Abstract
Background
The vast majority of acute diarrheal diseases are self-limiting and do not require treatment on a regular basis. Empirical antibiotics should only be used to treat dysenteric and invasive bacterial diarrhea. Antibiotic misuse in the treatment of acute diarrhea is widespread in clinical practice worldwide. Hence, the purpose of this study was to examine the pattern of antibiotic use for the acute diarrheal diseases at Hiwot Fana Specialized University Hospital, Harar, Ethiopia.
Methods
A retrospective, institution-based cross-sectional study was conducted to investigate the antibiotic utilization pattern for the treatment of acute diarrheal diseases from September 1 to September 30, 2022. Data were obtained retrospectively from patient cards treated for diarrheal disorders from August 1, 2021 to August 31, 2022, using standardized questionnaires, and the analysis was performed using IBM SPSS Statistics version 27.
Results
Among 332 patients in present study, 271 (81.63%) of them received nine different types of antibiotics, with the most commonly prescribed drugs were Cotrimoxazole (30.26%), Ciprofloxacin (19.19%), and Azithromycin (17.71%). Based on the presence of blood in the stools, 14.76% of the cases were invasive bacterial in nature. Antibiotics were prescribed about 2.55 times more frequently to patients under the age of 12 than to subjects 65 and older (AOR 2.55, 95% CI 1.45–3.87). Patients who received three or more medications were 2.77 times more likely to be prescribed antibiotics (AOR 2.77, 95% CI 1.84–7.56). For every unit increase in the number of drugs prescribed, the odds of prescribing antibiotics increased by 2.44 units (COR 2.44; 95% CI 2.06–4.32).
Conclusions
The current study found that antibiotics were overused in both adults and children with acute diarrheal diseases at Hiwot Fana Specialized University Hospital. The number of antibiotics prescribed was significantly associated with the patient’s age and the number of medications prescribed. To reduce antibiotic overuse, health professionals have to follow the national standard treatment guidelines.
Keywords: Diarrhea, Antibiotics utilization, Acute diarrheal disease, Cross-sectional study
Background
Antibiotics are medicines that are currently used worldwide to treat bacterial infections in both humans and animals [1, 2]. They work by killing the bacteria or making it difficult for bacteria to proliferate and flourish [3, 4]. A new antibiotic is brought onto the market frequently, leaving doctors little time to thoroughly familiarize themselves with the new medications while also allowing microbes plenty of opportunities to evolve various forms of resistance to secure their survival [5]. Antibiotics can be lifesaving in the treatment of bacterial infections and are the most commonly prescribed drugs among all medications. Their indiscriminate use increases the risk of antibiotic resistance, necessitating more cautious prescribing for the treatment of bacterial infections [6–8].
Antibiotics agent misuse raises therapy costs, adverse drug reactions (ADRs), and patient mortality [9]. Inappropriate antibiotic use is defined as using antibiotics in a way that minimizes the therapeutic effects while increasing toxicity and resistance development. In Ethiopia, there is evidence of antibiotic misuse by healthcare providers, unskilled practitioners, and drug consumers. These, together with the rapid spread of resistant bacteria and insufficient surveillance, will exacerbate the problem [10, 11]. Several studies have found various types of antibiotic misuse in hospital settings in both developing and developed countries, which raises the costs of treating bacterial infections and increases antibiotic resistance [12–16].
Diarrhea is regarded the passing of three or more loose or liquid stools per day. The passing of formed stools on a regular basis is not diarrhea, nor is the passing of loose, “pasty” stools by breastfed babies [17]. There are three distinct clinical kinds of diarrhea: acute bloody diarrhea, commonly known as dysentery; acute watery diarrhea, which lasts several hours or days; and persistent diarrhea, which lasts 14 days or longer [18]. Viruses are the main cause of acute diarrhea both in developed and underdeveloped nations, especially during the winter. No matter the etiology or severity of the process, supportive rehydration therapy is the cornerstone of treatment, and its fast and early adoption is linked to a positive outcome. It should also be combined with proper nutritional support [7, 19].
Since pathogens cannot be identified in more than 90% of diarrhea cases, empirical antibiotic therapy is advised. The clinical value of empiric antibiotic therapy should, however, be evaluated against the risk of side effects and the possibility of removing healthy bacteria [20]. Resistance is frequently linked to increased antibiotic use in hospitals. The rates of resistance shown in multidrug-resistant nosocomial infections are significantly influenced by the usage patterns of antibiotics [21–23]. The expense of treatment has gone up along with the increased morbidity and mortality in many patients due to the rising resistance [10, 22]. The ability of the underprivileged population to access contemporary healthcare will unquestionably be compromised by rising healthcare costs. Furthermore, most hospitals in developing countries had a higher than 30% rate of improper antibiotic use [24].
Antibiotic use is estimated to be inappropriate in 20–50% of cases, according to estimates [25]. This leads to more side effects, higher costs, and a high rate of antibiotic resistance (AMR) in community infections [25]. In severe diarrhea, antibiotics are most commonly misused for viral and self-limiting illnesses. About 70–80% of all diarrheal episodes are caused by viral infections, such as rotavirus [26]. Due to the self-limiting nature of acute diarrhea, complexity and length of time required to identify the pathogen, routine use of antibiotics is not advised in the majority of cases [27]. In a joint statement released in 2004, the World Health Organization (WHO) and the United Nations International Children’s Emergency Fund (UNICEF) suggested treating severe diarrhea in children with the low-osmolality oral rehydration solution (ORS) and zinc tablet [28].
Only in cases of serious bloody diarrhea or dysentery are antibiotics advised. Unfortunately, reports from several parts of the world indicated that improper use of antibiotics in the management of diarrhea is widespread [29]. To support the implementation of antibiotic stewardship programs (ASP) in various healthcare settings, antibiotics utilization pattern indicators could be assessed as useful standards [30–32]. For the purpose of developing a regional intervention program to encourage responsible use of antibiotics, prevent the spread of Antimicrobial Resistance (AMR), and lower the cost of acute diarrhea therapy, it is critical to understand the scope and pattern of antibiotic use for acute diarrhea in the community. As a result, this study was carried out at Hiwot Fana Specialized University Hospital, to analyze the pattern of antibiotic use for the treatment of acute diarrheal diseases.
Methods
Study setting and period
This study was carried out at Hiwot Fana Specialized University Hospital, a comprehensive teaching hospital for Haramaya University located in Harar town, 526 km to the east of Ethiopia’s capital, Addis Ababa. It is now the primary teaching and referral hospital in the country’s eastern region. Internal medicine, gynecology, obstetrics, surgery, dentistry, antenatal care, ophthalmology, hospital pharmacy, dermatology, and an antiretroviral therapy clinic are among the services provided by the hospital. From September 1 to September 30, 2022, a cross-sectional study design was used to assess the antibiotics utilization pattern for the management of acute diarrheal diseases in this hospital.
Study design
An institution-based cross-sectional study was conducted retrospectively, using quantitative approach to assess antibiotic utilization pattern for the treatment of acute diarrheal disease.
Source population
The source was all diarrheal patient records at Hiwot Fana Specialized University Hospital.
Study population
The study included patient charts used for the diagnosis and treatment of acute diarrheal disease at Hiwot Fana Specialized University Hospital from August 1, 2021 to August 31, 2022.
Sample size determination and sampling technique
To obtain the largest possible minimum sample size for this study, it was calculated using the single population proportion formula, assuming a 95% confidence interval, a 5% margin of error, and a prevalence of 50% and calculated with following formula:
where n = sample size, Z1−α/2 = standard normal variable at (1 − α) % confidence level and α (level of significance) was taken to be 5% (95% confidence level is used = 1.96), P = prevalence rate estimate for the population (50%), d = margin of the tolerated sampling error (0.5)
As a result, the n value was calculated and found to be around 384. The number of medical cards (population size, N) of patients who were treated for acute diarrheal disease within study period was 3752. Since the population size was less than 10,000 (N = 3752), a reduction formula was utilized using STAT CALC of Epi Info software and the actual sample size was found to be about 332. A systematic sampling technique was used to identify the patient charts. The sampling interval was determined by dividing the total number of patient charts by the sample size, yielding the interval (k = 11), and every 11th chart was selected. The first patient chart was chosen by lottery from the first to the eleventh patient chart, based on the time order of the records.
Data collection tools and procedures
Data abstraction formats were used to collect data retrospectively. The information acquired included the patients’ sociodemographic and clinical features, as well as patterns of antibiotic use over the study period. The patient chart, laboratory data, and medications were all utilized, as well as the prescriber profile. The data collection approach includes essential points that can quantitatively address main drug usage issues during antibiotic use. Every relevant fact was captured in the patient’s medication records.
Study variables
Explanatory variables included gender, age, duration, prescriber’s profession, and laboratory tests of stool characteristics, while the dependent variable was antibiotic utilization pattern.
Data processing and analysis
IBM SPSS Statistics version 27 was used to process and analyze the collected data. To provide the frequency and percentage distributions of the variables included in the study, descriptive statistics were used, followed by cross-tabulation. The outcome was presented in the form of narratives, tables, and figures.
Data quality control
A pretest was performed at the Jinela Health Center to determine whether the data collection format was valid and reliable, and the completeness of the data collection format was checked prior to the actual data collection. Data cleaning was also performed accordingly.
Results
Sociodemographic characteristics of the patients
There were 3752 patient records documented as diagnosed with acute diarrheal diseases within study period (August 1, 2022 to August 31, 2022). A total of 332 patient records were included in the study. Among 332 patients, 183 (55.12%) were males and 149 (44.88%) were females. Children under 5 years of age were 48.80% and patients > 65 years were 6.63% (Table 1).
Table 1.
Sociodemographic characteristics of the acute diarrhea patients at Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Study variables | Frequency | Percentage |
|---|---|---|
| Sex of patients | ||
| Male | 183 | 55.12 |
| Female | 149 | 44.88 |
| Age of patients | ||
| < 5 years | 162 | 48.80 |
| 5–12 years | 75 | 22.59 |
| 13–40 years | 38 | 11.45 |
| 41–65 years | 35 | 10.54 |
| > 65 years | 22 | 6.63 |
Clinical characteristics
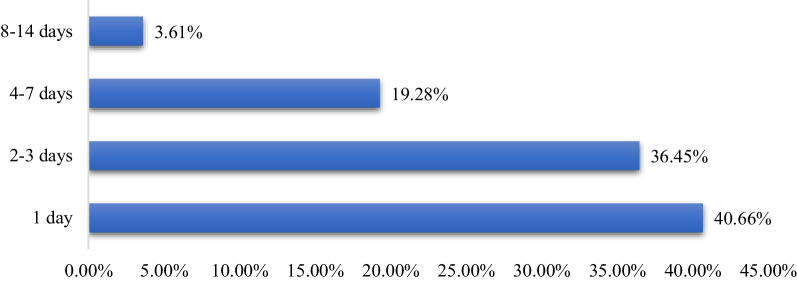
A review for the history of the cases shows that, 121 (36.45%) patients had experienced the illness for 2–3 days (Fig. 1). Most patients reported diarrhea-related illnesses, such as fever 142 (42.77%), vomiting 194 (58.43%), cough 23 (6.93%), chills 7 (2.11%), headache 29 (8.74%), abdominal cramps 109 (32.83%) and loss of appetite 26 (7.83%). From all patients, 84 (25.31%) of them had mild to moderate dehydration, while six patients (1.81%) had severe dehydration, which required intravenous fluid therapy.
Fig. 1.
Duration of diarrhea from onset to treatment for patients diagnosed with acute diarrhea at Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
Stool characteristics
From 332 patients, 237 (71.38%) patients had a stool examination ordered and 119 (50.21%) stool specimens were recorded positive as 81 (34.18%) were with unspecified bacteria and 57 (24.05%) contain amoeba, giardia, and ascariasis. Majority, 85.24% of stools were non-bloody and 14.76% have blood in stools (Fig. 2). The percentage of patients with bloody diarrhea that has received antibiotics was (100%), watery (86.26%) and mucoid (64.36%) (Table 2).
Fig. 2.
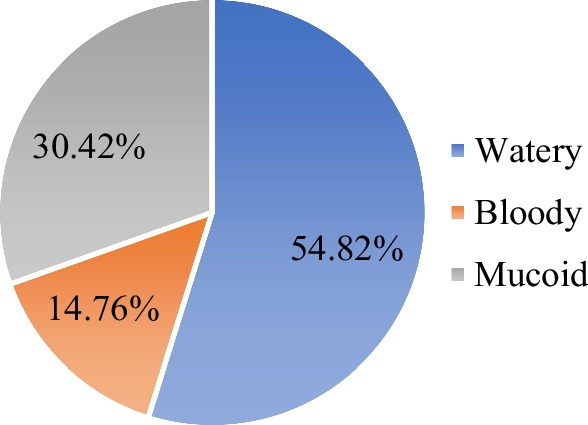
Stool characteristics of patients diagnosed with the acute diarrhea at Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
Table 2.
Antibiotics prescription by age groups and stool characteristics for acute diarrhea patients at Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Antibiotics prescribed | Age groups | Watery (N = 182) | Bloody (N = 49) | Mucoid (N = 101) | |||
|---|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | ||
| Yes (N = 553) | < 5 | 83 | 45.60 | 20 | 40.82 | 33 | 32.67 |
| 5–12 | 31 | 17.03 | 10 | 20.41 | 16 | 15.84 | |
| 13–40 | 17 | 9.34 | 5 | 10.20 | 9 | 8.91 | |
| 41–65 | 14 | 7.69 | 8 | 16.33 | 4 | 3.96 | |
| > 65 | 12 | 6.59 | 6 | 12.24 | 3 | 2.97 | |
| Sub-total | 157 | 86.26 | 49 | 100.00 | 65 | 64.36 | |
| No (N = 73) | < 5 | 14 | 7.69 | 0 | 0.00 | 12 | 11.88 |
| 5–12 | 5 | 2.75 | 0 | 0.00 | 13 | 12.87 | |
| 13–40 | 2 | 1.10 | 0 | 0.00 | 5 | 4.95 | |
| 41–65 | 4 | 2.20 | 0 | 0.00 | 5 | 4.95 | |
| > 65 | 0 | 0.00 | 0 | 0.00 | 1 | 0.99 | |
| Sub-total | 25 | 13.74 | 0 | 0.00 | 36 | 35.64 | |
| Total | 182 | 54.82 | 49 | 14.76 | 101 | 30.42 | |
N number of patients/records
Treatment patterns of acute diarrheal diseases
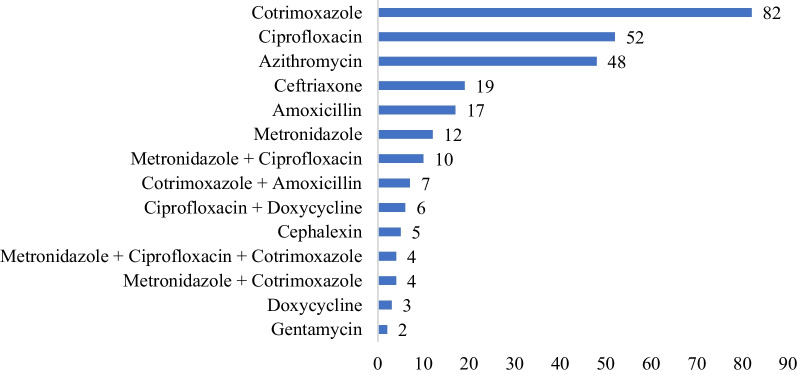
The patient’s record shows that, the number of antibiotics prescribed for single patient ranged from 1 to 3 drugs. About 81.63% of cases received at least one antibiotic drug, while 18.37% of them received no antibiotics. Specifically, 72.28% of patients received one, 8.13% received 2, and 1.21% received 3 antibiotics during the episode of diarrhea. There are nine types of antibiotics that prescribed for acute diarrhea treatment. Cotrimoxazole (30.26%), Ciprofloxacin (19.19%), Azithromycin (17.71%), Ceftriaxone (7.01%) and Amoxicillin (6.27%) were the most frequently prescribed antibiotics. In the same manner, of 162 under five children, 136 (83.95%) were prescribed with at least one antibiotic (Table 5). Of 332 patients, 151 (49.83) patients were prescribed with ORS, while 6 patients prescribed with IV fluid for treatment of dehydration. Other medications prescribed were; Paracetamol 139 (41.87%), Albendazole 23 (6.93%), Mebendazole 17 (5.12%), Ibuprofen 16 (4.83%), Diclofenac 14 (4.22%), Multivitamin 11 (3.31%), Tramadol 10 (3.01%), Metoclopramide 9 (2.71%), Omeprazole 7 (2.11%), Tinidazole 7 (2.111%), and Hyoscine 5 (1.51%) (Fig. 3).
Table 5.
Bivariate analysis of predictors of the prescribed antibiotics for acute diarrheal disease in Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Variables | Antibiotic prescribed | Bivariate analysis | ||||
|---|---|---|---|---|---|---|
| No | Percent | Yes | Percent | COR (95% CI) | P value | |
| Age of patients | 0.013 | |||||
| < 5 years | 26 | 16.05 | 136 | 83.95 | 2.17 (1.61–3.92) | 0.001 |
| 5–12 years | 18 | 24.00 | 57 | 76.00 | 2.46 (1.23–4.36) | 0.003 |
| 13–40 years | 7 | 18.42 | 31 | 81.58 | 1.04 (0.54–1.20) | 0.142 |
| 41–65 years | 9 | 25.71 | 26 | 74.29 | 1.01 (0.33–1.14) | 0.113 |
| > 65 years | 1 | 4.55 | 21 | 95.45 | Ref. | |
| Sex of patients | ||||||
| Male | 14 | 7.65 | 169 | 92.35 | 1.03 (0.81–1.25) | 0.136 |
| Female | 47 | 31.54 | 102 | 68.46 | Ref. | |
| Number of medication prescribed | 0.006 | |||||
| 1 drug | 38 | 84.44 | 7 | 15.56 | Ref. | |
| 2 drugs | 8 | 8.51 | 86 | 91.49 | 0.79 (0.26–1.27) | 0.132 |
| 3 drugs | 13 | 9.42 | 125 | 90.58 | 2.48 (1.39–22.31) | 0.002 |
| 4 drugs | 2 | 4.26 | 45 | 95.74 | 3.25 (1.51–33.52) | 0.001 |
| 5 drugs | 0 | 0.00 | 8 | 100.00 | 7.11 (1.79–36.48) | 0.017 |
COR crude odd ratio, CI confidence interval
*P < 0.05 was considered significant
Fig. 3.
Antibiotics prescribed for the acute diarrhea patients at Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
Adherence to standard treatment guidelines
The adherence to Standard Treatment Guideline (STG) was routinely assessed to show the appropriateness of antibiotics prescribing pattern. The result shows that 116 (34.94%) cases were treated in line with STG, while 216 (65.06%) cases were not treated according to National Standard Treatment Guideline recommendations (Table 3).
Table 3.
Antibiotic utilization patterns based on STG for acute diarrheal diseases in Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Antibiotic usage | Prescribed in line with STG | Not prescribed in line with STG | ||
|---|---|---|---|---|
| Given antibiotic for bloody diarrhea | 49 | 14.76% | 0 | 0.00% |
| Given IV fluid for severe diarrhea | 6 | 1.81% | 0 | 0.00% |
| Given antibiotic for non-bloody diarrhea | 0 | 0.00% | 216 | 65.06% |
| Not given antibiotic for non-bloody diarrhea | 61 | 18.37% | 0 | 0.00% |
| Total | 116 | 34.94% | 216 | 65.06% |
IV intravenous, STG Standard Treatment Guideline
Prescriber profile on acute diarrheal diseases
Most of the acute diarrheal patients were treated by Medical Interns, 149 (44.88%), General Practitioners, 61 (18.37%) and Nurses, 61 (18.37%). About 44 (13.25%) of patient records had no name of prescribers. The better proportion of antibiotic prescription in line with STG was among Senior Physicians (64.71%) and General Practitioners (55.74%), while Nurses (75.41%) and Medical Interns (65.77%) prescriptions were not in line with National Standard Treatment Guideline recommendations (Table 4).
Table 4.
Antibiotic usage for the acute diarrheal diseases of health professionals in Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Prescriber profession | Frequency | % | Antibiotic utilization pattern | |||
|---|---|---|---|---|---|---|
| In line with STG | Not in line with STG | |||||
| Frequency | % | Frequency | % | |||
| Specialist (MD) | 17 | 5.12 | 11 | 64.71 | 6 | 35.29 |
| General practitioners (GP) | 61 | 18.37 | 34 | 55.74 | 27 | 44.26 |
| Medical intern (MD) | 149 | 44.88 | 51 | 34.23 | 98 | 65.77 |
| Nurse | 61 | 18.37 | 15 | 24.59 | 46 | 75.41 |
| Unknown | 44 | 13.25 | 5 | 11.36 | 39 | 88.64 |
| Total | 332 | 116 | 216 | |||
MD medical doctor, STG Standard Treatment Guideline
Antibiotic prescribing predictors
At the bivariate level, the predictors of antibiotic prescribing, age (P = 0.013) and number of medicines prescribed (P < 0.006), were significantly associated with antibiotic prescribing. Antibiotic drugs were 2.46 times more likely to be given to patients under the age of 12 than to patients 65 and older (AOR 2.46, CI 1.23–4.36). When compared to those who received one or two antibiotics per prescription, those who received three or more drugs per prescription were more likely to receive an antibiotic. Hence, patient taking four drugs have more than three times probability of antibiotic prescribed for them (AOR 3.25, CI 1.51–33.52) (Table 5).
The full analysis model fitness test was performed to confirm the suitability and found analysis model containing all predictors was statistically significant, χ2 (5, N = 332) = 76.95, P < 0.001, indicated that the model was able to distinguish between the respondents who had been prescribed antibiotics and those who had not. Hosmer and Lemeshow test also supported the model fitness (χ2 = 6.382, df = 6, P = 0.613). The model as a whole also explained between 58.4% (Cox and Snell R square) and 78.1% (Nagelkerke R square) of the variance in antibiotic prescription and correctly classified 64.33% of those who had one. According to the model’s sensitivity, it correctly identified 59.6% of the group with antibiotic prescribed. Furthermore, the specificity was 67.4%. Age (P = 0.011) and number of medicines prescribed (P < 0.002) significantly associated with antibiotic prescribing after adjusting for potential confounders using multivariate logistic regression (Table 6). There is a significant increase in antibiotic prescribing with an increase in the number of medicines prescribed (P < 0.002). The odds of prescribing antibiotics increased by 2.44 units for every unit increase in the number of medicines prescribed (COR 2.44; 95% CI 2.06–4.32).
Table 6.
Multivariate analysis of predictors of the prescribed antibiotics for acute diarrheal disease in Hiwot Fana Specialized University Hospital; August 1, 2021 to August 31, 2022
| Study variable | Multivariate analysis | |
|---|---|---|
| COR (95% CI) | P value | |
| Age of patients | 0.011 | |
| < 5 years | 2.31 (1.73–3.12) | 0.001 |
| 5–12 years | 2.55 (1.45–3.87) | 0.014 |
| 13–40 years | 1.04 (0.54–1.20) | 0.142 |
| 41–65 years | 1.01 (0.33–1.14) | 0.113 |
| > 65 years | Ref. | |
| Sex of patients | ||
| Male | 1.06 (0.84–1.27) | 0.141 |
| Female | Ref. | |
| No of medication prescribed | 0.002 | |
| 1–2 drugs | Ref. | |
| 3–4 drugs | 2.77 (1.84–7.56) | < 0.001 |
| 5 or more drugs | 6.51 (1.89–47.22) | 0.012 |
COR crude odd ratio, CI confidence interval
*P < 0.05 was considered significant
Discussion
General prescribing pattern
This institution-based cross sectional study has investigated the pattern of antibiotic use for acute diarrheal diseases in Hiwot Fana Specialized University Hospital, Harar, Ethiopia. In the present study, 81.63% of patients have received at least one antibiotic drug. This result is slightly lower than study done at Bishoftu General Hospital, Ethiopia which was 86.8% [33] and far higher than the findings of the studies carried out in different parts of the world such as India with 71% [34], China 60.8% [35], and Thailand 45.1% [36] that had received an antibiotic drug for acute diarrheal disease. There could be a number of causes for the high prescription rate for antibiotics. The high level of routine empirical treatments observed in resource-poor nations is primarily a result of the increased occurrence of infectious diseases in developing countries, which increases the number of antibiotics prescribed [37]. The other factor can be patient pressure on doctors [38].
Antibiotic self-medication was reported to be common and about 44–45.1% in Ethiopia and Eritrea, according to several studies and a comprehensive review [39–41]. This finding may indicate that patients are more likely to directly or indirectly request antibiotic prescriptions from doctors as they are heavily involved in self-medicating with antibiotics [42]. The trend of King Chulalongkorn Memorial Hospital, Thailand with better prescribing pattern may be due to advanced practice and knowledge toward antibiotics rational use, enhanced education and control of over the counter drugs and better trend of following the standard treatment guideline recommendations [36].
For the 332 patients treated for acute diarrhea that were included in the current study, a total of 737 medications were prescribed, resulting in an average of 2.72 drugs per prescription, which is similar to study done in south India with 2.7 [43], but much higher than the WHO standard (1.6–1.8) [44], as well as some results from the comparable investigations carried out across Ethiopia, which revealed an average of 1.64–1.90 medications per encounter [45–52]. However, when compared to several other study results from Ethiopia, Sudan, India, and Saudi Arabia, which were found an average value of 2.02–4.2 medicines per encounter, this number is the comparable one [30, 31, 53–60]. This shows that, prescribers should restrict medicine prescriptions to only patients that are absolutely essential, because polypharmacy can expose patients to unfavorable drug effects and raise patient costs.
The percentage of encounters in this study when at least one antibiotic was prescribed was 41.52%, which is much higher than the WHO standard value of 20–26.2%. This result is comparable with study done in Bahawalpur, Pakistan which was 48.6% [61]. Similar studies carried out in various nations indicated that a percentage of encounters with antibiotics were between 9.1 and 38.4%, which is less than the result reported by the current study [31, 43, 47, 48, 56]. On the other hand, the result is lower than those of other comparable studies with a range of 52.3–75.1% [51, 52, 54, 55, 59, 61, 62].
In present study, 83.95% of children under 5 years with acute diarrhea have received at least one antibiotic drug which is lower than study done in Bishoftu General Hospital, Ethiopia with 92.6% [33]. However, higher than the findings of the studies conducted in Central Region Province of Thailand, Delhi, India, and Puducherry, India, where the percentage of patients prescribed on antibiotics were 72.6%, 64%, and 22%, respectively [63–65]. The percentage of acute diarrheal patients treated not in line with STG was 65.06%, which is slightly better than other study done in Bishoftu, Ethiopia with 72.3% [33]. However, the result is higher in percentage than the finding of the study carried out in China at 51.3% [35] and Thailand at 48.9% [36]. However, other study conducted in South Thailand, indicated that 73.8% of antibiotics prescribed were in line with STG for diarrheal disease treatment [66]. The most commonly prescribed drugs for acute diarrheal diseases were Cotrimoxazole (30.6%), Ciprofloxacin (19.19%) and Azithromycin (17.71%) in the present study. The finding is different from other study conducted in Thailand [63] and Ethiopia [33] as both studies indicated greater than 50% prescription was only Cotrimoxazole.
Antibiotic prescribing predictors
This study discovered a significant correlation between patient age and number of medications for antibiotics prescribed. Antibiotic prescriptions were found to be associated with being under the age of 12 as they got the highest proportion of antibiotics when compared to the other patient categories which is similar to research from Eritrea [40], Bangladesh [67], Yemen [68], and Cameroon [69]. According to the results of the current study, prescribing three or more medications per prescription was highly associated with prescribing antibiotics.
Antibiotics were about 2.55 times more likely to be prescribed to patients under the age of 12 than to subjects of 65 years and older (AOR 2.55, 95% CI 1.45–3.87). When compared to subjects who received one or two drugs per prescription, those who received more than two drugs were 2.77 times more likely to receive an antibiotic (AOR 2.77, 95% CI 1.84–7.56). The odds of prescribing antibiotics were increased by 2.44 units for every one unit increase in the number of medicines prescribed (COR 2.44; 95% CI 2.06–4.32). It is consistent with study done in Asmara, which found that probabilities increased by 2.02 for every one-unit increase (P < 0.001; OR 2.02; 95% CI 1.62–2.52) [40] and Zambia, where it was shown that odds rise by 2.7 for every one-unit increase (P < 0.001; OR 2.68, 95% CI 2.20–3.25) [70].
The current study limitation is that, it was conducted in only one hospital and so cannot be generalized to other facilities. However, because Hiwot Fana Specialized University Hospital is the only tertiary hospital in the research area, the current study can provide a picture of how antibiotics are used in East Ethiopia. This study discovered a significant incidence of incorrect antibiotic use, which may fuel rising antimicrobial resistance and associated costs on a national and worldwide scale. In general, the study determined the prevalence of antibiotic use, identified the types of antibiotics used in the treatment of acute diarrheal illness, and rated prescribers’ adherence to standard treatment guidelines.
Conclusion
The present study revealed that there was high overuse of antibiotics for both adults and children with acute diarrheal disease in Hiwot Fana Specialized University Hospital. The most common antibiotics prescribed were Cotrimoxazole, Ciprofloxacin and Azithromycin. The proportion of prescriptions containing an antibiotic was 41.52%, which is much higher than WHO-recommended standard (20–26.2%). The average number of prescriptions per encounter fell just short of WHO recommendations, and adherence to the Standard Treatment Guideline (STG) was also inadequate. Antibiotic prescribing revealed a strong correlation with patient age and the number of medications per prescription. Thus, to reduce antibiotics overuse, health professionals have to follow the national standard treatment guidelines.
Acknowledgements
The authors would like to thank the Hiwot Fana Specialized University Hospital Management and Record Department for their permission and support in carrying out the study, as well as all staff at the working card center who assisted us greatly in data collection. Our deep appreciation also goes to data collectors.
Abbreviations
- ADR
Adverse drug reaction
- AMR
Antimicrobial resistance
- ASP
Antimicrobial Stewardship Program
- EML
Essential Medicine List
- ORS
Oral rehydration salt
- STG
Standard treatment guideline
- UNICEF
United Nations International Children’s Emergency Fund
- WHO
World Health Organization
Author contributions
BD, SY, ZK and AM contributed to the original idea. BD and SY drafted the manuscript. ZK and AM participated in drafting the manuscript. BD and ZK developed the survey tool. BD and ZK collected the data with data collectors. BD performed data analysis and drafted the results. SY and AM critically reviewed and edited the manuscript. BD oversaw the entire project, from survey tool development to data analysis. The final manuscript was reviewed by all of the authors. All authors read and approved the final manuscript.
Funding
This study received no specific funding from public, commercial, or not-for-profit funding agencies.
Availability of data and materials
On reasonable request, the data used and analyzed during this study can be obtained from the corresponding author.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Research and Ethics Committee of Dire Dawa University’s College of Medicine and Health Science (DDU/REC/P/096/22). To protect study participants’ privacy and the confidentiality of personal data, the names of study participants were withheld from the data collection format and all others procedures were carried out in accordance with the applicable guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they do not have any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Beyene Dereje, Email: beyexderie23@gmail.com.
Shegaye Yibabie, Email: shegyg8@gmail.com.
Zenebe Keno, Email: zenebekano21@gmail.com.
Alemayehu Megersa, Email: aliemegersa@gmail.com.
References
- 1.Cunha CB. Antimicrobial Stewardship Programs: principles and practice. Med Clin North Am. 2018;102:797–803. doi: 10.1016/j.mcna.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Katzung BG. Basic and clinical pharmacology. 14. New York: McGraw-Hill Education; 2017. [Google Scholar]
- 3.Browne AJ, Chipeta MG, Haines-Woodhouse G, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Heal. 2021;5:e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Kotwani A, Wattal C, Joshi PC, Holloway K. Irrational use of antibiotics and role of the pharmacist: an insight from a qualitative study in New Delhi, India. J Clin Pharm Ther. 2012;37:308–312. doi: 10.1111/j.1365-2710.2011.01293.x. [DOI] [PubMed] [Google Scholar]
- 6.Nicolini G, Sperotto F, Esposito S. Combating the rise of antibiotic resistance in children. Minerva Pediatr. 2014;66:31–39. [PubMed] [Google Scholar]
- 7.Meisenheimer ES, Epstein C, Thiel D. Acute diarrhea in adults. Am Fam Physician. 2022;106:72–80. [PubMed] [Google Scholar]
- 8.Zinner SH. Antibiotic use: present and future. New Microbiol. 2007;30:321–325. [PubMed] [Google Scholar]
- 9.Patel N, Patel D, Desai H. Antimicrobial utilization pattern among pediatric inpatients of a tertiary care hospital in Central Gujarat. Natl J Physiol Pharm Pharmacol. 2019;9:1. [Google Scholar]
- 10.World Health Organization . WHO expert committee on specifications for pharmaceutical preparations. World Health Organization technical report series. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 11.DACA. Antimicrobial use, resistance and containment baseline survey syntheses of finding. 2009;1–160.
- 12.Silva ML, Cargnello C, Aulois-Griot M, Dumartin C. Antibiotic misuse: how to evaluate the costs? Med Mal Infect. 2019;49:485–494. doi: 10.1016/j.medmal.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Kaier K. Economic implications of the dynamic relationship between antibiotic use and hospital-acquired infections. Value Health. 2012;15:87–93. doi: 10.1016/j.jval.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Stein K, Farmer J, Singhal S, Marra F, Sutherland S, Quiñonez C. The use and misuse of antibiotics in dentistry: a scoping review. J Am Dent Assoc. 2018;149:869–884.e5. doi: 10.1016/j.adaj.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Machowska A, Stålsby Lundborg C. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health. 2018 doi: 10.3390/ijerph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Sidibi AM, Shen X, Dao K, Maiga A, Xie Y, Hesketh T. Lack of antibiotic knowledge and misuse of antibiotics by medical students in Mali: a cross-sectional study. Expert Rev Anti Infect Ther. 2021;19:797–804. doi: 10.1080/14787210.2021.1857731. [DOI] [PubMed] [Google Scholar]
- 17.Valerie Nemeth; Nicholas Pfleghaar. Diarrhea—StatPearls—NCBI Bookshelf. StatPearls Publishing. 2021. [PubMed]
- 18.Gessesse DN, Tarekegn AA. Prevalence and associated factors of diarrhea among under-five children in the Jawi district, Awi Zone Ethiopia, 2019. Community based comparative cross-sectional study. Front Pediatr. 2022;10:1–9. doi: 10.3389/fped.2022.890304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodgame RW. Viral causes of diarrhea. Gastroenterol Clin North Am. 2001;30:779–795. doi: 10.1016/S0889-8553(05)70210-7. [DOI] [PubMed] [Google Scholar]
- 20.Istúriz RE, Carbon C. Antibiotic use in developing countries. Infect Control Hosp Epidemiol. 2000;21:394–397. doi: 10.1086/501780. [DOI] [PubMed] [Google Scholar]
- 21.Yates RR. New intervention strategies for reducing antibiotic resistance. Chest. 1999;115:24S–27S. doi: 10.1378/chest.115.suppl_1.24S. [DOI] [PubMed] [Google Scholar]
- 22.Patterson JE. Antibiotic utilization. Chest. 2001;119:426S–430S. doi: 10.1378/chest.119.2_suppl.426S. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein RA. Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerg Infect Dis. 2001;7:188–192. doi: 10.3201/eid0702.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farthing M, Salam MA, Lindberg G, Dite P, Khalif IS-LE. Acute diarrhea in adults and children: a global perspective. World Gastroenterology Organisation global guidelines. J Clin Gastroenterol. 2012;47:12–20. doi: 10.1097/MCG.0b013e31826df662. [DOI] [PubMed] [Google Scholar]
- 25.Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991–1999. Ann Intern Med. 2003;138:1991–1999. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363:641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- 27.Wingate D, Phillips SF, Lewis SJ, Malagelada JR, Speelman P, Steffen R, Tytgat GNJ. Guidelines for adults on self-medication for the treatment of acute diarrhoea. Aliment Pharmacol Ther. 2001;15:773–782. doi: 10.1046/j.1365-2036.2001.00993.x. [DOI] [PubMed] [Google Scholar]
- 28.Walker CLF, Fontaine O, Young MW, Black RE. Zinc and low osmolarity oral rehydration salts for diarrhoea: a renewed call to action. Bull World Health Organ. 2009;87:780–786. doi: 10.2471/BLT.08.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatnagar S, Lodha R, Choudhury P, Sachdev HPS, Shah N, Narayan S, Wadhwa N, Makhija P, Kunnekel K, Ugra D. Recommendations IAP guidelines 2006 on management of acute diarrhea. Indian Pediatr. 2007;44:380. [PubMed] [Google Scholar]
- 30.Atif M, Azeem M, Saqib A, Scahill S. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control. 2017;6:1–12. doi: 10.1186/s13756-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atif M, Sarwar MR, Azeem M, Umer D, Rauf A, Rasool A, Ahsan M, Scahill S. Assessment of WHO/INRUD core drug use indicators in two tertiary care hospitals of Bahawalpur, Punjab, Pakistan. J Pharm Policy Pract. 2016;9:1–8. doi: 10.1186/s40545-016-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadi F, Zarei E. Prescribing patterns of rural family physicians: a study in Kermanshah Province, Iran. BMC Public Health. 2017;17:1–7. doi: 10.1186/s12889-017-4932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tulu S, Tadesse T, Alemayehu Gube A. Assessment of antibiotic utilization pattern in treatment of acute diarrhoea diseases in Bishoftu General Hospital, Oromia Ethiopia. Adv Med. 2018;2018:1–6. doi: 10.1155/2018/2376825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathak D, Pathak A, Marrone G, Diwan V, Lundborg CS. Adherence to treatment guidelines for acute diarrhoea in children up to 12 years in Ujjain, India—a cross-sectional prescription analysis. BMC Infect Dis. 2011;11:1–9. doi: 10.1186/1471-2334-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou FQ, Wang Y, Li J, Wang GQ, Liu Y. Management of acute diarrhea in adults in China: a cross-sectional survey. BMC Public Health. 2013 doi: 10.1186/1471-2458-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supcharassaeng S, Suankratay C. Antibiotic prescription for adults with acute diarrhea at King Chulalongkorn Memorial Hospital, Thailand. J Med Assoc Thail. 2011;94:545–550. [PubMed] [Google Scholar]
- 37.Castelnuovo G. Empirically supported treatments in psychotherapy: towards an evidence-based or evidence-biased psychology in clinical settings? Front Psychol. 2010;1:1–10. doi: 10.3389/fpsyg.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stivers T, Timmermans S. Arriving at no: patient pressure to prescribe antibiotics and physicians’ responses. Soc Sci Med. 2021;290:114007. doi: 10.1016/j.socscimed.2021.114007. [DOI] [PubMed] [Google Scholar]
- 39.Ateshim Y, Bereket B, Major F, Emun Y, Woldai B, Pasha I, Habte E, Russom M. Prevalence of self-medication with antibiotics and associated factors in the community of Asmara, Eritrea: a descriptive cross sectional survey. BMC Public Health. 2019;19:726. doi: 10.1186/s12889-019-7020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amaha ND, Weldemariam DG, Abdu N, Tesfamariam EH. Prescribing practices using WHO prescribing indicators and factors associated with antibiotic prescribing in six community pharmacies in Asmara, Eritrea: a cross-sectional study. Antimicrob Resist Infect Control. 2019;8:1–7. doi: 10.1186/s13756-019-0620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekonnen S, Getnet M, Dumessa E. Epidemiology of self-medication in Ethiopia: a systematic review and meta-analysis of observational studies. BMC Pharmacol Toxicol. 2018;19:1–12. doi: 10.1186/s40360-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tesfamariam S, Anand IS, Kaleab G, Berhane S, Woldai B, Habte E, Russom M. Self-medication with over the counter drugs, prevalence of risky practice and its associated factors in pharmacy outlets of Asmara, Eritrea. BMC Public Health. 2019;19:1–9. doi: 10.1186/s12889-019-6470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ps P, Rudra JT, Vasanthi P, Sushitha U, Sadiq MJ, Narayana G. Assessment of drug use pattern using World Health Organization core drug use indicators at Secondary Care Referral Hospital of South India. CHRISMED J Health Res. 2015;2:223. doi: 10.4103/2348-3334.158683. [DOI] [Google Scholar]
- 44.Ghei P. How to investigate drug use in health facilities. Selected drug use indicators. Health Policy (New York) 1995;34:73. doi: 10.1016/0168-8510(95)90068-3. [DOI] [Google Scholar]
- 45.Bekele NA, Tadesse J. Prescription auditing based on World Health Organization (WHO) prescribing indicators: a case of Dilla University referral hospital. J Drug Deliv Ther. 2018;8:21–25. doi: 10.22270/jddt.v8i6-s.2165. [DOI] [Google Scholar]
- 46.Admassie E, Begashaw B, Hailu W. Assessment of drug use practices and completeness of prescriptions in Gondar University Teaching Referral Hospital. IJPSR. 2013;4:265–275. [Google Scholar]
- 47.Asrade B. Assessment of completeness of prescription and rational drug use practice at Felege Hiwot Referral Hospital, North West Ethiopia. J Health Med Nurs. 2019;60:16–25. [Google Scholar]
- 48.Yilma Z, Mekonnen T, Siraj EA, Agmassie Z, Yehualaw A, Debasu Z, Tafere C, Ararsie M. Assessment of prescription completeness and drug use pattern in Tibebe-Ghion comprehensive specialized hospital, Bahir Dar, Ethiopia. Biomed Res Int. 2020 doi: 10.1155/2020/8842515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University teaching and referral hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res. 2013 doi: 10.1186/1472-6963-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tigist G, Yohannes T, Mekonnen S. Evaluation of the prescribing practice based on World Health Organization core prescribing indicators in Hiwot Fana Specialized University Hospital, Harar, eastern Ethiopia. J Drug Deliv Ther. 2016;6:25–30. [Google Scholar]
- 51.Dessie B, Atalaye G, Diress E, Getahun A. Practice towards rational drug use at Finotselam and Asirade Zewudie Hospitals based on WHO core drug use indicators, Northwest Ethiopia. Sci World J. 2020;2020:1–5. doi: 10.1155/2020/1634294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasahun GG, Demoz GT, Asayehegn AT, Gebrehiwot LG, Tesfay GM, Desta DM. Evaluation of pattern of drug use in tertiary health care setting in central Tigray using WHO prescribing indicators. Adv pharmacoepidemiol drug saf. 2020;9:228–5. [Google Scholar]
- 53.Mengistu G, Misganaw D, Tsehay T, Alemu BK, Bogale K. Assessment of drug use pattern using WHO core prescribing indicators at outpatient settings of governmental hospitals in dessie town. Drug Healthc Patient Saf. 2020;12:237–244. doi: 10.2147/DHPS.S266749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gashaw T, Sisay M, Mengistu G, Amare F. Investigation of prescribing behavior at outpatient settings of governmental hospitals in eastern Ethiopia: an overall evaluation beyond World Health Organization core prescribing indicators. J Pharm Policy Pract. 2018;11:1–11. doi: 10.1186/s40545-018-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabie D, Kheder SI. Assessment of prescribing and dispensing practices based on WHO core prescribing indicators in hospital and community pharmacies in Khartoum State—Sudan. 2020.
- 56.Mahmood A, Elnour AA, Ali AAA, Hassan NAGM, Shehab A, Bhagavathula AS. Evaluation of rational use of medicines (RUM) in four government hospitals in UAE. Saudi Pharm J. 2016;24:189–196. doi: 10.1016/j.jsps.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamo DB, Alemu BK. Rational drug-use evaluation based on world health organization core drug-use indicators in a Tertiary Referral Hospital, Northeast Ethiopia: a cross-sectional study. Drug Healthc Patient Saf. 2020;12:15–21. doi: 10.2147/DHPS.S237021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akodo J, Chijioke-Nwauche I. Evaluation of drug use pattern in Lulu Brigg’s Health Centre, University of Port Harcourt, Nigeria using WHO, prescribing indicators. Pharmainnov J. 2017;6:506–510. [Google Scholar]
- 59.Sisay M, Mengistu G, Molla B, Amare F, Gabriel T. Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross sectional study. BMC Health Serv Res. 2017;17:1–9. doi: 10.1186/s12913-017-2097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yousif BME, Supakankunti S. General practitioners’ prescribing patterns at primary healthcare centers in national health insurance, Gezira, Sudan. Drugs Real World Outcomes. 2016;3:327–332. doi: 10.1007/s40801-016-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atif M, Sarwar MR, Azeem M, Naz M, Amir S, Nazir K. Assessment of core drug use indicators using WHO/INRUD methodology at primary healthcare centers in Bahawalpur, Pakistan. BMC Health Serv Res. 2016;16:1–9. doi: 10.1186/s12913-016-1932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shelat PR, Kumbar SK. Analysis of out door patients’ prescriptions according to World Health Organization (WHO) prescribing indicators among private hospitals in Western India. J Clin Diagn Res. 2015;9:FC01-4. doi: 10.7860/JCDR/2015/12724.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howteerakul N, Higginbotham N, Dibley MJ. Antimicrobial use in children under five years with diarrhea in a central region province, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:181–187. [PubMed] [Google Scholar]
- 64.Singh J, Bora D, Sachdeva V, Sharma RS, Verghese T. Prescribing pattern by doctors for acute diarrhoea in children in Delhi, India. J Diarrhoeal Dis Res. 1995;13:229–231. [PubMed] [Google Scholar]
- 65.Priyadarshini K, Raj V, Balakrishnan S. Audit of use of antibiotics and zinc supplement in childhood diarrhea. J Pharmacol Pharmacother. 2013;4:204–205. doi: 10.4103/0976-500X.114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osatakul S, Puetpaiboon A. Appropriate use of empirical antibiotics in acute diarrhoea: a cross-sectional survey in southern Thailand. Ann Trop Paediatr. 2007;27:115–122. doi: 10.1179/146532807X192480. [DOI] [PubMed] [Google Scholar]
- 67.Syed A, Mosaddek M. Prescribing practice of antibiotics for outpatients in Bangladesh: rationality analysis. Am J Pharmacol. 2018;1:1008. [Google Scholar]
- 68.Alshakka M, Said K, Babakri M, Ansari M, Aldhubhani A, Azmi Hassali M, Mohamed Ibrahim MI. A study on antibiotics prescribing pattern at outpatient department in four hospitals in Aden-Yemen. J Pharm Pract Community Med. 2016;2:88–93. doi: 10.5530/jppcm.2016.3.5. [DOI] [Google Scholar]
- 69.Chem ED, Anong DN, Akoachere JFKT. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS ONE. 2018;13:1–18. doi: 10.1371/journal.pone.0193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lukali V, Michelo C. Factors associated with irrational drug use at a district hospital in Zambia: patient record-based observations. Med J Zambia. 2015;42:25–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On reasonable request, the data used and analyzed during this study can be obtained from the corresponding author.