prognosis for patients with ccRCC, and high HHLA2 was related to high survival rates and immune-related factors in ccRCC. Both HHLA2 and CD8 expression exhibited a consistent trend in ccRCC samples [74].
The following three studies reported a different outcome. Qianghua Zhou et al. studied patients who underwent surgical resection for ccRCC without receiving neoadjuvant therapy to form a training cohort and validation cohort comprising 206 and 197 patients, respectively, for retrospective analysis. Zhou and colleagues reported that HHLA2 expression was more prevalent than PD-L1 expression in ccRCC tissues. Positive HHLA2 expression was primarily related to shorter PFS and OS due to necrosis, advanced Fuhrman nuclei, microvascular invasion, and TNM stage. HHLA2 and PD-L1 positivity was related to the poorest prognosis and high density of CD8+ and CD4+ TIL infiltration [75]. Lujun Chen et al. enrolled 87 patients who underwent surgery and downloaded relevant information from TCGA database, and they reported that HHLA2 expression was higher in ccRCC tissue than in adjacent normal tissue at the mRNA level based on TCGA database. Moreover, HHLA2 expression was positively related to PD-L1, PD-L2, and B7H6 but negatively related to B7H3 at the mRNA level. Furthermore, high HHLA2 expression was associated with advanced TNM stage and larger tumour size. Thus, they suggested that HHLA2 can act as an independent risk factor to predict the prognosis of patients. Cell viability, migration, and invasion ability vastly decreased after knocking down HHLA2 expression in human ccRCC cell lines. Moreover, knockdown of HHLA2 inhibited cell cycle arrest at G1 phase and the expression of Cyclin D1, c-Myc, and Cyclin E1. The expression of epithelial-to-mesenchymal transition markers, including vimentin, E-cadherin, and N-cadherin, was also changed [76]. Chen et al. collected 92 patients diagnosed with ccRCC who underwent surgery as the primary treatment, and they reported that HHLA2 was high in ccRCC tissues compared to normal tissues. Moreover, the expression of HHLA2 was related to histological grade, tumour size and clinical stage. Chen and colleagues also identified the impact of HHLA2 on various biological processes and primary immune responses by mRNA, long noncoding RNA, and circular RNA [77].
Cervical adenocarcinoma
Jung Mi Byun et al. performed a retrospective study by analysing 78 patients who underwent hysterectomy for cervical adenocarcinoma, and they demonstrated that HHLA2 expression was negatively related to lymph node metastasis. High HHLA2 expression was associated with prolonged disease-free survival and improved OS. The proportion of high HHLA2 expression was higher than that of low HHLA2 expression (81.6 % vs 18.4 %). Furthermore, the expression of HHLA2 was positively related to PD-L1 [78].
Oesophageal cancer
Qijie Zhao et al. analysed data from TCGA, including 185 cases of oesophageal cancer, and they reported that high HHLA2 expression in oesophageal cancer was related to worse OS [29].
Conclusion and future perspectives
HHLA2 has an inhibitory receptor, namely, KIR3DL3, and a stimulatory receptor, namely, TMIGD2. The expression of KIR3DL3 and TMIGD2 is relatively independent at different stages and in subgroups of T cell and NK cell, respectively. TMIGD2 is expressed on cells with naïve characteristics, and KIR3DL3 is expressed on terminally differentiated cells. This review elucidated the KIR3DL3-HHLA2 and TMIGD2-HHLA2 interaction pathways, including intrinsic and extrinsic signal cascades. We also presented a possible mechanism by which KIR3DL3 and HHLA2 prevent immune cell overreaction by negative feedback in normal immune cells based on past studies. In the TME, expression of HHLA2, TMIGD2, and KIR3DL3 are all affected. Because the inhibitory function of KIR3DL3 is stronger than the stimulatory function of TMIGD2, the immune function is inhibited. However, TMIGD2 on tumour cells may act as an adhesion molecule to further promote tumour progression, such as cell–cell interactions, cell migration, and angiogenesis. These mechanisms are used by tumour cells to escape immune surveillance. Thus, HHLA2, TMIGD2, and KIR3DL3 antibodies may be targets for therapeutic development.
HHLA2 is more prevalent than PD-L1 and is expressed on many PD-L1-negative tumour cells (Table 2). Therefore, cancer immunotherapy for HHLA2 may have a potent therapeutic effect for patients who previously could not receive adequate treatment. In addition, HHLA2 binds to KIR3DL3 and TMIGD2 at different sites, potentially allowing the identification of HHLA2 antibodies that can distinctively block the KIR3DL3 inhibitory signal but persevere the TMIGD2 stimulatory signal [10]. Moreover, KIR3DL3 and PD-1 share similar mechanisms to regulate immune function, and immunotherapy combining KIR3DL3 and PD-1 may exert a better antitumour effect and benefit more tumour patients.
Table 2.
Relationship among HHLA2 expression, PD-L1 expression, TIL density, and other factors in different tumours.
| Human cancer type | Results | Ref. |
|---|---|---|
| Cervical adenocarcinoma | HHLA2 expression was positively related to PD-L1. | [78] |
| Clear cell renal cell carcinoma |
HHLA2 expression did not overlap with PD-L1 expression. | [10] |
| HHLA2 expression was positively and significantly associated with PD-L1 and PD-L2 at the mRNA level in whole tumour samples but individual cells were not examined | [76] | |
| HHLA2 expression was positively related to CD8. | [74] | |
| The positive rates of HHLA2 were more widespread than PD- L1. HHLA2/PD- L1 co-expression was related to a high density of CD8+ and CD4+ TILs. | [75] | |
| Glioma | HHLA2 was negatively related to specific genes, including IL-10, TGF-β, VEGF, DDL4, and other immune checkpoint molecules, including PD-L1, LAG3, and B7H3. | [71] |
| Hepatocellular carcinoma |
HHLA2 expression was related to the density of TILs but not to PD-L1 levels. | [66] |
| The expression levels of HHLA2 and PD-L1 were negatively correlated. | [67] | |
| Intrahepatic cholangiocarcinoma | HHLA2 expression was more frequent than PD-L1 expression. HHLA2 and PD-L1 co-expression were infrequent, and 50 % of PD-L1-negative samples had elevated HHLA2 expression. HHLA2 high expression was related to fewer CD3+ TILs and CD8+ TILs as well as a higher CD4+ Foxp3+/CD8+ TIL ratio. | [9] |
| Ovarian cancer | High HHLA2 expression was positively related to the density CD8+ TILs. | [60] |
| Osteosarcoma | TILs were present in 75 % of tumour specimens. | [62] |
| Lung cancer | Increased CD8 expression was related to more prolonged disease-free survival. | [36] |
| HHLA2 limited co-expression with PD-L1, and HHLA2 was commonly expressed in PD-L1 negative tumours. | [79] | |
| Colorectal carcinoma | The HHLA2 expression level was significantly associated with the invasion depth and CD8+T-cell infiltration status. | [73] |
The research about KIR3DL3-HHLA2 axis blockage to treat tumours are insufficient. Therefore, related preclinical and clinical research about KIR3DL3-HHLA2 axis blockage is needed. Future studies should focus on the tumoral therapeutic effects of mAbs targeting KIR3DL3 or HHLA2 to interrupt the KIR3DL3-HHLA2 signal axis. The possibility and feasibility of the KIR3DL3-HHLA2 axis as a therapeutic target alone or in combination with other cancer therapies should be further validated. The relationship between HHLA2 expression and the clinical prognosis in solid tumours is inconsistent. The expression levels of TMIGD2 and KIR3DL3 in tumour tissue should be further investigated to explain these contradictory results. Moreover, TMIGD2 participates in tumour progression by acting as an adhesion molecule, and intrinsic signalling cascades in which TMIGD2 acts as a stimulatory receptor need to be further investigated.
Ethics approval and consent to participate
Not applicable.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Yang Li: Investigation, Writing – original draft, Visualization. Chao Lv: Investigation, Writing – original draft. Yang Yu: Investigation, Visualization. Baokang Wu: Writing – original draft. Yizhou Zhang: Investigation. Qi Lang: Investigation, Visualization. Zhiyun Liang: Investigation, Visualization. Chongli Zhong: Investigation, Visualization. Yu Shi: Investigation, Visualization. Shukun Han: Investigation, Visualization. Feng Xu: Writing – review & editing. Yu Tian: Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The article was supported by the Natural Science Foundation of China (81974377), the Scientific Research Project of the Education Department of Liaoning Province (JC2019017), 345 Talent Project of Shengjing Hospital (2019–2021), and the Outstanding Scientific Fund of Shengjing Hospital.
Biographies

Yang Li is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on immune checkpoints, angiogenesis, and vasculature in cancers.He wrote the first draft of the manuscript and participate in completing the figures and tables in this review.

Chao Lv is a Ph.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on tumour immunotherapy, the correlation between immune checkpoint molecule expression and the clinical characters especially in gallbladder cancer. He wrote the first draft of the manuscript in this review.

Yang Yu is a M.D. candidate at Department of General Surgery, The First Affiliated Hospital of Jinzhou Medical University Her research focused on immune checkpoints. She organized the database and participated in completing the figures and tables.

Baokang Wu is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on Long Noncoding RNA, cancer immunotherapy and immune checkpoint signalling network. He participated in writing the first draft of the manuscript in this review.

Yizhou Zhang is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on Long Noncoding RNA and immune checkpoints. He organized the database in this review in this review.

Qi Lang is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on AI in cancer therapy and immune checkpoints. He completed the figures and tables in this review.

Zhiyun Liang is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. Her research focused on immune checkpoints especially in B7 family. She provided technical support for drawing, polished language and graphics in this review.

Chongli Zhong is a Ph.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on phenotypical and potential functional characteristics of different immune cells and cancer immunology. He provided technical support for drawing and polished language and graphics

Yu Shi is a M.D. candidate at Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. His research focused on tumor vasculature normalization and cancer immune checkpoint blockade. He participated in completing the figures and tables.

Shukun Han received the M.D. degree from China Medical University, Shenyang, China, in 2019. He is currently a Ph.D. candidate in Shengjing Hospital Affiliated to China Medical University. His research interests include immune checkpoint such as the correlation between B7 family and the clinical characters especially in colorectal cancer. He participated in Hospital Affiliated to China Medical University. His research focused on tumor vasculature.

Feng Xu received the B.S degree in Pediatrics and the M.D. degree in Surgery from China Medical University, Shenyang, China, in 2001 and 2006, respectively. He is currently a professor in Shengjing Hospital of China Medical University. His research interests include mechanisms of immune checkpoint action, tumor immunotherapy, and NK cell function. He managed the article design and reviewed the manuscript in this review.

Yu Tian is a professor of Department of General Surgery, Shengjing Hospital Affiliated to China Medical University. Hiis research group is involved in studies of several aspects of cancer immunology, including immune checkpoints, angiogenesis and vasculature in cancers, and AI in cancer therapy. Until now, more than 100 papers appeared in most distinguished journals at home and abroad, such as Oncoimmunology, Journal of Experimental & Clinical Cancer Research, Artificial Intelligence Reviews, and Laboratory Investigation. Current work is directed towards an understanding of the mechanisms of immune escape of cancer involved in B7 family in CD8+ TILs. The Group is also interested in the metabolism of bile acids in biliary diseases. He managed the article design, reviewed the manuscript and provided funding support in this review.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Levi-Schaffer F., Mandelboim O. Inhibitory and Coactivating Receptors Recognising the Same Ligand: Immune Homeostasis Exploited by Pathogens and Tumours. Trends Immunol. 2018;39(2):112–122. doi: 10.1016/j.it.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung K., Choi I. Emerging Co-signaling Networks in T Cell Immune Regulation. Immune Netw. 2013;13(5):184–193. doi: 10.4110/in.2013.13.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34(1):539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 4.Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res. 2015; 21(10): 2359–66. [DOI] [PMC free article] [PubMed]
- 5.Zhu Y., Yao S., Iliopoulou B.P., Han X., Augustine M.M., Xu H., et al. B7–H5 costimulates human T cells via CD28H. Nat Commun. 2013;4(1) doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y., Sun Y., Gao F., Koenig M.R., Sunderland A., Fujiwara Y., et al. CD28H expression identifies resident memory CD8 + T cells with less cytotoxicity in human peripheral tissues and cancers. Oncoimmunology. 2019;8(2) doi: 10.1080/2162402X.2018.1538440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byers J.T., Paniccia A., Kaplan J., Koenig M., Kahn N., Wilson L., et al. Expression of the Novel Costimulatory Molecule B7–H5 in Pancreatic Cancer. Ann Surg Oncol. 2015;22(S3):1574–1579. doi: 10.1245/s10434-014-4293-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhong C., Lang Q., Yu J., Wu S., Xu F., Tian Y. Phenotypical and potential functional characteristics of different immune cells expressing CD28H/B7-H5 and their relationship with cancer prognosis. Clin Exp Immunol. 2020;200(1):12–21. doi: 10.1111/cei.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing C.-Y., Fu Y.-P., Yi Y., Zhang M.-X., Zheng S.-S., Huang J.-L., et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. 2019;7(1) doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt RS, Berjis A, Konge JC, Mahoney KM, Klee AN, Freeman SS, et al. KIR3DL3 Is an Inhibitory Receptor for HHLA2 that Mediates an Alternative Immunoinhibitory Pathway to PD1. Cancer Immunol Res. 2021; 9(2): 156–69. [DOI] [PMC free article] [PubMed]
- 11.Wei Y., Ren X., Galbo P.M., Moerdler S., Wang H., Sica R.A., et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol. 2021;6(61) doi: 10.1126/sciimmunol.abf9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q., Wang J., Chen W., Zhang Q., Wei T., Zhou Y., et al. B7–H5/CD28H is a co-stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(2):530–539. doi: 10.1111/cas.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao R., Chinai J.M., Buhl S., Scandiuzzi L., Ray A., Jeon H., et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang X., Loke P'ng, Kim J., Murphy K., Waitz R., Allison J.P. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100(18):10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mager D.L., Hunter D.G., Schertzer M., Freeman J.D. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3) Genomics. 1999;59(3):255–263. doi: 10.1006/geno.1999.5877. [DOI] [PubMed] [Google Scholar]
- 16.Luu K, Schwarz H, Lundqvist A. B7-H7 Is Inducible on T Cells to Regulate Their Immune Response and Serves as a Marker for Exhaustion. Front Immunol. 2021; 12: 682627. [DOI] [PMC free article] [PubMed]
- 17.Campbell KS. Mystery Checkpoint Revealed: KIR3DL3 Finally Found a Ligand in HHLA2. Cancer Immunol Res 2021; 9(2): 128. [DOI] [PubMed]
- 18.Leaton LA, Shortt J, Kichula KM, Tao S, Nemat-Gorgani N, Mentzer AJ, et al. Conservation, Extensive Heterozygosity, and Convergence of Signaling Potential All Indicate a Critical Role for KIR3DL3 in Higher Primates. Front Immunol 2019; 10: 24. [DOI] [PMC free article] [PubMed]
- 19.Trundley A.E., Hiby S.E., Chang C., Sharkey A.M., Santourlidis S., Uhrberg M., et al. Molecular characterization of KIR3DL3. Immunogenetics. 2006;57(12):904–916. doi: 10.1007/s00251-005-0060-7. [DOI] [PubMed] [Google Scholar]
- 20.Trompeter H.-I., Gómez-Lozano N., Santourlidis S., Eisermann B., Wernet P., Vilches C., et al. Three structurally and functionally divergent kinds of promoters regulate expression of clonally distributed killer cell Ig-like receptors (KIR), of KIR2DL4, and of KIR3DL3. J Immunol. 2005;174(7):4135–4143. doi: 10.4049/jimmunol.174.7.4135. [DOI] [PubMed] [Google Scholar]
- 21.Nutalai R., Gaudieri S., Jumnainsong A., Leelayuwat C. Regulation of KIR3DL3 Expression via Mirna. Genes (Basel) 2019;10(8):603. doi: 10.3390/genes10080603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimi N., Rezazadeh K., Mahoney J.E., Hartsough E., Meyer R.D., Nusrat A. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell. 2012;23(9):1646–1656. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespo J., Vatan L., Maj T., Liu R., Kryczek I., Zou W. Phenotype and tissue distribution of CD28H(+) immune cell subsets. Oncoimmunology. 2017;6(12):e1362529. doi: 10.1080/2162402X.2017.1362529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang X, Long EO. CD28 Homolog Is a Strong Activator of Natural Killer Cells for Lysis of B7H7(+) Tumor Cells. Cancer Immunol Res 2019; 7(6): 939–51. [DOI] [PMC free article] [PubMed]
- 25.Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, et al. Sestrins induce natural killer function in senescent-like CD8(+) T cells. Nat Immunol 2020; 21(6): 684–94. [DOI] [PMC free article] [PubMed]
- 26.Moretta L. Dissecting CD56dim human NK cells. Blood. 2010;116(19):3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- 27.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 28.Ren X.Y., Chen X.J., Chen X.B., Wang C.Y., Liu Q., Pan X., et al. Immune Landscape of the B7 and TNFR Families in Oral Squamous Cell Carcinoma. Chin J Dent Res. 2020;23(2):109–117. doi: 10.3290/j.cjdr.a44747. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q., Hu F., Xiao Z., Li M., Wu X.u., Zhao Y., et al. Comprehensive molecular profiling of the B7 family in gastrointestinal cancer. Cell Prolif. 2018;51(5):e12468. doi: 10.1111/cpr.12468. https://doi.org/10.1111/cpr.2018.51.issue-510.1111/cpr.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D., Xiang S., Shen J., Xiao M., Zhao Y., Wu X.u., et al. Comprehensive understanding of B7 family in gastric cancer: expression profile, association with clinicopathological parameters and downstream targets. Int J Biol Sci. 2020;16(4):568–582. doi: 10.7150/ijbs.39769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pesce S, Squillario M, Greppi M, Loiacono F, Moretta L, Moretta A, et al. New miRNA Signature Heralds Human NK Cell Subsets at Different Maturation Steps: Involvement of miR-146a-5p in the Regulation of KIR Expression. Front Immunol 2018; 9: 2360. [DOI] [PMC free article] [PubMed]
- 32.Wang B., Ran Z., Liu M., Ou Y. Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Front Immunol. 2019;10:1573. doi: 10.3389/fimmu.2019.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingham R.J., Gish G., Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23(11):1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 34.Sun L., Amraei R., Rahimi N. NEDD4 regulates ubiquitination and stability of the cell adhesion molecule IGPR-1 via lysosomal pathway. J Biomed Sci. 2021;28(1):35. doi: 10.1186/s12929-021-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rude Voldborg B., Damstrup L., Spang-Thomsen M., Skovgaard Poulsen H. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8(12):1197–1206. doi: 10.1023/a:1008209720526. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Hu R., Li X., Shi Z., Tian H., Feng J., et al. B7–H4 and HHLA2, members of B7 family, are aberrantly expressed in EGFR mutated lung adenocarcinoma. Pathol Res Pract. 2020;216(10):153134. doi: 10.1016/j.prp.2020.153134. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, et al. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin Cancer Res 2017; 23(3): 825–32. [DOI] [PMC free article] [PubMed]
- 38.Farrag M., Ibrahim E., El-Hadidy T., Akl M., Elsergany A., Abdelwahab H. Human Endogenous Retrovirus-H Long Terminal Repeat- Associating Protein 2 (HHLA2) is a Novel Immune Checkpoint Protein in Lung Cancer which Predicts Survival. Asian Pac J Cancer Prev. 2021;22(6):1883–1889. doi: 10.31557/APJCP.2021.22.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Z., Zhang L., Xu W., Zhang G. EGFR may participate in immune evasion through regulation of B7H5 expression in nonsmall cell lung carcinoma. Mol Med Rep. 2018;18(4):3769–3779. doi: 10.3892/mmr.2018.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieder S.A., Wang J., White N., Qadri A., Menard C., Stephens G., et al. B7–H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol. 2021;18(6):1503–1511. doi: 10.1038/s41423-020-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kane L.P., Lin J., Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12(3):242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 42.owell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol 2011; 3(3). [DOI] [PMC free article] [PubMed]
- 43.van Rees D.J., Szilagyi K., Kuijpers T.W., Matlung H.L., van den Berg T.K. Immunoreceptors on neutrophils. Semin Immunol. 2016;28(2):94–108. doi: 10.1016/j.smim.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Getahun A., Cambier J.C. Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol Rev. 2015;268(1):66–73. doi: 10.1111/imr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billadeau D.D., Upshaw J.L., Schoon R.A., Dick C.J., Leibson P.J. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4(6):557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 46.Vivier E., Nunès J.A., Vély Frédéric. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 47.Molfetta R., Quatrini L., Zitti B., Capuano C., Galandrini R., Santoni A., et al. Regulation of NKG2D Expression and Signaling by Endocytosis. Trends Immunol. 2016;37(11):790–802. doi: 10.1016/j.it.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Gaud G., Lesourne R., Love P.E. Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol. 2018;18(8):485–497. doi: 10.1038/s41577-018-0020-8. [DOI] [PubMed] [Google Scholar]
- 49.Ivashkiv L.B. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10(4):340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Courtney A.H., Lo W.-L., Weiss A. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem Sci. 2018;43(2):108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawkins P.T., Stephens L.R., Suire S., Wilson M. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q., Qu J., Zhao M., Xu Q., Sun Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 2020;152:104595. doi: 10.1016/j.phrs.2019.104595. [DOI] [PubMed] [Google Scholar]
- 53.Varone A., Spano D., Corda D. Shp1 in Solid Cancers and Their Therapy. Front Oncol. 2020;10:935. doi: 10.3389/fonc.2020.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daëron M., Jaeger S., Du Pasquier L., Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224(1):11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 55.Long E.O., Sik Kim H., Liu D., Peterson M.E., Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31(1):227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Etxeberria I., Glez-Vaz J., Teijeira Á., Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2019;4:e000733. doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aznar MA, Labiano S, Diaz-Lagares A, Molina C, Garasa S, Azpilikueta A, et al. CD137 (4-1BB) Costimulation Modifies DNA Methylation in CD8(+) T Cell-Relevant Genes. Cancer Immunol Res 2018; 6(1): 69–78. [DOI] [PubMed]
- 58.Hu C., Xu Z., Chen S., Lv H., Wang Y., Wang X., et al. Overexpression of B7H5/CD28H is associated with worse survival in human gastric cancer. J Cell Mol Med. 2020;24(2):1360–1369. doi: 10.1111/jcmm.14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao Y., Li H., Yang L.-L., Mao L., Wu C.-C., Zhang W.-F., et al. The Expression Patterns and Associated Clinical Parameters of Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 and Transmembrane and Immunoglobulin Domain Containing 2 in Oral Squamous Cell Carcinoma. Dis Markers. 2019;2019:1–9. doi: 10.1155/2019/5421985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu G., Shi Y., Ling X., Wang D., Liu Y., Lu H., et al. HHLA2 predicts better survival and exhibits inhibited proliferation in epithelial ovarian cancer. Cancer Cell Int. 2021;21(1) doi: 10.1186/s12935-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolf N, Pearson BE, Bondzie PA, Meyer RD, Lavaei M, Belkina AC, et al. Targeting tumor multicellular aggregation through IGPR-1 inhibits colon cancer growth and improves chemotherapy. Oncogenesis. 2017; 6(9): e378. [DOI] [PMC free article] [PubMed]
- 62.Koirala P., Roth M.E., Gill J., Chinai J.M., Ewart M.R., Piperdi S., et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6(1) doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun W., Li S., Tang G., Sun S., Luo Y., Bai R., et al. HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Med. 2021;10(15):5256–5269. doi: 10.1002/cam4.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q., Li K., Lai Y., Yao K., Wang Q., Zhan X., et al. B7 score and T cell infiltration stratify immune status in prostate cancer. J Immunother Cancer. 2021;9(8):e002455. doi: 10.1136/jitc-2021-002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo M., Xiong Y., Lin Y., Liang R., Li Y., Ge L. H Long Terminal Repeat-Associating 2 (HHLA2) is a Biomarker of Advanced Stage Hepatocellular Carcinoma and Promotes Tumor Cell Development In Vitro. Med Sci Monit. 2021;27 doi: 10.12659/MSM.930215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo M., Lin Y., Liang R., Li Y., Ge L. Clinical Significance of the HHLA2 Protein in Hepatocellular Carcinoma and the Tumor Microenvironment. J Inflamm Res. 2021;14:4217–4228. doi: 10.2147/JIR.S324336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y., Huang Z., Yu X., Li Z., Zheng L., Xu J. HHLA2 Expression is Associated with Poor Survival in Patients with Hepatocellular Carcinoma. Biologics. 2021;15:329–341. doi: 10.2147/BTT.S325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan H., Qiu W., Koehne de Gonzalez A.K., Wei J.-S., Tu M., Xi C.-H., et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019;442:333–340. doi: 10.1016/j.canlet.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boor P.P.C., Sideras K., Biermann K., Hosein Aziz M., Levink I.J.M., Mancham S., et al. HHLA2 is expressed in pancreatic and ampullary cancers and increased expression is associated with better post-surgical prognosis. Br J Cancer. 2020;122(8):1211–1218. doi: 10.1038/s41416-020-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L.i., Tang L., Chang H., Huo S., Li Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell. 2020;33(1):116–122. doi: 10.1007/s13577-019-00280-2. [DOI] [PubMed] [Google Scholar]
- 71.Qi Y., Deng G., Xu P., Zhang H., Yuan F., Geng R., et al. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep. 2019 doi: 10.3892/or.2019.7343. https://doi.org/10.3892/or10.3892/or.2019.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin G., Ye H., Wang J., Chen S., Chen X., Zhang C. Immune Checkpoint Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 is Upregulated and Independently Predicts Unfavorable Prognosis in Bladder Urothelial Carcinoma. Nephron. 2019;141(4):256–264. doi: 10.1159/000495887. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Z., Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther. 2018;11:1563–1570. doi: 10.2147/OTT.S160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z., Liu J., Zhang C., Li F., Li L., Wang D., et al. Over-Expression and Prognostic Significance of HHLA2, a New Immune Checkpoint Molecule, in Human Clear Cell Renal Cell Carcinoma. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Q.-H., Li K.-W., Chen X.u., He H.-X., Peng S.-M., Peng S.-R., et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000157. doi: 10.1136/jitc-2019-000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L., Zhu D., Feng J., Zhou Y., Wang Q.i., Feng H., et al. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019;19(1) doi: 10.1186/s12935-019-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen D., Chen W., Xu Y., Zhu M., Xiao Y.i., Shen Y., et al. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet. 2019;56(1):43–49. doi: 10.1136/jmedgenet-2018-105454. [DOI] [PubMed] [Google Scholar]
- 78.Byun J.M., Cho H.J., Park H.Y., Lee D.S., Choi I.H., Kim Y.N., et al. The clinical significance of HERV-H LTR -associating 2 expression in cervical adenocarcinoma. Medicine (Baltimore) 2021;100(1):e23691. doi: 10.1097/MD.0000000000023691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng H, Borczuk A, Janakiram M, Ren X, Lin J, Assal A, et al. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1-Negative Human Lung Cancers. Clin Cancer Res. 2018; 24(8): 1954–64. [DOI] [PMC free article] [PubMed]