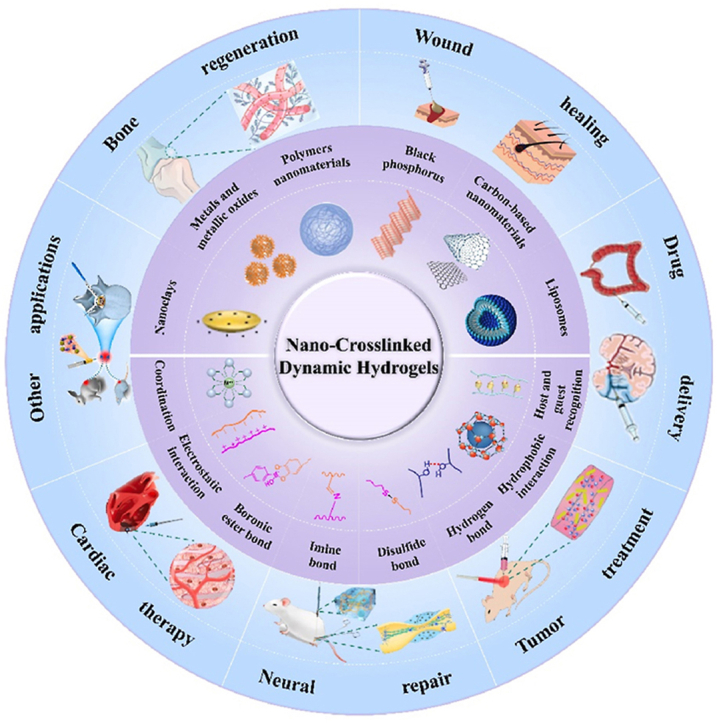
Abstract
Hydrogels resemble natural extracellular matrices and have been widely studied for biomedical applications. Nano-crosslinked dynamic hydrogels combine the injectability and self-healing property of dynamic hydrogels with the versatility of nanomaterials and exhibit unique advantages. The incorporation of nanomaterials as crosslinkers can improve the mechanical properties (strength, injectability, and shear-thinning properties) of hydrogels by reinforcing the skeleton and endowing them with multifunctionality. Nano-crosslinked functional hydrogels that can respond to external stimuli (such as pH, heat, light, and electromagnetic stimuli) and have photothermal properties, antimicrobial properties, stone regeneration abilities, or tissue repair abilities have been constructed through reversible covalent crosslinking strategies and physical crosslinking strategies. The possible cytotoxicity of the incorporated nanomaterials can be reduced. Nanomaterial hydrogels show excellent biocompatibility and can facilitate cell proliferation and differentiation for biomedical applications. This review introduces different nano-crosslinked dynamic hydrogels in the medical field, from fabrication to application. In this review, nanomaterials for dynamic hydrogel fabrication, such as metals and metallic oxides, nanoclays, carbon-based nanomaterials, black phosphorus (BP), polymers, and liposomes, are discussed. We also introduce the dynamic crosslinking method commonly used for nanodynamic hydrogels. Finally, the medical applications of nano-crosslinked hydrogels are presented. We hope that this summary will help researchers in the related research fields quickly understand nano-crosslinked dynamic hydrogels to develop more preparation strategies and promote their development and application.
Keywords: Dynamic hydrogels, Tissue engineering, Nano-crosslinking, Self-healing, Reversible covalent bonds
Graphical abstract
The construction and biomedical applications of nano-crosslinked dynamic hydrogels.
1. Introduction
Hydrogels are a class of polymers characterized by a three-dimensional (3D) network of crosslinked hydrophilic chains. Since hydrogels have a structure and function similar to those of the natural extracellular matrix (ECM), they can be widely used in the biomedical field [[1], [2], [3], [4], [5]] as drug-delivery platforms [[6], [7], [8], [9]], wound dressings [[10], [11], [12], [13]], and tissue engineering repair materials [[14], [15], [16], [17], [18], [19], [20], [21]]. Although hydrogels exhibit some remarkable properties, they still have many properties, such as a low mechanical strength, poor thermal stability, rapid degradation rate, and no self-healing ability after damage, that limit their biomedical applications.
Injectable hydrogels have attracted great interest because of their good biocompatibility, simple handling, easy formation, and noninvasive drug delivery by injection [[22], [23], [24]]. In addition, injectable hydrogels are potentially attractive for clinical applications because they can significantly reduce patient discomfort, risk of infection, recovery time, and treatment costs, especially in irregular wound repair [25]. Injectable hydrogels with shear-thinning properties are often subjected to mechanical forces that may disrupt the structural integrity of the hydrogel network. This disruption not only negatively affects hydrogel functional properties but also increases the risk of microbial invasion through cavities or fissures [26,27]. Therefore, biomedical hydrogels require a certain level of strain resistance. The mechanical properties of hydrogels can be regulated by their concentration and crosslinking density. Specifically, the mechanical strength and durability of hydrogels can be enhanced by increasing their concentration or crosslinking density; however, the functionality of the hydrogel may be affected. For example, the hydrogel porosity may decrease and the hydrogels may become more difficult to degrade, which is detrimental to the growth of cells and regenerative tissues [28]. Therefore, the key to obtaining good structural and functional integrity is to endow hydrogel materials with a self-healing ability.
Self-healing materials are a class of smart materials with an inherent ability to automatically repair damage without external intervention. Compared with conventional hydrogels, self-healing hydrogels possess dynamic and reversible 3D network structures that can restore their initial structure and function after damage. Due to their flexibility, biocompatibility, and easy functionalization, many self-healing hydrogels with unique properties and extended application durations have been developed through dynamic covalent or noncovalent interactions for different biomedical applications [29]. Injectable hydrogels with self-healing properties can be easily delivered in vivo without causing significant damage to the body [30]. Thus, injectable self-healing hydrogels have great promise for biomedical applications [31]. However, for dynamic hydrogels, a balance between the mechanical properties and injectability of the self-healing hydrogel must be achieved. Self-healing ability is usually exhibited by soft polymers and gels, which cannot provide stiffness to the hydrogel. Good mechanical properties, which are basic demands in medical applications, especially for tissue scaffold and drug-delivery materials, originate from relatively strong covalent bonds, which have relatively weak dynamic properties. The trade-off between these two factors should be considered for the construction of hydrogels with injectability and self-healing properties [32].
Nanocrosslinked dynamic hydrogels exhibit self-healing properties and can act as a responsive platform that resembles the natural cellular matrix. These nanomaterial hydrogels also exhibit better biocompatibility and good cell viability, proliferation, and differentiation. The incorporation of nanomaterials enhances the strength of hydrogels and their responses to external stimuli (e.g., pH [12,[33], [34], [35]], thermal [36,37], electromagnetic [38,39], etc.). The nature of the nanomaterials in the hydrogel also determines the type and function of stimuli-responsive hydrogels. In addition, the introduction of nanomaterials into hydrogels also improves their injectability and shear-thinning properties. Due to the porous microstructure of hydrogels and the various interactions between nanomaterials and hydrophobic polymer chains, nanomaterials can modulate the rheological response of hydrogels through unique interactions, resulting in better viscoelastic properties. The good dispersion of nanomaterials in hydrogels results in good biocompatibility and more effective functionality of nano-crosslinked dynamic hydrogels.
In this review, we focus on nanomaterials commonly used in hydrogels and summarize the nano-crosslinked dynamic hydrogels developed in recent years through different reversible crosslinking strategies between nanomaterials and polymers and the biomedical applications of these hydrogels.
2. Nanomaterials for dynamic hydrogel fabrication
Nanomaterials, with highly specific surfaces for achieving contact with other particles and special nanosized properties, have been widely used in medical applications, such as drug delivery, tumor treatment, and tissue regeneration. However, the easy agglomeration of nanosized materials inhibits their effect. Cytotoxicity should not be ignored for clinical applications. Hydrogels, especially hydrogels with self-healing ability and injectability, have attracted increasing attention from researchers. Since hydrogels have a 3D porous network structure identical to that of the ECM, they are one of the most competitive candidates for materials used in tissue engineering, wound repair, bone regeneration, etc. They are suitable for repairing irregular defect structures and vulnerable parts in particular. However, the balance between their mechanical properties and injectability has limited their use, and functionalized hydrogels still need to be further developed. Recent studies have shown the potential of incorporating nanoparticles (NPs) into hydrogels to enhance crosslinked networks or endow hydrogels with certain biological properties [40]. Nano-crosslinked dynamic hydrogels with both excellent mechanical properties and various functionalities have been developed. For these hydrogels, stiffness was significantly enhanced without sacrificing dynamics, which suggests the obvious superiority of these materials as tissue support materials used for tissue regeneration or drug delivery. Similar to other hydrogels, most of these nanoparticle-crosslinked hydrogels have been shown to have good biocompatibility and the ability to support cell adhesion and proliferation [41], and some were even capable of inducing cell differentiation [42]. The results showed that the mechanical properties may be stabilized by the introduction of NPs into the crosslinked network as a component of the gel skeleton, with the stiffness of the nanomaterial contributing to the hydrogel mechanical properties. The variation and modification of nanomaterials were feasible for the preparation various hydrogel materials with different functions for applications under the corresponding conditions. According to the different composites of the nanomaterials used in hydrogels, these nanomaterials are mainly classified as metals and metallic oxides, nanoclays, carbon-based nanomaterials, black phosphorus (BP), polymers, liposomes, etc. In the hydrogel matrix, these nanomaterials act as crosslinkers or crosslinking points, endowing hydrogels with many significant properties.
2.1. Metals and metallic oxides
Nanosized metals and metallic oxides have been widely used in the biomedical field. They are also used in the fabrication of injectable self-healing hydrogels. Current self-healing hydrogels exhibit good injectability under certain pressure (i.e., shear thinning) and rapidly return to their original gel state after injection (i.e., self-healing). However, the introduction of common crosslinking agents may lead to irreversible crosslinking (affecting self-healing properties), thus limiting their applications (such as in tissue scaffolds and drug delivery). Metals and metallic oxide nanomaterials can be used for the crosslinking of self-healing hydrogels [[43], [44], [45]]. In nano-crosslinked hydrogels, the introduction of metal/metallic oxide components can improve the rigidity of the hydrogel by reinforcing the skeleton. Moreover, the performance of the introduced nanocomposite can endow the hydrogel with functionality, which can produce hydrogels such as photothermal dynamic hydrogels used for photothermal therapy in tumor treatment, injectable self-healing antibacterial hydrogels used for wound repair, and self-healing magnetic double-network hydrogels used for image monitoring and controlling drug release. Among various metal and metallic oxides, nanogold, silver, and Fe3O4 are excellent candidates in this regard.
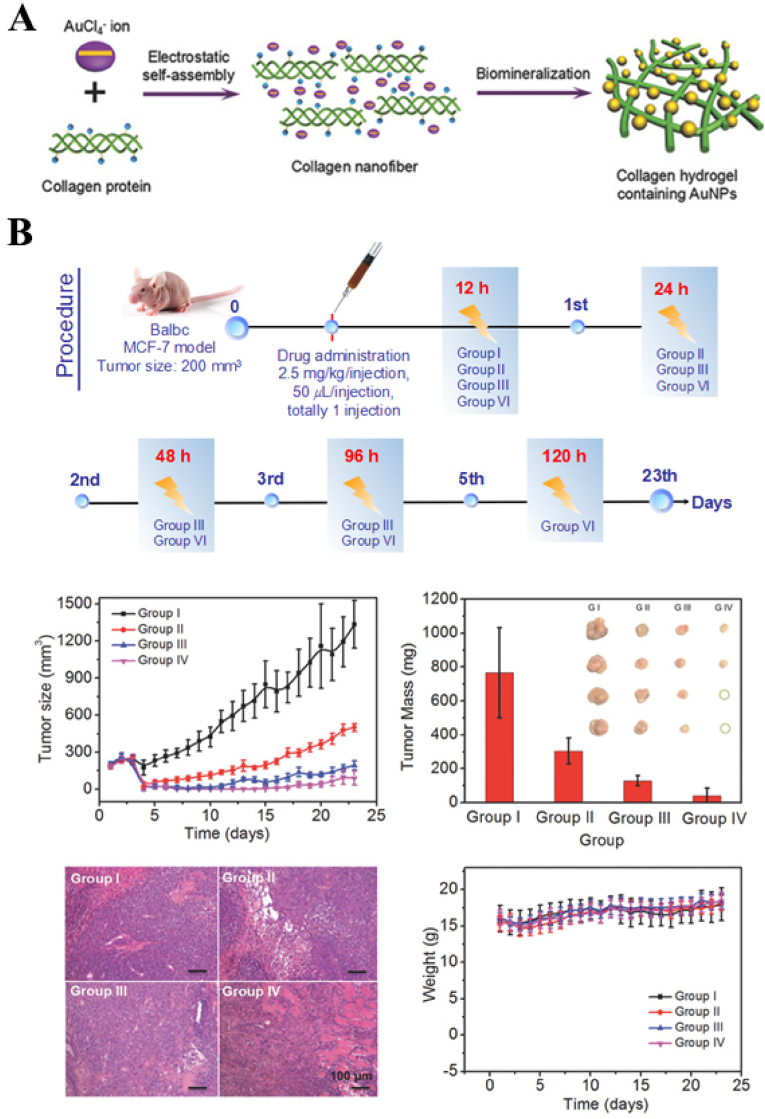
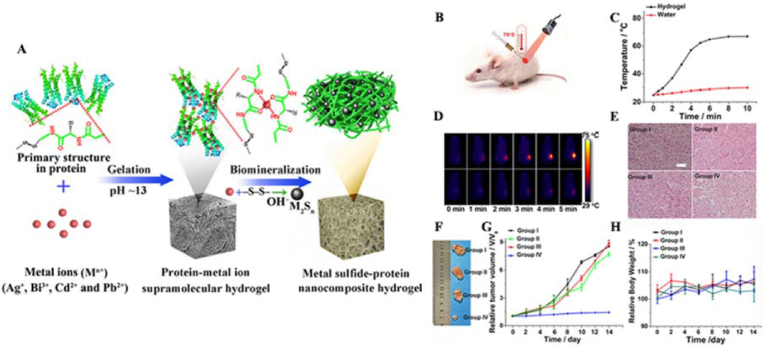
Xing et al. facilitated the in situ mineralization of collagen–gold hybrid hydrogels based on the self-assembly of biomolecules [46]. The assembly process was mainly related to the electrostatic interactions between the positively charged collagen molecular chains and the negatively charged [AuCl4]- ions (Fig. 1). Gold NPs (AuNPs), formed by in situ mineralization, played the role of regulating the mechanical properties of the hydrogels. The noncovalent interactions between AuNPs and collagen molecular chains conferred shear-thinning and self-healing properties to the hydrogels. This synthetic strategy is also applicable for other biopolymers (e.g., chitosan (CS), gelatin (Gel), etc.) to fabricate functional hydrogels. Such hydrogels can be used as drug-delivery carriers that can effectively deliver drugs to lesions and tumors, where the drugs can be retained in situ and a long and slow release can be achieved. Experiments have confirmed that during tumor treatment using hydrogels, the drug was enriched at the tumor site after 120 h. This resulted in a lower drug dosage and minimized damage to normal tissues. In addition, due to the presence of Au NPs, this hydrogel can also be used for photothermal therapy (PTT) for tumor treatment.
Fig. 1.
(A) Schematic diagram of the fabrication of an injectable collagen-based hydrogel containing gold nanoparticles based on a biomineralization-triggered self-assembly process (B) Tumor volumes of mice after different treatments; Tumor weights of different treatment groups (inset: photographs of the resected tumors from each group obtained after 23 d of treatment). All data are presented as mean ± SD (n = 4); Histology images of tumor slices (with H&E staining) from mice at different treatment groups. Scale is equal to original magnification × 100; Body weights of mice in different groups after treatment. Reproduced with permission [46]. Copyright 2016, John Wiley and Sons. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
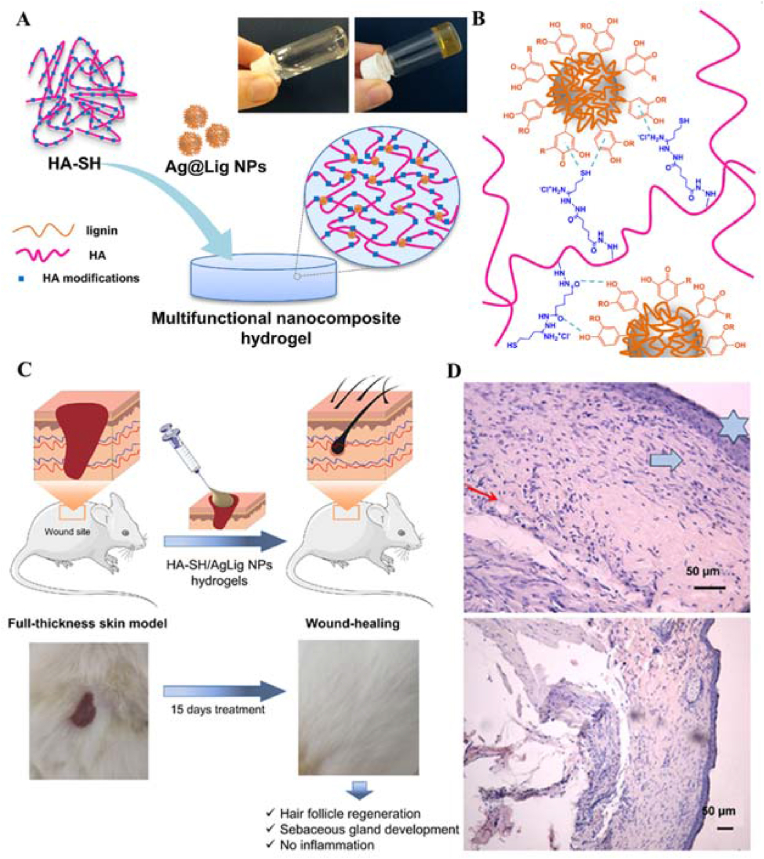
Perez-Rafael et al. developed multifunctional hydrogels for chronic wound management through the self-assembly of thiolated hyaluronic acid (HA-SH) and bioactive silver-lignin NPs (Ag@Lig NPs) [47]. The hydrogel showed not only shear-thinning and self-healing properties but also zero-order kinetics in the release of antimicrobial silver in response to infection-related hyaluronidase (Fig. 2). It inhibited the generation of the major enzymes myeloperoxidase and matrix metalloproteinases that respond to wound chronicity in wound exudate due to the antimicrobial property of silver. They also established a rat diabetes model, and the results showed that the HA-SH/Ag@Lig NP hydrogels induced complete tissue remodeling and restoration of skin integrity.
Fig. 2.
(A) Schematic representation of hydrogel formation (B)Possible polymer-NPs interaction leading to self-assembling. (C) Scheme on the in vivo mouse model (top) and representative photos of full thickness skin wounds before and after application of the nano-enabled hydrogels. (D) Hematoxylin-eosin-stained sections of the wound tissues at sacrifice (day 15 post-surgery). Blue six-pointed stars indicate the epidermis, blue arrows indicate new granulation tissue and the red arrows indicate the blood vessels. Reproduced with permission [47]. Copyright 2021, Elsevier B.V. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
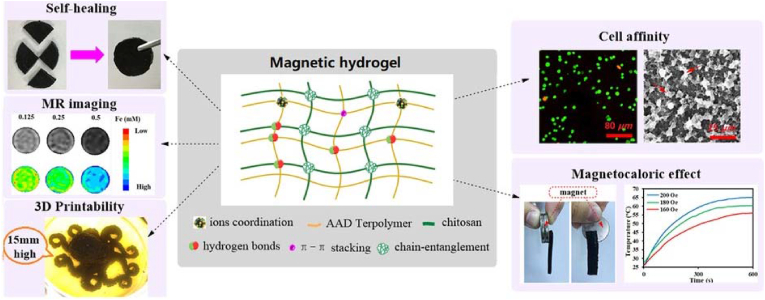
Functional composite materials made of NPs and hydrogels with unique magnetic properties can be used in image monitoring and controlled drug release after implantation. Therefore, they have attracted much attention in the biomedicine field [48]. Gang et al. utilized the interactions between magnetic Fe3O4 and a CS-polyolefin matrix to prepare novel self-healing magnetic double-network hydrogels (Fig. 3) [39]. Fe ions, which acted as binding sites on the HCl-etched magnetic Fe3O4 surface, underwent coordination with different carboxyl/hydroxyl groups, increasing the dispersion of Fe3O4 in the hydrogels. The interplay between ionic coordination, hydrogen bonding, and π-π stacking interactions enhanced the mechanical properties, self-healing, and anti-swelling abilities of the hydrogels. Moreover, nano-Fe3O4 endowed the hydrogel with excellent magneto-induced effects and magnetic resonance (MR) imaging capabilities.
Fig. 3.
A strategy for improving the strength and self-healing properties of magnetic hydrogels while endowing them with cytocompatibility and 3D printability. Reproduced with permission [39]. Copyright 2019, Royal Society of Chemistry.
2.2. Nanoclays
Nanoclays possess characteristics such as plasticity, large specific surface area, electronegativity, and nontoxicity, which make them a popular choice of material for medical applications due to their good biocompatibility, simple functionalization, low toxicity, and affordability [[49], [50], [51], [52]]. Recent studies have shown that the incorporation of nanoclay into dynamic hydrogels resulted in improved mechanical properties, such as increased strength and toughness, due to the integrity of the nanoclay [53].Additionally, the self-healing properties of these hydrogels were attributed to noncovalent interactions, such as hydrogen bonding, coordination, and electrostatic interactions [54].
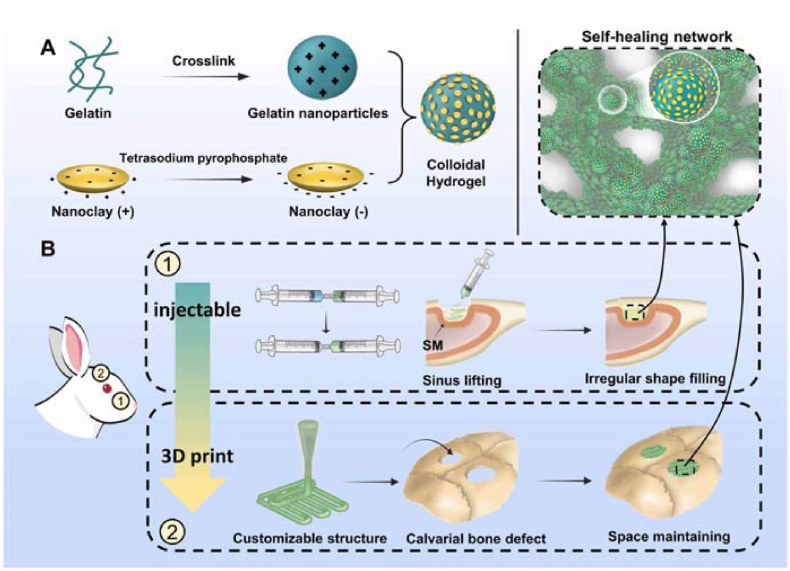
Nanosilicate (laponite XLS) is a synthetic nanoclay material with a disk shape, diameter of 20–30 nm and thickness of ∼1 nm. Nanosilicates are positively charged at the edges and negatively charged at the top and bottom surfaces. Thus, they can participate in reversible electrostatic interactions with charged polymers. The reversible properties of this electrostatic interaction allow the hydrogels to recover immediately after injection, thus restoring the prestrain modulus when the shear force is removed. Gao et al. synthesized stretchable, tough, and self-healing nanocomposite hydrogels by the in situ polymerization of acrylamide in the presence of exfoliated montmorillonite (MMT) platelets [55]. The hydrogels exhibited a fracture strain of approximately 11800% and a fracture energy of up to 10.1 MJ m−3. Due to the reversible desorption/reabsorption of polymer chains, the hydrogels recovered at room temperature after stretching or by a drying-reswelling procedure. Dou et al. obtained hydrogels that were self-healable and injectable via the electrostatic assembly of Gel NPs and nanoclay particles (Fig. 4) [56]. The hydrogel showed remarkable mechanical properties reflected by the maximal elastic modulus reaching ∼150 kPa and a high self-healing efficiency. They also revealed that the hydrogel showed excellent injectability and moldability.
Fig. 4.
Schematic illustration showing (A) the design rationale of the composite colloidal gels composed of gelatin nanoparticles and nanoclay particles and (B) their applications in bone regeneration. Reproduced with permission [56]. Copyright 2023, IOP Publishing.
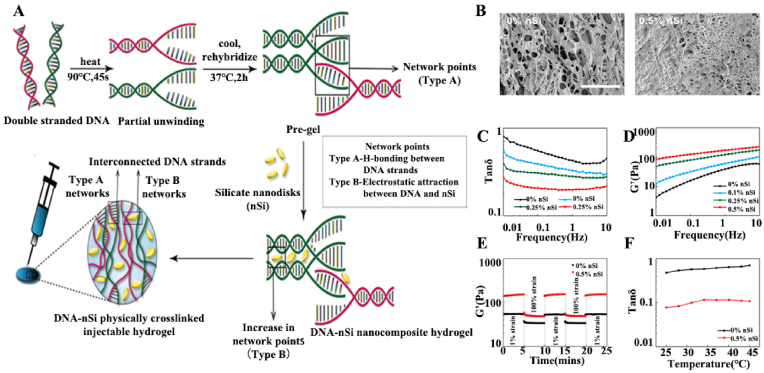
Nanosilicates easily participate in noncovalent interactions with charged particles and segments. They can be used for the controlled release of bioactive molecules, including drugs, proteins, and vaccines, because their high specific surface area and anisotropic charges enable the sustained release of loaded bioactive molecules. In addition, nanosilicates are degradable, and the degradation products can be readily absorbed by the body, facilitating bone tissue regeneration. Basu et al. synthesized an injectable hydrogel with sustained-drug-release properties utilizing the noncovalent interactions between deoxyribonucleic acid (DNA) and nanosilicates (Fig. 5) [57]. First, the DNA denaturation and rehybridization mechanism was used to form hydrogen bonds between complementary base pairs of adjacent DNA chains. Then, the attractive electrostatic interactions of the nanosilicates with the DNA backbones were used to generate additional crosslinking sites, which enhanced the mechanical properties of the hydrogels. The results showed that the addition of nanosilicates led to a decrease in the pore size of the hydrogels, which indicated a more compact network with a higher degree of physical crosslinking. The oscillatory shear rheology tests showed that the elasticity and storage modulus of the nanocomposite hydrogels increased due to the electrostatic interactions between the nanosilicates and DNA backbones. The rapid recovery of the hydrogels and the recovery of the energy storage modulus under dynamic oscillatory shear tests indicated their dynamic nature. The good thermal stability and minimal cytotoxic effects of the nanocomposite hydrogels showed that they are good candidates for medical applications. DNA-based nanosilicate hydrogels can be used to deliver drugs. The maintained shear-thinning properties of the hydrogel after drug loading confirmed its potential as an injectable drug-delivery system. The slow drug release was attributed to the addition of nanosilicates resulting in compact hydrogel structures with smaller pore sizes. The biological activity of dexamethasone release was confirmed in a rat cranial defect model by the osteogenic differentiation of human adipose stem cells in vitro and bone formation in vivo.
Fig. 5.
(A) Schematic representation of the design strategy for the development of DNA–nSi injectable hydrogels. (B) SEM images of the DNA-based hydrogel showing a highly porous structure (scale bar = 100 μm). (C) Tan δ (G″/G′) profiles for the nanocomposite hydrogels over a range of frequency from 0.01 to 10 Hz. (D) Frequency sweep experiments performed in the range of 0.01–10 Hz. (E) Recovery data obtained by monitoring the storage modulus of the nanocomposite hydrogels while subjecting them to alternating high (100%) and low (1%) strain conditions. (F) Tan δ values in the range of 25–45 °C. Reproduced with permission [57]. Copyright 2018, American Chemical Society.
2.3. Carbon-based nanomaterials
Carbon-based nanomaterials are one of the earliest developed nanomaterials. Carbon nanomaterials with different forms and particle sizes have since been developed. Nanosheets, nanospheres, nanotubes, nanorods, and nanodots are commonly and widely used in many fields. In the study of dynamic hydrogels for medical applications, carbon-based nanomaterials, such as carbon nanotubes (CNTs) [58] and graphene oxide (GO) [59], are also widely used [60,61]. GO is a type of carbon material with monolayer carbon atoms in a hexagonal lattice. It has attracted much attention because of its excellent optical and electronic properties. The multiple water-soluble functional groups, such as hydroxyl, carboxyl, and epoxide groups, in the crystal lattice allow them to easily disperse in water to form solutions with strong negative potentials. These properties enable GO to interact covalently or noncovalently with other materials. Many multifunctional, injectable and self-healing hydrogels with sensitivity to stimuli, such as pH, light, heat, and magnetism, have been fabricated and are useful in biomedical applications.
Recently, Bai et al. prepared pH-sensitive hydrogels using polyvinyl alcohol (PVA) and GO [33]. The large number of hydroxyl, epoxide, and carboxyl groups on the surface of GO sheets can form hydrogen bonds with the hydroxyl-rich PVA chains. One PVA chain can interact with two or more GO sheets to form crosslinking sites. GO/PVA hydrogels exhibit pH-induced gel-sol transition and can be used for the loading and selective release of drugs. Han et al. used GO nanosheets as crosslinking sites to obtain CS-based supramolecular hydrogels [62]. When the hydrogel was prepared with 8.0 wt% CS and 0.2 wt% GO, it underwent a thermally reversible sol-gel transition. CS/GO hydrogels that showed self-healing properties after damage were also prepared at room temperature by controlling the concentration and ratio of CS and GO (8.0 wt % CS/0.3 wt % GO). Wang et al. synthesized PVA/graphite oxide self-healing hydrogels with pH sensitivity and enhanced thermal properties [34]. Dual crosslinks were introduced in the hydrogels: one set of PVA crosslinks induced by freezing/thawing methods to confer self-healing properties to the obtained hydrogels, and the other set provided by GO as a two-dimensional crosslinker for enhancing pH sensitivity and thermal stability. The hydrogel showed not only self-healing ability without external stimuli or the addition of any healing agent but also pH sensitivity and thermal stability. The preparation method involving environmentally friendly components (PVA and GO) and a simple synthesis (freezing/thawing treatment) may pose little threat to the environment.
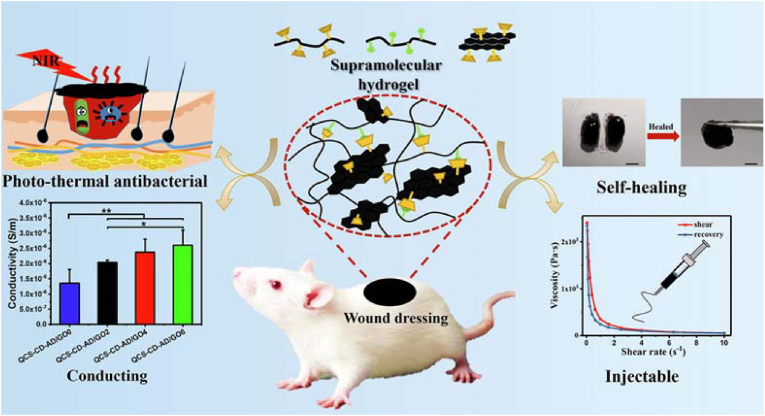
Zhang et al. synthesized injectable self-healing supramolecular hydrogels with electrical conductivity for skin regeneration based on the host–guest interactions of quaternized CS-graft-cyclodextrin (QCS-CD), quaternized CS-graft-adamantane (QCS-AD), and reduced graphene oxide (rGO) (Fig. 6) [63]. After incorporating rGO, which has good antibacterial activity, into the hydrogels, these hydrogels possessed electrical conductivity, which can modulate cellular activity, including cell adhesion, proliferation, and migration, and thus accelerate wound healing. In addition, full contact between the sharp nanowalls of rGO and bacterial cell membranes, as well as good charge transfer between bacteria and nanowalls, led to bacterial cell membrane damage and thus inhibited bacterial activity. The rGO incorporated into hydrogels eliminated pathogenic bacteria from the wound area due to its good antibacterial ability, maintained a suitable moist and sterile wound-healing environment, greatly reduced the risk of bacterial infection, and recruited more fibroblasts during the wound-healing process to promote the wound repair process.
Fig. 6.
Schematic illustration of injectable self-healing photo-thermal antibacterial hydrogel and its characterization of self-healing, injectable, and conductivity. Reproduced with permission [63]. Copyright 2020, Elsevier B.V.
2.4. Black phosphorus
BP has been developed as a new two-dimensional material in recent years. Due to its inherent semiconductor properties, BP shows excellent photothermal conversion efficiency and has attracted the attention of biomedical researchers. BP nanosheets also have good biocompatibility and can degrade into nontoxic phosphates and phosphonates in water, providing good nucleation sites for biomineralization in vivo and further regulating bone formation [64]. The homo-layers of BP nanosheets are not in the same plane as the atoms but have an anisotropic folded honeycomb structure. This high-specific-surface-area structure facilitates drug loading and modification for the development of multifunctional biomaterials. BP nanosheets require proper modification for biomedical use, as BP itself tends to precipitate in water or organismic microenvironments, leading to inhomogeneous photothermal effects. Therefore, some typical strategies have been developed, such as the modification of polymers by simple physical mixing or chemical treatment [65].
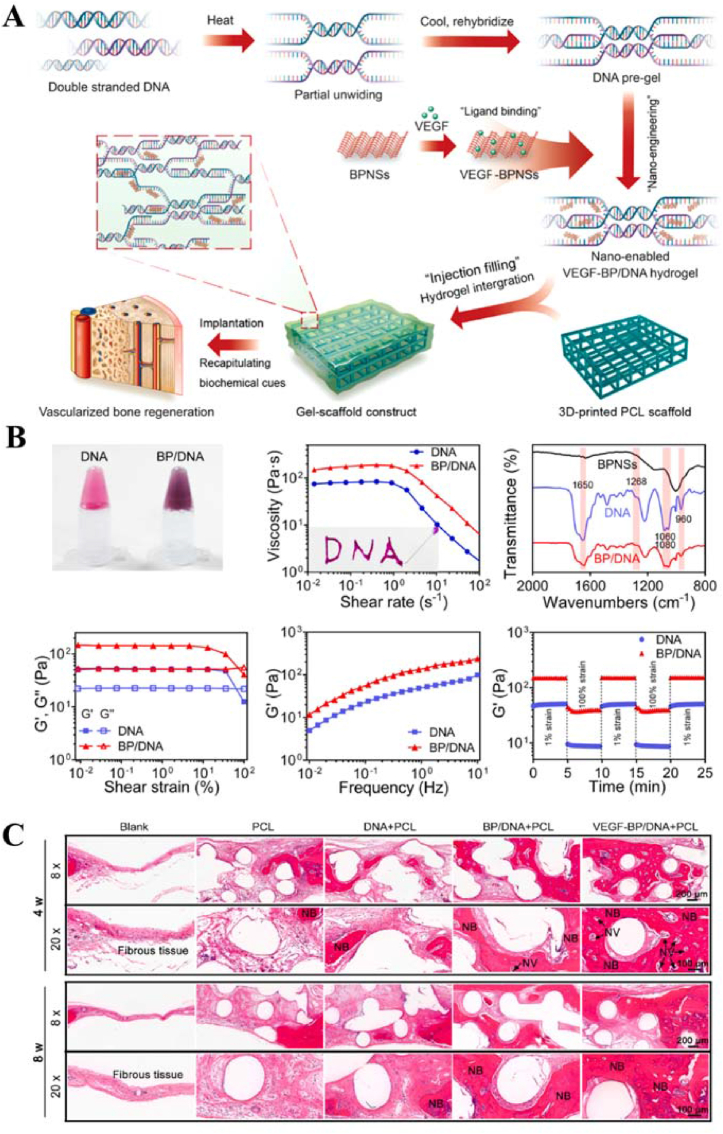
Miao et al. developed a dynamic DNA hydrogel supporting BP nanosheets (BPNSs) integrated with 3D-printed polycaprolactone (PCL) scaffolds to construct bioactive hydrogel scaffold structures for enhanced angiogenesis and bone regeneration (Fig. 7) [66]. The average lateral size of the BPNSs was approximately 200 nm, and they had good crystallinity. The BPNSs were dispersed into DNA hydrogels and then vortexed and mixed to produce nano-DNA hydrogels. As BPNSs were incorporated into the DNA hydrogel matrix, the viscosity of the hydrogel increased accordingly. In addition, the increase in shear rate led to a decrease in viscosity, indicating the shear-thinning properties of the nano-DNA hydrogels, which are essential for injectable hydrogels. Fourier transform infrared (FTIR) spectra of the hydrogels indicated changes in the DNA molecular environment and the presence of interactions between the BPNSs and the DNA backbone. Oscillatory rheological analysis showed that the incorporation of BPNSs significantly increased the storage modulus of the DNA hydrogels. Oscillatory strain amplitude tests showed the complete recovery of the storage modulus of the DNA and BP/DNA hydrogels within a few seconds after several cycles of strain changes, indicating the self-healing ability of the physically crosslinked network. These results were possibly attributed to physical interactions, including π-π stacking between the six-membered BPNS ring and the DNA backbone. These interactions modulated the mechanical stability of the BP/DNA hydrogels, and the formation of additional crosslinking sites may have been responsible for the observed increase in storage module (G’) values. The introduction of BPNSs led to a significant decrease in the pore size of the hydrogels, suggesting the formation of a dense nanocomposite network with a higher degree of physical crosslinking. The addition of BPNSs to the physical crosslinking networks allowed their stability to be maintained in physiological environments. In physiological environments, vascular endothelial growth factor (VEGF) was prone to degradation and loss of its biological activity. VEGF was adsorbed on the BPNS surface or embedded in the interlayer space through electrostatic interactions, and encapsulation of VEGF with biomaterials not only maintained its activity but also lead to the sustainable release of VEGF at specific defect sites. Injectable BP/DNA nano-crosslinked hydrogels could be used as a promising VEGF delivery vehicle and biocoating. The in vivo results from a rat cranial defect model showed that the hydrogel scaffold structures were able to promote the growth of mature blood vessels and induce osteogenesis to promote new bone formation, indicating that the strategy of integrating dynamic nano-crosslinked hydrogels with 3D-printed scaffolds has high application potential in bone tissue engineering.
Fig. 7.
(A) Schematic illustration of the integration of a 3D-printed PCL scaffold with BPNS-containing DNA hydrogels loaded with VEGF for vascularized bone regeneration. (B) The photograph, FTIR spectra, and rheological analysis of BP/DNA hydrogels (C) H&E staining images of cranial defects after the implantation of gel-scaffold constructs for 4 and 8 weeks, NV indicated by the arrow represented for new blood vessels, and NB represented new bone tissue. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Reproduced with permission [66]. Copyright 2023, Elsevier B.V.
2.5. Polymers nanomaterials
Polymers such as polyethylene glycol (PEG), alginate (ALG), polylactic-co-glycolic acid (PLGA), polycaprolactone (PCL), and hyaluronic acid (HA) have good biocompatibility, nontoxicity, degradability, and rigidity. They are widely used in medical applications. Nanoparticle polymers can also be used as crosslinkers in self-healing hydrogels for improved mechanical properties and multifunctionality [67,68].
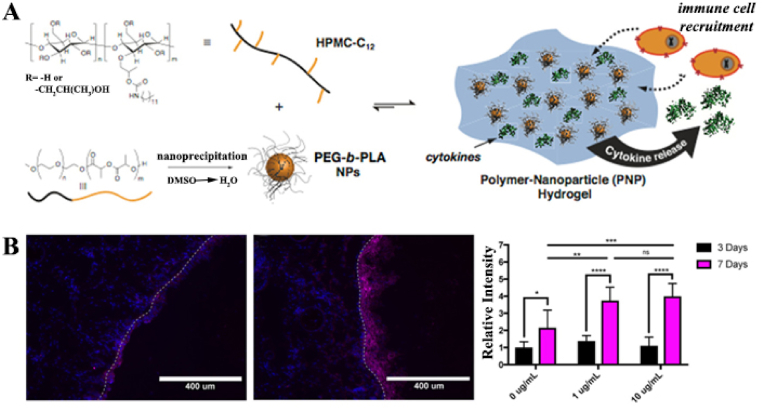
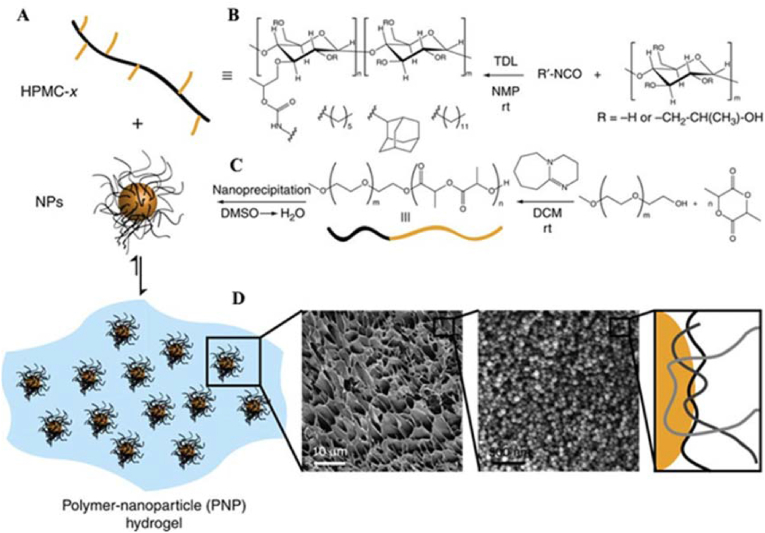
Fenton et al. synthesized a class of injectable and self-healing hydroxypropylmethylcellulose (HPMC) polymer nanoparticle (PNP) hydrogels for cytokine release and recruitment of dendritic cells (DCs) [69]. PNP hydrogels were formed utilizing hydrophobically modified dodecyl HPMC (HPMC-C12) and PEG-b-PLA polymers of different block molecular weights (specifically PEG2k-b-PLA16k and PEG5k-b-PLA20k) (Fig. 8). Each PEG-b-PLA polymer was transformed into NPs by nanoprecipitation. Both PEG-b-PLA NPs were similar in diameter (Dh ~ 80 nm), but the PEG brushes differed in length (2 or 5 kDa) and were small enough to facilitate hydrogel formation by inducing the polymer bridging of multiple NPs. In addition, the higher-molecular-weight PEG block improved the mechanical properties of the PNP hydrogels and resulted in a shorter self-healing time. Model protein therapies were released from the hydrogels in vitro for approximately 1 week and in vivo for approximately 5 days, with subtle differences in the release rates between several of the hydrogels. The effects of hydrogel recruitment on immune cells were tested by subcutaneously injecting PNP hydrogels loaded with C–C motif ligand 21 (CCL21) in a mouse model. The experimental results showed that the CCL21-loaded PNP hydrogels preferentially recruited DCs to the injection site in vivo compared to the non-CCL21-loaded hydrogels. In summary, this PNP hydrogel system, which is capable of recruiting specific immune cell populations to the injection site, has the potential to be applied for in vivo immunomodulation after minimally invasive subcutaneous injection.
Fig. 8.
(A) Schematic representation of PNP hydrogels comprising hydrophobically modified hydroxypropylmethylcellulose (HPMC-C12) and PEG-b-PLA NPs. (B) Localized recruitment of DCs into a subcutaneously injected cytokine-free or CCL21-impregnated PNP hydrogel. Dashed line represents the border between tissue (left of dashed line) and the PNP hydrogel (right of dashed line). (blue = DAPI, purple = anti-CD11c Alexa Fluor 647). Reproduced with permission [69]. Copyright 2019, American Chemical Society. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
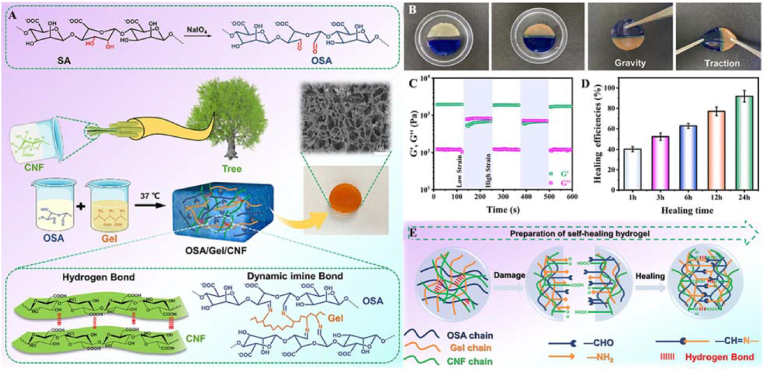
Cellulose nanofibers (CNFs) have nanoscale characteristics, good biocompatibility, suitable rheological properties, and high mechanical strength. They have been used as an enhancer and hydrophilic interpenetrating polymer in hydrogels [70]. Cui et al. designed a hydrogel with a CNF-reinforced oxidized alginate (OSA)/Gel semi-interpenetrating network through a facile one-step approach without a crosslinker [71]. The hydrogel showed good injectability and self-healing ability because of its dynamic imine bonds and hydrogen bonds. Due to the introduction of CNFs, the notable compressive modulus of the OSA/Gel/CNF hydrogel reached 361.3 KPa. By combining this result with its enhanced preosteoblast cell (MC3T3-E1) viability (>96%), proliferation, osteogenic differentiation, and splendid biomineralization (Ca/P ≈ 1.69), it was concluded that the OSA/Gel/CNF hydrogel is an excellent candidate for bone regeneration, especially in minimally invasive conditions.
2.6. Liposomes
Liposomes are artificial membranes made from lecithin and ceramide. They have a hollow structure and a bimolecular layer that is the same as that of the skin cell membrane. These spherical vesicles self-assembled from phospholipid bilayers endow hydrogels with good biocompatibility, low toxicity, and good drug loading with both hydrophilic and hydrophobic drugs. Such bilayers can be formulated from a variety of phospholipids that may even have a targeting component or respond to external stimuli (e.g., temperature or shear stress). Hydrogels crosslinked by liposomes have been well fabricated and used for drug delivery by modifying drugs that target the liposome surface. Drug release could be controlled by external stimulation. Stalder et al. formulated an on-demand drug-delivery hydrogel system containing liposomes for osteoarthritis treatment. The synthetic liposomes were 100 nm large unilamellar vesicles extruded from 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) and 20 mol% of admixed 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine (DPPE) [72]. The addition of DPPE coated the surface of the liposomes with amine groups that could react with functional groups such as aldehydes. The hydrogels were formed by the reaction between aldehyde-containing oxidized dextran, the amines on the surface of the liposome and copolymer polyethyleneimine (PEI) to form imine bonds. The imine bonds were tolerant to an aqueous environment at physiological pH, the reaction produced no byproducts, and the formed chemical bonds were dynamic, allowing the hydrogels to degrade over time. The hydrogels were mechanically responsive. When subjected to mechanical forces, the hydrogels with liposomes loaded with drugs transferred the applied mechanical forces to the bound liposomes, thus triggering the release of the drugs carried within the liposomes and allowing the drug to act for a longer period of time. Uchida et al. developed a novel hydrogel composed of three layers of polymeric micelles and PEI for the sustained release of hydrophilic compounds [73]. The hydrogels were formed from PEI (as the main chains of the hydrogels) and vesicular trilayer polymeric micelles (as the crosslinkers and carriers of the hydrophilic compound). The viscoelastic properties of the hydrogels were altered by adjusting the molecular weight, concentration, and pH of the constituent components of the hydrogels. In addition, the structural differences of the micelles affected the gelation properties of the hydrogels. Hydrogels formed from three-layer polymeric micelles with vesicle-like flexible structures exhibited a higher storage modulus than those formed from two-layer polymeric micelles with highly stacked hard structures. The microstructural differences of the crosslinkers induced macroscopic changes in the properties of the hydrogels. These hydrogels can encapsulate hydrophilic drugs for sustained drug release in medical applications.
3. Strategies for nano-crosslinked dynamic hydrogel fabrication
3.1. Reversible covalent interactions
3.1.1. Imine bonds
Imine bonds are some of the most active and common dynamic covalent bonds. They can be formed by Schiff base reactions. Nondissociative or dissociative dynamic interactions occur under the stimulation of heat, water, and acid. Therefore, self-healing imine bonds are usually used for preparing self-healing materials. Regarding nano-crosslinked hydrogels, dynamic imine bonds have recently been used for crosslinking to form porous network structures by Schiff base reactions between amine groups and active carbonyl groups (aldehydes and ketones). The hydrogel materials showed self-healing properties due to the intrinsic self-healing property conferred by the imine bonds.
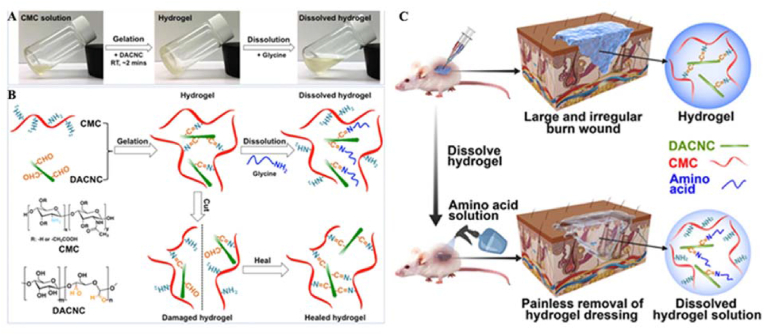
Many dynamic hydrogels with different functions have been prepared by imine bond interactions. Huang et al. prepared a novel injectable nanocomposite self-healing hydrogel using water-soluble carboxymethyl chitosan (CMC) and rigid rod-like dialdehyde-modified cellulose nanocrystals (DACNCs) (Fig. 9) [74]. The hydrogel was crosslinked by dynamic imine bonds between the amine groups of CMC and the aldehyde groups of the DACNCs. The large aspect ratio and specific surface area of the DACNCs increased the number of crosslinking sites within the hydrogel, which could be easily damaged and reorganized, thus enabling the hydrogel to rapidly self-heal. The DACNCs as nanoreinforced fillers improved the strength of the hydrogels. The hydrogel exhibited high self-healing efficiency (∼5 min), injectability, good mechanical strength, and a high equilibrium swelling rate of 350%. In addition, the hydrogel had the unique ability to dissolve on demand in an amino acid solution, resulting in painless removal during wound dressing changes. Moreover, 3D cell encapsulation demonstrated that the hydrogel had the potential to be used as an ECM to support cell growth, with 97.3% cell viability after 7 days of incubation. The hydrogel was injected to cover large and irregularly shaped wounds to maintain a moist environment and absorb large amounts of wound exudate, which was then easily removed from the wound by dissolving the hydrogel on demand. In vivo trials showed that the self-healing hydrogel effectively treated deep partial-thickness burn wounds, with only 0.6% of wounds unclosed and without scar formation after 2 weeks of healing. In conclusion, on-demand dissolvable nanocomposite self-healing hydrogels have multiple advantages and good potential as wound dressing materials for patients with deep partial-thickness burns.
Fig. 9.
(A) Pictures of the hydrogel gelation and dissolution process. (B) Schematic illustration of reversible and dynamic imine bonds (−C=N−) formation and self-healing in the CMC/DACNC hydrogel. (C) Schematic diagram of the fabrication of an on-demand dissolvable self-healing hydrogel for deep partial-thickness burn wound healing. Reproduced with permission [74]. Copyright 2018, American Chemical Society.
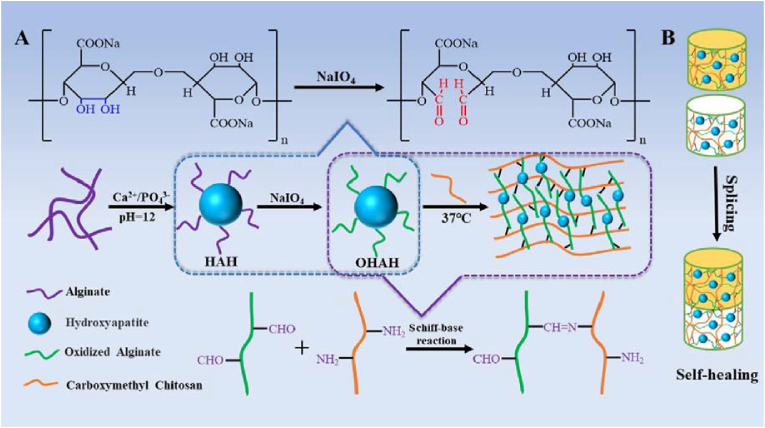
The presence of amino groups on DNA can react with the aldehyde groups on OSA to form imine bonds. Basu et al. designed a nanocomposite DNA-based hydrogel crosslinked with OSA via the formation of reversible imine linkages [75]. The formulated hydrogel functioned as an injectable carrier for the sustained delivery of a small-molecule drug, simvastatin. The hydrogel exhibited self-healing and shear-thinning properties due to the reversible nature of the covalent imine bonds formed between the aldehyde groups of OSA and the amine groups present in the DNA nucleotides. Ma et al. designed and fabricated injectable hydrogels based on OSA-modified HA NPs and CMC via imine bonds (Fig. 10) [76]. The hydrogel showed good mechanical properties and the interface compatibility of the modified HA particles to organic matrices, suggesting its potential as a material for bone regeneration. Lin et al. reported the fabrication of a self-healing hydrogel by crosslinking biodegradable difunctional polyurethane (DFPU) nanoparticle dispersions and CS [77]. The self-healing property of the hydrogel originated from the imine bonds between DFPU and CS at room temperature. The low immune response in a rat 14-d implantation model revealed that these hydrogels are promising new materials for biomedical applications.
Fig. 10.
Schematic illustration of a dynamic hydrogel formed by oxidized alginate-modified HA nanoparticles and carboxymethyl chitosan through imine bonds. Reproduced with permission [76]. Copyright 2020, Elsevier B.V.
3.1.2. Disulfide bonds
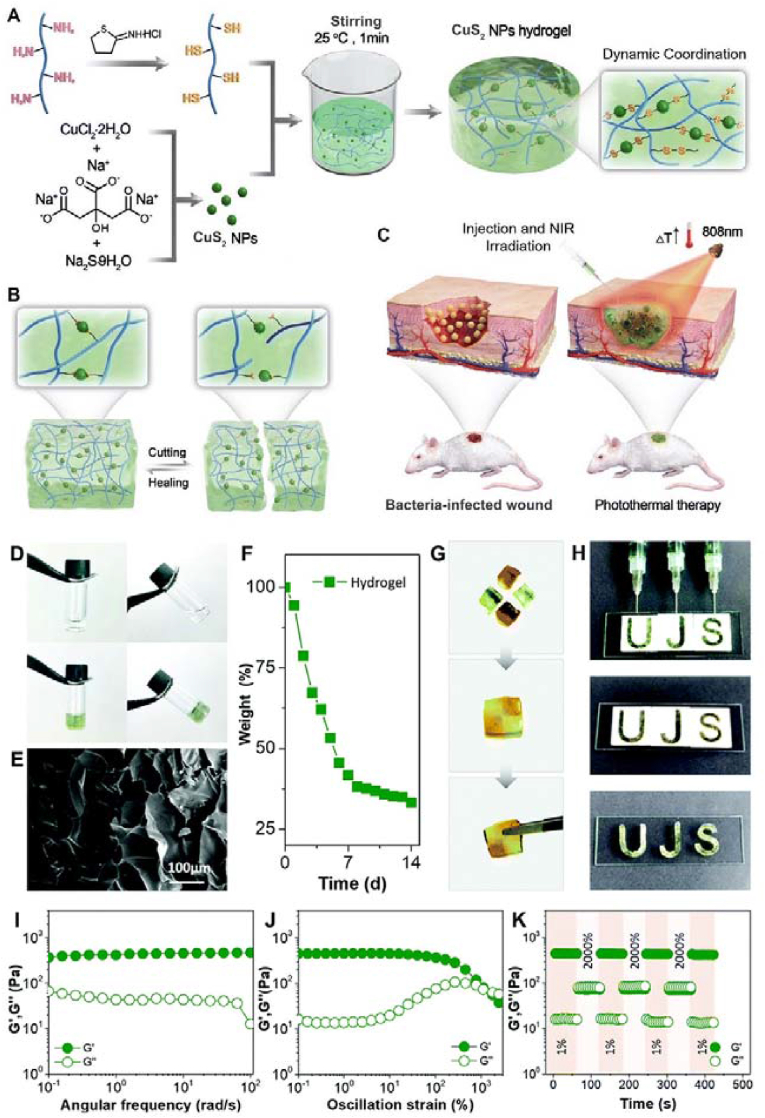
Disulfide bonds, essentially those based on the thiol/disulfide dynamic exchange reaction, are another type of dynamic covalent bond for self-healing hydrogels. To achieve disulfide bond crosslinking, thiolation/sulfhydrylation is needed. Sulfhydrylation treatment of biological macromolecules such as Gel and CS has been used to provide thiol groups for crosslinking. Our group designed a dynamic hydrogel based on CuS2 NPs and thiolated Gel (Fig. 11) [78]. By introducing CuS2 NPs into the hydrogel as the main skeleton, the mechanical properties were enhanced. Rapid photothermal sterilization and accelerated wound healing were realized because of the photothermal properties of copper sulfide under infrared light irradiation (808 nm).
Fig. 11.
Schematic illustration of the preparation (A), self-healing ability (B), and application in infected skin tissue repair (C) of the dynamic nano-crosslinked protein hydrogel. (D) The gelation of the SH-Gel solution after the addition of CuS2 NPs. (E) SEM of the hydrogel. (F) In vitro degradation of the hydrogel. (G) Self-healing process of hydrogel blocks. (H) Injectability and remolding process of the hydrogel. (I) Dynamic oscillatory frequency sweeps (strain = 1%), (J) strain amplitude sweeps (frequency = 1 rad/s), and (K) step-strain sweeps (strain = 1% or 2000%, frequency = 1 rad/s) of the dynamic hydrogel. Reproduced with permission [78]. Copyright 2022, Royal Society of Chemistry.
3.1.3. Boronic ester bonds
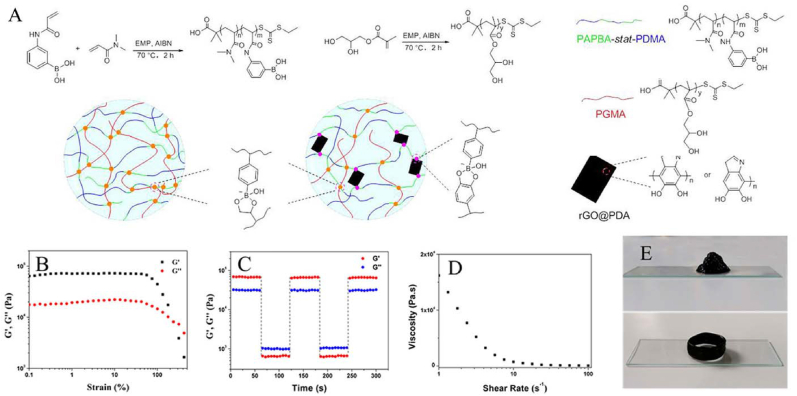
Through the formation of reversible boronic ester bonds, self-healing hydrogels can be obtained. Boronic ester bonds are generally formed by condensation reactions of compounds containing boric acid (phenylboronic acid) groups with alcohols (phenols) containing hydroxyl groups. They exhibit good stability under neutral or alkaline conditions, while they dissociate under acidic conditions. Therefore, these bonds facilitate the preparation of dynamic hydrogels. Many pH-responsive hydrogels have been developed using this strategy and used as platforms for controlled drug release and tumor treatment. He et al. developed high-quality magnetic self-healing hydrogels through the Kabachnik−Fields (KF) reaction [79]. Dynamic borate ester linkages formed between phenylboronic acid (PBA) and phosphonic acid (PA) groups. The surface of iron oxide NPs (IONPs) strongly interacts with PA groups. The hydrogel was obtained by mixing PA groups and PVA containing IONPs. Huang et al. described injectable self-healable nanocomposite (NC) hydrogels with good mechanical strength and mussel-inspired adhesive properties (Fig. 12) [60]. These hydrogels were obtained by solution-mixing poly(N,N-dimethylacrylamide-stat-3-acrylamidophenylboronic acid) (PDMA-stat-PAPBA), poly(glycerol monomethacrylate) (PGMA), and poly(dopamine)-coated chemically reduced graphene oxide (rGO@PDA) in an alkaline environment. During the network formation process, boronic ester dynamic covalent bonds (DCBs) played a major role, while rGO nanosheets homogeneously dispersed in the hydrogel matrix acted as both 2D crosslinkers and reinforcing fillers.
Fig. 12.
(A) Illustration of the nano-crosslinked hydrogels by dynamic borate ester bond formation. (B) dependence of moduli on strain amplitude sweep (γ = 0.1–400%) at a fixed frequency of 1 rad/s; (C) step-strain test at a fixed frequency of 1 rad/s (1% or 400% of strain); (D) viscosity measurement at 1% of strain; (E) printed cone and hollow cylinder 3D patterns. Reproduced with permission [60]. Copyright 2019, American Chemical Society.
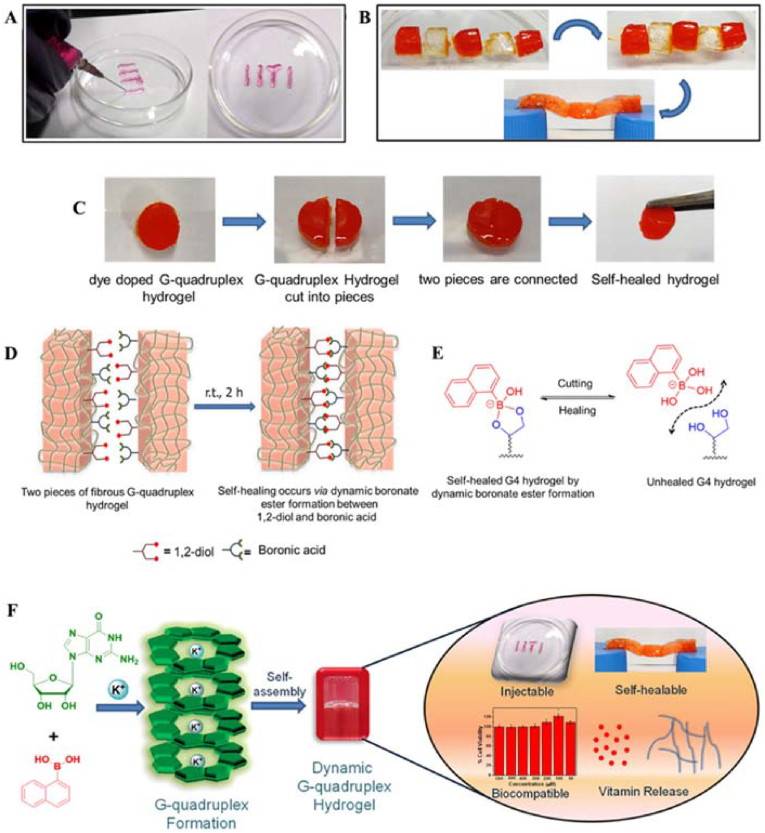
Ghosh et al. prepared injectable, self-healable, and biocompatible dynamic hydrogels by molecular self-assembly and reversible covalent bond formation with low-molecular-weight hydrogelators. (Fig. 13) [80]. They prepared G-NapBA/K+ hydrogels with a columnar structure by multicomponent self-assembly and reversible bond formation between guanosine (G) and 1-naphthaleneboronic acid (1-NapBA) in the presence of the monovalent cation K+. G-quartets were formed by the supramolecular Hoogsten-type hydrogen bonding interactions between the guanine units, and the K+ ions exerted a stabilizing effect. Self-assembly occurred for 1-NapBA and the cis diols of guanosine units by the formation of cyclic boronate esters. Furthermore, stronger supramolecular G-quadruplex nanofibrous hydrogels with columnar structures were obtained by π−π stacking interactions between adjacent naphthalene moieties and cation-templated hydrogen-bonded G-quartet motifs. Rheological experiments confirmed that the hydrogels had good mechanical and thixotropic properties, injectability and printability. Its good biocompatibility and controlled drug release of important biomolecules/drugs such as vitamin B 2, vitamin B 12, and doxorubicin suggested its potential application in the medical field.
Fig. 13.
(A) Injectability and printability properties of the G–NapBA/K+ hydrogel (vitamin B12-loaded gel was extruded through a 24 G syringe). (B) Demonstration of the self-healing ability of the G-quadruplex hydrogel. (C) Optical images of the self-healing process. Sequence of self-healing observed by the methyl red dye-doped hydrogel, and a hang test confirms the healed gel. (D) Schematic representation of the self-healing process in the presence of dynamic reversible boronate esters. (E) Self-healing mechanism. (F) Graphical representation of the formation of G-quadruplex, leading to the formation of a self-supporting hydrogel (G/1-NapBA/KOH = 50:25:25 mM). Reproduced with permission [80]. Copyright 2020, American Chemical Society. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Noncovalent interactions
3.2.1. Coordination interactions
Coordination interactions occur between ligands (donate electrons) and one positively charged transition-metal ion. The covalent coordination bond is strong and has been used to prepare highly adhesive, elastic, and self-healable materials. The sticky feet of mussels, which are attributed to the coordination of Fe3+ and catechol ligands, are a typical example of the strength of this bond in nature. Reversible metal-ligand coordination is also widely used in designing self-healing hydrogels. Au and Ag ions with good biocompatibility and special medical functions are commonly used in nano-crosslinked hydrogels. They usually form dynamic thiolate-Au/Ag interactions. These functional groups can also be used to modify fragments to produce functionalized dynamic hydrogels.
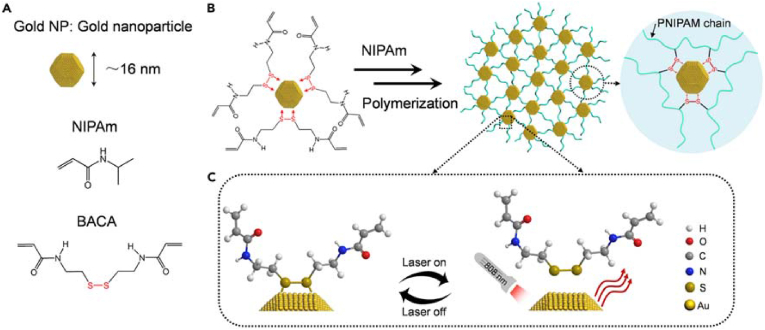
The coordination between metal and ligands are commonly used to prepare dynamic hydrogels [45,[81], [82], [83]]. Qin et al. developed a dynamic hydrogel strategy by taking advantage of the coordination between noble metal ions and ligands [82]. First, they developed gold NP/PNIPAM (GNP) hydrogels using the dynamic thiolate-Au (RS-Au) interaction. Due to the RS-Au coordination and the remarkable photothermal effect of the NPs, the hydrogels achieved rapid self-healing within 1 min under near-infrared (NIR) light (808 nm). The design and synthesis of GNP hydrogels are shown in Fig. 14. N,N-Bis(acryloyl)cystamine (BACA) containing a disulfide bond reacted with Au NPs to form RS-Au bonds. The hydrogels were used as photo-controlled drug carriers for biomedical applications due to the thermally responsive PNIPAM polymer chains and optothermal gold NPs in the hydrogels. The hydrogel showed shear-thinning performance. The hydrogel recovered quickly after injection, suggesting its usefulness in drug delivery. Due to the photothermal effect, the hydrogels enabled the intelligent control of Dox release under NIR laser irradiation.
Fig. 14.
Schematic illustrations of gold-thiolate interaction-triggered self-healing nanocomposite hydrogels. (A) Schematic structure of gold NPs and a list of comonomers used for the polymerization of hydrogels. (B) Schematic formation of GNP hydrogels with modified gold NPs as large crosslinkers in situ free-radical polymerizations. (C) Schematic mechanism for the dynamic and reversible RS–Au bonding under NIR laser irradiation (808 nm). Reproduced with permission [82]. Copyright 2017, Elsevier B.V. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
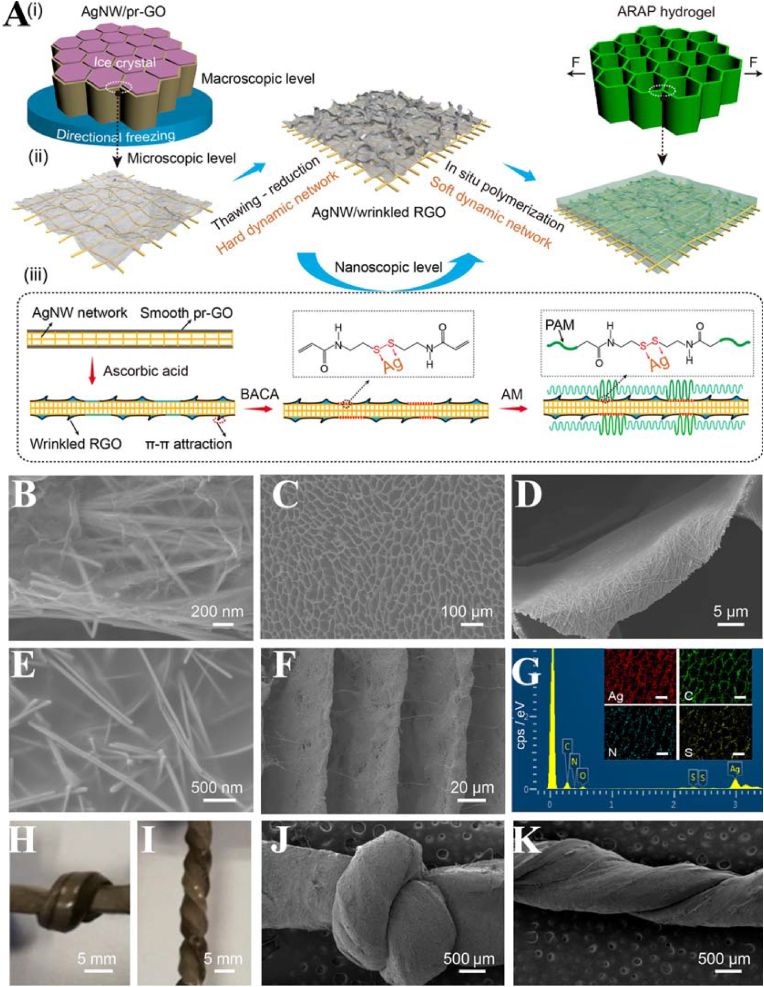
Subsequently, anisotropic and self-healing silver (Ag) NP/polyacrylamide (PAM) (SNPP) hydrogels with rapid and efficient multiresponsiveness were synthesized [84]. The SNPP hydrogels showed excellent stretchable behaviors and mechanical anisotropy. The mechanical anisotropy of the hydrogels was attributed to the coordination interactions between Ag NPs and sulfur-containing molecules as well as surface-induced S–S bond cleavage. The fast and efficient self-healing properties of the SNPP hydrogels were attributed to the dynamic coordination interaction of RS-Ag in the hydrogel network under NIR irradiation and low pH conditions. Recently, ultrastretchable, self-healing, and conductive hydrogels as conductors were prepared with Ag nanowires (AgNWs), wrinkled rGO nanosheets, and PAM (Fig. 15) [85]. The AgNW/rGO aerogel-PAM (ARAP) hydrogels possessed great stretchability with an elongation of 3250%, strong endurance to knotted and twisted deformations, and excellent stability with a resistance change of 223% at 2000% strain. The addition of AgNWs and rGO greatly increased the sliding friction of the hydrogels and improved the crack resistance during the stretching process. The rGO nanosheets, as crack bridges, prevented severe crack propagation and stabilized the structure. The Ag-SR coordination bonds in the ARAP hydrogels endowed them with excellent response healing properties. Since the Ag-SR bonds were temperature-sensitive, the heat generated by the photothermal conversion of AgNWs under NIR irradiation triggered the breaking/generation transition of the Ag-SR bonds. With this reversible crosslinking of dynamic Ag-SR bonds, the hydrogel network can be rebuilt, allowing for healing of the ruptured region of the hydrogels. The hydrogels can be applied in stretchable conductors and electronic devices.
Fig. 15.
(A) Schematic illustrations of the fabrication process. (i) Macroscopic level: cellular monolith. (ii) Microscopic level: film-like cell wall. (iii) Nanoscopic level: AgNW network covered with the wrinkled RGO nanosheets and PAM chains. (B) SEM image of the compartmental wall of the AgNW/RGO aerogel. (C) Top-view SEM image of the ARAP hydrogel. (D, E) Magnified SEM images in (C). (f) Side-view SEM image and (G) EDS spectra of the hydrogel. Scale bars: 50 μm. Optical and SEM images of the knotted (H, J) and twisted (I, K) hydrogels. Reproduced with permission [85]. Copyright 2021, American Chemical Society.
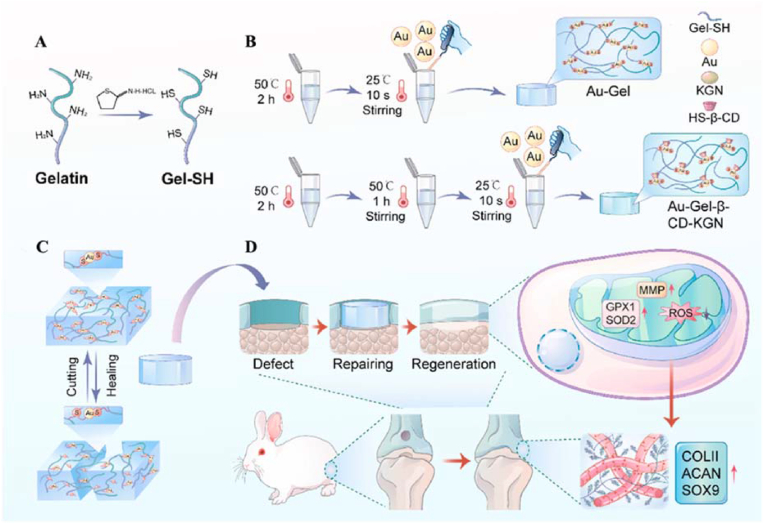
Coordination interactions can also be used to fabricate functional dynamic hydrogels. Recently, our group prepared dynamic hydrogels with small-molecule compounds by using the coordination of Au and S to boost chondrogenesis [86]. The hydrogel design mainly involved the coordination between collagen and metal ions (Fig. 16). The chondroinductive factor kartogenin (KGN) was enveloped via CD by supramolecular interactions for medical applications. The good injectability and self-healing ability of hydrogels suggested their suitability for minimally invasive procedures. The results showed that the hydrogel significantly facilitated the expression of cartilage matrix components (aggrecan and type II collagen) in articular chondrocytes and did not affect cell proliferation and migration. The functional hydrogel repaired bone defects by mitochondrial reinforcement, suggesting its application potential in cartilage repair.
Fig. 16.
Schematic illustration of metal ions and collagen-based dynamic hydrogel loaded with KGN promoting cartilage regeneration by enhanced mitochondrial functions. (A) Gelatin is mercaptoized with Traut's reagent to form mercapto gelatin (SH-Gel). (B) Self-healing ability is endowed by introducing gold chlorate, followed by mixing with cyclodextrin-encapsulated KGN. (C) The “cutting and healing” remoldable network structure was achieved through an “Au–S″a dynamic bond. (D) In vivo injection of hydrogel facilitates cartilage repair following injury through refinement of mitochondrial polarization and redox balance. Reproduced with permission [86]. Copyright 2022, Elsevier B.V. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Electrostatic interactions
The design and preparation of dynamic hydrogels by electrostatic interactions is an alternative strategy. Electrostatic interaction refers to the interaction between two charged particles. This interaction can also be used in the formation of hydrogels. Hydrogels contain components with positive charges on the surface and components with negative charges. Two charged segments can undergo crosslinking to form hydrogels by electrostatic interactions. The intrinsic self-healing property of the hydrogels makes them dynamic. Charged polymer, particle, sheet, and even macromolecular fragments can be used as reactants for preparing hydrogels. Functionalization and modification can also be carried out. Due to fast complexation reaction kinetics and reactivity to a variety of external stimuli (e.g., temperature, pH, cations, anions, etc.), electrostatic interactions are one of the most intriguing dynamic bonds used in hydrogel fabrication.
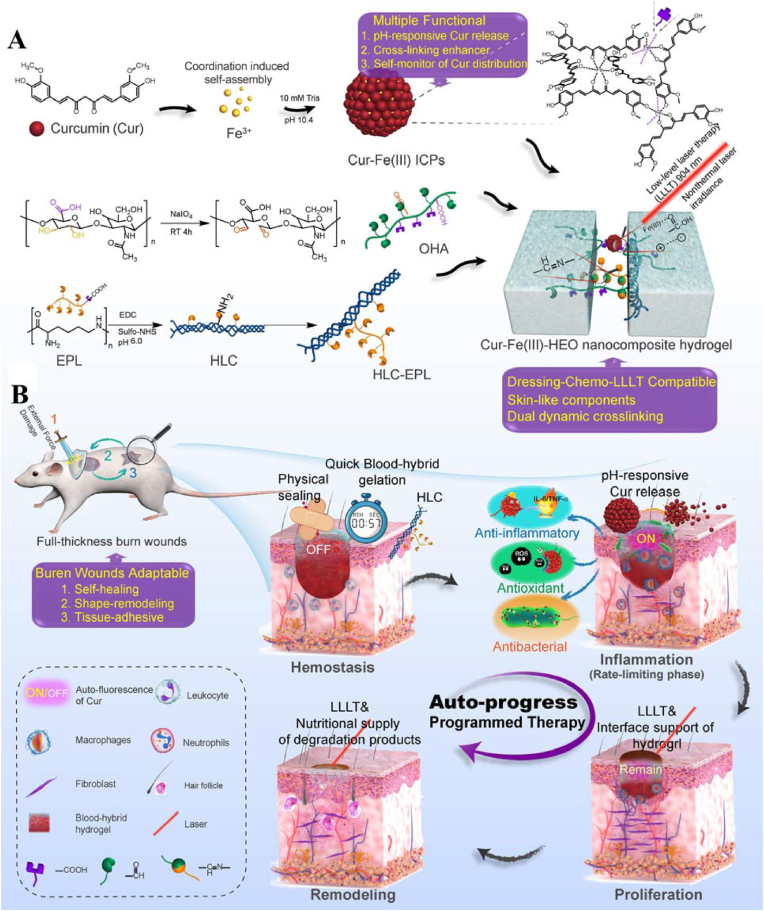
Shen et al. proposed a new strategy combining dressing management and chemotherapy with low-intensity high-pulse nonthermal laser therapy (LLLT) by designing a highly integrated and structurally simple ultrasmall drug-composite hydrogel wound dressing (Fig. 17) [87]. This nanocomposite hydrogel consisted of oxidized hyaluronic acid (OHA), ε-polylysine-grafted human-like collagen (HLC-EPL), and curcumin-Fe (III) coordination polymer nanodrugs (Cur-Fe (III) ICPs) doubly crosslinked by imine bonds and NP–polymer interactions. NP–polymer interactions were mainly dynamic interactions between the positively charged Cur-Fe (III) ICP NPs and the negatively charged hydrogel backbone molecules. Cur-Fe (III) ICPs, the nanodrugs in the hydrogels with nearly uniform small sizes, enhanced the biological activity of Cur. The carrier-free and ultrasmall (9.14 ± 1.25 nm) Cur-Fe (III) ICPs exhibited minimal light scattering and light absorption, which allowed the drug-loaded hydrogels to retain good light transmission (>90.1%). Due to the coordination structure of Cur-Fe (III) ICPs, this nanocomposite hydrogel (Cur-Fe (III)-HEO) depolymerized and released Cur during the inflammatory phase in response to the acidic microenvironment of the wound. The “off-on” fluorescence change before and after drug release allowed visual monitoring of chemotherapy during wound healing. The electrostatic interactions and imine bonding in the hydrogels allowed the nanocomposite dressings to achieve 84.6% crosslinking reconstruction efficiency in 10 min, making them more adaptable to burns. Animal experiments showed in a major reduction in closure time from 21 days to 9 days for surface full-layer burn wounds and the accelerated reconstruction of skin structures. This rapid self-healing hydrogel wound dressing offers a new strategy for burn treatment.
Fig. 17.
Schematic illustration of the preparation of the Cur-Fe(III)-HEO nanocomposite hydrogel (A) and the programmed dressing-chemo-LLLT combination therapy of burn wounds based on it (B). Reproduced with permission [87]. Copyright 2021, Elsevier B.V.
3.2.3. Hydrogen bonds
Hydrogen bonds are the most common noncovalent interactions in nature. They occur between positive hydrogen atoms and electronegative acceptor atoms, such as oxygen, nitrogen, or fluorine. The association and dissociation of hydrogen bonds can occur in a matter of picoseconds or even less than a picosecond. As a common physical interaction, hydrogen bonds are commonly used in preparing hydrogels. Hydrogel materials can be prepared by the crosslinking of components with terminal hydrogen atoms and negative particles. Due to its self-healing property, the resulting hydrogel can have dynamic properties. Hydrogen bonds also contribute to the mechanical properties of hydrogels. However, these bonds are always used together with other interactions for the preparation of dynamic hydrogels due to the weakness of the hydrogen bond.
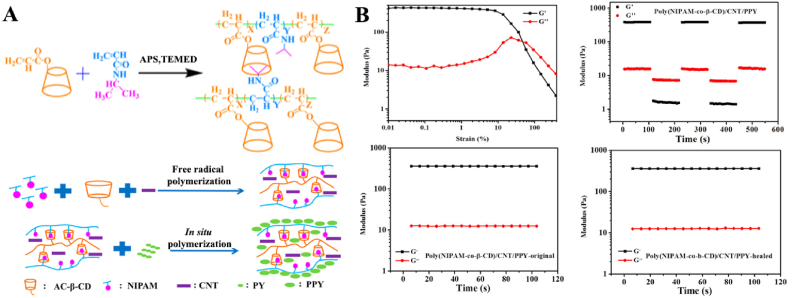
Deng et al. reported self-healing conductive hydrogels based on β-CD, N-isopropylacrylamide (NIPAM), multiwalled CNTs, and nanostructured polypyrrole (PPY) [88]. The addition of CNTs and PPY in the hydrogels lead to the formation of hydrogen bonds with NIPAM, which together with the host-guest interactions endowed the hydrogels with self-healing properties and improved the electrical conductivity of the hydrogels. The characterized thermal response properties of the PNIPAM hydrogels typically resulted in sharp phase transitions and fast swelling/drying rates. These hydrogels showed expansion rates with temperature similar to those of pure PNIPAM hydrogels. The hydrogels showed a rapid response not only to thermal radiation but also to NIR light. Conductive nanomaterials, such as CNTs or PPYs, exhibit the ability to absorb NIR light and efficiently convert NIR light into heat. In the hydrogel, the CNTs served as a physical crosslinking agent, and the soft PNIPAM chains formed a good elastic network to prevent mechanical damage and fracture of the rigid PPY nanoaggregates. Hydrogels can also exhibit photothermal properties when these conductive components are doped into the hydrogels. When the temperature exceeded the volumetric phase variable (VPTT), the PNIPAM-based hydrogels contracted and exhibited significant photothermal behavior. When the laser exposure was turned off, the shrinkage of poly (NIPAM-co-β-CD)/CNT hydrogels ceased accordingly, and these hydrogels gradually recovered to their original shape after water absorption. The hydrogels exhibited good reversibility during compression and relaxation. The mechanism of the good elasticity and fast recovery properties of the hydrogels was attributed to the elastic macroporous and soft-hard bicontinuous network structures of the poly (NIPAM-co-β-CD)/CNT/PPY hydrogels, consisting of rigid CNT and soft PNIPAM chains. In addition, the compressive stress of the hydrogels increased from 2.9 kPa to 10.2 and then to 20.5 kPa with the sequential addition of the rigid conductive component (CNT/PPY) under 70% stretch. Due to their excellent properties, these conductive hydrogels have a wide range of promising applications in the fields of smart electronics and biomedicine, such as pressure sensors, large-scale human motion monitoring sensors, and self-healing electronics.
As mentioned above, the hydrogen bond strategy is used together with other interaction strategies to obtain dynamic hydrogels. Cui et al. synthesized OSA/Gel/CNF hydrogels through a CNF-reinforced OSA/Gel semi-interpenetrating network under the synergistic effects of dynamic imine bonds and hydrogen bonds without a crosslinker (Fig. 18) [71]. The results showed that when the hydrogels were cut in half, the dynamic imine and hydrogen bonds broke and produced free reactive groups at the fractured interface. These groups exhibited strong propensities for conformational rearrangement. Then, the dissociated reactive groups reacted with the cleaved surfaces to form new imine bonds and hydrogen bonds. Therefore, the self-healing of hydrogels has been realized under the synergistic effects of hydrogen bonds and imine bonds. Due to the incorporation of CNFs, the swelling, water uptake capacity, degradation behavior, and compressive modulus of the hydrogels were improved. By combining these results with the bioactivity, biomineralization capacity, and osteogenic activity results, it was concluded that the OSA/Gel/CNF hydrogels are promising bone injury repair materials.
Fig. 18.
(A) Schematic representation of the synthesized OSA/Gel/CNF and suggested chemistry of the materials. Self-healing behaviors (B), cyclic G′ and G″ values of the hydrogel (C), healing efficiency test (D), and the synergistic self-healing mechanism (E) of hydrogels. Reproduced with permission [71]. Copyright 2022, Elsevier B.V.
3.2.4. Hydrophobic interactions
Hydrophobicity endows aqueous solutions of nonpolar compounds with unusual properties and plays a key role in a variety of chemical and biophysical phenomena, such as the folding of proteins or the self-assembly of amphiphilic compounds into micelles and membranes. Hydrophobic interactions differ from other noncovalent interactions in that they do not depend on direct intermolecular attraction between interacting species and instead are driven by the tendency of water molecules to retain their hydrogen bonds network around nonpolar solutes. They vary according to temperature, the presence of cosolutes, and the size of nonpolar species. Hydrogels formed by hydrophobic interactions can be obtained by introducing hydrophobic sequences within or at the termini of hydrophilic polymer chains. The transient network formed by interchain interactions depends on the polymer concentration, the ratio of hydrophobic molecules, and the polymer structure. Due to the dynamic and reversible nature of the junctions, the resulting hydrogels may exhibit self-healing capabilities.
Hydrogels cross-linked by hydrophobic interaction can also be used for drug delivery [89]. The hydrogel exerted a self-healing effect through hydrophobic interactions between HPMC-x derivative polymer chains and NPs. Specifically, the hydrophobic interaction between the hydrophobic part of the HPMC chains and the hydrophobic chains of poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA) NPs was enhanced by the hydrophobic modification of HPMC-x, where x refers to hexyl (C6), adamantyl (AD) or dodecyl (C12) functional groups, which enhanced the adsorption of HPMC on NPs and thus generated polymer-NP (PNP) hydrogels (Fig. 19). The presence of poly(ethylene glycol) chains on the PEG-b-PLA NPs significantly reduced the inherent affinity between HPMC and NPs and increased the shear storage modulus by a factor of 30 compared to that of pure HPMC. Therefore, the strong adhesion properties between HPMC-x polymers and PEG-b-PLA NPs and the consequent formation of hydrogels require the presence of HPMC with sufficiently long hydrophobic chain segments. In addition, dynamic noncovalent interactions occurred within these materials, resulting in stress-induced flow properties and self-healing properties. Transient and reversible interactions between NPs and HPMC chains dominated the self-assembly of PNP hydrogels, allowing flow under applied stress and full recovery of their structural properties. The viscosity of the hydrogels varied considerably from low to high shear rates, indicating that the hydrogels possessed good injection properties. Since the PEG-b-PLA NPs in the PNP hydrogels contained both hydrophilic and hydrophobic structures, these materials can double encapsulate and control the release of therapeutic drugs. The PNP hydrogels contained PEG-b-PLA NPs that acted as structural components of the materials as well as carriers, thus enabling the simultaneous encapsulation of multiple therapeutic drugs and their release in different ways.
Fig. 19.
(A) Schematic representation of the preparation of polymer–nanoparticle (PNP) hydrogels utilizing noncovalent interactions between core-shell nanoparticles (NPs) and (B) hydrophobically modified hydroxypropylmethylcellulose. (C) NPs composed of either poly(styrene) (PS; nondegradable) or poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA; biodegradable). Reproduced with permission [89]. Copyright 2015, Nature Publishing Group.
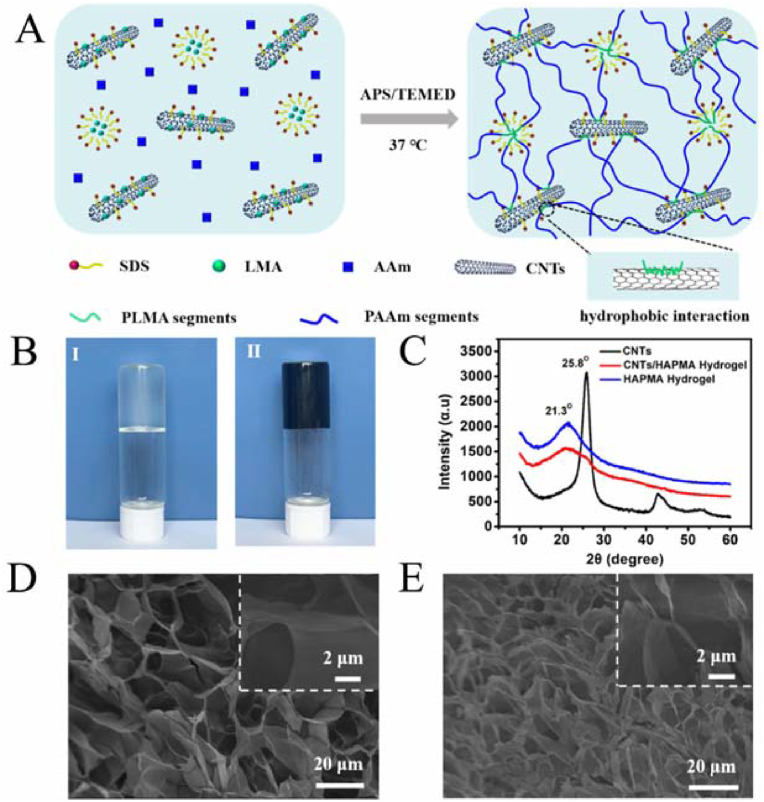
Nanomaterials (e.g., CNTs) can enhance the interfacial interaction with the hydrogel matrix through hydrophobic interactions, resulting in an extremely scalable, highly resilient, and fatigue-resistant conductive nanocomposite hydrogel (Fig. 20) [90]. CNTs were integrated into poly (acrylamide-co-lauryl methacrylate)-based hydrophobically associating polyacrylamide (HAPAAm) hydrogels, and poly (lauryl methacrylate) (PLMA) fragments formed hydrophobic interactions with carbon nanotubes. The carbon nanotubes enhanced electrical conductivity while enhancing the mechanical properties of the material. The hydrogel possessed excellent mechanical properties and good electrical conductivity and could be used as a reliable biosensor.
Fig. 20.
Preparation and characterization of the CNTs/HAPAAm hydrogels. (A) Schematic illustration of the preparation process for the CNTs/HAPAAm hydrogel. (B) The photographs of (Ⅰ) the HAPAAm hydrogel and (Ⅱ) the CNTs/HAPAAm hydrogel. (C) XRD patterns of CNTs, HAPAAm hydrogel, and CNTs/HAPAAm hydrogel. SEM images of (D) the HAPAAm hydrogel and (E) the CNTs/HAPAAm hydrogel. Reproduced with permission [90]. Copyright 2020, American Chemical Society.
3.2.5. Host–guest recognition
Host–guest recognition is ubiquitous in organisms. The specific identification involved in this recognition makes it advantageous to use in targeted drug delivery and disease treatment. It has also been used in designing nanomaterial dynamic hydrogels. Taking advantage of the large specific surface area of nanomaterials, nanomaterials can be used as the host agent, and the guest agent can be used to modify the surface, thus resulting in the formation of a hydrogel through the interactions between the host and guest. Due to the intrinsic self-healing ability endowed by noncovalent host–guest recognition, the hydrogel can have excellent dynamicity. Many hydrogels with different functions have been obtained owing to the easy modification of nanostructures.
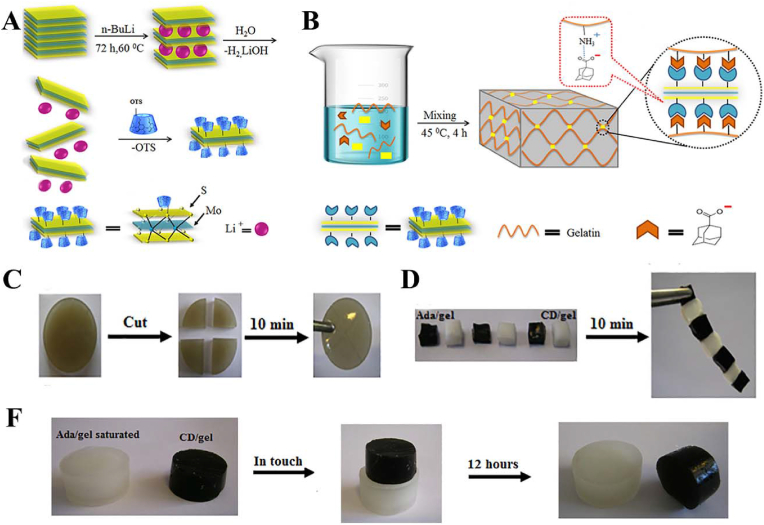
Zohreband et al. constructed an injectable, self-healable, and flexible supramolecular hydrogel by host-guest interactions between an adamantane-modified Gel matrix and CD-functionalized exfoliated MoS2 (Fig. 21) [91]. The mechanical properties of the hydrogels were enhanced. The incorporation of almost 1 wt% CDMoS2 into a Gel matrix with a 1 cm2 cross-section produced a hydrogel that was able to tolerate one hundred grams. Additionally, the storage modulus (G′) and loss modulus (G″) of the obtained hydrogel were 10 and 25 times higher than those of neat Gel, respectively.
Fig. 21.
(A) Schematic representation of the functionalization of MoS2 by β-cyclodextrin. (B) Schematic representation of functional supramolecular hydrogel based on the host-guest interactions between an adamantane-modified gelatin matrix and cyclodextrin-functionalized exfoliated MoS2. (C) Self-healing of MoS2gel. (D, F) Investigation of the host-guest interactions and molecular recognition macroscopically. Reproduced with permission [91]. Copyright 2021, Elsevier B.V.
Deng et al. synthesized multifunctional stimuli-responsive hydrogels based on β-CD, NIPAM, multi-walled CNTs, and nanostructured PPY (Fig. 22) [88]. Among these components, β-CD served as the host molecule, NIPAM served as the guest molecule, CNT served as the physical crosslinker and conducting substrate, and PPY served as the highly conductive component. The obtained hydrogels exhibited flexible and elastic mechanical properties, self-healing properties, high conductivity, and rapid stimuli-responsive properties for both temperature and NIR light. Multifunctional hydrogels are excellent candidates for application in stimuli-responsive artificial organs and electrical devices.
Fig. 22.
(A) Designation and preparation of multifunctional stimuli-responsive hydrogels. (B) Strain amplitude sweep test (γ = 0.1%–500%) at a fixed angular frequency (10 rad/s) at 25 °C of poly(NIPAM-co-β-CD)/CNT/PPY hydrogels; Alternate step strain sweep test with small strain (γ = 1.0%) to subsequent large strain (γ = 400%) with 100 s for every strain interval at a fixed angular frequency (10 rad/s) at 25 °C; Storage modulus and loss modulus profiles for original and healed hydrogel. Reproduced with permission [88]. Copyright 2018, American Chemical Society.
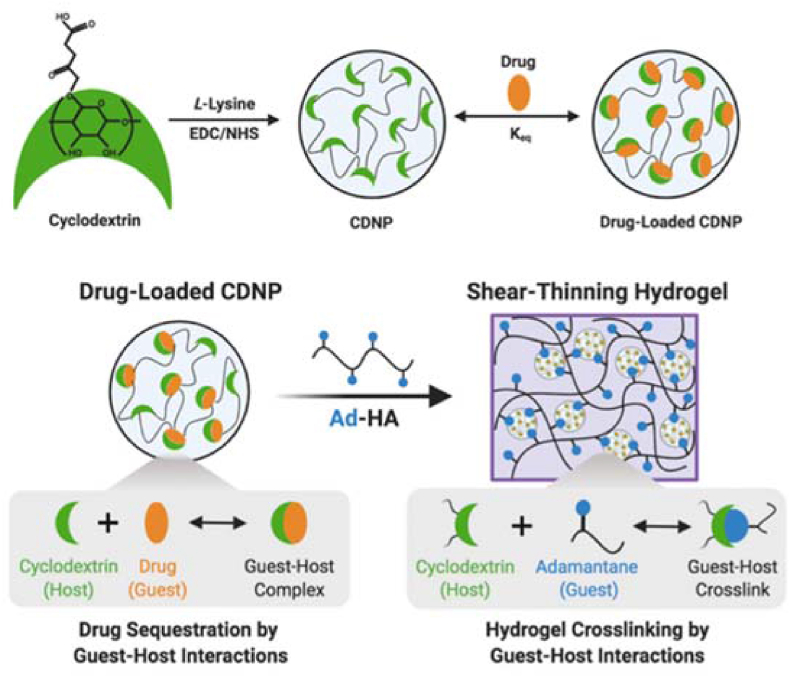
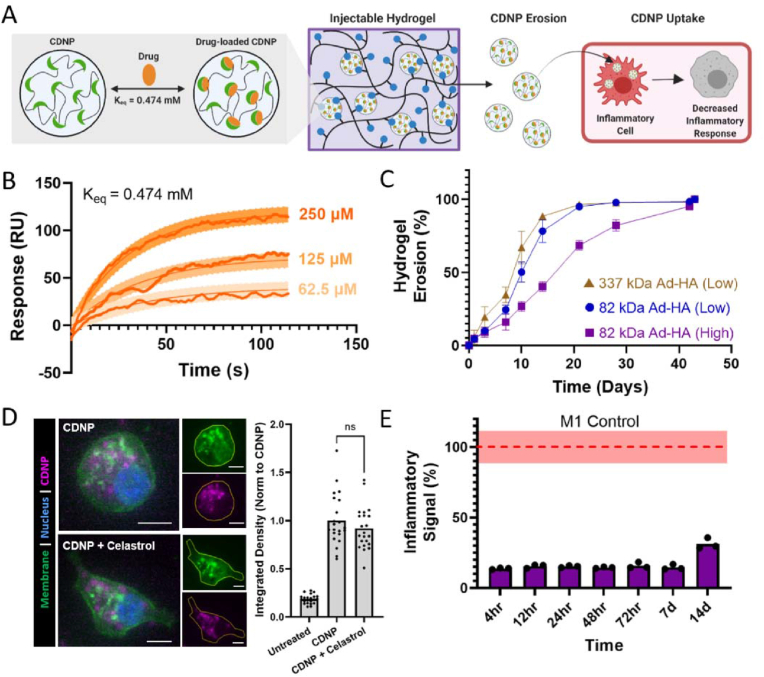
Through supramolecular assembly by host–guest interactions, cyclodextrin nanoparticles (CDNPs) were crosslinked by adamantane-modified hyaluronic acid (AD-HA) to yield an injectable self-healing hydrogel [92]. The hydrogel was formed by the supramolecular association of the guest and host components, while the host–guest interactions of the CDNPs allowed host sites to form in the nanoparticle interior for drug retention (Fig. 23). The NP hydrogel locally modulated cell-specific phenotypes and was capable of a wide range of sustained drug release.
Fig. 23.
Schematic of drug-loaded hydrogel assembly by host–guest interactions. Reproduced with permission [92]. Copyright 2022, Royal Society of Chemistry.
4. Biomedical applications of nano-crosslinked dynamic hydrogels
4.1. Bone regeneration
Bone defects are a common ailment in daily life. They are always caused by severe trauma, osteonecrosis, osteochondritis, or end-stage osteoarthritis [93]. Patients with bone injury suffer from extreme pain, joint limitation, or movement limitation. The regeneration of bone is particularly important [94]. However, the self-repair and self-regeneration of bone are almost impossible to realize due to the lack of blood vessels, lymph, or innervation of cartilage bone [95,96]. According to the mobility of the articulation, high-strength hydrogel scaffolds with self-healing properties, such as dynamic hydrogels, show special advantages in bone regeneration. Their injectability makes them suitable for minimally invasive treatment and irregular trauma. Their 3D network structure similar to that of the natural ECM of normal tissue facilitates cell migration, differentiation, and nutrient transfer to peripheral tissues [[97], [98], [99]]. By adding growth factors or nanomaterials with special functions into hydrogels, the hydrogels can be introduced into the damaged site to accelerate repair and regeneration.
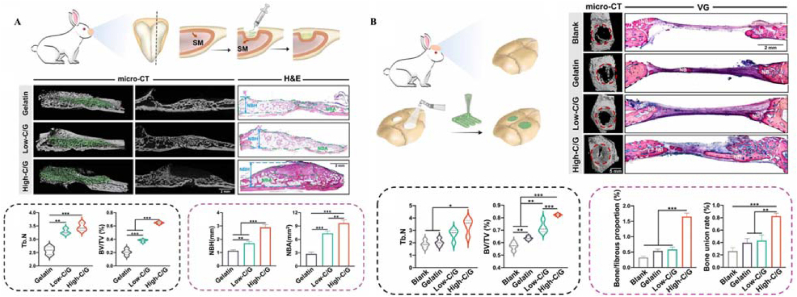
Dou et al. developed adaptable and osteogenic composite colloidal gels with high self-healing efficiency and injectability via the electrostatic assembly of Gel NPs and nanoclay particles (Fig. 24) [56]. A sinus bone augmentation model and critical-sized cranial defect model were designed. The results confirmed that the composite hydrogels were able to adapt to local complexity, including irregular or customized defect shapes and continuous on-site mechanical stimuli, and to realize osteointegrity with the surrounding bone tissues and eventually be replaced by newly formed bone.
Fig. 24.
Diagram, micro-CT, and VG staining images, quantitative analysis, evaluation of bone formation in New Zealand rabbit (A) sinus augmentation model and (B)cranial defect model treated with gelatin/clay hydrogels. Diagram of the sinus augmentation model. (∗ p < 0.05, ∗∗ p < 0.01 and ∗∗∗ p < 0.001) Reproduced with permission [56]. Copyright 2023, IOP Publishing.
Moreover, 3D bioprinting technology can be customized to personalize tissue-engineered scaffolds for precise and personalized treatment [100]. Because the molecular structure of hydrogels is similar to that of the natural ECM in living organisms, hydrogels can provide structure and nutrients to surrounding living cells. Hydrogels can be applied as 3D-printed bioinks for bone regeneration. Zhai et al. synthesized nanocomposite hydrogels using N-acryloyl glycinamide (NAGA) and nanoclay, which is the first report of the application of 3D-printed high-strength hydrogel scaffolds in bone regeneration [101]. The printed hydrogel scaffold could be bent at will and could even withstand being crushed by a car and recovered its original shape quickly, and the structure of the scaffold was well maintained after bearing high pressure. Furthermore, the hydrogel scaffold did not swell significantly and had good stability after long-term immersion in PBS solution, which is essential for the maintenance of the shape of 3D-printed hydrogel scaffolds after printing. In vitro and in vivo experiments showed that the active ions (e.g., Mg2+, Si4+, etc.) contained in the nanoclay promoted the proliferation and differentiation of osteoblasts on the scaffold and effectively promoted the growth of new bone tissue at the defect site or even inside the scaffold, which provides the possibility of developing a variety of bioinks for the 3D printing of biological scaffolds to achieve the personalized repair of degenerated load-bearing tissues.
4.2. Wound dressing
Wound dressing is frequently used for trauma treatment. Ideally, it should be used throughout the entire wound-healing process. Wound repair includes the processes of hemostasis, immune reaction, anti-inflammation, granulation generation, and vascellum formation, which last a long time. The study of wound dressings focuses on attachment capacity, regulation of macrophage polarization, anti-infection ability, and acceleration of granulation and angiogenesis. Nanomaterial hydrogels with cell mobility, nutrient transportation, air/water permeability, and multifunctional agents have been used in wound treatment [43,47,87,102]. The results showed that they are the most promising wound dressing material, even for patients with diabetes.
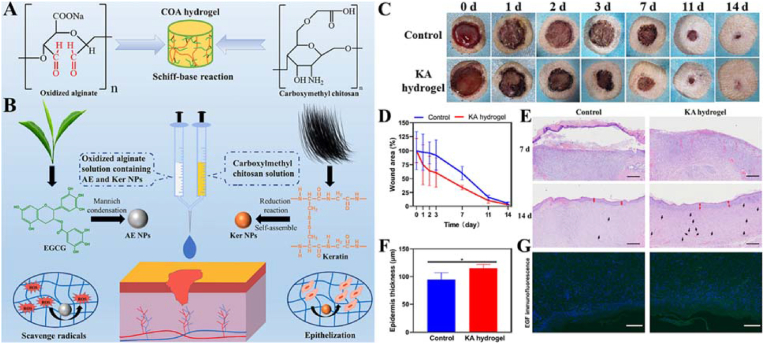
Ma et al. functionalized an injectable OSA/CMC hydrogel (KA hydrogel) [102]. The hydrogel demonstrated injectability and self-healing ability and contained keratin NPs (Ker NPs), which facilitate epithelization capability, and nanosized EGCG covered with Ag NPs (AE NPs), which have radical scavenging capability (Fig. 25).
Fig. 25.
(A) Formation of injectable hydrogel based on oxidized alginate and carboxymethyl chitosan. (B) Representative photographs of the wound of SD rats treated with or without KA hydrogel; Statistical results of wound area at different times; H&E staining result of the renascent skin (scale bar: 1 mm); Thickness of renascent epidermis. (Red arrow: the renascent epidermis. Black arrow: the blood capillary); EGF immunofluorescence (FITC, green fluorescence) images of the renascent skin at 14 days (Scale bar is 200 μm), and the blue fluorescence was the cell nucleus labeled by DAPI. Reproduced with permission [102]. Copyright 2022, Elsevier B.V. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
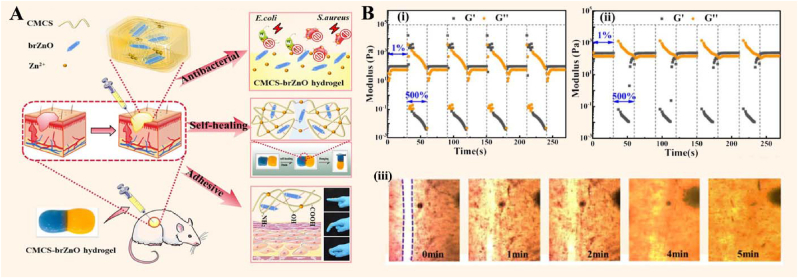
Hu et al. synthesized an injectable multifunctional hydrogel (CMCS-brZnO) by incorporating fusiform-like zinc oxide nanorods (brZnO) into CMCS (Fig. 26) [43]. In the hydrogel, brZnO acted as not only a crosslinker but also a nanofiller. The study showed that the hydrogels significantly promoted wound healing and reduced the inflammatory response by controlling the sustainable release of antibacterial Zn2+.
Fig. 26.
(A) Antibacterial activity, self-healing, and adhesion of CMCS-brZnO hydrogel. (B) G′ and G″ of CMCS-brZnO-1 (i) and CMCS-brZnO-2(ii) hydrogel under alternate low (1%) and high (500%) strain at a fixed angular frequency of 10 rad/s; (iii) Self-healing process of the CMCS-brZnO-2 hydrogel monitored by differential interference microscope. Reproduced with permission [43]. Copyright 2022, Elsevier B.V.
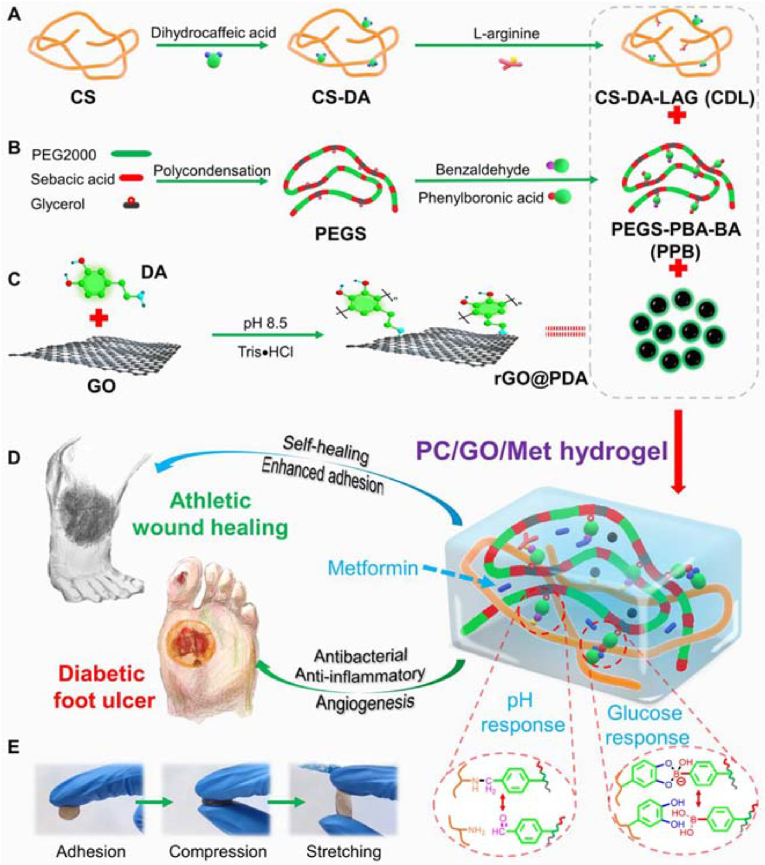
Diabetes is the largest chronic disease in the world and poses a serious risk to human health. Diabetic foot wounds take longer to heal, and the resulting chronic wounds may even lead to amputation. Some hydrogels can be used as dressings for the treatment of diabetic foot wounds. Liang et al. developed pH/glucose dual-responsive multifunctional phenylboronic acid and benzaldehyde bifunctional poly (ethylene glycol)-co-poly (glycerol sebacic acid)/dihydrocaffeic acid and L-arginine cografted CS (PEGS-PBA-BA/CS-DA-LAG, denoted as PC) hydrogel dressings (Fig. 27) [12]. The hydrogel displayed self-healing properties due to its double dynamic bonding, imine bonds, and phenylboronic ester. The imine bond structures in the hydrogels were pH-sensitive and unstable under acidic conditions, which led to increased drug release. The dynamic phenylboronic ester structure in the hydrogel was responsive to glucose. Therefore, hydrogel dressings facilitated the repair of low-pH and high-glucose diabetic wounds. In addition, the double dynamic covalent bonds conferred good self-healing properties to the hydrogel, which allowed potential damage associated with hydrogel rupture to be avoided and showed advantages in the repair of foot wounds. In a rat type II diabetic foot model, PC hydrogels promoted wound healing by reducing inflammation and enhancing angiogenesis. It was confirmed that PC hydrogels with the addition of metformin (Met) and GO possessed electrical conductivity and exerted hemostatic effects, which contributed to the transmission of electrical signals in biological tissues and promoted wound healing. In conclusion, stimuli-responsive PC/GO/Met hydrogels have been shown to promote wound healing in chronic athletic diabetic wounds and provide a therapeutic strategy for the controlled release of medication for type II diabetic foot wounds.
Fig. 27.
Schematic diagram of the preparation and application of the PC/GO/Met hydrogel. (A) Preparation of dihydrocaffeic acid and L-arginine-cografted chitosan (CS-DA-LAG) and (B) phenylboronic acid and benzaldehyde difunctionalized polyethylene glycol-co-poly (glycerol sebacic acid) (PEGS-PBA-BA) and (C) polydopamine-coated rGO (rGO@PDA). (D) Schematic diagram of the structure, pH, and glucose-responsive mechanism of the PC hydrogel and its application in diabetic foot ulcer and athletic wound healing. (E) Representative pictures of the PC hydrogel adhesion, compression, and stretching. Reproduced with permission [12]. Copyright 2022, American Chemical Society.
4.3. Drug delivery
Drug delivery plays a vital role in disease treatment. During the drug delivery process, several factors affect treatment efficiency. The hydrophobicity of a drug can prevent it from reaching the lesion site. The storm release of the drug within a certain period can seriously affect treatment efficacy and increase treatment cost by necessitating multiple administrations. The toxicity and side effects of drugs also need to be considered. Therefore, effective drug loading, biocompatibility, and biodegradability of drug carrier systems are necessary aspects to be evaluated. Hydrogels are also widely used in drug delivery, especially hydrogels that utilize liposomes [[103], [104], [105]]. When drugs are placed inside the liposome core and the liposomes are contained in the hydrogel network, the drugs can reach the specific site with ease because of the bilayer structure of the liposome and the 3D network structure of the hydrogels, which allows the drugs to maintain their structural and functional integrity and biological activity. In addition, the drugs can be continuously released, thus reducing the drug dosage and toxicity to normal tissues [35,75,106]. Additionally, crosslinking of the drug in the hydrogel network is beneficial for its controllable release. Nanomaterial hydrogels with dynamic properties are superior in drug delivery due to their injectable and self-healing properties. They can realize local drug delivery, which decreases drug side effects on organs.
Lee et al. developed injectable hybrid hydrogels based on the interaction between hydrophobically modified chitosan (hmC) and liposomes (Fig. 28) [107]. The hydrophobic chain segments of hmC were embedded in the hydrophobic interior of liposomes to form injectable hydrogels. Dox solution and Doxil (a commercial adriamycin-encapsulated liposome formulation) solution were mixed with hmC. Dynamic rheology tests showed that hmC converted the liposome-containing Doxil solution into hydrogel, while the Dox solution remained in solution. The steady-shear rheology showed that Doxil+hmC hydrogels exhibited shear-thinning behavior, which made them injectable, and the hydrogels could be successfully passed through a 30G1/2″ syringe needle without difficulty. Moreover, the hydrogels rapidly recovered from shear force and achieved a sufficiently high elastic modulus to ensure positioning at the injection site. Cationic solutes, such as the anticancer drug adriamycin (Dox), could be encapsulated in these liposomal hydrogels. Kinetic studies showed that solute release from these hydrogels could last more than a week. Thus, hmC-linked liposome hydrogels have been proven to be a promising class of injectable biomaterials.
Fig. 28.
(A) Structure of an injectable liposome gel at rest (left) and under shear force (right). (B) Schematic of the experimental setup. SK-BR-3 cells are plated per well, and a given sample is added to the upper chamber. The samples are (1) DMEM media (control); (2) solution of 13 mg/mL hmC; (3) solution of hmC+ Dox (180 μg/mL); (4) gel of hmC+ Doxil (Dox, 400 μg/mL); (5) gel of hmC+ Doxil (Dox, 320 μg/mL); and (6) gel of hmC+ Doxil (Dox, 280 μg/mL). (C) Cell viability results and photographs from trypan blue staining after 11 days. Reproduced with permission [107]. Copyright 2012, American Chemical Society. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A shear-thinning and self-healing hydrogel was formed through the simple mixing of host NPs (CDNPs) and a guest-modified polymer (AD-HA) in aqueous conditions (Fig. 29) [92]. The release of celastrol-loaded NPs from the hydrogel arrested proinflammatory signaling in MF reporter assays for two weeks, indicating its great potential for long-term immune modulation.
Fig. 29.
Therapeutic nanoparticle erosion enables long-term modulation of the MF phenotype. (A) Schematic of drug-loaded CDNP release from hydrogels and uptake by MF. (B) Binding sensorgrams between celastrol and CD, assessed at increasing concentrations of celastrol. (C) Cumulative erosion of hydrogels. (D) RAW264.7 cell uptake of unloaded (CDNP) and drug-loaded (CDNP-Cel) nanoparticles. (E) Anti-inflammatory activity of drug-release samples, investigated with RAW Blue™ cells. Reproduced with permission [92]. Copyright 2022, Royal Society of Chemistry. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.4. Tumor treatment
Tumors are the most serious detriment to human health. They are composed of a new tissue that is different from normal body tissue and are caused by abnormal gene changes induced by multiple factors. Treatment focuses on tumor elimination and prevention of recurrence. During the treatment process, hydrogel materials have the best performance. Its structural similarity to ECM makes it suitable for clinical application due to its good biocompatibility, cell fluidity, and easy functionalization. Dynamic hydrogels with functional agents can provide nutrients required for normal tissue and also functional groups to kill tumor cells. The mechanisms of tumor treatment include photothermal therapy and magnetotherapy. Dynamic hydrogels produced from nanomaterials with photothermal properties have been investigated.
Dynamic hydrogels containing Au NPs can be used for tumor therapy. Xing et al. fabricated collagen protein hydrogels with shear-thinning and self-healing properties through the self-assembly of biomolecules such as peptides and proteins [46]. The hydrogel formed by the intercalation of electrostatic complexation between inorganic anionic cluster [AuCl4]- ions and positively charged collagen chains. During the formation of these hydrogels, Au NPs acted as crosslinkers, not only providing flexible and controllable mechanical properties but also acting as photothermal agents owing to their high absorbability under laser irradiation at 635 nm. The collagen–gold hybrid hydrogel effectively suppressed tumor growth after one intratumoral injection with laser irradiation without the pathologic lesion of normal tissue.
Wang et al. proposed and fabricated Bi2S3–BSA nanocomposite hydrogels with tunable networks and injectability and self-healing ability (Fig. 30) [108]. The dynamic hydrogels were formed by the rapid formation of a protein–metal ion dynamic network. The high photothermal efficiency of the dynamic hydrogels was attributed to the formation of metal sulfides. The strong near-infrared (NIR) absorbance of the hydrogels suggests their application potential as injectable photothermal therapy agents for tumor treatment. The temperature of tumorous sites was increased to approximately 70 °C, and the tumor tissues were seriously damaged after NIR laser irradiation for 5 min, while negligible side effects were observed.
Fig. 30.
Schematic of the synthesis and antitumor property of the Bi2S3-BSA nanocomposite hydrogel: (A) Preparation of the metal sulfide-protein nanocomposite hydrogel in situ; (B) Bi2S3–BSA nanocomposite hydrogel-based PTT agents against tumors; (C, D) Photothermal property of Bi2S3–BSA nanocomposite hydrogels; (E, F, G) H&E staining of the tumor tissues, representative tumors and tumor volumes of mice after different treatments: Group I PBS (control), Group II (Hydrogel), Group III (808 laser irradiation, 0.75 Wcm−2), Group IV (Hydrogel+808 nm laser irradiation, scale bar = 100 mm); (H) Body weight. Reproduced with permission [108]. Copyright 2018, John Wiley and Sons.
4.5. Other applications
With studies being undertaken all over the world, great progress in nanodynamic hydrogels has been made. It has been used in many medical fields. In addition to the above applications, it has also been used for preventing epidural adhesion after laminectomy [109], prolonging the codelivery of vaccine components to the immune system [110], neural repair [111], myocardial infarction treatment [112], and inhibiting peritoneal adhesions [113].
Myocardial infarction, especially acute myocardial infarction, severely compromises human health and even life. In addition to primary angioplasty, thrombolysis by drug is the first-line treatment. Drug delivery through dynamic hydrogels has become the preferred treatment. Chen et al. prepared SaB-PDA/EMH drug-loaded hydrogels for the local delivery of salvianolic acid B (SaB) (Fig. 31) [112]. With the addition of SaB-loaded polydopamine NPs (SaB-PDA) to the hydrogel, the self-healing ability of the elastin-mimic peptide hydrogel (EMH) was enhanced. Injectability caused by the low viscosity and biocompatibility was also achieved. In vitro and in vivo experiments showed that intramyocardial hydrogel injection is a good method for myocardial infarction treatment.
Fig. 31.
Scheme of the preparation of peptide-constructed pre-EMH and SaB-PDA NPs for the intramyocardial injection of SaB-PDA/pre-EMH with an in situ TGase-responsive crosslinking process of EMH to achieve myocardial infarction treatment. Reproduced with permission [112]. Copyright 2021, Elsevier B.V.
The SaB-PDA/EMH hydrogel was produced with SaB-PDA and EMH. In the gelation process, SaB-PDA provided crosslinking sites, and its catechol groups and benzene rings were crosslinked to the EMH meshes and fibers by hydrogen bonding and π-π stacking. The coexistence of chemical crosslinking and physical crosslinking in the hydrogels resulted in their excellent self-healing ability and improved mechanical properties. The elastic modulus (G′) and viscous modulus (G″) of the SaB-PDA/EMH hydrogel increased with the action of upregulated transglutaminase (TGase) in heart tissue post-myocardial infarction, while the storage modulus, which contributes to injectability, was significantly reduced. Due to the superior dynamic ability of the hydrogel, the release of SaB was dramatically slow, and the SaB-PDA/EMH hydrogel showed a sustained release of ∼17% at 96 h at the ventricular wall. The SaB-PDA/EMH hydrogel inhibited ventricular remodeling and accelerated angiogenesis for myocardial infarction treatment.
5. Conclusion and outlook
As discussed above, the dynamic nature of hydrogel biomaterials including self-healing and injectable properties can be biomimetic designed by dynamic crosslinking interaction of nanomaterials in the hydrogel network. As tissue scaffolds or substitutes, these ECM-like dynamic hydrogel biomaterials thus show potentials in field including tissue engineering, drug delivery, tumor treatment and so on. As known, the reversible interactions in traditional dynamic hydrogels enable rapid structural recovery of damaged regions that would be beneficial in injection applications, while a proper balance between mechanical properties and recovery rates remains a great challenge. Fortunately, this problem could be well addressed by introducing nanomaterials into hydrogels, in particular, nanomaterials-crosslinked polymer network. In this review, we highlighted the use of nanomaterials for fabrication of self-healing and injectable hydrogels, and then discussed their dynamic crosslinking mechanism and biomedical applications. The various organic or inorganic nanomaterials, synthetic and fabrication strategies, and their applications of nano-crosslinked dynamic hydrogels were listed in Table 1. Conceivably, increasing nanomaterials will show promise for dynamic nanocomposite hydrogels in medical diagnosis and therapy. We expect further efforts will be made for not only novel nanomaterials but also polymer network and even functional biomolecules in the future.
Table 1.
Nanomaterials, synthetic strategies, and biomedical applications of nano-crosslinked dynamic hydrogels.
| Nanomaterials | Reversible interactions | Function in the hydrogel (besides as crosslinker) | Application | Reference | |
|---|---|---|---|---|---|
| Metals and metallic oxides | Au | Coordination interaction | Improving the mechanical properties; Photothermal properties; Catalytic properties |
Drug delivery; Tumor treatment; Wound healing; Cartilage regeneration |
[[71], [72], [73],86] |
| Ag | Coordination interaction | Antioxidant properties and antibacterial activity; Multi-responsive actuating capability |
Wound healing; Artificial muscle | [47,84,85,102] | |
| Fe3O4 | Coordination interaction | Magnetic property | Tissue regeneration; Cancer treatment; Image monitoring |
[39,48,79] | |
| brZnO | Coordination interaction | Antibacterial | Tissue repair | [34] | |
| MgO | Coordination interaction | Antibacterial | Wound healing | [44] | |
| Nanoclays | nSi | Electrostatic interaction; Hydrogen bond |
Enhancing mechanical properties | Tissue engineering; Bone regeneration; Drug delivery |
[53,54,56,57,75,101] |
| MMT | Hydrogen bond | Reinforce chemically cross-linked | Artificial muscle | [55] | |
| Carbon-based nanomaterials | GO/rGO | Electrostatic interaction; Hydrogen bond; π-π stacking; Host-guest interaction |
Improving mechanical properties; Conductivity; Photothermal property and pH sensitivity |
Drug delivery; Wound repair |
[12,33,34,[59], [60], [61], [62], [63]] [85] |
| CNTs | Borate ester bond; Hydrogen bond; Hydrophobic interactions |
Electrical conductivity; Photothermal property; Improving mechanical properties |
Wound treatment | [58,88,90] | |
| Black phosphorus | BPNS | π-π stacking | Improving mechanical properties; Photothermal property |
Photothermal cancer therapy; Bone tissue engineering |
[66] |
| Polymers nanomaterials | PEG-b-PLA NPs | Hydrophobic interactions | Improving rheological properties | Drug delivery; Inhibiting peritoneal adhesions |
[69,89,110,113] |
| PPY | Hydrogen bond | Photothermal property; Electrical conductivity; Improving mechanical properties |
Neural repair; Monitoring sensors | [88,111] | |
| PDA | Hydrogen bond; Electrostatic interaction; π-π stacking |
Adhesive property; Photothermal effect |
Tissue engineering; Arthrodial cartilage; Artificial skin; Myocardial infarction treatment |
[53,59,68,112] | |
| CNC/CNF | Hydrogen bond; Imine bonds |
Improving mechanical properties | Artificial electronic skins; Bone regeneration; Wound healing |
[70,71,74] | |
| CD | Host-guest interaction | High drug loading capacity | Drug delivery | [92] | |
| Gel | Electrostatic interaction | Biocompatibility and biodegradability | Bone regeneration | [56] | |
| Liposomes | Liposomes | Imine bond | Excellent biocompatibility and low toxicity | Drug delivery | [72,73,107] |
Despite the rapid development and vast perspective, there are still some issues that need to be solved in the development of nano-crosslinked dynamic hydrogels dynamic nanocomposite hydrogels, such as, the biodegradation of hydrogels (e.g., carbon-based nanomaterials), insufficient means to form reversible interactions, and the nanotoxicity in long-term use. In fact, current developed dynamic nanocomposite hydrogels cannot meet the growing demand in medical diagnosis and therapy. We speculate that new fabrication strategies should be continuously proposed, and currently existed means should be collaboratively applied and combined with the modification of hydrogels with functional groups or agents. These two concerns are highly depended on the advances of chemistry and material engineering. As ECM-mimicking material scaffolds, the trade-off between dynamic property and mechanical properties of nano-crosslinked hydrogels, in particular, during the degradation process in vivo, greatly affect the outcome of diagnosis and therapy, thus restricting its clinic application. Therefore, how to design the dynamic nanocomposite hydrogels with proper mechanical properties or tissue adaptability is still a key problem. This is not only for the scientists working in field of nano-crosslinked hydrogels, but also for others in traditional dynamic hydrogel design. Although nano-crosslinking can improve the mechanical properties of hydrogels, the potential nanotoxicity of these hydrogels may be even more harmful than that pure nanomaterial-based drug delivery, mainly due to the longer in vivo retention time of hydrogel scaffolds. Thus, the biocompatibility of nano-crosslinked dynamic hydrogels should be the first concern during biomedical applications. The authors suggest to design nanomaterials from biogenic molecules or matrices, and combinate current biogenic polymers (nature-derived polymers) to form dynamically crosslinked hydrogel networks. In spite of these obvious difficulties, the development of nano-crosslinked dynamic hydrogels has been witnessed with a great success, they have been considered as a now promising platform for building dynamic biomimetic devices and systems, especially with 3D printing innovations, in biomedical fields. We anticipated that, with the development of nano-crosslinked dynamic hydrogels and advanced biotechnologies, their combination may synergistically bring more promise for the design of ECM-like biomaterials with tailored physicochemical properties and biological functions, which will greatly promote the progress in the field of medical diagnosis and therapy.
Author contribution statement
Qinghe Wang: Investigation, writing-original draft. Yan Zhang: Investigation, discussion, review, editing. Yue Ma: Investigation, visualization, writing-review & editing. Miao Wang: Investigation, writing-review & editing. Guoqing Pan: Conceptualization, supervision, writing-review & editing, funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the National Natural Science Foundation of China (32222041 and 21875092), the National Natural Science Foundation of Jiangsu Province (BK20220059), the National Key Research and Development Program of China (2019YFA0112000), the Innovation and Entrepreneurship Program of Jiangsu Province, and the Jiangsu Specially Appointed Professor Program.
Contributor Information
Miao Wang, Email: wangmiao@ujs.edu.cn.
Guoqing Pan, Email: panguoqing@ujs.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Wang Z., Zhang Y., Yin Y., Liu J., Li P., Zhao Y., Bai D., Zhao H., Han X., Chen Q. High-strength and injectable supramolecular hydrogel self-assembled by monomeric nucleoside for tooth-extraction wound healing. Adv. Mater. 2022 doi: 10.1002/adma.202108300. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y., Diana D.D., Wu D., Wang W., Peng Y., Asha A.B., Hall D.G., Ishihara K., Narain R. Bioinspired self-healing hydrogel based on benzoxaborole-catechol dynamic covalent chemistry for 3d cell encapsulation. ACS Macro Lett. 2018;7:904–908. doi: 10.1021/acsmacrolett.8b00434. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y., Yin Y.M., Ni L., Miao H.H., Wang Y.J., Pan C., Tian X.H., Pan J.M., You T.Y., Li B., Pan G.Q. Thermo-responsive imprinted hydrogel with switchable sialic acid recognition for selective cancer cell isolation from blood. Bioact. Mater. 2021;6:1308–1317. doi: 10.1016/j.bioactmat.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding F., Zhang L., Chen X., Yin W., Ni L., Wang M. Photothermal nanohybrid hydrogels for biomedical applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1066617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He W., Bai J., Chen X., Suo D., Wang S., Guo Q., Yin W., Geng D., Wang M., Pan G., Zhao X., Li B. Reversible dougong structured receptor-ligand recognition for building dynamic extracellular matrix mimics. Proc. Natl. Acad. Sci. U.S.A. 2022;119 doi: 10.1073/pnas.2117221119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu M., Wei K., Lin S., Chen X., Wu C.C., Li G., Bian L. Bioadhesive polymersome for localized and sustained drug delivery at pathological sites with harsh enzymatic and fluidic environment via supramolecular host-guest complexation. Small. 2018;14 doi: 10.1002/smll.201702288. [DOI] [PubMed] [Google Scholar]
- 7.Mi B., Chen L., Xiong Y., Yang Y., Panayi A.C., Xue H., Hu Y., Yan C., Hu L., Xie X., Lin Z., Zhou W., Cao F., Xiao X., Feng Q., Liu G. Osteoblast/osteoclast and immune cocktail therapy of an exosome/drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano. 2022 doi: 10.1021/acsnano.1c08284. [DOI] [PubMed] [Google Scholar]
- 8.Huebsch N., Kearney C.J., Zhao X., Kim J., Cezar C.A., Suo Z., Mooney D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9762–9767. doi: 10.1073/pnas.1405469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Liu H., Yu A., Lin D., Bao Z., Wang Y., Li X. Bioinspired self-assembly supramolecular hydrogel for ocular drug delivery. Chin. Chem. Lett. 2021;32:3936–3939. doi: 10.1016/j.cclet.2021.03.037. [DOI] [Google Scholar]
- 10.Qu J., Zhao X., Liang Y., Zhang T., Ma Peter X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y., He J., Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021 doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y., Li M., Yang Y., Qiao L., Xu H., Guo B. Ph/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano. 2022;16:3194–3207. doi: 10.1021/acsnano.1c11040. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y., Li Z.H., Song S.L., Yang K.R., Liu H., Yang Z., Wang J.C., Yang B., Lin Q. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201901474. [DOI] [Google Scholar]
- 14.Pan P., Chen X., Xing H., Deng Y., Chen J., Alharthi F.A., Alghamdi A.A., Su J. A fast on-demand preparation of injectable self-healing nanocomposite hydrogels for efficient osteoinduction. Chin. Chem. Lett. 2021;32:2159–2163. doi: 10.1016/j.cclet.2020.12.001. [DOI] [Google Scholar]
- 15.Wang H.Y., Heilshorn S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27:3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty P., Oved H., Bychenko D., Yao Y., Tang Y., Zilberzwige-Tal S., Wei G., Dvir T., Gazit E. Nanoengineered peptide-based antimicrobial conductive supramolecular biomaterial for cardiac tissue engineering. Adv. Mater. 2021;33 doi: 10.1002/adma.202008715. [DOI] [PubMed] [Google Scholar]
- 17.Tang J.D., Mura C., Lampe K.J. Stimuli-responsive, pentapeptide, nanofiber hydrogel for tissue engineering. J. Am. Chem. Soc. 2019;141:4886–4899. doi: 10.1021/jacs.8b13363. [DOI] [PubMed] [Google Scholar]
- 18.Gan D., Jiang Y., Hu Y., Wang X., Wang Q., Wang K., Xie C., Han L., Lu X. Mussel-inspired extracellular matrix-mimicking hydrogel scaffold with high cell affinity and immunomodulation ability for growth factor-free cartilage regeneration. J Orthop Translat. 2022;33:120–131. doi: 10.1016/j.jot.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruvinov E., Tavor Re'em T., Witte F., Cohen S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: a 6-month study in a mini-pig model of osteochondral defects. J Orthop Translat. 2019;16:40–52. doi: 10.1016/j.jot.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W., Zhang H., Zhou Q., Zhou F., Zhang Q., Su J. Smart hydrogels for bone reconstruction via modulating the microenvironment. Research. 2023;6:89. doi: 10.34133/research.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Ma J., Feng Q., Xie E., Meng Q., Shu W., Wu J., Bian L., Han F., Li B. Building osteogenic microenvironments with a double-network composite hydrogel for bone repair. Research. 2023;6 doi: 10.34133/research.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L., Qin R., Zhang J., Liu J., Liu S., Li F., Gong A., Hanliang Q., Du F., Zhang M. Mussel-inspired in situ fabrication of a photothermal composite hydrogel for mr-guided localized tumor ablation. RSC Adv. 2021;11:19461–19469. doi: 10.1039/D1RA00903F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Liu Y., Hsu S.H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules. 2019;24:3005. doi: 10.3390/molecules24163005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Zou X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019;4:120–131. doi: 10.1016/j.bioactmat.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posadowska U., Brzychczy-Wloch M., Pamula E. Injectable gellan gum-based nanoparticles-loaded system for the local delivery of vancomycin in osteomyelitis treatment. J. Mater. Sci. Mater. Med. 2016;27:9. doi: 10.1007/s10856-015-5604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Zhu D., Paul A., Cai L., Enejder A., Yang F., Heilshorn S.C. Covalently adaptable elastin-like protein - hyaluronic acid (elp - ha) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., Patsis P.A., Hauser S., Voigt D., Rothe R., Gunther M., Cui M.Y., Yang X.G., Wieduwild R., Eckert K., Neinhuis C., Akbar T.F., Minev I.R., Pietzsch J., Zhang Y.X. Cytocompatible, injectable, and electroconductive soft adhesives with hybrid covalent/noncovalent dynamic network. Adv. Sci. 2019;6 doi: 10.1002/advs.201802077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin S.R., Aghaei-Ghareh-Bolagh B., Dang T.T., Topkaya S.N., Gao X., Yang S.Y., Jung S.M., Oh J.H., Dokmeci M.R., Tang X.S., Khademhosseini A. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv. Mater. 2013;25:6385–6391. doi: 10.1002/adma.201301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saez Talens V., Davis J., Wu C.H., Wen Z., Lauria F., Gupta K., Rudge R., Boraghi M., Hagemeijer A., Trinh T.T., Englebienne P., Voets I.K., Wu J.I., Kieltyka R.E. Thiosquaramide-based supramolecular polymers: aromaticity gain in a switched mode of self-assembly. J. Am. Chem. Soc. 2020;142:19907–19916. doi: 10.1021/jacs.0c02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Z., Gerecht S. A self-healing hydrogel as an injectable instructive carrier for cellular morphogenesis. Biomaterials. 2018;185:86–96. doi: 10.1016/j.biomaterials.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor D.L., Panhuis M.I.H. Self-healing hydrogels. Adv. Mater. 2016;28:9060–9093. doi: 10.1002/adma.201601613. [DOI] [PubMed] [Google Scholar]
- 32.Yu C., Yue Z., Shi M., Jiang L., Chen S., Yao M., Yu Q., Wu X., Zhang H., Yao F., Wang C., Sun H., Li J. An intrapericardial injectable hydrogel patch for mechanical-electrical coupling with infarcted myocardium. ACS Nano. 2022;16:16234–16248. doi: 10.1021/acsnano.2c05168. [DOI] [PubMed] [Google Scholar]
- 33.Bai H., Li C., Wang X., Shi G. A ph-sensitive graphene oxide composite hydrogel. Chem. Commun. (Camb.). 2010;46:2376–2378. doi: 10.1039/c000051e. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Zhang Z., Chen B., Shao J., Guo Z. Self-healing hydrogel of poly(vinyl alcohol)/graphite oxide with ph-sensitive and enhanced thermal properties. J. Appl. Polym. Sci. 2018;135 doi: 10.1002/app.46143. [DOI] [Google Scholar]
- 35.Wu M., Chen J., Huang W., Yan B., Peng Q., Liu J., Chen L., Zeng H. Injectable and self-healing nanocomposite hydrogels with ultrasensitive ph-responsiveness and tunable mechanical properties: implications for controlled drug delivery. Biomacromolecules. 2020;21:2409–2420. doi: 10.1021/acs.biomac.0c00347. [DOI] [PubMed] [Google Scholar]
- 36.Yong Ho Y., Won Ho P. Dual-crosslinked, self-healing and thermo-responsive methylcellulose/chitosan oligomer copolymer hydrogels. Carbohydr. Polym. 2021 doi: 10.1016/j.carbpol.2021.117705. [DOI] [PubMed] [Google Scholar]
- 37.Kweon O.Y., Samanta S.K., Won Y., Yoo J.H., Oh J.H. Stretchable and self-healable conductive hydrogels for wearable multimodal touch sensors with thermoresponsive behavior. ACS Appl. Mater. Interfaces. 2019;11:26134–26143. doi: 10.1021/acsami.9b04440. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y., Wang M., Ma C., Wang Y., Li X., Yu G. A conductive self-healing hybrid gel enabled by metal-ligand supramolecule and nanostructured conductive polymer. Nano Lett. 2015;15:6276–6281. doi: 10.1021/acs.nanolett.5b03069. [DOI] [PubMed] [Google Scholar]
- 39.Gang F., Yan H., Ma C., Jiang L., Gu Y., Liu Z., Zhao L., Wang X., Zhang J., Sun X. Robust magnetic double-network hydrogels with self-healing, mr imaging, cytocompatibility and 3d printability. Chem. Commun. (Camb.). 2019;55:9801–9804. doi: 10.1039/c9cc04241e. [DOI] [PubMed] [Google Scholar]
- 40.Janeček E.-R., McKee J.R., Tan C.S.Y., Nykänen A., Kettunen M., Laine J., Ikkala O., Scherman O.A. Hybrid supramolecular and colloidal hydrogels that bridge multiple length scales. Angew. Chem. Int. Ed. 2015;54:5383–5388. doi: 10.1002/anie.201410570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin T., Gu Y., Cheng R., Tang J., Sun Z., Cui W., Chen L. Inorganic strengthened hydrogel membrane as regenerative periosteum. ACS Appl. Mater. Interfaces. 2017;9:41168–41180. doi: 10.1021/acsami.7b13167. [DOI] [PubMed] [Google Scholar]
- 42.Tavares M., Santos S., Custódio C., Farinha J., Baleizão C., Mano J. Platelet lysates-based hydrogels incorporating bioactive mesoporous silica nanoparticles for stem cell osteogenic differentiation. Materials Today Bio. 2021;9 doi: 10.1016/j.mtbio.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu T., Wu G., Bu H., Zhang H., Li W., Song K., Jiang G. An injectable, adhesive, and self-healable composite hydrogel wound dressing with excellent antibacterial activity. Chem. Eng. J. 2022;450 doi: 10.1016/j.cej.2022.138201. [DOI] [Google Scholar]
- 44.Tang X., Wang X., Sun Y., Zhao L., Li D., Zhang J., Sun H., Yang B. Magnesium oxide-assisted dual-cross-linking bio-multifunctional hydrogels for wound repair during full-thickness skin injuries. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202105718. [DOI] [Google Scholar]
- 45.Huang C., Ye Q., Dong J., Li L., Wang M., Zhang Y., Zhang Y., Wang X., Wang P., Jiang Q. Biofabrication of natural Au/bacterial cellulose hydrogel for bone tissue regeneration via in-situ fermentation. Smart Mater. Med. 2023;4:1–14. doi: 10.1016/j.smaim.2022.06.001. [DOI] [Google Scholar]
- 46.Xing R., Liu K., Jiao T., Zhang N., Ma K., Zhang R., Zou Q., Ma G., Yan X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016;28:3669–3676. doi: 10.1002/adma.201600284. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Rafael S., Ivanova K., Stefanov I., Puiggali J., Del Valle L.J., Todorova K., Dimitrov P., Hinojosa-Caballero D., Tzanov T. Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment. Acta Biomater. 2021;134:131–143. doi: 10.1016/j.actbio.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Shi L., Zeng Y., Zhao Y., Yang B., Ossipov D., Tai C.W., Dai J., Xu C. Biocompatible injectable magnetic hydrogel formed by dynamic coordination network. ACS Appl. Mater. Interfaces. 2019;11:46233–46240. doi: 10.1021/acsami.9b17627. [DOI] [PubMed] [Google Scholar]
- 49.Wu C., Gaharwar A.K., Chan B.K., Schmidt G. Mechanically tough pluronic F127/laponite nanocomposite hydrogels from covalently and physically cross-linked networks. Macromolecules. 2011;44:8215–8224. doi: 10.1021/ma200562k. [DOI] [Google Scholar]
- 50.Waters R., Pacelli S., Maloney R., Medhi I., Ahmed R.P., Paul A. Stem cell secretome-rich nanoclay hydrogel: a dual action therapy for cardiovascular regeneration. Nanoscale. 2016;8:7371–7376. doi: 10.1039/c5nr07806g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lokhande G., Carrow J.K., Thakur T., Xavier J.R., Parani M., Bayless K.J., Gaharwar A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018;70:35–47. doi: 10.1016/j.actbio.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerativitayanan P., Gaharwar A.K. Elastomeric and mechanically stiff nanocomposites from poly(glycerol sebacate) and bioactive nanosilicates. Acta Biomater. 2015;26:34–44. doi: 10.1016/j.actbio.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Han L., He Y., An R., Wang X., Zhang Y., Shi L., Ran R. Mussel-inspired, robust and self-healing nanocomposite hydrogels: effective reusable absorbents for removal both anionic and cationic dyes. Colloids Surf., A. 2019;569:18–27. doi: 10.1016/j.colsurfa.2019.02.054. [DOI] [Google Scholar]
- 54.Cao X., Liu H.Z., Yang X.H., Tian J.H., Luo B.H., Liu M.X. Halloysite Nanotubes@Polydopamine reinforced polyacrylamide-gelatin hydrogels with nir light triggered shape memory and self-healing capability. Compos. Sci. Technol. 2020;191 doi: 10.1016/j.compscitech.2020.108071. [DOI] [Google Scholar]
- 55.Gao G., Du G., Sun Y., Fu J. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption. ACS Appl. Mater. Interfaces. 2015;7:5029–5037. doi: 10.1021/acsami.5b00704. [DOI] [PubMed] [Google Scholar]
- 56.Dou Z., Tang H., Chen K., Li D., Ying Q., Mu Z., An C., Shao F., Zhang Y., Zhang Y., Bai H., Zheng G., Zhang L., Chen T., Wang H. Highly elastic and self-healing nanostructured gelatin/clay colloidal gels with osteogenic capacity for minimally invasive and customized bone regeneration. Biofabrication. 2023;15 doi: 10.1088/1758-5090/acab36. [DOI] [PubMed] [Google Scholar]
- 57.Basu S., Pacelli S., Feng Y., Lu Q., Wang J., Paul A. Harnessing the noncovalent interactions of DNA backbone with 2d silicate nanodisks to fabricate injectable therapeutic hydrogels. ACS Nano. 2018;12:9866–9880. doi: 10.1021/acsnano.8b02434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng P., Liang X., Chen F., Chen Y., Zhou J. Novel multifunctional dual-dynamic-bonds crosslinked hydrogels for multi-strategy therapy of mrsa-infected wounds. Appl. Mater. 2022;26 doi: 10.1016/j.apmt.2022.101362. [DOI] [Google Scholar]
- 59.Han L., Lu X., Wang M., Gan D., Deng W., Wang K., Fang L., Liu K., Chan C.W., Tang Y., Weng L.T., Yuan H. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017;13 doi: 10.1002/smll.201601916. [DOI] [PubMed] [Google Scholar]
- 60.Huang W., Qi C., Gao Y. Injectable self-healable nanocomposite hydrogels with mussel-inspired adhesive properties for 3d printing ink. ACS Appl. Nano Mater. 2019;2:5000–5008. doi: 10.1021/acsanm.9b00936. [DOI] [Google Scholar]
- 61.Liu J., Song G., He C., Wang H. Self-healing in tough graphene oxide composite hydrogels. Macromol. Rapid Commun. 2013;34:1002–1007. doi: 10.1002/marc.201300242. [DOI] [PubMed] [Google Scholar]
- 62.Han D., Yan L. Supramolecular hydrogel of chitosan in the presence of graphene oxide nanosheets as 2d cross-linkers. ACS Sustain. Chem. Eng. 2013;2:296–300. doi: 10.1021/sc400352a. [DOI] [Google Scholar]
- 63.Zhang B., He J., Shi M., Liang Y., Guo B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020;400 doi: 10.1016/j.cej.2020.125994. [DOI] [Google Scholar]
- 64.Miao Y., Shi X., Li Q., Hao L., Liu L., Liu X., Chen Y., Wang Y. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019;7:4046–4059. doi: 10.1039/c9bm01072f. [DOI] [PubMed] [Google Scholar]
- 65.Xing C., Chen S., Qiu M., Liang X., Liu Q., Zou Q., Li Z., Xie Z., Wang D., Dong B., Liu L., Fan D., Zhang H. Conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701510. [DOI] [PubMed] [Google Scholar]
- 66.Miao Y., Chen Y., Luo J., Liu X., Yang Q., Shi X., Wang Y. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3d-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023;21:97–109. doi: 10.1016/j.bioactmat.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu C., Yue Z., Zhang H., Shi M., Yao M., Yu Q., Liu M., Guo B., Zhang H., Tian L., Sun H., Yao F., Li J. Ultra-histocompatible and electrophysiological-adapted pedot-based hydrogels designed for cardiac repair. Adv. Funct. Mater. 2023 doi: 10.1002/adfm.202211023. [DOI] [Google Scholar]
- 68.Yang L., Wang Z.H., Fei G.X., Xia H.S. Polydopamine particles reinforced poly(vinyl alcohol) hydrogel with nir light triggered shape memory and self-healing capability. Macromol. Rapid Commun. 2017;38 doi: 10.1002/marc.201700421. [DOI] [PubMed] [Google Scholar]
- 69.Fenton O.S., Tibbitt M.W., Appel E.A., Jhunjhunwala S., Webber M.J., Langer R. Injectable polymer-nanoparticle hydrogels for local immune cell recruitment. Biomacromolecules. 2019;20:4430–4436. doi: 10.1021/acs.biomac.9b01129. [DOI] [PubMed] [Google Scholar]
- 70.Ma W., Cao W., Lu T., Jiang Z., Xiong R., Samal S.K., Huang C. Healable, adhesive, and conductive nanocomposite hydrogels with ultrastretchability for flexible sensors. ACS Appl. Mater. Interfaces. 2021;13:58048–58058. doi: 10.1021/acsami.1c20271. [DOI] [PubMed] [Google Scholar]
- 71.Cui S., Zhang S., Coseri S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023;300 doi: 10.1016/j.carbpol.2022.120243. [DOI] [PubMed] [Google Scholar]
- 72.Stalder E., Zumbuehl A. Liposome-containing mechanoresponsive hydrogels. Macromol. Mater. Eng. 2017;302 doi: 10.1002/mame.201600549. [DOI] [Google Scholar]
- 73.Uchida Y., Fukuda K., Murakami Y. The hydrogel containing a novel vesicle-like soft crosslinker, a “trilayered” polymeric micelle, shows characteristic rheological properties. J. Polym. Sci., Part B: Polym. Phys. 2013;51:124–131. doi: 10.1002/polb.23187. [DOI] [Google Scholar]
- 74.Huang W., Wang Y., Huang Z., Wang X., Chen L., Zhang Y., Zhang L. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl. Mater. Interfaces. 2018;10:41076–41088. doi: 10.1021/acsami.8b14526. [DOI] [PubMed] [Google Scholar]
- 75.Basu S., Pacelli S., Paul A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020;105:159–169. doi: 10.1016/j.actbio.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 76.Ma L., Su W., Ran Y., Ma X., Yi Z., Chen G., Chen X., Deng Z., Tong Q., Wang X., Li X. Synthesis and characterization of injectable self-healing hydrogels based on oxidized alginate-hybrid-hydroxyapatite nanoparticles and carboxymethyl chitosan. Int. J. Biol. Macromol. 2020;165:1164–1174. doi: 10.1016/j.ijbiomac.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Lin T.W., Hsu S.H. Self-healing hydrogels and cryogels from biodegradable polyurethane nanoparticle crosslinked chitosan. Adv. Sci. 2020;7 doi: 10.1002/advs.201901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin W., Wang Q., Zhang J., Chen X., Wang Y., Jiang Z., Wang M., Pan G. A dynamic nano-coordination protein hydrogel for photothermal treatment and repair of infected skin injury. J. Mater. Chem. B. 2022;10:8181–8185. doi: 10.1039/d2tb01146h. [DOI] [PubMed] [Google Scholar]
- 79.He X., Zeng Y., Liu G., Tian Y., Wei Y., Zhao L., Yang L., Tao L. Magnetic self-healing hydrogel from difunctional polymers prepared via the kabachnik-fields reaction. ACS Macro Lett. 2022;11:39–45. doi: 10.1021/acsmacrolett.1c00720. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh T., Biswas A., Gavel P.K., Das A.K. Engineered dynamic boronate ester-mediated self-healable biocompatible G-quadruplex hydrogels for sustained release of vitamins. Langmuir. 2020;36:1574–1584. doi: 10.1021/acs.langmuir.9b03837. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Q.X., Mu S.D., Liu X., Qiu G.R., Astruc D., Gu H.B. Gallol-tethered injectable aunp hydrogel with desirable self-healing and catalytic properties. Macromol. Chem. Phys. 2019;220 doi: 10.1002/macp.201800427. [DOI] [Google Scholar]
- 82.Qin H., Zhang T., Li H., Cong H., Antonietti M., Yu S. Dynamic Au-thiolate interaction induced rapid self-healing nanocomposite hydrogels with remarkable mechanical behaviors. Chem. 2017;3:691–705. doi: 10.1016/j.chempr.2017.07.017. [DOI] [Google Scholar]
- 83.Wang C., Liu X., Wulf V., Vazquez-Gonzalez M., Fadeev M., Willner I. DNA-based hydrogels loaded with Au nanoparticles or Au nanorods: thermoresponsive plasmonic matrices for shape-memory, self-healing, controlled release, and mechanical applications. ACS Nano. 2019;13:3424–3433. doi: 10.1021/acsnano.8b09470. [DOI] [PubMed] [Google Scholar]
- 84.Qin H., Zhang T., Li N., Cong H., Yu S. Anisotropic and self-healing hydrogels with multi-responsive actuating capability. Nat. Commun. 2019;10:2202. doi: 10.1038/s41467-019-10243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang P.P., Qin H., Dai J., Yu S.H., Cong H.P. Ultrastretchable and self-healing conductors with double dynamic network for omni-healable capacitive strain sensors. Nano Lett. 2022;22:1433–1442. doi: 10.1021/acs.nanolett.1c03618. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Yin W., Liu Y., Hou M., Shi Q., Liu T., Wang M., Yang H., Pan G., He F., Zhu X. Dynamic protein hydrogel with supramolecularly enveloped kartogenin promotes cartilage regeneration through mitochondrial activation. Compos. B Eng. 2022;246 doi: 10.1016/j.compositesb.2022.110257. [DOI] [Google Scholar]
- 87.Shen S., Fan D., Yuan Y., Ma X., Zhao J., Yang J. An ultrasmall infinite coordination polymer nanomedicine-composited biomimetic hydrogel for programmed dressing-chemo-low level laser combination therapy of burn wounds. Chem. Eng. J. 2021;426 doi: 10.1016/j.cej.2021.130610. [DOI] [Google Scholar]
- 88.Deng Z., Guo Y., Zhao X., Ma P.X., Guo B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018;30:1729–1742. doi: 10.1021/acs.chemmater.8b00008. [DOI] [Google Scholar]
- 89.Appel E.A., Tibbitt M.W., Webber M.J., Mattix B.A., Veiseh O., Langer R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015;6:6295. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin Z., Sun X., Yu Q., Zhang H., Wu X., Yao M., Liu W., Yao F., Li J. Carbon nanotubes/hydrophobically associated hydrogels as ultrastretchable, highly sensitive, stable strain, and pressure sensors. ACS Appl. Mater. Interfaces. 2020;12:4944–4953. doi: 10.1021/acsami.9b21659. [DOI] [PubMed] [Google Scholar]
- 91.Zohreband Z., Adeli M., Zebardasti A. Self-healable and flexible supramolecular gelatin/mos(2) hydrogels with molecular recognition properties. Int. J. Biol. Macromol. 2021;182:2048–2055. doi: 10.1016/j.ijbiomac.2021.05.106. [DOI] [PubMed] [Google Scholar]
- 92.Soni S.S., D'Elia A.M., Alsasa A., Cho S., Tylek T., O'Brien E.M., Whitaker R., Spiller K.L., Rodell C.B. Sustained release of drug-loaded nanoparticles from injectable hydrogels enables long-term control of macrophage phenotype. Biomater. Sci. 2022;10:6951–6967. doi: 10.1039/d2bm01113a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li M., Fang F., Sun M., Zhang Y., Hu M., Zhang J. Extracellular vesicles as bioactive nanotherapeutics: an emerging paradigm for regenerative medicine. Theranostics. 2022;12:4879–4903. doi: 10.7150/thno.72812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupte M.J., Swanson W.B., Hu J., Jin X., Ma H., Zhang Z., Liu Z., Feng K., Feng G., Xiao G., Hatch N., Mishina Y., Ma P.X. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1–11. doi: 10.1016/j.actbio.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valipour F., Valipour F., Rahbarghazi R., Navali A.M., Rashidi M.R., Davaran S. Novel hybrid polyester-polyacrylate hydrogels enriched with platelet-derived growth factor for chondrogenic differentiation of adipose-derived mesenchymal stem cells in vitro. J. Biol. Eng. 2021;15:6. doi: 10.1186/s13036-021-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ao Y., Zhang E., Liu Y., Yang L., Li J., Wang F. Advanced hydrogels with nanoparticle inclusion for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.951513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao H., Wang X., Jin A., Wang M., Wang Z., Huang X., Dai J., Wang X., Lin D., Shen S.G. Reducing relapse and accelerating osteogenesis in rapid maxillary expansion using an injectable mesoporous bioactive glass/fibrin glue composite hydrogel. Bioact. Mater. 2022;18:507–525. doi: 10.1016/j.bioactmat.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li D., Zhao L., Cong M., Liu L., Yan G., Li Z., Li B., Yu W., Sun H., Yang B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent. Mater. 2020;36:e229–e240. doi: 10.1016/j.dental.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 99.Guan P., Liu C., Xie D., Mao S., Ji Y., Lin Y., Chen Z., Wang Q., Fan L., Sun Y. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property facilitates/promotes growth plate injury repair. Bioact. Mater. 2022;10:145–158. doi: 10.1016/j.bioactmat.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding Y., Zhang X., Mi C., Qi X., Zhou J., Wei D. Recent advances in hyaluronic acid-based hydrogels for 3d bioprinting in tissue engineering applications. Smart Mater. Med. 2023;4:59–68. doi: 10.1016/j.smaim.2022.07.003. [DOI] [Google Scholar]
- 101.Zhai X., Ma Y., Hou C., Gao F., Zhang Y., Ruan C., Pan H., Lu W.W., Liu W. 3d-Printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater. Sci. Eng. 2017;3:1109–1118. doi: 10.1021/acsbiomaterials.7b00224. [DOI] [PubMed] [Google Scholar]
- 102.Ma L., Tan Y., Chen X., Ran Y., Tong Q., Tang L., Su W., Wang X., Li X. Injectable oxidized alginate/carboxylmethyl chitosan hydrogels functionalized with nanoparticles for wound repair. Carbohydr. Polym. 2022;293 doi: 10.1016/j.carbpol.2022.119733. [DOI] [PubMed] [Google Scholar]
- 103.Javvaji V., Dowling M.B., Oh H., White I.M., Raghavan S.R. Reversible gelation of cells using self-assembling hydrophobically-modified biopolymers: towards self-assembly of tissue. Biomater. Sci. 2014;2:1016–1023. doi: 10.1039/c4bm00017j. [DOI] [PubMed] [Google Scholar]
- 104.Chen Y., Javvaji V., MacIntire I.C., Raghavan S.R. Gelation of vesicles and nanoparticles using water-soluble hydrophobically modified chitosan. Langmuir. 2013;29:15302–15308. doi: 10.1021/la4037343. [DOI] [PubMed] [Google Scholar]
- 105.Liang Y., Kiick K.L. Liposome-cross-linked hybrid hydrogels for glutathione-triggered delivery of multiple cargo molecules. Biomacromolecules. 2016;17:601–614. doi: 10.1021/acs.biomac.5b01541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ziemczonek P., Gosecka M., Gosecki M., Marcinkowska M., Janaszewska A., Klajnert-Maculewicz B. Star-shaped poly(furfuryl glycidyl ether)-block-poly(glyceryl glycerol ether) as an efficient agent for the enhancement of nifuratel solubility and for the Formation of injectable and self-healable hydrogel platforms for the gynaecological therapies. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee J.H., Oh H., Baxa U., Raghavan S.R., Blumenthal R. Biopolymer-connected liposome networks as injectable biomaterials capable of sustained local drug delivery. Biomacromolecules. 2012;13:3388–3394. doi: 10.1021/bm301143d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L., Liang K., Jiang X., Yang M., Liu Y.N. Dynamic protein-metal ion networks: a unique approach to injectable and self-healable metal sulfide/protein hybrid hydrogels with high photothermal efficiency. Chemistry. 2018;24:6557–6563. doi: 10.1002/chem.201705841. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y., Li L., Ma Y., Tang Y., Zhao Y., Li Z., Pu W., Huang B., Wen X., Cao X., Chen J., Chen W., Zhou Y., Zhang J. Multifunctional supramolecular hydrogel for prevention of epidural adhesion after laminectomy. ACS Nano. 2020;14:8202–8219. doi: 10.1021/acsnano.0c01658. [DOI] [PubMed] [Google Scholar]
- 110.Saouaf O.M., Roth G.A., Ou B.S., Smith A.A.A., Yu A.C., Gale E.C., Grosskopf A.K., Picece V., Appel E.A. Modulation of injectable hydrogel properties for slow Co-delivery of influenza subunit vaccine components enhance the potency of humoral immunity. J. Biomed. Mater. Res. A. 2021;109:2173–2186. doi: 10.1002/jbm.a.37203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J., Wong C., Hsu S. An injectable, electroconductive hydrogel/scaffold for neural repair and motion sensing. Chem. Mater. 2020;32:10407–10422. doi: 10.1021/acs.chemmater.0c02906. [DOI] [Google Scholar]
- 112.Chen R., Zhu C., Xu L., Gu Y., Ren S., Bai H., Zhou Q., Liu X., Lu S., Bi X., Li W., Jia X., Chen Z. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120855. [DOI] [PubMed] [Google Scholar]
- 113.Stapleton L.M., Lucian H.J., Grosskopf A.K., Smith A.A.A., Totherow K.P., Woo Y.J., Appel E.A. Dynamic hydrogels for prevention of post-operative peritoneal adhesions. Adv. Ther. 2021;4 doi: 10.1002/adtp.202000242. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.