Abstract
Importance
Autism and eating disorders cooccur at high rates, with autism impacting the efficacy of eating disorder treatments and outcomes. Females are underdiagnosed with autism and diagnosed later in life than their male counterparts.
Objective
The purpose of this study was to define the incidence of eating disorder professionals identifying autism in female adolescents and young adults engaged in treatment for an eating disorder.
Design
The research design is a cross-sectional retrospective records review. The charts reviewed were from the medical records of forty assigned-at-birth females between the ages of 13–25 who received treatment for an eating disorder at the partial hospitalization program (PHP) level of care between 2020 and 2022.
Main outcomes
Upon entering PHP for an eating disorder, 10% of the study participants had a pre-existing diagnosis of autism. A total of 27.5% of participants had clinical suspicion of autism. The number of autism traits that an individual possessed directly correlated with the number of calendar days in treatment.
Conclusion
Eating disorder professionals in this study identified autism in 17.5% of adolescent and young adult females who entered PHP treatment for an eating disorder who had not previously been diagnosed with autism. Eating disorder professionals can anticipate that individuals with autism may be in treatment for a longer duration. Further studies should explore effective treatment measures for those with autism in treatment for an eating disorder.
Keywords: Feeding and eating disorder, Autism, Female, Adolescent, Diagnosis
It is well established that autism and eating disorders often co-occur. Females are less likely to be diagnosed with autism and tend to receive a diagnosis later in life than males. Therefore, during eating disorder treatment at higher levels of care, eating disorder professionals may be the first to recognize autism in their female patients. A review of chart records from female teens and young adults receiving eating disorder treatment at the partial hospitalization level of care between 2020–2022, revealed that 10% had a diagnosis of autism upon entering treatment and an additional 17.5% received a new diagnosis during treatment. The number of autism traits present in an individual directly correlated to longer treatment durations.
Introduction
The link between autism and eating disorder was first described in 1983 by Swedish child and adolescent psychiatry professor Christopher Gillberg. He observed that three boys with autism had female cousins with an eating disorder and hypothesized that anorexia nervosa (AN) was the female version of autism [1]. Once thought to be a male-dominant diagnosis, autism has shifted to a more realistic gender ratio of 3:1 [2] in recent decades and affects one in 44 children [3].
However, autism is over-represented in individuals receiving treatment for an eating disorder, with co-occurrence rates of 23–32% [4, 5]. Potential autism-specific mechanisms underlying eating difficulties include deficits in social interaction and relationships, poor sense of self and identity, difficulties with emotions, and a need for control and predictability [6]. Additionally, individuals with autism have higher rates of picky eating, feeding problems, gastrointestinal symptoms, and sensory issues [7].
Females with autism continue to be underdiagnosed and diagnosed later in life than their male counterparts [8, 9]. Theories about the underdiagnosis in females include that the Diagnostic and Statistical Manual, Fifth Edition (DSM-5) may favor male-typical presentations, females are more likely to “mask” or “camouflage” their autism traits, or there is a gender bias in diagnosis even when the diagnostic criteria are met [10]. A functional MRI study of males and females with autism revealed that their brains are functionally organized differently, contributing to their clinical symptoms in distinct ways [11].
In the United States, one in five females develops an eating disorder by the age of 40 [12].
A 2018 longitudinal study evaluated the hypothesis that starvation, due to prolonged restriction, can induce autism traits that would resolve with adequate nutritional rehabilitation. However, the authors demonstrated that autism traits and diagnosis persisted after 12 months of treatment [13]. There is, therefore, a benefit to identifying autism in individuals with an eating disorder early in the treatment course. Individuals with an eating disorder and autism are more likely to have difficulties with cognitive behavioral therapy and group therapy, which are eating disorder treatment standards of care [14]. Additionally, these individuals have poorer outcomes regarding their eating disorder recovery and prognosis of mental health outcomes and socioeconomic function [13, 15]. These findings underscore the importance of identifying autism in individuals with an eating disorder as a prognostic factor.
Partial hospitalization treatment for an individual with an eating disorder is often facilitated as a day program through a multidisciplinary team of professionals that interact with their patients intensively for more than forty hours per week, across the span of several months. Therefore, eating disorder professionals may be the first clinicians to identify autism traits in their patients, particularly females. The purpose of this study was to define the incidence of eating disorder professionals identifying autism in female adolescents and young adults engaged in treatment for an eating disorder.
The article most closely related to this research study was a 2020 review article in which Brown and Stokes [16] discuss the reasons why eating disorder professionals may be the first providers to recognize autism in their female patients. Additionally, the authors reviewed autism and eating disorder comorbidity, treatment, and priorities for research and clinical practice. However, no original research exists on the subject. In a 2021 mixed-methods study of females with autism, parents of teenagers with autism, and their health care providers, Babb et al. [17] had an “unexpected finding” that all adult and adolescent females had received their autism diagnosis after they engaged in eating disorder services. The average age of AN diagnosis in the group was 17, but the average autism diagnosis was 29 years old. Similarly, in a semi-structured interview study of individuals with AN and autism, the majority of participants diagnosed with autism received their diagnosis after receiving eating disorder treatment, with the average age of diagnosis being 23.5 years of age [18].
In a large retrospective study, individuals with AN between the ages of 8–32 and matched controls, non-eating disorder diagnoses were monitored for a median of nine years [19]. The non-eating disorder diagnoses evaluated were substance use disorders, schizophrenia or psychosis, affective disorders, phobia or anxiety disorders, obsessive–compulsive disorders, adjustment disorders, personality disorders, and autism. For the individuals diagnosed with AN, 25% received at least one of those non-eating disorder diagnoses after two years [19]. At 20 years, 55% had received a non-eating disorder diagnosis [19]. At the onset of the study, 0.6% of the individuals with AN had a pre-existing diagnosis of autism (vs. 0.2% of the controls). The follow-up data at all time intervals yielded too few autism observations to be statistically significant. Therefore, the authors did not include follow-up data in the findings.
Currently, a gap exists in the literature defining the incidence of a new autism diagnosis in adolescent or young adult females after engaging in treatment of an eating disorder. To this author’s knowledge, no original research has been conducted on this topic, nor has the literature grossly estimated this incidence. In the present study, this author aimed to answer the following research question: “What is the incidence of new or suspected autism diagnosis in female adolescents and young adults after initiating eating disorder treatment?” Additionally, this study identified the risk factors associated with a new autism diagnosis and evaluated the impact of the presence of autism traits on treatment outcomes. It was hypothesized that the presence of autism in the general population.
Methods
Participants
The research design was a cross-sectional retrospective records review. The charts reviewed were from the medical records of 40 individuals that concluded treatment for an eating disorder at the Recovery and Wellness Center of Eastern Washington at the partial PHP level of care between 2020 to 2022. Eligible study participants were assigned-at-birth-females between the age of 13 and 25 at the time of PHP admission for an eating disorder that met the DSM-5-TR [20] criterion of AN, bulimia nervosa, binge eating disorder, atypical anorexia nervosa, avoidant-restrictive food intake disorder, purging disorder, or unspecified feeding and eating disorder.
The Recovery and Wellness Center of Eastern Washington’s PHP level of care is a day program run 8.5 h per day, five days per week. Determination for this level of care is made in accordance with the American Psychological Association’s eating disorder level of care guidelines. A transition from PHP to the intensive outpatient program (IOP) level of care is made at the recommendation of the individual’s treatment team once the individual has met treatment goals related to reduction of eating disorder behaviors. The IOP level of care is four hours a day three to five days per week.
Data collection
Patient age, ethnicity, and eating disorder diagnosis at the time of admission were collected from the patient charts. The presence of co-morbid mental health conditions from the medical discharge summary were collected, including major depressive disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive compulsive disorder, attention deficit hyperactivity disorder, and mood disorder. Mood disorder for the purposes of this study includes bipolar I disorder, bipolar II disorder, disruptive mood dysregulation disorder, and cyclothymic disorder.
The presence of a historical diagnosis of autism, family history of autism, family history of autism in a first-degree relative, and history of developmental delays was collected from the intake medical assessment interview of the patient and a parent or guardian of minors. Autism traits for the purposes of this study are the individual components of the diagnostic criteria for autism per the DSM-5-TR [21], including deficits in social-emotional reciprocity, deficits in nonverbal communicative behaviors, deficits in developing, maintaining, and understanding relationships, stimming, rigidity, hyper-fixated interests, and sensory processing disorder. The presence of these traits was collected from chart notes from the medical provider and psychotherapists throughout the course of treatment.
The clinical suspicion of autism, referral for neuropsychological testing, and a new autism diagnosis was included from medical chart notes. The number of days in treatment at the PHP and intensive outpatient program level of care and the total number of calendar days from admission to discharge is reported. Individuals that were engaged in treatment for less than 15 calendar days were excluded, due to less clinician opportunity to observe autism traits. Whether or not the patient has previously been engaged in a higher level of care (residential, PHP, or IOP) for an eating disorder or whether they were a step-down from a residential treatment center to PHP was collected from the admission assessment.
The type of discharge is noted as graduation, discharge, or completion. A designation of graduation indicates that a patient met all expected treatment goals. Completion indicates that a patient reduced eating disorder behaviors and was appropriate for a transition to an outpatient level of care but did not meet all standards of treatment. A discharge refers to either a patient self-discharging from either PHP or IOP or discharged by the facility’s staff at any point in treatment.
Statistical analysis
With the assistance of a statistician, the data sets were run through IBM SPSS software. A one-sample proportions test with Jeffreys 95% CI was run on the sample of 40 patients to answer the primary research question to determine if female adolescents and young adults receiving treatment for an eating disorder will have a higher prevalence of pre-existing, clinical suspicion, and new autism diagnosis than the general population.
An independent-samples t-test was run to determine if there were different percentages of autistic traits exhibited between the two groups. There were no outliers in the data, as assessed by inspection of a boxplot. The homogeneity of variances was assessed by Levene's test for equality of variances. A bivariate analysis using chi-square tests for association was conducted between family history of autism and autism variables. The homogeneity of variances was assessed by Levene's test of homogeneity of variances and a one-way ANOVA was conducted to determine if the percentage of autism traits exhibited were different between the three graduation groups.
Results
Descriptive characteristics
The study participants’ information was assessed using descriptive statistical techniques. Frequencies (n) and percentages (%) of the sample characteristics are presented in Table 1.
Table 1.
Descriptive Characteristics
| Variable | n | % |
|---|---|---|
| White | 29 | 72.5 |
| Hispanic | 10 | 25 |
| Asian | 1 | 2.5 |
| 13–15 years | 17 | 42.5 |
| 16–18 years | 12 | 30 |
| 19–21 years | 4 | 10 |
| 22–25 years | 7 | 17.5 |
| Anorexia nervosa | 11 | 27.5 |
| Atypical anorexia nervosa | 16 | 40 |
| Bulimia nervosa | 5 | 12.5 |
| Avoidant restrictive food intake disorder | 3 | 7.5 |
| Binge eating disorder | 1 | 2.5 |
| Purging disorder | 2 | 5 |
| Unspecified feeding or eating disorder | 2 | 5 |
| Previous eating disorder treatment in higher level of care | 4 | 10 |
| Step-down from a residential treatment center | 7 | 17.5 |
| Graduation | 18 | 45 |
| Discharge | 11 | 27.5 |
| Program completion | 11 | 27.5 |
| Depression | 16 | 40 |
| Anxiety | 23 | 57.5 |
| Post traumatic stress disorder | 5 | 12.5 |
| Obsessive compulsive disorder | 3 | 7.5 |
| Attention deficit hyperactivity disorder | 9 | 22.5 |
| Other mood disorder | 10 | 25 |
| Historical diagnosis of autism | 4 | 10 |
| Family history of autism | 11 | 27.5 |
| First degree relative with autism | 5 | 12.5 |
| History of developmental delays | 8 | 20 |
| Deficits in social-emotional reciprocity | 13 | 32.5 |
| Deficits in nonverbal communicative behaviors | 20 | 50 |
| Deficits in developing, maintaining, an understanding relationships | 21 | 52.5 |
| Stimming | 4 | 10 |
| Rigidity | 16 | 40 |
| Special interests | 7 | 17.5 |
| Sensory processing disorder | 11 | 27.5 |
| Clinical suspicion of autism | 11 | 27.5 |
| Referral for neuropsychological testing | 4 | 10 |
| New autism diagnosis | 5 | 12.5 |
Autism in eating disorder treatment versus general population
A historical diagnosis of autism upon admission to PHP was 10% (95% CI of 3.5% to 22%) and was statistically significantly higher than the estimate of the general population, p < 0.001. The clinical suspicion of autism in eating disorder patients was 27.5% (95% CI of 15.6% to 42.5%) and was statistically significantly higher than the estimate of the general population, p < 0.001. The proportion of new autism diagnoses was 12.5% (95% CI of 4.9% to 25.2%) and was statistically significantly higher than the estimate of the general population, p < 0.001. Figure 1 depicts these values.
Fig. 1.
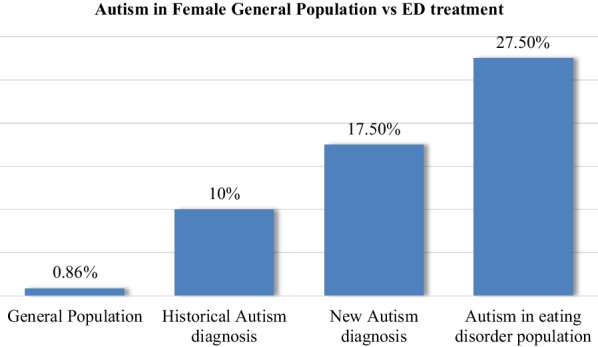
Autism in female general population versus eating disorder treatment
Risk associations
Thirty of the participants had restrictive eating disorders, while 10 had non-restrictive eating disorders. No statistically significant difference was seen in the percentage of autism traits exhibited by individuals with a restrictive eating disorder (M = 33.750, SD = 30.645) and non-restrictive eating disorders (M = 23.750, SD = 22.399), t(38) = 0.947, p = 0.349).
A statistically significant association between clinical suspicion of autism and the lack of a other mood disorder was present, χ2(1) = 5.057, p = 0.025. None of the individuals with a clinical suspicion of autism had a diagnosed other mood disorder versus 34.5% of those with no clinical suspicion of autism.
A statistically significant association between family history of autism and developmental delays was present, χ2(1) = 11.317, p < 0.001. Approximately 55% of those with a family history of autism reported developmental delays versus 7% of those without a family history of autism.
Treatment outcomes
A statistically significant, moderate, positive correlation between the percentage of autism traits exhibited and the number of calendar days of treatment was present, r = 0.388, p = 0.013. Approximately 15.08% of the variation in calendar days of treatment can be attributed to the percentage of autism traits exhibited by the patients. Figure 2 displays a scatterplot of the percent of autism traits and calendar days of treatment that visually depicts the relationship.
Fig. 2.
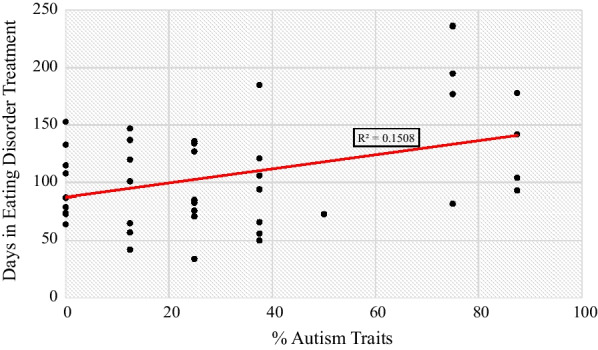
Scatterplot relationship between the percentage of autism traits and days in eating disorder treatment
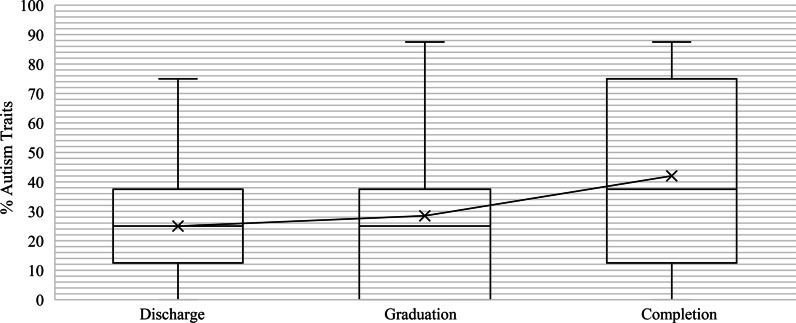
Participants were classified into three groups: discharge (n = 11), graduation (n = 18) and completion (n = 11). The differences in the percentage of autism traits exhibited were not statistically significantly different between the groups, p = 0.338, and is pictured in the boxplot in Fig. 3.
Fig. 3.
Distribution of the percentage of autism traits and treatment outcomes
Discussion
The findings of this study support existing literature that autism is overrepresented in the eating disorder population. The CDC reports that autism is present in 1 in 116 girls (0.88%) in the United States [3]. However, upon entering the PHP level of care for an eating disorder, 10% of the study participants had a pre-existing diagnosis of autism. An additional 12.5% of study participants were given a new autism diagnosis. A total of 27.5% of participants had clinical suspicion of autism. This aligns with the previously reported autism and eating disorder co-occurrence rates of 23–32% [4, 5].
Autism traits in this study were characterized by the components of the autism diagnostic criterion outlined in the (DSM-5-TR) [20]. The number of autism traits that an individual possessed directly correlated with the number of calendar days in treatment, with no autism traits being associated with a mean of 98 (SD = 31) days in treatment and 6 autism traits associated with 173 (SD = 65) days in treatment. This supports the previous findings that individuals with autism have a poorer prognosis in terms of their eating disorder [5]. However, the presence of autism traits in this study did not impact whether a participant met the requirements to graduate from the eating disorder program.
The presence of autism traits was not limited to those with a restrictive eating disorder. Despite a preponderance of literature on the presence of autism in AN and avoidant-restrictive food intake disorder, autism traits have been seen in non-restrictive eating disorders [22, 23]. Additionally, none of the individuals in this study identified to have autism were diagnosed with bipolar I disorder, bipolar II disorder, disruptive mood dysregulation disorder, or cyclothymic disorder. A 7.5% cooccurrence rate of autism and bipolar disorder has been seen in adults [24]. The predominance of adolescents in this study may contribute to lack of other mood disorder diagnoses.
Recommendations and practical applications
Further research is needed on adaptions to care for those with autism undergoing treatment for an eating disorder. However, the literature does suggest that individuals with autism are less likely to respond to cognitive behavioral therapy [14]. Neurodiversity-affirming care can also be applied to this patient population, such as avoiding sarcasm and euphemisms, providing concise verbal and written instructions, allowing the use of sensory fidgets and safe stimming, and breaking tasks down into small steps. Treatment goals should be tailored to the individual in a way that meets the individual’s needs, rather than normalizing behaviors to neurotypical social standards [18]. Returning the individual’s relationship to food or exercise to what it was before the onset of the eating disorder is a suggested measure to apply.
Limitations
Limitations of this study are the small sample size and that the participants are from a single ED treatment center. Evaluating data from a single treatment center allows for diagnostic consistency across the sample population but is open to an internal bias of the diagnosing clinicians. However, since the sample size yielded statistically significant findings, it is worth evaluating in a larger sample across multiple treatment locations.
While this particular treatment center serves participants from the surrounding areas of eastern Washington and Oregon, it is worth noting that the economy in the region where the Recovery and Wellness Center is located is anchored in research, development, and technology [25]. More scientists and engineers per capita are located in the region than anywhere else in the nation [25]. Known as the "Silicone Valley Phenomenon," an area rich in technical professionals is linked to higher autism rates in those professionals and their children [26], however no studies have evaluated this phenomenon in this area.
Conclusions
Eating disorder professionals in this study identified autism in 17.5% of adolescent and young adult females who entered treatment at the PHP level of care for an eating disorder who had not previously been diagnosed with autism. Recognizing undiagnosed autism may benefit the patient, their family, and the clinical team to have a greater understanding of the individual and their treatment needs. Eating disorder professionals can anticipate that individuals with autism may be in treatment for a longer duration, as supported by the increasing number of days of treatment associated with the number of autism traits.
Acknowledgements
The author would like to thank her faculty advisor, Michelle McMoon, Ph.D., PA-C, and Katie Klute, the director of the Recovery and Wellness Center of Eastern Washington. A special thanks to Kirstie Eastwood at Dissertation by Design for the statistical analyses.
Abbreviations
- AN
Anorexia Nervosa
- CDC
Centers for Disease Control and Prevention
- DSM-5-TR
Diagnostic and Statistical Manual, 5th edition, text revision
- IOP
Intensive outpatient program
- PHP
Partial hospitalization program
Author contributions
MAP is responsible for the conceptualization, development of methodology, data collection, statistical analysis, drafting of the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data that support the findings of this study are available on reasonable written request to the author.
Declarations
Ethics approval and consent to participate
The patient records from the Recovery and Wellness Center of Eastern Washington required no additional consent, as the researcher is a covered entity on the signed HIPPA agreement. This research was IRB-exempt because it involves secondary research using identifiable private information. The data points were deidentified and collected by a single researcher. Written permission was granted by the Recovery and Wellness Center of Eastern Washington and was exempt from A. T. Still University’s Internal Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gillberg C. Are autism and anorexia nervosa related? Br J Psych. 1983;142:428. doi: 10.1192/bjp.142.4.428b. [DOI] [PubMed] [Google Scholar]
- 2.Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child and Adolesc Psych. 2017;56(6):466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Maernner MJ, Shaw KA, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years- autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveil Summ. 2021;70(11):1–16. doi: 10.15585/mmwr.ss7011a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numata N, Nakagawa A, Yoshioka K, et al. Associations between autism spectrum disorder and eating disorders with and without self-induced vomiting: an empirical study. J Eat Disord. 2021 doi: 10.1186/s40337-020-00359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentz M, Westwood H, Jepsen JRM, Plessen KJ, Tchanturia K. The autism diagnostic observation schedule: patterns in individuals with anorexia nervosa. Eur Eat Disord Rev. 2020;28(5):571–579. doi: 10.1002/erv.2757. [DOI] [PubMed] [Google Scholar]
- 6.Brede J, Babb C, Jones C, et al. “For me, the anorexia is just a symptom, and the cause is the autism”: investigating restrictive eating disorders in autistic women. J Autism Dev Disord. 2020;50(12):4280–4296. doi: 10.1007/s10803-020-04479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leader G, O’Reilly M, Gilroy SP, Chen JL, Ferrari C, Mannion A. Comorbid feeding and gastrointestinal symptoms, challenging behavior, sensory issues, adaptive functioning and quality of life in children and adolescents with autism spectrum disorder. Dev Neurorehab. 2021;24(1):35–44. doi: 10.1080/17518423.2020.1770354. [DOI] [PubMed] [Google Scholar]
- 8.Tang JW, Li JW, Baulderstone D, Jeyaseelan D. Presenting age and features of females diagnosed with autism spectrum disorder. J Paed Child Health. 2021;57(8):1182–1189. doi: 10.1111/jpc.15417. [DOI] [PubMed] [Google Scholar]
- 9.Corscadden P, Casserly AM. Identification of autism in girls: role of trait subtleties, social acceptance and masking. Reach J. 2021;34(1);18–28. Accessed 26 Jan 2022. https://reachjournal.ie/index.php/reach/article/view/313
- 10.Hull L, Petrides KV, Mandy W. The female autism phenotype and camouflaging: a narrative review. Rev J Autism Dev Disord. 2020;7(4):306–317. doi: 10.1007/s40489-020-00197-9. [DOI] [Google Scholar]
- 11.Supekar K, de los Angeles C, Ryali S, Cao K, Ma T, Menon V. Deep learning identifies robust gender differences in functional brain organization and their dissociable links to clinical symptoms in autism. Br J Psychiatr. 2022;220:202–209. doi: 10.1192/bjp.2022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward ZJ, Rodriquez P, Wright DR, Austin SB, Long MW. Estimation of eating disorders prevalence by age and associations with mortality in a simulated nationally representative US cohort. JAMA. 2019;2(10):12925. doi: 10.1001/jamanetworkopen.2019.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazar BP, Peynenburg V, Rhind C, Hibbs R, Schmidt U, Gowers S. An examination of the clinical outcomes of adolescents and young adults with broad autism spectrum traits and autism spectrum disorder and anorexia nervosa: a multi centre study. Int J Eat Disord. 2018;51(2):174–179. doi: 10.1002/eat.22823.29331075. [DOI] [PubMed] [Google Scholar]
- 14.Atwood ME, Friedman A. A systematic review of enhanced cognitive behavioral therapy (CBT-E) for eating disorders. Int J Eat Disord. 2020;53(3):311–330. doi: 10.1002/eat.23206. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen S, Dobrescu SR, Dinkler L, et al. Effects of autism on 30-year outcome of anorexia nervosa. J Eat Disord. 2022;10(1):1–14. doi: 10.1186/s40337-021-00518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CM, Stokes MA. Intersection of eating disorders and the female profile of autism. Psychiatr Clin North Am. 2020;43(4):735–743. doi: 10.1016/j.psc.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Babb C, Brede J, Jones CRG, et al. ‘It’s not that they don’t want to access the support … it’s the impact of the autism:’ The experience of eating disorder services from the perspective of autistic women, parents and healthcare professionals. Autism. 2021;25(5):1409–1421. doi: 10.1177/1362361321991257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinnaird E, Norton C, Stewart C, Tchanturia K. Same behaviours, different reasons: What do patients with co-occurring anorexia and autism want from treatment? Inter Rev Psychiatr. 2019;31(4):308–317. doi: 10.1080/09540261.2018.1531831. [DOI] [PubMed] [Google Scholar]
- 19.Steinhausen HC, Villumsen MD, Hørder K, Winkler LAD, Bilenberg N, Støving RK. Comorbid mental disorders during long-term course in a nationwide cohort of patients with anorexia nervosa. Int J Eat Disord. 2021;54(9):1608–1618. doi: 10.1002/eat.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual. 5th ed, text revision. 2022:311–332.
- 21.American Psychiatric Association. Neurodevelopmental disorders. In: Diagnostic and Statistical Manual. 5th ed, text revision. 2022:38–46.
- 22.Gesi C, Carmassi C, Luciano M, et al. Autistic traits in patients with anorexia nervosa, bulimia nervosa or binge eating disorder: a pilot study. Eur Psychiatry. 2017;41(Supplement):S100. doi: 10.1016/j.eurpsy.2017.01.310. [DOI] [Google Scholar]
- 23.Numata N, Nakagawa A, Yoshioka K, et al. Associations between autism spectrum disorder and eating disorders with and without self-induced vomiting: an empirical study. J Eat Disord. 2021;9(1):1–9. doi: 10.1186/s40337-020-00359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varcin KJ, Herniman SE, Lin A, et al. Occurrence of pyschosis and bipolar disorder in adults with autism: a systematic review and meta-analysis. Nerurosci Biobehav Rev. 2022;134:104543. doi: 10.1016/j.neubiorev.2022.104543. [DOI] [PubMed] [Google Scholar]
- 25.Tri-City Development Council. Tri-cities economy. TRIDEC website. 2015. Accessed March 29, 2022. https://www.tridec.org/tri-cities-economy
- 26.Baron-Cohen S. Autism and the technical mind: children of scientists and engineers may inherit genes that not only confer intellectual talents but also predispose them to autism. SciAm. 2012;307(5):72–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable written request to the author.