Summary
Background
Due to the high risk of severe infection among pediatric hematology and oncology patients, antimicrobial use is particularly high. With our study, we quantitatively and qualitatively evaluated, based on institutional standards and national guidelines, antimicrobial usage by employing a point-prevalence survey with a multi-step, expert panel approach. We analyzed reasons for inappropriate antimicrobial usage.
Methods
This cross-sectional study was conducted at 30 pediatric hematology and oncology centers in 2020 and 2021. Centers affiliated to the German Society for Pediatric Oncology and Hematology were invited to join, and an existing institutional standard was a prerequisite to participate. We included hematologic/oncologic inpatients under 19 years old, who had a systemic antimicrobial treatment on the day of the point prevalence survey. In addition to a one-day, point-prevalence survey, external experts individually assessed the appropriateness of each therapy. This step was followed by an expert panel adjudication based upon the participating centers’ institutional standards, as well as upon national guidelines. We analyzed antimicrobial prevalence rate, along with the rate of appropriate, inappropriate, and indeterminate antimicrobial therapies with regard to institutional and national guidelines. We compared the results of academic and non-academic centers, and performed a multinomial logistic regression using center- and patient-related data to identify variables that predict inappropriate therapy.
Findings
At the time of the study, a total of 342 patients were hospitalized at 30 hospitals, of whom 320 were included for the calculation of the antimicrobial prevalence rate. The overall antimicrobial prevalence rate was 44.4% (142/320; range 11.1–78.6%) with a median antimicrobial prevalence rate per center of 44.5% (95% confidence interval [CI] 35.9–49.9). Antimicrobial prevalence rate was significantly higher (p < 0.001) at academic centers (median 50.0%; 95% CI 41.2–55.2) compared to non-academic centers (median 20.0%; 95% CI 11.0–32.4). After expert panel adjudication, 33.8% (48/142) of all therapies were labelled inappropriate based upon institutional standards, with a higher rate (47.9% [68/142]) when national guidelines were taken into consideration. The most frequent reasons for inappropriate therapy were incorrect dosage (26.2% [37/141]) and (de-)escalation/spectrum-related errors (20.6% [29/141]). Multinomial, logistic regression yielded the number of antimicrobial drugs (odds ratio, OR, 3.13, 95% CI 1.76–5.54, p < 0.001), the diagnosis febrile neutropenia (OR 0.18, 95% CI 0.06–0.51, p = 0.0015), and an existing pediatric antimicrobial stewardship program (OR 0.35, 95% CI 0.15–0.84, p = 0.019) as predictors of inappropriate therapy. Our analysis revealed no evidence of a difference between academic and non-academic centers regarding appropriate usage.
Interpretation
Our study revealed there to be high levels of antimicrobial usage at German and Austrian pediatric oncology and hematology centers with a significant higher number at academic centers. Incorrect dosing was shown to be the most frequent reason for inappropriate usage. Diagnosis of febrile neutropenia and antimicrobial stewardship programs were associated with a lower likelihood of inappropriate therapy. These findings suggest the importance of febrile neutropenia guidelines and guidelines compliance, as well as the need for regular antibiotic stewardship counselling at pediatric oncology and hematology centers.
Funding
European Society of Clinical Microbiology and Infectious Diseases, Deutsche Gesellschaft für Pädiatrische Infektiologie, Deutsche Gesellschaft für Krankenhaushygiene, Stiftung Kreissparkasse Saarbrücken.
Keywords: Antimicrobial resistance, Antimicrobial stewardship, Cancer, Expert panel, Pediatric hematology, Pediatric oncology, Point-prevalence survey
Research in context.
Evidence before this study
Previous point-prevalence studies, audits, and studies using case vignettes have documented high usage of broad-spectrum antibiotics. They also have reported mixed results regarding implementation of national guidelines on febrile neutropenia in pediatric oncology centers. There remains a dearth of data regarding the appropriateness of antimicrobial therapy in this patient cohort.
The above synopsis is based upon a search of PubMed/Medline for articles using the keywords (antibiotic∗ OR antimicrobial∗ OR antibacterial∗ OR antifungal∗) AND (children [tiab] OR pediatric∗ [tiab]) AND (oncol∗ OR hematol∗) through August 8, 2022, without application of date or language restrictions.
Added value of this study
Ours is the first multi-center, point-prevalence study in pediatric oncology centers to use a multi-step, expert panel process to qualitatively assess antimicrobial treatment based upon institutional (local) standards and national guidelines. A commissioned, expert panel facilitated the adjudication process to determine whether or not a given therapy was appropriate. We found that nearly half of all antimicrobial treatments was inappropriate with respect to local standards and/or national guidelines. Our findings indicate insufficiencies in the implementation of local standards, as well as discrepancies between local practice and national guidelines. Findings were comparable between academic and non-academic hospitals. A higher number of antimicrobials was predictive of inappropriate therapy, while the diagnosis of febrile neutropenia was negatively associated with inappropriate therapy.
Implications of all the available evidence
Our data suggest that implementing a modified expert panel within a point-prevalence study may be beneficial when qualitatively assessing antimicrobial therapy on pediatric oncology and hematology units—an approach that also may be useful for other medical or pediatric subspecialties. Additionally, our data indicate that better alignment between institutional and national guidelines is required. Among pediatric oncology centers, there is a clear need for antimicrobial stewardship programs.
Introduction
Pediatric cancer patients face a particularly high risk of severe infection because of the immune deficiency associated with their underlying disease, as well as due to immunosuppression related to their anticancer treatments.1 These risks, along with the wide spectrum of opportunistic pathogens, lead to high antimicrobial usage rates at pediatric hematology and oncology and hematology centers (PHOC). This, in turn, drives antimicrobial resistance.2,3 In this instance, however, high antimicrobial usage often is triggered by fear of undertreatment4—a concern that needs to be balanced against overtreatment and its adverse effects.3,5
Evidence-based guidelines at national and international levels offer guidance for clinically-relevant decision-making. These guidelines provide physicians with information about the best care options for pediatric cancer patients with febrile neutropenia (FN). Although German guidelines for the diagnostic and therapeutic management of pediatric cancer patients with febrile neutropenia were published in 2016,6 implementation of these guidelines in the form of a written standard for clinical practice is not compulsory. The implementation of a local antimicrobial stewardship (AMS) program also is not required.7 Both interventions have the goal of providing evidence-based, best-practice diagnostics and treatment to patients with FN.
The effects of such interventions can be approximated indirectly by assessing antimicrobial usage in a given setting and at a given time point, i.e., by means of a point-prevalence survey (PPS).8 However, most PPS describe local patterns of antimicrobial usage, but do not additionally include a qualitative, external assessment regarding appropriateness at the patient and antimicrobial substance level.9
In our cross-sectional study—a PPS across German and Austrian PHOCs—we aimed to: (i) assess antimicrobial usage at both the center and the patient level; (ii) to adjudicate the appropriateness of each treatment by employing a modified multi-step expert panel approach and analyze reasons for inappropriate therapy; (iii) to determine and compare the degree of adherence to institutional standards and national guidelines for the management of FN, and as an exploratory approach; (iv) to compare academic and non-academic centers.
Methods
Study design
We conducted a multi-center, cross-sectional study that made use of a point-prevalence survey to observe antimicrobial prescribing at 30 PHOCs across Germany and Austria in December 2020. This was followed by a multi-step, expert panel process throughout the year 2021 to evaluate the appropriateness of treatment with respect to institutional and national guidelines. Our study protocol recently has been published.10 The study is reported in adherence with the STROBE statement (Table S1, Appendix p. 12–13).
Study population
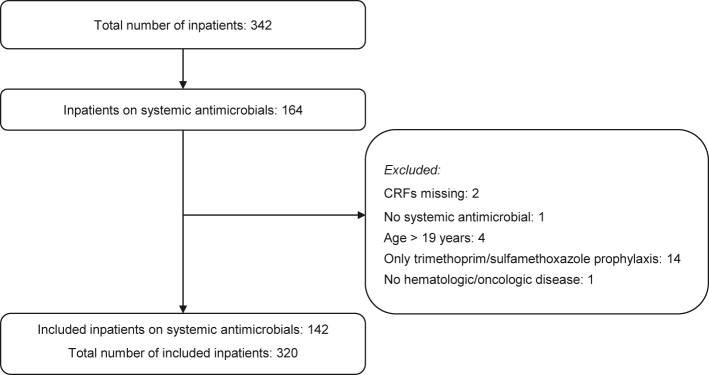
All pediatric hematology and oncology centers within the German Society for Pediatric Oncology and Hematology (GPOH) and German Society for Pediatric Infectious Diseases (DGPI) were invited (N = 60) and eligible for inclusion if they employed an institutional standard for the management of FN. We included inpatients under 19 years old with an oncologic or hematologic disease who had received a prescription for systemic antimicrobials (i.e., intravenous or oral antibiotics and/or antifungals), at 08:00 a.m. local time on the day of the PPS. Reasons for exclusion were: (i) patients receiving only cotrimoxazole (TMP-SMX, trimethoprim/sulfamethoxazole) prophylaxis or else only taking locally-acting antimicrobials (e.g., amphotericin B lozenges) because the appropriateness of prescribing TMP-SMX was not the objective of this study; (ii) patients being 19 years of age or older; and (iii) patients who did not have an oncologic or hematologic disease (Fig. 1).
Fig. 1.
Reasons for individual patient exclusion.
All experts involved in the expert panel approach were specialists in pediatric oncology/hematology and/or pediatric infectious diseases and were members of the Working group for Febrile Neutropenia within the GPOH and/or the DGPI. GPOH and DGPI recruited the experts for the study. Although an expert's institution could contribute to the PPS, experts were excluded from evaluating data from their own institutions. The 15 experts, of whom 14 are employed in Germany and one in Austria, formed five panels with three adjudicators each for the multi-step, expert panel process.11,12 The configuration of the expert panels was randomly selected (Appendix p. 5).
Data collection
The single-day PPS was conducted at 30 German and Austrian hospitals on a selected weekday (in order to match average patient activity on surveyed centers) between November 30, 2020 and December 17, 2020.
All participating centers received a standardized form for basic hospital data and a case report form for patient and antimicrobial data (Appendix p. 6–7). Each participating center provided their institutional standard for antimicrobial treatment to the study team. All patient-related data and institutional standards were anonymized.
Hospital-based physicians collected general data pertaining to the pediatric hematology/oncology unit, including: number of total beds, number of hospitalized patients, number of patients on source isolation (due to colonization with a multidrug-resistant pathogen), number of patients on protective isolation (due to very severe immunodeficiency/immunosuppression), number of patients on antimicrobial therapy, antimicrobial stewardship programs (yes/no), AMS member composition (infectious disease (ID) specialists, clinical microbiologist, pharmacist, non ID physician; multiple selection possible) type of AMS activities (regular AMS rounds, regular AMS meetings, consultation service; single choice), number of physicians, number of nursing staff, and ID consultation service (pediatric ID specialists, ID specialists, pediatrician with ID expertise; single choice).
Likewise, data about the patients’ characteristics were collected, including: age (months), weight (kg), height (cm), oncologic/hematologic disease, state of disease (first diagnosis vs. relapse), trimethoprim/sulfamethoxazole prophylaxis (yes/no), granulocytopenia <0.5 × 109/L (yes/no), mucositis grade III according to the World Health Organization oral mucositis grading scale (yes/no), severe graft-versus-host disease grade III to IV (yes/no), subcutaneously tunneled or implanted long-term central venous access device (yes/no), creatinine clearance <50 mL/min per 1.73 m2 (yes/no), high risk for fungal infections defined as acute myeloid leukemia undergoing induction therapy, leukemia relapse/not in remission, allogeneic stem cell transplantation, prolonged neutropenia for ≥10 days and steroid therapy, or graft-versus-host-disease grade III-IV (yes/no), colonization with multidrug-resistant organisms (yes/no), and reason for antimicrobial treatment (i.e., type of infectious syndrome and identified microorganisms).
Further information about each antimicrobial drug was documented, including: name of antimicrobial, indication (i.e., therapy, prophylaxis, surgical prophylaxis, indeterminate), duration of therapy until PPS, initial/first-line vs. escalation therapy, dosage (mg, g, IU), route of administration (intravenous/oral), therapeutic drug monitoring for aminoglycosides/glycopeptides/antifungals (yes/no, and if yes peak levels/through levels). In the event of a suspected infection without apparent focus, treatment was defined “empirical”; in cases with a clinical infectious syndrome without pathogen detection, treatment was defined “calculated”; and in instances of a clinical infectious syndrome with a pathogen detection, treatment was defined “targeted”.
The multi-step, expert-panel process was conducted throughout the year of 2021. In a first step, the experts individually adjudicated each antimicrobial treatment in comparison with the institutional standards. Each patient's treatment regimen was labelled as appropriate, inappropriate, or indeterminate. When labelled as inappropriate, reasons were given a specific code (e.g., incorrect dosing, defined as over 20% deviation from the corresponding recommendation of the institutional standard and/or the national guideline) (Table S2, Appendix p. 14–15). In a second step, all experts from one panel again adjudicated the cases, unless in the first step all three experts unanimously had considered the therapy to be appropriate. Adjudication was conducted via video conference among the three adjudicators of a panel in the presence of a moderator (CP or AS). In addition to the institutional standard, the expert panels took national guidelines (ones valid at the time of the study), into consideration when adjudicating therapy in a third step. Expert panels aimed to reach consensus when labelling a therapy as appropriate, inappropriate, or indeterminate (i.e., due to missing information). When no consensus could be reached, the case was classified as “no consensus reached”. Each adjudication was conducted on three different levels: (i) assessment by the individual expert regarding alignment with institutional standards; (ii) expert-panel adjudication regarding application of institutional standards; and (iii) expert panel adjudication regarding application of national guidelines.
Outcome measures
The focus of the study was to analyze antimicrobial prevalence rate (defined as the number of patients treated with systemic antimicrobial drugs and included in the study divided by the number of all included, hospitalized patients, overall and per center), along with the rate of appropriate, inappropriate, and indeterminate antimicrobial therapies with regard to institutional standards and national guidelines. A therapy was assessed as appropriate if it complied with institutional standards/national guidelines. Accordingly, a therapy was inappropriate if it did not comply with institutional standards/national guidelines. Reasons for inappropriate therapies were further assessed using the given codes. A therapy was indeterminate if, for example, it was not included in the institutional standard/national guidelines or the patients’ medical history was too complex (i.e. missing information) for the therapy to be assessed appropriately. To make the adjudications before and after the expert panel process comparable, treatments adjudicated beforehand were recorded as appropriate/inappropriate/indeterminate when at least two out of three experts individually made the same adjudication. If each expert adjudicated differently, the treatment was considered “no consensus reached”.
Additionally, we individually analyzed each adjudication before the expert panel process. In an explorative approach, we compared academic and non-academic centers and the adherence to institutional standards and national guidelines. Finally, we sought to find predictors of inappropriate therapy using center- and patient-related data.
Data analysis
Patient and hospital data collected during the PPS were reported descriptively. We present the results with counts and percentages. Means with standard deviations (SD) or medians with interquartile ranges (IQR) were provided, regardless of whether the data were normally distributed (Shapiro-Wilk-Test performed). The 95% confidence intervals (CI) were also calculated. Significant differences (p value < 0.05) were compared using either Fisher's exact-test, t-test, Mann-Whitney-U-test or McNemar-test, as appropriate. Missing data was specified in tables and figures. When calculating percentages, number of missing values were excluded in the denominator. We performed multinomial logistic regression to identify predictors of inappropriate therapy. Factors selected for multinomial logistic regression were based on clinical relevance and most common characteristics of centers (academic vs. non-academic, AMS programs) and patients (age, central venous access device, multidrug resistant organism colonization, three most common infectious disease syndromes, number of antimicrobials). Multinomial logistic regression was performed in one step with all factors mentioned above. We additionally conducted sensitivity analysis on the statistical model based on cluster-adjusted standard errors. Statistical analyses were performed using SPSS (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY) and Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Ethics approval
The local ethics committee (Ärztekammer des Saarlandes, number 33/20) approved this study. Patient consent was not needed since all patient and center specific data were pseudonymized.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation or in the writing of the report.
Results
Study population and characteristics
Thirty hospitals—29 (96.7%) located in Germany and one (3.3%) in Austria—with a maximum capacity of 452 inpatients, participated in the point-prevalence study. Of these, 24 (80.0%) were academic hospitals and six (20.0%) were non-academic hospitals (Table 1; Table S3, Appendix p. 16). Pediatric AMS activities were performed at 18 (66.7%) of all participating centers (Table S4, Appendix p. 17). At the time of the point-prevalence study, 164/342 (48.0%) inpatients were receiving antimicrobial therapy. We obtained case report forms for 162 patients, of which 142 (87.7%) were included in the study (Fig. 1). Accordingly, the total number of included inpatients to calculate the antimicrobial prevalence rate was 320.
Table 1.
Characteristics of centers participating in the point-prevalence survey.
| Characteristics | Total N | Academic centers | Non-academic centers | p-value |
|---|---|---|---|---|
| Hospitals, n (%) | 30 | 24 (80.0) | 6 (20.0) | |
| AMS programsa, n (%) | 18 (66.7) | 15 (62.5) | 3 (50.0) | 0.66 |
| Bed, n (%) | 452 | 375 | 77 | |
| Per center, median (IQR) | 15 (11.8–19.0) | 15 (12.3–19.0) | 12.5 (5.8–19.8) | 0.38 |
| Physiciansb,c, n (%) | 146.8 | 120.2 | 26.6 | |
| Per center, median (IQR) | 4.0 (3.5–6.4) | 4.0 (3.5–6.5) | 4.0 (2.7–8.7) | 0.76 |
| Nursing staffb,c, n (%) | 525.8 | 439.3 | 86.5 | |
| Per center, median (IQR) | 16.9 (15.0–21.0) | 17.0 (15.0–21.0) | 15.0 (13.3–22.5) | 0.49 |
| Hospitalized patients, n (%) | 342 | 297 | 45 | |
| Per center, median (IQR) | 11.5 (7.0–15.0) | 12 (9.3–15.8) | 7.5 (5.0–9.0) | 0.027 |
| Patients on source isolation, n (%) | 38 | 36 | 2 | |
| Per center, median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.082 |
| Patients on protective isolation, n (%) | 24 | 23 | 1 | |
| Per center, median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.3) | 0.23 |
| Patients on antimicrobial therapy, n (%) | 164 | 153 | 11 | |
| Per center, median (IQR) | 5.0 (2.0–9.0) | 6.0 (3.0–9.0) | 1.0 (1.0–3.3) | 0.0013 |
| Patients included, n (%) | 142 | 133 | 9 | |
| Per center, median (IQR) | 4.5 (2.0–7.0) | 6.0 (2.3–7.8) | 1.0 (1.0–2.3) | <0.001 |
Bold values indicate statistically significant p-values.
Antimicrobial stewardship in pediatric centers.
Information about two centers missing.
Number of full-time employees.
The median age of all included patients was 6.8 (IQR 3.1–12.5) years. The most common underlying condition was acute lymphoblastic leukemia (ALL) (52/142 [36.6%]), followed by solid malignant tumors excluding brain tumors (44/142 [31.0%]). In 29/142 (20.4%) there was a relapse of the underlying hematologic/oncologic disease. In addition, more than half of all patients (71/140 [50.7%]) had granulocytopenia, 126/141 (89.4%) had a central venous access device and 47/141 (33.3%) were at high risk for fungal infections (Table 2). The most commonly reported infectious disease diagnosis for antimicrobial treatment was fever without a source during neutropenia (febrile neutropenia, FN) (67/142 [47.2%]), followed by skin and soft tissue infection, bloodstream infection (BSI), and fever without a source without neutropenia (Fig. S1, Appendix p. 8; Table S5, Appendix p. 18). In 25/142 (17.6%) of patients, pathogens were detected.
Table 2.
Patient demographics and characteristics.
| Patients, N = 142 | Patients at academic hospitals, n = 133 | Patients at non-academic hospitals, n = 9 | p-value | |
|---|---|---|---|---|
| Demographics, median (IQR) | ||||
| Age (years) | 6.8 (3.1–12.5) | 6.2 (3.0–12.3) | 8.3 (5.6–14.3) | 0.16 |
| Weight (kg) | 22.1 (24.5–42.3) | 22.0 (13.9–42.5) | 24.3 (19.8–46.8) | 0.25 |
| Height (cm)a | 120.0 (96.0–154.5) | 120.0 (95.2–154.6) | 130.0 (112.8–162.0) | 0.26 |
| Oncologic/hematologic disease, n/N (%) | ||||
| ALL | 52/142 (36.6) | 49/133 (36.8) | 3/9 (33.3) | 1.00 |
| AML | 18/142 (12.7) | 16/133 (12.0) | 2/9 (22.2) | 0.32 |
| Hodgkin's lymphoma | 1/142 (0.7) | 1/133 (0.8) | 0/9 (0.0) | 1.00 |
| MDS/aplastic anemia | 2/142 (1.4) | 2/133 (1.5) | 0/9 (0.0) | 1.00 |
| Non-Hodgkin lymphoma | 12/142 (8.5) | 12/133 (9.0) | 0/9 (0.0) | 1.00 |
| Solid malignant tumor outside brain | 44/142 (31.0) | 42/133 (31.6) | 2/9 (22.2) | 0.72 |
| Malignant brain tumor | 7/142 (4.9) | 6/133 (4.5) | 1/9 (11.1) | 0.37 |
| Other | 6/142 (4.2) | 5/133 (3.8) | 1/9 (11.1) | 0.33 |
| State of disease, n/N (%) | ||||
| First diagnosis | 113/142 (79.6) | 106/133 (79.7) | 7/9 (77.8) | 1.00 |
| Relapse | 29/142 (20.4) | 27/133 (20.3) | 2/9 (22.2) | 1.00 |
| Additional data of interest, n/N (%) | ||||
| Neutropeniab | 71/140 (50.7) | 68/131 (51.9) | 3/9 (33.3) | 0.32 |
| Mucositis grade IIIc | 16/141 (11.3) | 16/132 (12.1) | 0/9 (0) | 0.60 |
| Severe graft-versus-host diseasec | 1/141 (0.7) | 0/132 (0) | 1/9 (11.1) | 0.064 |
| Central venous access devicec | 126/141 (89.4) | 119/132 (90.2) | 7/9 (77.8) | 0.25 |
| High-risk for fungal infectionc | 47/141 (33.3) | 46/132 (34.8) | 1/9 (11.1) | 0.27 |
| TMP/SMX prophylaxisc | 127/141 (90.1) | 120/132 (90.9) | 7/9 (77.8) | 0.22 |
| Colonization with multidrug-resistant organism, n/N (%) | ||||
| MRSA | 1/142 (0.7) | 1 (0.8) | 0/9 (0) | 1.00 |
| VRE | 1/142 (0.7) | 0 (0.0) | 1/9 (11.1) | 0.063 |
| Multi-drug resistant gram-negative organisms | 11/142 (7.7) | 11 (8.3) | 0/9 (0.0) | 1.00 |
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; IQR: interquartile range; MDS: myelodysplastic syndrome; MRSA: methicillin-resistant Staphylococcus aureus; TMP/SMX: trimethoprim/sulfamethoxazole; VRE: vancomycin-resistant enterococci.
3 cases unknown.
2 cases unknown.
1 case unknown; determinators were the total number of patients, when patients value was missing the patient was excluded for that value.
Antimicrobial use
The overall antimicrobial prevalence rate (APR) was 44.4% (142/320) (Table 3). The APR per center varied between 11.1% and 78.6% (mean 44.5, IQR 26.3–56.0, 95% CI 35.9–49.9). The APR was higher in the 277 patients treated at academic centers (133/277 [48.0%]) than in the 43 patients at non-academic centers (9/43 [20.9%]; p < 0.001).
Table 3.
Data on antimicrobials reported in the point-prevalence survey illustrated on patient level.
| Total N (%) | Academic centers | Non-academic centers | p-values | |
|---|---|---|---|---|
| Antimicrobial prevalence rate overall, n/N (%) | ||||
| Antimicrobial | 142/320 (44.4) | 133/277 (48.0) | 9/43 (20.9) | <0.001 |
| Antibiotic | 126/320 (39.4) | 119/277 (43.0) | 7/43 (16.3) | <0.001 |
| Antifungal | 59/320 (18.4) | 54/277 (19.5) | 5/43 (11.6) | 0.291 |
| Antimicrobial prevalence rate per center (%), median (95%-CI) | ||||
| Antimicrobial | 44.5 (35.9–49.9) | 50.0 (41.2–55.2) | 20.0 (11.0–32.4) | <0.001 |
| Antibiotic | 40.0 (31.8–45.0) | 48.5 (36.6–49.9) | 16.3 (7.0–30.8) | 0.0013 |
| Antifungal | 17.9 (12.1–22.8) | 17.9 (12.9–25.3) | 10.0 (−1.8–23.4) | 0.38 |
| Prescribed antimicrobial per patient, median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 1.0 (1.0–2.5) | 0.42 |
| Antibiotics | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (0.5–2.0) | 0.19 |
| Antifungals | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 1.0 (0.0–1.0) | 0.61 |
| Indication, n/N (%) | ||||
| Antibiotics | 126/142 (88.7) | 119/133 (89.5) | 7/9 (77.8) | 0.243 |
| Therapy | 114/142 (80.3) | 108/133 (81.2) | 6/9 (66.7) | 0.380 |
| Medical prophylaxis | 9/142 (6.3) | 7/133 (5.3) | 2/9 (22.2) | 0.102 |
| Surgical prophylaxis | 7/142 (4.9) | 7/133 (5.3) | 0/9 (0.0) | 1.000 |
| Antifungals | 59/142 (41.5) | 54/133 (40.6) | 5/9 (55.6) | 0.490 |
| Therapy | 18/142 (12.7) | 17/133 (12.8) | 1/9 (11.1) | 1.000 |
| Medical prophylaxis | 40/142 (28.2) | 36/133 (27.1) | 4/9 (44.4) | 0.270 |
| Unknown | 1/124 (0.0) | 0/133 (0.0) | 1/9 (11.1) | |
| Point of therapy, n/N (%) | ||||
| First-line | 49/142 (34.5) | 46/133 (34.6) | 3/9 (33.3) | 1.00 |
| Escalation | 93/142 (65.5) | 87/133 (65.4) | 6/9 (66.7) | 1.00 |
| Therapy regimen, n/N (%) | ||||
| Monotherapy | 60/142 (42.3) | 55/133 (41.4) | 5/9 (55.6) | 0.49 |
| Antibiotic | 58/126 (46.0) | 54/119 (45.4) | 4/7 (57.1) | 0.70 |
| Antifungal | 54/59 (91.5) | 49/54 (90.7) | 5/5 (100.0) | 1.00 |
| Combination | 82/142 (57.7) | 78/133 (58.6) | 4/9 (44.4) | 0.49 |
| Antibiotic | 68/126 (54.0) | 65/119 (54.6) | 3/7 (42.9) | 0.70 |
| ≥3 antibiotics | 13/68 (19.1) | 13/65 (20.0) | 0/3 (0.0) | 1.00 |
| Antifungal | 5/59 (8.5) | 5/54 (9.3) | 0/5 (0.0) | 1.00 |
Bold values indicate statistically significant p-values.
In total, 274 antimicrobials were prescribed in 142 patients included in the study. The median number of antimicrobial drugs per patient was 2.0 (IQR 1.0–3.0). A single antimicrobial drug was administered to 60/142 (42.3%) patients, and a combination of antimicrobial drugs to 82/142 (57.7%) patients.
Antibiotic therapy was given to 126/142 (88.7%) of patients. Indication for antibiotic therapy was therapeutic for 114/142 (80.3%) of patients, medical prophylactic for 9/142 (6.3%) of patients and surgical prophylactic for 7/142 (4.9%) of patients. In 68 of 126 (54.0%) antibiotic treatment regimens, a combination therapy was applied (Table S6, Appendix p. 18). A dual antibiotic treatment regimen was given to 55/126 (43.7%) of patients. In 13/126 (10.3%) of patients, three or more antibiotics were given in combination. Meropenem plus a glycopeptide (16/54 [29.6%]) and piperacillin/tazobactam plus a glycopeptide (13/54 [24.1%]) were the most frequent dual treatment regimens.
Of all 142 patients, 59 (41.5%) received an antifungal therapy. Indication for antifungal therapy was therapeutic for 18/142 (12.7%) of patients and medical prophylactic in 40/142 (28.2%) of patients. A combination of two antifungals was used in 4/59 (6.8%) of antifungal treatment regimens, while a combination of three antifungals was used in one instance (Table S7, Appendix p. 19–20).
Antibacterial agents (antibiotics) accounted for 209 (76.3%), and antifungal agents for 65 (23.7%) of all 274 antimicrobial prescriptions. The most frequently used antibacterial class was broad-spectrum penicillins (65/209 [31.1%]), followed by glycopeptides (45/209 [21.5%]) and carbapenems (36/209 [17.2%]) (Fig. S2, Appendix, p. 9; Table S8, Appendix p. 21–22). Half of all therapeutic antibiotics (96/192 [50.0%]) were labelled as escalation therapy, the other half (96/192 [50.0%]) as initial therapy. When given with a therapeutic indication, duration of therapy up to the time point of the PPS varied between 1 and 23 days (median 3, IQR 1.0–7.0). Therapeutic drug monitoring (TDM) was performed in 17/21 prescribed aminoglycosides (81.0%) and in 16/19 vancomycin treatments (84.2%). In both situations, only trough levels were used (Table S7, Appendix p. 19–20).
Regarding systemic antifungal agents, liposomal amphotericin B was used in 37/65 (56.9%) instances, followed by voriconazole (8/65 [12.3%]) and caspofungin (8/65 [12.3%]) (Fig. S3, Appendix p. 9; Table S9, Appendix p. 23). Medical prophylaxis accounted for 40 (63.5%) prescriptions, and therapy for 23 (36.5%) (two cases had an unknown indication). Antifungal treatment was employed as targeted therapy against a confirmed fungal Infection in 9/23 (39.1%) of the therapeutic utilizations of antifungal agents. Therapeutic antifungal agents were labelled as first-line therapy in 8/23 (34.8%) of therapeutic antifungals up to the time point of the PPS, duration of antifungal prophylaxis was between one and 254 days (median 10.5, IQR 3–33.5) (Table S7, Appendix p. 19–20).
Multi-step adjudication process
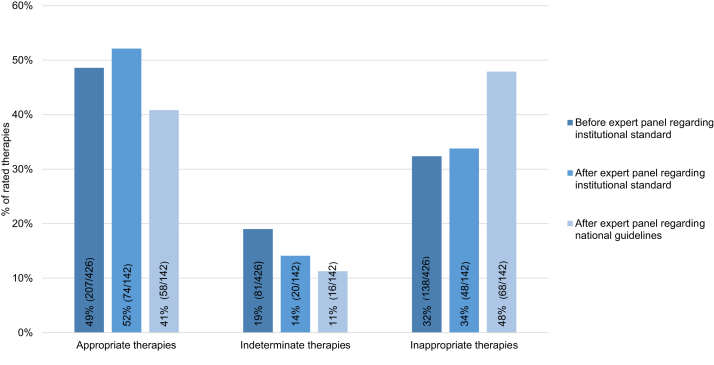
In total, we obtained three adjudication processes from each expert panel (Fig. 2, Fig. 3). The first step (i.e., individual expert adjudication) yielded 426 adjudications (3 adjudications × 142 patients) with respect to institutional standards. Of all antimicrobial therapies appropriate therapy accounted for 207/426 (48.6%), inappropriate therapy accounted for 138/426 (32.4%), and indeterminate therapy for 81/426 (19.0%). Regarding majority vote (i.e., two or more experts in the first adjudication voting congruently), 72 (50.7%) of all 142 therapies were labelled as appropriate, 38 (26.8%) as inappropriate, and 20 (14.1%) as indeterminate. In 12 (8.5%) of all 142 therapies, experts were unable to reach consensus.
Fig. 2.
Rate of appropriate, indeterminate, and inappropriate therapies; individual adjudication regarding institutional standards, and expert panel adjudication regarding institutional standards and national guidelines, respectively. Number of adjudications before expert panel: 426 (3 experts × 142 therapies); Number of adjudications after expert panel: 142 (3 experts agreed on adjudication of 142 therapies).
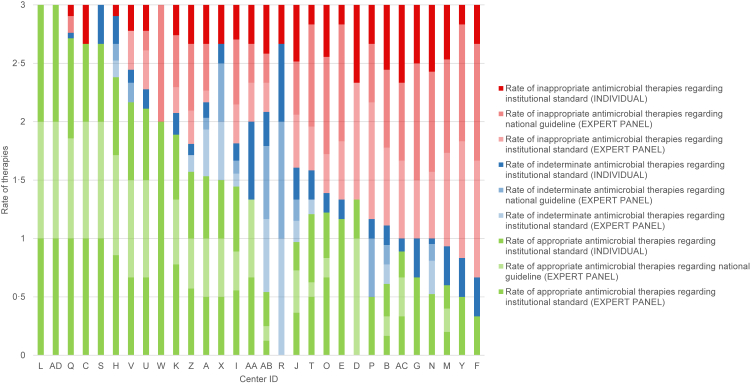
Fig. 3.
Rate of appropriate, inappropriate, and indeterminable therapies according to individual adjudication, expert panel adjudications with institutional standards as reference, and expert panel adjudication with national guideline as reference. Each letter code ID indicates one center. ∗y-axis runs between 0 and 3 since 3 independent adjudications are shown which all sum up to 100% (i.e., 1).
In the second adjudication step, the expert panels provided assessments with respect to institutional standards. For the third adjudication step, the expert panels took national guidelines into account. These steps resulted in 142 adjudications each.
After the final expert panel conference, the rate of indeterminate therapy with respect to institutional standards was 4.9% lower (20/142 [14.1%]) than the individual adjudication before the final expert panel (81/426 [19.0%]).
When referring to national guidelines, the rate of inappropriate therapy significantly increased—from 48/142 (33.8%) to 68/142 (47.9%; +14.1%, p = 0.0003). Correspondingly, the rate of appropriate therapy significantly decreased—from 52.1% to 40.9% (−11.5%, p = 0.0015) (Fig. 2). No statistically significant differences in appropriateness between academic and non-academic centers was observed (Fig. 4; Fig. S4, Fig. S5, Appendix p. 10–11).
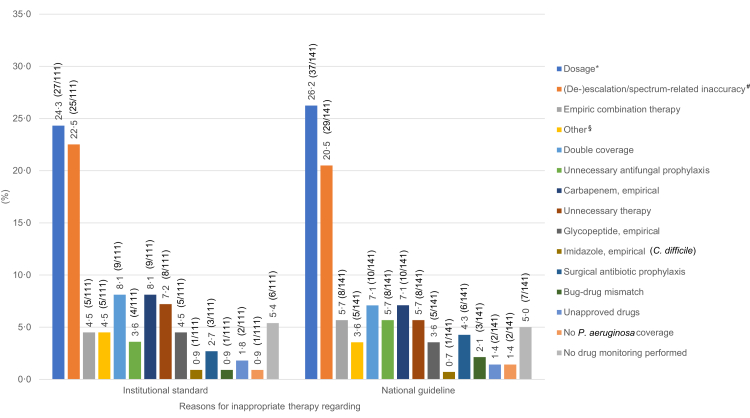
Fig. 4.
Reasons for inappropriate therapy after expert panel process. More than one reason for inappropriate therapy could be named: 111 reasons for inappropriate therapy regarding institutional standard; 141 reasons for inappropriate therapy regarding national guideline; ∗defined as more than 20% deviation from the corresponding recommendation of the institutional standard or the national guideline; #including no de-escalation, early escalation, delayed escalation, broad-spectrum therapy; §including prolonged therapy, missing indication.
Multinomial logistic regression across all centers yielded the number of antimicrobial drugs (odds ratio, OR, 3.13, 95% CI 1.76–5.54, p < 0.001), the diagnosis category for febrile neutropenia (OR 0.18, 95% CI 0.06–0.51, p = 0.0015) and an existing pediatric AMS program (OR 0.35, 95% CI 0.15–0.84, p = 0.019) as predictors of inappropriate therapy (Table S10, Appendix p. 23).
Sensitivity analysis confirmed the two former variables, number of antimicrobial drugs and the diagnosis category for febrile neutropenia as significantly associated with inappropriate therapy, in addition to fever without neutropenia, while the existence of a pediatric AMS program slightly missed statistical significance (OR 0.35, 95% CI 0.12–1.09, p = 0.07) (Table S11, Appendix p. 24).
Regarding national guidelines, 141 reasons for inappropriate therapy were recorded. The most frequent reasons for inappropriate therapy with regard to national guidelines were incorrect dosing (37/141 [26.2%]) and (de-)escalation/spectrum-related errors (29/141 [20.6%]). The latter included unreasonable use of broad-spectrum therapy, no de-escalation (narrowing according to a detected pathogen), and premature as well as delayed escalation (Tables S2–S8, Appendix p. 8–9, p. 15). Other common reasons for inappropriate therapy were double coverage (10/141 [7.1%]), unreasonable first-line carbapenem use (10/141 [7.1%]), unnecessary empirical combination therapy (8/141 [5.7%]), unnecessary antifungal prophylaxis (8/141 [5.7%]), and unnecessary therapy overall (8/141 [5.7%]) (Fig. 4; Table S12, Appendix p. 24).
Discussion
In this cross-sectional, point-prevalence survey including 30 PHOCs from Germany and Austria, the overall antimicrobial prevalence rate was 44.4%—a level lower than the 63.5% previously reported by the Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point-prevalence survey.2 Importantly, however, our study showed an inappropriate therapy rate that ranged from 33.8% to 47.9%, depending upon whether institutional standards or national guidelines were employed as reference. This finding indicates a strong discrepancy between the institutional standards of the PHOCs participating in our PPS and the German national guidelines pertaining to the management of febrile neutropenia in pediatric oncology and hematology patients.6
Among the most frequent reasons for inappropriate therapy, incorrect dosing ranked first, followed by premature escalation during empiric treatment, lack of de-escalation,13 and unnecessary use of antibiotic combinations for empirical therapy.14 In accordance with other single center studies,15,16 we found a high proportion of empirical carbapenem use (approximately 19%),12 although a substantial proportion of carbapenem treatments were deemed inappropriate by expert panel adjudication. The higher prevalence of severe infections in pediatric oncology and hematology may explain this finding, as may carbapenem use in escalation therapy and/or high local resistance patterns (data not collected). Of note, prevalence of carbapenem-resistant organisms has been on the rise Europe, although prevalence in Germany still is comparatively lower than in other European countries.17 For this reason, German guidelines do not recommend the first-line use of carbapenem in febrile neutropenia.6
Our study found aminoglycosides were prescribed in just one-tenth of empirical treatments. This laudable finding indicates a partial adherence to the national guidelines, which recommend monotherapy in clinically-stable children with febrile neutropenia.14 Nevertheless, to improve patient safety in light of aminoglycosides' adverse effects, a restrictive prescribing pattern should be additionally encouraged.18, 19, 20
Due to the immunodeficiency inherent to our patient cohort, along with their high risk for invasive fungal infections, the proportion of prophylactic antibacterial and antifungal prescribing was higher in our study than in other pediatric cohorts.21 This may be explained by the fact that invasive fungal diseases (IFD) strongly impact morbidity and mortality in immunocompromised patients.22,23
We found antimicrobial dosage errors to be the most frequent reason for inappropriate therapy. This contrasts sharply with a large pediatric PPS from the United States in which bug-drug mismatch ranked first among reasons for inappropriateness, whereas dosage errors represented only a minor proportion.24 Appropriate dosing of antibiotics—one taking into account specific characteristics of pharmacokinetics in the FN patient group and treatment situation—is necessary in order to optimize clinical efficacy. Inadequate dosing may impair efficacy and foster the selection of resistant pathogens.22 The same applies to the lack of de-escalation and premature escalation, an issue our study frequently encountered. Although some pediatric oncologists may be reluctant to implement local antimicrobial stewardship programs,25 these are urgently needed in order to improve antimicrobial prescribing at PHOCs.26,27 Regular participation in national, point-prevalence surveys including external expert panels, as shown in our study, may pave the way for sustained improvements in clinical practice.
Although patient characteristics in academic and non-academic settings were similar, antimicrobials more frequently were prescribed in academic centers than in non-academic centers. In our regression analyses, we found that the higher the number of antimicrobials, the higher the probability of inappropriate therapy. In addition, a febrile neutropenia diagnosis and AMS activities, defined as regular AMS rounds, meetings, or consultation services, were associated with a lower rate of inappropriate therapy. However, sensitivity analysis yielded a weakened explanatory role of AMS activities when taking into account cluster effects at the center level, i.e., altering the model assumption that observations are independent within centers. Guidelines for febrile neutropenia, which, when adhered to, serve to inform clinicians of best practices for diagnosis and therapy, were in place prior to our study. By contrast, however, other clinical syndromes reported in our PPS, had no specific, preexisting guidelines. This may have led to clinicians making more individualized—and therefore less evidence-based treatment—decisions in some cases.
Our study has several strengths that deserve mention. First, the multi-center set-up yielded generalizability on a national level. Second, we employed an extended expert panel approach which allowed for an in-depth assessment of each patient and each antimicrobial treatment, thereby generating high-quality, robust data. Third, we scrutinized local standards with regard to alignment with national guidelines on febrile neutropenia in pediatric oncology (unpublished data on file)—an approach enabling us to inform participating centers about possible quality improvements.
Limitations of the study include the exclusive invitations for centers affiliated to the GPOH (60 centers from Germany, seven centers from Austria, and nine centers from Switzerland), and the prerequisite that hospitals have institutional standards on FN (which may have introduced a selection bias). In addition, we did not collect or obtain information on local antimicrobial invasive pathogen and resistance patterns. However, we anticipate that differences between centers would be small, reflecting the national average. Another limitation is the lack of data on non-use cases, which would have been necessary to explore factors associated with potential undertreatment. This important aspect was beyond the scope of this work, but should be studied in future projects. Furthermore, our data, which stem from two central European, high-income countries, may not be applicable to settings where access to certain antibiotics is limited and/or where prescribing patterns differ.28
In summary, the high antimicrobial prescription rate and the high rate of inappropriate therapies shown by our study indicate the importance of AMS programs in PHOCs. Likewise, our findings indicate that up-to-date, local standards of care based on national guidelines—along with adherence to them—are paramount to improving antimicrobial use in pediatric oncology and hematology. Future studies should aim to analyze the facilitators and barriers to guideline adherence, as well as to implementing AMS in pediatric oncology and hematology units, and should include qualitative and mixed-methods approaches.29,30
Contributors
CP, MH, and AS conceived and conceptualized the study. CP, KR, KL, MH, and AS collected the data provided by the participating centers, and accessed and verified all data. CP, KR, GW, MH, and AS conducted the statistical analyses. AA, NG, AHG, JH, JL, TL, JGL, LM, TT, SV, UVB, SW, CP, MH, and AS served as expert panel members. All authors interpreted the data. CP and KR wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final version for submission.
Data sharing statement
De-identified data obtained during this study are available immediately following publication for researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author.
Declaration of interests
AA reports having received payments or honoraria by Saarland University (Homburg). HJL reports having received payments or honoraria by CSL Behring, and support for attending meetings and/or travel by Pfizer, Roche, Bayer, Sobi, and CSL Behring. TL reports having received consulting fees by Gilead Sciences, Merck/MSD, Pfizer, Mundipharma, Roche; and payments or honoraria by Merck/MSD, Sanofi, Gilead Sciences, and Pfizer. LM reports being on the advisory board of and having received payments by Shionogi, and having received payments or honoraria by the German Pediatric Society for Infectious Diseases and the Professional Association of Pediatricians. The remaining authors declare no conflicts of interest.
Acknowledgments
This study has been funded by a 2021 CAREer Grant from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) to CP. Additional funding was provided by the Deutsche Gesellschaft für Pädiatrische Infektiologie (DGPI), the Deutsche Gesellschaft für Krankenhaushygiene (DGKH), and the Stiftung Kreissparkasse Saarbrücken.
The AB-PPS PedOnc Study Group
Jan Baier, Stefan Balzer, Ümmügül Behr, Benedikt Bernbeck, Karin Beutel, Claudia Blattmann, Konrad Bochennek, Holger Cario, Angelika Eggert, Karoline Ehlert, Simone Göpner, Udo Kontny, Dieter Körholz, Christof Kramm, Melchior Lauten, Lienhard Lessel, Christin Linderkamp, Stephan Lobitz, Volker Maas, Rainer Misgeld, Urs Mücke, Jennifer Neubert, Lisa Nonnenmacher, Manon Queudeville, Antje Redlich, Martina Rodehüser, Sarah Schober, Meinolf Siepermann, Thorsten Simon, Hadi Souliman, Martina Stiefel, Verena Wiegering, Beate Winkler.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100599.
Contributor Information
Cihan Papan, Email: cihan.papan@ukbonn.de.
AB-PPS PedOnc Study Group:
Jan Baier, Stefan Balzer, Ümmügül Behr, Benedikt Bernbeck, Karin Beutel, Claudia Blattmann, Konrad Bochennek, Holger Cario, Angelika Eggert, Karoline Ehlert, Simone Göpner, Udo Kontny, Dieter Körholz, Christof Kramm, Melchior Lauten, Lienhard Lessel, Christin Linderkamp, Stephan Lobitz, Volker Maas, Rainer Misgeld, Urs Mücke, Jennifer Neubert, Lisa Nonnenmacher, Manon Queudeville, Antje Redlich, Martina Rodehüser, Sarah Schober, Meinolf Siepermann, Thorsten Simon, Hadi Souliman, Martina Stiefel, Verena Wiegering, and Beate Winkler
Appendix A. Supplementary data
References
- 1.Lehrnbecher T. Treatment of fever in neutropenia in pediatric oncology patients. Curr Opin Pediatr. 2019;31:35–40. doi: 10.1097/MOP.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 2.Versporten A., Sharland M., Bielicki J., Drapier N., Vankerckhoven V., Goossens H. The antibiotic resistance and prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32:e242–e253. doi: 10.1097/INF.0b013e318286c612. [DOI] [PubMed] [Google Scholar]
- 3.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papan C., Willersinn M., Weiß C., Karremann M., Schroten H., Tenenbaum T. Antibiotic utilization in hospitalized children under 2 years of age with influenza or respiratory syncytial virus infection - a comparative, retrospective analysis. BMC Infect Dis. 2020;20:606. doi: 10.1186/s12879-020-05336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon A., Mock M., Graf N., von Müller L. Investigation of clostridium difficile ribotypes in symptomatic patients of a German pediatric oncology center. Eur J Pediatr. 2018;177:403–408. doi: 10.1007/s00431-017-3070-1. [DOI] [PubMed] [Google Scholar]
- 6.Lehrnbecher T., Groll A., Agyeman P., et al. Recommendations for diagnostics and therapy of children with cancer presenting with fever and neutropenia - comparison of two current guidelines. Klin Pediatr. 2018;230:115–121. doi: 10.1055/s-0044-101953. [DOI] [PubMed] [Google Scholar]
- 7.Gerber J.S., Jackson M.A., Tamma P.D., Zaoutis T.E. Policy statement: antibiotic stewardship in pediatrics. J Pediatric Infect Dis Soc. 2021;10:641–649. doi: 10.1093/jpids/piab002. [DOI] [PubMed] [Google Scholar]
- 8.Hufnagel M., Versporten A., Bielicki J., Drapier N., Sharland M., Goossens H. High rates of prescribing antimicrobials for prophylaxis in children and neonates: results from the antibiotic resistance and prescribing in European children point prevalence survey. J Pediatric Infect Dis Soc. 2019;8:143–151. doi: 10.1093/jpids/piy019. [DOI] [PubMed] [Google Scholar]
- 9.Blinova E., Lau E., Bitnun A., et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14:e280–e288. doi: 10.1097/PCC.0b013e31828a846d. [DOI] [PubMed] [Google Scholar]
- 10.Papan C., Reifenrath K., Last K., et al. Antimicrobial use in pediatric oncology and hematology: protocol for a multicenter point-prevalence study with qualitative expert panel assessment. JMIR Res Protoc. 2022;11 doi: 10.2196/35774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Houten C.B., Naaktgeboren C.A., Ashkenazi-Hoffnung L., et al. Expert panel diagnosis demonstrated high reproducibility as reference standard in infectious diseases. J Clin Epidemiol. 2019;112:20–27. doi: 10.1016/j.jclinepi.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Papan C., Argentiero A., Porwoll M., et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: a prospective, multicentre cohort study. Clin Microbiol Infect. 2022;28:723–730. doi: 10.1016/j.cmi.2021.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Reinecke J., Lowas S., Snowden J., Neemann K. Blood stream infections and antibiotic utilization in pediatric leukemia patients with febrile neutropenia. J Pediatr Hematol Oncol. 2019;41:251–255. doi: 10.1097/MPH.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 14.Wattier R.L., Levy E.R., Sabnis A.J., Dvorak C.C., Auerbach A.D. Reducing second gram-negative antibiotic therapy on pediatric oncology and hematopoietic stem cell transplantation services. Infect Control Hosp Epidemiol. 2017;38:1039–1047. doi: 10.1017/ice.2017.118. [DOI] [PubMed] [Google Scholar]
- 15.Horikoshi Y., Suwa J., Higuchi H., et al. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int J Infect Dis. 2017;64:69–73. doi: 10.1016/j.ijid.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Ockfen S., Egle L., Sauter K., et al. Meropenem use in pediatric oncology - audit on indication, appropriateness and consumption comparing patient derived and pharmacy dispensing data. Klin Padiatr. 2021;233:278–285. doi: 10.1055/a-1481-8905. [DOI] [PubMed] [Google Scholar]
- 17.Castagnola E., Bagnasco F., Mesini A., et al. Antibiotic resistant bloodstream infections in pediatric patients receiving chemotherapy or hematopoietic stem cell transplant: factors associated with development of resistance, intensive care admission and mortality. Antibiotics (Basel) 2021;10:266. doi: 10.3390/antibiotics10030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMullan B.J., Haeusler G.M., Hall L., et al. Aminoglycoside use in paediatric febrile neutropenia - outcomes from a nationwide prospective cohort study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Af Sandeberg M., Johansson E., Wettergren L., Bjork O., Hertting O., Nilsson A. Antibiotic use during infectious episodes in the first 6 months of anticancer treatment-a Swedish cohort study of children aged 7-16 years. Pediatr Blood Cancer. 2017;64:e26397. doi: 10.1002/pbc.26397. [DOI] [PubMed] [Google Scholar]
- 20.Herberger S., Oberkircher N., Wenzel G.I., et al. Prospektives Audit des Gentamicin Drug Monitorings in einem Kinderkrebszentrum. Klin Padiatr. 2021;233:123–126. doi: 10.1055/a-1352-5053. [DOI] [PubMed] [Google Scholar]
- 21.Ferreras-Antolín L., Irwin A., Atra A., et al. Pediatric antifungal prescribing patterns identify significant opportunities to rationalize antifungal use in children. Pediatr Infect Dis J. 2022;41:e69–e74. doi: 10.1097/INF.0000000000003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groll A.H., Pana D., Lanternier F., et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021;22:e254–e269. doi: 10.1016/S1470-2045(20)30723-3. [DOI] [PubMed] [Google Scholar]
- 23.Yeoh D.K., Haeusler G.M., McMullan B.J., et al. Antifungal use in children with acute leukaemia: state of current evidence and directions for future research. J Antimicrob Chemother. 2022;77:1508–1524. doi: 10.1093/jac/dkac060. [DOI] [PubMed] [Google Scholar]
- 24.Tribble A.C., Lee B.R., Flett K.B., et al. Appropriateness of antibiotic prescribing in United States children's hospitals: a national point prevalence survey. Clin Infect Dis. 2020;71:e226–e234. doi: 10.1093/cid/ciaa036. [DOI] [PubMed] [Google Scholar]
- 25.Wolf J., Sun Y., Tang L., et al. Antimicrobial stewardship barriers and goals in pediatric oncology and bone marrow transplantation: a survey of antimicrobial stewardship practitioners. Infect Control Hosp Epidemiol. 2016;37:343–347. doi: 10.1017/ice.2015.295. [DOI] [PubMed] [Google Scholar]
- 26.MacBrayne C.E., Williams M.C., Levek C., et al. Sustainability of handshake stewardship: extending a hand is effective years later. Clin Infect Dis. 2020;70:2325–2332. doi: 10.1093/cid/ciz650. [DOI] [PubMed] [Google Scholar]
- 27.Horikoshi Y., Kaneko T., Morikawa Y., et al. The North wind and the sun: pediatric antimicrobial stewardship program combining restrictive and persuasive approaches in hematology-oncology ward and hematopoietic stem cell transplant unit. Pediatr Infect Dis J. 2018;37:164–168. doi: 10.1097/INF.0000000000001746. [DOI] [PubMed] [Google Scholar]
- 28.Hsia Y., Lee B.R., Versporten A., et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 29.Haeusler G.M., Slavin M.A., Bryant P.A., Babl F.E., Mechinaud F., Thursky K.A. Management of fever and neutropenia in children with cancer: a survey of Australian and New Zealand practice. J Paediatr Child Health. 2018;54:761–769. doi: 10.1111/jpc.13899. [DOI] [PubMed] [Google Scholar]
- 30.Morgan J.E., Phillips B., Haeusler G.M., Chisholm J.C. Optimising antimicrobial selection and duration in the treatment of febrile neutropenia in children. Infect Drug Resist. 2021;14:1283–1293. doi: 10.2147/IDR.S238567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.