Summary
Background
People with HIV who inject drugs experience intersecting forms of stigma that adversely impact care access. This RCT aimed to evaluate effects of a behavioral intersectional stigma coping intervention on stigma and care utilization.
Methods
We recruited 100 participants with HIV and past-30-day injection drug use at a non-governmental harm reduction organization in St. Petersburg, Russia, and randomized them 1:2 to receive usual services only or an additional intervention of three weekly 2-h group sessions. Primary outcomes were change in HIV and substance use stigma scores at one month after randomization. Secondary outcomes were initiation of antiretroviral treatment (ART), substance use care utilization, and changes in frequency of past-30-days drug injection at six months. The trial was registered as NCT03695393 at clinicaltrials.gov.
Findings
Participant median age was 38.1 years, 49% were female. Comparing 67 intervention and 33 control group participants recruited October 2019–September 2020, the adjusted mean difference (AMD) in change in HIV and substance use stigma scores one month after baseline were 0.40, (95% CI: −0.14 to 0.93, p = 0.14) and −2.18 (95% CI: −4.87 to 0.52, p = 0.11), respectively. More intervention participants than control participants initiated ART (n = 13, 20% vs n = 1, 3%, proportion difference 0.17, 95% CI: 0.05–0.29, p = 0.01) and utilized substance use care (n = 15, 23% vs n = 2, 6%, proportion difference 0.17, 95% CI: 0.03–0.31, p = 0.02). The adjusted median difference in change in injecting drug use frequency 6 months after baseline was −3.33, 95% CI: −8.51 to 1.84, p = 0.21). Five not intervention-related serious adverse events (7.5%) occurred in the intervention group, one (3.0%) serious adverse event in the control group.
Interpretation
This brief stigma-coping intervention did not change stigma manifestations or drug use behaviors in people with HIV and injection drug use. However, it seemed to reduce stigma's impact as an HIV and substance use care barrier.
Funding
R00DA041245, K99DA041245, P30AI042853.
Keywords: Acceptance and commitment therapy, Stigmatization, Discrimination, Injection drug use, Opioid use disorder, Russian Federation
Research in context.
Evidence before this study
Oppressive power structures and systems, criminalization of HIV and key populations, and the resulting HIV stigma are central barriers to ending the HIV epidemic. HIV stigma adversely affects the entire HIV care cascade and health of PWH. HIV stigma interventions in generalized HIV epidemics have targeted structural and provider stigma, and have sought to empower PWH to cope with stigma. Key populations for ending the HIV epidemic such as people who inject drugs often experience intersecting stigma manifestations: In addition to HIV stigma, they are also affected by stigma related to their substance use and other traits and conditions, and by the intersection of these stigma manifestations. Key population communities have long called to act on stigma. However, there is a lack of interventions specifically targeting HIV stigma among people who inject drugs, or other forms of stigma such as substance use stigma and its intersection with HIV stigma.
We conducted a PubMed search using the keywords “stigma,” “HIV,” “substance use,” and “intervention,” up to August 2022, which yielded no stigma intervention evaluations for people with HIV who inject drugs. One previous study conducted in a residential substance use treatment setting in the U.S.A. found Acceptance and Commitment Therapy (ACT) effective in increasing engagement in substance use care.
Added value of this study
This study provides evidence on a brief, effective stigma intervention that specifically targets people with HIV who inject drugs, complementing existing stigma interventions for other populations. Findings demonstrate that a three-session group stigma intervention is feasible to implement in Russia and improves key HIV and substance use outcomes, including increasing ART initiation and increasing engagement in substance use care among people with HIV who inject drugs. This study recruited clients at a harm reduction organization who are not in substance use care or on ART, i.e., participants who at least partly due to stigma have not been connected to the formal healthcare setting in Russia. Our findings reinforce that in settings where stigmatizing attitudes remain pervasive, stigma-coping interventions empowering affected people can attenuate stigma's impact on substance use and HIV care.
Implications of all the available evidence
Getting key populations for HIV into care is paramount to ending the HIV epidemic. This behavioral group intervention, integrated into existing clinical and community services, could be an effective strategy to connect people with HIV who inject drugs to HIV and substance use care. Future studies need to investigate whether such empowerment intervention can improve HIV and substance use outcomes in the long term. Given that we observed no impact on stigma scores, multi-level interventions need to also address provider and structural stigma affecting this population. To get to zero AIDS, policies are needed for stigma-informed prevention and care specifically targeting key populations involving integrated multi-level interventions that also address the various manifestations of public, structural and individual-level stigma. Ultimately, addressing intersectional stigma will require not only stigma coping interventions grounded in intersectionality, but sharing power with communities in interlocking systems of power that extend beyond HIV and substance use.
Introduction
Stigma, the negative labeling and devaluation of people based on an undesired social category, is a well-documented barrier to care and has negative health effects.1 Stigma is rooted in power imbalances and social inequalities,2 and negatively impacts health care and health of people with HIV, including those who inject drugs.
HIV stigma manifestations, including enacted (experienced), anticipated (expected) and internalized stigma (endorsing stigmatizing attitudes),1 have adverse effects on the entire HIV care cascade. These range from delayed testing and care to low rates of antiretroviral treatment (ART) initiation and adherence, thereby impeding viral load suppression.3
In addition to HIV stigma, the key population of people who inject drugs also faces widespread stigma related to their substance use.4 Experiences of substance use stigma and negative perceptions about addiction treatment are associated with adverse healthcare outcomes such as impaired utilization of and retention in addiction treatment, less access to harm reduction services, and reduced ART adherence.5, 6, 7, 8 Substance use stigma is also linked with increased drug use and other HIV risk behaviors.5
Intersectionality theory suggests that people experience multiple forms of stigma in interlocking systems and structures of oppressive power.9 Intersectional stigma results in reciprocally constructed stigma manifestations that are not unitary, mutually exclusive entities.10 As people with HIV who inject drugs often avoid care to avoid stigmatization due to both living with HIV and substance use, intersectional stigma reduces engagement in medical care and thus impedes ending the global HIV epidemic. In Russia and other settings in Eastern Europe and Central Asia, HIV has been primarily transmitted through unsafe injection of opioids, more recently predominantly of illicitly produced methadone.11 The composition, quality, pharmacokinetics and clinical characteristics of street methadone used in St. Petersburg are heterogeneous and largely unknown.12
HIV incidence and mortality continue to increase in spite of global progress.13 We previously found that people with HIV who inject drugs in Russia and who manifested high intersectional stigma had less access to and utilization of HIV and addiction treatment than those with less manifestations, which was not explained by either form of stigma alone.14 Given the various other structural barriers to HIV and substance use care in Russia, such as the need to register with the government authorities to receive care for HIV or substance use disorders in the public health services, the separation of care systems for these and other conditions, or the legal prohibition of agonist treatment for opioid use disorder, stigma substantially impedes this key population's care engagement.
As negative societal attitudes toward key HIV populations such as PWID persist in Russia and globally, a primary target of an intervention to reduce stigma as a care barrier may be to help people cope with stigma manifestations through acceptance-based approaches. Rather than trying to directly dispute internalized stigmatizing attitudes, acceptance-based approaches serve to reduce the conditioned link between internalized attitudes, fears, and shame and resultant avoidance behaviors that interfere with healthcare seeking and other adaptive behaviors. A previous randomized controlled trial of Acceptance and Commitment Therapy (ACT) to help people with addictions in residential treatment address internalized stigma demonstrated improvements in treatment engagement and reduction in shame.15 Given stigma's previously identified adverse impact on health and care, particularly important targets for an acceptance-based stigma intervention are health care engagement, mental health, and injection drug use. While there are manifold efforts aiming to reduce providers' and the public's stigmatizing attitudes,16,17 evidence on the effectiveness of stigma interventions specifically targeting people with HIV who inject drugs in order to facilitate care engagement is limited.1,18
As part of the SCRIPT (Stigma Coping to Reduce HIV Risks and Improve substance use Prevention and Treatment) study, we therefore designed an intervention to help people cope with intersectional HIV and substance use stigma manifestations and with stigma-related care avoidance. We modified an ACT-based substance use stigma intervention approach4 to target people with HIV who inject drugs. ACT is a type of cognitive-behavioral therapy, which we adapted for a non-therapy community context, as this study's stigmatized potential participants usually have limited access to formal care sectors. We hypothesized that providing people who are not on ART or in substance use care due to intersectional stigma with stigma-coping skills would reduce their stigma manifestations and improve ART initiation, substance use care utilization and substance use. The SCRIPT study aimed to evaluate the intervention's effects in this population on HIV and substance use stigma, care engagement, and injection drug use frequency; and to study this novel intervention's implementation assessing participant satisfaction, intervention fidelity and uptake.
Methods
Study design
SCRIPT was an open label, randomized, controlled clinical trial to evaluate a behavioral intervention aimed at empowering people with HIV who inject drugs in St. Petersburg, Russia, cope with HIV and substance use stigma. The behavioral intervention, adapted by members of the community, practitioners and researchers, consisted of mindfulness, acceptance, and values-focused group sessions based on Acceptance and Commitment Therapy (ACT)19 that aimed to improve coping with stigma-related shame and fear, reduce HIV and substance use risk behaviors, and improve care engagement with the ultimate goal of improving overall health. Recruitment took place at a civil society organization that provides free harm reduction and HIV prevention services in outreach buses in St. Petersburg. Due to lack of space on the buses, we conducted trial assessments and intervention group sessions at a community clinic that provides outpatient substance use treatment services. The study protocol was described in detail elsewhere20 and was approved by Institutional Review Boards at Boston University Medical Campus and Pavlov University (clinicaltrials.gov NCT03695393). We report our trial following the extended CONSORT guidelines.
Participants
We recruited 100 participants (see Table 1) between October 2019 and September 2020 at the outreach buses. Eligibility criteria were: age 18 years or older; HIV-positive status by self-report; injection drug use in the past 30 days; not currently on ART; provision of two contacts to assist with follow-up; address within 100 km of the study site; not enrolled in any other research studies; possession of a phone; ability and willingness to comply with study protocols and procedures over six months. Exclusion criteria were: not fluent in Russian; cognitive impairment precluding informed consent; acute severe psychiatric illness (i.e., hallucinations, suicidal plans, or psychosis). Participants used a range of drugs with details summarized in the Appendix. All participants provided written informed consent.
Table 1.
Baseline characteristics of participants in the SCRIPT study, 2019–2021.
| Total N = 100 | Intervention N = 67 | Control N = 33 | |
|---|---|---|---|
| Age in years, mean (SD; min-max) | 38.1 (5.3; 19.0–55.0) | 38.3 (4.9; 19.0–55.0) | 37.7 (6.4; 22.0–50.0) |
| Gender, n (%) | |||
| Male | 51 (51%) | 34 (51%) | 17 (52%) |
| Female | 49 (49%) | 33 (49%) | 16 (48%) |
| Race | |||
| White | 96 (96%) | 65 (97%) | 31 (94%) |
| Asian | 1 (1%) | 0 (0%) | 1 (3%) |
| Other | 3 (3%) | 2 (3%) | 1 (3%) |
| Marital status, n (%) | |||
| Married/Domestic partnership | 67 (67%) | 43 (64%) | 24 (73%) |
| Long-term relationship but not living together | 6 (6%) | 4 (6%) | 2 (6%) |
| Single/Divorced/Widowed/Separated | 27 (27%) | 20 (30%) | 7 (21%) |
| Education, n (%) | |||
| Middle school or less | 27 (27%) | 17 (25%) | 10 (30%) |
| High school/College | 70 (70%) | 47 (70%) | 23 (70%) |
| Higher Education | 3 (3%) | 3 (4%) | 0 (0%) |
| Employment status, n (%) | |||
| Employed full- or part-time | 42 (42%) | 26 (39%) | 16 (48%) |
| Looking for work, unemployed | 38 (38%) | 27 (40%) | 11 (33%) |
| Disabled | 10 (10%) | 7 (10%) | 3 (9%) |
| Other (Temporarily laid off/Sick leave/Maternity leave/Retired/Homemaker/Student) | 10 (10%) | 7 (10%) | 3 (9%) |
| Individual monthly income from all legal and non-legal sources, before taxes, n (%) | |||
| No individual income | 6 (6%) | 4 (6%) | 2 (6%) |
| ≤20,000 rubles (∼273 USD) | 43 (43%) | 28 (42%) | 15 (45%) |
| 20,001–40,000 rubles (∼273–546 USD) | 36 (36%) | 25 (37%) | 11 (33%) |
| >40,001 rubles (∼546 USD) | 15 (15%) | 10 (15%) | 5 (15%) |
| Stable place to live in the past 30 days, n (%) | 97 (97%) | 66 (99%) | 31 (94%) |
| History of any criminal arrests, n (%) | 94 (94%) | 62 (93%) | 32 (97%) |
| Ever on ART, n (%) | 39 (39%) | 30 (45%) | 9 (27%) |
| HIV care utilization (visited infection disease physician for HIV within inpatient or outpatient setting in the past 6 months) | 37 (37%) | 26 (39%) | 11 (33%) |
| Substance use care utilization (treated for a substance use disorder in the inpatient/outpatient setting or participated in AA, NA, or any other alcohol or drug 12 step self-recovery program in the past 6 months) | 13 (13%) | 10 (15%) | 3 (9%) |
| Depressive symptoms—PHQ-9a | |||
| Mean score (SD; min-max) | 8.42 (5.73; 0.00–21.00) | 8.55 (5.79; 0.00–21.00) | 8.15 (5.69; 0.00–19.00) |
| Minimal/mild, n (%) | 64 (64%) | 42 (63%) | 22 (67%) |
| Moderate/severe, n (%) | 36 (36%) | 25 (37%) | 11 (33%) |
| Anxiety symptoms—GAD-7b | |||
| Mean (SD; min-max) | 5.37 (4.16; 0.00–17.00) | 5.49 (3.92; 0.00–16.00) | 5.12 (4.66; 0.00–17.00) |
| Minimal/mild, n (%) | 84 (84%) | 57 (85%) | 27 (82%) |
| Moderate/severe, n (%) | 16 (16%) | 10 (15%) | 6 (18%) |
| Marijuana use in the past 30 days, n (%) | 24 (24%) | 17 (25%) | 7 (21%) |
| Injecting drug use frequency in the past 30 days (number of injections), median (IQR; min-max) | 15.00 (10.00–28.50; 2.00–100.00) | 15.00 (9.50–30.00; 2.00–100.00) | 15.00 (10.00–20.00; 4.00–60.00) |
| Shared injection works/a cooker/mix with someone when injecting drugs, n (%) | 54 (54%) | 37 (55%) | 17 (52%) |
| HIV internalized stigma, mean (SD; min-max) | 3.38 (1.72; 0.00–7.00) | 3.31 (1.78; 1.00–7.00) | 3.52 (1.60; 0.00–7.00) |
| Substance use stigma, mean (SD; min-max) | 31.75 (7.28; 12.00–46.00) | 31.40 (7.27; 12.00–46.00) | 32.45 (7.35; 16.00–46.00) |
| Alcohol use disorder (AUDIT-C), n (%) | |||
| Low risk, score below n | 50 (50%) | 32 (48%) | 18 (55%) |
| Moderate risk, score n–n | 17 (17%) | 12 (18%) | 5 (15%) |
| High/severe risk, score above n | 23 (23%) | 23 (34%) | 10 (30%) |
PHQ-9: Patient Health Questionnaire-9.
GAD-7: Generalized Anxiety Disorder-7.
Randomization and masking
Participants were randomized in a 2:1 ratio using R, version 4.0.5 (package ‘randomizeR’21) to generate the random allocation sequence to assign participants to receive the stigma intervention plus usual care, or only care as usual in permuted blocks of random size ranging from one to six. We chose an unequal randomization ratio to increase the number of intervention participants and thus examine the intervention implementation in a higher number of participants. There was no masking. The study assessor who enrolled participants assigned participants to the intervention or control group.
Interventions
Usual care
All participants had access to usual care from the harm reduction civil society organization consisting of provision of sterile equipment for safer drug injection, distribution of opioid overdose reversal medications, counseling, navigation services, and referral to addiction and HIV treatment clinics, as well as informational handouts on HIV care, drug harm reduction, and safer sex.
Stigma intervention
Participants randomized to the stigma interventions received usual care plus ACT, consisting of three 2-h weekly group sessions, with three to eight participants attending each group, scheduled over the course of a month. The group sessions involved a combination of didactic components, educational stories, experiential activities, and homework assignments. Guided by a health stigma framework,1 we designed these activities to help people with HIV who inject drugs respond more effectively to internalized, experienced, and anticipated stigma manifestations. As a result, this particular intervention applied ACT processes to help participants cope with stigma, aiming to accept stigma-related negativity so as to reduce suffering; and commit to their values to encourage healthy behavior and care engagement.
We trained three psychologists to lead the intervention groups in pairs. Interventionists practiced their skills with two groups during which we reviewed the study protocol and all study procedures prior to the trial. During the trial, interventionists attended monthly clinical supervision sessions with NP that included feedback based on review of audio recorded sessions. We also provided a refresher training following a three-month pause in recruitment and group intervention sessions at the beginning of the COVID-19 pandemic from March to June 2020.
With participants’ consent, we audio-recorded all group sessions for supervision and intervention fidelity monitoring. We offered individual makeup sessions to participants who had missed a session. We reported detailed descriptions of the intervention and development process separately.20
Study visit schedule for participant assessments
Study visits occurred at baseline, one-month post-baseline, and six-month post-baseline. We conducted all study visits in person until the onset of the COVID-19 pandemic in Russia in March 2020, after which baseline visits and all intervention sessions were conducted in-person and all follow-up visits as phone interviews. Participants received 2000 rubles (approximately 32 USD at time of study) as time compensation at each of the intervention sessions.
Outcomes
Primary outcomes
The two primary effectiveness outcomes were mean change in HIV and substance use stigma scores between baseline and one month.
We measured internalized HIV stigma via the internalized AIDS-Related Stigma Scale (IA-RSS).22 Total score was the sum of seven items with yes or no responses. We measured substance use stigma by summing 12 items from the Substance Abuse Self-Stigma Scale (SASSS).23 To validate this abbreviated SASSS scale, we had previously selected the four highest loading items from each of the three subscales (self-devaluation, stigma avoidance, and values disengagement), to be rated using a five-point Likert-type scale, with higher stigma scores indicating more stigma.24 We calculated changes as the difference between one-month and baseline estimates. Negative score change indicated a decrease in stigma while positive change represented an increase in stigma at follow up.
The primary implementation outcome was participant satisfaction, measured in the intervention group at one-month post-intervention and defined as an average score of three or greater on a five-point scale (1 = low, 5 = high) of three items that signified overall satisfaction (“1. How much did you enjoy attending the ACT trainings?”, “2. Did the ACT trainings meet or exceed your expectations?”, and “3. Do you think the ACT trainings would be useful in helping others with HIV and substance use?”).
Secondary outcomes
The secondary effectiveness outcomes were initiation of HIV care, engagement in substance use care, and changes in frequency of injection drug use, all assessed at the six-month visit. Initiation of HIV care was defined as self-reported antiretroviral treatment (ART) initiation in the preceding 6 months. Engagement in substance use care was a binary variable defined as self-reported engagement in any of the following services in the preceding six months: treatment for a substance use disorder in an outpatient or inpatient setting; participation in alcohol or substance use recovery support such as a twelve-step program.
We measured frequency of injection drug use via the question, “During the past 30 days, about how many times did you inject drugs?” Change was computed as number of days at follow up minus the number of days at baseline.
Secondary implementation outcomes assessed among participants who received the group intervention were intervention uptake, measured as rates of complete participation, and fidelity of intervention delivery, measured as adherence to the study intervention manual. Complete participation was defined as attending all three ACT sessions fully. We considered those who were over 20 min late or left more than 20 min early during at least one session as having “attended all sessions but not fully.” Intervention fidelity was rated from session audio recordings using a coding manual adapted from Luoma et al.15 The first 40 min of the first session were excluded from coding as it was a general orientation, with the remainder of the sessions divided into 40-min segments. The coding manual assesses ACT processes, stigma content, interventions related to interpersonal process, and non-adherent interventions. Each code was rated from one (target process not observed) to five (great frequency and very in-depth coverage). Additional codes rated overall fidelity and competence. Interventionists completed an adherence checklist and session summary after each session. Two raters were trained to code sessions. N.P. served as a rater for inter-rater consistency and a second rater coded all reviewed segments.
Exploratory outcome
We assessed engagement in any care at six months (either substance use or HIV care) as an exploratory outcome. We also assessed additional measures of satisfaction and fidelity as exploratory outcomes. We measured length of time of attendance in each session as an exploratory outcome.
Statistical approach
Sample size justification
We designed this feasibility study to investigate whether the SCRIPT stigma intervention involving people with HIV who inject drugs was feasible. In order to estimate the standard deviation for sample size calculations for a sufficiently powered subsequent effectiveness and implementation trial, sample sizes between n = 2425 and 5026 have been recommended. Exceeding the conservatively recommended minimum of n = 50 participants, i.e., 25 intervention participants vs 25 control participants, we planned to recruit 67 intervention participants and 33 control group participants. We chose a 2:1 ratio so as to achieve more stable estimates of feasibility and acceptability outcomes for the intervention.
Statistical analyses
We conducted intention to treat analyses for all outcomes. As a sensitivity analysis, we also conducted per protocol analyses comparing intervention completers to controls on primary and secondary outcomes (reported in the Appendix). For primary efficacy outcomes, we used linear regression adjusting for the baseline stigma scores and included the following covariates chosen a priori based on their known association with stigma: injection frequency, history of ART, and depressive symptoms. For secondary efficacy and exploratory outcomes we used linear regression for continuous outcomes and linear probability models for categorical outcomes.
As diagnostic analyses showed several outliers resulting in a distribution with excess kurtosis of the outcome of change in injection drug use frequency, we used quantile regression estimating the conditional median model of change in injecting drug use frequency to reduce the impact of outliers. Secondary analyses were unadjusted with the exception of change in injection frequency and substance use stigma subscales, which we adjusted for the baseline scores. We used robust standard errors to account for potential clustering of individuals who received the intervention together. All effect sizes are reported on unadjusted outcomes as no widely recognized means is available to for calculating adjusted effect sizes. Effect sizes use Cohen's d for numeric outcomes and Cohen h for categorical outcomes, considering an estimate near 0.2 as a small effect, near 0.5 as a medium effect, and near 0.8 as a large effect.27 The effect size for change in injecting drug use frequency was calculated as the difference in medians divided by the pooled median absolute deviation, with confidence intervals by bootstrap. We opted for a complete case analysis considering low attrition (2% (n = 2) at 1 month and 5% (n = 5) at 6 months) and no missing data among the participants who completed the follow up assessments. We set the level of significance at p < 0.05 and conducted all analyses in R, version 4.0.5.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Baseline characteristics
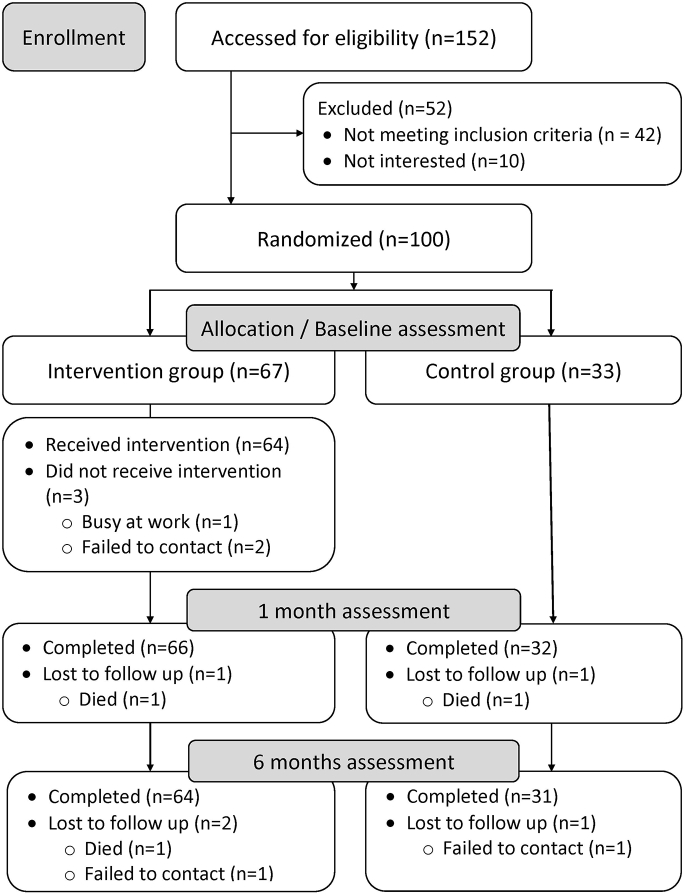
Of 152 individuals screened, 110 (72.4%) were eligible, and 100 were enrolled and randomized (Fig. 1). Among the randomized participants (67 in the intervention arm and 33 in the usual care arm), 98% completed the one-month assessment and 95% completed the six-month assessment.
Fig. 1.
Flow chart of theSCRIPT study. ∗Cumulative; total lost to follow up across the study was 5 individuals (3 died and 2 failed to contact).
Participants had a mean age of 38 years at baseline, and had been living with HIV for a mean of 10 years (Table 1). About half were female, two thirds lived with a partner, and over half had never initiated ART. About a third were above the Patient Health Questionnaire28 cutoff of ≥10 for moderate to severe depressive disorder, 16% were above the Generalized Anxiety Disorder (7 item scale) cutoff of ≥10 for moderate to severe anxiety29 and about a quarter (23%) were at risk for hazardous drinking or alcohol use disorder using the AUDIT-C score of ≥four for men and ≥three for women.30 Participants used a range of substances, with details of ever and recent (past 30 days) substance use summarized in the Appendix Tables. Results are consistent with recent trends in Russia, where injection use of historically primarily heroin has more recently transitioned to injection primarily of illicitly manufactured methadone. Polysubstance use was common in this cohort, again reflecting trends in Russia.
Primary efficacy outcomes
On primary adjusted analyses (Table 2A; Forrest plots in Appendix Fig. S4), change in HIV stigma scores from pre-intervention to one-month assessments did not differ in the treatment and control groups (adjusted mean difference (AMD): 0.40, 95% CI −0.14 to 0.93, p = 0.14). There were no between-group differences in change from baseline to six-month follow-up either (AMD: 0.19, 95% CI −0.52 to 0.89, p = 0.60). Change in substance use self-stigma from pre-to-one-month did not differ in the treatment and control groups (AMD: −2.18, 95% CI −4.87 to 0.52, p = 0.11). There were also no between-group differences in change from baseline to 6 month follow up on adjusted analyses either (AMD: 1.40, 95% CI −1.30 to 4.00, p = 0.31).
Table 2.
Primary and secondary outcomes at 1 and 6-months.
| A. At 1 month | Intervention N = 66 | Control N = 32 | Unadjusted estimatea [95% CI], p | Adjustedb estimate [95% CI], p | Effect sizec [95% CI] |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Changed in substance use stigma score from baseline, mean (SD; min-max) | −1.42 (7.14; −20.00 to 15.00) | 0.06 (7.81; −16.00 to 16.00) | −1.49 [−4.73; 1.75], p = 0.364 | −2.18 [−4.87; 0.52], p = 0.112 | 0.20 [−0.22, 0.62] |
| Changed in internalized HIV stigma score from baseline, mean (SD; min-max) | 0.45 (1.42; −3.00 to 4.00) | −0.06 (1.05; −3.00 to 3.00) | 0.52 [0.01; 1.02], p = 0.044 | 0.40 [−0.14; 0.93], p = 0.141 | −0.40 [−0.82, 0.03] |
| Secondary outcomes | |||||
| Changed in injecting drug use frequency from baseline, median (IQR; min-max) | 0.00 (−5.00 to 2.75; −50.00 to 40.00) | 0.00 (−3.50 to 5.25; −15.00 to 13.00) | 0.00 [−3.35; 3.35], p = 1.000 | 0.00 [−3.43; 3.43], p = 1.000 | 0.00 [−0.57, 0.24] |
| Fully attended all 3 sessions (n, %) | 36 (54%) | – | – | – | – |
| Participated in all 3 sessions, but not fully (n, %) | 18 (27%) | – | – | – | – |
| B. At 6 months | Intervention N = 64 | Control N = 31 | Unadjusted estimatea [95% CI], p | Adjusted estimateb [95% CI], p | Effect sizec [95% CI] |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Changed in substance use stigma score from baseline, mean (SD; min-max) | −0.80 (7.95; −26.00 to 20.00) | −2.55 (5.42; −15.00, 12.00) | 1.75 [−1.01; 4.51], p = 0.210 | 1.40 [−1.3; 4.0], p = 0.31 | −0.24 [−0.67, 0.19] |
| Changed in internalized HIV stigma score from baseline, mean (SD; min-max) | 0.44 (1.52; −2.00 to 5.00) | 0.16 (1.68; −4.00 to 4.00) | 0.28 [−0.43; 0.98], p = 0.438 | 0.19 [−0.52; 0.89], p = 0.599 | −0.18 [−0.60, 0.25] |
| Secondary outcomes | |||||
| Changed in injecting drug use frequency from baseline, median (IQR; min-max) | 0.00 (−8.25 to 5.00; −70.00 to 30.00) | 0.00 (−4.00 to 9.50; −14.00 to 105.00) | 0.00 [−5.88; 5.88], p = 1.000 | −3.33 [−8.51; 1.84], p = 0.210 | 0.00 [−0.67, 0.25] |
| ART initiation, n (%) | 13 (20%) | 1 (3%) | 0.17 [0.05; 0.29], p = 0.005 | — | 0.58 [0.15, 1.00] |
| Engagement in substance use care, n (%) | 15 (23%) | 2 (6%) | 0.17 [0.03; 0.31], p = 0.017 | 0.16 [0.02; 0.30], p = 0.022 | 0.51 [0.08, 0.93] |
| Engagement in substance use or HIV care, n (%) | 20 (31%) | 3 (10%) | 0.21 [0.06; 0.37], p = 0.008 | 0.21 [0.05; 0.37], p = 0.010 | 0.54 [0.11, 0.97] |
Bold indicates statistically significant difference between groups at p < 0.05.
Estimate is a mean difference for numeric outcomes and risk difference for categorical outcomes. Confidence intervals and p-values for the estimates were derived based on robust standard errors.
Changes in HIV and substance use stigma were adjusted for respective baseline stigma score, injecting frequency, history of ART (yes/no), and depressive symptoms (moderate or severe symptoms vs no or mild symptoms). Change in injecting drug use frequency and engagement in substance use care (including a composite outcome “Engagement in substance use or HIV care”) were adjusted for respective baseline scores. We reported unadjusted estimates for the “ART initiation” because all participants were not on ART at baseline.
Cohen d for mean differences and Cohen h for proportion differences. An estimate near 0.2 is a small effect, an estimate near 0.5 is a medium effect, and an estimate near 0.8 is a large effect. The effect size for change in injecting drug use frequency was calculated as the difference in medians divided by the pooled median absolute deviation, with confidence intervals by bootstrap (Mangiafico's d). All effect sizes are for unadjusted analyses.
Change was calculated as a follow up estimate minus baseline estimate. Negative score represents a decrease in the outcome while positive score indicates an increase in the outcome at the follow up.
Secondary efficacy outcomes
The proportion of intervention participants who initiated ART within six months after baseline was 17 percentage points higher compared with the control participants (13/64, 20% vs 1/31, 3%, proportion difference (PD) = 0.17, 95% CI: 0.05–0.29, p = 0.005) (Table 2B). Substance use treatment within six months post-baseline was higher by 17 percentage points among intervention participants relative to participants in the control condition (15/64, 23% vs 2/31, 6%, PD = 0.17, 95% CI: 0.03–0.31, p = 0.017). The proportion of intervention participants who initiated either HIV or substance use treatment within six months post-baseline was 21 percentage points higher than the control participants (20/64, 31% vs 3/31, 10%, proportion difference (PD) = 0.21, 95% CI: 0.06–0.37, p = 0.008).
There were no between-group differences in the median change of drug use frequency in the past 30 days, adjusted for their baseline use frequency, at 1 month (adjusted median difference (AMdnD) = 0.00, 95% CI −3.43 to 3.43, p = 1.000) and at 6 months (AMdnD = −3.33, 95% CI −8.51 to 1.84, p = 0.210). There were no between-group differences in participants’ sharing of potentially contaminated drug using equipment (29/64, 47% vs 13/31, 43%, proportion difference (PD) = 0.03, 95% CI: −0.18 to 0.25, p = 0.758).
Primary implementation outcome
Almost all participants (95%) were overall satisfied with the intervention according to the three items of the satisfaction scale (Appendix). Exploratory satisfaction outcomes indicated that 83% were satisfied with other group participants, 88% were satisfied with the interventionists, and 92% felt the intervention was relevant to their concerns. A little over half (58%) reported utilizing some of the skills taught in the intervention, and almost 3/4 (73%) reporting their health seeking motivation had increased due to the intervention.
Secondary implementation outcomes
The majority of participants 81% attended all three sessions, with 54% attending all sessions without ever arriving late or leaving early (Table 2A). Few participants (15%) attended only one or two sessions, and 4% attended no sessions (Appendix). A per-protocol analysis, comparing the 54% participants who received the full intervention with the control group participants, using the same analytic strategy otherwise, yielded similar results than the intent-to-treat analyses for primary and secondary outcomes and is presented in the Appendix.
Random coding of 33 of 104 session audio recording segments available for fidelity assessments showed that fidelity to the manual was excellent, with 100% of segments obtaining a score of 5 (excellent) on the overall adherence item (Appendix). Furthermore, 97% of segments were rated as at least 3 (adequate) on the overall competence rating item, with 67% of sessions rated as adequate competence and 30% with good competence, and none as excellent. Overall inter-rater agreement across the 13 rated items was good (ICC = 0.79).
Exploratory outcomes
Additional exploratory analyses to better characterize fidelity to the treatment manual showed that the five ACT processes outlined in the fidelity manual were used frequently. All recorded segments had at least one ACT process targeted with moderate depth (3 or higher rating) and on average 2.6 (SD = 0.8) ACT processes were covered per segment in at least a moderate amount of depth (rating of 3 of higher) suggesting ACT processes were targeted frequently and in depth. The two target processes that were not specific to ACT, focusing on the interpersonal process and stigma, occurred less frequently, averaging a rating of 1.2 (SD = 0.4, range 1–2) and 1.7 (SD = 0.9, range 1–4).
Adverse events
There were six serious adverse events over the course of the study. Four participants died (one due to cancer [intervention arm, never attended a session], one due to stroke [control arm], and two due to cerebral edema [intervention arm, both occurred sometime between the 1-month and 6-month assessments]). One participant was hospitalized due to pulmonary tuberculosis (intervention arm, occurred after second session). One participant was hospitalized due to HIV progression (intervention arm, occurred after first session). None of the serious adverse events were determined to be related to the study.
Discussion
This randomized controlled trial of people with HIV and injection drug use examined the efficacy and implementation of a 6-h behavioral intervention based on Acceptance and Commitment Therapy. The study aimed to examine the feasibility and acceptability of an intervention aimed to empower participants in St. Petersburg, Russia to cope with HIV and substance use stigma in order to improve their ART initiation and substance use care utilization. We did not detect a change in intervention participants’ stigma manifestations, but improvements in healthcare engagement. Participants were highly satisfied with the intervention, which was implemented with high uptake and fidelity.
We observed no statistically significant differences between groups at 1 month or six-month post-intervention in the primary outcomes of changes in HIV or substance use internalized stigma. In interpreting this effect, it is important to note that the study was primarily a feasibility study and only had sufficient power (at 80% power) to detect medium-to-large (d = 0.60) between-groups differences. Thus, it is possible that clinically meaningful differences might result from this intervention but were not of sufficient magnitude to be detected in this study. Sensitivity analyses using unadjusted scores showed that there was a statistically significant increase in HIV stigma scores in the intervention group relative to controls at the one month follow up. Some studies have found that interventions targeting stigma may initially cause an increase in stigma relative to control conditions.15 This may be due to participants not being fully aware of their own and society's stigma. Stigma discussions may increase awareness, which could be reflected in stigma assessments. Other studies suggest that in settings of ongoing stigmatization, internalized stigma attitudes may be relatively resistant to change.31 Consistently, a prior study assessing a multi-level, 4-session stigma intervention to reduce risks of PWH who inject drugs in Vietnam found no change in either HIV or substance use stigma over two years.32 Notably, the study from which this intervention was derived among people with substance use disorders in residential treatment showed that internalized shame did not lower directly after the intervention but at a four month follow-up15 and was associated with attendance of outpatient treatment. The ongoing stigmatization in Russia of people with HIV and substance use in care settings might have contributed to maintaining high levels of stigma manifestations even among those who accessed care. A systematic review of internalized HIV stigma interventions for key populations and PWH found methodologically strong evidence for the effect of interventions targeting HIV stigma at both the structural and individual levels.33 This study's findings and available evidence suggest that changes in stigma-related shame and fear affecting PWH who inject drugs might require multi-level interventions also addressing care workers and changes in structural stigma, thereby allowing referral to systems that provide stigma-informed care. Such multi-level intersectional stigma interventions supporting affected people, while also addressing provider attitudes and structural stigma sources, will need to be examined in randomized trials with sufficient follow-up periods.
We detected a higher likelihood that intervention group participants would initiate ART (20% vs 3% compared to controls) and engage with substance use treatment (23% compared to 7%). The increase in participants’ care engagement in the absence of a reduction in internalized stigma might be due to the acceptance-based nature of the intervention, i.e., the reduction in avoidance of health care settings. The intervention was not intended to directly reduce shame and fears related to internalized societal stigma, but was rather focused on improving stigma coping and thereby increasing healthy behavior and care seeking.
Implementation assessments showed that participants were satisfied with the intervention and attended group sessions at high rates: 81% attended all three sessions. Additionally, fidelity analyses showed that our relatively brief training program resulted in overall good adherence to the intervention manual and adequate competence. Interventionists appeared to target ACT processes frequently and in depth, while specific discussion of stigma occurred with less frequency. It appears possible that interventionists may have under-emphasized the stigma-targeting components of the intervention relative to the components targeting values-based behavior change and health care seeking; this may partially account for the lack of an observed effect on internalized stigma scores. Community members will be crucial in the development and testing of appropriate implementation strategies for stigma interventions for this population, possibly supported by mobile phones or other technologies as previously demonstrated.35
While none of the serious adverse events (SAEs) observed in the study were found to be related to the study, they were relatively common, with approximately 5% of the sample experiencing an SAE during the six month period assessed. Our interpretation is that this represents how difficult it is to be a member of this severely ill and marginalized population with untreated HIV. Future studies with similar samples should be careful to monitor SAEs, attempt to prevent them, and assess their relationship to the study.
We observed no statistically significant between-group differences in median change in past 30-day injection frequency. As with the stigma outcomes, the intervention may have been underpowered to detect changes in this outcome, particularly since changing to quantile regression instead of our planned linear regression may have further reduced statistical power. More detail on this analysis, along with sensitivity analyses using winsorized linear regression, are included in the online appendix materials.
These limitations notwithstanding, an important strength of this study is that it targeted a population with very limited access to healthcare who have developed stigma-related care avoidance. Participants received services from a mobile harm reduction unit. Almost a third of participants receiving the intervention (31%) engaged with either HIV or substance use services over a period of six months, i.e., an about three times higher care engagement rate than the 10% found in control group participants. Additionally, in the absence of stigma score changes, the intervention appeared to be intersectional in nature: of the 31% who engaged in healthcare, about 40% sought both HIV and substance use care, thus successfully navigating the separately located and vertically organized HIV and substance use care clinics in St. Petersburg.
Limitations
The lack of an attention control represents a potential weakness, although it seems unlikely that this alone would foster care seeking. The separate organization of HIV care from substance use care in Russia makes it unlikely that health care utilization results were simply due to intervention participants' familiarity with a substance use treatment site. This would not account for the increased utilization of HIV care, given that HIV care is delivered in a different location and with separate registration processes. We also did not conduct tests to verify HIV status or ART initiation during the study. Routine HIV testing and referrals for ART treatment are conducted on the outreach bus. HIV and ART status data thus relied on self-reported confirmations of potential participants who were referred to us by the NGO based on their HIV and ART status. This study may have also benefitted from a follow-up period longer than six months. In addition, intervention effects may have been stronger if interventionist training were improved, as overall competence was typically rated as adequate (M = 3.3) compared to the trial from which this intervention was adapted, where competence was typically rated as excellent (M = 4.7).15 This study's interventionists may have needed more training or the intervention manual may have needed continued, stigma-focused adaptation. Furthermore, this study was underpowered to identify effects larger than medium-to-large and therefore may have not identified smaller effects that may still be clinically meaningful. In addition, due to limited statistical power and study design we were unable to model whether observed effects on treatment seeking occurred via changes in stigma or stigma coping. Additionally, it is unclear how results would generalize to populations that were recruited in settings other than a community service like we utilized in this study or to what extent the results would generalize outside the context of the COVID-19 pandemic during which much of the follow up occurred.34 While we made a purposive effort to recruit participants at an outreach service serving individuals who tend to avoid care in the formal sector because of stigma-related avoidance, results cannot necessarily be generalized to populations in settings other than a community service like in this study. Finally, the intervention focused primarily on increasing motivation to seek care and helping participants cope with and overcome psychological barriers toward taking those actions; for example, participants were not explicitly coached on how to interact with the health care system or whether to disclose their drug use to ART providers. This may be important in that ART providers may harbor stigma against drug users that could further increase intersectional stigmatization. Disclosure of methadone use to ART providers is also relevant because of potential interactions with ART medications.
Conclusions
Intersectional stigma affecting people with HIV and current substance use contributes to substantial barriers to healthcare engagement. This stigma-coping intervention, based on acceptance, mindfulness, and orientation towards values, empowered this HIV key population to engage in healthcare in the face of internalized stigma and structural barriers. While the intervention did not change negative attitudes and emotions related to internalized stigma or injection drug use, it appeared to reduce stigma's impact as an HIV and substance use care barrier: it promoted engagement in HIV and substance use treatment. In the context of pervasive intersectional stigma, helping people with HIV and injection drug use recognize, accept and cope with stigma could empower them to take actions toward supporting their care and health.
Contributors
Jason B. Luoma: Writing—original draft; Conceptualization; Methodology; Resources; Supervision; Writing—review & editing. Sarah L. Rossi: Writing—original draft; Visualization; Project administration. Yuliia Sereda: Data curation; Formal analysis; Methodology; Software; Writing—review & editing; Verified the data. Nikolai Pavlov: Conceptualization; Resources; Supervision. Olga Toussova: Investigation; Writing—review & editing. Sally Bendiks: Project administration; Writing—review & editing. Tetiana Kiriazova: Writing—review & editing. Marina Vetrova: Writing—review & editing. Elena Blokhina—Conceptualization; Project administration; Supervision; Writing—review & editing. Evgeny Krupitsky—Conceptualization. Dmitry Lioznov—Conceptualization; Resources; Supervision. Sara Lodi—Conceptualization; Formal analysis; Methodology. Karsten Lunze—Conceptualization; Funding acquisition; Methodology; Supervision; Writing—original draft. Writing—review & editing; Verified the data.
Data sharing statement
De-identified data and study documents can be made available upon request to the URBAN ARCH repository by contacting Natalia Gnatienko (natalia.gnatienko@bmc.org).
Declaration of interests
We declare no conflicts of interests.
Acknowledgements
We thank all participants for dedicating their time to this trial, Lemma Romneva and colleagues from the Andrey Rylkov Foundation for their input to intervention adaptation, Ilia Nadareishvili and David Tvildiani Medical University in Tbilisi, Georgia, for hosting the intervention training, Janna Vasileva and Kristina Abramova for their data collection efforts, and Pavel Trachuk and Evgeniya Mitrofanova Dashkova for their help with fidelity coding. This work was supported by the National Institute on Drug Abuse (NIDA) under Grant R00DA041245 and Grant K99DA041245; and by the National Institute of Allergy and Infectious Disease (NIAID) through Grant P30AI042853 to the Providence/Boston Center for AIDS Research. The funding source had no role in the writing of the manuscript or the decision to submit it for publication, or any aspect pertinent to the study. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100611.
Appendix A. Supplementary data
References
- 1.Stangl A.L., Earnshaw V.A., Logie C.H., et al. The Health Stigma and Discrimination Framework: a global, crosscutting framework to inform research, intervention development, and policy on health-related stigmas. BMC Med. 2019;17(1):31. doi: 10.1186/s12916-019-1271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman S.R., Williams L.D., Guarino H., et al. The stigma system: how sociopolitical domination, scapegoating, and stigma shape public health. J Community Psychol. 2022;50(1):385–408. doi: 10.1002/jcop.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heylen E., Chandy S., Shamsundar R., Nair S., Ravi Kumar B.N., Ekstrand M.L. Correlates of and barriers to ART adherence among adherence-challenged people living with HIV in southern India. AIDS Care. 2020;33:1–8. doi: 10.1080/09540121.2020.1742862. Published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luoma J.B., Twohig M.P., Waltz T., et al. An investigation of stigma in individuals receiving treatment for substance abuse. Addict Behav. 2007;32(7):1331–1346. doi: 10.1016/j.addbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Stringer K.L., Marotta P., Baker E., et al. Substance use stigma and antiretroviral therapy adherence among a drug-using population living with HIV. AIDS Patient Care STDS. 2019;33(6):282–293. doi: 10.1089/apc.2018.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idrisov B., Lunze K., Cheng D.M., et al. Food insecurity, HIV disease progression and access to care among HIV-infected Russians not on ART. AIDS Behav. 2017;21(12):3486–3495. doi: 10.1007/s10461-017-1885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korthuis P.T., Edelman E.J. Substance use and the HIV care continuum: important advances. Addict Sci Clin Pract. 2018;13 doi: 10.1186/s13722-018-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go V.F., Frangakis C., Minh N.L., et al. Increased survival among HIV-infected PWID receiving a multi-level HIV risk and stigma reduction intervention: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2017;74(2):166–174. doi: 10.1097/QAI.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins P.H. Intersectionality's definitional dilemmas. Annu Rev Sociol. 2015;41(1):1–20. doi: 10.1146/annurev-soc-073014-112142. [DOI] [Google Scholar]
- 10.Turan J.M., Elafros M.A., Logie C.H., et al. Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med. 2019;17 doi: 10.1186/s12916-018-1246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimer R., Lyubimova A., Barbour R., Levina O.S. Emergence of methadone as a street drug in St. Petersburg, Russia. Int J Drug Policy. 2016;27:97–104. doi: 10.1016/j.drugpo.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solonin S.A., Belova M.V., Bazhenov A.I., Tyurin I.A., Potskhveria M.M., Godkov M.A. Acute methadone poisonings in patients in an emergency hospital. HIV Infect Immunosuppr Disord. 2020;12(2):69–78. doi: 10.22328/2077-9828-2020-12-2-69-78. [DOI] [Google Scholar]
- 13.Beyrer C., Wirtz A.L., O'Hara G., Léon N., Kazatchkine M. The expanding epidemic of HIV-1 in the Russian Federation. PLoS Med. 2017;14(11) doi: 10.1371/journal.pmed.1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetrova M.V., Cheng D.M., Bendiks S., et al. HIV and substance use stigma, intersectional stigma and healthcare among HIV-positive PWID in Russia. AIDS Behav. 2021;25:2815. doi: 10.1007/s10461-021-03172-5. Published online January 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luoma J.B., Kohlenberg B.S., Hayes S.C., Fletcher L. Slow and steady wins the race: a randomized clinical trial of acceptance and commitment therapy targeting shame in substance use disorders. J Consult Clin Psychol. 2012;80(1):43–53. doi: 10.1037/a0026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielenberg J., Swisher G., Lembke A., Haug N.A. A systematic review of stigma interventions for providers who treat patients with substance use disorders. J Subst Abuse Treat. 2021;131 doi: 10.1016/j.jsat.2021.108486. [DOI] [PubMed] [Google Scholar]
- 17.Geter A., Herron A.R., Sutton M.Y. HIV-related stigma by healthcare providers in the United States: a systematic review. AIDS Patient Care STDS. 2018;32(10):418–424. doi: 10.1089/apc.2018.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston J.D., Milne T., Fang M.L., Amari E. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction. 2011;107(1):39–50. doi: 10.1111/j.1360-0443.2011.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luoma J.B., Kohlenberg B.S., Hayes S.C., Bunting K., Rye A.K. Reducing self-stigma in substance abuse through acceptance and commitment therapy: model, manual development, and pilot outcomes. Addict Res Theory. 2008;16(2):149–165. doi: 10.1080/16066350701850295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S., Sereda Y., Luoma J., et al. Addressing intersectional stigma as a care barrier for HIV-positive people who inject drugs: design of an RCT in St. Petersburg, Russia. Contemp Clin Trials Commun. 2021;24 doi: 10.1016/j.conctc.2021.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uschner D., Schindler D., Hilgers R.D., Heussen N. randomizeR: an R package for the assessment and implementation of randomization in clinical trials. J Stat Softw. 2018;85:1–22. doi: 10.18637/jss.v085.i08. [DOI] [Google Scholar]
- 22.Simbayi L.C., Strebel A., Cloete A., Henda N., Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med. 2007;64(9):1823–1831. doi: 10.1016/j.socscimed.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luoma J.B., Nobles R.H., Drake C.E., et al. Self-stigma in substance abuse: development of a new measure. J Psychopathol Behav Assess. 2013;35(2):223–234. doi: 10.1007/s10862-012-9323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sereda Y., Kiriazova T., Makarenko O., et al. Stigma and quality of co-located care for HIV-positive people in addiction treatment in Ukraine: a cross-sectional study. J Int AIDS Soc. 2020;23(5) doi: 10.1002/jia2.25492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 26.Sim J., Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65(3):301–308. doi: 10.1016/j.jclinepi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 30.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 31.Vetrova M., Lodi S., Rateau L., et al. Stigma and ART initiation among people with HIV and a lifetime history of illicit drug use in Saint-Petersburg, Russia-A prospective cohort analysis. Int J Drug Policy. 2022;102 doi: 10.1016/j.drugpo.2022.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go V.F., Frangakis C., Minh N.L., et al. Efficacy of a multi-level intervention to reduce injecting and sexual risk behaviors among HIV-infected people who inject drugs in Vietnam: a four-arm randomized controlled trial. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantelic M., Steinert J.I., Park J., Mellors S., Murau F. ‘Management of a spoiled identity’: systematic review of interventions to address self-stigma among people living with and affected by HIV. BMJ Glob Health. 2019;4(2) doi: 10.1136/bmjgh-2018-001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll J.J., Rossi S.L., Vetrova M.V., Kiriazova T., Lunze K. Supporting the health of HIV-positive people who inject drugs during COVID-19 and beyond: lessons for the United States from St. Petersburg, Russia. Am J Public Health. 2022;112(S2):S123–S127. doi: 10.2105/AJPH.2022.306727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batchelder A.W., Moskowitz J.T., Jain J., Cohn M., Earle M.A., Carrico A.W. A novel technology-enhanced internalized stigma and shame intervention for HIV-positive persons with substance use disorders. Cogn Behav Pract. 2020;27(1):55–69. doi: 10.1016/j.cbpra.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.