Abstract
Background
Synaptogyrin-2 (SYNGR2), as a member of synaptogyrin gene family, is overexpressed in several types of cancer. However, the role of SYNGR2 in pan-cancer is largely unexplored.
Methods
From the TCGA and GEO databases, we obtained bulk transcriptomes, and clinical information. We examined the expression patterns, prognostic values, and diagnostic value of SYNGR2 in pan-cancer, and investigated the relationship of SYNGR2 expression with tumor mutation burden (TMB), microsatellite instability (MSI), immune infiltration, and immune checkpoint (ICP) genes. The gene set enrichment analysis (GSEA) software was used to perform pathway analysis. Besides, we built a nomogram of liver hepatocellular carcinoma patients (LIHC) and validated its prediction accuracy.
Results
SYNGR2 was highly expressed in most cancers. The high expression of SYNGR2 significantly reduced the overall survival (OS), disease-specific survival (DSS), disease-free interval (DFI), and progression-free interval (PFI) in multiple types of cancer. Also, receiver operating characteristic (ROC) curve analysis demonstrated that SYNGR2 showed high accuracy in distinguishing cancerous tissues from normal ones. Moreover, SYNGR2 expression was correlated with TMB, MSI, immune scores, and immune cell infiltrations. We also analyzed the association of SYNGR2 with immunotherapy response in LIHC. Finally, a nomogram including SYNGR2 and pathologic T, N, M stage was built and exhibited good predictive power for the OS, DSS, and PFI of LIHC patients.
Conclusion
Overall, SYNGR2 is a critical oncogene in various tumors. SYNGR2 participates in the carcinogenic progression, and may contribute to the immune infiltration in tumor microenvironment. Our study suggests that SYNGR2 can serve as a predictor related to prognosis in pan-cancer, especially LIHC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12859-023-05323-y.
Keywords: SYNGR2, Pan-cancer, LIHC, Prognosis, Immune analysis
Introduction
Cancer is a major disease that seriously threatens human health and life worldwide, and the incidence and mortality are rapidly increasing globally [1]. The treatment of cancers has always been a pressing challenge for the medical community. Cancer patients have experienced significant improvements thanks to advances in surgery, molecular-targeted therapy and immunotherapy in recent years. However, the 5-year survival rate of cancer patients after diagnosis remains discouraging [2]. Consequently, there is a dire need to clarify the molecular mechanisms elucidating patterns of cancer pathogenesis and to identify reliable biomarkers for the early detection, diagnosis and treatment of cancers [3].
Synaptogyrin-2 (SYNGR2), also known as Cellugyrin, belongs to the synaptogyrin gene family. SYNGR2 gene is located in chromosome 17q25.3, encoding a protein product composed of 224 amino acids [4]. Previous studies have found that SYNGR2 plays an important role in cellular exocytosis, the storage and transport of GLUT4 at the cytoplasmic membrane, and the formation and maturation of microvesicles in neuronal cells [5]. At present, the studies on synaptogyrin gene family are still in their infancy. SYNGR3 was found to be expressed in the cytoplasm of chromophobe renal cell carcinoma, but not in the cytoplasm of renal oncocytoma. Accordingly, SYNGR3 may be used as the basis for the diagnosis of renal cell carcinoma in the future [6]. With the continuous progress of research, Bin Li et al. [7] found that SYNGR2 was associated with poorer overall survival, poorer disease-specific survival and T stage in ESCC. SYNGR2 may be used as a biomarker for determining prognosis and immune infiltration in ESCC. However, the effects of enhanced SYNGR2 gene expression on prognosis have not been systematically evaluated across different cancer types. In the last twenty years, comprehensive genomic characterization of tumors has become a major goal in the field of cancer research. The development of molecular histology and next-generation sequencing technologies has dramatically changed the study of cancer [8]. Large-scale genomics projects provide matched molecular and clinical data of various cancers, which helps systematically analyze the survival impact of single gene expression. Therefore, this is advantageous for analyzing and revealing potential biomarkers’ prognostic values with Pan-cancer analysis.
In this study, we examined the expression of SYNGR2 across 33 cancer types. Meanwhile, we also evaluated the prognostic value of SYNGR2 in pan-cancer based on multiple databases. Moreover, this study totally explored the potential association of SYNGR2 with clinical characteristics, tumor mutation burden (TMB), microsatellite instability (MSI), immune infiltration, and immune checkpoint genes. Finally, we found that the expression of SYNGR2 was significantly associated with survival prognosis in liver hepatocellular carcinoma (LIHC), and had a high diagnostic value in LIHC. Therefore, we constructed a SYNGR2-related prognostic risk-score model for LIHC patients and identified signal pathways that SYNGR2 regulates the development of LIHC. The current study indicates that SYNGR2 has promise as a prognostic biomarker in various cancers. These findings may have important implications in guiding basic research as well as clinical practice.
Materials and methods
Data collection
The RNA sequencing and clinical information for 33 types of cancers were acquired from the Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/), and the Genotype-Tissue Expression (GTEx, https://www.genome.gov/Funded-Programs-Projects/Genotype-Tissue-Expression-Project). The Single‑cell data of SYNGR2 was obtained from the Tabula Muris (https://tabula-muris.ds.czbiohub.org/). To verify the predictive value of SYNGR2 in LIHC, gene expression data and clinical data of GSE14520 were downloaded from the GEO (https://www.ncbi.nlm.nih.gov/geo) database. First, the expression levels of SYNGR2 in normal and tumor tissues were compared using the Wilcoxon rank sum test function in the “ggplot2” R package. Additionally, an online tool, the Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/), was adopted to visualize the SYNGR2 expression levels in different stages of all tumors. GEPIA comprised gene expression information from TCGA and gene expression profiles for normal tissues from the GTEx database [9]. Finally, we explored the protein expression level of SYNGR2 between primary tumors and normal tissues through the UALCAN portal (http://ualcan.path.uab.edu/analysis-prot.html) [10, 11].
Analysis of survival and prognosis
The pan-cancer samples were divided into SYNGR2high and SYNGR2low expression groups based on the minimum p-value approach. The Kaplan–Meier survival curves were utilized to exhibit the correlation of SYNGR2 expression with the prognosis of patients’ overall survival (OS), disease-specific survival (DSS), progression-free interval (PFI), and disease-free interval (DFI). We also implemented univariate Cox regression analysis to estimate the prognostic value of SYNGR2 by calculating the hazard ratio (HR) and 95% confidence interval (CI) by executing the “survival” and “forestplot” R packages.
SYNGR2’s capacity to distinguish tumor from non-tumor tissues
A ROC analysis of SYNGR2 expression levels was performed using the “pROC” R package to examine whether SYNGR2 expression levels can separate tumors and normal tissue across the 33 types of cancer, and the area under curve (AUC) was calculated [12].
Gene set enrichment analyses
To access the biological functions and pathways, the Gene Set Enrichment Analysis (GSEA) software (https://www.gsea-msigdb.org/gsea/downloads.jsp) was used to perform pathway analysis [13]. The gene sets h.all.v7.4.symbols.gmt and c2.cp.kegg.v7.4.symbols.gmt were chosen as the reference gene set. The normalized enrichment score (|NES|> 1), nominal p value < 0.05 (NOM p value), and FDR adjusted q-value < 0.25 were considered as significant pathway enrichment. The top five significantly enriched signaling pathways were demonstrated.
Implication of SYNGR2 expression in tumor immune microenvironment
The ESTIMATE algorithm was used to analysis the difference of stromal score, and immune score by the R package “estimate” [14]. The CIBERSORT algorithm was applied to assess the levels of 22 infiltrating immune cell subtypes [15, 16]. CIBERSORT can compute the abundance of specific cell types in a mixed sample based on the bulk expression. The R packages “limma” and “CIBERSORT” were used.
Correlation analysis of SYNGR2 with TMB, MSI, checkpoint genes, and immunophenotype scores
We used the “maftools” package in R software to organize the single-nucleotide variants (SNV) data downloaded from the TCGA database in multiple alignment format. We also assessed tumor mutation burden (TMB) for each sample. Microsatellite instability (MSI) refers to the nucleotide insertions or deletions in the microsatellite loci. Spearman’s rank method was used to determine the correlation of SYNGR2 with TMB and MSI. The correlation results for TMB and MSI were visualized in radar maps. To explore the prognostic value of SYNGR2 on immunotherapy, we analyzed the relationship between SYNGR2 and 29 common immune checkpoint genes. The immunophenotype scores (IPS) downloaded from The Cancer Immunome Atlas (TCIA, https://tcia.at/home) were leveraged to predict the clinical response to immunotherapy in SYNGR2high and SYNGR2low expression groups [17].
Establishment and evaluation of the nomogram
SYNGR2 expression and the pathologic T, N, M stage were used to build a nomogram, which is an effective and convenient approach for estimating the survival in individual patients [18]. The calibration curve was performed to verify the prediction accuracy of the nomogram.
Statistical analysis
R version 4.1.3 was used for all statistical studies. The survival curve was plotted by K–M plotter. Wilcoxon test was used to compare the differences between two groups, and Spearman analysis was used to calculate the correlation coefficients. Double-tailed p < 0.050 was considered statistically significant.
Result
SYNGR2 expression analysis in human pan‑cancer
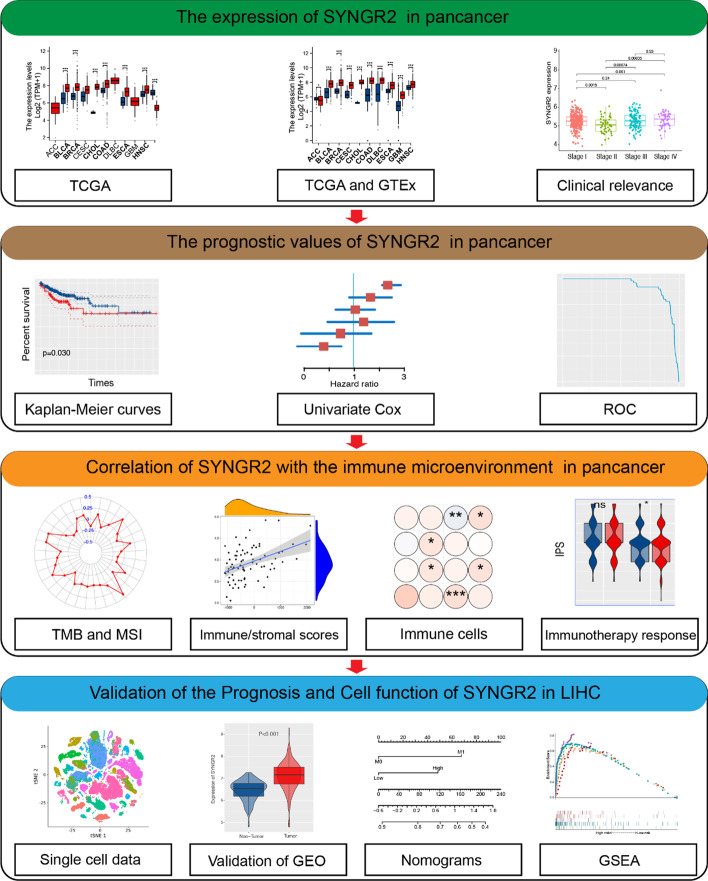
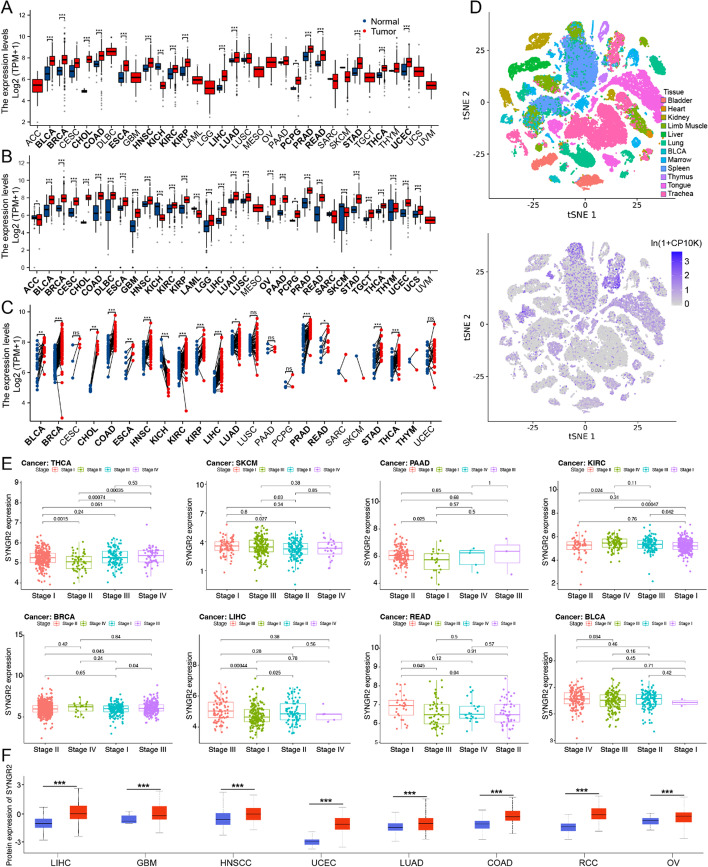
The flowchart of our study in Fig. 1. The RNA expression of SYNGR2 in pan-cancer data was first evaluated using the TCGA database. The results revealed that SYNGR2 mRNA level was significantly higher expressed in BLCA, BRCA, CHOL, LIHC, and other tumor tissues in comparison to corresponding normal tissues (Fig. 2A). Taking into account the lack of normal samples in the TCGA database for some cancer types, we integrated the GTEx database for further analysis and the results showed that the expression level of SYNGR2 in most tumor tissues are much higher than the corresponding control tissues (Fig. 2B). In paired samples, the expression of SYNGR2 in tumor tissues of BLCA, BRCA, CHOL, and others are significantly higher than the corresponding control tissues (Fig. 2C). Overall, SYNGR2 expression was significantly upregulated in various cancers. Next, human tissue were classified into 12 types as reported previously using the dimensional reduction method which was called t-distributed stochastic neighbor embedding (t-SNE), including bladder, heart, kidney, limb muscle, liver, lung, BLCA, marrow, spleen, thymus, tongue, trachea. The results suggested that SYNGR2 was highly expressed in liver, lung, marrow, spleen and tongue (Fig. 2D). Additionally, GEPIA-based analysis showed that SYNGR2 was differentially expressed in different stages in THCA, SKCM, PAAD, KIRC, BRCA, LIHC, READ, and BLCA (Fig. 2E). The UALCAN online tool confirmed that SYNGR2 protein levels were significantly upregulated in LIHC, GBM, HNSCC, UCEC, LUAD, COAD, RCC, and OV (Fig. 2F).
Fig. 1.
The flowchart for comprehensive analysis
Fig. 2.
The expression of SYNGR2 in pan-cancer and different pathological stages. A. Differential expression of SYNGR2 in normal and tumor samples of 33 tumors in The Cancer Genome Atlas (TCGA) database. B. Data from TCGA and Genotype-Tissue Expression (GTEx) database showed differential expression of SYNGR2 in multiple cancers. C. Differential expression of SYNGR2 in cancers and normal tissues from TCGA dataset. D. T-SNE plot showing the expression of SYNGR2 in human tissue. E. Expression of SYNGR2 in different pathological stages of indicated tumors. F. SYNGR2 protein expression levels between tumor and respective normal tissues for 8 types of cancers. *p < 0.05, **p < 0.01, and ***p < 0.001
Prognostic potential of SYNGR2 in pan-cancer
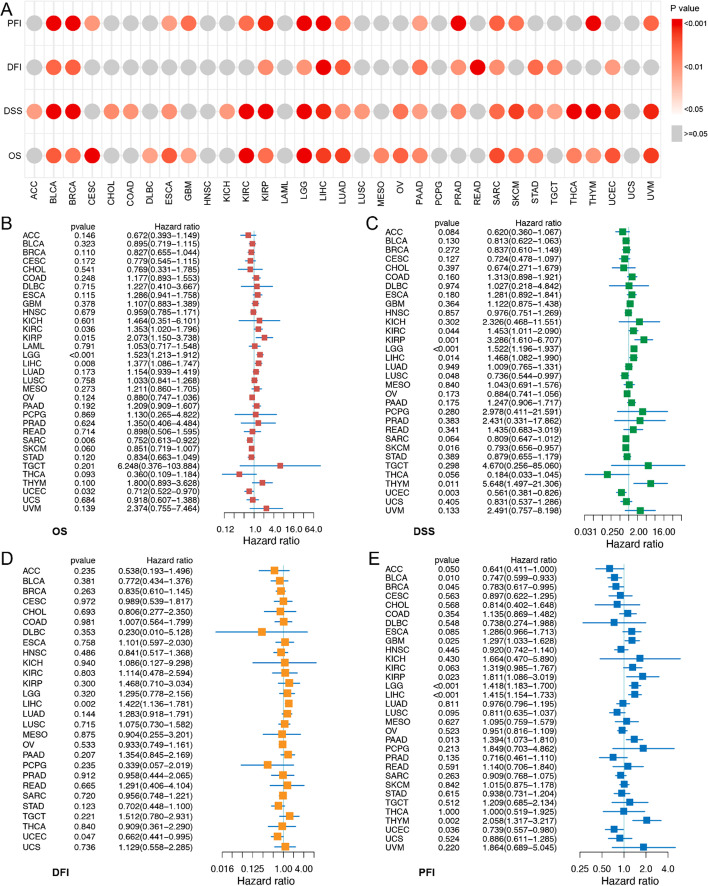
We investigated the clinical relevance of SYNGR2 in the different tumor types using Kaplan–Meier survival analysis (Fig. 3A; Additional file 1: Figs. S1–S4) regarding OS, DSS, DFI, and PFI. For example, the results showed that high SYNGR2 levels were associated with poor OS in BRCA, DLBC, GBM, MESO, PAAD, SKCM, STAD, THCA, and THYM (all p < 0.05), BLCA, ESCA, KIRP, LUAD, OV, SARC, and UCEC (all p < 0.01), CESC, KIRC, LGG, LIHC, and UVM (all p < 0.001). It is worth noting that SYNGR2 was most associated with the survival of LIHC (all p < 0.001). Univariate Cox regression analyses were further conducted to assess the associations of SYNGR2 with prognosis (Fig. 3B–E). Univariate Cox regression analysis showed that high expression of SYNGR2 significantly reduces the OS in 6 types of cancers including KIRC, KIRP, LGG, LIHC, SARC, and UCEC (Fig. 3B). Results for DSS, DFI, and PFI are shown in Fig. 3C–E. These results suggested that SYNGR2 expression had a powerful prognostic ability in different tumors.
Fig. 3.
Correlation between SYNGR2 gene expression and prognosis in pan-cancer. A. Kaplan–Meier survival analyses were performed to determine the association between SYNGR2 and Overall Survival (OS), Disease-Specific Survival (DSS), Disease-Free Interval (DFI), Progression-Free Interval (PFI). B–E. The forest plots of univariate Cox regression analysis for OS (B), DSS (C), DFI (D), and PFI (E)
Diagnostic value of SYNGR2 for pan-cancer
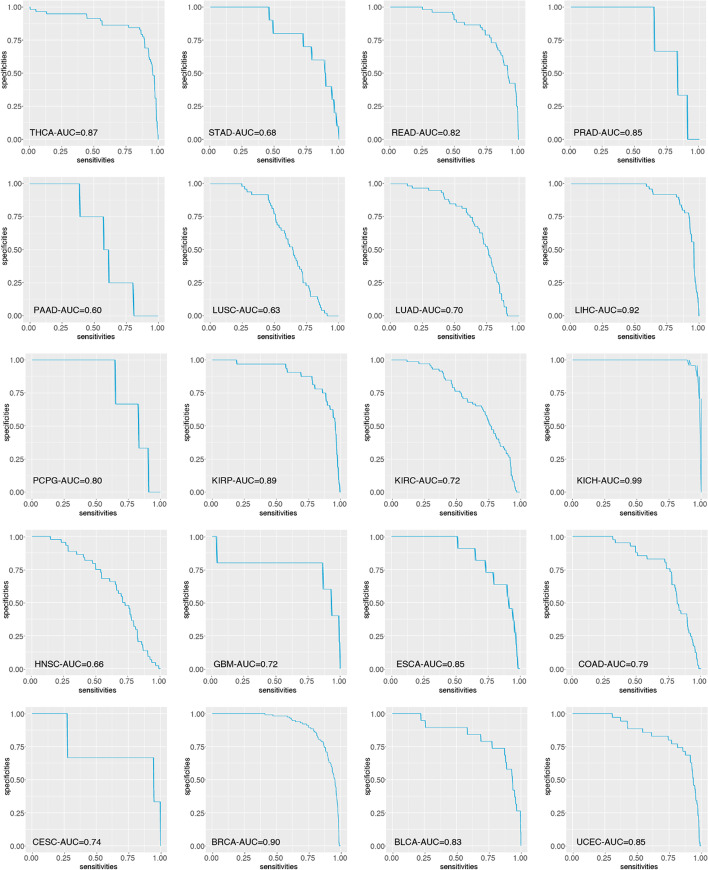
Furtherly, we used receiver operating characteristic (ROC) curve to assess the diagnostic Value of SYNGR2 expression levels between tumor and normal tissues. The area under curve (AUC) values for ROC analysis in each cancer are shown in Fig. 4. The AUC values suggested that SYNGR2 could reliably distinguish tumor from normal tissues for various types of cancer, especially THCA (AUC = 0.87), PRAD (AUC = 0.85), LIHC (AUC = 0.92), KIRP (AUC = 0.89), KICH (AUC = 0.99), ESCA (AUC = 0.85), BRCA (AUC = 0.90), and UCEC (AUC = 0.85).
Fig. 4.
The ROC curve was used to assess the diagnostic value of SYNGR2 expression levels between tumor and normal tissues
Correlations of SYNGR2 expression with TMB, MSI, and immune score in pan-cancer
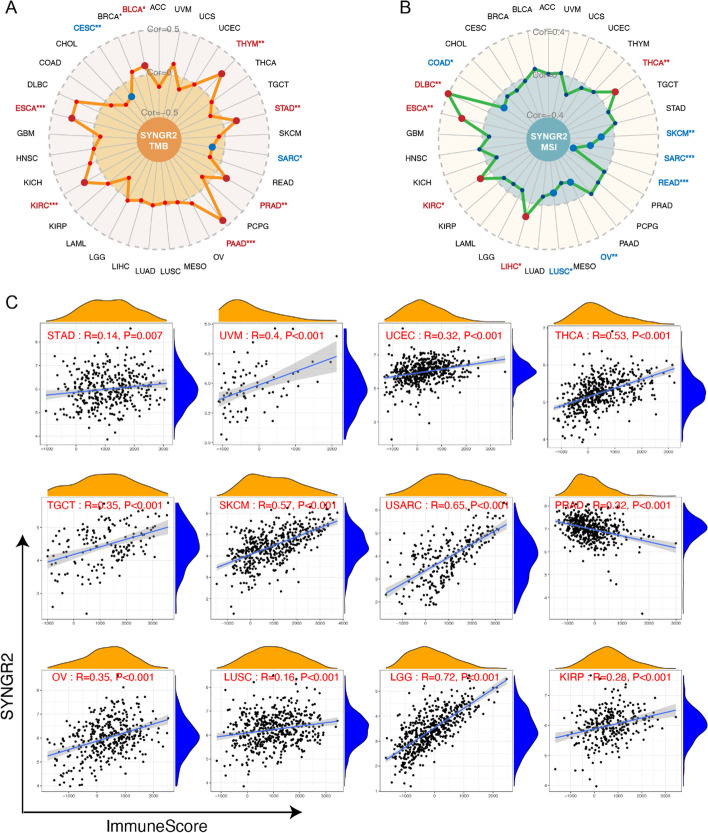
Both tumor mutation burden (TMB) and microsatellite instability (MSI) are pivotal characteristics of tumors. TMB, which counts the number of somatic mutations per megabase (mut/Mb), is an emerging potential biomarker for immunotherapy [19]. MSI refers to an abnormal DNA mismatch repair function where microsatellite replication errors are not corrected and accumulate, causing changes in microsatellite sequence length or base composition. MSI can be used as a prognostic marker for cancer patients [20]. Here, we evaluated the correlation of SYNGR2 expression with TMB and MSI (Fig. 5A–B). Results showed that SYNGR2 expression was positively related to TMB in BLCA, STAD, PRAD, and other tumor tissues; while negatively associated with TMB in SARC, and CESC. In terms of MSI, the SYNGR2 expression was positively related to MSI in DLBC, ESCA, KIRC, LIHC, and THCA; while negatively related to MSI in COAD, LUSC, OV, and other tumor tissues.
Fig. 5.
Correlation between the expression of SYNGR2 with TMB, MSI, and immune scores in pan-cancer. A–B. Radar maps of correlations between SYNGR2 expression and TMB (A) or MSI (B). C. The scatter plots of correlation between SYNGR2 expression and immune scores in multiple cancers. *p < 0.05, **p < 0.01, and ***p < 0.001
The tumor microenvironment (TME) plays an important role in the development of cancer. The TME consists of tumor cells, immune cells, stromal cells, etc. The Estimation of Stromal and Immune cells in Malignant Tumor tissues using Expression data (ESTIMATE) algorithm was used to evaluate infiltrating immune and stromal cells by calculating immune scores and stromal scores. SYNGR2 expression was found to positively correlate with the immune score in STAD, UVM, UCEC, and other tumor tissues, but negatively correlated with the immune score in PRAD (Fig. 5C). Regarding stromal score, a positive correlation with SYNGR2 expression was seen in GBM, KICH, SARC, SKCM, TGCT, THCA, UVM, and a negative correlation in BRCA, COAD, PRAD, and PAAD (Additional file 1: Fig. S5).
Correlation between SYNGR2 and immune cell infiltration, immunophenotype scores in different tumors
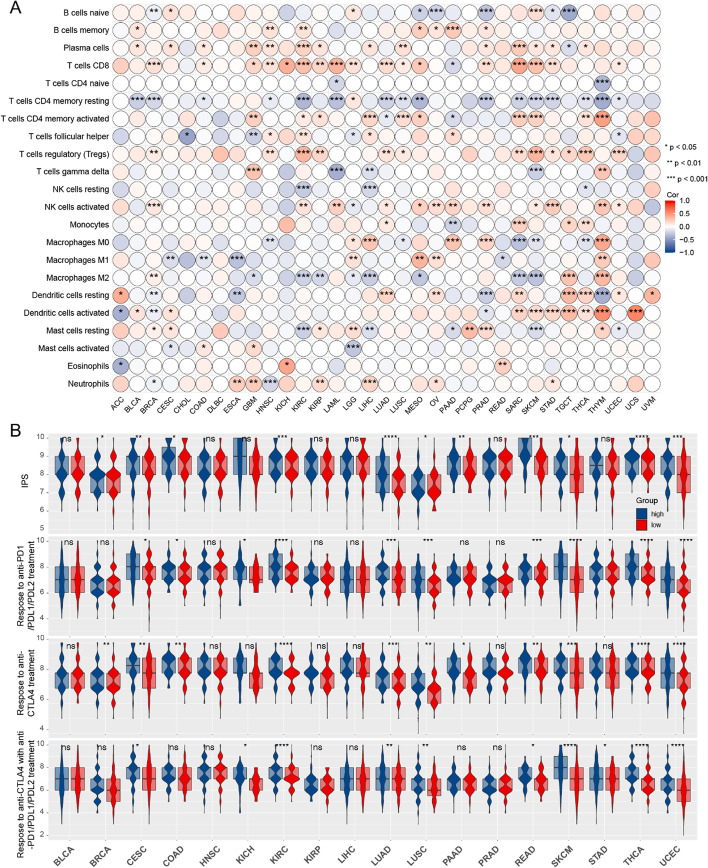
Previous studies have found that the expression of SYNGR2 in esophageal cancer is related to immune cell infiltration [7]. Based on this, the Estimating Relative Subsets of RNA Transcripts (CIBERSORT) algorithm was used to determine the composition of 22 immune cell subsets based on gene expression profiles. Results indicated that SYNGR2 was significantly associated with immune cell subsets in BRCA, GBM, HNSC, and other tumor tissues. SYNGR2 exhibited positive associations with Plasma cells, T regulatory cells (Tregs), CD8+ T cells, activated memory CD4+ T cells, activated NK cells, activated Dendritic cells, and negative associations with native B cells, resting memory CD4+ T cells, M2 Macrophages in the majority of tumors (Fig. 6A).
Fig. 6.
Correlation between the expression of SYNGR2 and immune infiltration in pan-cancer. A. The abundance of 22 immune cells calculated by CIBERSORT. B. The difference of IPS between SYNGR2high and SYNGR2low expression groups. *p < 0.05, **p < 0.01, and ***p < 0.001
As presented in Fig. 6B, we determined the sensitivity to immune checkpoint inhibitors for the different tumors. Our results showed that the SYNGR2high group possessed a significantly higher immunophenotype scores (IPS) than the SYNGR2low group in BRCA, CESC, COAD, KIRC, and other tumor tissues. Moreover, the SYNGR2high group was more likely to gain benefits from anti-PD1/PDL1/PDL2 therapies in CESC, COAD, KICH, other tumor tissues, and respond to anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) therapy in BRCA, CESC, COAD, and other tumor tissues. Finally, the SYNGR2high group was prone to respond to the combination of the two immunotherapies in CESC, KICH, KIRC, and other tumor tissues.
Correlations of SYNGR2 expression with HLA, immune checkpoint genes, and chemokines in LIHC
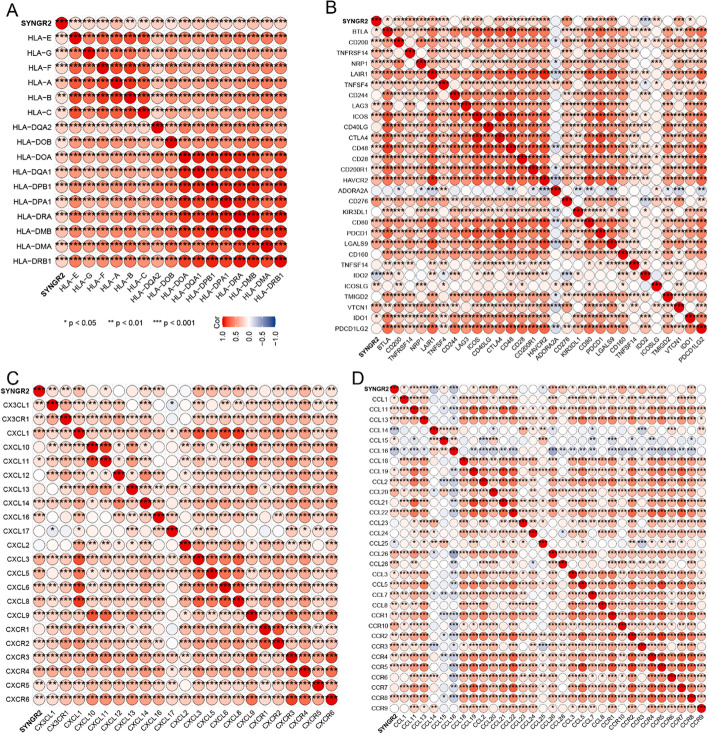
In the above analysis, we discovered a correlation between SYNGR2 expression and prognosis of multiple tumors and immune cell infiltration. We found that SYNGR2 was significantly associated with LIHC, especially in terms of prognosis and diagnostic value. Therefore, to further understand the role of SYNGR2 in LIHC, we analyzed the expression of human leukocyte antigen (HLA), immune checkpoint genes, and chemokines.
Class I HLA is the expression product of the human major histocompatibility complex (MHC) and is located on the short arm of chromosome 6 [21]. The deficiency of HLA may impair cells’ ability to present neoantigens and cause immune tolerance [22]. The results showed that SYNGR2 was positively correlated with all HLA members (Fig. 7A). Cancer immunotherapy represented by immune checkpoint inhibitors (ICIs) offers a promising treatment option for LIHC [23]. To further investigate the predictive role of SYNGR2 Expression in immunotherapy, we examined the correlation between SYNGR2 and immune checkpoint (ICP) genes, (Fig. 7B). Many ICP genes were positively correlated with SYNGR2, especially in CD200, CD200R1, CTLA4, LAIR1, ICOS, CD276, PDCD1, CDD80, and VTCN1. Chemokines and chemokine receptors play an important role in the development and progression of LIHC. Different chemokines and receptors have different roles in hepatocellular carcinoma [24]. Chemokines can be classified into 4 categories according to the position of the two conserved N-terminal cysteine residues: CC, CXC, C and CX3C [25]. Chemokines and chemokine receptors mediate the movement of immune cells in the TME [26]. We found that SYNGR2 was highly positive correlated with CXCL1, CXCL3, CXCL5, CXCL8, CXCR4, CCL2, CCL20, CCL26, CCL28, CCL7, CCR10, CCR3, and CCR8 expression in LIHC samples (Fig. 7C–D).
Fig. 7.
Assessment of immunotherapy response in LIHC. A. Correlations of SYNGR2 Expression with HLA gene family. B. Correlation of SYNGR2 Expression with immune checkpoint genes. C, D. Correlation between SYNGR2 and chemokines in tumors. *p < 0.05, **p < 0.01, and ***p < 0.001
The expression, prognostic value and signaling pathway of SYNGR2 in LIHC
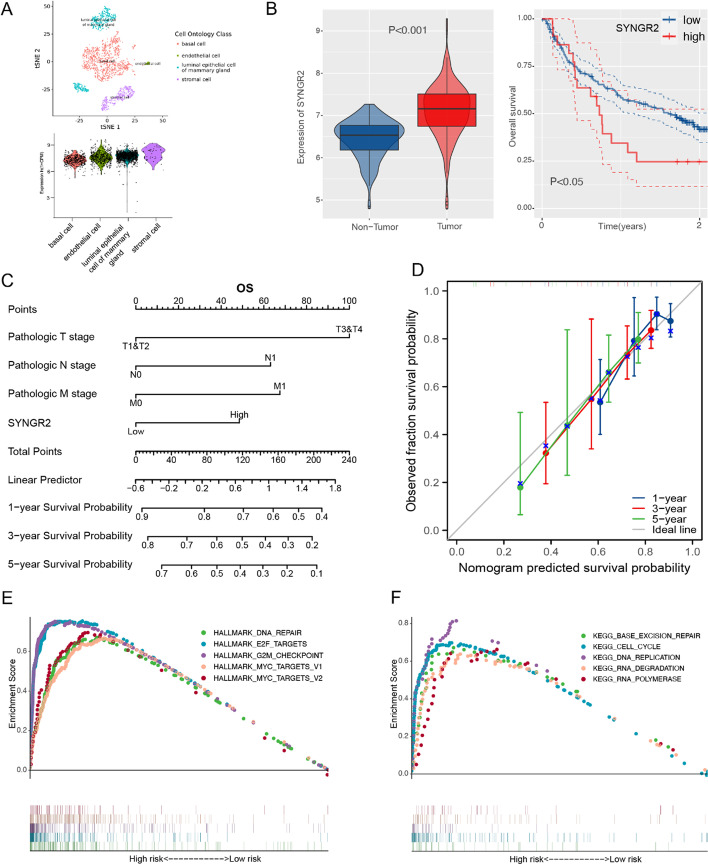
First, we use the t-SNE method to classify Cell Ontology Class into 4 types based on the expression level of SYNGR2, including basal cell, endothelial cell, luminal epithelial cell of mammary gland, stromal cell. The results suggested that SYNGR2 was highly expressed in stromal cell (Fig. 8A). Additionally, results above were verified in GSE14520 dataset to further demonstrate the expression and prognosis of SYNGR2 in LIHC. The expression of SYNGR2 in tumor tissue is higher than that in non-tumor tissue (p < 0.001), and the SYNGR2high group had a worse OS compared with the SYNGR2low group in K-M curve (p < 0.05; Fig. 8B).
Fig. 8.
GSEA regarding SYNGR2 for LIHC and construction of a SYNGR2-based prognostic prediction model. A. T-SNE plot showing the expression of SYNGR2 in Cell Ontology Class. B. The SYNGR2 expression and prognosis analysis in GSE14520 dataset. C. Nomogram for predicting the proportion of patients with OS. D. Calibration curves of the nomogram for 1, 3 and 5 years. E, F. Enrichment results of HALLMARK and KEGG signaling pathways
In order to investigate the application of SYNGR2 in cancer prognosis, we built a nomogram for predicting the OS, DSS, PFI of LIHC patients (Fig. 8C, Additional file 1: Fig. S6A, C). The pathologic T, N, M stage and SYNGR2 were included as prognostic factors in the nomogram. The calibration curve showed that the nomogram had a good ability in predicting possibility of 1-, 3-, 5-years OS, DSS, and PFI in LIHC (Fig. 8D, Additional file 1: Fig. S6B, D). These results demonstrated that the nomogram combining SYNGR2 expression and pathologic T, N, M stage had better predictive power for the OS, DSS, and PFI of LIHC patients, which might contribute to assess the prognosis of patients.
To explore the potential function of SYNGR2 in LIHC, we carried out a gene set enrichment analysis (GSEA). The top five signaling pathways significantly associated with SYNGR2 are exhibited in Fig. 8E–F. SYNGR2 was associated with DNA repair, E2F targets, MYC targets, cell cycle, DNA replication, and RNA polymerase. These results suggest that SYNGR2 plays a vital role in tumorigenesis and progression.
Discussion
SYNGR2 belongs to the synaptogyrin gene family. The function of synaptogyrin is related to the release of neurotransmitters and the early neurons development [27]. The SYNGR2 gene is expressed at high levels in all tissues, except brain, and it plays an important role in biological processes such as cell exocytosis, the storage and transport of GLUT4 in the cytoplasmic membrane, and the formation and maturation of microvesicles in neuronal cells [5]. To date, the evidence of SYNGR2’s implications in cancer is very preliminary and lacks experimental validation. Li et al. [7] reported the elevated expression of SYNGR2 in ESCC. High SYNGR2 expression was associated with poorer OS in ESCC. SYNGR2 has an important reference value for the diagnosis of ESCC. Collectively, SYNGR2’s potential roles in carcinogenesis and cancer development are worthwhile to be further disclosed.
In the present study, we explored the pan-cancer expression profiles of SYNGR2, and the correlation between SYNGR2 aberrant expression and patient prognosis in different cancers. A pan-cancer analysis revealed that compared to normal tissues, significantly elevated mRNA expression levels of SYNGR2 were observed in 26 cancers, including BLCA, BRCA, CESC, and other tumor tissues. Furthermore, SYNGR2 expression levels increased with tumor progression in THCA, SKCM, PAAD, and other tumor tissues. In terms of prognosis, Kaplan–Meier survival and univariate Cox regression analysis demonstrated that patients with SYNGR2high tumors were more likely to suffer inferior survival in BRCA, PAAD, BLCA, and other tumor tissues.
Cancer is a complicated disease involving complex reciprocal networks between tumor cells and the immune system. The tumor microenvironment (TME) is the internal environment in which the tumor cells are located, which includes not only the tumor cells themselves, but also the stromal cells, immune cells and other components [28]. The TME plays an important role in the proliferation, invasion and metastasis of cancer [29]. On the one hand, immune cells are primarily responsible for the elimination of tumor cells. The infiltration density and activity of immune cells have been demonstrated to be not only predictive of the response to ICBs [30], but also independent prognostic markers for tumor patients [31, 32]. On the other hand, tumor cells can recruit immunosuppressive cells to evade immune killing by altering their immunogenicity and biological properties [33]. We adopted ESTIMATE algorithm to determine stromal and immune scores for samples across 33 cancer types. Our exploration demonstrated that SYNGR2 is positively correlated with immune scores in most cancer types. However, the association of SYNGR2 with stromal scores varied among different tumors. This suggests that the role of SYNGR2 is tumor-specific. We also used the CIBERSORT algorithm to estimate the proportion of 22 immune cell subsets in each tumor sample. The correlation test indicated that SYNGR2 was extensively correlated to immune cell infiltrates in BRCA, GBM, HNSC, and other tumor tissues. In addition, we also found that aberrant SYNGR2 expression was correlated with increased immune cell infiltration of T regulatory cells (Tregs) in the majority of cancers. Tregs is an immunosuppressive cell that is abundant in the TME. Tregs inhibits the T cells activation and proliferation through various mechanisms, such as inhibition of MHC molecules and co-stimulatory molecules (CD80 and CD86) on the surface of antigen-presenting cells (APCs) to inhibit APC maturation and thus attenuate the interaction between APCs and T cells [34]. This further suggests that SYNGR2 may play an important regulatory role in the TME.
Given the correlation between SYNGR2 and TME, we further explored whether SYNGR2 is related to the response to immunotherapy. Tumor mutation burden (TMB) and microsatellite instability (MSI) have been described as powerful predictors of tumor behavior and response to immunotherapy [19, 20]. In this study, we evaluated the correlation of SYNGR2 expression with TMB and MSI. Our pan-cancer analysis revealed that SYNGR2 expression was positively related to TMB in BLCA, STAD, PRAD, and other tumor tissues. In terms of MSI, the SYNGR2 expression was positively related to MSI in DLBC, ESCA, KIRC, LIHC, and THCA. Next, in the study for IPS it was found that SYNGR2high tumors exhibited a significantly higher IPS than the SYNGR2low tumors. The IPS is the most comprehensive estimator of tumor immunogenicity. The IPS has shown remarkable performance in terms of predicting response to immunotherapies blocking CTLA and PD1 [17]. Overall, these results indicated that SYNGR2 might be related to the response to immunotherapy.
In this study, we observed the most robust relationship of aberrant SYNGR2 expression with patient prognosis in LIHC. Therefore, we validated the role of SYNGR2 in LIHC by constructing a prognostic risk score model, as well as evaluating the relationship between SYNGR2 and immune checkpoints. The nomogram including SYNGR2 and pathologic T, N, M stage showed good prognostic predictive performance. To further clarify the role of SYNGR2 in tumorigenesis and progression, we conducted a gene set enrichment analysis. The results suggested SYNGR2 was associated with DNA repair, E2F targets, MYC targets, cell cycle, DNA replication, and RNA polymerase. E2F, an important regulator of the cell cycle and apoptosis, plays an important role in the development of cancer [35]. Chen et al. [36] found that overexpression of E2F1 accelerated the proliferation of hepatocellular carcinoma cells and promoted tumor formation in vitro hepatocellular carcinoma cell lines and mouse models. MYC is one of the most widely investigated cancer-causing genes, being implicated in the formation, maintenance and progression of several different cancer types [37, 38]. Moreover, many ICP genes were positively correlated with SYNGR2 in LIHC. ICIs have caused a revolution in cancer care by reversing the immunosuppressive tumor microenvironment. In our research, CD200, CD200R1, CTLA4, LAIR1, ICOS, CD276, PDCD1, CDD80, and VTCN1 were correlated to SYNGR2. Notably, CTLA4 and PDCD1 /PD1 have received considerable attention as a putative immune checkpoint in TME. To sum up, the above analysis demonstrated that SYNGR2 played a tumor-promoting role and related to the response to ICP therapies in LIHC.
Although our pan-cancer analysis exhibited great performance, there were some limitations in our research. First, our findings should be explained cautiously due to the retrospective nature of the study. Second, all data for the article were obtained from public databases. Therefore, more clinical data are needed to validate the above results. Finally, the mechanism of SYNGR2 in LIHC prognosis remains unknown, a more in-depth investigation will be undertaken in vivo or in vitro.
In conclusion, SYNGR2 can be considered as a critical oncogene. Moreover, SYNGR2 is differentially expressed in a variety of tumors and aberrant expression is associated with the progression of the tumor, especially in LIHC. The aberrant SYNGR2 expression is associated with immune cell infiltration, immune scores, ICP genes, IPS, TMB, and MSI. Our findings demonstrated that SYNGR2 could be a potential biomarker, as well as a predictor of survival and immunotherapy in cancer treatment.
Supplementary Information
Additional file 1: Fig. S1. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and overall survivalof pan-cancer. Fig. S2. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and disease-specific survivalof pan-cancer. Fig. S3. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and disease-free intervalof pan-cancer. Fig. S4. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and progression-free intervalof pan-cancer. Fig. S5. Correlation between the expression of SYNGR2 and stromal scores in pan-cancer. Fig. S6. Prediction of DSS and PFI by SYNGR2-based prognostic models. (A, C). Nomogram for predicting the proportion of patients with DSS, and PFI. (B, D). Calibration curves of the nomogram for 1, 3, and 5 years.
Acknowledgements
Not applicable.
Abbreviations
- SYNGR2
Synaptogyrin-2
- TCGA
The cancer genome atlas
- GTEx
Genotype-tissue expression
- GEPIA
Gene expression profiling interactive analysis
- TCIA
The cancer immunome atlas
- GSEA
Gene set enrichment analysis
- ACC
Adrenocortical carcinoma
- BLCA
Bladder urothelial carcinoma
- BRCA
Breast invasive carcinoma
- CESC
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL
Cholangiocarcinoma
- COAD
Colon adenocarcinoma
- DLBC
Lymphoid neoplasm diffuse large b-cell lymphoma
- ESCA
Esophageal carcinoma
- GBM
Glioblastoma multiforme
- HNSC
Head and neck squamous cell carcinoma
- KICH
Kidney chromophobe
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney renal papillary cell carcinoma
- LAML
Acute myeloid leukemia
- LGG
Brain lower grade glioma
- LIHC
Liver hepatocellular carcinoma
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- MESO
Mesothelioma
- OV
Ovarian serous cystadenocarcinoma
- PAAD
Pancreatic adenocarcinoma
- PCPG
Pheochromocytoma and paraganglioma
- PRAD
Prostate adenocarcinoma
- READ
Rectum adenocarcinoma
- SARC
Sarcoma
- SKCM
Skin cutaneous melanoma
- STAD
Stomach adenocarcinoma
- TGCT
Testicular germ cell tumors
- THYM
Thymoma
- THCA
Thyroid carcinoma
- UCS
Uterine carcinosarcoma
- UCEC
Uterine corpus endometrial carcinoma
- UVM
Uveal melanoma
- OS
Overall survival
- DSS
Disease-specific survival
- DFI
Disease-free interval
- PFI
Progression free interval
- IPS
Immunophenotype scores
- TMB
Tumor mutation burden
- TME
The tumor microenvironment
- MSI
Microsatellite instability
- ICP
Immune checkpoints
- ROC
Receiver operating characteristic
- ICIs
Immune checkpoint inhibitors
- T-SNE
T-distributed stochastic neighbor embedding
- SNV
Single-nucleotide variants
- AUC
Area under curve
- HR
Hazard ratio
- CI
Confidence interval
- APCs
Antigen-presenting cells
- Tregs
T regulatory cells
- HLA
Human leukocyte antigen
- MHC
Major histocompatibility complex
- CTLA-4
Anti-cytotoxic T-lymphocyte associated protein 4
Author contributions
All authors contributed to the study conception and design. CXL and ZWQ drafted the manuscript. CXL and PW performed the analyses and interpreted all the data. CXL, HRZ, and CZ prepared the figures and tables. YBZ reviewed and revised the manuscript. All authors approved the final manuscript.
Funding
This study was supported by Beijing Medical Award Foundation (grant number: YXJL-2022-0080-0168) and the Haiyan Foundation of Harbin Medical University Cancer Hospital (Grant number: JJZD2014-06).
Availability of data and materials
The original contributions presented in the study are included in the article/Additional file. Further inquiries can be directed to the corresponding authors.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators GBDCRF. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10352):563-91 [DOI] [PMC free article] [PubMed]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20(4):209–215. doi: 10.1038/s41577-019-0264-y. [DOI] [PubMed] [Google Scholar]
- 4.Kedra D, Pan HQ, Seroussi E, et al. Characterization of the human synaptogyrin gene family. Hum Genet. 1998;103(2):131–141. doi: 10.1007/s004390050795. [DOI] [PubMed] [Google Scholar]
- 5.Kioumourtzoglou D, Pryor PR, Gould GW, et al. Alternative routes to the cell surface underpin insulin-regulated membrane trafficking of GLUT4. J Cell Sci. 2015;128(14):2423–2429. doi: 10.1242/jcs.166561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan MH, Wong CF, Tan HL, et al. Genomic expression and single-nucleotide polymorphism profiling discriminates chromophobe renal cell carcinoma and oncocytoma. BMC Cancer. 2010;10:196. doi: 10.1186/1471-2407-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Ren MY, Chen YZ, et al. SYNGR2 serves as a prognostic biomarker and correlates with immune infiltrates in esophageal squamous cell carcinoma. J Gene Med. 2022;24(8):e3441. doi: 10.1002/jgm.3441. [DOI] [PubMed] [Google Scholar]
- 8.Cieslik M, Chinnaiyan A. Global genomics project unravels cancer's complexity at unprecedented scale. Nature. 2020;578(7793):39–40. doi: 10.1038/d41586-020-00213-2. [DOI] [PubMed] [Google Scholar]
- 9.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Chandrashekar D, Varambally S, et al. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun. 2019;10(1):5679. doi: 10.1038/s41467-019-13528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrashekar D, Bashel B, Balasubramanya S, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao K, Ma Z, Zhang W. SPP1 comprehensive analysis to identify as a prognostic biomarker in cervical cancer. Front Genet. 2021;12:732822. doi: 10.3389/fgene.2021.732822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian A, Tamayo P, Mootha V, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshihara K, Shahmoradgoli M, Martinez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Khodadoust MS, Liu CL, et al. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charoentong P, Finotello F, Angelova M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi: 10.1016/j.jtcvs.2017.12.107. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal C, Thompson JC, Chien AL, et al. Baseline plasma tumor mutation burden predicts response to pembrolizumab-based therapy in patients with metastatic non-small cell lung cancer. Clin Cancer Res. 2020;26(10):2354–2361. doi: 10.1158/1078-0432.CCR-19-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baretti M, Le D. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62. doi: 10.1016/j.pharmthera.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Trowsdale J, Knight J. Major histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mcgranahan N, Rosenthal R, Hiley C, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171(6):1259–1271. doi: 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anwanwan D, Singh SK, Singh S, et al. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han KQ, He XQ, Ma MY, et al. Inflammatory microenvironment and expression of chemokines in hepatocellular carcinoma. World J Gastroenterol. 2015;21(16):4864–4874. doi: 10.3748/wjg.v21.i16.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagarsheth N, Wicha M, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dangaj D, Bruand M, Grimm A, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885–900.e10. doi: 10.1016/j.ccell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janz R, Südhof T. Cellugyrin, a novel ubiquitous form of synaptogyrin that is phosphorylated by pp60c-src. J Biol Chem. 1998;273(5):2851–2857. doi: 10.1074/jbc.273.5.2851. [DOI] [PubMed] [Google Scholar]
- 28.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goliwas KF, Deshane JS, Elmets CA, et al. Moving immune therapy forward targeting TME. Physiol Rev. 2021;101(2):417–425. doi: 10.1152/physrev.00008.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ock CY, Keam B, Kim S, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res. 2016;22(9):2261–2270. doi: 10.1158/1078-0432.CCR-15-2834. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu S, Hiratsuka H, Koike K, et al. Tumor-infiltrating CD8 T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019;8(1):80–93. doi: 10.1002/cam4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Q, Chen N, Ge C, et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019;8(7):1593806. doi: 10.1080/2162402X.2019.1593806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10(1):41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 34.Shan F, Somasundaram A, Bruno TC, et al. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. 2022;8(11):944–961. doi: 10.1016/j.trecan.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9(11):785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YL, Uen YH, Li CF, et al. The E2F transcription factor 1 transactives stathmin 1 in hepatocellular carcinoma. Ann Surg Oncol. 2013;20(12):4041–4054. doi: 10.1245/s10434-012-2519-8. [DOI] [PubMed] [Google Scholar]
- 37.Beaulieu M, Castillo F, Soucek L. Structural and biophysical insights into the function of the intrinsically disordered myc oncoprotein. Cells. 2020;9(4):1038. doi: 10.3390/cells9041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy M, O’grady S, Tang M, et al. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94:102154. doi: 10.1016/j.ctrv.2021.102154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and overall survivalof pan-cancer. Fig. S2. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and disease-specific survivalof pan-cancer. Fig. S3. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and disease-free intervalof pan-cancer. Fig. S4. Kaplan–Meier survival analysis for the association between the expression of SYNGR2 and progression-free intervalof pan-cancer. Fig. S5. Correlation between the expression of SYNGR2 and stromal scores in pan-cancer. Fig. S6. Prediction of DSS and PFI by SYNGR2-based prognostic models. (A, C). Nomogram for predicting the proportion of patients with DSS, and PFI. (B, D). Calibration curves of the nomogram for 1, 3, and 5 years.
Data Availability Statement
The original contributions presented in the study are included in the article/Additional file. Further inquiries can be directed to the corresponding authors.