Abstract
A 63-year-old man with hypertrophic cardiomyopathy (HCM), mid-ventricular obstruction, and an apical aneurysm had an episode of cardiac arrest due to sustained ventricular tachycardia (VT). He was resuscitated and an implantable cardioverter-defibrillator (ICD) was implanted. In the following years, several episodes of VT and ventricular fibrillation were successfully terminated by antitachycardia pacing or ICD shocks. Three years after ICD implantation, he was re-admitted because of refractory electrical storm (ES). Since aggressive pharmacological treatments, direct current cardioversions, and deep sedation were not effective, he underwent epicardial catheter ablation which was successful to terminate ES. However, because of the recurrence of refractory ES after one year, he proceeded to surgical left ventricular myectomy with apical aneurysmectomy which provided him a relatively stable clinical course for six years. Although epicardial catheter ablation may be an acceptable option, surgical resection of apical aneurysm seems to be most efficacious for ES in patients with HCM and an apical aneurysm.
Learning objectives
In patients with hypertrophic cardiomyopathy (HCM), implantable cardioverter-defibrillators (ICDs) are the gold standard of therapy for prophylaxis against sudden death. Electrical storm (ES) caused by recurrent episodes of ventricular tachycardia can cause sudden death even in patients with ICDs. Although epicardial catheter ablation may be an acceptable option, surgical resection of apical aneurysm is most efficacious for ES in patients with HCM, mid-ventricular obstruction, and an apical aneurysm.
Keywords: Electrical storm, Epicardial catheter ablation, Hypertrophic cardiomyopathy, Left ventricular apical aneurysm, Surgical left ventricular aneurysmectomy
Introduction
Patients with hypertrophic cardiomyopathy (HCM) have an increased risk of death from several causes such as sudden cardiac death, heart failure, and stroke. In particular, sudden unexpected death is the most feared complication of HCM [1]. Since ventricular tachyarrhythmias are the cause of cardiac arrest and sudden death in patients with HCM [2], implantable cardioverter-defibrillators (ICDs) are now the mainstay of therapy for prophylaxis against sudden death [2], [3].
However, electrical storm (ES) caused by recurrent episodes of ventricular tachycardia (VT) can cause sudden death even in patients with ICDs.
Case report
A 63-year-old man with HCM, mid-ventricular obstruction, and an apical aneurysm experienced several episodes of near syncope and was referred to our hospital because of frequent non-sustained VT (NSVT) documented in a local hospital. He had also been diagnosed as having systemic hypertension and paroxysmal atrial fibrillation with a previous episode of cerebral embolism and had been put on warfarin, amiodarone, and bisoprolol.
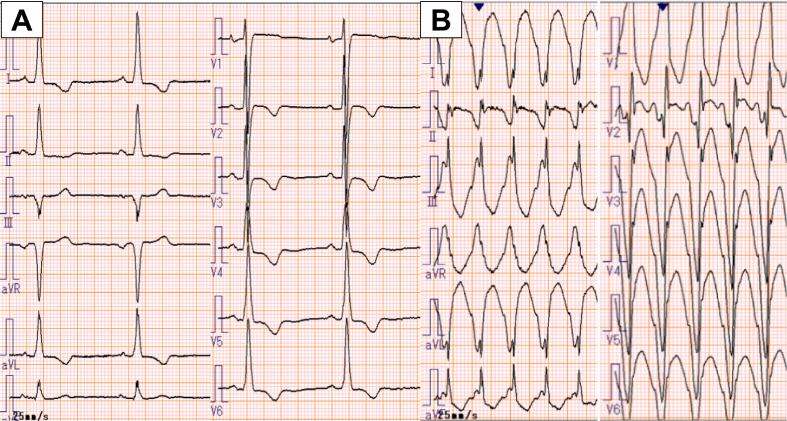
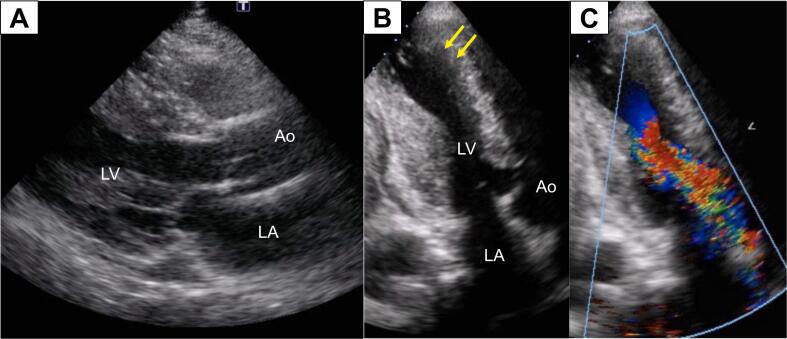
On admission in the year of 2011, he was alert with normal blood pressure at 120/70 mmHg and regular heart rate at 60 bpm. On examination, he had a fourth heart sound and a grade 3/6 ejection systolic murmur at the apex. Electrocardiography showed normal sinus rhythm at 52 bpm and prominent left ventricular hypertrophy with T-wave inversions (Fig. 1A). Chest radiograph revealed no cardiac enlargement or pulmonary congestion. B-type natriuretic peptide (626 pg/mL) was significantly elevated and high-sensitivity cardiac troponin T (0.075 ng/mL) was mildly elevated. Echocardiography showed diffuse left ventricular hypertrophy with maximum wall thickness of 18 mm at mid-ventricular level and severe apical hypokinesis suggestive of an apical aneurysm (Fig. 2). Mid-ventricular obstruction with pressure gradient of 170 mmHg was also documented. During hospitalization, he had an episode of cardiac arrest due to sustained VT during treadmill exercise test (Fig. 1B). He was resuscitated and an ICD was implanted. His pharmacological treatment with warfarin, amiodarone (200 mg, b.i.d.), and bisoprolol (2.5 mg, s.i.d.) was continued at discharge because of several episodes of NSVT on exercise after ICD implantation.
Fig. 1.
(A) Electrocardiogram on admission showing sinus rhythm, and prominent left ventricular hypertrophy. (B) Electrocardiogram showing sustained monomorphic ventricular tachycardia which led to an episode of cardiac arrest.
Fig. 2.
Echocardiogram on admission. (A) A long-axis view showing diffuse left ventricular hypertrophy. (B) An apical two-chamber view showing maximum wall thickening at mid-ventricular level and an apical aneurysm (arrows). (C) A color-Doppler echocardiogram in apical two-chamber view during systole showing mosaic color-flow from mid-ventricular level, indicating significant pressure gradient.
Ao, aorta; LA, left atrium; LV, left ventricle.
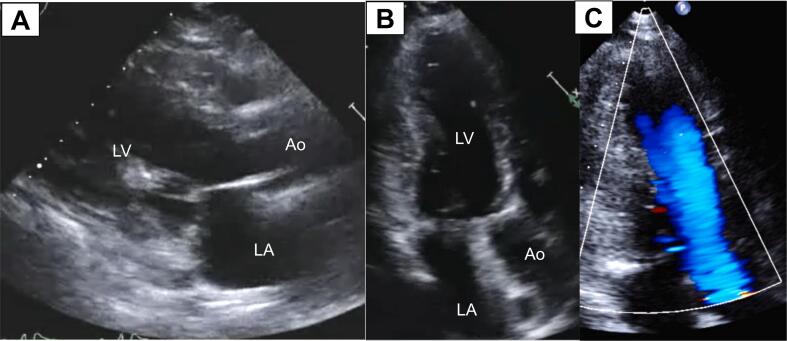
In the following years, several episodes of sustained VT were successfully terminated by antitachycardia pacing or ICD shocks. Three years after ICD implantation, he was re-admitted because of refractory ES. Aggressive pharmacological treatment with several antiarrhythmic drugs, cibenzoline, and beta blockade, frequent direct current cardioversions and deep sedation were not effective. Therefore, he proceeded to an emergency and rescue catheter ablation. Since sustained VT of epicardial origin was clinically suspected based on the morphology of VT on a 12‑lead electrocardiogram associated with the presence of apical aneurysm, epicardial access was first achieved using the percutaneous subxiphoid approach. Three-dimensional electroanatomic voltage maps revealed low-amplitude regions with a voltage ≤1.5 mV suggestive of electrophysiological scar, involving the lateral left ventricular epicardium. Ventricular tachycardia terminated reproducibly by imposing a catheter on the site. Thereafter, endocardial mapping was performed but failed to show any activation prior to the electric potential at the low-amplitude regions of the epicardium. Thus, we considered the exit of VT probably existed at the epicardial site, and decided to perform radiofrequency catheter ablation with epicardial approach. While VT was hemodynamically stable, epicardial radiofrequency ablation was attempted using a 4-mm electrode catheter. Ventricular tachycardia finally became non-inducible after 38th session of radiofrequency energy application. Refractory ES was thus successfully terminated. However, after one uneventful year, he again developed refractory ES. Therefore, the decision was made to perform surgical left ventricular myectomy with apical aneurysmectomy, left ventricular reconstruction, and endocardial cryoablation (Fig. 3). Since then, together with pharmacological treatment, he has been relatively well without episodes of refractory ES for six years.
Fig. 3.
Echocardiogram after surgical treatment. (A) A long-axis view showing somewhat broadened left ventricular cavity. (B) An apical two-chamber view showing disappearance of an apical aneurysm. (C) A color-Doppler echocardiogram in apical two-chamber view during systole showing laminar color-flow in the absence of mosaic flow at mid-ventricular level.
Ao, aorta; LA, left atrium; LV, left ventricle.
Discussion
This HCM patient with mid-ventricular obstruction and an apical aneurysm is unique and exceptional because he developed ES refractory to aggressive trials of antiarrhythmic drugs, frequent direct current cardioversions, and other modalities, despite previous ICD implantation. Second, although epicardial catheter ablation was successful to terminate ES, the uneventful period lasted only for one year after the procedure. Third, surgical left ventricular myectomy with apical aneurysmectomy was needed to finally terminate refractory ES.
Patients with HCM are at increased risk for sudden cardiac death [1], [3]. Since ventricular tachyarrhythmias are the cause of cardiac arrest and sudden death [2], and with availability of ICD, risk stratification for ICD implantation is critical in this population. There is general agreement that an ICD is the gold standard therapy for secondary prevention in survivors of cardiac arrest or sustained spontaneous VT [4]. For primary prevention, the weight of evidence also supports use of an ICD in high-risk patients [4]. The presence of a left ventricular apical aneurysm, the presence of extensive gadolinium enhancement, and end-stage HCM with ejection fraction <50 % may influence the decision to place an ICD for primary prevention [4], [5], [6]. Our patient received an ICD for secondary prevention with cardiac arrest due to sustained and pulseless VT.
Although an ICD significantly enhances survival in patients with sustained VT and ventricular fibrillation, the recurrence of these malignant ventricular tachyarrhythmias can still be a cause of death. Electrical storm, characterized by recurrent episodes of VT resulting in appropriate ICD shocks, is a frightening event associated with poor prognosis. Electrical storm has been recognized as an independent predictor of cardiac death. Previous data indicate that catheter ablation is an effective treatment in a broad population of patients suffering from ES in whom the complete suppression of all clinical VTs was achieved [7]. The protective role of catheter ablation over the long-term is also suggested. Since an apical aneurysm represents an important example of anatomic re-entry for monomorphic VT, it also provides the opportunity to consider catheter-ablation-based treatment for refractory recurrent VT. Since the ventricular wall in patients with HCM can be quite thick and thus endocardial ablation alone may be of limited value, an epicardial approach or combined epicardial and endocardial approach should be considered.
Epicardial catheter ablation was acutely successful to terminate ES in our patient. However, after one year of uneventful period, he again developed refractory ES. Because of the recurrence of ES and the presence of an apical aneurysm, he finally underwent surgical left ventricular myectomy with apical aneurysmectomy, left ventricular reconstruction and endocardial cryoablation. By terminating ES and preventing ES recurrence, surgical left ventricular aneurysmectomy has provided the patient a relatively stable clinical course for six years without recurrent episodes of refractory ES. Thus, it is postulated that surgical left ventricular aneurysmectomy may favorably affect cardiac mortality in this particular patient population [8], [9], [10].
In conclusion, if refractory to aggressive trials of antiarrhythmic drugs and other modalities including epicardial catheter ablation, surgical left ventricular aneurysmectomy is a most efficacious option for refractory ES in patients with HCM and apical aneurysm.
Declaration of competing interest
All authors have no conflict of interest to disclose.
Acknowledgments
Acknowledgment
We thank Dr. Kazuhiro Satomi for his helpful participation in epicardial catheter ablation procedure. We also thank Dr. Shuichiro Takanashi and his surgical team for their excellent surgical work in this patient.
Patient permission/consent statement
Informed consent was obtained from the patient for publication of this case report, including accompanying images.
References
- 1.Maron B.J. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. doi: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J., Shen W.K., Link M.S., Epstein A.E., Almquist A.K., Daubert J.P., Brady G.H., Favale S., Rea R.F., Boriani G., Estes N.A.M., Spirito P. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 3.Maron B.J., Rowin E.J., Maron M.S. Paradigm of sudden death prevention in hypertrophic cardiomyopathy. Circ Res. 2019;125:370–378. doi: 10.1161/CIRCRESAHA.119.315159. [DOI] [PubMed] [Google Scholar]
- 4.Ommen S.R., Mital S., Burke M.A., Day S.M., Deswal A., Elliott P., Evanovich L.L., Hung J., Joglar J.A., Kantor P., Kimmelstiel C., Kittleson M., Link M.S., Maron M.S., Martinez M.W., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on practice guidelines. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 5.Rowin E.J., Maron B.J., Haas T.S., Garberich R.F., Wang W., Link M.S., Maron M.S. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69:761–773. doi: 10.1016/j.jacc.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Green J.J., Berger J.S., Kramer C.M., Saleno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–377. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Carbucicchio C., Santamaria M., Trevisi N., Maccabelli G., Giraldi F., Fassini G., Riva S., Moltrasio M., Cireddu M., Veglia F., Della Bella P. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short-and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 8.Hang D., Schaff H.V., Ommen S.R., Dearani J.A., Nishimura R.A. Combined transaortic and transapical approach to septal myectomy in patients with complex hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg. 2018;155:2096–2102. doi: 10.1016/j.jtcvs.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 9.Kunkala M.R., Schaff H.V., Nishimura R.A., Abel M.D., Sorajja P., Dearani J.A., Ommen S.R. Transapical approach to myectomy for midventricular obstruction in hypertrophic cardiomyopathy. Ann Thorac Surg. 2013;96:564–570. doi: 10.1016/j.athoracsur.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 10.Sun D., Schaff H.V., Nishimura R.A., Geske J.B., Dearani J.A., Ommen S.R. Transapical septal myectomy for hypertrophic cardiomyopathy with midventricular obstruction. Ann Thorac Surg. 2021;111:836–844. doi: 10.1016/j.athoracsur.2020.05.182. [DOI] [PubMed] [Google Scholar]