Abstract
The ability of the widespread avian pathogen Mycoplasma gallisepticum to invade cultured human epithelial cells (HeLa-229) and chicken embryo fibroblasts (CEF) was investigated by using the gentamicin invasion assay and a double immunofluorescence microscopic technique for accurate localization of cell-associated mycoplasmas. The presence of intracellular mycoplasmas in both cell lines was clearly demonstrated, with organisms entering the eukaryotic cells within 20 min. Internalized mycoplasmas have the ability to leave the cell, but also to survive within the intracellular space over a 48-h period. Frequencies of invasion were shown to differ between the two cell lines, but were also considerably dependent on the mycoplasma input population. Of the prototype strain R, a low-passage population in artificial medium, Rlow, was capable of active cell invasion, while a high-passage population, Rhigh, showed adherence to but nearly no uptake into HeLa-229 and CEF. By passaging Rlow and Rhigh multiple times through HeLa-229 cells, the invasion frequency was significantly increased. Taken together, these findings demonstrate that M. gallisepticum has the capability of entering nonphagocytic host cells that may provide this pathogen with the opportunity for resisting host defenses and selective antibiotic therapy, establishing chronic infections, and passing through the respiratory mucosal barrier to cause systemic infections.
The genus Mycoplasma, now numbering over 100 species, represents wall-less prokaryotes known to cause chronic diseases in humans and animals. The avian pathogen Mycoplasma gallisepticum induces severe chronic respiratory disease in chickens (18) and sinusitis in turkeys (9, 42), which cause significant economic loss to the poultry industry (29). Like a large number of other pathogenic mycoplasmas, this agent colonizes its host via the mucosal surface of the respiratory tract. One crucial, initial step for the establishment of the disease is the adhesion of M. gallisepticum to its host target cell. Following the colonization of the respiratory tract, M. gallisepticum disease may progress to systemic infection, resulting in salpingitis, arthritis, and passage of the organism through the egg (33, 34). Isolation of the pathogen from the hock of chicken with polyarthritis induced by experimental infection (20) and from diverse body sites of naturally infected birds, such as the urogenital tract, the bile (4), or the brain (7), implies that M. gallisepticum has the ability to translocate across the respiratory mucosal barrier, to enter the bloodstream, and to disseminate throughout the body.
Our current understanding of the virulence factors that may promote M. gallisepticum infection and induce disease is limited. Earlier studies revealed that M. gallisepticum strains differ markedly in their pathogenicity for chickens (22, 23, 30, 34) and that in vitro passages in artificial medium of a particular M. gallisepticum strain affect its virulence (24). More specifically, experimental infection studies with chickens showed that a low-passage population (Rlow) and a high-passage population (Rhigh) of the M. gallisepticum prototype strain R colonize the trachea, while only Rlow induces air sac lesions (22).
In the mid-1960s, studies of the interaction of M. gallisepticum with animal cells indicated that this prokaryote is an extracellular parasite that adheres to the epithelial cell surface by a terminal bleb structure (39, 44). A few years later, reports noting the presence of M. gallisepticum within epithelial cells (5, 37) seemed to conflict with this description. These early observations of intracellular M. gallisepticum were not further pursued until the present study, although several hallmarks associated with M. gallisepticum infections may argue for its intracellular localization: the usual establishment of chronic disease once the bird is infected with M. gallisepticum (36), including the possibility that the infection may be dormant until the bird is stressed (33), as well as the limited effects of antibiotic treatment of infected birds, which usually does not result in the total elimination of infection (36).
In 1989, the discovery of the human mycoplasma species M. penetrans inside eukaryotic cells (25) stimulated interest in determining whether other mycoplasmas were able to invade epithelial cells. Since then, three other human mycoplasmas that colonize the respiratory and/or the genital tract, M. fermentans (35, 38), M. genitalium, and M. pneumoniae (3, 27), were shown to be facultative intracellular organisms. These findings offered a new perspective regarding the strategies employed by these organisms to survive and persist within their complex immunocompetent hosts. For these very simple prokaryotes, invasion may offer access to almost unlimited nutrients for growth and protection against the host immune defense. This phenomenon may also play a crucial role in allowing pathogenic mycoplasmas to reach more favorable niches and cause systemic infections by passing the mucosal barrier.
In light of these new findings and the advantage of recent imaging technologies combining confocal laser scanning microscopy (CLSM) with immunofluorescent techniques, we have reexamined the ability of M. gallisepticum to function as an intracellular organism. One approach that may enable a better understanding of the interaction occurring between M. gallisepticum and its host cell is the use of cultured monolayers of established cell lines, which offers a less complex environment than that of the actual target tissue. In the present study, we used human epithelial cells and chicken embryo fibroblasts (CEF) as a model system to demonstrate that M. gallisepticum strain R may indeed act as a facultative intracellular microorganism, with the virulent low-passage population Rlow and the avirulent high-passage population Rhigh showing differences in their invasion frequency that increase after multiple passages through cultured cells.
MATERIALS AND METHODS
Mycoplasma strains and growth conditions.
The M. gallisepticum laboratory passage populations Rlow and Rhigh used in this study were kindly provided by S. Levisohn, Kimron Veterinary Institute, Bet Dagan, Israel. Rlow and Rhigh correspond to the prototype strain R propagated 10 and 160 times in artificial medium, respectively (24). Prior to infection, mycoplasma cultures were grown at 37°C in modified Hayflick medium (41) containing 20% (vol/vol) heat-inactivated horse serum (Life Technologies, Inc., Rockville, Md.) to mid-exponential phase, as indicated by the metabolic color change of the medium. The number of viable mycoplasmas in a suspension was determined by plating serial dilutions on Hayflick medium containing 1% (wt/vol) agar, followed by incubation at 37°C. After 6 to 8 days, the number of CFU was counted by using an SMZ-U stereomicroscope (Nikon Corp., Tokyo, Japan).
Cell culture.
All cell culture reagents were obtained from Gibco BRL, Life Technologies. The human epithelial-like cell line HeLa-229 (ATCC CCL-2.1) and the CEF (ATCC CRL-1590), both purchased from the American Type Culture Collection (ATCC; Manassas, Va.), were certified to be free of mycoplasmas. Cells were grown in a 5% CO2 atmosphere at 37°C in minimum essential medium (MEM) containing 2 mM l-glutamine and Earl's balanced salts, supplemented with 7.5% (vol/vol) fetal calf serum, 5% (vol/vol) tryptose phosphate broth, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10 mM HEPES buffer. This formulation is designated throughout this report as MEMS. Propagation of the cell lines was performed in cell culture flasks (Iwaki Glass Co., Ltd., Gyoda, Japan). Cell monolayers were detached from cell culture vials by trypsinization as recommended by the ATCC and seeded at 10 to 20% confluency into Lab Tech II chamber slides (Nalge Nunc International, Naderville, Ill.) for confocal microscopy (see below) 24 h prior to mycoplasma infection and at 30 to 40% confluency into 24-well microdilution dishes (Corning Costar Europe, Badhoeverdorp, The Netherlands) for the gentamicin assay (see below) 3 days prior to infection. Cell cultures were regularly shown to be free of mycoplasma contamination by plating the eukaryotic cells on mycoplasma agar medium as described above.
Infection experiments.
Mycoplasma invasion experiments were carried out in 24-well microdilution dishes containing MEMS. Cell monolayers were infected with mid-exponential-phase cultures of mycoplasmas resuspended in MEMS at a multiplicity of infection (MOI) of approximately 20 (i.e., approximately 107 CFU per 5 × 105 eukaryotic cells) and incubated for 5 min to 48 h at 37°C with 5% CO2. Prior to infection, mycoplasma suspensions were forced 15 times through a 23-gauge needle to disrupt mycoplasma aggregates without affecting mycoplasma viability.
Gentamicin invasion assay.
The sensitivity of M. gallisepticum to gentamicin was assayed in 96-well microtiter plates (Corning) by using checkerboard arrays of 10-fold dilutions of the mycoplasma inocula seeded in modified Hayflick medium to reach a final concentration of 10−4 to 10−7 CFU per ml. In the second dimension, twofold dilutions of a gentamicin (Sigma, St. Louis, Mo.) solution were added to the various inocula, with a final concentration of 12.5 to 200 μg/ml. After 3 h of incubation at 37°C, aliquots of cultures were plated on Hayflick agar medium without gentamicin. Plates were incubated at 37°C for 6 days, before the number of CFU was determined. Using this procedure, no survivor was detected in a culture seeded with an initial inoculum of 106 organisms and grown in the presence of 100 μg of gentamicin per ml. To ensure the reliability of the assays described below, a working concentration of 400 μg/ml was further used.
The gentamicin invasion assay, which was performed to determine the internalization of M. gallisepticum by eukaryotic cells, was derived from the procedure reported by Elsinghorst (11) and originally developed by Kihlström (19). Briefly, infected cell monolayers were washed three times at room temperature with phosphate-buffered saline (PBS; 2.7 mM KCl, 1.47 mM KH2PO4, 137 mM NaCl, 8.0 mM Na2HPO4 [pH 7.4]) to remove nonadherent mycoplasmas. The cells were then trypsinized, and extracellular mycoplasmas were killed by incubation of the infected cells in MEMS supplemented with 400 μg of gentamicin per ml for an additional 3-h period at 37°C in a 5% CO2 atmosphere. After gentamicin treatment, the infected cells were collected by centrifugation at 1,000 × g for 10 min and washed with PBS. They were then resuspended in modified Hayflick medium, and appropriate dilutions were plated on agar medium to allow intracellular mycoplasmas to form colonies. The number of CFU was counted and compared to the number of CFU inoculated per well to determine the invasion frequencies. The morphology of the infected cells was assessed at various time points by using a Nikon Diaphot 300 phase-contrast microscope. In addition, the number of dead cells stained with 0.5% (wt/vol) nigrosin solubilized in PBS was determined by using a standard light microscope. The persistence and replication of mycoplasmas within the eukaryotic intracellular space were also assessed by the same procedure, except that after 2 h of infection, the monolayers were incubated for 24 or 48 h in MEMS containing 100 μg of gentamicin per ml in order to prevent the multiplication of mycoplasmas outside the eukaryotic cells. The gentamicin invasion assay was also used to assess whether intracellular mycoplasmas were able to escape from the cell. For this purpose, cell monolayers were infected, and extracellular mycoplasmas were killed with gentamicin after 2 h of infection as described above. Cells were then overlaid with fresh MEMS without the antibiotic, and after 2 additional h of incubation, the number of CFU in the supernatant and in the cell fraction was determined by plating of successive dilutions on agar plates and compared to the number of CFU in the supernatant and in the cell fraction after incubation with medium containing gentamicin, as determined in parallel control experiments. Each experiment was performed in triplicate.
Invasion assay in the presence of eukaryotic cytoskeleton inhibitors.
The role of eukaryotic cytoskeletal components in M. gallisepticum cell invasion was assessed by coincubating the infected cells with microfilament or microtubule inhibitors. Inhibition assays were performed in the presence of the microfilament inhibitor cytochalasin D (5 μg/ml) (Sigma) or the microtubule inhibitor nocodazole (10 μg/ml) (Sigma), as described elsewhere (31). Briefly, cytochalasin D was preincubated with the cells for 30 min at 37°C prior to infection. Nocodazole was preincubated with the cells for 1 h at 4°C and then for an additional 30 min at 37°C prior to infection. In both experiments, inhibitors were present throughout the 2-h infection period, until the medium containing nonadherent mycoplasmas was replaced by fresh MEMS containing 400 μg of gentamicin per ml. Invasion assays were then performed as described above. Possible adverse effects of the inhibitors on the viability of eukaryotic cells were assessed by nigrosin staining as described above. Colony plate counts of mycoplasma suspensions with or without inhibitors demonstrated that the chemicals did not significantly affect M. gallisepticum viability.
Propagation of M. gallisepticum in HeLa-229 cells.
Enrichment of invasive mycoplasmas was achieved by performing successive infection experiments, as described above. Briefly, after 2 h of infection, HeLa-229 cell monolayers were washed with PBS to remove nonadherent mycoplasmas and incubated overnight at 37°C. Viable membrane-bound and intracellular mycoplasmas obtained by plating the infected, trypsinized cells on mycoplasma solid medium were recovered in PBS, diluted 1:100 in MEMS, and subjected to an additional cycle of infection. Mycoplasma populations resulting from 3 or 10 cycles of HeLa cell infection with Rlow (designated as Rlowp3 and Rlowp10, respectively) or from 10 cycles of infection with Rhigh (designated as Rhighp10) were used in further experiments.
Antibodies.
Anti-M. gallisepticum serum was generated by inoculating rabbits subcutaneously with 1010 CFU of M. gallisepticum Rlow suspended in 1 ml of PBS, followed by three monthly injections of an identical suspension. The presence of specific antibodies in the serum, collected 2 weeks after the last injection, was monitored by immunostaining of M. gallisepticum colonies and by Western blot analysis of M. gallisepticum whole-cell extracts. For this purpose, various serum dilutions in PBS containing 1% (wt/vol) bovine serum albumin (PBS-BSA) were used. Rabbit preimmune serum was used as a negative control. For the double immunofluorescence experiments described below, sera were diluted 1:150 in PBS-BSA. Texas red-labeled and fluorescein isothiocyanate (FITC)-labeled goat antibodies to rabbit immunoglobulins (Ig) (Harlan Sera-Lab, Ltd., Loughborough, England) were diluted 1:150 in PBS-BSA for immunostaining.
Double immunofluorescence microscopy technique.
Detection of mycoplasmas within eukaryotic cells was performed by using the double immunofluorescence microscopy procedure described by Heeseman and Laufs (17). In all experiments, four washes were performed with PBS-BSA. Cell lines, propagated overnight into four-well Lab Tech II chamber slides (Nunc), were infected with a mycoplasma suspension as described above and incubated for an additional 2 or 14 h. After washing, the chamber slides were overlaid with 0.3 ml of rabbit anti-M. gallisepticum serum and gently shaken at 20°C for 20 min. The excess antiserum was removed by successive washes, and the cells were covered with 0.3 ml of FITC-labeled antirabbit Ig as a secondary antibody at 20°C for 20 min to stain extracellularly located mycoplasmas. Air-dried cells were then fixed and permeabilized for antibody diffusion by using successively 50, 75, and 96% (vol/vol) ethanol solutions and finally 100% methanol. After air drying at 20°C, the monolayers were again overlaid with anti-M. gallisepticum serum, followed by Texas red-labeled antirabbit Ig as a secondary antibody to stain extracellular and intracellular mycoplasmas. Finally, the chambers were removed, and the cells were rinsed with PBS and mounted under a glass coverslip in 1:1.7 (vol/vol) glycerol-PBS containing 13% (wt/vol) Mowiol (Clariant, Muttenez, Switzerland) and 0.5% (wt/vol) n-propyl gallate (Sigma). The cells were examined by CLSM with a Leica TCN-NT confocal laser scanning microscope (Leica Microsystems Heidelberg, Heidelberg, Germany) with an oil immersion lens (magnification, ×63). Extracellular and intracellular mycoplasmas were examined through the appropriate FITC and Texas red filter sets, respectively.
Statistical analysis.
Invasion frequencies are expressed as the mean ± standard deviation of n independent values. The significance of differences between means of experiments was calculated by Student's t test. Differences with P < 0.05 were considered significant.
RESULTS
Invasion of M. gallisepticum into cultured eukaryotic cells.
To investigate the capability of M. gallisepticum to invade cultured eukaryotic cells, HeLa-229 cell monolayers were infected with a low-passage population of the prototype strain R, Rlow. After 2 h of infection, the infected monolayers were exposed to 400 μg of gentamicin per ml, trypsinized, and directly plated on Hayflick agar medium to allow intracellular mycoplasmas to form colonies. The results of this experiment were expressed as the percentage of CFU obtained after gentamicin treatment relative to the initial inoculum (frequency of invasion) and are summarized in Table 1. The data revealed that approximately 4.17% of the initial inoculum had survived the gentamicin treatment. Plating of the infected cells on agar medium containing 200 μg of gentamicin per ml showed that this value was not due to the selection of gentamicin-resistant mutants, but indicated that M. gallisepticum strain Rlow is capable of invading nonphagocytic cells. Identical sets of experiments were performed with CEF monolayers, and comparison of the invasion frequencies showed that the invasion rate of M. gallisepticum strain Rlow is significantly higher in CEF than in HeLa-229 cells (Table 1; P < 0.05).
TABLE 1.
Frequencies of invasion of cultured HeLa and CEF cells by M. gallisepticum strain R populations
| M. gallisepticum strain R populationa | % Invasionb
|
|
|---|---|---|
| HeLa-229 | CEF | |
| Rlow | 4.17 ± 0.72 | 5.48 ± 1.12 |
| Rlowp3 | 4.98 ± 1.29 | 8.96 ± 1.10 |
| Rlowp10 | 6.02 ± 0.68 | 9.33 ± 0.88 |
| Rhigh | 0.35 ± 0.24 | 1.11 ± 0.34 |
| Rhighp10 | 4.72 ± 1.16 | 5.69 ± 1.52 |
Described in Materials and Methods.
Percentage of mycoplasmas forming colonies after gentamicin treatment relative to the initial inoculum. Values represent the means of at least three independent experiments performed in triplicate ± standard deviations.
There are currently no data available regarding factors influencing cell internalization of mycoplasmas; however, attachment of the mycoplasma to the eukaryotic cell is certainly a prerequisite of the invasion process. Previous reports have shown that M. gallisepticum possesses a battery of genetic systems that generate high-frequency variation in expression of surface components within propagating clonal populations (2, 16, 21, 26, 43). Because some of these variable products are involved in M. gallisepticum-host cell interactions (2, 26) and because the inoculum population used as in this study is not per se a clonal population, Rlow was subjected to three successive cycles of HeLa-229 cell infection as described in Materials and Methods to define whether the invasion process could be improved by preadapting the mycoplasmas to the cell culture environment, thereby enriching or selecting mycoplasma subpopulations presenting a more adhesive or invasive phenotype. The invasion frequency of the resulting passage, designated Rlowp3, was shown to be higher with HeLa-229 cells, as well as with CEF, than the invasion frequency of Rlow (Table 1; P < 0.05). Finally, the frequency of invasion of mycoplasmas recovered after seven additional passages of Rlowp3 in a HeLa-229 cell culture, designated as Rlowp10, was significantly increased in HeLa cells (P < 0.001) but not in CEF (P < 0.5) when compared to Rlowp3 (Table 1).
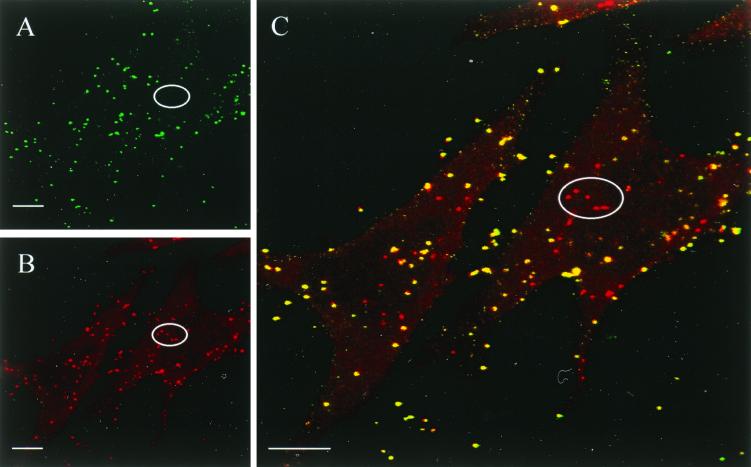
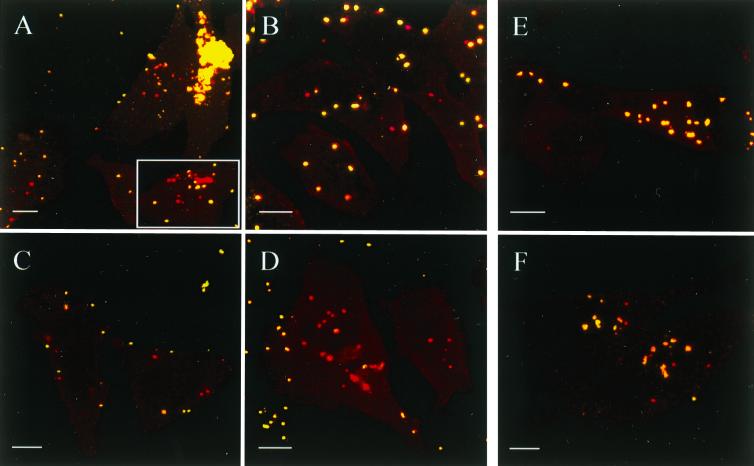
Entry of M. gallisepticum into nonphagocytic cells was confirmed by the double immunofluorescence assay developed by Heesemann and Laufs (17) and modified in the present study as described in Materials and Methods. CLSM analysis of double-immunostained HeLa and CEF monolayers infected with Rlow, Rlowp3, and Rlowp10, respectively, demonstrated the presence of mycoplasmas within the eukaryotic cells and confirmed the results obtained with the gentamicin invasion assay. This is illustrated in Fig. 1, which shows three CLSM micrographs of the same area of a CEF monolayer infected for 14 h with Rlowp3, each image representing the superimposition of four optical sections through the infected cell monolayer. Visualization of extracellular mycoplasmas (green fluorescent flask shapes) was obtained with the FITC filter set (Fig. 1A), while Texas red filtering (Fig. 1B) revealed both extracellular and intracellular mycoplasmas (red fluorescent flask shapes). Finally, Fig. 1C, representing the computer-generated superimposition of the two previous images, clearly indicates the localization of the extracellular mycoplasmas (yellow) and the intracellular mycoplasmas (red) which parasitize the entire cell target. No immunofluorescence was detected when the same experiment was performed with noninfected monolayers (data not shown). In agreement with the results obtained by using the gentamicin invasion assay, the estimation of the number of intracellular immunofluorescent mycoplasmas showed that the frequency of invasion of Rlowp3 was higher in CEF (Fig. 1C) than in HeLa-229 cells (Fig. 2C). Interestingly, confocal micrographs indicated that mycoplasmas in contact with HeLa cells or CEF are mostly isolated when Rlowp3 (Fig. 2B and C) or Rlowp10 (Fig. 2D) was used as inoculum, while Rlow appeared to form aggregates on the cell surface as well as in the intracellular space (Fig. 2A). This may explain the observed differences in calculating the frequency of invasion by using the gentamicin assay. Attempts at reducing the aggregate formation of the original Rlow population by sonication resulted in an increase in the CFU counts but a decrease of the invasion frequencies, suggesting this procedure may affect the invasive capability by stressing the organisms or by altering their surface architecture.
FIG. 1.
Confocal micrographs depicting the interaction of M. gallisepticum strain R with CEF cells. Panels A to C represent the same area of a CEF monolayer infected for 14 h with M. gallisepticum Rlowp3 and immunostained as described in Materials and Methods. (A) FITC fluorescence showing mycoplasmas which are extracellularly located. (B) Texas red fluorescence showing extracellular and intracellular mycoplasmas. (C) Superimposed images of panels A and B indicating the localization of extracellular (yellow) and intracellular (red) mycoplasmas. For illustration, the localization of five intracellular mycoplasmas has been circled. Each micrograph is the result of four focal sections through the monolayer. Bars, 10 μm.
FIG. 2.
Confocal micrographs depicting the interaction of M. gallisepticum strain R with HeLa-229 cells. Panels A to F represent HeLa-229 cell monolayers infected with Rlow (A), Rlowp3 (B and C), Rlowp10 (D), Rhigh (E), and Rhighp10 (F). Cell monolayers were infected for 2 h (B) or 14 h (A, C, D, E, and F) and immunostained as described in Materials and Methods. Each micrograph represents superimposed images sequentially obtained by using the FITC and Texas red filter sets, each image corresponding to four focal sections through the respective cell monolayer. The insert in panel A illustrates the intracellular aggregation of Rlow. Bars, 10 μm.
Time course of M. gallisepticum cell invasion and survival within the cell.
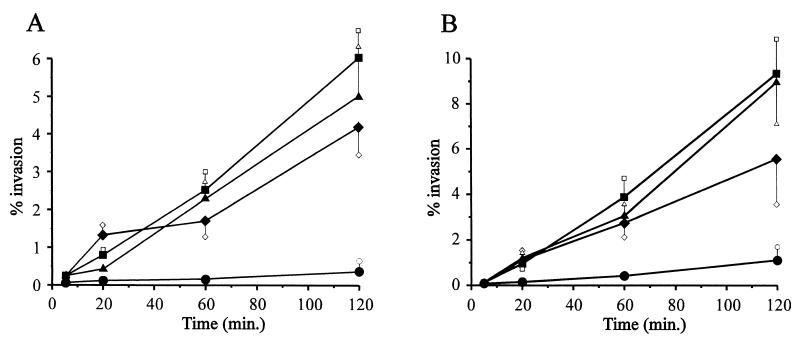
To determine the time required for M. gallisepticum to enter the eukaryotic cell, HeLa-229 (Fig. 3A) and CEF (Fig. 3B) cell monolayers were infected with Rlowp3 and treated with gentamicin at different time points ranging from 5 min to 48 h in order to kill extracellular mycoplasmas. The results indicated that penetration of M. gallisepticum into cultured cells occurred as early as 5 min after infection and that the number of intracellular mycoplasmas increased rapidly within the first 2 h.
FIG. 3.
Time course of M. gallisepticum invasion into cultured HeLa-229 (A) or CEF (B) cells. Gentamicin invasion assays were performed as described in Materials and Methods with HeLa or CEF monolayers infected with Rlow (⧫), Rlowp3 (▴), Rlowp10 (■), and Rhigh (●). Each value represents the mean ± standard deviation of a minimum of three independent experiments performed in triplicate.
Intracellular persistence of M. gallisepticum cells was examined by incubating Rlowp3-infected HeLa-229 monolayers for 48 h in the presence of gentamicin to prevent extracellular growth of mycoplasmas (data not shown). The percentage of intracellular mycoplasmas after 24 h (6.8%) was higher than that after 2 h (5.6%) and then decreased within the following 24 h to 3.9%. The morphology and viability of infected cells were examined by light microscopy and staining of dead cells, indicating that infection with M. gallisepticum at an MOI of approximately 100 did not affect the HeLa-229 monolayer, but induced the progressive vacuolation of CEF 8 h after infection, resulting in 100% lethality 48 h after infection. Survival of the internalized mycoplasmas past 48 h could not be assessed because the infected HeLa cells started detaching after this time period.
The decrease in internalized organisms after 24 h raised the question of whether the mycoplasmas are dying intracellularly or whether they are killed after leaving the cells by the gentamicin in the medium. To further assess the capability of the internalized mycoplasmas to leave the cell, the presence of organisms in the supernatant of infected HeLa cell monolayers following gentamicin treatment was determined as described in Materials and Methods. The results showed that 37% ± 6.4% of Rlowp3 organisms internalized 2 h after inoculation had escaped from the cells within the following 2 h, with 86% of the escaped mycoplasmas associated with the cell membrane.
Role of eukaryotic cytoskeletal components in M. gallisepticum cell invasion.
The role of host cytoskeletal components in the invasion process was examined by performing the gentamicin invasion assay in the presence of the microfilament inhibitor cytochalasin D and the microtubule inhibitor nocodazole, respectively. Reproducible results indicated that the invasion ability of Rlowp3 in the presence of nocodazol was reduced by 72%, whereas cytochalasin D had no significant inhibitory effect on the invasion process. Neither of the inhibitors affected the viability of the eukaryotic cells nor reduced the viability of mycoplasmas more than 26%.
Variability of M. gallisepticum invasion frequencies.
As shown by immunofluorescent staining of HeLa-229 cell monolayers infected with Rlowp10 (Fig. 2D), most of the mycoplasmas in contact with the cells are located intracellularly (red fluorescence), with the intracellular organisms parasitizing the entire cytoplasmic space. In contrast, no intracellular mycoplasma could be detected when the same experiment was performed with the Rhigh population (Fig. 2E). Similarly, the frequency of invasion of M. gallisepticum Rhigh as assessed by the gentamicin assay was shown to be extremely low compared to that of Rlow, Rlowp3, or Rlowp10 (Table 1). This was observed independently of the cell line, although the number of mycoplasmas surviving the gentamicin treatment was higher in CEF (1.11%) than in HeLa-229 cells (0.35%) (P < 0.01). From these data, it can be concluded that the inability of Rhigh to efficiently invade eukaryotic cells resulted from its successive passaging in artificial media (i) by complete loss of genetic information necessary for the invasion process and/or (ii) by selection of populations that have switched on and/or off variable components directly or indirectly involved in the invasion process. To further assess whether minor subpopulations presenting invasive capabilities could be selected, Rhigh was propagated 10 times through HeLa-229 monolayers as described in Materials and Methods, and the resulting population, Rhighp10, was examined for its cell invasiveness by using the gentamicin assay. The results showed (i) that the invasion frequency of Rhighp10 in HeLa-229 cells (4.72%) or in CEF (5.69%) was significantly higher (P < 0.001) than that of Rhigh (0.35% and 1.11%, respectively) and (ii) that Rlowp10 and Rhighp10 had similar levels of gentamicin survival (Table 1). Invasion of HeLa-229 cells by Rhighp10 was further confirmed by the double immunofluorescence assay (Fig. 2F), in which red fluorescent flask-shaped organisms corresponding to internalized mycoplasmas were observed by CLSM. In contrast to infection with Rlow, infection of cell monolayers with Rhigh did not result in any mycoplasma aggregates inside or outside the eukaryotic cells. Further experiments revealed that (i) 5.8% of the Rhighp10 inoculum survived intracellularly after 24 h, while no intracellular organism was detected after 48 h, and (ii) 15% of the mycoplasmas internalized 2 h after inoculation had the ability to leave the cell within the following 2 h.
DISCUSSION
The data presented in this report provide evidence that the ability of mycoplasmas to enter nonphagocytic cells is not restricted to the human mycoplasmas M. penetrans (25), M. fermentans (35, 38), M. pneumoniae (3), and M. genitalium (27), because it is also shared by the avian mycoplasma species M. gallisepticum. In this study, the presence of M. gallisepticum organisms within HeLa-229 or CEF cells was clearly demonstrated by two different approaches: (i) the gentamicin assay, which provides a semiquantitative method for comparison of invasion frequencies among different mycoplasma populations; and (ii) the double immunofluorescence labeling technique combined with CLSM, which offers a simple and accurate differentiation between intracellular and extracellular mycoplasmas. In addition, the utilization of CLSM revealed that intracellular M. gallisepticum cells parasitize the entire target cell from close to the cell membrane to the perinuclear regions. A similar observation was reported for internalized M. penetrans in WI-38 human lung cells (3).
Interestingly, the invasive capability of M. gallisepticum was altered by serial multiplication of the original strain R in artificial medium: while the high-passage population, Rhigh, although adhering to HeLa-229 and CEF cells, does not show a significant invasion potential, the low-passage population, Rlow, is capable of both adhesion and invasion. Even though the factors distinguishing Rlow from Rhigh have yet to be identified, the difference in internalization between the two mycoplasma populations suggests that mycoplasma entry into eukaryotic cells may be a phenomenon clearly distinct from attachment. This is in agreement with the previous report of Baseman et al. on the isolation of M. pneumoniae mutants that have retained their cytoadherent property, but are deficient in invasion (3).
Whether the increase in the percentage of organisms presenting an invasive phenotype after passage of Rlow through cultured HeLa-229 cells is due to the loss of mycoplasma autoaggregation (as seen in Rlow) rather than to a selection of the “invasive phenotype” remains to be elucidated. Since Rhigh does not aggregate, the presence of mycoplasmas presenting an “invasive phenotype” within the total Rhigh population after passage through HeLa-229 cell monolayers raised the question of to what extent the invasion process is linked to the existence of multiple genetic systems spontaneously generating phenotypic variants in M. gallisepticum (2, 26, 43). In an attempt to address this question, a clonal population derived from Rhigh that was randomly selected and shown to be noninvasive was passaged 10 times through cell culture. Since this procedure did not result in the selection of an invasive population (data not shown), it is more likely that the original Rhigh population was composed of phenotypically mixed organisms, most of which have lost their capacity for cell invasion. Comparison by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the protein profiles of Rlow, Rlowp3, Rlowp10, Rhigh, Rhighp3, and Rhighp10 revealed variation in several components, none of which directly correlated with the variation in invasion frequency (A. Lugmair, F. Winner, R. Rosengarten, and C. Citti, unpublished data). Considering that Neisseria meningitidis serogroup B undergoes concurrent phase switching of multiple surface components in order to invade nasopharyngeal epithelial cells (10), a similar role of such variable surface components of M. gallisepticum in the invasion process cannot be ruled out, in particular because it is very likely that certain variable components provide the organism with an enhanced ability to adhere to the eukaryotic cell (2).
Several invasive bacteria were shown upon contact with the host cell surface to trigger cytoskeletal rearrangements that result in bacterial internalization via microtubule- and/or microfilament-based mechanisms (8, 13–15, 28, 32). In a recent paper by Borovsky et al., it was demonstrated that both microfilaments and microtubules play a crucial role in M. penetrans invasion of HeLa cells (6). In our study, the microfilament inhibitor cytochalsin D did not significantly decrease the frequency of invasion of M. gallisepticum Rlowp10 into HeLa cells, while the microtubule inhibitor nocodazole reduced the cell entry, suggesting that M. gallisepticum may use a different strategy from that of M. penetrans to reach the intracellular space.
One remarkable finding of the present study is the ability of the internalized mycoplasmas to survive within the eukaryotic cell. Indeed, M. gallisepticum remains viable during extended periods of intracellular residence, although a reduction in gentamicin survivors occurred between 24 and 48 h postinfection. Persistence of the human pathogen M. penetrans within WI-38 human lung cells has also been observed over a 24-h period, with a decrease in the number of internalized organisms by 90% between 24 and 48 h postinfection (3). Even though digestion of internalized M. gallisepticum during intracellular residence cannot be ruled out, the capacity of internalized organisms to leave the cell may account for the decrease in intracellular organisms observed between 24 and 48 h in the presence of gentamicin. In the course of our study, the presence of internalized fluorescent structures representing two connected M. gallisepticum cell bodies with their opposed terminal bleb structures directed outwards was observed several times (data not shown). Whether this observation reflects dividing or aggregating organisms cannot be defined at this point. However, the increase in the number of internalized mycoplasmas during incubation of HeLa cells with Rlowp3 in the presence of gentamicin over a 24-h period indicates that M. gallisepticum may indeed replicate intracellularly.
Although the ability of internalized M. gallisepticum to multiply within the host cell remains to be convincingly demonstrated, the results presented in this study offer new insights into the potential virulence strategies employed by this avian pathogen. Invasion of nonphagocytic host cells may provide M. gallisepticum with the ability to cross the respiratory mucosal barrier and gain access to the bloodstream, but may also allow this pathogen to avoid host protective mechanisms as well as selective antibiotic treatment. Even though virulence is the result of multiple events that involve both the pathogen and its host, the incapacity of the avirulent Rhigh population to enter HeLa-229 or CEF cells is one argument to support the hypothesis presented above. Several other successful bacterial pathogens have been shown to colonize their hosts by translocating across the respiratory epithelium after invading the epithelial cells. One example is Bordetella avium, which translocates across the epithelium overlying the bronchus-associated lymphoid tissue of turkeys via cell invasion (12). Another example is Escherichia coli 078, which crosses the air-blood barrier of turkeys by vacuole transportation through the cells (1), while Haemophilus influenzae was shown in in vitro studies to cross-polarized human epithelial cell layers by entering the intercellular space (40). Whether M. gallisepticum host cell invasion also occurs in vivo and whether this capability provides the organism indeed with a means to translocate across the respiratory epithelium and successfully colonize its host remain to be elucidated. Nevertheless, the finding that M. gallisepticum is capable of both entering and escaping eukaryotic cells along with the reported isolation of M. gallisepticum from multiple body sites (4, 7) supports the speculation that the systemic dissemination of this particular pathogen from the respiratory tract may in fact be associated with its translocation across various host cell layers via cell invasion.
ACKNOWLEDGMENTS
This work was supported in part by grant P13215-GEN (C.C. and R.R.) from the Fonds zur Förderung der wissenschaftlichen Forschung.
We thank Sharon Levisohn for providing M. gallisepticum strains, Peter Böck and Ingrid Walter for using the confocal laser scanning microscope, Andreas Hensel for the immunization of rabbits, and Karin Siebert-Gulle for excellent technical assistance.
REFERENCES
- 1.Ackermann M R, Cheville N F. Ultrastructural studies of the lung of turkeys (Meleagris gallopavo) inoculated intratracheally with Escherichia coli. Vet Pathol. 1991;28:183–191. doi: 10.1177/030098589102800301. [DOI] [PubMed] [Google Scholar]
- 2.Athamna A, Rosengarten R, Levisohn S, Kahane I, Yogev D. Adherence of Mycoplasma gallisepticum involves variable surface membrane proteins. Infect Immun. 1997;65:2468–2471. doi: 10.1128/iai.65.6.2468-2471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baseman J B, Lange M, Criscimagna N L, Giron J A, Thomas C A. Interplay between mycoplasmas and host target cells. Microb Pathog. 1995;19:105–116. doi: 10.1006/mpat.1995.0050. [DOI] [PubMed] [Google Scholar]
- 4.Bencina D, Dorrer D. Demonstration of Mycoplasma gallisepticum in tracheas of healthy carrier chickens by fluorescent-antibody procedure and the significance of certain serologic tests in estimating antibody response. Avian Dis. 1984;28:574–578. [PubMed] [Google Scholar]
- 5.Boam G W, Sanger V L. Electron microscope studies of turkey sinus epithelial cells infected with Mycoplasma gallisepticum. Avian Dis. 1970;14:503–513. [PubMed] [Google Scholar]
- 6.Borovsky Z, Tarshis M, Zhang P, Rottem S. Protein kinase C activation and vacuolation in HeLa cells invaded by Mycoplasma penetrans. J Med Microbiol. 1998;47:915–922. doi: 10.1099/00222615-47-10-915. [DOI] [PubMed] [Google Scholar]
- 7.Chin R P, Daft B M, Meteyer C U, Yamamoto R. Meningoencephalitis in commercial meat turkeys associated with Mycoplasma gallisepticum. Avian Dis. 1991;35:986–993. [PubMed] [Google Scholar]
- 8.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson W R, Nettles V F, Couvillion C E, Yoder H W., Jr Infectious sinusitis in wild turkeys. Avian Dis. 1982;26:402–405. [PubMed] [Google Scholar]
- 10.de Vries F P, van der Ende A, van Putten J P M, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 12.Fagerland J A, Myers R K, Arp L H. Uptake of ferritin and Bordetella avium in bronchus-associated lymphoid tissue of turkeys. Vet Immunol Immunopathol. 1994;40:367–377. doi: 10.1016/0165-2427(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 13.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;80:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 16.Gorton T S, Geary S J. Antibody-mediated selection of a Mycoplasma gallisepticum phenotype expressing variable proteins. FEMS Microbiol Lett. 1997;155:31–38. doi: 10.1111/j.1574-6968.1997.tb12682.x. [DOI] [PubMed] [Google Scholar]
- 17.Heesemann J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan F T W. Avian mycoplasmas. In: Tully J G, Whitcomb R F, editors. The mycoplasmas. II. Human and animal mycoplasmas. New York, N.Y: Academic Press; 1979. pp. 1–48. [Google Scholar]
- 19.Kihlström E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977;17:290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamas da Silva J M, Adler H E. Pathogenesis of arthritis induced in chickens by Mycoplasma gallisepticum. Pathol Vet. 1969;6:385–395. doi: 10.1177/030098586900600502. [DOI] [PubMed] [Google Scholar]
- 21.Levisohn S, Rosengarten R, Yogev D. In vivo variation of Mycoplasma gallisepticum antigen expression in experimentally infected chickens. Vet Microbiol. 1995;45:219–231. doi: 10.1016/0378-1135(95)00039-d. [DOI] [PubMed] [Google Scholar]
- 22.Levisohn S, Dykstra M J, Lin M Y, Kleven S H. Comparison of in vivo and in vitro methods for pathogenicity evaluation for Mycoplasma gallisepticum in respiratory infection. Avian Pathol. 1986;15:233–246. doi: 10.1080/03079458608436284. [DOI] [PubMed] [Google Scholar]
- 23.Levisohn S, Glisson J R, Kleven S H. In ovo pathogenicity of Mycoplasma gallisepticum strains in the presence and absence of maternal antibody. Avian Dis. 1985;29:188–197. [PubMed] [Google Scholar]
- 24.Lin M Y, Kleven S H. Evaluation of attenuated strains of Mycoplasma gallisepticum as vaccines in young chickens. Avian Dis. 1984;28:88–99. [PubMed] [Google Scholar]
- 25.Lo S C, Dawson M S, Wong D M, Newton III P B, Sonoda M A, Engler W F, Wang R Y, Shih J W, Alter H J, Wear D J. Identification of Mycoplasma incognitus infection in patients with AIDS: an immunohistochemical, in situ hybridization and ultrastructural study. Am J Trop Med Hyg. 1989;41:601–616. doi: 10.4269/ajtmh.1989.41.601. [DOI] [PubMed] [Google Scholar]
- 26.Markham P F, Glew M D, Brandon M R, Walker I D, Whithear K G. Characterization of a major hemagglutinin protein from Mycoplasma gallisepticum. Infect Immun. 1992;60:3885–3891. doi: 10.1128/iai.60.9.3885-3891.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mernaugh G R, Dallo S F, Holt S C, Baseman J B. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin Infect Dis. 1993;17(Suppl. 1):69–78. doi: 10.1093/clinids/17.supplement_1.s69. [DOI] [PubMed] [Google Scholar]
- 28.Meyer D H, Lippmann J E, Fives-Taylor P M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed H O, Carpenter T E, Yamamoto R. Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Dis. 1987;31:477–482. [PubMed] [Google Scholar]
- 30.Power J, Jordan F T W. A comparison of the virulence of three strains of Mycoplasma gallisepticum and one strain of Mycoplasma gallinarum in chicks, turkey poults, tracheal organ cultures and embryonated fowl eggs. Res Vet Sci. 1976;21:41–46. [PubMed] [Google Scholar]
- 31.Rosenshine I, Ruschkowski S, Finlay B B. Inhibitors of cytoskeletal function and signal transduction to study bacterial invasion. Methods Enzymol. 1994;236:467–476. doi: 10.1016/0076-6879(94)36035-9. [DOI] [PubMed] [Google Scholar]
- 32.Schramm N, Wyrick P B. Cytoskeletal requirements in Chlamydia trachomatis infection of host cells. Infect Immun. 1995;63:324–332. doi: 10.1128/iai.63.1.324-332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtländer C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–415. [Google Scholar]
- 34.Soeripto K, Whithear G, Cottew G S, Harrigan K E. Virulence and transmissibility of Mycoplasma gallisepticum. Aust Vet J. 1989;66:65–72. doi: 10.1111/j.1751-0813.1989.tb09746.x. [DOI] [PubMed] [Google Scholar]
- 35.Stadtländer C T, Watson H L, Simecka J W, Cassell G H. Cytopathogenicity of Mycoplasma fermentans (including strain incognitus) Clin Infect Dis. 1993;17(Suppl. 1):289–301. doi: 10.1093/clinids/17.supplement_1.s289. [DOI] [PubMed] [Google Scholar]
- 36.Stipkovits L, Kempf I. Mycoplasmoses in poultry. Rev Sci Tech Off Int Epizoot. 1996;15:1495–1525. doi: 10.20506/rst.15.4.986. [DOI] [PubMed] [Google Scholar]
- 37.Tajima M, Nunoya T, Yagihashi T. An ultrastructural study on the interaction of Mycoplasma gallisepticum with the chicken tracheal epithelium. Am J Vet Res. 1979;40:1009–1014. [PubMed] [Google Scholar]
- 38.Taylor-Robinson D, Davies H A, Sarathchandra P, Furr P M. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int J Exp Pathol. 1991;72:705–714. [PMC free article] [PubMed] [Google Scholar]
- 39.Uppal P K, Chu H P. Attachment of Mycoplasma gallisepticum to the tracheal epithelium of fowls. Res Vet Sci. 1977;22:259–260. [PubMed] [Google Scholar]
- 40.van Schilfgaarde M, van Alphen L, Eijk P, Everts V, Dankert J. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect Immun. 1995;63:4729–4737. doi: 10.1128/iai.63.12.4729-4737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise K S, Watson R K. Mycoplasma hyorhinis GLD surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983;41:1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoder H W., Jr . Mycoplasma gallisepticum infection. In: Calnek B W, Beard C W, Barnes H J, Reid W M, Yoder H W Jr, editors. Diseases of poultry. Ames, Iowa: Iowa State University Press; 1991. pp. 198–212. [Google Scholar]
- 43.Yogev D, Menaker D, Strutzberg K, Levisohn S, Kirchhoff H, Hinz K-H, Rosengarten R. A surface epitope undergoing high-frequency phase variation is shared by Mycoplasma gallisepticum and Mycoplasma bovis. Infect Immun. 1994;62:4962–4968. doi: 10.1128/iai.62.11.4962-4968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucker Franklin D, Davidson M, Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966;124:521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]