Abstract
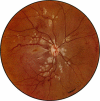
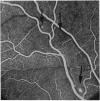
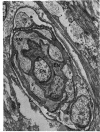
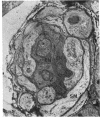
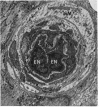
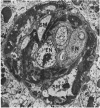
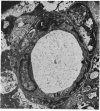
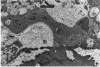
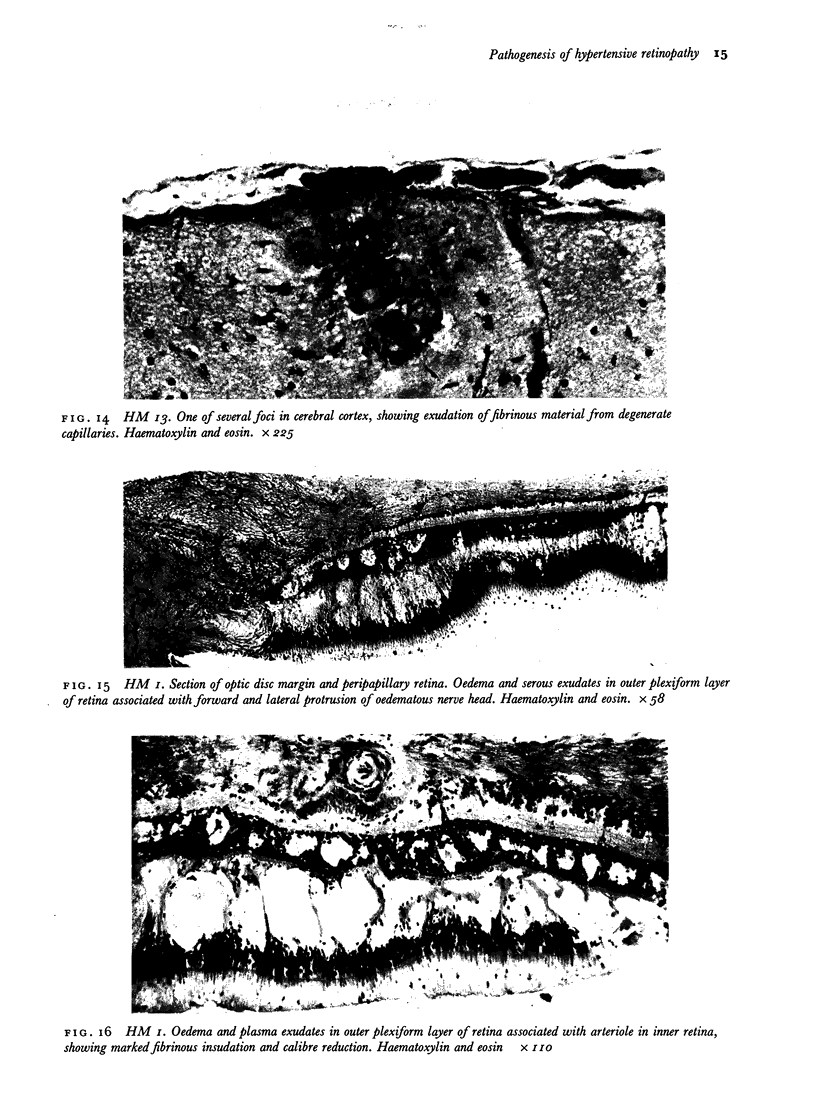
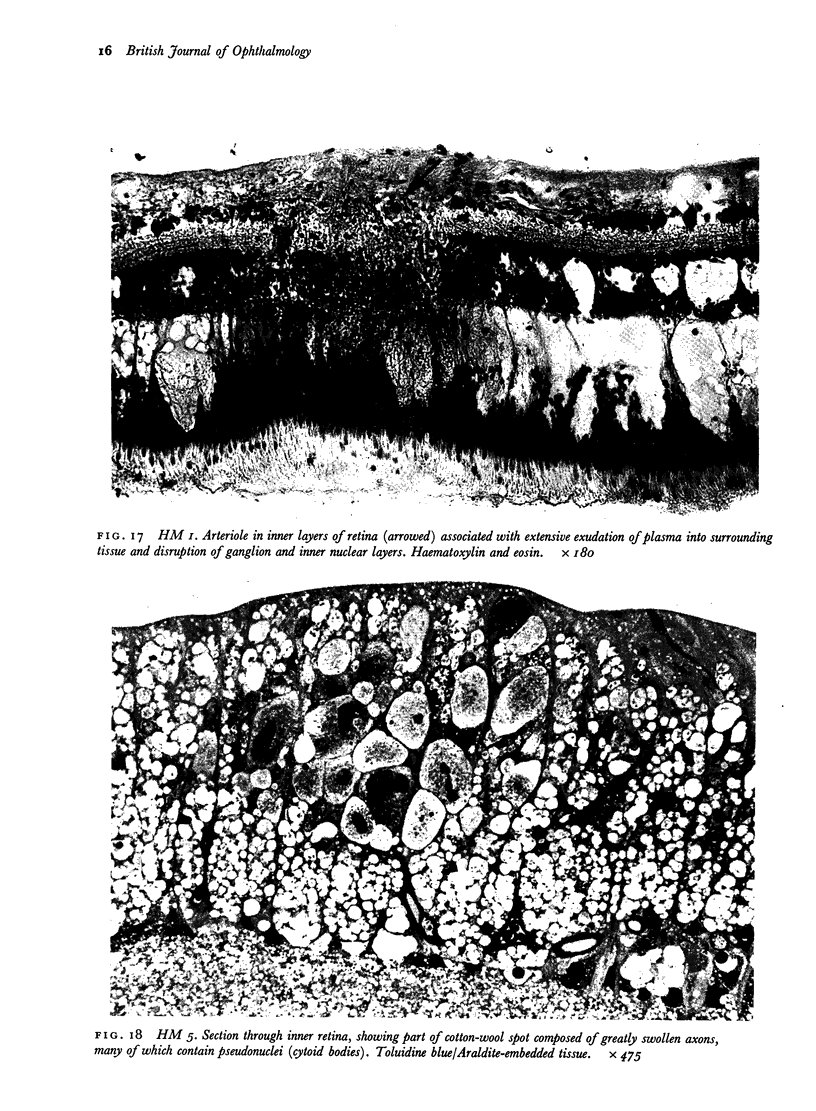
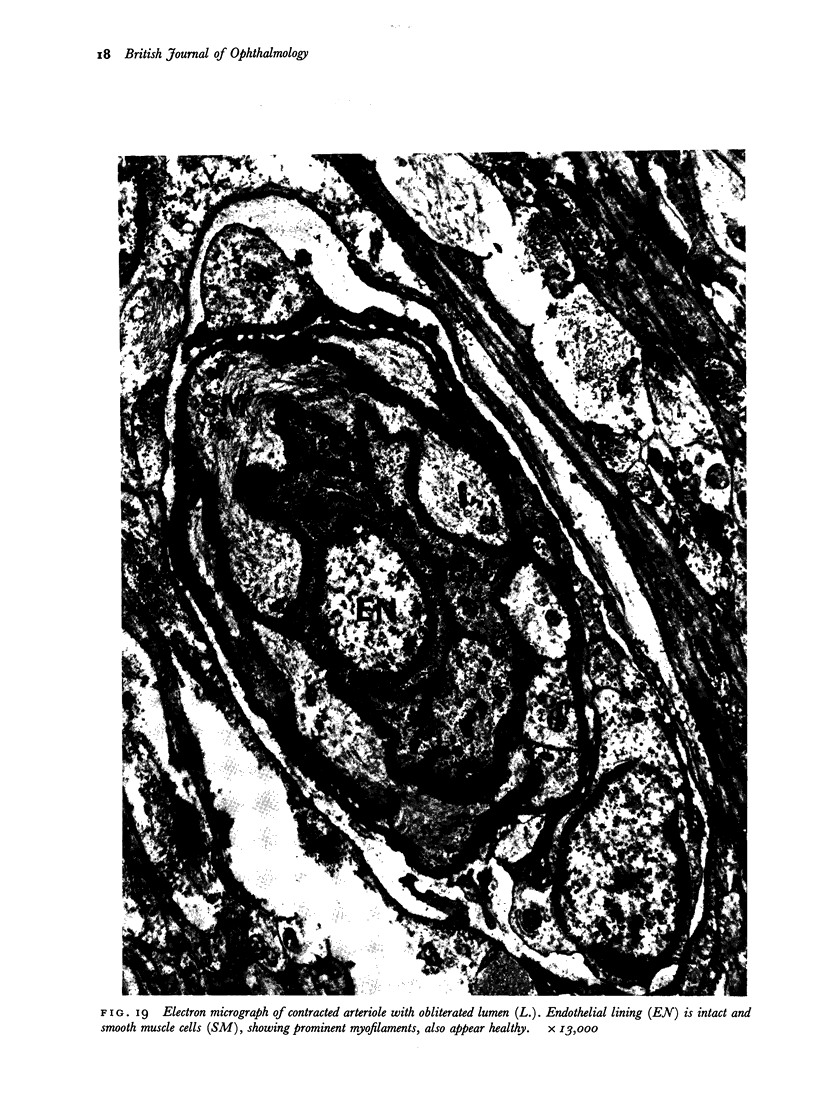
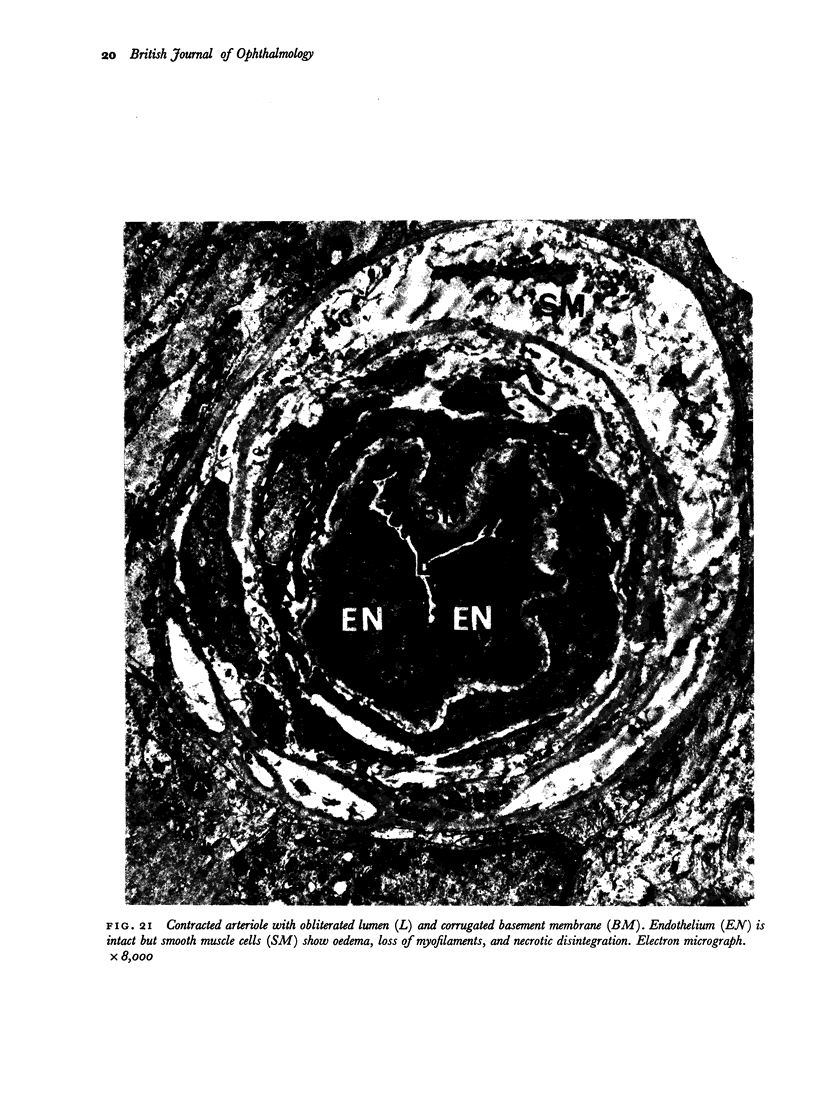
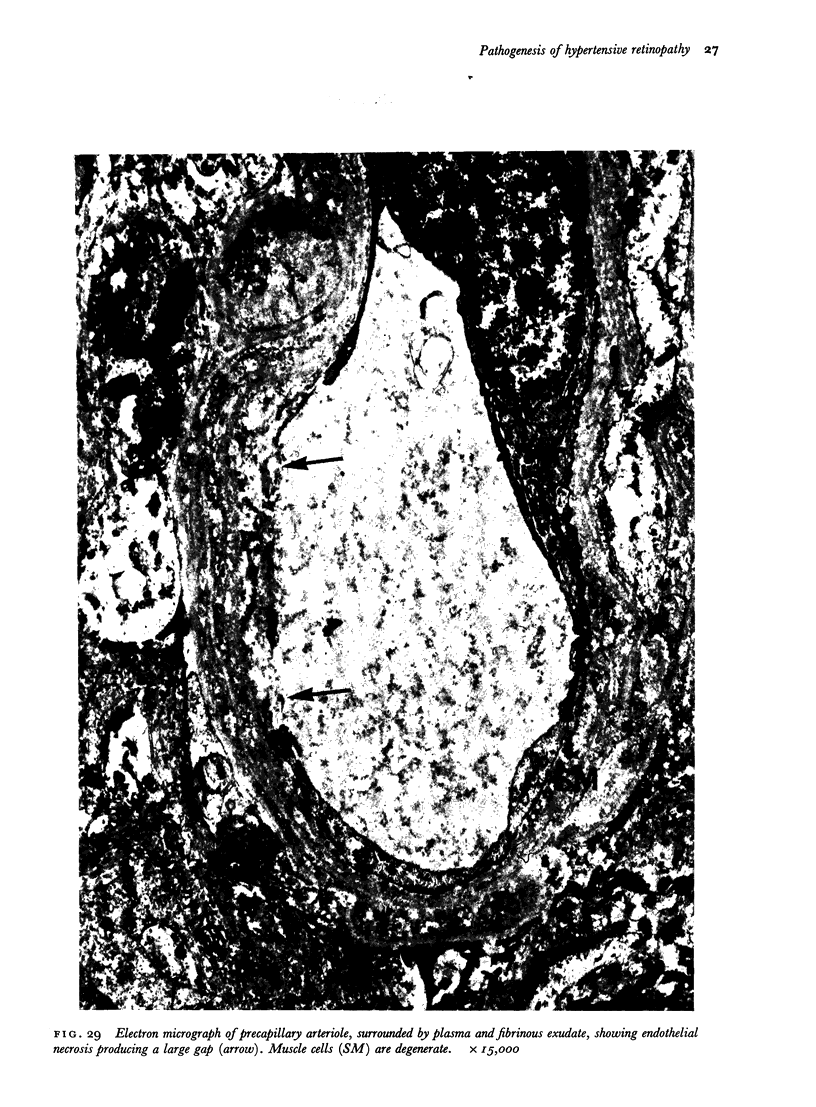
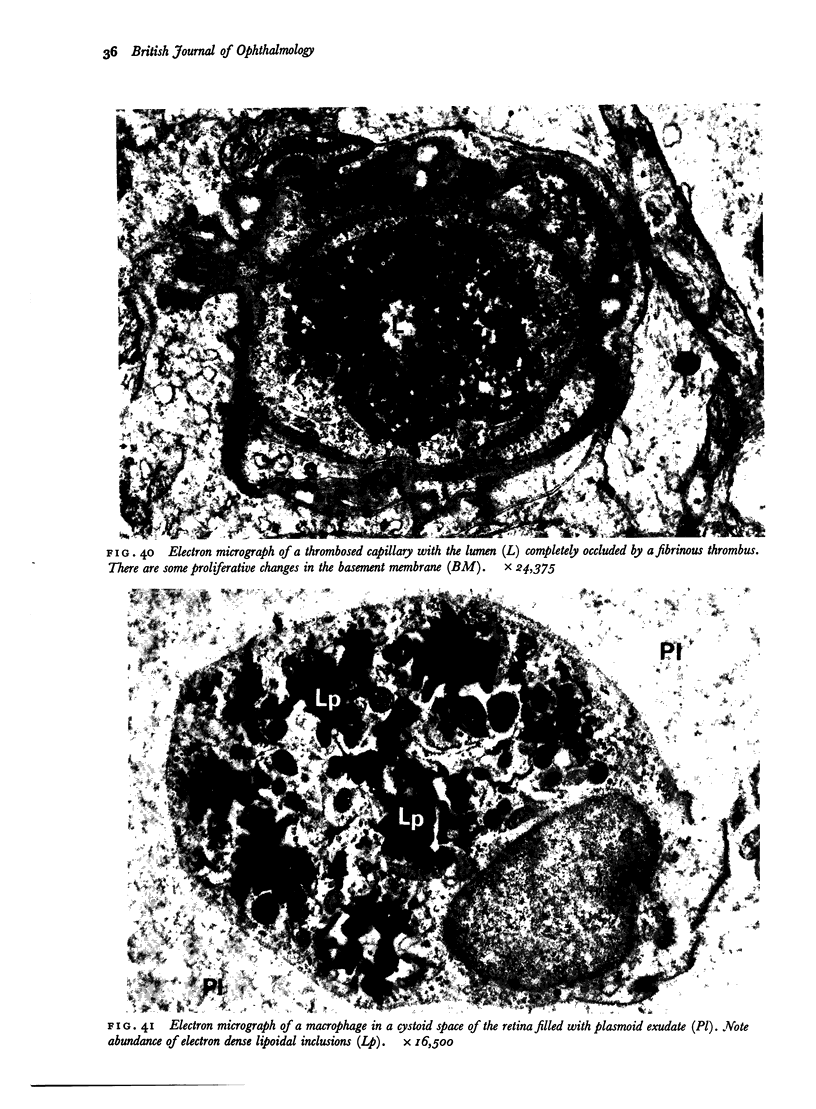
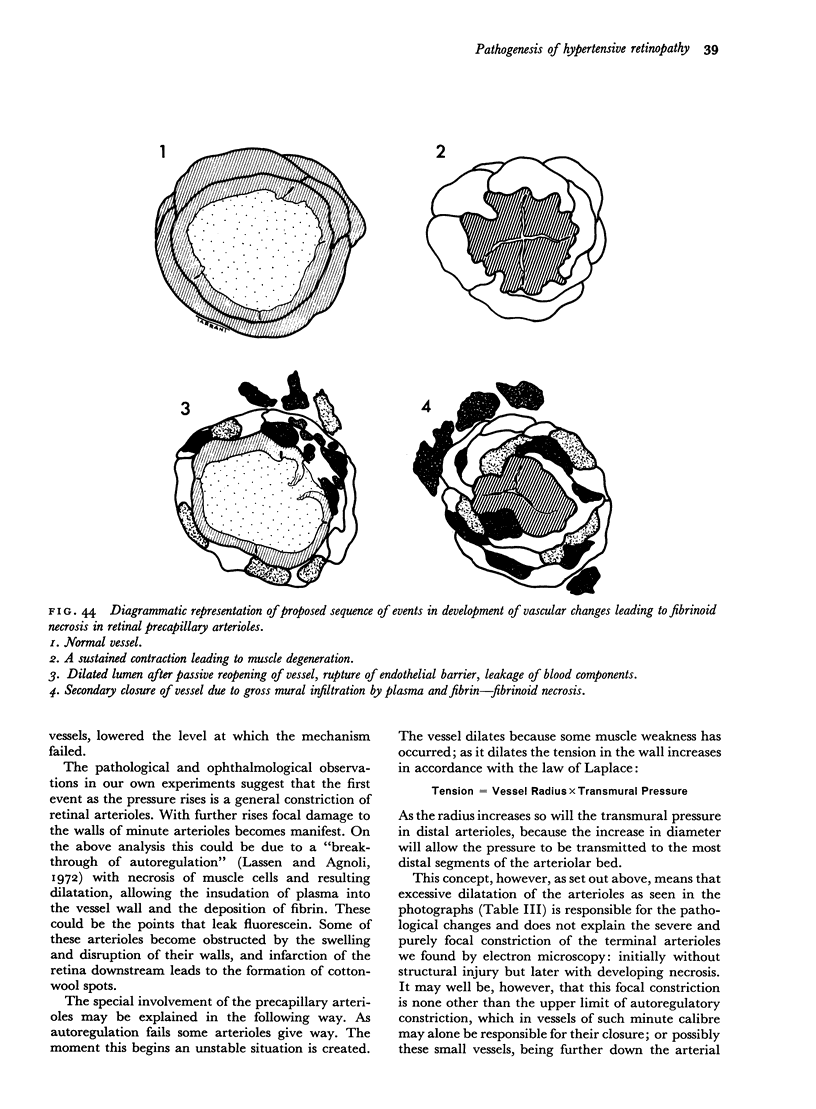
Retinal changes in accelerated hypertension were studied in seventeen monkeys with experimental hypertension by means of ophthalmoscopy and colour and flourescence photography during life, and by injection and digest preparations and light and electron microscopy after the animals had been killed. Cotton-wool spots developed in all but three monkeys. The arteries became tortuous and dilated and the light reflex decreased in those animals that became hypertensive. The earliest abnormality was a development of many points of fluorescein leakage on terminal arterioles or small arteries. Such leaking points were always present in relation to cotton-wool spots but were not confined to such areas. Focal narrowing of arteries was not observed but arteriolar occlusion and retrograde filling of the distal segment was present in three animals. Superficial linear haemorrhages were noted in five animals. Light microscopy revealed cotton-wool spots which were identical to those observed in man with a collection of swollen axons containing densely staining pseudonuclei. Study of the arterioles by electron microscopy showed findings ranging from normality to extensive necrosis. Many precapillary arteries were constricted and some were virtually occluded. Degenerative changes were present in smooth muscle cells in the wall of many of the constricted arterioles. Many arteries also showed insudation into their wall of plasma which had seeped into the muscular coat displacing and sometimes entirely replacing the smooth muscle cells. Except for arterioles with advanced necrosis, there was no indication of how plasma insudation occurred. Two arterioles with extensive necrosis showed a break within the endothelial cell cytoplasm through which penetration of plasma proteins had probably occurred. The extravascular tissues showed collections of amorphous material, sone of it with the typical banded configuration of fibrin. The sequence of events proposed to explain these features is as follows: (1) The arterioles constrict as the pressure rises, most likely as a result of vascular autoregulation. This may head to occlusion of the precapillary arterioles and is associated with necrosis of vascular smooth muscle. (2) Dilatation then occurs with insudation of plasma into the unsupported wall through a damaged endothelium. This stage probably corresponds to the autoregulatory break-point and is evidenced clinically by focal leakage of fluorescein. (3) Progressive plasma insudation into the vessel wall with further muscle necrosis results in secondary occlusion and the typical picture of advanced fibrinoid necrosis.
Full text
PDF































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABT K., BRUCKNER R. Netzhautgefässspasmen bei artifiziell hypertonischen Ratten. Ophthalmologica. 1950 Jan;119(1):17-43, pl. doi: 10.1159/000300803. [DOI] [PubMed] [Google Scholar]
- ASHTON N. Diabetic retinopathy: a new approach. Lancet. 1959 Oct 24;2(7104):625–630. doi: 10.1016/s0140-6736(59)91407-2. [DOI] [PubMed] [Google Scholar]
- ASSCHER A. W., ANSON S. G. A vascular permeability factor of renal origin. Nature. 1963 Jun 15;198:1097–1098. doi: 10.1038/1981097a0. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Koletsky S. Arteriosclerosis of the mesenteric arteries of rats with renal hypertension. Electron microscopic observations. Am J Pathol. 1970 Dec;61(3):293–322. [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- Ashton N., Coomes E. N., Garner A., Oliver D. O. Retinopathy due to progressive systemic sclerosis. J Pathol Bacteriol. 1968 Oct;96(2):259–268. doi: 10.1002/path.1700960202. [DOI] [PubMed] [Google Scholar]
- Becker C. G., Murphy G. E. Demonstration of contractile protein in endothelium and cells of the heart valves, endocardium, intima, arteriosclerotic plaques, and Aschoff bodies of rheumatic heart disease. Am J Pathol. 1969 Apr;55(1):1–37. [PMC free article] [PubMed] [Google Scholar]
- Breckenridge A., Dollery C. T., Parry E. H. Prognosis of treated hypertension. Changes in life expectancy and causes of death between 1952 and 1967. Q J Med. 1970 Jul;39(155):411–429. [PubMed] [Google Scholar]
- Bulpitt C. J., Dollery C. T., Kohner E. M. The marginal plasma zone in the retinal microcirculation. Cardiovasc Res. 1970 Apr;4(2):207–212. doi: 10.1093/cvr/4.2.207. [DOI] [PubMed] [Google Scholar]
- Chiang J., Kowada M., Ames A., 3rd, Wright R. L., Majno G. Cerebral ischemia. III. Vascular changes. Am J Pathol. 1968 Feb;52(2):455–476. [PMC free article] [PubMed] [Google Scholar]
- Cunha-Vaz J. G., Shakib M. Ultrastructural mechanisms of breakdown of the blood-retina barrier. J Pathol Bacteriol. 1967 Apr;93(2):645–652. doi: 10.1002/path.1700930225. [DOI] [PubMed] [Google Scholar]
- DUNCAN L. E., Jr, CORNFIELD J., BUCK K. The effect of blood pressure on the passage of labeled plasma albumin into canine aortic wall. J Clin Invest. 1962 Jul;41:1537–1545. doi: 10.1172/JCI104610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollery C. T., Henkind P., Paterson J. W., Ramalho P. S., Hill D. W. I. Ophthalmoscopic and circulatory changes in focal retinal ischaemia. Br J Ophthalmol. 1966 Jun;50(6):285–324. doi: 10.1136/bjo.50.6.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGERMAN R. L., MEYER R. K., BUESSELER J. A. EFFECTS OF ALLOXAN DIABETES AND STEROID HYPERTENSION ON RETINAL VASCULATURE. Am J Ophthalmol. 1964 Dec;58:965–978. [PubMed] [Google Scholar]
- FENNELL R. H., Jr, REDDY C. R., VAZQUEZ J. J. Progressive systemic sclerosis and malignant hypertension. Immunohistochemical study of renal lesions. Arch Pathol. 1961 Aug;72:209–215. [PubMed] [Google Scholar]
- Fisher E. R., Perez-Stable E., Pardo V. Ultrastructural studies in hypertension. I. Comparison of renal vascular and juxtaglomerular cell alterations in essential and renal hypertension in man. Lab Invest. 1966 Sep;15(9):1409–1433. [PubMed] [Google Scholar]
- GAY A. J., GOLDOR H., SMITH M. CHORIORETINAL VASCULAR OCCLUSIONS WITH LATEX SPHERES. Invest Ophthalmol. 1964 Dec;3:647–656. [PubMed] [Google Scholar]
- GIESE J. Acute vascular disease caused by severe renal ischaemia. Acta Pathol Microbiol Scand. 1962;56:399–408. doi: 10.1111/j.1699-0463.1962.tb04191.x. [DOI] [PubMed] [Google Scholar]
- GORDON D. B., NOGUEIRA A. Increased vascular reactivity in experimental hypertension. Circ Res. 1962 Mar;10:269–273. doi: 10.1161/01.res.10.3.269. [DOI] [PubMed] [Google Scholar]
- Giacomelli F., Wiener J., Spiro D. The cellular pathology of experimental hypertension. V. Increased permeability of cerebral arterial vessels. Am J Pathol. 1970 Apr;59(1):133–160. [PMC free article] [PubMed] [Google Scholar]
- Goldby F. S., Beilin L. J. How an acute rise in arterial pressure damages arterioles. Electron microscopic changes during angiotensin infusion. Cardiovasc Res. 1972 Sep;6(5):569–584. doi: 10.1093/cvr/6.5.569. [DOI] [PubMed] [Google Scholar]
- HARRY J., ASHTON N. THE PATHOLOGY OF HYPERTENSIVE RETINOPATHY. Trans Ophthalmol Soc U K. 1963;83:71–90. [PubMed] [Google Scholar]
- HATT P. Y., DONTCHEFF A. Contribution de la microscopie électronique à l'étude du mécanisme de l'hypertension artérielle expérimentale d'origine rénale chez le rat; les lésions des artères du rein. Arch Mal Coeur Vaiss. 1959 May;52(5):490–503. [PubMed] [Google Scholar]
- Häggendal E., Johansson B. Effect of increased intravascular pressure on the blood-brain barrier to protein in dogs. Acta Neurol Scand. 1972;48(3):271–275. doi: 10.1111/j.1600-0404.1972.tb07548.x. [DOI] [PubMed] [Google Scholar]
- Hüttner I., Jellinek H., Kerényi T. Fibrin formations in vascular fibrinoid change in experimental hypertension: an electron microscopic study. Exp Mol Pathol. 1968 Dec;9(3):309–321. doi: 10.1016/0014-4800(68)90022-1. [DOI] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960 Dec;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Kohner E. M., Dollery C. T., Shakib M., Henkind P., Paterson J. W., De Oliveira L. N., Bulpitt C. J. Experimental retinal branch vein occlusion. Am J Ophthalmol. 1970 May;69(5):778–825. doi: 10.1016/0002-9394(70)93420-3. [DOI] [PubMed] [Google Scholar]
- Kowada M., Ames A., 3rd, Majno G., Wright R. L. Cerebral ischemia. I. An improved experimental method for study; cardiovascular effects and demonstration of an early vascular lesion in the rabbit. J Neurosurg. 1968 Feb;28(2):150–157. doi: 10.3171/jns.1968.28.2.0150. [DOI] [PubMed] [Google Scholar]
- LORINC J., GORACZ G. Y. New method of inducing experimental hypertension in the rat. Acta Physiol Acad Sci Hung. 1954;5(3-4):489–494. [PubMed] [Google Scholar]
- Lassen N. A., Agnoli A. The upper limit of autoregulation of cerebral blood flow--on the pathogenesis of hypertensive encepholopathy. Scand J Clin Lab Invest. 1972 Oct;30(2):113–116. doi: 10.3109/00365517209081099. [DOI] [PubMed] [Google Scholar]
- Linton A. L., Gavras H., Gleadle R. I., Hutchison H. E., Lawson D. H., Lever A. F., Macadam R. F., McNicol G. P., Robertson J. I. Microangiopathic haemolytic anaemia and the pathogenesis of malignant hypertension. Lancet. 1969 Jun 28;1(7609):1277–1282. doi: 10.1016/s0140-6736(69)92221-1. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. A., Gardner D. L. The fine structure of the mesenteric arteries of the rat. Angiology. 1966 Dec;17(12):902–931. doi: 10.1177/000331976601701203. [DOI] [PubMed] [Google Scholar]
- Northover A. M., Northover B. J. The effect of vaso-active substances on rat mesenteric blood vessels. J Pathol. 1970 Jun;101(2):99–108. doi: 10.1002/path.1711010205. [DOI] [PubMed] [Google Scholar]
- OONEDA G., OOYAMA Y., MATSUYAMA K., TAKATAMA M., YOSHIDA Y., SEKIGUCHI M., ARAI I. ELECTRON MICROSCOPIC STUDIES ON THE MORPHOGENESIS OF FIBRINOID DEGENERATION IN THE MESENTERIC ARTERIES OF HYPERTENSIVE RATS. Angiology. 1965 Jan;16:8–17. doi: 10.1177/000331976501600102. [DOI] [PubMed] [Google Scholar]
- PEASE D. C., MOLINARI S. Electron microscopy of muscular arteries; pial vessels of43 the cat and monkey. J Ultrastruct Res. 1960 Jun;3:447–468. doi: 10.1016/s0022-5320(60)90022-8. [DOI] [PubMed] [Google Scholar]
- Rodda R., Denny-Brown D. The cerebral arterioles in experimental hypertension. I. The nature of arteriolar constriction and its effects on the collateral circulation. Am J Pathol. 1966 Jul;49(1):53–76. [PMC free article] [PubMed] [Google Scholar]
- Röhlich P., Oláh I. Cross-striated fibrils in the endothelium of the rat myometral arterioles. J Ultrastruct Res. 1967 Jun;18(5):667–676. doi: 10.1016/s0022-5320(67)80212-0. [DOI] [PubMed] [Google Scholar]
- Strandgaard S., Olesen J., Skinhoj E., Lassen N. A. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973 Mar 3;1(5852):507–510. doi: 10.1136/bmj.1.5852.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener J., Lattes R. G., Meltzer B. G., Spiro D. The cellular pathology of experimental hypertension. IV. Evidence for increased vascular permeability. Am J Pathol. 1969 Feb;54(2):187–207. [PMC free article] [PubMed] [Google Scholar]