Abstract
Background:
Obesity at diagnosis of childhood acute lymphoblastic leukemia (ALL) is associated with greater risk of relapse; whether this association extends to obesity during maintenance is unstudied.
Methods:
This study used data from AALL03N1 to calculate median body mass index (BMI) for 676 children over 6 consecutive months during maintenance therapy; BMI percentile (BMI%ile) were operationalized as normal/underweight (<85% ile), overweight/obese (85%–98%ile), and extreme obesity (≥99%ile). Hazard of relapse was estimated using multivariable proportional subdistributional hazards regression after adjusting for all relevant demographic and clinical predictors.
Results:
Median age at study enrollment was 6 years and median length of follow-up was 7.9 years. Overall, 43.3% of the cohort was underweight/normal weight, 44.8% was overweight/obese, and 11.8% had extreme obesity. Cumulative incidence of relapse at 4 years from study enrollment was higher among those with extreme obesity (13.6% ± 4.5%) compared to those with underweight/normal weight (9.0% ± 2.1%). Multivariable analysis revealed that children with extreme obesity had a 2.4-fold (95% confidence interval [CI], 1.1–5.0; p = .01) greater hazard of relapse compared to those who were underweight/normal weight. Overweight/obese patients were at comparable risk to those who were underweight/normal weight (hazard ratio, 0.8; 95% CI, 0.4–1.6). Erythrocyte thioguanine nucleotide (TGN) levels were significantly lower among children with extreme obesity compared to those with underweight/normal weight (141.6 vs. 168.8 pmol/8 × 108 erythrocytes; p = .0002), however, the difference in TGN levels did not explain the greater hazard of relapse among those with extreme obesity.
Conclusions:
Extreme obesity during maintenance therapy is associated with greater hazard of relapse in children with ALL. Underlying mechanisms of this association needs further investigation.
Keywords: child, drug metabolites, leukemia, obesity, recurrence
Lay summary:
• Findings from this study demonstrate that extreme obesity during maintenance therapy is associated with a greater hazard of relapse among children with acute lymphoblastic leukemia.
• We show that children with obesity have lower levels of erythrocyte thioguanine nucleotides even after adjusting for adherence to oral chemotherapy.
• However, these lower levels do not explain the greater hazard of relapse, paving the way for future studies to explore this association.
INTRODUCTION
Five-year disease-free survival rates among children with acute lymphoblastic leukemia (ALL) now approach 90%.1–3 Predictors of relapse include disease biology, treatment response, and nonadherence to 6-mercaptopurine (6MP) during maintenance.4,5 An association between higher body mass index (BMI) at diagnosis and relapse risk has been reported by some,6,7 but not others.8,9 These conflicting reports likely stem from the use of a single BMI measurement at diagnosis to determine risk of relapse that occurs months to years later; however, BMI changes significantly over the course of ALL therapy.10–12 This point is highlighted by a study that found an association between extremes in body weight during the post-induction but pre-maintenance phase and relapse risk in children with high-risk ALL.13 However, evidence for an association between BMI during maintenance therapy and relapse risk is lacking.
The Children’s Oncology Group (COG) study AALL03N1 enrolled children with ALL entering maintenance in first remission to determine the association between adherence to oral 6MP and relapse risk.4 As part of this study, monthly height and weight, methotrexate and 6MP dose intensity, and erythrocyte 6MP metabolite levels were measured for 6 months and patients were followed for up to 10 years. We used this unique resource to test our hypothesis that a higher BMI during maintenance is associated with a greater hazard of relapse. Additionally, we explored whether variations in erythrocyte thioguanine nucleotide (TGN; anti-leukemia metabolite of 6MP) exist between BMI categories, and whether the association between BMI and relapse risk is explained in part by erythrocyte TGN levels.
MATERIALS AND METHODS
The eligibility criteria for enrollment on COG-AALL03N1 included: (1) age ≤21 years at diagnosis of ALL, and (2) first remission when entering maintenance. All participating sites had approval from local institutional review boards and written informed consent and/or assent was obtained from patients and/or parents or legal guardians before enrollment on AALL03N1. The study schema is provided in Figure S1. Additional details of AALL03N1 and its primary findings have been previously described.4,5,14–16 For this secondary analysis, we excluded patients >19 years because BMI percentiles (BMI%ile) are not available for these individuals through the Centers for Disease Control and Prevention (CDC).
Clinical characteristics (age at ALL diagnosis, sex, age at study enrollment, National Cancer Institute [NCI] risk grouping [standard risk: age, ≤9.99 years and white blood cell count <50,000/mm3 at ALL diagnosis; high risk if otherwise], ALL subtype, leukemic blasts cytogenetics [favorable: t(12;21), hyperdiploidy, trisomy 4 and 10, or trisomy 4, 10, and 17; unfavorable: t(9;22), t(4;11), hypodiploidy, or extreme hypodiploidy; neutral: neither favorable nor unfavorable]) were provided by participating sites. Patients and/or parents self-reported race/ethnicity, parental education, and annual household income. The monthly height and weight measurements provided by the participating sites were used to calculate the BMI (weight in kilograms/height in meters)2 and averaged across the 6 months. Dose intensity of 6MP (6MPDI) and methotrexate (MTXDI) were calculated by dividing monthly prescribed doses (as reported by participating sites) by planned protocol doses (6MP = 75 mg/m2/day; MTX = 20 mg/m2/week). Participating sites reported the dates when oral chemotherapy was held for illness or toxicity. Erythrocyte TGN (in pmol/8 × 108 erythrocytes) was measured from monthly blood samples submitted by participating sites and averaged across the 6 months. Intrapatient coefficient of variation (CV%) in erythrocyte TGN was calculated as a ratio of standard deviation to the mean of all measurements for an individual patient.4 Annual updates on clinical outcomes (vital status, relapse, or second neoplasm) were collected from the sites for up to 10 years from diagnosis. A subgroup of patients (63%) used the electronic medication monitoring device (TrackCap Medication Events Monitoring System [MEMS]; MWV Switzerland, Ltd) for measuring 6MP adherence; this group was labeled as the MEMS subcohort. Patients with heterozygous or homozygous TPMT-deficiency or mutant NUDT15 genotype (8.9%) were excluded from the current analysis to ensure homogeneity of the study population.
Statistical analysis
Absolute BMI was converted into BMI percentiles using the CDC age- and sex-adjusted data.17 Patients were classified into three groups based on BMI percentiles: underweight/normal weight (<85% ile), overweight/obese (85%–98%ile), and extremely obese (≥99%ile) per American Academy of Pediatrics definitions.18 Because definitions of extreme obesity have evolved, we also conducted sensitivity analysis defining extreme obesity as BMI >120% of 95%ile for age and sex.19 Patients from households with annual household income <$50,000 and parental education ≤high school were classified as having low socioeconomic status (SES). Demographic and clinical data were compared across BMI categories. Logistic regression analyses were used to determine the association between extreme obesity and age at study entry, sex, race/ethnicity, and SES.
Cumulative incidence of relapse was estimated across BMI categories by treating second neoplasms and death as competing risks. We examined the hazard of relapse using proportional sub-distribution hazard regression models by BMI%ile category (<85%ile [reference], 85%–98%ile, and ≥99%ile) after adjusting for age at study entry, sex, race/ethnicity, SES, NCI risk group, blast cytogenetics, 6MPDI, MTXDI, number of days chemotherapy was held due to toxicity/illness, erythrocyte TGN levels, and time from start of maintenance therapy to study enrollment. For patients in the MEMS subcohort, models for relapse risk included 6MP adherence (calculated as average of monthly adherence across the 6 months on study) in addition to the above variables.
Mean erythrocyte TGN levels were compared across the three BMI categories, after adjusting for age at study participation, race/ethnicity, sex, 6MPDI, and time from start of maintenance therapy to study enrollment, using generalized estimating equations to account for repeated measurements. The same analysis was also conducted in the MEMS subcohort, adding 6MP adherence to the above variables. The effect of erythrocyte TGN metabolites in explaining relapse risk was assessed using proportional sub-distribution hazard regression models.
All statistical analyses were performed using SAS software v9.4 (SAS Institute Inc) and two-sided testes with p < .05 were considered statistically significant.
Data sharing statement
Requests for AALL03N1 de-identified data set should be directed to Dr. Smita Bhatia (smitabhatia@uabmc.edu).
RESULTS
Patient characteristics
Overall, 676 patients were included in this analysis. Table 1 highlights the sociodemographic and disease characteristics of the patients, overall and by BMI category. The median age at ALL diagnosis was 5 years (range, 1–18 years) and at study enrollment was 6 years (2–19 years). The cohort was followed for a median of 7.9 years from study entry (0.1–13 years). The majority of patients were male (68.6%); 33.3% were non-Hispanic White, 33.3% Hispanic, 17.8% were African American, and 15.7% were Asian. Overall, 29.8% met criteria for low SES. Most patients had B-lymphoblastic leukemia (88.8%), NCI standard risk disease (58.0%), and neutral cytogenetics (51.7%). The demographic and clinical characteristics of the 435 patients in the MEMS subcohort are summarized in Table S1. Table S2 compares characteristics of patients who participated in the MEMS subcohort to those who did not. Patients in the MEMS subcohort were younger compared to those not in the MEMS subcohort (4 years vs. 5 years, p = .04); there were no other differences between the two groups.
TABLE 1.
Sociodemographic and disease characteristics of patients with ALL, overall and by BMI category
| Variable | Entire cohort (n = 676) | <85%ile (n = 293) | 85%–98%ile (n = 303) | ≥99%ile (n = 80) | P |
|---|---|---|---|---|---|
|
| |||||
| Age at diagnosis, year | |||||
| Median (range) | 5 (1–18) | 5 (1–17) | 4 (1–18) | 4 (1–18) | .01 |
| Mean (±SD) | 6.1 (4.4) | 6.7 (4.8) | 5.7 (4.0) | 5.8 (4.5) | |
| Age at study enrollment, year | |||||
| Median (range) | 6 (2–19) | 7 (2–19) | 6 (2–19) | 6 (2–19) | .01 |
| Mean (±SD) | 7.7 (4.4) | 8.2 (4.8) | 7.2 (4.0) | 7.4 (4.5) | |
| Length of follow-up, year | |||||
| Median (range) | 7.9 (0.1–13.0) | 7.7 (0.2–11) | 8.0 (0.1–13.0) | 7.4 (0.2–9.5) | .05 |
| Sex, No. (%) | |||||
| Male | 464 (68.6) | 202 (68.9) | 205 (67.7) | 57 (71.3) | .8 |
| Race/ethnicity, No. (%) | |||||
| Non-Hispanic White | 225 (33.3) | 115 (39.3) | 92 (30.4) | 18 (22.5) | .0001 |
| Hispanic | 225 (33.3) | 78 (26.6) | 106 (35.0) | 41 (51.3) | |
| African American or Black | 120 (17.8) | 43 (14.7) | 65 (21.5) | 12 (15.0) | |
| Asian | 106 (15.7) | 57 (19.5) | 40 (13.2) | 9 (11.3) | |
| Parental education, No. (%) | |||||
| ≤High school | 227 (34.6) | 92 (32.2) | 97 (33.1) | 38 (49.4) | .01 |
| Yearly household income, No. (%) | |||||
| <$50,000 | 372 (59.3) | 149 (55.6) | 164 (57.8) | 59 (78.7) | .001 |
| Socioeconomic strata, No. (%) | |||||
| Low SES | 186 (29.8) | 74 (27.7) | 78 (21.6) | 34 (45.9) | .03 |
| ALL subtype, No. (%) | |||||
| B-lymphoblastic leukemia | 594 (88.1) | 255 (87.0) | 267 (88.7) | 72 (90.0) | .7 |
| T-lymphoblastic leukemia | 75 (11.1) | 34 (11.6) | 33 (11.0) | 8 (10.0) | |
| NCI risk group, No. (%) | |||||
| High risk | 282 (42.0) | 140 (47.9) | 109 (36.3) | 33 (42.0) | .02 |
| Cytogenetics, No. (%) | |||||
| Favorable | 269 (42.3) | 110 (39.0) | 126 (45.0) | 33 (44.6) | .3 |
| Neutral | 329 (51.7) | 154 (54.6) | 141 (50.4) | 34 (46.0) | |
| Unfavorable | 38 (6.0) | 18 (6.4) | 13 (4.6) | 7 (9.4) | |
| BMI%ile | |||||
| Median, range | 88.5 (0–100) | 61.9 (0.0–84.9) | 94.6 (85.2–99.0) | 99.5 (99–100) | <.0001 |
| 6MP dose intensity | |||||
| Median (range) | 0.87 (0.03–2.97) | 0.84 (0.03–2.97) | 0.88 (0.2–2.1) | 0.87 (0.4–1.45) | .06 |
| Erythrocyte TGN, pmol/8 × 108 erythrocytes | |||||
| Median (range) | 141 (0–474) | 138.9 (0.3–474.0) | 144.0 (0.7–432.9) | 138 (0–306.2) | .3 |
| CV%, median (range) | 29.0 (0–241.2) | 30.4 (1.4–241.2) | 28.0 (1.7–222.1) | 31.2 (0–156.1) | .06 |
| Methotrexate dose intensity | |||||
| Median (range) | 0.87 (0.1–2.73) | 0.85 (0.1–2.73) | 0.89 (0.2–2.0) | 0.87 (0.3–1.5) | .2 |
| Days oral chemotherapy held for toxicity or illness | |||||
| Median (range) | 0 (0–102) | 0 (0–56) | 0 (0–102) 0 (0–48) | .3 | |
Note: Low SES was defined as median household income <$50,000 and parental education ≤high school. Favorable cytogenetics included t(12; 21); hyperdiploidy; trisomy 4 and 10; or trisomy 4, 10, and 17. Unfavorable cytogenetics included t(9; 22), t(4; 11), hypodiploidy, or extreme hypodiploidy. Neutral cytogenetics implied absence of favorable or unfavorable cytogenetics. p value compares means.
Abbreviations: 6MP, 6-mercaptopurine; %ile, percentile; ALL, acute lymphoblastic leukemia; BMI, body mass index; CV, coefficient of variation; NCI, National Cancer Institute; SD, standard deviation; SES, socioeconomic status; TGN, thioguanine nucleotide.
BMI during maintenance therapy
The median BMI%ile for all patients was 88.5 (0–100). Using the predefined BMI cutoffs, 43.3% (n = 293) were underweight/normal weight (BMI <85%ile), 44.8% (n = 303) were overweight/obese (BMI = 85%–98%ile), and 11.8% (n = 80) had extreme obesity (BMI ≥99%ile). On univariate comparison, patients with extreme obesity were significantly more likely to be younger at time of diagnosis (p = .01), Hispanic (p = .0001), and from lower SES (p = .03) when compared with those in the other two BMI categories. No significant differences were observed in 6MPDI (0.84 vs. 0.88 vs. 0.87, p = .06) and MTXDI (0.85 vs. 0.88 vs. 0.87, p = .2) by BMI categories (<85% ile, 85%–98%ile, and ≥99%ile, respectively) (Table 1). Similarly, among patients in the MEMS subcohort, neither 6MPDI (0.80 vs. 0.87 vs. 0.90, p = .4), MTXDI (0.85 vs. 0.89 vs. 0.89, p = .7), nor adherence to 6MP (91.6% vs. 91.7% vs. 89.8%, p = .4) varied by BMI categories (<85%ile, 85%–98%ile, and ≥99%ile, respectively) (Table S1). Median number of days maintenance chemotherapy was held due to toxicity or illness was also not significantly different between BMI categories among all patients (p = 0.3, Table 1) and those in the MEMS subcohort (p = 0.7, Table S1).
In a logistic regression model examining factors associated with extreme obesity, Hispanic ethnicity was associated with twofold greater odds of extreme obesity (95% confidence interval [CI], 1.03–3.9; p = .02) compared to non-Hispanic White race/ethnicity (Table S3).
Relapse risk by BMI category
In this cohort of 676 children with ALL followed for a median of 7.9 years (0.1–13.0 years) from study entry, 63 (9.3%) children experienced a relapse. The cumulative incidence of relapse at 4 years from study enrollment was highest among patients with extreme obesity (13.6% ± 4.5%) when compared to those who were overweight/obese (7.4% ± 1.9%) or underweight/normal weight (9.0% ± 2.1%) (Figure 1). As shown in Figure 2, patients with extreme obesity had a 2.4-fold (95% CI, 1.1–5.1; p = .02) greater hazard of relapse when compared to patients who were underweight/normal weight after adjusting for clinical characteristics, race/ethnicity, SES, chemotherapy dose intensity, and median TGN levels. Hazard of relapse among patients who were overweight/obese was comparable to patients who were under/normal weight (hazard ratio [HR], 0.8; 95% CI, 0.4–1.6; p = .6). After adjusting for adherence in addition to above covariates, we found a significantly greater hazard of relapse among patients with extreme obesity in the MEMS subcohort (HR, 3.5; 95% CI, 1.5–8.5; p = .005) (Table S4). Again, the hazard of relapse for those who were overweight/obese was comparable to those who were underweight/normal weight (HR, 0.8; 95% CI, 0.3–2.1; p = .7). Similarly, sensitivity analysis demonstrated an elevated hazard of relapse among patients with BMI ≥120% of the 95%ile (HR, 2.9; 95% CI, 1.5–5.7; p = .002) in the entire cohort as well the MEMS cohort (HR, 3.8; 95% CI, 1.6–8.6; p = .002) compared to those who were under/normal weight (Table S5).
FIGURE 1.
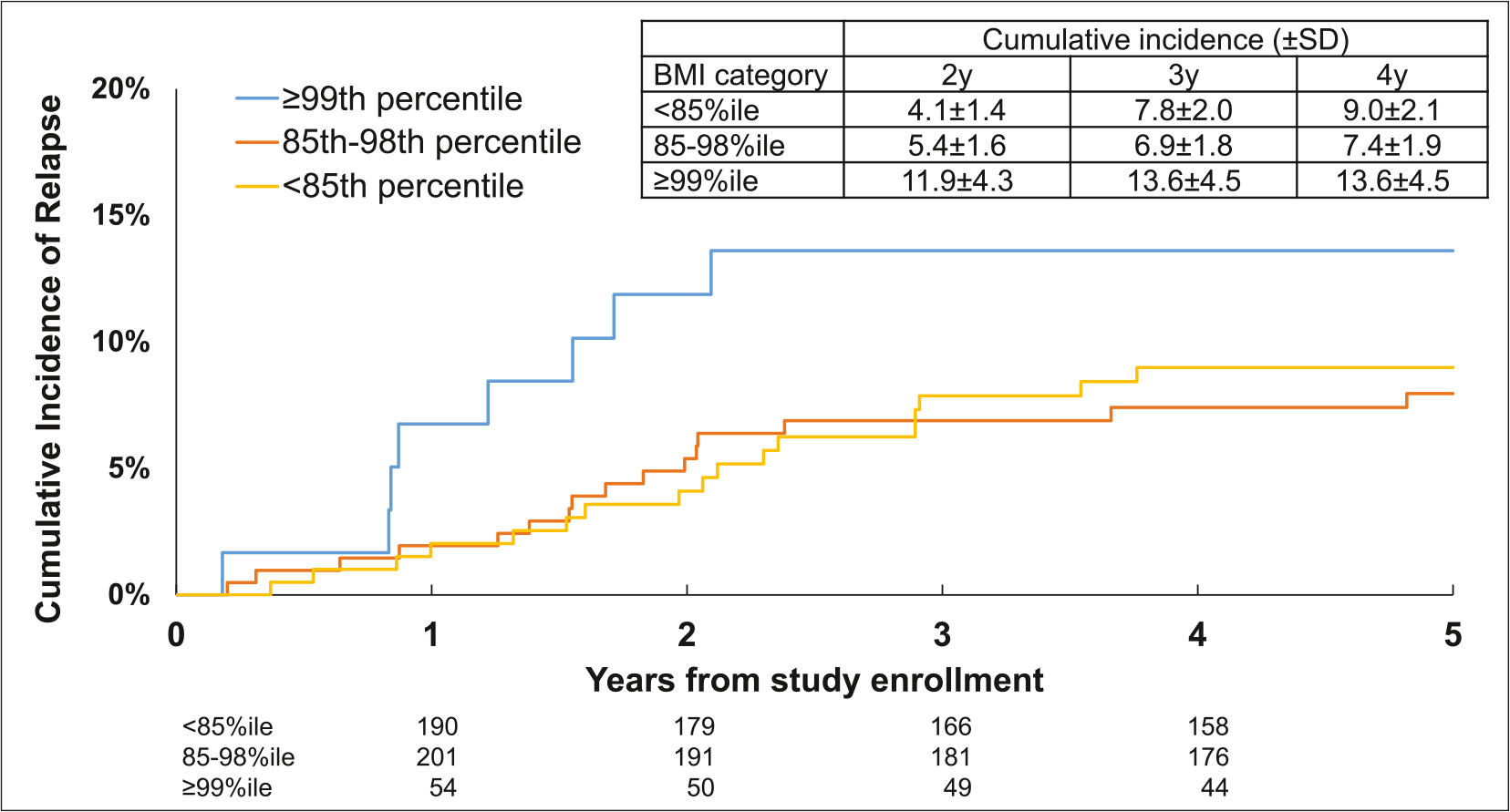
Cumulative incidence of relapse after study enrollment by BMI category in children with acute lymphoblastic leukemia. BMI indicates body mass index; SD, standard deviation.
FIGURE 2.
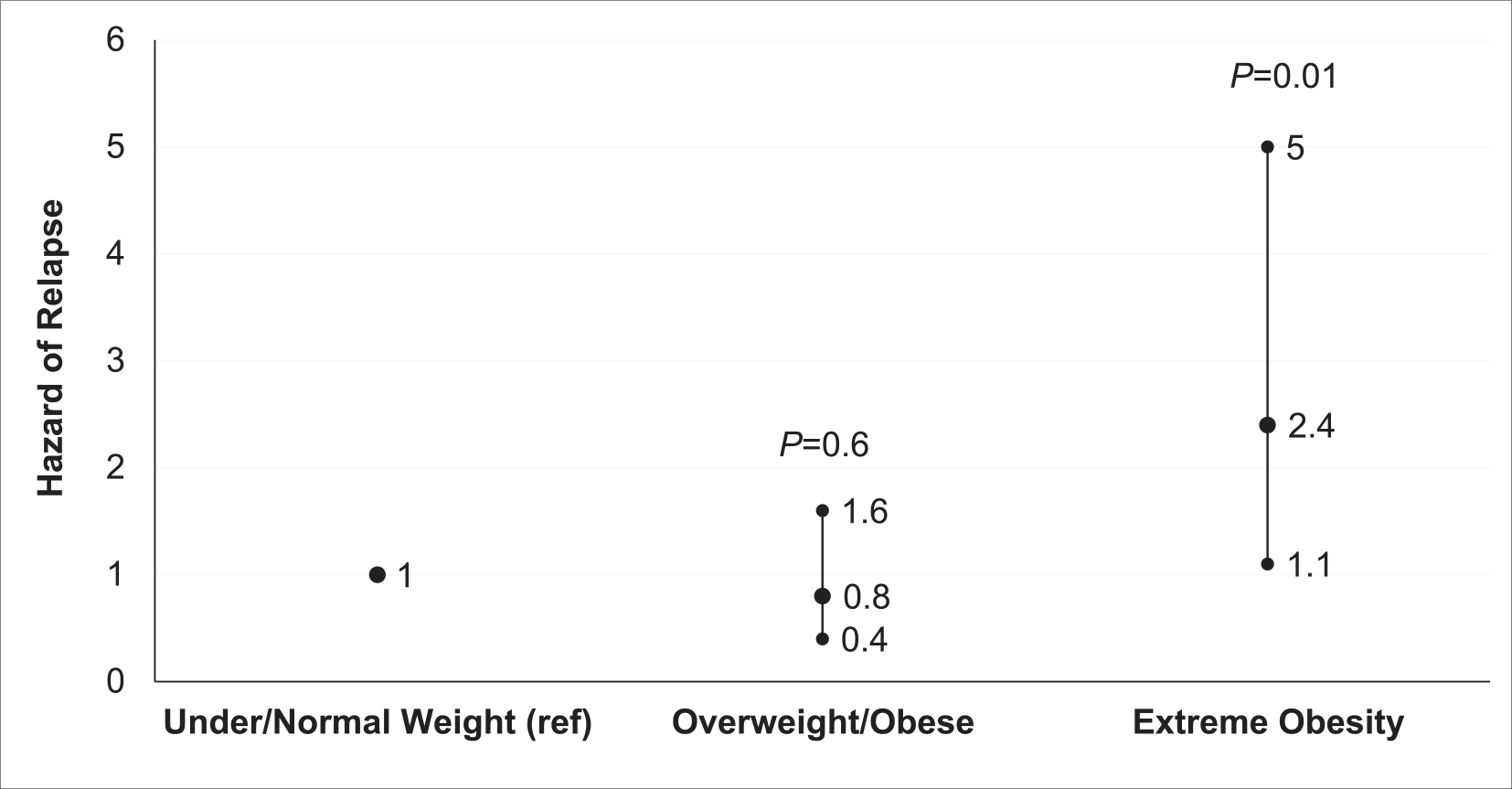
Hazard of relapse by body mass index (BMI) category. Proportional subdistribution hazard regression showing hazard of relapse by BMI category after adjusting for age at study entry, sex, race/ethnicity, socioeconomic status, National Cancer Institute risk group, cytogenetics, median 6MP dose intensity, median methotrexate dose intensity, median thioguanine nucleotide levels, and time from start of maintenance.
Erythrocyte TGN by BMI category
Compared to patients who were underweight/normal weight, lower erythrocyte TGN levels were observed in patients who were overweight/obese (mean difference: −13.7 ± 5.8 pmol/8 × 108 erythrocytes, p = .02) as well as in those with extreme obesity (mean difference: −27.2 ± 7.4 pmol/8 × 108 erythrocytes, p = .0002) after adjusting for age at study enrollment, race/ethnicity, sex, 6MPDI, and time from start of maintenance therapy to study enrollment (Figure 3). The same trends were observed in the MEMS subcohort (Figure S2), where patients with extreme obesity and those who were overweight/obese had significantly lower TGN levels (mean difference: −30.8 ± 9.4 pmol/8 × 108 erythrocytes, p = .001 and −17.2 ± 9.4 pmol/8 × 108 erythrocytes, p = .03, respectively) after adjusting for age at study enrollment, race/ethnicity, sex, 6MPDI, time from start of maintenance to study enrollment, and 6MP adherence.
FIGURE 3.
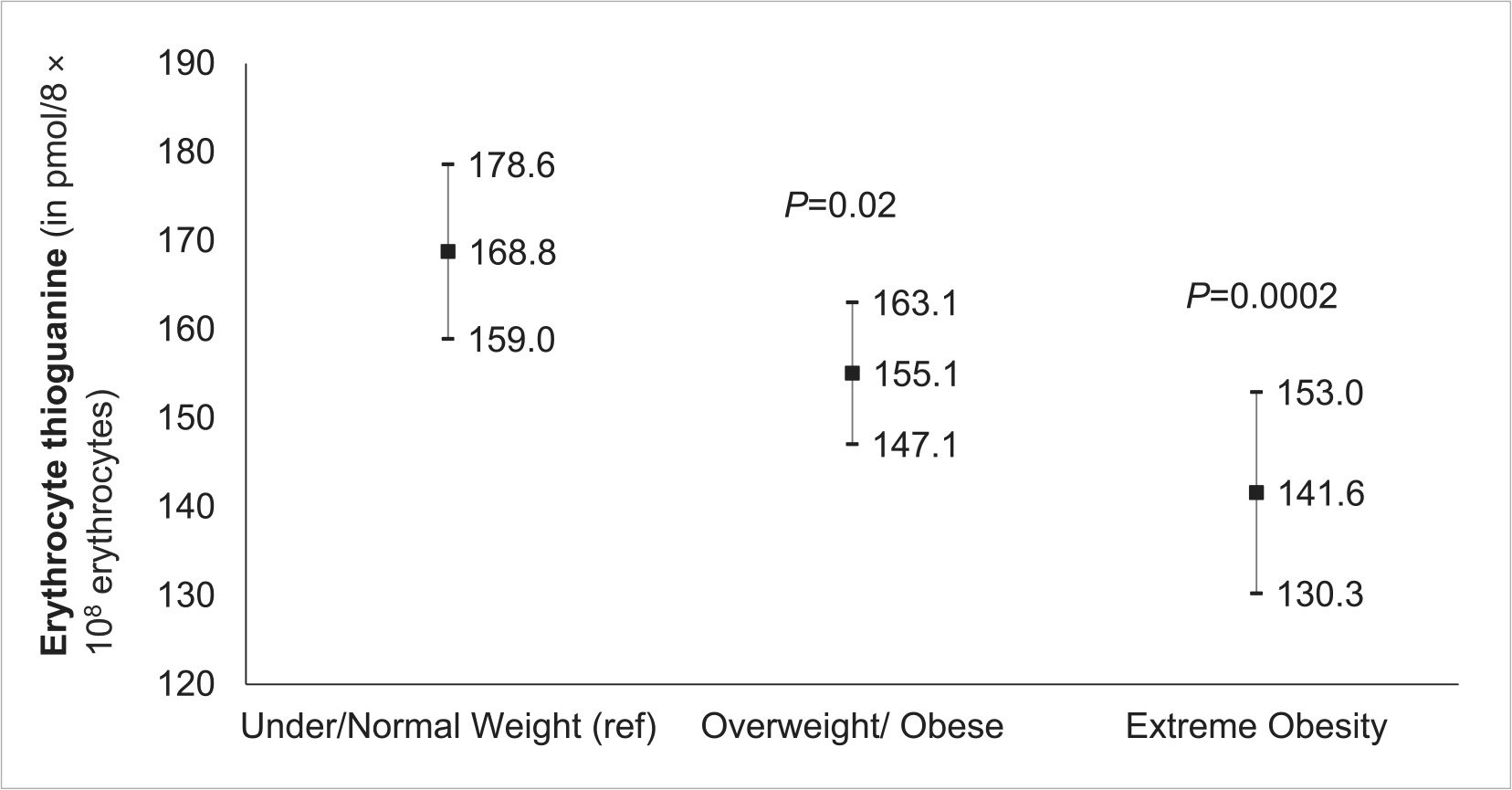
Fitted means of erythrocyte thioguanine nucleotide in all patients. Model adjusted for age at study enrollment, race/ethnicity, sex, 6MP dose intensity, and time from start of maintenance to study enrollment.
TGN levels and relapse risk by BMI category
Table 2 highlights our attempt to examine the role of erythrocyte TGN levels in the association between BMI categories and relapse risk. Patients with extreme obesity had a 2.4-fold greater hazard of relapse (95% CI, 1.2–4.7) in an unadjusted model (model 1) when compared with underweight/normal weight patients. After adjusting for age at study entry, sex, NCI risk group, blast cytogenetics, 6MPDI and MTXDI, number of days chemotherapy held due to toxicity/illness, and time from start of maintenance to study entry (model 2), patients with extreme obesity continued to have a significantly greater hazard of relapse compared to those who were underweight/normal weight (HR, 2.7; 95% CI, 1.3–5.7; p = .007). Successive addition of SES (model 3: HR, 2.8; 95% CI, 1.3–5.7; p = .006), race/ethnicity (model 4: HR, 2.4; 95% CI; 1.1–5.1, p = .02) did not materially alter the association between BMI and relapse risk. Finally, adding TGN levels (model 5: HR, 2.4; 95% CI, 1.1–5.1; p = .02) or TGN CV% (model 6: HR, 2.5; 95% CI, 1.2–5.5; p = .02) to model 4 did not explain the association between extreme obesity and the hazard of relapse (Table 2). Because there was significant collinearity between NCI risk group and disease phenotype in our cohort (p < .001) that precluded inclusion of both variables simultaneously in a model, we replaced NCI risk group with disease phenotype (T-cell vs. B-cell [reference]) and found similarly elevated hazard of relapse among patients with extreme obesity when compared to those who were underweight/normal weight (model 7: HR, 2.6; 95% CI, 1.2–5.5; p = .02). Table S6 highlights effects of covariates on the full model. In the MEMS subcohort (Table S4), similar findings were observed, even after inclusion of adherence to the model (unadjusted model: HR, 3.0; 95% CI, 1.3–6.5; p = .007; final model: HR, 3.5; 95% CI, 1.5–8.5; p = .005).
TABLE 2.
Hazard of relapse by BMI%ile category among all participants
| BMI ≥99%ile vs. <85% ile |
BMI 85%–98%ile vs. <85%ile |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
|
| ||||
| Model 1a | 2.4 (1.2–4.7) | .01 | 0.9 (0.5–1.6) | .7 |
| Model 2b | 2.7 (1.3–5.7) | .007 | 1.0 (0.5–1.8) | .9 |
| Model 3c | 2.8 (1.3–5.7) | .006 | 0.9 (0.5–1.7) | .8 |
| Model 4d | 2.4 (1.1–5.1) | .02 | 0.8 (0.4–1.6) | .6 |
| Model 5e | 2.4 (1.1–5.1) | .02 | 0.8 (0.4–1.6) | .6 |
| Model 6f | 2.5 (1.2–5.5) | .02 | 0.9 (0.4–1.7) | .7 |
| Model 7g | 2.6 (1.2–5.5) | .02 | 0.9 (0.4–1.7) | .7 |
Abbreviations: %tile, percentile; BMI, body mass index; CI, confidence interval; CV, coefficient of variation; HR, hazard ratio; NCI, National Cancer Institute; TGN, thioguanine nucleotide.
Unadjusted HR for relapse risk by BMI category.
Adjusted HR for relapse risk by BMI category, adjusting for age at study entry, sex, NCI risk group, blast cytogenetics, median 6MP dose intensity, median methotrexate dose intensity, number of days chemotherapy held due to toxicity/illness and time from start of maintenance.
Adds to socioeconomic strata to covariates included in model 2.
Adds race/ethnicity (ref: non-Hispanic White) to covariates included in model 3.
Adds median TGN levels to covariates included in model 4.
Removes median TGN and adds TGN CV% to covariates included in model 4.
Substitutes NCI risk with disease phenotype (B-cell vs. T-cell [reference]) in model 5.
DISCUSSION
Prior work has examined the association between obesity at diagnosis and relapse risk in children with ALL.6–9,13 To our knowledge, this is the first study to show that extreme obesity during maintenance therapy is independently associated with relapse in children with ALL entering maintenance in first remission. We also find that erythrocyte TGN levels decline with increasing BMI. However, erythrocyte TGN levels fail to explain the greater hazard of relapse in children with extreme obesity.
The prevalence of obesity has increased over the past several decades among children and adolescents in the United States.20 Although direct comparisons are not feasible, data from CDC indicates that prevalence of extreme obesity during the time frame of this study (2000–2010) averaged approximately 5%, which is lower than almost 12% observed in this cohort.21 Children and adolescents with ALL have unique therapeutic exposures (e.g., corticosteroids10), which coupled with limited mobility due to peripheral neuropathy or during hospitalizations may disproportionately place them at higher risks of obesity compared to the general population. Almost 60% of the patients in this cohort were at least overweight or obese, a proportion that appears to be higher than that reported in the general population, but similar to previous observations among children with ALL during maintenance therapy.11
Obesity at ALL diagnosis is associated with a greater likelihood of end of induction minimal residual disease (MRD) (a strong determinant of relapse risk) and lower disease-free survival.6,7 BMI increases significantly over the course of ALL treatment due to therapeutic exposures10–12; it is possible that there is a concomitant change in the relation between obesity and relapse risk. Our data indicate that extreme obesity (i.e., BMI ≥99%ile) during maintenance is independently associated with a significantly greater hazard of ALL relapse in children entering maintenance in first remission. Several mechanisms can be postulated to explain this association between obesity and inferior outcomes in children with ALL.22 First, it has been shown in mice and in vitro models that adipose tissue deposits exert a survival advantage to the leukemic blasts.23,24 Thus, it is conceivable that patients with extreme obesity have a greater burden of “protected” leukemic blasts in adipose tissue that subsequently leads to disease relapse. Second, drug pharmacokinetics in patients with extreme obesity may be different compared to those who have lower BMIs.25 It has been shown that mice with diet-induced obesity have lower systemic exposure to vincristine, a key component of ALL therapy during maintenance, due to altered pharmacokinetics.26 Obesity also induces inflammation, which may contribute to tumorigenesis, drug resistance, and propagation of leukemic blasts.27,28 Finally, obesity is associated with obstructive sleep apnea, which is associated with intermittent hypoxemia and resistance of cancer cells to treatment.29 These findings are important because targeted weight-management interventions are feasible and effective in reducing gain in adipose tissue and improving disease outcomes in children with ALL and should be tested on larger scale.30
The relationship between BMI and erythrocyte TGN levels has remained unclear in children with ALL.8,31 We show that erythrocyte TGN levels vary by BMI category, with the lowest levels being observed in those with extreme obesity. This finding confirms previous observations among patients with inflammatory bowel disease of an inverse relationship between BMI and erythrocyte TGN levels.32,33 Lower erythrocyte TGN levels were previously shown to increase relapse risk in children with ALL.34,35 However, work done through the COG-AALL03N1 study has shown that intra-individual variations in TGN levels, rather than absolute TGN levels, are associated with relapse risk.4 We show that neither absolute erythrocyte TGN levels nor intra-individual variations in the TGN levels explain the association between BMI categories hazard of relapse, presenting a need to explore other mechanisms to explain the association between extreme obesity and relapse risk. Given these data, physicians should continue to dose chemotherapy on blood counts and organ toxicity, rather than TGN levels even among individuals with obesity.
Our study is limited in not having access to BMI at diagnosis or MRD at the end of induction. Obesity at diagnosis is associated with greater likelihood of MRD-positivity at end of induction.6 It is possible that children enrolled on COG-AALL03N1, despite being in morphological remission when entering maintenance, had detectable MRD at end of induction that contributed to greater relapse among those with extreme obesity. It has also been recently shown that obese children are more likely to develop ALL with CRLF2 rearrangements, a recently recognized high-risk subtype that was not tested for at the time of AALL03N1.36 This is particularly important because both CRLF2-rearranged ALL37 and obesity38,39 are more prevalent in children of Hispanic ethnicity; further studies that account for these variables are needed. Despite these limitations, our study is the first to show variations in 6MP metabolites by BMI in a large, racially/ethnically, and geographically diverse patient population. Additionally, we were able to control for adherence to 6MP and had BMI and 6MP metabolite measurements across several months of observation, rather than at a single time point. Finally, AALL03N1 was conducted during the time when ALL protocols administered monthly corticosteroid pulses. With recent treatment strategies administering corticosteroids less frequently, the prevalence of obesity may change in this population, thus altering this relationship between obesity and ALL relapse.
In summary, we show that extreme obesity during maintenance therapy for ALL is independently associated with greater hazard of relapse. We also find that BMI is inversely related to erythrocyte TGN levels in children with ALL. Further studies are needed to understand the mechanism of these observations, such that interventional studies aimed at reducing relapse risk could be designed.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by National Institutes of Health/National Cancer Institute (R01CA096670 to S.B.), the National Cancer Institute Community Oncology Research Program (UG1CA189955), and St. Baldrick’s Foundation.
Footnotes
CONFLICTS OF INTEREST
David Dickens reports consulting fees from Tempus. A. Kim Ritchey reports fees for data and safety monitoring for the Children’s Oncology Group. The other authors made no disclosures.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Angiolillo AL, Schore RJ, Kairalla JA, et al. Excellent outcomes with reduced frequency of vincristine and dexamethasone pulses in standard-risk B-lymphoblastic leukemia: results from Children’s Oncology Group AALL0932. J Clin Oncol. 2021:JCO2 000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salzer WL, Burke MJ, Devidas M, et al. Impact of intrathecal triple therapy versus intrathecal methotrexate on disease-free survival for high-risk B-lymphoblastic leukemia: Children’s Oncology Group study AALL1131. J Clin Oncol. 2020;38(23):2628–2638. doi: 10.1200/jco.19.02892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunsmore KP, Winter SS, Devidas M, et al. Children’s Oncology Group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38(28):3282–3293. doi: 10.1200/jco.20.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a Children’s Oncology Group study. JAMA Oncol. 2015;1(3):287–295. doi: 10.1001/jamaoncol.2015.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic White children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30(17):2094–2101. doi: 10.1200/jco.2011.38.9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orgel E, Tucci J, Alhushki W, et al. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood. 2014;124(26): 3932–3938. doi: 10.1182/blood-2014-08-595389 [DOI] [PubMed] [Google Scholar]
- 7.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25(15): 2063–2069. doi: 10.1200/jco.2006.07.7792 [DOI] [PubMed] [Google Scholar]
- 8.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108(13):3997–4002. doi: 10.1182/blood-2006-05-024414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eissa HM, Zhou Y, Panetta JC, et al. The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood Cancer J. 2017;7(2):e531. doi: 10.1038/bcj.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320. doi: 10.1002/cncr.23050 [DOI] [PubMed] [Google Scholar]
- 11.Esbenshade AJ, Simmons JH, Koyama T, Lindell RB, Friedman DL. Obesity and insulin resistance in pediatric acute lymphoblastic leukemia worsens during maintenance therapy. Pediatr Blood Cancer. 2013;60(8):1287–1291. doi: 10.1002/pbc.24489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: a report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7): 1249–1254. doi: 10.1002/pbc.22237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orgel E, Sposto R, Malvar J, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2014;32(13):1331–1337. doi: 10.1200/jco.2013.52.6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landier W, Hageman L, Chen Y, et al. Mercaptopurine ingestion habits, red cell thioguanine nucleotide levels, and relapse risk in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group study AALL03N1. J Clin Oncol. 2017;35(15):1730–1736. doi: 10.1200/jco.2016.71.7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. doi: 10.1182/blood-2014-01-552166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landier W, Chen Y, Hageman L, et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: a Children’s Oncology Group study. Blood. 2017;129(14):1919–1926. doi: 10.1182/blood-2016-07-726893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). Accessed February 5, 2021. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 18.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329c [DOI] [PubMed] [Google Scholar]
- 19.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/cir.0b013e3182a5cfb3 [DOI] [PubMed] [Google Scholar]
- 20.Fryar CDCM, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018; 2020.
- 21.Cheryl D, Fryar MDC, Joseph A. Prevalence of Overweight, Obesity, and Severe Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2017–2018. NCHS Health E-Stats; 2020. [Google Scholar]
- 22.Orgel E, Sea JL, Mittelman SD. Mechanisms by which obesity impacts survival from acute lymphoblastic leukemia. J Natl Cancer Inst Monogr. 2019;2019(54):152–156. doi: 10.1093/jncimono-graphs/lgz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramanik R, Sheng X, Ichihara B, Heisterkamp N, Mittelman SD. Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res. 2013;37(5):503–509. doi: 10.1016/j.leukres.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behan JW, Yun JP, Proektor MP, et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69(19):7867–7874. doi: 10.1158/0008-5472.can-09-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GR, Al-Obaidi M, Rower J, et al. Does oxaliplatin pharmacokinetics (PKs) explain associations between body composition and chemotherapy toxicity risk in older adults with gastrointestinal (GI) cancers? J Clin Oncol. 2021;39(15_suppl):3095. doi: 10.1200/jco.2021.39.15_suppl.3095 [DOI] [Google Scholar]
- 26.Behan JW, Avramis VI, Yun JP, Louie SG, Mittelman SD. Diet-induced obesity alters vincristine pharmacokinetics in blood and tissues of mice. Pharmacol Res. 2010;61(5):385–390. doi: 10.1016/j.phrs.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239–12. doi: 10.1155/2013/139239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado-Martin C, Meyer LK, Huang BJ, et al. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia. 2017;31(12):2568–2576. doi: 10.1038/leu.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83–92. doi: 10.2147/hp.s93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orgel E, Framson C, Buxton R, et al. Caloric and nutrient restriction to augment chemotherapy efficacy for acute lymphoblastic leukemia: the IDEAL trial. Blood Adv. 2021;5(7):1853–1861. doi: 10.1182/bloodadvances.2020004018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuccaro P, Guandalini S, Pacifici R, et al. Fat body mass and pharmacokinetics of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Ther Drug Monit. 1991;13(1):37–41. doi: 10.1097/00007691-199101000-00004 [DOI] [PubMed] [Google Scholar]
- 32.Poon SS, Asher R, Jackson R, et al. Body mass index and smoking affect thioguanine nucleotide levels in inflammatory bowel disease. J Crohns Colitis. 2015;9(8):640–646. doi: 10.1093/ecco-jcc/jjv084 [DOI] [PubMed] [Google Scholar]
- 33.Meijer B, Wilhelm AJ, Mulder CJJ, Bouma G, van Bodegraven AA, de Boer NKH. Pharmacology of thiopurine therapy in inflammatory bowel disease and complete blood cell count outcomes: a 5-year database study. Ther Drug Monit. 2017;39(4):399–405. doi: 10.1097/ftd.0000000000000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7(12):1816–1823. doi: 10.1200/jco.1989.7.12.1816 [DOI] [PubMed] [Google Scholar]
- 35.Schmiegelow K, Schroder H, Gustafsson G, et al. Risk of relapse in childhood acute lymphoblastic leukemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. Nordic Society for Pediatric Hematology and Oncology. J Clin Oncol. 1995;13(2):345–351. doi: 10.1200/jco.1995.13.2.345 [DOI] [PubMed] [Google Scholar]
- 36.Mittelman SD, Kim J, Raca G, Li G, Oberley MJ, Orgel E. Increased prevalence of CRLF2 rearrangements in obesity-associated acute lymphoblastic leukemia. Blood. 2021;138(2):199–202. doi: 10.1182/blood.2021011106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. doi: 10.1182/blood-2009-09-245944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo JC, Maring B, Chandra M, et al. Prevalence of obesity and extreme obesity in children aged 3–5 years. Pediatr Obes. 2014;9(3):167–175. doi: 10.1111/j.2047-6310.2013.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. JAMA. 2001;286(22):2845–2848. doi: 10.1001/jama.286.22.2845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


