Abstract
To produce chronic infection, microbial pathogens must escape host immune defenses. Infection with the human pathogenic fungus Cryptococcus neoformans is typically chronic. To understand the mechanism by which C. neoformans survives in tissue after the infection of immunocompetent hosts, we systematically studied the course of pulmonary infection in mice by electron microscopy. The macrophage was the primary phagocytic cell at all times of infection, but neutrophils also ingested yeast. Alveolar macrophages rapidly internalized yeast cells after intratracheal infection, and intracellular yeast cells were noted at all times of infection from 2 h through 28 days. However, the proportion of yeast cells in the intracellular and extracellular spaces varied with the time of infection. Early in infection, yeast cells were found predominantly in the intracellular compartment. A shift toward extracellular predominance occurred by 24 h that was accompanied by macrophage cytotoxicity and disruption. Later in infection, intracellular persistence in vivo was associated with replication, residence in a membrane-bound phagosome, polysaccharide accumulation inside cells, and cytotoxicity to macrophages, despite phagolysosomal fusion. Many phagocytic vacuoles with intracellular yeast had discontinuous membranes. Macrophage infection resulted in cells with a distinctive appearance characterized by large numbers of vacuoles filled with polysaccharide antigen. Similar results were observed in vitro using a macrophage-like cell line. Our results show that C. neoformans is a facultative intracellular pathogen in vivo. Furthermore, our observations suggest that C. neoformans occupies a unique niche among the intracellular pathogens whereby survival in phagocytic cells is accompanied by intracellular polysaccharide production.
Cryptococcus neoformans is the causative agent of cryptococcosis, a life-threatening fungal infection (reviewed in reference 45). C. neoformans infections can occur in individuals with both normal and impaired immune function, but most cases are found in patients with immune deficiency. C. neoformans infections are acquired from the environment, presumably by inhalation of infectious particles (17). In immune-competent hosts, most primary infections are believed to be asymptomatic (41). Evidence that exposure to C. neoformans is common comes from serological studies that demonstrate the presence of antibodies in the majority of normal individuals (7, 12). Many infections are believed to result from reactivation of latent infection (25). Hence, this organism has adapted for persistence in tissue, but the mechanism by which it resists immune clearance is not well understood (5).
The traditional view of cryptococcal pathogenesis is that, as with encapsulated bacteria, its polysaccharide capsule prevents phagocytosis and that disease is caused by extracellular accumulations of organisms and polysaccharide, resulting in host tissue compression (41, 58). When provided with complement or antibody opsonins, phagocytic cells are able to ingest C. neoformans in vitro. In humans, phagocytosis of C. neoformans by monocytes is followed by phagosomal acidification and phagolysosomal fusion (40). However, phagocytosis is not always accompanied by fungal killing, and there is convincing in vitro evidence that this pathogen can replicate intracellularly (13, 14, 36, 38). Although in vitro studies can provide important insights into the mechanisms of microbial pathogenesis, it is essential to validate in vitro observations in vivo before these are accepted as physiologically relevant. Today, C. neoformans is suspected of being capable of intracellular pathogenesis in vivo, but this has never been convincingly demonstrated. The demonstration that a pathogen can replicate intracellularly in vivo is a difficult experimental task because techniques for dynamic studies of infected tissues are not available. For many microbes classically thought to be intracellular pathogens, this property has been inferred but not rigorously proven.
The immunologic mechanisms of host defense against pulmonary C. neoformans infections have been extensively studied in experimental animals (reviewed in reference 5). The inflammatory response to primary cryptococcal infection in the lung has been studied primarily by light microscopy. The literature contains only limited ultrastructural information, most of which is derived from in vitro systems (36, 51). In tissue, ultrastructural studies of biopsies of human cutaneous cryptococcosis have described budding yeast within macrophages (27) and vacuolation of macrophage cytoplasm (48). Studies with experimental animals have examined macrophage morphologic and cytoplasmic changes in peritoneal exudates of guinea pigs, rats, and rabbits after intraperitoneal infection with C. neoformans (34, 51) and in hepatic granulomas in rats after intravenous (i.v.) infection (54). The ultrastructural appearance of intravascular granulomas in the lungs in rats after i.v. inoculation has also been described (63). These studies have each provided important information about host cell-cryptococcus interactions. However, there has not been a systematic ultrastructural study of experimental pulmonary cryptococcosis over the course of infection.
We approached the question of whether C. neoformans is a facultative intracellular pathogen by systematically studying pulmonary infection in mice by light microscopy and electron microscopy (EM). The results showed that the capsule did not prevent phagocytosis in vivo and that the yeast cells survived and replicated inside macrophages, despite phagolysosomal fusion. Intracellular replication was associated with cytotoxicity and profuse intracellular polysaccharide production. The results demonstrated that pulmonary infection is a highly dynamic process whereby the location of C. neoformans cells changes during the course of infection as a function of intracellular replication and the degree of inflammation.
MATERIALS AND METHODS
C. neoformans.
ATCC strain 24067 (serotype D) was used for most experiments (21). This strain was selected because it has been used in previous studies of pulmonary pathology (18–20). One experiment was done with strain H99 (serotype A), the type strain for C. neoformans var. grubii (22). Another experiment was done with the acapsular strain Cap 67 or its parent strain 3501 (serotype D) (23). Isolates were maintained at −80°C. Cultures were started by inoculation of Sabouraud dextrose broth (Difco, Detroit, Mich.) with a loopful of frozen stock and incubated for 48 h at 30°C with moderate shaking. Cells were washed three times in sterile phosphate-buffered saline (PBS) and counted using a hemacytometer, and the count was confirmed by plating on Sabouraud dextrose agar. For one experiment, organisms were killed by heating to 55°C for 30 min. Plating of the culture after heating demonstrated >99.9% killing.
Infection.
Specific-pathogen-free C57BL/6 mice, A/JCr mice, and 129/SvEv mice were obtained from the National Cancer Institute (Bethesda, Md.), Jackson Laboratories (Bar Harbor, Maine), or Taconic Laboratories (Germantown, N.Y.). Within each experiment, mice were of the same sex. Mice were 6 to 10 weeks old at the time of infection. Mice were infected intratracheally as described earlier (18). Briefly, mice were anesthetized with 65 mg of sodium pentobarbital per kg and then inoculated intratracheally with 104 or 106 organisms in 0.05 ml of sterile PBS via a midline neck incision, except where indicated. The higher inoculum was used for experiments in which mice were studied prior to 24 h because it allowed organisms to be seen in sufficient numbers by EM early after infection. In the following sets of experiments, mice were infected concurrently and were killed by cervical dislocation at the indicated times: (i) 2 h and 14 days (repeated twice); (ii) 24 h, 48 h, 7 days, and 28 days; (iii) 8, 16, and 24 h after infection with live yeast of strain 24067, 24 h after infection with strains 3501 and Cap 67 and the heat-killed yeast of strain 24067 (106 organisms for all), and 14 days after infection with strains Cap 67 (108 yeast cells) and 3501 (104 yeast cells); and (iv) 2 h, 7 days, and 14 days after infection with strain H99. To evaluate whether our findings in C57BL/6 mice were present in other mouse strains, A/JCr mice and 129/SvEv mice infected with strain 24067 were studied on days 14 and 26 (A/JCr mice) or on days 13 and 28 (129/SvEv mice) after infection. In each experiment, two mice were studied for each group. Their lungs were fixed in Trump's fixative (4% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer) for EM. One lobe of the lung was fixed in 10% buffered formalin and embedded in paraffin for light microscopic comparison. In three additional experiments, lungs of C57BL/6 mice infected for 2 h and 14 days were processed for acid phosphatase cytochemistry as described below. In total, the study included analysis of 249 blocks from 74 mice, 105 of which were studied by EM, and includes data from three mouse strains and four cryptococcal strains, representing two serotypes, three of which are encapsulated. At least three 1-μm toluidine blue-stained sections of all blocks were examined by light microscopy, and representative blocks were selected for examination by EM.
Microscopy.
EM of lung tissue was performed according to a published procedure (19). Immunogold histochemistry was performed using either monoclonal antibody (MAb) 2H1 or MAb 18B7, murine immunoglobulin G1 (IgG1) antibodies that bind to the glucuronoxylomannan component of C. neoformans capsular polysaccharide (CNPS) (4) as described earlier (3). For primary MAb staining, grids were incubated overnight at 4°C in 5 μg of MAb 2H1 or murine IgG (Sigma) per ml as a control. Acid phosphatase cytochemistry was performed on lung sections using either cytidine monophosphate or sodium trimetaphosphate (Sigma, St. Louis, Mo.), as previously described (15, 50). Tissue from uninfected mice and infected tissue that was not incubated in substrate served as controls. To demonstrate colocalization of acid phosphatase activity and CNPS, sections incubated in trimetaphosphate were subsequently stained with MAb 2H1, as described above, except that phosphate-free buffers were used, the H2O2 incubation and etching were eliminated, and sections were imaged without subsequent uranyl or lead staining.
The percentage of intact intracellular yeast was calculated at 7, 14, and 28 days after infection by counting ca. 50 to 100 intracellular yeast cells on random micrographs obtained at each time point. Yeasts were considered to be “intact” if their cytoplasm and intracellular organelles were clearly visible and the shape of the cell wall was regular. Phagosome and capsule diameters were measured, and the volumes were calculated. For the calculation of phagosome volume, only phagosomes containing one yeast cell were included.
Budding index.
The number of cryptococci with buds whose cell wall was continuous with that of a parent yeast cell was divided by the total number of yeast cells in an oil immersion field of a 1-μm section stained with toluidine blue and magnified ×1,000. Five fields were counted for each mouse. Statistical analysis was performed using the two-tailed paired t test and was calculated using Microsoft Excel 97 (Redmont, Wash.).
In vitro experiments.
Phagocytosis assays were performed with the murine macrophage-like cell line J774.16. A total of 105 cells were plated per well on 96-well tissue culture plates and were stimulated with gamma interferon (100 U/ml) and lipopolysaccharide (0.2 μg/ml) for 28 h. Allowing for doubling of cells and to achieve an effector/target ratio of 1:2, 4 × 105 yeast cells of strain Cap 67 or 3501 were then added to the wells in medium containing 10% fresh normal mouse serum from C57BL/6 mice. The plates were incubated at 37°C with 10% CO2 for 3 h. The wells were washed three times with fresh medium to remove extracellular yeast and mouse serum, preventing further phagocytosis. At this time and after total incubation times of 18, 42, and 66 h, five wells for each strain were washed, fixed with methanol, and stained with Giemsa. A fifth well was stained with trypan blue to assess J774 cell viability; three to eight determinations in which 50 to 100 cells were counted were made both for total cell viability (all J774 counted) and for viability of J774 cells with intracellular yeast. In Giemsa-stained wells, the number of yeast cells per J774 cell that contained intracellular yeast cells was counted for >100 J774 cells per well. The percentage of phagocytic cells was determined by counting the number of J774 cells with intracellular yeast in 100 cells. Four fields were counted per well and were averaged. For each time, assays were also performed in wells of chamber slides (Nalge Nunc International, Naperville, Ill.), and immunohistochemistry for CNPS was performed using MAb 2H1 as described elsewhere (46). Statistical analysis was performed by Newman Keuls test after analysis-of-variance determination (Primer of biostatistics: the program, version 3.01; McGraw-Hill, Inc.).
RESULTS
Overview of pulmonary infection at the ultrastructural level.
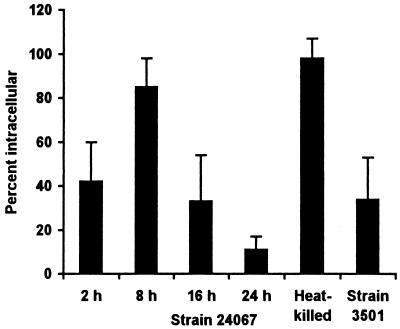
The course of pulmonary infection with ATCC strain 24067 in C57BL/6 and A/JCr mice has been described previously at the light microscopic level by us and by others (18, 20, 31). Alveolar macrophages are known to be the major phagocytic cell in the lung (20). Preliminary experiments revealed that a substantial number of yeast cells were located inside macrophages at 2 h after infection, but by 24 h most yeast cells were extracellular. To gain insight into this observation, we determined the proportion of intracellular yeast at additional times in the first 24 h after infection (Fig. 1). The percentage of yeast cells found inside phagocytic cells was maximal at 8 h and then declined until 24 h. Many macrophages containing C. neoformans had a low electron density relative to the adjacent cells, a finding consistent with cell damage (Fig. 2). Neutrophils were often observed in close proximity to dead or dying macrophages. The shift from intracellular predominance to extracellular predominance coincided with the appearance of cellular debris in the extracellular space in close proximity to yeast cells (data not shown), which may have resulted from host cell or cryptococcal cell destruction. Hence, the initial course of pulmonary infection in mice involves rapid phagocytosis of C. neoformans cells, macrophage toxicity, neutrophil influx, and cellular disruption.
FIG. 1.
Location of C. neoformans. The percentage of intracellular yeast for each group was determined by counting all yeast cells on 4 or 5 noncontiguous grids for each of two mice (8 to 10 grids per group). Heat-killed yeast of strain 24067 and live yeast of strain 3501 were studied 24 h after infection. Bars represent means; error bars denote standard deviations. All pairwise comparisons between tissues infected with strain 24067 were statistically significantly different as determined by Student's t test with the Bonferroni correction except for comparison between the tissues obtained at 2 and 16 h and between the tissue obtained at 8 h and tissue from mice infected with heat-killed yeast.
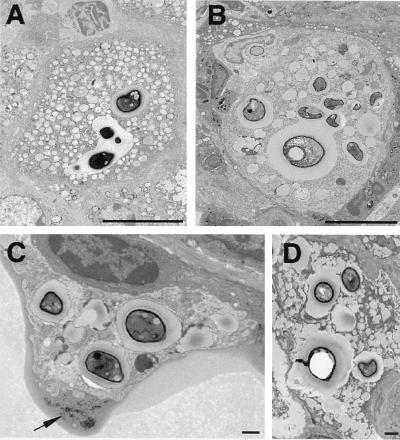
FIG. 2.
Intracellular replication and cytopathic effects of C. neoformans. (A and B) Phagosomes containing multiple yeast cells, demonstrating heterogeneity of size within the phagosome and budding forms. Bars, 10 μm. Time after infection: A, 28 days; B, 7 days. (C) Macrophage containing several intracellular C. neoformans cells at 7 days shows cytoplasmic disruption. The arrow points to membrane-bound cellular debris seen in proximity to this cell, suggesting that cellular destruction is a consequence of infection. (D) Multinucleated giant cell at 28 days demonstrating numerous intracellular yeast cells and abundant cytoplasmic vacuolation. Bars, 1 μm.
At 48 h and 7 days after infection, C. neoformans cells were found in both intracellular and extracellular spaces. Beginning 7 days after infection, there was a shift toward intracellular predominance that coincided with the appearance of multinucleated cells and granulomatous inflammation. Nevertheless, there was considerable inter- and intraexperimental variation, such that in some tissue blocks there was extracellular predominance, whereas in others most yeast cells were inside phagocytic cells. Granulomatous inflammation, as defined by the presence of organized collections of macrophages (1), was present in lung tissue at 14 and 28 days. By 28 days, the location of C. neoformans was predominantly intracellular, and most yeast cells were found inside multinucleated giant cells. No yeast cells were observed inside blood vessels.
Neutrophil infiltration.
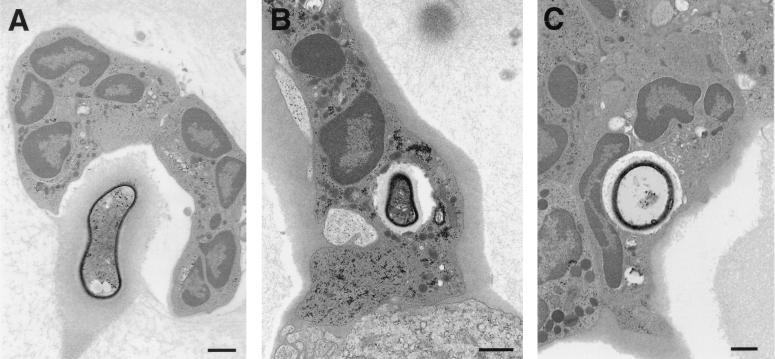
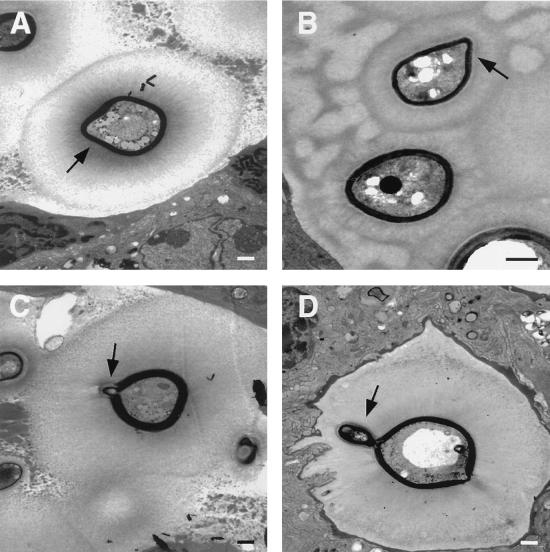
At 2 and 8 h after infection, occasional neutrophils were present adjacent to extracellular cryptococci (Fig. 3). Neutrophils were observed only in infected areas of the lung and were the only inflammatory cells noted in contact with yeast apart from macrophages. By 16 h after infection, clusters of one to three neutrophils were present near extracellular yeast cells and adjacent to macrophages containing intracellular yeast. At 48 h after infection, occasional collections of neutrophils consisting of one to four cells abutted extracellular yeast. Neutrophils were present through day 7, after which time they were rarely seen. On day 7 after infection, collections of inflammatory cells were more prominent, and larger groups of neutrophils were seen, some of which contained intracellular yeast. Others surrounded dead macrophages with intracellular yeast. Cryptococcal CNPS was only detected by immunoelectron microscopy in phagosomes that contained yeast, suggesting that neutrophils did not phagocytose shed polysaccharide (data not shown).
FIG. 3.
Neutrophils in close contact with C. neoformans in tissue. (A) A collection of neutrophils abuts an extracellular yeast in the alveolar space 48 h after infection. (B and C) At 7 days after infection, neutrophil phagocytosis of C. neoformans is seen, suggesting a role for neutrophils in host defense in vivo. Bars, 1 μm.
Phagolysosomal fusion.
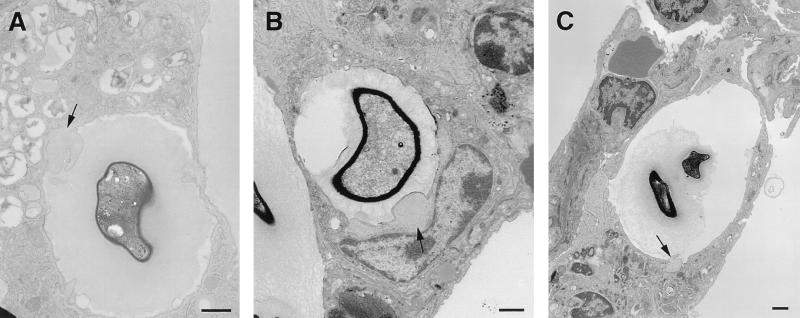
Phagolysosomal fusion was observed in alveolar macrophages 2 h after infection (Fig. 4). In addition, acid phosphatase-positive vesicles and tubular structures were in close proximity to phagosomes containing yeast cells, and acid phosphatase activity at the periphery of some phagosomes demonstrated phagolysosomal fusion. At 14 days, acid phosphatase reaction product lined the inside of many phagosomes. In phagosomes that contained acid phosphatase activity, most staining was present at the outer surface of the capsule. In uninfected macrophages, acid phosphatase reaction product was limited to well-demarcated lysosomal structures. No reaction product was seen in infected control tissue incubated without substrate. Consistent with prior studies, acid phosphatase-positive vacuoles were present inside C. neoformans (33, 43), but no activity was present in the capsule of extracellular yeast or at the phagosome periphery when lysosomes were not in contact with phagosomes. These findings suggest that the acid phosphatase lining the phagosomes originated from macrophages.
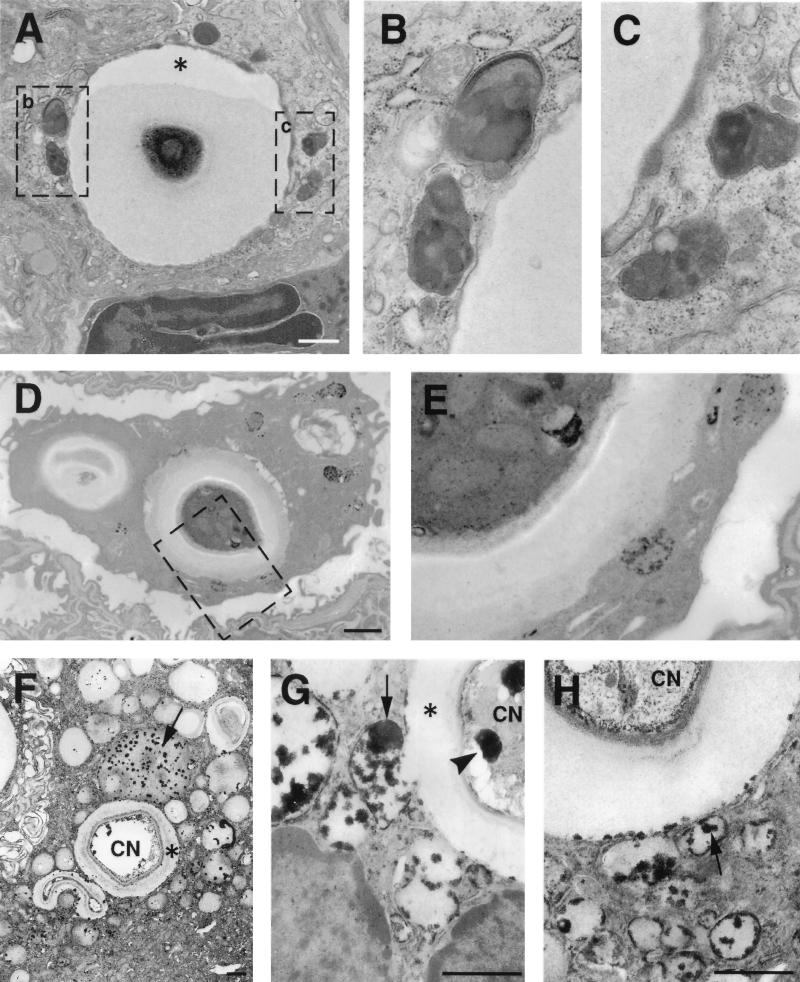
FIG. 4.
Phagolysosomal fusion. (A) Intracellular cryptococcus 2 h after infection. Macrophage lysosomes have moved toward the yeast and appear to fuse with the phagosome. Asterisk denotes artifactual contraction of the cryptococcal capsule from the wall of the phagosome. (B and C) Higher magnification of areas contained within rectangles in panel A demonstrates the fusion of lysosomes with the phagosome. Electron-dense material at the periphery of the phagosome likely represents entry of lysosomal components into phagosome. (D and E) Acid phosphatase cytochemistry 2 h after infection demonstrating lysosomal fusion with the phagosome, with entry of the black reaction product into the phagosome. Panel E is a higher-magnification view of the rectangular region shown in panel D. (F to H) Vacuoles containing black deposits representing acid phosphatase activity (arrows) are fused with phagosomes containing C. neoformans on day 14. Many small cytoplasmic vacuoles without yeast have acid phosphatase activity, suggesting that they are lysosomes. Some of these vacuoles are in direct continuity with the phagosome (E), while others are discontinuous. (F) Acid phosphatase reaction product is also visible at the periphery of the phagosome. Bars, 1 μm.
Intracellular replication.
Examination of lung tissue sections provided strong evidence for intracellular replication of C. neoformans in macrophages. During the first 24 h, most phagosomes contained one organism, but after this time both the number of yeast cells per phagosome and the number of phagosomes per macrophage or multinucleated giant cell increased (Fig. 2; Table 1). The sizes of yeast cells within single phagosomes became increasingly heterogeneous, a finding consistent with ongoing budding. Yeast cells at a variety of stages in the budding process were observed intracellularly (data not shown). Analysis of lung sections from mice on day 14 with sizable collections of both intracellular and extracellular yeast showed that the mean budding index was higher for intracellular than for adjacent extracellular yeast (6.8 ± 5.3 versus 1.3 ± 1.4; P = 0.005 by paired Student t test). Although limited ultrastructural study of C. neoformans budding in vitro suggests that the capsule is thin at the leading edge of the bud (6, 35), budding extracellular yeast cells in the lung were contained within large masses of CNPS (Fig. 5). We also noted that, consistent with intracellular survival, the percentage of intact yeast cells in macrophages was 60 to 76% at 7, 14, and 28 days of infection.
TABLE 1.
Characteristics of macrophage phagosomes containing C. neoformans
| Time after infectiona | No. of yeast cells/macrophageb | No. of yeast cells/ phagosomec | No. of phagosomes/ macrophage | Yeast size variable within phagosome |
|---|---|---|---|---|
| 2 h | 1–2 | 1–2 | 1 | No |
| 8 h | 1–2 | 1–2 | 1–2 | +/−d |
| 16 h | 1–2 | 1–2 | 1–2 | Yes |
| 24 h | 1–5 | 1–5 (>3 rare) | 1–2 | No |
| 48 h | 1–3 | 1–3 | 1 | Yes |
| 7 days | 1–6 | 1–4 | 1–6 | Yes |
| 14 days | 1–11 | 1–9 | 1–5 | Yes |
| 28 days | 1–18 | 1–4 | 1–13 | Yes |
Results are from all experiments described in Materials and Methods.
Macrophage denotes a macrophage or multinucleated giant cell. Numbers indicate the range.
A phagosome was defined as a membrane-bound vacuole containing yeast in an interconnecting space. Phagosomes in which connection between vacuoles was not visible were counted as separate phagosomes.
Variability was present in some tissue blocks.
FIG. 5.
Geometry of extracellular budding. Extracellular organisms at various stages of budding. Photos were obtained in 129/SvEv mice 13 days after infection. Bars, 1 μm. Arrows point to buds. At all stages, the buds are encased in structures that label with MAb to CNPS. This phenomenon was seen in all three mouse strains and with all three encapsulated cryptococcal strains in C57BL/6 mice.
Effects of C. neoformans on macrophages.
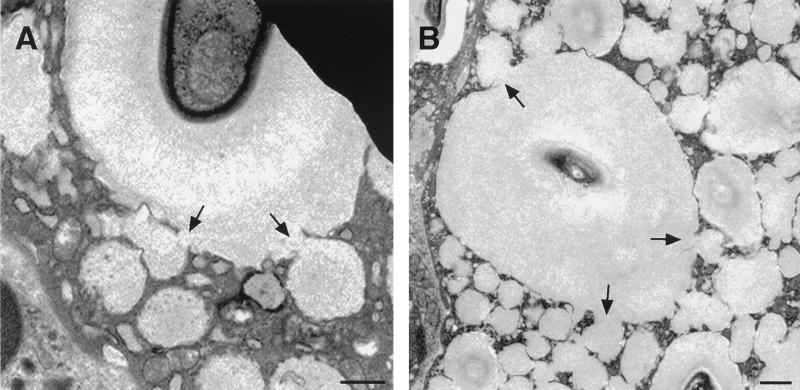
C. neoformans intracellular residence was associated with cytotoxicity to host phagocytic cells. At 2 h after infection macrophages with intracellular yeast appeared intact, but by 8 h after infection the cell membrane of some macrophages with intracellular yeast was disrupted, a result consistent with cell destruction as a consequence of phagocytosis of yeast. By 16 h after infection many macrophages had cytoplasmic areas with few organelles and lower electron density in regions of contact with extracellular and intracellular cryptococci (blebs) (Fig. 6).
FIG. 6.
Cytoplasmic blebs in macrophages with internalized C. neoformans. Adjacent to phagosomes containing yeast, the macrophage cytoplasm developed hypolucent areas (blebs) with few organelles (arrows) at regions of contact with extracellular cryptococci. These may represent a precursor for the increasing cytoplasmic disruption seen with increasing time after infection. Bars, 1 μm. Times after infection: A, 48 h; B, 8 h; C, 24 h.
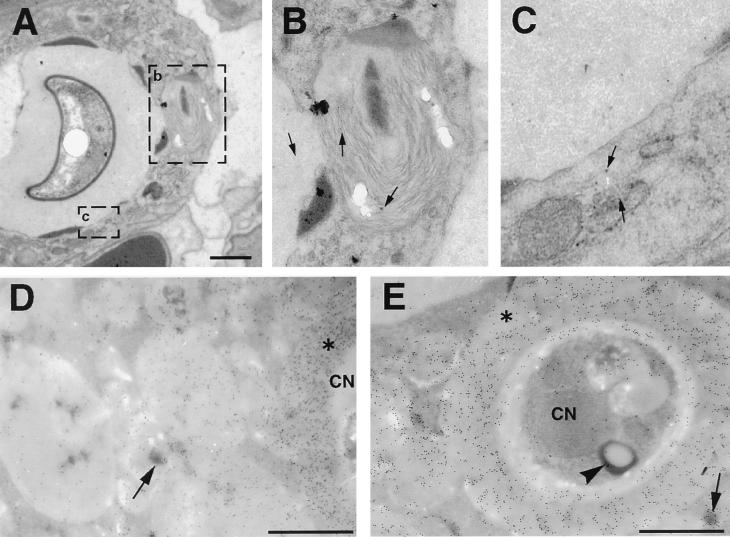
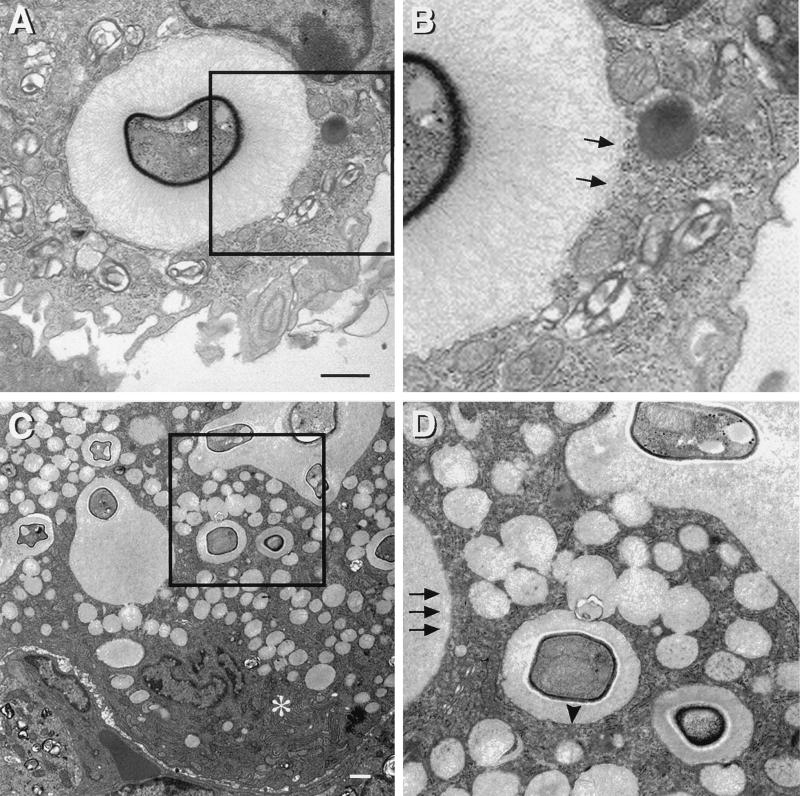
After 48 h, there was a gradual and progressive increase in the number of cytoplasmic vacuoles in phagocytic cells containing yeast. Some membrane-bound vacuoles contained lamellar-body-like structures that are seen in lysosomes, and others contained amorphous material. At 7 days vacuolation of the macrophage cytoplasm was more prominent, and by 14 and 28 days extensive vacuolation resulted in cytoplasmic disruption (Fig. 2). By light microscopy, the vesicles appear in toluidine blue-stained sections as holes in the macrophage cytoplasm (data not shown). Ultrastructural characteristics of cell death accompanied these findings, including rounding of macrophage nuclei and cytoplasmic disruption. Staining with MAb to CNPS was used to determine the vacuole contents and the intracellular location of cryptococcal CNPS (Fig. 7). No gold was present in sections incubated in normal mouse IgG or sections of uninfected lung incubated in MAb 2H1, indicating that staining was specific. Many gold particles were located inside vesicular structures that were 12 to 15 nm in diameter. By 48 h, gold was localized predominantly in intracytoplasmic vacuoles or in the small vesicular structures. Continuity between the CNPS-containing vacuoles and the phagosomes was apparent (Fig. 8). Many of these vacuoles also contained acid phosphatase activity, suggesting that they originated from lysosomes. The cytoplasmic disruption seen in association with progressive vacuolation and the presence of CNPS inside these vacuoles indicate a cytotoxic pathologic process. However, in areas of the lung in which large numbers of macrophages were present, cells appeared to be of two types—those that contained large numbers of yeast cells with the cytopathic changes described above and those that contained single yeast cells but were otherwise comparatively intact (data not shown). These findings were observed in all of the conditions studied.
FIG. 7.
Immunoelectron microscopy for CNPS. (A) Two hours after infection, lysosomes fuse with the phagosome containing C. neoformans. (B and C) Higher magnification of the areas within rectangles seen in panel A. Gold particles inside lysosomes indicate that the CNPS has entered the lysosome (arrows). (D and E) Colocalization of gold particles demonstrating the presence of CNPS, with acid phosphatase activity in cytoplasmic vacuoles 14 days after infection. Note the continuity between spaces containing CNPS and lysosomes. Sections were not counterstained with uranyl or lead. CN, C. neoformans; Asterisks indicate cryptococcal capsules. Arrows point to representative substrate deposition indicative of lysosomal acid phosphatase activity. Arrowhead denotes acid phosphatase activity inside C. neoformans. Bars, 1 μm.
FIG. 8.
Vacuole formation. (A and B) Pulmonary macrophages from two C57BL/6 mice infected for 14 days demonstrating the network of cytoplasmic vacuoles. Direct continuity is seen between the contents of cytoplasmic vacuoles and phagosomes (arrows). Bars, 1 μm.
Phagosome volume and membrane.
Phagosome volume in vivo and in vitro was a function of capsule volume for internalized C. neoformans cells (data not shown). The average phagosome volume increased with the time of infection, reflecting an increase in the average capsule size of C. neoformans cells in the lung. Several giant phagosomes having volumes of >10,000 μm3 were observed. Phagosome size increased incrementally with the time of infection from day 7 onward such that at days 7, 14, and 28 the volume of phagosomes containing single yeast cells were 98 ± 134 (n = 17 phagosomes), 254 ± 263 (n = 22), and 2,296 ± 2,383 (n = 10) μm3, respectively (P < 0.001; Kruskal-Wallis test). Analysis of several hundred phagosomes containing cryptococci revealed that in many cells the phagocytic membrane was discontinuous, even when nearby nuclear, plasma, and endoplasmic reticulum membranes appeared intact (Fig. 9). Macrophages with discontinuous phagosomal membranes were observed at all times of infection.
FIG. 9.
Phagosomal membrane disruption. Macrophage phagosomes containing C. neoformans in a C57BL/6 mouse 48 h after infection (A and B) and in an A/JCr mouse 14 days after infection (C and D) demonstrate areas in which the phagosome membrane is discontinuous (arrows), despite the appearance of well-defined membranes in cytoplasmic organelles. Panels B and D are enlargements of areas contained in the rectangles in panels A and C, respectively. The asterisk in panel C demonstrates well-preserved membranes in the Golgi apparatus. The arrowheads in panels C and D demonstrate sharply defined phagosomal membrane coexisting in a cell in which a phagosomal membrane is disrupted. Bars, 1 μm.
Fate of live versus dead C. neoformans.
The observation that at 24 h the majority of yeast cells were in the extracellular space suggested profuse extracellular replication, intracellular replication with lysis, or both. To investigate these possibilities, we compared the locations of live and heat-killed yeast cells instilled into mouse lungs. When heat-killed yeast cells were used, examination of lung tissue 24 h later revealed that all yeast cells were intracellular. However, cytopathic changes were seen inside cells containing heat-killed yeast, and neutrophils were prominent in the inflammatory infiltrate surrounding these macrophages (data not shown).
CNPS in intracellular pathogenesis.
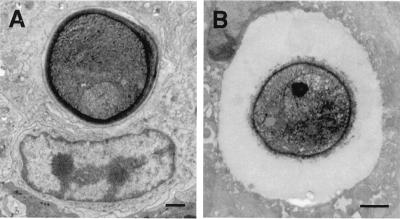
To investigate the requirement for capsule production for C. neoformans intracellular pathogenesis, we compared the outcome of pulmonary infection with the acapsular strain Cap 67 and its encapsulated parental strain 3501 (23). When the acapsular strain was instilled into the lungs, almost all yeast cells were found intracellularly in macrophages and neutrophils at 24 h after infection (data not shown). Macrophages contained one or two phagosomes per cell, with one yeast cell per phagosome. The macrophage cytoplasm and nuclei appeared normal. All yeast cells were approximately the same size, and no budding forms were found. At 14 days after infection, the lungs of mice infected with the acapsular strain were normal and no organisms were seen, a finding consistent with the clearance of pulmonary infection. In contrast, the appearance of lung tissue infected with strain 3501 for 24 h or 14 days was similar to that seen with strain 24067. Many macrophages contained multiple phagosomes with more than one yeast cell, and the macrophage cytoplasm contained vacuoles similar to those seen with strain 24067. In addition to intracellular polysaccharide synthesis, the polysaccharide capsule may contribute to intracellular survival by providing a buffer space between the site of lysosome-phagosome fusion and the fungal cell wall (Fig. 10). At 24 h after infection, the distance between the phagosome membrane and cell wall for phagocytosed encapsulated cells was 8.6 times that for the nonencapsulated strain (0.11 ± 0.03 μm [n = 8 cells] and 0.95 ± 0.05 μm [n = 12 cells; P <0.001 by Student's t test], for strains Cap 67 and 3501, respectively).
FIG. 10.
The distance between the phagosome membrane and the C. neoformans cell wall is dependent on the size of the capsule. (A) Phagosome containing a nonencapsulated Cap 67 mutant has the phagosomal membrane in close apposition to the fungal cell wall. (B) In a phagosome containing the encapsulated strain 3501, the polysaccharide capsule is interposed between the phagosomal membrane and the cell wall. Bars, 1 μm.
Studies with A/JCr and 129/SvEv mice and C. neoformans H99.
To establish whether the observations made were dependent on the mouse strain, experiments were performed with two additional murine strains. In both of these strains, intracellular budding yeast and extensive cytoplasmic vacuolation were observed at 14 and 28 days after infection. Variability occurred in the proportion of intracellular yeast and in the size of extracellular yeast. For A/JCr mice, intracellular replication and macrophage cytotoxicity were extensive, while in 129/SvEv mice, phagosomes containing multiple yeast cells were less commonly seen than in C57BL/6 mice. Hence, the phenomena of intracellular replication and macrophage cytotoxicity seen in C57BL/6 mice are found in other mouse strains, but there may be variability among macrophages from different mouse strains to control intracellular infection.
To establish whether the observations made with the serotype D strains 24067 and 3501 were restricted to this serotype, we conducted limited studies with the serotype A strain H99. At 2 h after infection, the majority of H99 cells in the alveolar space were ingested by macrophages (data not shown), a result paralleling what was found early during infection with strain 24067. At 14 days, cells of strain H99 were found inside macrophages, most of which showed signs of cytotoxicity, as represented by extensive vacuolar formation, lower electron density and, in some cases, disruption of plasma membranes. Hence, the results with all three strains representing both serotypes were qualitatively similar.
In vitro study.
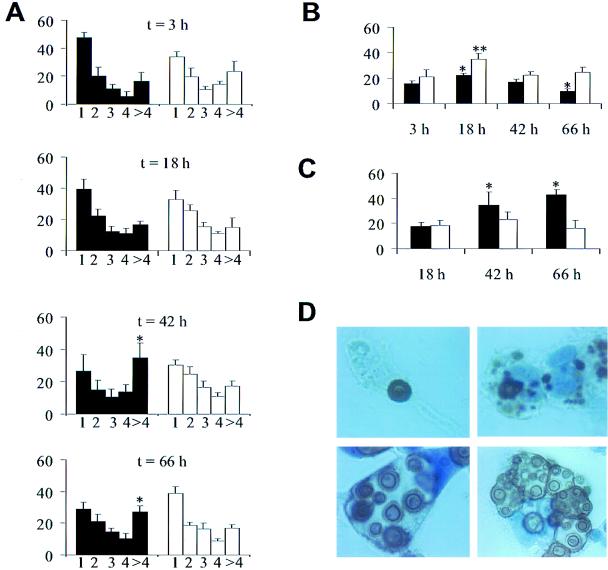
In vitro interactions of C. neoformans and macrophages were studied using J774 cells and complement-derived opsonins. Complement was used as an opsonin because C3 deposition has been demonstrated in the C. neoformans capsule in the alveolar space (18). In vitro experimentation confirmed that encapsulated, but not acapsular, yeast cells replicated intracellularly and were toxic to phagocytic cells. With increased incubation times, the number of yeast cells per phagocytic J774 cell increased for the encapsulated, but not the acapsular strain (Fig. 11). Furthermore, higher percentages of J774 cells with intracellular encapsulated yeast were dead or dying, but this was not the case with acapsular yeast cells. J774 cells with intracellular encapsulated yeast cells but not with intracellular acapsular yeast cells accounted for a disproportionately high percentage of dead J774 cells at 42 and 66 h (data not shown). Immunohistochemistry confirmed the presence of cytoplasmic CNPS, intracellular replication, and massively distended phagosomes in J774 cells with intracellular encapsulated yeast (Fig. 11).
FIG. 11.
In vitro phagocytosis assay with J774 cells and C. neoformans strains 3501 (encapsulated, black bars) and Cap 67 (acapsular, white bars). Values represent means; error bars denote standard deviations. (A) Percentage of J774 cells with number of yeast cells (n = 1, 2, 3, 4, or >4) per J774 cell after various times (t) of incubation. Statistical analysis compared the percentage of J774 cells with >4 yeast cells per cell. Asterisks denote P values of <0.05 compared to values at 3 h. (B) Percentage of J774 cells with intracellular C. neoformans after various times of incubation. An asterisk denotes a P value of <0.05 compared to the value at 3 h of incubation with strain 3501. A double asterisk denotes a P value of <0.05 compared to the value at 3 h of incubation with strain Cap 67. (C) Percentage of dead J774 cells that contained intracellular C. neoformans. An asterisk denotes a P value of <0.05 compared to the value at 18 h of incubation with strain 3501. The percentage of dead cells may be underestimated because dead cells become unattached and are washed off during the staining process. The loss of dead cells likely explains the decrease in the percentage of J774 cells with intracellular yeast at 66 h of incubation with strain 3501 seen in panel B. (D) Immunohistochemistry for CNPS using MAb 2H1. Top left, single cell of strain 3501 inside an intact J774 cell after 3 h of incubation; top right, J774 cell after 18 h of incubation with collections of CNPS and single intracellular yeast demonstrating that, in some cases, yeast cells appeared to be destroyed; bottom, J774 cells after 66 h of incubation with strain 3501 show cells with large vacuoles containing multiple cryptococci with budding forms (left) and cells with multiple phagosomes containing yeast cells heterogeneous in size, consistent with active replication (right). All panels were photographed at a magnification of ×1,000.
DISCUSSION
A long-held view in the field of C. neoformans pathogenesis is that the capsule promotes virulence by being antiphagocytic. This concept is based largely on the observation that phagocytic cells rarely ingest encapsulated cells without opsonins in vitro and on pathological studies that frequently show extracellular yeast cells in tissue. In the present study, we investigated the location of yeast cells at various times of infection by EM, which allows accurate localization of yeast to intra- and extracellular compartments through visualization of cellular landmarks. Practically all acapsular yeast cells were inside macrophages, but a small proportion of encapsulated cells were extracellular at all of the times of infection examined. This observation supports a role for the capsule in inhibiting phagocytosis in vivo. However, the more significant result appears to be that the capsule does not prevent phagocytosis in vivo. Phagocytosis in vivo may be explained by the fact that the lung contains opsonins for C. neoformans, such as complement and collectins (18, 55). The view that the polysaccharide capsule of encapsulated bacteria is not antiphagocytic in vivo was proposed almost 50 years ago (reviewed in reference 62) but is not widely accepted.
The location of C. neoformans cells in the lung is a function of the time of infection. Shortly after intratracheal infection, C. neoformans cells are found primarily inside alveolar macrophages. By 24 h, the majority of C. neoformans cells were in the extracellular space. The higher percentage of yeast cells found in the extracellular space at 24 h relative to that found at 2 h is consistent with intracellular replication followed by phagocytic cell lysis and release of live yeast into the extracellular space. The possibility of intracellular growth followed by cell lysis is supported by the present observation of cell debris in close proximity to yeast cells and also to reports that C. neoformans can replicate and lyse macrophage lineage cells in vitro (14, 36). In mice given heat-killed C. neoformans, the majority of yeast cells at 24 h were inside macrophages, demonstrating that limited clearance of dead yeast cells occurs by this time. Consequently, clearance of intracellular yeast is an unlikely explanation for our finding. The alternative explanation of extracellular predominance resulting from more rapid replication of C. neoformans in the extracellular space cannot be excluded, but in vitro data has shown that growth inside macrophages is more rapid than it is extracellularly (14).
The initial transition from intracellular predominance to extracellular predominance was associated with macrophage cytotoxicity and disruption, as indicated by (i) low cytoplasmic electron density and membrane disruption and (ii) the appearance of cell debris in the alveolar space in close proximity to extracellular yeast cells. Although EM cannot directly determine cell viability, the findings of reduced cytoplasmic electron density, rounding of nuclei, and disrupted cellular membranes are accepted EM criteria for cell damage and death. The fungal cell components responsible for macrophage cytotoxicity were not characterized. These cytotoxic changes observed early in the course of infection were unlike those described for later in infection. A second form of cellular alteration consisted of the formation of cytoplasmic blebs. Cytoplasmic blebs protruded into phagosomes and were apparent in macrophage luminal surfaces in close proximity to extracellular yeast cells. In 1993, Sakaguchi reported disorganization of actin filaments in cytoplasmic areas in contact with C. neoformans in hepatic granulomas (54). A similar phenomenon may be responsible for the cytoplasmic blebs observed in alveolar macrophages in this study. The occurrence of cytoplasmic blebs suggests that phagocytic cell contact with the C. neoformans capsule results in alteration of the cell cytoskeleton.
Mice developed granulomatous inflammation, a finding consistent with prior literature, which is indicative of a cell-mediated immune response (28, 47). However, in several murine pulmonary infection models, cellular immunity is insufficient to control infection in the lung, and mice die with large pulmonary fungal burdens (18, 20, 32). At later stages of infection (7 to 28 days), progression of the inflammatory response toward granulomatous inflammation was accompanied by a shift toward a preponderance of yeast cells being inside phagocytic cells. Phagocytosis of microorganisms can lead to killing of the microbe or to microbial replication inside phagocytic cells. Demonstrating that a pathogen replicates inside host cells in vivo remains a formidable undertaking because the technology does not exist for serial observations of undisturbed infected tissue in vivo. For C. neoformans, in vitro studies provide evidence for both intracellular killing and intracellular survival followed by replication (26, 36, 37, 61). Furthermore, pathological studies have been equivocal regarding the outcome of phagocytosis in vivo, since intracellular localization has been associated with both control and persistence of infection (57). Our results demonstrated that intracellular residence was associated with multiple yeast cells per phagosome, a higher budding index, and the emergence of a yeast cell population that was heterogeneous in size. We interpret these findings to be indicative of intracellular replication in vivo. Preferential phagocytosis of budding forms is an unlikely explanation for the measured higher budding index inside cells since there was a relative paucity of budding cells in the extracellular space and the extracellular buds were well encapsulated. The most straightforward explanation of the data is that C. neoformans replicates inside macrophages in vivo, thus validating similar observations previously made in vitro (14, 36, 38).
Intracellular residence is believed to confer significant advantages to microbes by insulating them from immune effector mechanisms and providing access to the nutrient-rich cellular interior but requires evasion of microbicidal mechanisms of phagocytic cells. Other facultative intracellular pathogens have specialized strategies for intracellular survival, such as prevention of phagolysosomal fusion (29), blockage of phagosomal acidification (2, 16), and escape from the phagosomal vacuole (24). For C. neoformans, these strategies are not applicable, since yeast cells are always in phagosomes, lysosomes fuse with the phagosome, and phagosomal acidification occurs normally in vitro (40). For C. neoformans, growth is enhanced at the acidic pH of the lysosome relative to the neutral pH of serum (39), and this may provide a growth advantage relative to yeast cells in the extracellular space. Survival of C. neoformans in the phagolysosome may be aided by two unique characteristics of this fungal pathogen: melanin synthesis and the polysaccharide capsule. Melanin synthesis has been demonstrated in yeast cells in lung tissue (49) and could potentially protect the fungal cell against oxidative burst products and microbicidal proteins in vitro (49, 60). The capsule separates the phagolysosomal membrane from the yeast cell wall, and this separation could limit the fungicidal effects of lysosomal products. Furthermore, the average phagosomal volume increased with time of infection because of increased capsule size. Large-volume phagosomes may promote intracellular survival by causing a greater dilution of antimicrobial substances released after lysosomal fusion.
This study demonstrates for the first time that macrophages ingesting C. neoformans in vivo are filled with vesicles containing capsular polysaccharide. The result is a very distinctive appearance for macrophages that, to our knowledge, has no counterpart in any other pathologic process. We have named these macrophages hueco cells (“hueco” means “hole” in Spanish). Inspection of micrographs from earlier studies suggests that cells resembling hueco cells also occur in human C. neoformans infection (27, 30, 48). The polysaccharide inside the vesicles may originate from phagocytosis of soluble extracellular polysaccharide or from intracellular polysaccharide production by ingested yeast. Our analysis of micrograph images strongly suggests that at least some of these vesicles arise from intracellular polysaccharide production. Direct connections were observed repeatedly between phagolysosomes containing C. neoformans and polysaccharide-containing vesicles, suggesting origin in association with the phagolysosome. Further, the size of individual vesicles was relatively homogeneous, suggesting that they arise from defined structures rather than from phagocytic events. We propose that the vesicles originate from lysosomes and develop following exchange of lysosomal contents with phagosomal polysaccharide after phagolysosomal fusion. Regardless of the mechanism by which vesicles arise, intracellular persistence of C. neoformans in vivo was accompanied by the accumulation of polysaccharide antigen in the cytoplasm of macrophages. This observation strongly suggests a role for capsular polysaccharide in intracellular pathogenesis.
Our study also yielded the first evidence that C. neoformans is cytotoxic to phagocytic cells in vivo. Host cell cytotoxicity is not generally considered to be a characteristic of C. neoformans pathogenesis. Although EM cannot directly indicate the vitality of a cell, inspection of macrophages containing yeast cells revealed that such cells had reduced cytoplasmic electron density and disruption. These findings are accepted EM correlates of cytotoxicity and cell death. In addition, we noted discontinuous phagosomal membranes in several vacuoles containing C. neoformans in cells with apparently intact nuclear and endoplasmic reticular membranes. Although we cannot exclude the possibility that the discontinuities in the vacuolar membrane resulted from fixation and processing, the simultaneous presence of intact membranes around other organelles in those cells argues against an artifactual effect. Furthermore, C. neoformans produces several enzymes that are potentially toxic to macrophages, including phospholipases and proteases (8, 9).
We found neutrophil phagocytosis of strain 24067 in C57BL/6 mice 7 days after infection, but not at earlier or later times, despite intimate association between these cells and yeast. The reason that the timing of ingestion appears to be limited is unclear, but one possibility is that neutrophils require a narrowly defined effector/target ratio, as they do in vitro (11). The function of neutrophils in the host response to cryptococcal infection is unclear. A role for neutrophils as antifungal effector cells has been suggested by in vitro and ex vivo studies (34, 56). In vitro, yeast cells must be ingested to be killed by neutrophils (13, 44, 53, 59). Here we report the first demonstration of phagocytosis of C. neoformans by neutrophils in vivo, supporting the possibility that neutrophils contribute to cryptococcal killing. Data from human control nervous system autopsy series suggests that neutrophils may be recruited in response to tissue damage resulting from infection rather than in response to the yeast itself (42). Our finding that neutrophils were commonly associated with damaged macrophages containing intracellular heat-killed yeast may represent such neutrophil recruitment to damaged tissue. CNPS stimulates human neutrophils to produce proinflammatory cytokines (52), suggesting a potential immunomodulatory role.
Several studies have shown that C. neoformans can replicate inside human monocytes and microglia in vitro, but comparable studies have not been done with murine-derived cells. To investigate whether the process observed in mice could be simulated in vitro, we infected J774 cells with encapsulated and acapsular C. neoformans and monitored the course of infection over 48 h. This cell line displays many macrophage properties and kills C. neoformans when provided with antibody opsonins. In our experiment, we used complement as the opsonin because C3 is available in the alveolar space, whereas antibody responses are seldom made during murine infection (18). Acapsular cells did not replicate intracellularly and were not cytotoxic for J774 cells. In contrast, encapsulated cells replicated intracellularly and killed the phagocytic cell. These results support the proposal that the capsule is important for intracellular survival and replication and that C. neoformans cells can be cytotoxic to phagocytic cells.
In summary, our results indicate that C. neoformans can replicate inside phagocytic cells in vivo, thus establishing this fungus as a facultative intracellular pathogen in vivo. We propose that C. neoformans intracellular replication and phagocytic cell destruction are major components of the pathogenic process for cryptococcal infection in the lung. Persistence of infection in lung tissue may involve repeating cycles of phagocytosis, intracellular residence, and phagocytic cell destruction. Fungal replication in murine C. neoformans pulmonary infection appears to occur both intra- and extracellularly. Intracellular replication in vivo was associated with the accumulation of macrophage cytoplasmic vesicles that were filled with capsular polysaccharide and phagocytic cytotoxicity. The C. neoformans capsule appears to have a very complex role in pathogenesis, functioning both offensively and defensively. These observations, together with prior studies, imply that C. neoformans occupies a special niche among intracellular pathogens, since the combined association of intracellular polysaccharide production, melanin synthesis, phagocytic cell vacuolation, and cytotoxicity represents a unique strategy for intracellular persistence. These results are exciting because they suggest many new directions for the study of C. neoformans pathogenesis.
ACKNOWLEDGMENTS
M.F. is supported by NIH grant AI01341. P.N. is supported by NIH grant CA06576. A.C. is supported by NIH grants AI22774, AI13342, and HL59842. A. Casadevall is a recipient of a Burroughs-Wellcome Fund Scholar Award in Experimental Therapeutics.
We thank Jorge Bermudez for assistance with histopathology, Clemen Cayetano and Valentin Storovoytov for assistance with electron microscopy, and the Graphic Arts Center of the Albert Einstein College of Medicine. We also thank Gregory Serdahl, without whose assistance this work could not have been completed.
REFERENCES
- 1.Adams D O. The granulomatous inflammatory response. Am J Pathol. 1976;84:164–191. [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine J-C, Prina E, Jouanne C, Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L-A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: American Society for Microbiology; 1998. [Google Scholar]
- 6.Cassone A, Simonetti N, Strippoli V. Wall structure and bud formation in Cryptococcus neoformans. Arch Microbiol. 1974;95:205–212. [Google Scholar]
- 7.Chen L C, Goldman D L, Doering T L, Pirofski L, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67:2218–2224. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L-C, Blank E S, Casadevall A. Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol. 1996;3:570–574. doi: 10.1128/cdli.3.5.570-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S C A, Muller M, Zhou J Z, Wright L C, Sorrell T C. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175:414–420. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- 10.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 11.Davies S F, Clifford D P, Hoidal J R, Repine J E. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1982;145:870–874. doi: 10.1093/infdis/145.6.870. [DOI] [PubMed] [Google Scholar]
- 12.DeShaw M, Pirofski L-A. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond R D, Root R K, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 14.Diamond R D, Bennett J E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973;7:231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doty S B, Smith C E, Hand A R, Oliver C. Inorganic trimetaphosphatase as a histochemical marker for lysosomes in light and electron microscopy. J Histochem Cytochem. 1977;25:1381–1384. doi: 10.1177/25.12.200672. [DOI] [PubMed] [Google Scholar]
- 16.Eissenberg L G, Goldman W E, Schlesinger P H. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med. 1993;177:1605–1611. doi: 10.1084/jem.177.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis D H, Pfeiffer T J. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet. 1990;336:923–925. doi: 10.1016/0140-6736(90)92283-n. [DOI] [PubMed] [Google Scholar]
- 18.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 19.Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect Immun. 1997;65:1899–1907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmesser M, Kress Y, Casadevall A. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J Infect Dis. 1998;177:1639–1646. doi: 10.1086/515314. [DOI] [PubMed] [Google Scholar]
- 21.Franzot S P, Mukherjee J, Cherniak R, Chen L-C, Hamdan J S, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franzot S P, Salkin I F, Casadevall A. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J Clin Microbiol. 1999;37:838–840. doi: 10.1128/jcm.37.3.838-840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromtling R A, Shadomy H J, Jacobson E S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia. 1982;79:23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- 24.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger D L, Perfect J R, Durack D T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986;136:672–680. [PubMed] [Google Scholar]
- 27.Granier F, Kanitakis J, Hermier C, Zhu Y Y, Thivolet J. Localized cutaneous cryptococcosis successfully treated with ketoconazole. J Am Acad Dermatol. 1987;16:243–249. doi: 10.1016/s0190-9622(87)80073-7. [DOI] [PubMed] [Google Scholar]
- 28.Graybill J R, Alford R H. Cell-mediated immunity in cryptococcosis. Cell Immunol. 1974;14:12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- 29.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hino H, Takizawa K, Asboe-Hansen G. Ultrastructure of Cryptococcus neoformans. Acta Dermatovenerol Ingosl. 1982;62:113–117. [PubMed] [Google Scholar]
- 31.Huffnagle G B, Boyd M B, Street N E, Lipscomb M F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 32.Huffnagle G B, Chen G-H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 33.Kalina M, Bubis J J, Kletter Y, Shahar A, Aronson M. Ultrastructural localization of acid phosphatase in the yeast Cryptococcus neoformans. Experientia. 1970;26:287–288. doi: 10.1007/BF01900099. [DOI] [PubMed] [Google Scholar]
- 34.Kalina M, Kletter Y, Shahar A, Aronson M. Acid phosphatase release from intact phagocytic cells surrounding a lare-sized parasite (35275) Proc Soc Exp Biol Med. 1971;136:407–410. doi: 10.3181/00379727-136-35275. [DOI] [PubMed] [Google Scholar]
- 35.Kozel T R, Wilson M A, Murphy J W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991;59:3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S C, Kress Y, Zhao M-L, Dickson D W, Casadevall A. Cryptococcus neoformans survive and replicate in human microglia. Lab Investig. 1995;73:871–879. [PubMed] [Google Scholar]
- 37.Levitz S M, DiBenedetto D J. Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J Immunol. 1989;142:659–665. [PubMed] [Google Scholar]
- 38.Levitz S M, Farrell T P. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990;58:1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitz S M, Nong S-H, Seetoo K F, Harrison T S, Speizer R A, Simons E R. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun. 1999;67:885–890. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Littman M L. Cryptococcosis (torulosis) Am J Med. 1959;27:976–988. doi: 10.1016/0002-9343(59)90181-0. [DOI] [PubMed] [Google Scholar]
- 42.Lovchik J A, Lipscomb M F. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1993;9:617–627. doi: 10.1165/ajrcmb/9.6.617. [DOI] [PubMed] [Google Scholar]
- 43.Mahvi T A, Spicer S S, Wright N J. Cytochemistry of acid mucosubstance and acid phosphatase in Cryptococcus neoformans. Can J Microbiol. 1974;20:833–838. doi: 10.1139/m74-128. [DOI] [PubMed] [Google Scholar]
- 44.Miller G P G, Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983;131:1455–1459. [PubMed] [Google Scholar]
- 45.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy J W. Cryptococcal immunity and immunostimulation. Adv Exp Med Biol. 1992;319:225–230. doi: 10.1007/978-1-4615-3434-1_23. [DOI] [PubMed] [Google Scholar]
- 48.Noble R C, Fajardo L F. Primary cutaneous cryptococcosis: review and morphologic study. Am J Clin Pathol. 1971;57:13–22. doi: 10.1093/ajcp/57.1.13. [DOI] [PubMed] [Google Scholar]
- 49.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Novikoff P M, Yam A. Sites of lipoprotein particles in normal rat hepatocytes. J Cell Biol. 1978;76:1–11. doi: 10.1083/jcb.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papadimitriou J M, Robertson T A, Kletter Y, Aronson M, Walters M N-I. An ultrastructural examination of the interaction between macrophages and Cryptococcus neoformans. J Pathol. 1978;124:103–109. doi: 10.1002/path.1711240206. [DOI] [PubMed] [Google Scholar]
- 52.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel T R. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson M D, White L J, McKay T C, Shankland G S. Differential binding of acapsulate and encapsulated strains of Cryptococcus neoformans to human neutrophils. J Med Vet Mycol. 1993;31:189–199. [PubMed] [Google Scholar]
- 54.Sakaguchi N. Ultrastructural study of hepatic granulomas induced by Cryptococcus neoformans by quick-freezing and deep-etching method. Virchows Arch. 1993;64:57–66. doi: 10.1007/BF02915096. [DOI] [PubMed] [Google Scholar]
- 55.Schelenz S, Malhotra R, Sim R B, Holmskov U, Bancroft G J. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect Immun. 1995;63:3360–3366. doi: 10.1128/iai.63.9.3360-3366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneerson-Porat S, Shahar A, Aronson M. Formation of histiocyte rings in response to Cryptococcus neoformans infection. J Reticuloendothelial Soc. 1965;2:249–255. [PubMed] [Google Scholar]
- 57.Schwartz D A. Characterization of the biological activity of Cryptococcus infections in surgical pathology. The budding index and carminophilic index. Ann Clin Lab Sci. 1988;18:388–397. [PubMed] [Google Scholar]
- 58.Sheppe W M. Torula infection in man. Am J Med Sci. 1924;167:91–108. [Google Scholar]
- 59.Tacker J R, Farhi F, Bulmer G S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972;6:162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberg P B, Becker S, Granger D L, Koren H S. Growth inhibition of Cryptococcus neoformans by human alveolar macrophages. Am Rev Respir Dis. 1987;136:1242–1247. doi: 10.1164/ajrccm/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 62.Wood W B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;XLVII:72–98. [PubMed] [Google Scholar]
- 63.Yamaoka H, Sakaguchi N, Sano K, Ito M. Intravascular granuloma induced by intravenous inoculation of Cryptococcus neoformans. Mycopathologia. 1996;133:149–158. doi: 10.1007/BF02373022. [DOI] [PubMed] [Google Scholar]